Abstract
Violence exposure during childhood is common and associated with poor cognitive and academic functioning. However, little is known about how violence exposure influences cognitive processes that might contribute to these disparities, such as working memory, or their neural underpinnings, particularly for cognitive processes that occur in emotionally salient contexts. We address this gap in a sample of 54 participants aged 8 to 19 years (50% female), half with exposure to interpersonal violence. Participants completed a delayed match to sample task for emotional faces while undergoing functional magnetic resonance imaging scanning. Violence-exposed youth performed worse than controls on happy and neutral, but not angry, trials. In whole-brain analysis, violence-exposed youth had reduced activation in the left middle frontal gyrus and right intraparietal sulcus during encoding and the left superior temporal sulcus and temporal–parietal junction during retrieval compared to control youth. Reduced activation in the left middle frontal gyrus during encoding and the left superior temporal sulcus during retrieval mediated the association between violence exposure and task performance. Violence exposure influences the frontoparietal network that supports working memory as well as regions involved in facial processing during working memory for emotional stimuli. Reduced neural recruitment in these regions may explain atypical patterns of cognitive processing seen among violence-exposed youth, particularly within emotional contexts.
More than one in five children in the United States will be exposed to violence by the time they reach adulthood (Finkelhor, Ormrod, & Turner, 2009; McLaughlin et al., 2013). Exposure to violence is associated with myriad negative outcomes across the life span, including psychopathology (McLaughlin et al., 2012), social difficulties (Shonk & Cicchetti, 2001), and poor academic functioning (Holt, Finkelhor, & Kantor, 2007). Most prior research has focused on disruptions in emotional processing as a possible mechanism linking violence exposure to downstream negative outcomes (Hanson et al., 2010; McLaughlin, Busso, et al., 2014). Less is known about how violence exposure influences cognitive processes. Animal models demonstrate that experiences of stress influence prefrontal cortex (PFC) structure and function (Lupien, McEwen, Gunnar, & Heim, 2009), and several studies have observed alterations in PFC structure among children who have experienced violence (Gold et al., 2016; Hanson et al., 2010). Considerably less research has examined the link between violence exposure and PFC function, particularly within emotionally salient contexts. Here, we investigate how exposure to violence in childhood influences neural systems supporting working memory (WM), a core cognitive process that underlies executive functions and relies heavily on PFC circuitry, with a task examining memory for emotional facial expressions.
WM is a core domain of executive functioning that is involved in many other higher order cognitive processes, including inhibition, planning, problem solving, and rule-based learning (Barrett, Tugade, & Engle, 2004; Miyake & Friedman, 2012). Conceptual models of the neurodevelopmental consequences of childhood adversity posit that experiences involving deprivation, or an absence of cognitive stimulation and enrichment in the early environment, should have particularly pronounced effects on cognitive development, including executive functioning and WM, and the frontoparietal networks that support these processes (McLaughlin, Sheridan, & Lambert, 2014; Sheridan & McLaughlin, 2014). Behavioral studies support these predictions and have consistently found disruptions in WM for nonemotional information among individuals who have experienced forms of childhood adversity involving deprivation, such as institutional rearing (Tibu et al., 2016), poverty (Lipina et al., 2013), and low family socioeconomic status (SES; Sarsour et al., 2011). These behavioral differences are likely mediated by atypical development of neural structure and function following deprivation. Deprivation-related experiences, including poverty and institutional rearing, are associated with widespread reductions in cortical thickness and surface area (Mackey et al., 2015; McLaughlin, Sheridan, Winter, et al., 2014; Noble et al., 2015); these reductions in cortical surface area and volume mediate the association between SES and performance on WM tasks (Noble et al., 2015). Functionally, deprivation related to low SES is associated with atypical recruitment in frontoparietal networks during WM tasks, including the middle frontal gyrus (MFG) and intraparietal sulcus (IPS; Finn et al., 2016; Sheridan, Peverill, Finn, & McLaughlin, 2017). In one of these studies, associations of SES with WM were observed after adjusting for violence exposure (Sheridan et al., 2017). It is critical that these behavioral and magnetic resonance imaging (MRI) studies have all focused on “cold” cognitive, or nonemotional, forms of WM.
In contrast, studies of childhood experiences of threat (i.e., violence) and WM have produced mixed findings, with some studies finding poor WM ability following violence exposure after adjusting for co-occurring deprivation (DePrince, Weinzierl, & Combs, 2009; Gould et al., 2012; Vasilevski & Tucker, 2016), others reporting associations that fail to control for deprivation experiences known to be strongly linked to WM (Augusti & Melinder, 2013), and some finding no association between violence exposure and WM (Twamley, Hami, & Stein, 2004). Given the high degree of overlap between experiences of threat and deprivation (McLaughlin, Sheridan, & Lambert, 2014), studies measuring and accounting for these exposures are necessary to better characterize the distinct and overlapping associations with WM. For example, a recent study from our lab found that parental education is strongly associated with spatial WM and superior parietal cortex recruitment during encoding after controlling for violence exposure, but violence exposure exhibits no association with WM performance or neural recruitment after adjusting for parental education (Sheridan et al., 2017).
One possible explanation for these discrepancies is that violence exposure primarily influences WM performance in the context of emotional information, particularly in the presence of emotional cues that signal potential threat. This is consistent with theoretical conceptualizations of violence exposure as representing an environmental threat that exists along a continuum from witnessing violence to being directly victimized (McLaughlin, Sheridan, & Lambert, 2014; Sheridan & McLaughlin, 2014); experiences along this continuum should have strong influences on social information processing in the presence of salient emotional cues, particularly those involving potential threat (McLaughlin & Lambert, 2016). Studies have consistently shown that children who have experienced or witnessed violence exhibit preferential attention and heightened perception to socially threatening cues, such as angry faces (Pollak, Cicchetti, Hornung, & Reed, 2000; Pollak & Tolley-Schell, 2003; Swartz, Graham-Bermann, Mogg, Bradley, & Monk, 2011) and a variety of other information processing biases that facilitate the rapid identification of threat-related information in the environment (Lambert et al., 2017; McLaughlin & Lambert, 2016). Thus, WM might be particularly influenced by violence exposure when applied in the context of emotionally salient cues, particularly threat cues (i.e., angry faces). However, it is unclear whether information processing biases for threatening information would facilitate or disrupt WM performance among youth who have experienced violence. One possibility is that violence exposure would produce heightened memory for threat-related stimuli. Consistent with this, a recent study shows that adults exposed to violence as children have WM deficits for happy, but not angry, faces (Cromheeke, Herpoel, & Mueller, 2014). Alternatively, heightened emotional reactivity to threat cues and generalization of threat responses to other stimulus types (e.g., neutral cues) is well documented in children exposed to violence (Lambert et al., 2017; McLaughlin & Lambert, 2016; McLaughlin, Peverill, Gold, Alves, & Sheridan, 2015), which could interfere with WM for threat-related stimuli or emotional stimuli more broadly. To our knowledge, no research has examined WM for emotionally salient stimuli, or the neural systems that support this process, among children who have experienced violence.
We do so in the present study. We investigate whether childhood violence exposure influences behavioral performance and neural activation during an emotional WM task. We examined this question by adapting a delayed match to sample WM task for emotional faces previously used in studies of adults (Braunlich, Gomez-Lavin, & Seger, 2015; Lo-Presti et al., 2008). We expected that children and adolescents who experienced violence would perform worse on the WM task compared to youth without violence exposure, particularly when the emotional expression was neutral or happy. In contrast, based on work showing enhanced attention to threatening information among violence-exposed youths (Pollak et al., 2000; Pollak & Tolley-Schell, 2003), we anticipated that youth who experienced violence would perform as well as children who had never experienced violence on trials involving angry faces. In addition, we expected that violence exposure would be associated with reduced activation during encoding in areas that support WM, including the MFG and IPS, specifically on happy and neutral trials, and that these neural differences would be a mechanism linking violence exposure to poor task performance on happy and neutral trials. Finally, we evaluated whether associations between violence exposure and WM persisted after controlling for family SES, as measured by parental education, and youth psychopathology.
Method
Participants
A sample of 66 participants aged 6 to 19 years (M = 13.58 years, SD = 3.25 years; 32 male) without MRI contraindications (e.g., orthodontic braces) participated. The sample was recruited from schools, after-school and prevention programs, medical clinics, and the general community in Seattle, Washington, between February 2014 and February 2015. Recruitment efforts aimed to recruit a sample with variation in violence exposure. To do so, we recruited from neighborhoods with high levels of violent crime, clinics that serve a predominantly low-SES catchment area, and agencies that work with families who have experienced violence (e.g., domestic violence shelters and programs for parents mandated to receive intervention by Child Protective Services). Approximately half (n = 32) were exposed to violence and half (n = 34) were gender- and age-matched controls. Participants in the control group had no violence exposure but were not excluded for exposure to other forms of trauma, such as accidents and injuries.
Eight participants (3 male, mean age: 10.23, SD = 3.26) were excluded from analyses due to below chance performance (i.e., <50% accuracy). One participant (female, 15 years) was excluded due to an incidental finding, and 3 participants (2 male, mean age: 8.57, SD = 2.09) did not complete the task in the scanner. The final analytic sample included 54 participants (n = 26 with violence exposure). See Table 1 for the sociodemographic characteristics of the final sample as a function of violence exposure.
Table 1.
Distribution of sociodemographics and psychopathology by violence exposure
| Violence Exposed (n = 26) | Controls (n = 28) | |||||
|---|---|---|---|---|---|---|
| % | n | % | n | χ2 | p | |
| Female | 50.0 | 13 | 46.4 | 13 | 0.07 | .79 |
| Race/ethnicity | 7.45 | .11 | ||||
| White | 50.0 | 13 | 71.4 | 20 | ||
| Black | 19.2 | 5 | 0.0 | 0 | ||
| Latino | 19.2 | 5 | 10.7 | 3 | ||
| Asian/Pacific Islander | 7.7 | 2 | 10.7 | 3 | ||
| Biracial/other | 3.8 | 1 | 7.1 | 2 | ||
| Parent education | 17.91 | <.001 | ||||
| High school or less | 56.0 | 14 | 3.8 | 1 | ||
| Some college | 12.0 | 3 | 11.5 | 3 | ||
| College degree | 12.0 | 3 | 38.5 | 10 | ||
| Graduate degree | 20.0 | 5 | 46.2 | 12 | ||
| M | SD | M | SD | t | p | |
| Age | 14.64 | 2.69 | 13.90 | 2.95 | −0.96 | .34 |
| Internalizing symptoms | 55.36 | 10.52 | 45.29 | 11.41 | −3.33 | .002 |
| Externalizing symptoms | 54.68 | 10.99 | 43.71 | 10.85 | −3.65 | .001 |
Note: Internalizing and externalizing symptoms were measured by the Youth Self-Report of the Child Behavior Checklist.
Procedure
The Institutional Review Board at the University of Washington approved all procedures. Participants were compensated, and written informed consent was obtained from legal guardians, while youths provided written assent.
Measures
Violence exposure.
Violence exposure was defined using the DSM-5 (American Psychiatric Association, 2013) definition of trauma and included physical or sexual abuse, witnessing chronic domestic violence, and direct exposure to other violence (e.g., physically assaulted by a stranger). Violence exposure was assessed with the Childhood Experiences of Care and Abuse (CECA) interview (Bifulco, Brown, Lillie, & Jarvis, 1997), the Childhood Trauma Questionnaire (CTQ; Bernstein, Ahluvalia, Pogge, & Handelsman, 1997), and the University of California at Los Angeles Post-Traumatic Stress Disorder Reaction Index (PTSD-RI) trauma screen (Steinberg, Brymer, Decker, & Pynoos, 2004). The CECA assesses care-giving experiences, including exposure to physical and sexual abuse (i.e., coded as present or absent); we modified the interview to ask additional questions about witnessing domestic violence (i.e., directly observing violence directed at a care-giver). Interrater reliability for reports of violence exposure is excellent, and validation studies suggest high agreement between siblings on reports of violence (Bifulco et al., 1997). The CTQ is a 28-item scale that assesses the frequency of childhood physical and sexual violence and has good convergent and discriminant validity (Bernstein et al., 1997) and demonstrated good reliability within the present sample (α = 0.83). The child- and parent-report versions of the 13-item PTSD-RI trauma screen assessed for other violence exposure (e.g., victimization outside the home).
We used the CECA, CTQ, and PTSD-RI trauma screen to create a dichotomous indicator of violence exposure as our primary independent variable. Participants who reported physical abuse, sexual abuse, witnessing more than two incidents of domestic violence, directly experiencing other violence, or who had a score on the CTQ physical or sexual abuse subscales above a validated threshold (Walker et al., 1999) were classified as exposed to violence. We additionally created continuous indicators of maltreatment frequency using the CTQ physical and sexual abuse subscales (i.e., how often each participant experienced abuse-related violence) and a violence severity score reflecting the number of discrete types of violence exposure each participant experienced.
Psychopathology.
Internalizing and externalizing symptoms were reported by youth on the Youth Self Report of the Child Behavior Checklist (Achenbach, 1991). The Child Behavior Checklist scales are among the most widely used measures of youth emotional and behavioral problems and use extensive normative data to generate age-standardized estimates of symptom severity.
WM task.
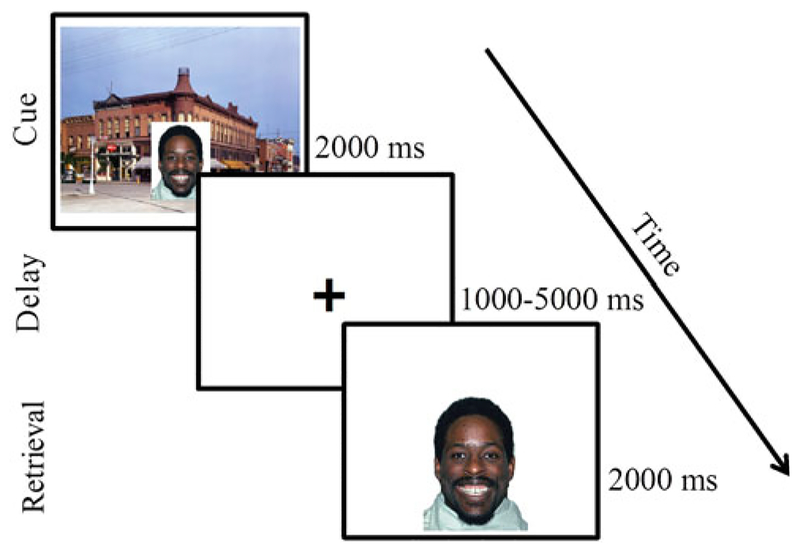
Participants completed a WM task (Figure 1) using a delayed match to sample design with emotional faces. This design is similar to studies examining WM for faces in adults (LoPresti et al., 2008). All stimuli were faces drawn from a standardized stimulus set (Tottenham et al., 2009). Stimuli were neutral, happy, and angry faces, distributed evenly across trials and presented in a counterbalanced order across participants. Participants were instructed to encode the faces and their emotional expressions.
Figure 1.
(Color online) Delayed match to sample emotional working memory task. Participants were presented with a face embedded in a realistic scene and instructed to hold the face in memory over a variable delay period. Participants were told to indicate “yes” or “no” with a button press whether the cue matched the probe on both emotional expression and identity.
The task consisted of two runs of 50 trials. Each trial involved an encoding (2000 ms), delay (1000–5000 ms), and retrieval (2000 ms) phase and an intertrial interval (ITI) of 500 ms (67% of trials) or 2000 ms (33% of trials). Each actor was presented 6–7 times for each facial expression. During encoding, facial stimuli were embedded in realistic background scenes to make encoding more similar to real-world facial encoding and to allow us to examine context encoding for a separate study that involved a memory test for implicitly encoded contextual information outside of the scanner (Lambert et al., 2017). During the retrieval phase, an image of a face without a background scene was presented (probe), and participants were asked to indicate whether the probe face was identical to the encoding face in terms of both identity and emotional expression. On one-third of trials, the probe face presented matched the encoding face (i.e., was the same actor showing the same emotion) and on the other two-thirds of trials, the probe did not match the encoding face in either identity (1/3) or emotion (1/3). Subjects completed two runs of the task, with the exception of one subject who completed only one run.
Image acquisition and processing
Before undergoing scanning, children 12 years and younger and any older children exhibiting anxiety about the scan were trained to minimize head movements in a mock scanner. They watched a movie with a head-mounted motion tracker that stopped playing if a movement of over 2 mm occurred. This method has been shown to significantly reduce head motion once children are in the scanner (Raschle et al., 2012). In addition, in the scanner we used an inflatable head-stabilizing pillow to restrict movement.
Scanning was performed on a 3T Phillips Achieva scanner at the University of Washington Integrated Brain Imaging Center using a 32-channel head coil. T1-weighted multiecho MPRAGE volumes were acquired (repetition time = 2530 ms, echo time s, = 1640–7040 μs, flip angle = 7 degrees, field of view = 256 mm2, 176 slices, in-plane voxel size = 1 mm3). Blood oxygenation level dependent (BOLD) signal during functional runs was acquired using a gradient-echo T2*-weighted EPI sequence. Thirty-two 3-mm thick slices were acquired parallel to the AC-PC line (repetition time = 2000 ms, echo time = 30 ms, flip angle = 90 degrees, band-width = 2300, echo spacing = 0.5, field of view = 256×256, matrix size = 64×64). Prior to each scan, four images were acquired and discarded to allow longitudinal magnetization to reach equilibrium.
Functional MRI (fMRI) preprocessing.
Preprocessing and statistical analysis of fMRI data was performed in a pipeline using Make, a software development tool designed for building executables from source files that can be used to create neuroimaging workflows that rely on multiple software packages (Askren et al., 2016). Simultaneous motion and slice-time correction was performed in NiPy (Roche, 2011). Spatial smoothing with a Gaussian kernel (6-mm full width at half-maximum) was performed in FSL (Jenkinson, Beck-mann, Behrens, Woolrich, & Smith, 2012). Data were inspected for artifacts, and volumes with motion >2 mm or >3 SD change in signal intensity were excluded from analysis using volume-specific covariates of no interest. Six rigid-body motion regressors were included in person-level models. All but two subjects (one male, 12 years and one female, 9 years) had very little motion; those with the highest motion had fewer than 10% of volumes with framewise displacement outliers across both runs, with the next highest being 3.6% of volumes. A component-based anatomical noise correction method (Behzadi, Restom, Liau, & Liu, 2007) was used to reduce noise associated with physiological fluctuations. Person- and group-level models were estimated in FSL. Following estimation of person-level models, the resulting contrast images were normalized into standard space, and anatomical coregistration of the functional data with each participant’s T1-weighted image was performed using surface-based registration in FreeSurfer version 5.3 (Dale, Fischl, & Sereno, 1999), which provides better alignment than other methods in children (Ghosh et al., 2010). Normalization was implemented in Advanced Normalization Tools software, version 2.1.0 (Avants et al., 2011).
Statistical analysis
WM performance.
Behavioral performance on the emotional WM task was assessed using d′, which was calculated using the following formula:
where z is the standardized score as a measure of the sensitivity to detect mismatches. To examine WM performance as a function of emotion type and violence exposure, we conducted a 3×2 repeated measures analysis of variance (ANOVA) with emotion (angry, happy, neutral) as a within-subjects factor and group (violence exposed, control) as a between-subjects factor with d′ serving as the dependent variable.
fMRI.
FMRI data processing was performed using FEAT (FMRI Expert Analysis Tool) Version 6.00, part of FSL (FMRIB’s Software Library, http://www.fmrib.ox.ac.uk/fsl). Regressors were created by convolving a boxcar function of phase duration with the standard double-gamma hemodynamic response function for each phase of the task (encoding, delay, and retrieval). A general linear model was constructed for each participant. Higher level analysis was carried out using FLAME1 (FMRIB’s Local Analysis of Mixed Effects; Woolrich, 2008). Individual-level estimates of BOLD activity were submitted to group-level random effects models of encoding, delay, and retrieval periods, each compared to baseline (ITI). Whole-brain analyses were conducted using only correct trials. Based on recent simulation work suggesting cluster-level correction in widely used fMRI software packages leads to increased probability of false-positive results (Eklund, Nichols, & Knutsson, 2016), we applied a conservative approach to cluster-level correction available that does not elevate risk of false positive findings in recent simulations, while also not being overly conservative and producing false negatives in event-related designs (see Eklund et al., 2016). Specifically, we applied cluster-level correction in FSL (z > 2.3, p <.01) to our models run in FSL FLAME. We examined differences in BOLD response during contrasts of interest as a function of violence exposure in whole-brain analysis. Results were then projected onto the cortical surface for visualization purposes using Connectome Workbench (Marcus et al., 2013).
Finally, to determine whether group differences varied across the stimulus emotion type we conducted a repeated-measures ANOVA examining an Emotion (angry, happy, neutral)×Group (violence exposed, control) interaction. Because FSL does not have the functionality to perform a within-subjects ANOVA, we conducted this analysis in AFNI using the 3dLME function. Preprocessed individual-level contrasts were converted for use in AFNI, and the results from 3dLME were cluster-corrected using the 3dClustSim tool in AFNI.
Region of interest (ROI) analysis.
ROI analyses examining brain–behavior associations were conducted on all trials to ensure behavioral variability. ROIs were created by masking functional activation during a contrast of interest (e.g., encoding > ITI) in the entire sample with an anatomical mask from the Harvard–Oxford cortical atlas in FSL. We created ROIs for three regions that were recruited during the task across the entire sample, including the MFG and IPS, which have been previously shown to be recruited during WM and to correlate with WM performance (Curtis & D’Esposito, 2003; Soto, Rotshtein, & Kanai, 2014), and STS due to associations with facial processing and social cognition (Hein & Knight, 2008). Parameter estimates were extracted for these ROIs for each participant during the encoding, delay, and retrieval periods. We conducted linear regression to determine whether the frequency or severity of violence exposure was associated with BOLD signal in those regions; we adjusted for age as analyses with continuous measures of violence exposure were not group matched for age. In addition, we examined whether activation in these ROIs explained the association between violence exposure and d′ using standard tests of statistical mediation. We tested the significance of indirect effects using a bootstrapping approach that provides confidence intervals for the indirect effect (Hayes, 2013).
Sensitivity analyses.
We conducted sensitivity analyses for each behavioral, whole-brain, and ROI analysis controlling for SES (highest parent educational attainment) and current internalizing and externalizing psychopathology symptoms to evaluate whether associations of violence exposure with neural recruitment and task performance were the result of confounding by SES or psychopathology.
Results
WM performance
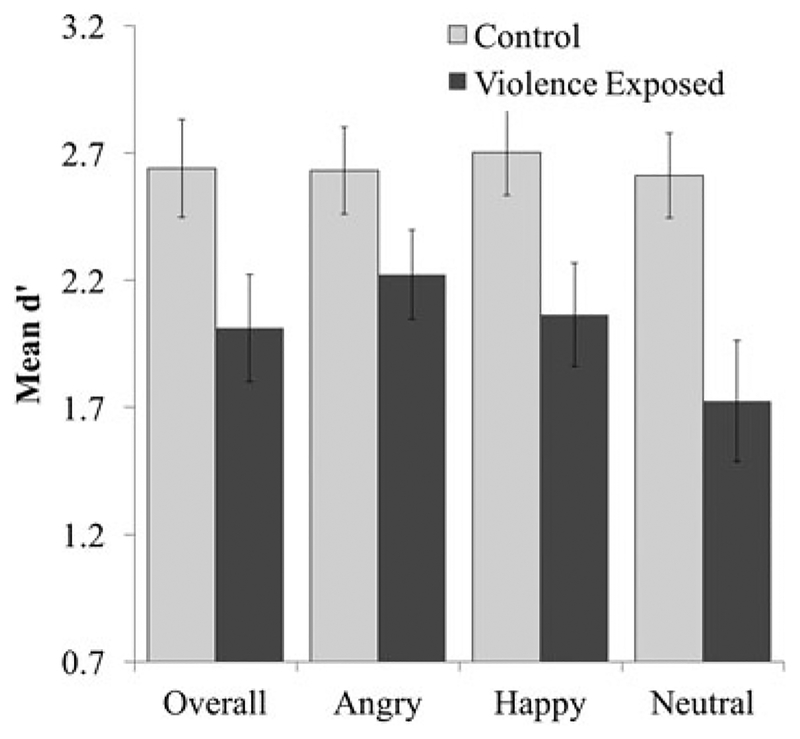
We found main effects of both emotion, F (2, 104) = 4.83, p = .01, partial η2 = 0.08, and group, F (1, 52) = 6.37, p = .02, partial η2 = 0.12, on WM performance. Specifically, performance was lower on neutral trials than happy (p = .003) and angry (p = .02) trials, and violence-exposed participants performed worse overall compared to those without violence exposure (Table 2). However, these main effects were qualified by a significant Emotion×Group interaction, F (2, 104) = 3.46, p = .04, partial η2= 0.06 (Figure 2), whereby violence-exposed participants performed worse than participants with no history of violence exposure on happy (p = .02, Cohen d = 0.66) and neutral (p = .003, Cohen d = 0.83) trials, but only performed marginally worse on angry trials (p = .10, Cohen d = 0.46). When comparing differences in performance across emotion trials within each group, youth with no violence exposure showed no differences in performances across emotion type (ps > .43, Cohen ds < 0.10) whereas violence-exposed youth demonstrated relatively worse performance on neutral compared to angry (p = .001, Cohen d = 0.46) and happy (p = .007, Cohen d = 0.30) trials.
Table 2.
Working memory performance overall and across emotion type in the total sample and as a function of group
| Overall | Angry | Happy | Neutral | |||||
|---|---|---|---|---|---|---|---|---|
| M | SD | M | SD | M | SD | M | SD | |
| Total sample | 2.34 | 1.08 | 2.43 | 0.91 | 2.40 | 1.01 | 2.18 | 1.14 |
| Violence exposed | 2.01 | 1.07 | 2.22 | 0.89 | 2.07 | 1.04 | 1.73 | 1.21 |
| Controls | 2.64 | 1.01 | 2.63 | 0.90 | 2.70 | 0.89 | 2.61 | 0.88 |
Figure 2.
Delayed match to sample emotional working memory task performance. There was a significant Emotion×Group interaction whereby violence exposed youth performed worse (d’) on happy and neutral, but not angry, trials compared to control youth. Error bars represent standard error.
Neural recruitment
Whole-brain neural recruitment in full sample.
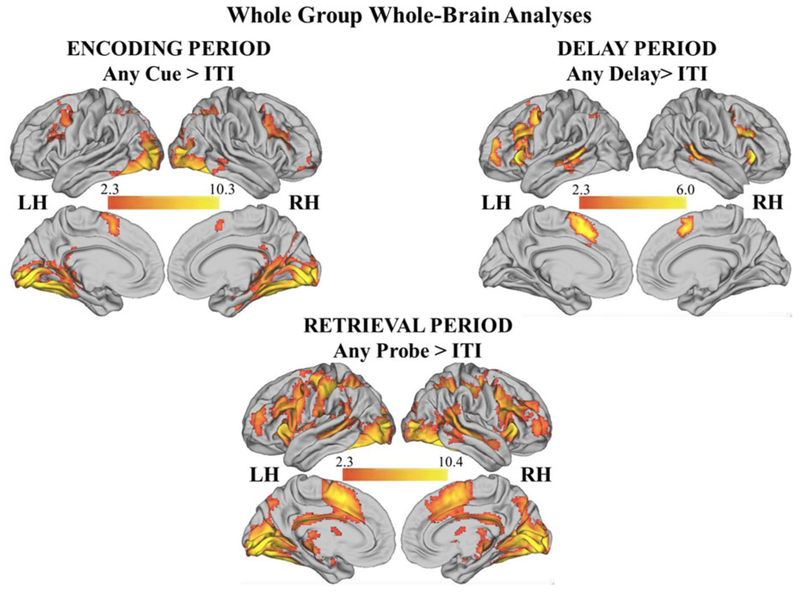
To examine task-related BOLD activation, we performed whole-brain general linear model analyses in the entire sample for the encoding, delay, and retrieval periods, each compared to ITI (see online-only supplemental Table S.1 and Figure 3).
Figure 3.
(Color online) Significant clusters during the delayed match to sample emotional working memory task in the entire sample during encoding, delay, and retrieval periods. See online-only supplementary Table S.1 for details.
Encoding.
The contrast of encoding > ITI showed activation in the frontoparietal cortex including the bilateral MFG/inferior frontal sulcus and IPS. This analysis also revealed bilateral activation in the dorsal anterior cingulate cortex, striate and extrastriate cortex, and inferior temporal cortex, including the fusiform and parahippocampal gyri. Finally, this contrast revealed activation bilaterally in the posterior hippocampal cortex, which is consistent with encoding of spatially complex information.
Delay.
The contrast of delay > ITI demonstrated activation in the prefrontal cortex including the MFG/inferior frontal sulcus, anterior insula, frontal pole, and dorsal anterior cingulate cortex. In addition, it revealed activation within the bilateral STS and anterior IPS.
Retrieval.
The contrast of probe > ITI revealed activation bilaterally in the MFG/inferior frontal sulcus, IPS, frontal pole, anterior cingulate cortex, anterior insula, posterior cingulate cortex, precuneus, striate cortex, and inferior temporal cortex, including the fusiform gyrus and STS. Subcortically, this contrast revealed significant recruitment of the thalamus, caudate, putamen, pallidum, and cerebellum.
Violence exposure and neural recruitment.
The omnibus test for Emotion × Group revealed no significant clusters in whole-brain analysis. As such, all remaining analyses were conducted in FSL as previously described and examined group differences collapsed across emotion type.
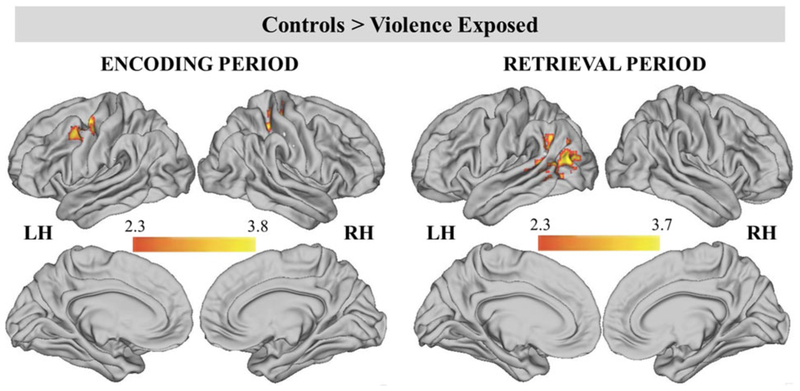
During encoding, participants with no history of violence exposure had greater BOLD signal than children with violence exposure in two clusters (Table 3; Figure 4). These included the left precentral gyrus/MFG and right postcentral gyrus/IPS extending into the right precentral gyrus. BOLD activation in the left MFG was negatively associated with violence severity (β = −0.30, p = .04), but not frequency of exposure to violence (β = −0.18, p = .22). BOLD activation in the right IPS was marginally negatively associated with violence severity (β = −0.27, p = .06), but not frequency of exposure to violence (β = −0.21, p = .14). No group differences were found during the delay period.
Table 3.
Whole-brain analysis by group (control > violence exposed)
| Anatomical Region | Brodmann Area | x | y | z | Voxels | z Max | p |
| Encoding Period | |||||||
| Right postcentral gyrus/intraparietal sulcus | 4 | 28 | −28 | 62 | 161 | 3.60 | .003 |
| Right precentral gyrus | NA | 22 | −22 | 64 | |||
| Left precentral gyrus/middle frontal gyrus | 6 | −42 | −6 | 56 | 252 | 3.84 | <.0001 |
| Retrieval Period | |||||||
| Left lateral occipital cortex/temporal parietal junction | 19 | −52 | −72 | 12 | 700 | 3.71 | <.0001 |
| Superior temporal sulcus | 39 | −56 | −58 | 14 | |||
| Angular gyrus | 39 | −60 | −58 | 22 | |||
Note: Significantly different clusters activated during encoding (any facial cue > ITI) and retrieval (any facial probe > ITI) periods for participants exposed to violence versus control participants. Cluster-level correction applied in FSL, z > 2.3 was the primary threshold, and p <.01 was the cluster-level threshold.
Figure 4.
(Color online) Significant clusters during encoding and retrieval periods for the contrast of controls > violence exposed. Cluster-level correction applied in FSL, z > 2.3 was the primary threshold, and p < .01 was the cluster-level threshold.
During the retrieval period, control participants exhibited greater BOLD signal than violence-exposed children in one large cluster that encompassed the left lateral occipital cortex/temporal parietal junction (TPJ), left STS, and left angular gyrus. Both violence frequency (β = −0.31, p = .02) and severity (β = −0.45, p = .001) were negatively associated with left STS BOLD activation.
Violence-exposed participants did not exhibit greater BOLD signal than control participants in any clusters across encoding, delay, and retrieval periods.
Brain–behavior associations.
We conducted ROI analyses to investigate whether the associations between violence exposure and emotional WM performance were mediated by reduced BOLD signal in the left MFG and right IPS during encoding and left STS during the retrieval period (i.e., regions that were significantly task active in the entire sample and demonstrated group differences as a function of violence exposure in whole-brain analysis). Only regions that were task active in the entire sample were examined, as ROIs were defined by masking the contrast of interest in the full sample with a structural mask to avoid “double-dipping” by defining ROIs solely based on whole-brain group differences (Vul, Harris, Winkielman, & Pashler, 2009; see Methods section for details on ROI definition). While all three ROIs were initially examined simultaneously within the mediation model, the right IPS during encoding did not significantly mediate the association between violence exposure and WM performance and was removed from the model. In the final model, violence exposure predicted left MFG BOLD signal during encoding (b = −0.49, p = .02), which in turn predicted left STS BOLD signal during retrieval (b = 0.51, p = .002). Higher activation in both the left MFG during encoding (b = .33, p = .10) and the left STS during retrieval (b = 0.28, p = .11) were marginally associated with WM performance (overall d′), and the association between violence exposure and WM performance was reduced to nonsignificance in the final model (b = −0.34, p = .24). The indirect effect of violence exposure on WM performance through reduced BOLD signal was significant for the left MFG during encoding and the left STS during the retrieval period: indirect effect = −0.07, 95% confidence interval [−0.22, −0.008].
Sensitivity analyses
We conducted sensitivity analyses controlling for highest parental education and internalizing and externalizing symptoms across behavioral, whole-brain group comparisons, and brain–behavior analyses. The Emotion×Group interaction in predicting WM performance remained significant after adjustment for potential confounders, F (2, 90) = 3.22, p < .05.
Group differences (controls > violence-exposed) in the left MFG and right IPS during encoding were no longer significant in whole-brain analyses after controls for family SES and psychopathology were included, although a significant cluster emerged in the left IPS (see online-only supplementary Table S.2 and Figure S.1). Violence severity was no longer associated with left MFG recruitment during encoding (β = −0.13, p = .42).
The results were largely unchanged during the retrieval period, with a large cluster encompassing the TPJ and STS persisting, and additional clusters in the right MFG and right IPS emerging. The association between violence frequency and left STS recruitment during retrieval reduced to nonsignificance once controlling for confounders (β = −0.22, p = .18), while the association of violence severity with the left STS remained (β = −0.30, p = .04). Finally the indirect effect of violence exposure on WM performance through reduced BOLD signal in the left MFG during encoding and the left STS during retrieval remained marginally significant: indirect effect = −0.05, 90% confidence interval [−0.20, −0.0004]. Together, these findings indicate that family SES and co-occurring psychopathology are not fully explaining the associations of violence exposure with WM performance or neural recruitment.
Discussion
Little research has examined how violence exposure influences neural recruitment during WM, particularly for emotional stimuli. Here, we show that children who have been exposed to violence have worse WM for happy and neutral faces than youth who have never experienced violence, but exhibit similar WM ability for angry faces. Violence exposure was associated with reduced neural recruitment in regions known to be involved in WM during encoding, including the MFG and IPS, as well as regions involved in facial processing and social cognition during retrieval, including the TPJ and STS, independent of stimulus emotion type. Reduced BOLD signal in the MFG and STS mediated the association between violence exposure and poor WM performance.
WM behavioral performance varied as a function of violence exposure and emotion type. Specifically, children who experienced violence performed worse on happy and neutral trials, but relatively better on angry trials, compared to control youth. These effects were medium to large (Cohen ds = 0.66–0.83) and robust when controlling for internalizing and externalizing psychopathology and SES, suggesting that patterns in behavioral performance were not solely due to confounders known to be associated with violence exposure and WM. In addition, we found that violence-exposed youth performed relatively worse on neutral trials compared to angry and happy trials, though these effects were small (Cohen ds = 0.30–0.46). While caution is needed when interpreting null findings, these behavioral results are consistent with extensive evidence documenting patterns of preferential information processing and heightened salience of threat cues among children who have experienced violence (see McLaughlin, 2016; McLaughlin & Lambert, 2016, for a review). Such patterns are likely adaptive for children being raised in environments characterized by legitimate danger. Exposure to violence may increase the salience of anger and facilitate information processing for angry faces due to the relevance of anger in others as a signal of potential threat in the environment. Much of the existing work on information processing in youth exposed to violence has focused on perceptual processing of facial emotion and attention (Pollak & Tolley-Schell, 2003; Shackman, Shackman, & Pollak, 2007) with little work examining WM specifically. Here we show that this attentional bias can influence other forms of information processing, including WM. This pattern is also broadly consistent with findings of poor spatial WM for happy, but not angry, faces in adult women with a history of child abuse (Cromheeke et al., 2014). Of note, Cromheeke et al. contrasted responses to happy and angry faces with neutral faces (i.e., women with an abuse history performed worse on happy compared to neutral trials), whereas our findings show better WM performance for happy and angry faces when compared to neutral faces among violence-exposed youth. It is possible the discrepant findings are due to the tendency for youth to perceive neutral faces as more emotionally ambiguous than adults (Marusak, Zundel, Brown, Rabinak, & Thomason, 2016; Thomas et al., 2001). This emotional ambiguity might have resulted in greater cognitive effort being required to determine the emotional expression on neutral faces, interfering with memory performance. Greater research is needed to explore how emotional WM varies across development following exposure to violence and other forms of adversity.
Youth with a history of violence exposure showed reduced neural activation relative to controls in regions associated with WM including the left MFG and right IPS during encoding. Furthermore, reduced activation in the left MFG during encoding mediated the association between violence exposure and poor WM performance. Group differences during encoding remained significant in the IPS even after controlling for family SES and the presence of internalizing and externalizing psychopathology. The MFG and IPS are integral to the control processes necessary for WM, such as encoding and maintaining representations of visual stimuli (Curtis & D’Esposito, 2003), and increases in MFG and IPS activation are associated with improved WM performance across development (Geier, Garver, Terwilliger, & Luna, 2009; Scherf, Sweeney, & Luna, 2006). Conceptual models highlight the potential harmful effects of stress on PFC development and function (Lupien et al., 2009), which has been posited to contribute to impairment in academic and cognitive functioning following violence exposure (DePrince et al., 2009; Holt et al., 2007). Yet, few studies have examined these assertions empirically in developmental studies of humans. The pattern of findings in the present study supports the theory that violence exposure influences neural systems underlying WM when the stimuli to be remembered are emotional in nature and that reductions in neural recruitment in frontoparietal regions account for emotional WM deficits associated with violence exposure.
Reduced neural recruitment in regions associated with facial processing and social cognition during the retrieval period, including the left STS, was observed in youth with a history of violence exposure relative to controls. These differences were unchanged when controlling for psychopathology and SES. Furthermore, reduced activation in the left STS during the retrieval period mediated the association between violence exposure and WM performance. The STS is associated with facial processing and social cognition (Deen, Koldewyn, Kanwisher, & Saxe, 2015; Hein & Knight, 2008), and greater STS recruitment has been specifically linked to the processing of changeable facial features, such as emotional expressions (Haxby, Hoffman, & Gobbini, 2000). The ability to perceive and remember facial expressions is thought to facilitate social communication (Haxby, Hoffman, & Gobbini, 2002) and related neural systems (i.e., STS), and behavioral responses are likely impacted by interpersonal stress (Nolte et al., 2013). For example, Nolte et al. observed reduced activation in the left STS and subsequent disruptions in social cognitive ability (specifically identifying mental state when only observing an individual’s eyes) following an interpersonal stress induction in adults. This is consistent with behavioral studies showing delayed social cognition development among maltreated children (Cicchetti, Rogosch, Maughan, Toth, & Bruce, 2003). The present study extends these findings by highlighting reduced STS recruitment as a potential mechanism linking violence exposure to poorer WM for emotional faces.
Although we found an Emotion×Group interaction predicting WM performance, we did not find a similar interaction in neural response across emotion types. Given our relatively small sample size, this may be a result of lack of power and should be interpreted with caution. Alternatively, this pattern could suggest a general reduction in neural recruitment during WM as opposed to reduced recruitment specifically in response to emotional WM. Future research examining neural responses during emotional and nonemotional WM among larger samples of children with and without violence exposure are needed before firm conclusions can be drawn about whether recruitment of neural systems underlying WM vary as a function of violence exposure differentially across various emotional stimuli.
The limitations of the current study include, first, a cross-sectional design that does not allow us to determine the temporal direction between reductions in neural recruitment and disruptions in emotional WM ability. Second, as noted above, the relatively small sample size may have impacted the ability to find group differences in neural recruitment as a function of emotion type. Given that behavioral performance varied as a function of emotion type in the violence-exposed group, it would be useful to study these processes among larger samples of children and adolescents and with nonemotional stimuli to better compare neural recruitment in emotional versus nonemotional contexts. Third, we did not adjust for intelligence (IQ) within our models. While some previous studies in the WM literature have included a measure of intelligence as a covariate, more recently it has been demonstrated that including IQ as a covariate in cognitive research is inappropriate and leads to anomalous findings due to the high degree of statistical overlap between IQ and other types of cognition (Dennis et al., 2009), including WM. It is hard to imagine what the construct of WM even represents after removing variance associated with IQ (Miller & Chapman, 2001). Fourth, while the control and violence-exposed groups did not statistically differ on racial/ethnic makeup, the control group did not include any African American youth, which could limit generalizability of our findings. Fifth, the present study utilized a task-based measure of WM ability; it will be important to establish whether neural and behavioral measures of emotional WM impacts functional outcomes like academic performance and symptoms of psychopathology.
Violence exposure is associated with WM performance and neural function in emotionally salient contexts. Violence-exposed youths performed worse than children never exposed to violence on WM for happy and neutral, but not angry, emotional faces. In addition, violence exposure was associated with reduced neural recruitment in regions associated with WM, facial processing, and social cognition, and this reduced neural activation explained disruptions in WM for emotional faces. Overall, these findings suggest disruptions in neural and behavioral emotional WM ability may contribute to poorer academic and cognitive functioning observed among youth with a history of violence exposure.
Supplementary Material
Acknowledgments
This research was supported by National Institute of Child Health and Human Development Grant T32-HD057822 and National Institute of Mental Health Grants K01-MH092526, R01-MH103291, and R01-MH106482.
Footnotes
Supplementary Material
To view the supplementary material for this article, please visit https://doi.org/10.1017/S0954579417001638.
References
- Achenbach TM (1991). Manual for the Child Behavior Checklist/4–18 and 1991 profile. Burlington, VT: University of Vermont, Department of Psychiatry. [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (DSM-5). Washington, DC: Author. [Google Scholar]
- Askren MK, McAllister-Day TK, Koh N, Mestre Z, Dines JN, Korman BA, … Madhyastha TM (2016). Using make for reproducible and parallel neuroimaging workflow and quality-assurance. Frontiers in Neuroinformatics. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augusti E-M, & Melinder A (2013). Maltreatment is associated with specific impairments in executive functions: A pilot study: Executive functions in maltreated children. Journal of Traumatic Stress, 26, 780–783. doi: 10.1002/jts.21860 [DOI] [PubMed] [Google Scholar]
- Avants BB, Tustison NJ, Song G, Cook PA, Klein A, & Gee JC (2011). A reproducible evaluation of ANTs similarity metric performance in brain image registration. NeuroImage, 54, 2033–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Tugade MM, & Engle RW (2004). Individual differences in working memory capacity and dual-process theories of the mind. Psychological Bulletin, 130, 553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behzadi Y, Restom K, Liau J, & Liu TT (2007). A component based noise correction method (CompCor) for BOLD and perfusion based fMRI. NeuroImage, 37, 90–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein DP, Ahluvalia T, Pogge D, & Handelsman L (1997). Validity of the Childhood Trauma Questionnaire in an adolescent psychiatric population. Journal of the American Academy of Child & Adolescent Psychiatry, 36, 340–348. [DOI] [PubMed] [Google Scholar]
- Bifulco A, Brown GW, Lillie A, & Jarvis J (1997). Memories of childhood neglect and abuse: Corroboration in a series of sisters. Journal of Child Psychology and Psychiatry, 38, 365–374. [DOI] [PubMed] [Google Scholar]
- Braunlich K, Gomez-Lavin J, & Seger CA (2015). Frontoparietal networks involved in categorization and item working memory. Neuro-Image, 107, 146–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA, Maughan A, Toth SL, & Bruce J (2003). False belief understanding in maltreated children. Development and Psychopathology, 15, 1067–1091. [DOI] [PubMed] [Google Scholar]
- Cromheeke S, Herpoel L-A, & Mueller SC (2014). Childhood abuse is related to working memory impairment for positive emotion in female university students. Child Maltreatment, 19, 38–48. doi: 10.1177/1077559513511522 [DOI] [PubMed] [Google Scholar]
- Curtis CE, & D’Esposito M (2003). Persistent activity in the prefrontal cortex during working memory. Trends in Cognitive Sciences, 7, 415–423. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, & Sereno MI (1999). Cortical surface-based analysis: I. Segmentation and surface reconstruction. NeuroImage, 9, 179–194. [DOI] [PubMed] [Google Scholar]
- Deen B, Koldewyn K, Kanwisher N, & Saxe R (2015). Functional organization of social perception and cognition in the superior temporal sulcus. Cerebral Cortex, 25, 4596–4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M, Francis DJ, Cirino PT, Schachar R, Barnes MA, & Fletcher JM (2009). Why IQ is not a covariate in cognitive studies of neurodevelopmental disorders. Journal of the International Neuropsychological Society, 15, 331. doi: 10.1017/S1355617709090481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePrince AP, Weinzierl KM, & Combs MD (2009). Executive function performance and trauma exposure in a community sample of children. Child Abuse and Neglect, 33, 353–361. [DOI] [PubMed] [Google Scholar]
- Eklund A, Nichols TE, & Knutsson H (2016). Cluster failure: Why fMRI inferences for spatial extent have inflated false-positive rates. Proceedings of the National Academy of Sciences. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkelhor D, Ormrod RK, & Turner HA (2009). The developmental epidemiology of childhood victimization. Journal of Interpersonal Violence, 24, 711–731. [DOI] [PubMed] [Google Scholar]
- Finn AS, Minas JE, Leonard JA, Mackey AP, Salvatore J, Goetz C, … Gabrieli JD (2016). Functional brain organization of working memory in adolescents varies in relation to family income and academic achievement. Developmental Science. Advance online publication. doi: 10.1111/desc.12450/full [DOI] [PubMed] [Google Scholar]
- Geier CF, Garver K, Terwilliger R, & Luna B (2009). Development of working memory maintenance. Journal of Neurophysiology, 101, 84–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh SS, Kakunoori S, Augustinack J, Nieto-Castanon A, Kovelman I, Gaab N, … Fischl B (2010). Evaluating the validity of volume-based and surface-based brain image registration for developmental cognitive neuroscience studies in children 4 to 11 years of age. Neuro-Image, 53, 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold AL, Sheridan MA, Peverill M, Busso DS, Lambert HK, Alves S, … McLaughlin KA (2016). Childhood abuse and reduced cortical thickness in brain regions involved in emotional processing. Journal of Child Psychology and Psychiatry, 57, 1154–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould F, Clarke J, Heim C, Harvey PD, Majer M, & Nemeroff CB (2012). The effects of child abuse and neglect on cognitive functioning in adulthood. Journal of Psychiatric Research, 46, 500–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Chung MK, Avants BB, Shirtcliff EA, Gee JC, Davidson RJ, & Pollak SD (2010). Early stress is associated with alterations in the orbitofrontal cortex: A tensor-based morphometry investigation of brain structure and behavioral risk. Journal of Neuroscience, 30, 7466–7472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, & Gobbini MI (2000). The distributed human neural system for face perception. Trends in Cognitive Sciences, 4, 223–233. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Hoffman EA, & Gobbini MI (2002). Human neural systems for face recognition and social communication. Biological Psychiatry, 51, 59–67. [DOI] [PubMed] [Google Scholar]
- Hayes AF (2013). Introduction to mediation, moderation, and conditional process analysis: A regression-based approach. New York: Guilford Press. [Google Scholar]
- Hein G, & Knight RT (2008). Superior temporal sulcus—It’s my area: Or is it? Journal of Cognitive Neuroscience, 20, 2125–2136. [DOI] [PubMed] [Google Scholar]
- Holt MK, Finkelhor D, & Kantor GK (2007). Multiple victimization experiences of urban elementary school students: Associations with psychosocial functioning and academic performance. Child Abuse and Neglect, 31, 503–515. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, & Smith SM (2012). NeuroImage, 62, 782–790. [DOI] [PubMed] [Google Scholar]
- Lambert HK, Sheridan MA, Sambrook KA, Rosen ML, Askren MK, & McLaughlin KA (2017). Hippocampal contribution to context encoding across development is disrupted following early-life adversity. Journal of Neuroscience, 37, 1925–1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipina S, Segretin S, Hermida J, Prats L, Fracchia C, Camelo JL, & Colombo J (2013). Linking childhood poverty and cognition: Environmental mediators of non-verbal executive control in an Argentine sample. Developmental Science, 16, 697–707. [DOI] [PubMed] [Google Scholar]
- LoPresti ML, Schon K, Tricarico MD, Swisher JD, Celone KA, & Stern CE (2008). Working memory for social cues recruits orbitofrontal cortex and amygdala: A functional magnetic resonance imaging study of delayed matching to sample for emotional expressions. Journal of Neuroscience, 28, 3718–3728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, & Heim C (2009). Effects of stress throughout the life span on the brain, behaviour and cognition. Nature Reviews Neuroscience, 10, 434–445. [DOI] [PubMed] [Google Scholar]
- Mackey AP, Finn AS, Leonard JA, Jacoby-Senghor DS, West MR, Gabrieli CF, & Gabrieli JD (2015). Neuroanatomical correlates of the income-achievement gap. Psychological Science, 26, 925–933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus DS, Harms MP, Snyder AZ, Jenkinson M, Wilson JA, Glasser MF, … et al. (2013). Human Connectome Project informatics: Quality control, database services, and data visualization. Neuro-Image, 80, 202–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marusak HA, Zundel CG, Brown S, Rabinak CA, & Thomason ME (2016). Convergent behavioral and corticolimbic connectivity evidence of a negativity bias in children and adolescents. Social Cognitive and Affective Neuroscience. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA (2016). Future directions in childhood adversity and youth psychopathology. Journal of Clinical Child and Adolescent Psychology, 45, 361–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Busso DS, Duys A, Green JG, Alves S, Way M, & Sheridan MA (2014). Amygdala response to negative stimuli predicts PTSD symptom onset following a terrorist attack. Depression and Anxiety, 31, 834–842. doi: 10.1002/da.22284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Green JG, Gruber MJ, Sampson NA, Zaslavsky AM, & Kessler RC (2012). Childhood adversities and first onset of psychiatric disorders in a national sample of US adolescents. Archives of General Psychiatry, 69, 1151–1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Koenen KC, Hill ED, Petukhova M, Sampson NA, Zaslavsky AM, & Kessler RC (2013). Trauma exposure and posttraumatic stress disorder in a national sample of adolescents. Journal of the American Academy of Child & Adolescent Psychiatry, 52, 815–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, & Lambert HK (2016). Child trauma exposure and psychopathology: Mechanisms of risk and resilience. Current Opinion in Psychology, 14, 29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Peverill M, Gold AL, Alves S, & Sheridan MA (2015). Child maltreatment and neural systems underlying emotion regulation. Journal of the American Academy of Child & Adolescent Psychiatry, 54, 753–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, & Lambert HK (2014). Childhood adversity and neural development: Deprivation and threat as distinct dimensions of early experience. Neuroscience & Biobehavioral Reviews, 47, 578–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, Winter W, Fox NA, Zeanah CH, & Nelson CA (2014). Widespread reductions in cortical thickness following severe early-life deprivation: A neurodevelopmental pathway to attention-deficit/hyperactivity disorder. Biological Psychiatry, 76, 629–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GA, & Chapman JP (2001). Misunderstanding analysis of covariance. Journal of Abnormal Psychology, 110, 40. [DOI] [PubMed] [Google Scholar]
- Miyake A, & Friedman NP (2012). The nature and organization of individual differences in executive functions four general conclusions. Current Directions in Psychological Science, 21, 8–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble KG, Houston SM, Brito NH, Bartsch H, Kan E, Kuperman JM, … Sowell ER (2015). Family income, parental education and brain structure in children and adolescents. Nature Neuroscience, 18, 773–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolte T, Bolling DZ, Hudac CM, Fonagy P, Mayes L, & Pelphrey KA (2013). Brain mechanisms underlying the impact of attachment-related stress on social cognition. Frontiers in Human Neuroscience, 7. doi: 10.3389/fnhum.2013.00816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollak SD, Cicchetti D, Hornung K, & Reed A (2000). Recognizing emotion in faces: Developmental effects of child abuse and neglect. Developmental Psychology, 36, 679–688. doi: 10.1037/0012-1649.36.5.679 [DOI] [PubMed] [Google Scholar]
- Pollak SD, & Tolley-Schell SA (2003). Selective attention to facial emotion in physically abused children. Journal of Abnormal Psychology, 112, 323. [DOI] [PubMed] [Google Scholar]
- Raschle N, Zuk J, Ortiz-Mantilla S, Sliva DD, Franceschi A, Grant PE, … Gaab N (2012). Pediatric neuroimaging in early childhood and infancy: Challenges and practical guidelines. Annals of the New York Academy of Sciences, 1252, 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche A (2011). A four-dimensional registration algorithm with application to joint correction of motion and slice timing in fMRI. IEEE Transactions on Medical Imaging, 30, 1546–1554. [DOI] [PubMed] [Google Scholar]
- Sarsour K, Sheridan M, Jutte D, Nuru-Jeter A, Hinshaw S, & Boyce WT (2011). Family socioeconomic status and child executive functions: The roles of language, home environment, and single parenthood. Journal of the International Neuropsychological Society, 17, 120–132. [DOI] [PubMed] [Google Scholar]
- Scherf KS, Sweeney JA, & Luna B (2006). Brain basis of developmental change in visuospatial working memory. Journal of Cognitive Neuroscience, 18, 1045–1058. doi: 10.1162/jocn.2006.18.7.1045 [DOI] [PubMed] [Google Scholar]
- Shackman JE, Shackman AJ, & Pollak SD (2007). Physical abuse amplifies attention to threat and increases anxiety in children. Emotion, 7, 838. [DOI] [PubMed] [Google Scholar]
- Sheridan MA, & McLaughlin KA (2014). Dimensions of early experience and neural development: Deprivation and threat. Trends in Cognitive Sciences, 18, 580–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheridan MA, Peverill MA, Finn AS, & McLaughlin KA (2017). Dimensions of childhood adversity have distinct associations with neural systems underlying executive functioning. Development and Psychopathology. Advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shonk SM, & Cicchetti D (2001). Maltreatment, competency deficits, and risk for academic and behavioral maladjustment. Developmental Psychology, 37, 3. [PubMed] [Google Scholar]
- Soto D, Rotshtein P, & Kanai R (2014). Parietal structure and function explain human variation in working memory biases of visual attention. NeuroImage, 89, 289–296. [DOI] [PubMed] [Google Scholar]
- Steinberg AM, Brymer MJ, Decker KB, & Pynoos RS (2004). The University of California at Los Angeles Post-Traumatic Stress Disorder Reaction Index. Current Psychiatry Reports, 6, 96–100. [DOI] [PubMed] [Google Scholar]
- Swartz JR, Graham-Bermann SA, Mogg K, Bradley BP, & Monk CS (2011). Attention bias to emotional faces in young children exposed to intimate partner violence. Journal of Child and Adolescent Trauma, 4, 109–122. doi: 10.1080/19361521.2011.573525 [DOI] [Google Scholar]
- Thomas KM, Drevets WC, Whalen PJ, Eccard CH, Dahl RE, Ryan ND, & Casey BJ (2001). Amygdala response to facial expressions in children and adults. Biological Psychiatry, 49, 309–316. [DOI] [PubMed] [Google Scholar]
- Tibu F, Sheridan MA, McLaughlin KA, Nelson CA, Fox NA, & Zeanah CH (2016). Disruptions of working memory and inhibition mediate the association between exposure to institutionalization and symptoms of attention deficit hyperactivity disorder. Psychological Medicine, 46, 529–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N, Tanaka JW, Leon AC, McCarry T, Nurse M, Hare TA, … Nelson C (2009). The NimStim set of facial expressions: Judgments from untrained research participants. Psychiatry Research, 168, 242–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twamley EW, Hami S, & Stein MB (2004). Neuropsychological function in college students with and without posttraumatic stress disorder. Psychiatry Research, 126, 265–274. doi: 10.1016/j.psychres.2004.01.008 [DOI] [PubMed] [Google Scholar]
- Vasilevski V, & Tucker A (2016). Wide-ranging cognitive deficits in adolescents following early life maltreatment. Neuropsychology, 30, 239–246. doi: 10.1037/neu0000215 [DOI] [PubMed] [Google Scholar]
- Vul E, Harris C, Winkielman P, & Pashler H (2009). Puzzlingly high correlations in fMRI studies of emotion, personality, and social cognition. Perspectives on Psychological Science, 4, 274–290. [DOI] [PubMed] [Google Scholar]
- Walker EA, Unutzer J, Rutter C, Gelfand A, Saunders K, VonKorff M, … Katon W (1999). Costs of health care use by women HMO members with a history of childhood abuse and neglect. Archives of General Psychiatry, 56, 609–613. [DOI] [PubMed] [Google Scholar]
- Woolrich M (2008). Robust group analysis using outlier inference. Neuro-Image, 41, 286–301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.