Abstract
The proteasome inhibitor carfilzomib is highly effective in the treatment of multiple myeloma. It irreversibly binds the chymotrypsin-like active site in the β5 subunit of the 20S proteasome. Despite impressive response rates when carfilzomib is used in combination with immunomodulatory agents in newly diagnosed multiple myeloma patients; no biomarker exists to accurately predict response and clinical outcomes. We prospectively assessed the activity in peripheral blood of the chymotrypsin-like (CHYM), caspase-like (CASP) and trypsin-like (TRYP) proteolytic sites in 45 newly diagnosed multiple myeloma patients treated with eight cycles of carfilzomib, lenalidomide and dexamethasone (CRd) (NCT01402284). Samples were collected per protocol and proteasome activity measured through a fluorogenic assay. Median CHYM levels after one dose of carfilzomib decreased by >70%. CHYM and CASP activity decreased throughout treatment reaching a minimum after eight cycles of treatment. Higher levels of proteasome activity associated with higher disease burden (r>0.30; p<0.05) and higher disease stage (0.10 < p <0.20). No association was found with the probability of achieving a complete response, minimal residual disease negativity or time to best response. Further studies evaluating proteasome activity in malignant plasma cells may help elucidate how proteasome activity can be used as a biomarker in multiple myeloma.
Introduction
The 26S proteasome is part of the ubiquitin-proteasome system which plays a critical role in protein degradation.[1] In this system, unneeded or damaged proteins are degraded through the proteasome’s catalytic 20S subunits termed the chymotrypsin-like (CHYM), trypsin-like (TRYP) and post-glutamyl peptide hydrolase-like (PGPH, caspase-like (CASP)).
Clinically, based on high rates of rapid, deep and durable responses, 20S proteasome inhibitors have become a cornerstone in the treatment of multiple myeloma patients.[2–4] Although 20S proteasome inhibitors have become part of modern anti-myeloma standard of care, unfortunately, some newly diagnosed multiple myeloma (MM) patients have inadequate responses to this class of drugs.[3,4] Moreover, among patients with relapsed disease the response rates are substantially lower.[2] Ideally, access to biomarkers predictive of clinical responses could help clinicians tailor treatment for myeloma patients.
Based on 101 cases, compared to healthy controls, newly diagnosed MM patients have been reported to have elevated free circulating proteasomes (cProt) detectable in peripheral blood.[5] Inspired by this observation, a retrospective study of 50 NDMM patients that were non-uniformly treated, showed that patients who maintained high total cProt levels post therapy had poorer response to therapy and shorter overall survival.[5] Furthermore, prior retrospective studies focusing on other hematologic malignancies have reported proteasome activity in plasma to be a predictor of treatment response and survival.[6,7]
To test the hypothesis that enzymatic activities of plasma cProt is a biomarker associated with clinical outcomes in NDMM patients, we assessed peripheral plasma collected on a prospective clinical trial including 45 newly diagnosed MM patients treated in a uniform way with carfilzomib, lenalidomide and dexamethasone.[8]
Methods
Patients and samples
We assessed 45 newly diagnosed MM patients treated with CRd therapy.[8] Briefly, patients received eight 28-day cycles of combination therapy with carfilzomib (20 mg/m2, 36 mg/m2) intravenously on Days 1,2,8,9,15,16; lenalidomide 25 mg orally on Days 1–21 and dexamethasone 20/10 mg orally on Days 1,2,8,9,15,16,22,23. Cycle 1 Day 1 lenalidomide and dexamethasone were omitted in all the patients. After combination therapy, patients went on to receive up to twenty-four 28-day cycles of extended dosing lenalidomide 10 mg orally on Days 1–21. Median follow-up (from on-study date until date of analysis) for the study is 17.3 months. Baseline demographic, clinical and laboratory parameters were collected. Plasma samples from peripheral blood were collected at time intervals as specified in the protocol [C1D1 (baseline), C1D2 (24 h post single dose carfilzomib 20mg/m2), C2D1, C4D1, C8D1 and post cycle 8 of treatment] and frozen to −80 °C. Patient responses were assessed per current IMWG guidelines.[9]
Measurement of proteasome activity
CHYM, CASP and TRYP activities from cProt were assayed by continuously monitoring the production of 7-amino-4-methylcoumarin (AMC) from fluorogenic peptides in plasma.
To measure CHYM and CASP activity, 45 μL of plasma were mixed with 5% sodium dodecyl sulfate (SDS) at room temperature for 15 min to activate the plasma. The assay buffer was 25 mmol/L SDS/N-(2-hydroxethyl) piperazine-NA-2-ethanesulfonic acid (HEPES). To measure TRYP activity, 50 μL of plasma were mixed with 2.5 μL 10% Tween-20 to activate the plasma. The assay buffer was 0.05% Tween/HEPES. The reaction wells contained 30 μL of assay buffer, 10 μL. activated sample, and 10 μL. of the prospective fluorogenic peptide-AMC substrate. The fluorogenic peptides used for the reactions were Suc-LLVY-AMC for CHYM, BZ-VGR-AMC for TRYP and Z-LLE-AMC for CASP.
To measure the fluorescence release of free AMC with time, the SpectraMax M5 (Molecular Devices, Sunnyvale, CA) instrument was used with a read interval of 1 min during 30 min at 37 °C. Samples were analyzed per triplicate. Enzymatic activities were quantified (pmol AMC/s/mL plasma) by generating a standard curve of AMC (range, 0–8 μM). The slope of this curve was then used as a conversion factor to calculate the absolute specific activity of each individual samples as pmol AMC/second/mL of plasma.
Minimal residual disease testing using flow cytometry
Bone marrow aspirate specimens were processed within 12 h of collection. Red blood cells were prelysed by incubating with hypertonic ammonium chloride solution (150mM NH4CI, 10mM KHCO3, 0.1 mM EDTA) for 10 min at room temperature (maintained at 21–23 °C) at a ratio of 1:9 (volume of sample: volume of lysing solution). Specimens were then washed twice with phosphate buffered saline (PBS) to remove cytophilic antibodies before determining cell number. Cells were then stained for 30 min at room temperature with a panel of antibodies against surface and intracellular kappa and lambda (Polyclonal Rabbit Anti-Human, F(ab’)2, Dako) and surface CD13PE-Cy7 (clone L138), CD19APC (clone SJ25C1), CD20APC-H7 (clone L27), CD27PE (clone L128), CD38v450 (clone HB7), CD45v500 (clone HI30), CD56PE-Cy7 (clone NCAM16.2), CD81FITC (clone JS-81), CD117PE (clone 104D2), CD138 PerCP-Cy5.5 (clone MI15) (BD Biosciences, San Jose, CA) and CD16FITC (clone DJ130c) (Dako, Carpinteria, CA) as previously described.[10,11] Cellular viability of lysed and washed bone marrow aspirate cells was assessed by staining with the fluorescent dye 7-amino-actinomycin D (7-AAD) for 10 min at room temperature (7-AAD only stains non-viable cells). For intracellular light chain evaluation, cells were stained with antibodies against surface antigens and then permeabilized with Fix and Perm reagent (Invitrogen, Frederick, MD) followed by incubation with anti-kappa and anti-lambda antibodies or isotype controls. All cells were fixed in 1.0% paraformaldehyde and stored at 4°C for up to 12 h before acquisition. Specimens were acquired using an 8-color multipara-metric approach on a 3-laser FACS Canto II (BD Biosciences, San Jose, CA) with DiVa 6.1.1 software and analyzed by FCS Express 3 software (DeNovo Software, Los Angeles, CA). Approximately 3–4 million cells were acquired for each tube. The fraction of plasma cells among all nucleated cells was determined by quantitating the percent of bright CD38 and CD138 positive cells in an analysis gate devoid of cellular debris.
Statistical methods
A Wilcoxon signed rank test was used to test the significance of paired comparisons of cProt values between two different times. The significance of the difference in cProt values when classified into two groups according to stage, MRD negativity, or development of a complete response were determined using an exact Wilcoxon rank sum test. Spearman’s rank correlation was used to assess the correlation between cProt levels and clinical laboratory parameters, as well as being used to determine the correlation between time to best response and 15 early assay parameters (baseline, C1D2, C2D1 for each of CHYM, CASP and TRYP activity, as well as the change from baseline to C1D2 and C2D1 for each of the three activities). Spearman correlations are interpreted as follows: if ∣r∣ > 0.70, the correlation is strong; if 0.50 < ∣r∣ < 0.70, the correlation is moderately strong; if 0.30 < ∣r∣ < 0.50, the correlation is weak to moderately strong; and if ∣r∣ <0.30, the correlation is weak. The p-value associated with a correlation coefficient tests whether r = 0 and thus is less informative than the magnitude of the correlation. The probability of developing a complete response as a function of the number of cycles of treatment received was estimated using the method of Kaplan–Meier.[12] A log-rank test was used to determine the significance of the difference in probability of developing a complete response as a function of baseline cProt values divided into three approximately equal-sized categories. All p-values are two-tailed and presented without any formal adjustment for multiple comparisons. Since many exploratory associations were performed, to be conservative, results with p-values <0.005 should be interpreted as reflecting significant associations while if 0.005 < p < 0.05, the difference would suggest a trend toward significance. Analyses were performed using GraphPad Prism version 6.01 (La Jolla, CA) or SAS version 9.3 (Cary, NC).
Results
Forty-five patients were evaluated in the study. Patients had a median age of 60 years (range 40–88; 40% females). The median M-spike for all patients was 2.9 g/dL. Most patients had early stage disease: International Staging System (ISS) Stage I (n = 22, 49%), Stage II (n = 22, 49%), Stage III (n = 1, 2%). All multiple myeloma isotypes were present in the study, with about 50% of the patients having an IgG kappa subtype (n = 23, 51%). All the patients presented with end-organ damage according to the IMWG criteria.[13] In concordance with other series, most patients presented with bony lytic lesions.[14] A majority of patients had normal cytogenetics by standard metaphase karyotyping; however, fluorescence in situ hybridization (FISH) showed that about half of the patients were hyperdiploid and about 10% had deletion 17p (Table 1).
Table 1.
Baseline clinical, laboratory and genetic characteristics of enrolled patients.
| Characteristics | |
|---|---|
| Female, n(%) | 18 (40%) |
| ISS Stage, n(%) | |
| I | 22 (49%) |
| II | 22 (49%) |
| III | 1 (2%) |
| Median age (range) years | 60 (40–88) |
| Isotype, n(%)[median, range] | |
| IgG kappa g/dL | 23 (51%)[3.1,0–7.1] |
| IgG lambda g/dL | 7 (15.5%)[2.8, 0–7.6] |
| IgA kappa g/dL | 6 (13.3%)[1.35,0–1.8) |
| IgA lambda g/dL | 4 (8.88%)[1.6,1.4–2.8] |
| Kappa light chain mg/dL | 4 (8.88%)[243,39.5–1620] |
| Lambda light chain[actual value] mg/dL | 1 (2%)[51.6] |
| Clinical end-organ damage, n(%) | |
| Lytic lesions | 37 (82%) |
| Anemia | 12 (27%) |
| Hypercalcemia | 3 (7%) |
| Renal insufficiency | 0 |
| Cytogenetics*, n(%) | |
| Normal | 33 (78%) |
| Hyperdiploidy | 5 (11%) |
| t(11;14) | 1 (2%) |
| del(11) | 2 (4.5%) |
| del(17p) | 1 (2%) |
| FISH#, n(%) | |
| Normal | 9 (20%) |
| Hyperdiploidy | 17 (38%) |
| t(11,14) | 4 (9%) |
| del(13q) | 11 (24.5%) |
| del(17p) | 5 (11%) |
ISS: international staging system; M-spike: monoclonal spike; FISH: fluorescence in-situ hybridization.
missing 1,
missing 6,
none of the patients had the following genetic abnormalities: t(4;14), t(14;16),t(14;20).
Proteasome activity in plasma throughout treatment
Median baseline levels of CHYM, CASP, and TRYP activity for all patients were 0.83 (0.12–3.89), 0.75 (0.19–2.28) and 2.95 (0.77–7.39) pmol AMC/s/ml plasma, respectively.
Median CHYM levels after an initial single dose of carfilzomib 20 mg/m2 were reduced by 72% (p < 0.0001), whereas CASP and TRYP levels were increased by 20% (p = 0.016) and 5% (p = 0.02), respectively. After one month of combination treatment, all three measured cProt activity levels had increased when compared to baseline values. CHYM and TRYP levels increased significantly (p = 0.014 and p = 0.021, respectively) whereas CASP levels did not (p = 0.078). As combination treatment continued, the levels of the three measurable enzymatic activities of the proteasome decreased significantly. After eight cycles of combination treatment, all three proteasome activities were statistically significantly reduced when compared to baseline activity values (Table 2, Figure 1).
Table 2.
Chymotrypsing-like, caspase-like and trypsin-like activity (pmol 7-amino-4 methylcoumarin/second/mL) variations throughout treatment in newly diagnosed patients with multiple myeloma.
| CHYMOTRYPSIN |
CASPASE |
TRYPSIN |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Median | Mean ± SD | p-value | Median | Mean ± SD | p-value | Median | Mean ± SD | p-value | |
| Baseline | 0.83 | 1.02 ± 0.75 | p < 0.0001* | 0.75 | 0.88 ± 0.54 | p = 0.016* | 2.95 | 3.16 ± 1.35 | p = 0.022* |
| 24 hour | 0.23 | 0.35 ± 0.28 | 0.90 | 1.05 ± 0.59 | 3.10 | 3.56 ± 1.63 | |||
| C2D1 | 1.07 | 1.30 ± 0.97 | 0.91 | 1.03 ± 0.61 | 3.60 | 3.73 ± 1.56 | |||
| C4D1 | 0.79 | 0.97 ± 0.64 | 0.67 | 0.80 ± 0.45 | 3.00 | 3.38 ± 1.58 | |||
| C8D1 | 0.65 | 0.77 ± 0.59 | 0.65 | 0.74 ± 0.38 | 3.20 | 3.21 ± 1.18 | |||
| C9D1 | 0.23 | 0.24 ± 0.09 | p < 0.0001* | 0.39 | 0.41 ± 0.14 | p < 0.0001* | 2.40 | 2.55 ± 0.95 | p = 0.0064* |
Baseline: values obtained C1D1 before treatment start; C: cycle; D: day; 24 h: values 24 h post one single dose of 20 mg/m2 of Carfilzomib; SD: standard deviation. Baseline, 24 hour and C2D1 activity values include analysis in 45 patients. Due to early median follow up of 17 months 42 patients were available for analysis of C4D1 activities, 38 patients for analysis of C8D1 activities and 34 patients for analysis of C9D1 activities.
p-value: Wilcoxon signed rank test of 24 h and C9D1 values compared to baseline values of each activity.
Figure 1.
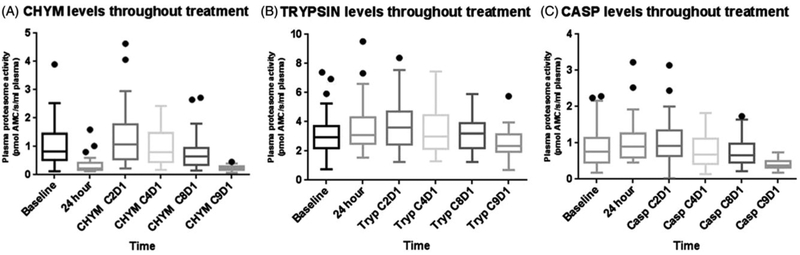
Box and whisker plot of CHYM, CASP and TRYP levels throughout treatment. Whiskers plotted at the 5th and 95th percentile levels.
Association with clinical parameters and stage
Pretreatment CHYM and CASP activity levels were weak to moderately well correlated with the baseline values of serum monoclonal protein, serum total protein and serum IgG. Additionally, baseline CASP levels were also weak to moderately-well correlated with baseline Beta-2 microglobulin values. Table 3 shows these results with associated correlation coefficients and p-values. No correlations were found between CHYM and CASP and age, serum free lambda, serum free kappa, serum free light chain ratio, serum IgA, serum IgM, creatinine, hemoglobin, calcium, albumin, the percentage of abnormal plasma cells by flow cytometry in bone marrow aspirate and the percentage of bone marrow core biopsy plasma cell infiltration. TRYP baseline values were not correlated with clinical parameters (r < 0.30).
Table 3.
Correlations of proteasome activities with relevant clinical variables in newly diagnosed multiple myeloma patients.
| Spearman’s Rank Correlation (r) and p-value |
||
|---|---|---|
| Laboratory parameters | CHYM | CASP |
| Serum M-protein | r = 0.45; p = 0.0032 | r = 0.43; p = 0.0051 |
| Serum total protein | r = 0.44; p = 0.0030 | r = 0.42; p = 0.0045 |
| Serum IgG | r = 0.37; p = 0.021 | r = 0.37; p = 0.011 |
| β-2 Microglobulin | r = 0.28; p = 0.071 | r = 0.33; p = 0.028 |
CHYM: chymotrypsin-like; CASP: caspase-like. p < 0.05 for tests of r = 0 for all correlations except as noted.
Correlation coefficient (r) such that 0.3<r<0.5 suggests a correlation that is weak to moderately strong; r < 0.30 is a weak correlation.
CHYM and CASP baseline activity values increased somewhat with increasing ISS stage (p = 0.17 and p = 0.10, respectively), although this did not reach statistical significance. ISS stage II and III were grouped together for the purpose of analysis, as only one patient in the study had stage III disease. TRYP baseline values were somewhat lower (p = 0.14) in stage II/III compared to stage I patients, although this did not reach statistical significance (Table 4, Figure 1 in Supplementary material).
Table 4.
Baseline values of chymotrypsin-like, caspase-like and trypsin-like activity (pmol 7-amino-4 methylcoumarin/second/mL) by international staging system (ISS) stage.
| ISS STAGE I | ISS STAGE II AND IIIa | ||||
|---|---|---|---|---|---|
| Median | Mean ± SEM | Median | Mean ± SEM | p-valueb | |
| Chymotrypsin | 0.70 | 0.93 ± 0.19 | 0.91 | 1.10 ± 0.13 | 0.17 |
| Caspase | 0.66 | 0.74 ± 0.10 | 0.79 | 1.01 ± 0.12 | 0.10 |
| Trypsin | 3.04 | 3.60 ± 0.35 | 2.90 | 2.76 ± 0.20 | 0.14 |
Only one patient has stage III ISS.
Wilcoxon rank sum test.
Association with clinical outcomes
Based on a Kaplan-Meier analysis, the median number of cycles delivered to achieve a CR was 8 (Figure 2 in Supplementary material). We determined the association of the 15 early assay parameters based on CHYM, CASP and TRYP activity (as defined in the statistical methods section) with best response, minimal residual disease negativity and time to achieve the best response (among the 44 patients with at least a VGPR). No correlations (all r < 0.30) were identified for the number of cycles to achieve a best response, or associations with developing a CR or not, or of obtaining minimal residual disease (MRD) negativity by eight cycles of treatment, with the exception of TRYP at the beginning of cycle 2, or the difference in this parameter between baseline and beginning of cycle 2.
Baseline cProt activities were also studied in relation to the probability of developing a complete response as a function of the number of cycles of therapy. Kaplan–Meier curves for CHYM, CASP and TRYP activities at baseline showed no statistical difference in the probability of achieving a complete response [global p-values of 0.70, 0.76 and 0.19 for these three measures, respectively] (Figure 3 in Supplementary material).
Discussion
Based on strong clinical responses, proteasome inhibitors have become part of modern anti-myeloma standard of care.[15] These agents selectively and reversibly (Bortezomib) or irreversibly (carfilzomib) inhibit the β5 (chymotrypsin-like) proteolytic sites of the proteasome. Furthermore, it has been reported that free cProt can be found at elevated levels in peripheral blood of MM patients compared to healthy individuals.[16] Inspired by these observations, a retrospective study included 50 newly diagnosed MM patients treated with different induction regimens and found higher levels of cProt measured by enzyme-linked immunosorbent assay (ELISA) to be associated with higher disease burden (from MGUS to SMM to MM), as well as response to treatment and overall survival.[5] Also, cProt activity measured through a fluorogenic-based assay has been found to be associated with survival and response to treatment in other hematologic malignancies; however, to our knowledge, it has never been evaluated in patients with MM.[7,8]
In this prospective clinical study including 45 patients with uniformly treated newly diagnosed MM, we assessed patterns of cProt activity at baseline, during and after treatment with eight cycles of combination CRd. Similarly to what has been reported in a smaller series of acute myeloid leukemia (AML) and acute lymphoblastic leukemia (ALL) patients, we found cProt activity to decline in relation to treatment.[17] For example, at the first measurement, at 24 h after a single dose of carfilzomib (without lenalidomide or dexamethasone), the median reduction in CHYM activity was over 70%; however, at the same time, a small but significant increase in CASP and TRYP activity also occurred. This observation is important because it demonstrates that carfilzomib specifically inhibits its target in vivo. Additionally, the small initial increase in CASP and TRYP activity may be interpreted as an attempt to maintain homeostatic balance, carfilzomib is a much stronger inhibitor of the CHYM activity (IC50 5 nM) than TRYP (IC50 3600 nM) and CASP activity (IC50 2400 nM).[18] Furthermore, we found both CHYM and CASP activity to decrease throughout CRd therapy, suggesting that not only is the carfilzomib IC50 important for cProt inhibition but that continued exposure to therapy effectively inhibited all three measured proteasome activities. Carfilzomib may not be the only agent contributing to cProt level inhibition. For example, lenalidomide binds to an E3 ubiquitin ligase complex and modulates its substrate specificity, resulting in proteasomal degradation of specific proteins. Thus, lenalidomide could modulate proteasome activity.[19] cProt activities measured after the initial C1D2 time point may have been affected by lenalidomide or dexamethasone. Future studies should address the contribution of other agents to proteasome inhibition in myeloma patients.
Higher levels of cProt have been reported in patients with MM than those with MGUS. Additionally, increased cProt activity is associated with disease states with increased cell turn over (such as in AML). [5–7] In our study, CHYM and CASP activity were weakly to moderately correlated with laboratory parameters indicative of increased tumor burden in MM. When ISS stage was analyzed, higher levels of activity were seen in stage II and III than stage I, although it did not reach statistical significance.
In this prospectively designed, uniformly treated cohort of newly diagnosed MM patients, no association was found between proteasome activities with time to best clinical response, including CR or MRD negativity by flow cytometry. Study limitations include a relatively low number of patients of whom only one was International Staging System (ISS) stage 3. It has been postulated that cProt are reflective of cell turn over, tumor burden, inflammation and may be released via secretion from dying or injured cells or through increased immunological activity.[16,20,21] Thus, the lack of association between baseline cProt activity and clinical response suggests that cProt activity in plasma may not be reflective of the activity inside malignant plasma cells. The contribution of bone marrow microenvironment cells or peripheral leukocytes to the levels of cProt should also be considered. This has to be clarified in future studies. Moreover, in this clinical trial, newly diagnosed MM patients achieved high rates of very deep clinical responses (i.e. MRD negativity). It is possible that cProt activity levels may play a role in predicting clinical outcomes in the setting of therapies with lower rates of deep clinical responses (e.g. in newly diagnosed MM patients treated with less effective drugs and/or in relapsed/refractory patients). Future studies are needed to address these aspects.
In summary, based on 45 newly diagnosed MM patients treated uniformly with highly effective combination therapy (CRd) we did not find baseline cProt activity to be associated with clinical response (neither CR nor MRD negativity). However, we found that CHYM and CASP plasma activity levels correlated with disease burden. Also, 24-h post-single dose carfilzomib, we found the plasma activity to dramatically decrease and the cProt activities continued to decrease throughout the treatment. Although less convenient, proteasome activity measured in the bone marrow clonal plasma cells may be more sensitive and specific for MM patients and may be a better marker for clinical outcome. Future studies are needed to test whether an increase in cProt activity levels after treatment is associated with recurrent disease.
Supplementary Material
References
- [1].Manasanch EE, Korde N, Zingone A, et al. The proteasome: mechanisms of biology and markers of activity and response to treatment in multiple myeloma. Leuk Lymphoma. 2014;55:1707–1714. [DOI] [PubMed] [Google Scholar]
- [2].Richardson PG, Xie W, Jagannath S, et al. A phase II trial of lenalidomide, bortezomib and dexamethasone in patients with relapsed and relapsed/refractory myeloma. Blood. 2014;123:1461–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Richardson PG, Weller E, Lonial S, et al. Lenalidomide, bortezomib, and dexamethasone combination therapy in patients with newly diagnosed multiple myeloma. Blood. 2010; 116:679–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Jakubowiak AJ, Dytfeld D, Griffith KA, et al. A phase 1/2 study of carfilzomib in combination with lenalidomide and low-dose dexamethasone as a frontline treatment for multiple myeloma. Blood. 2012;120:1801–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Jakob C, Egerer K, Liebisch P, et al. Circulating proteasome levels are an independent prognostic factor for survival in multiple myeloma. Blood. 2007;109:2100–2105. [DOI] [PubMed] [Google Scholar]
- [6].Ma W, Kantarjian H, O'Brien S, et al. Enzymatic activity of circulating proteasomes correlates with clinical behavior in patients with chronic lymphocytic leukemia. Cancer. 2008;112:1306–1312. [DOI] [PubMed] [Google Scholar]
- [7].Ma W, Kantarjian H, Bekele B, et al. Proteasome enzymatic activities in plasma as risk stratification of patients with acute myeloid leukemia and advanced-stage myelodysplastic syndrome. Clin Cancer Res. 2009;15:3820–3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Korde N, Roschewski M, Zingone A, et al. Treatment with carfilzomib-lenalidomide-dexamethasone with lenalidomide extension in patients with smoldering or newly diagnosed multiple myeloma. JAMA Oncol. 2015;1:746–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Durie BG, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–1473. [DOI] [PubMed] [Google Scholar]
- [10].Jasper GA, Arun I, Venzon D, et al. Variables affecting the quantitation of CD22 in neoplastic B cells. Cytometry Part B, Clin Cytom. 2011;80:83–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Tembhare PR, Yuan CM, Venzon D, et al. Flow cytometric differentiation of abnormal and normal plasma cells in the bone marrow in patients with multiple myeloma and its precursor diseases. Leukemia Res. 2014;38:371–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Amer Statist Assn. 1958;53:457–481. [Google Scholar]
- [13].International Myeloma Working G Criteria for the classification of monoclonal gammopathies, multiple myeloma and related disorders: a report of the International Myeloma Working Group. Br J Haematol. 2003;121:749–757. [PubMed] [Google Scholar]
- [14].Greenberg AJ, Rajkumar SV, Therneau TM, et al. Relationship between initial clinical presentation and the molecular cytogenetic classification of myeloma. Leukemia. 2014;28:398–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ocio EM, Mitsiades CS, Orlowski RZ, et al. Future agents and treatment directions in multiple myeloma. Exp Rev Hematol. 2014;7:127–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Wada M, Kosaka M, Saito S, et al. Serum concentration and localization in tumor cells of proteasomes in patients with hematologic malignancy and their pathophysiologic significance. J Lab Clin Med. 1993;121:215–223. [PubMed] [Google Scholar]
- [17].Ostrowska H, Hempel D, Holub M, et al. Assessment of circulating proteasome chymotrypsin-like activity in plasma of patients with acute and chronic leukemias. Clin Biochem. 2008;41:1377–1383. [DOI] [PubMed] [Google Scholar]
- [18].Demo SD, Kirk CJ, Aujay MA, et al. Antitumor activity of PR-171, a novel irreversible inhibitor of the proteasome. Cancer Res. 2007;67:6383–6391. [DOI] [PubMed] [Google Scholar]
- [19].Fink EC, Ebert BL. The novel mechanism of lenalidomide activity. Blood. 2015;126:2366–2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Roth GA, Moser B, Krenn C, et al. Heightened levels of circulating 20S proteasome in critically ill patients. Eur J Clin Invest. 2005;35:399–403. [DOI] [PubMed] [Google Scholar]
- [21].Egerer K, Kuckelkorn U, Rudolph PE, et al. Circulating proteasomes are markers of cell damage and immunologic activity in autoimmune diseases. J Rheumatol. 2002;29:2045–2052. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


