Abstract
HHLA2 is a newly identified member of the B7 immune checkpoint family, but its function and crosstalk with immune cells is not fully understood. To gain insights to HHLA2 expression profile and to determine the clinical significance and function of HHLA2 in pancreatic cancer, we performed immunohistochemistry (IHC) analyses on tissue microarrays (TMAs) of pancreatic ductal adenocarcinoma (PDAC, n=92) with matched peritumoral tissues as well as in cohorts of precancerous lesions: pancreatic intraepithelial neoplasia (PanIN) and intraductal papillary mucinous neoplasm (IPMN). We found that HHLA2 was rarely detected in normal acinar, islet, and ductal cells but widely expressed from early pancreatic precancerous lesions to invasive PDAC. The overall HHLA2 positivity was 95% (19/20) in low grade PanIN and 70.73% (29/41) in IPMN. HHLA2 expression was detected in 77.17% (71/92) of the PDAC cases and was significantly associated with better prognosis in this cohort. Our findings suggest that HHLA2 may behave as a costimulatory ligand in pancreatic cancer, which differs from other B7 family members that are largely characterized as checkpoint inhibitors. Further investigation of the HHLA2 signaling pathway and its receptors is warranted by our data and may lead to novel therapeutic interventions.
Keywords: HHLA2, checkpoint costimulator, pancreatic ductal adenocarcinoma, IPMN, prognostic significance
1. Introduction
Pancreatic ductal adenocarcinoma (PDAC) is a malignancy with an overall 5-year survival of 8% for all stages combined. The majority of patients present with stage IV disease at diagnosis, precluding surgical therapy, and these patients have an overall 5-year survival of 3%. Other therapeutic approaches such as chemotherapy are generally not effective; this is thought to be due largely to abundant desmoplasia in this tumor type. Desmoplasia is a producer of the cytokines and chemokines that orchestrate rapid and silent tumor progression to allow tumor cells to be isolated within extensive fibrosis which results in inefficient drug delivery [1]. Given this innate resistance to chemotherapy agents, new therapeutic approaches aiming to improve the native immune response are urgently needed in this area.
Immune checkpoint proteins are surface molecules on immune effector cell populations that activate/inhibit effector function when engaged to their cognate ligand(s). Expression of the co-inhibitory ligands on cancer cells has been suggested as a mechanism by which these cells evade the immune response [2]. Thus, therapeutics (such as immune checkpoint inhibitors) that block the interaction between the immune checkpoint protein and its ligand(s) can restore the effector function of immune cells and promote tumor regression. At present, clinical trials such as LAG3 (CD233), TIM3, B7H3 (CD276), CD39, CD73, and adenosine A2a receptors have been reported in addition to the CTLA4 and PD1 antibodies which have already been approved [3-5], and clinical trials for other checkpoint receptors and ligand targets are also forthcoming. Most of these immunological checkpoints therapies were developed in combination with the PD-1 pathway-suppressing antibodies including checkpoints co-expressed with PD-L1, in an effort to create a ‘double-block’ therapy.
Immune-based therapy for pancreatic cancer has gained attention in every preceding decade and therefore the enthusiasm now generated tends to be short-lived [6]. In addition to the resistance conferred by desmoplasia, pancreatic cancer is characterized by a highly immunosuppressive environment, with multiple components and pathways inhibiting effective pancreatic cancer targeted immune responses [7]. Therefore, there is great potential in targeting these mechanisms of immunosuppression in order to reverse them and create an environment that supports the infiltration of anti-tumor immune responses and enables the generation of T cells capable of killing pancreatic tumor cells. Each of these components and pathways represents a potential target for pancreatic cancer therapy. Moreover, the analysis of immune infiltrates in human tumors has revealed a strong association between better prognosis and the presence of a humoral response to pancreatic tumor antigens, such as MUC-1 and mesothelin, and of tumor-infiltrating cytotoxic T lymphocyte and Th1 cells [8]. In a mouse model in which an activating KRAS mutation is expressed in the pancreas, pre-invasive pancreatic lesions are characterized by the infiltration of immune suppressor cells rather than immune effector cells, suggesting that tumor immunity may be an early event in pancreatic cancer development [9]. Targeting negative T-cell regulators, such as cytotoxic T-lymphocyte-associated (CTLA) protein-4 and B- and T-lymphocyte attenuator (BTLA) presents another therapeutic option. Vaccines tested on mice were able to reverse the blockage of T-cell response mediated by CTLA4 and BTLA4, confirming the elicitation of anti-tumor immune response is possible [10, 11].
Although the above data suggest that an antitumor immune response may be elicited in PDAC, unfortunately this response is insufficient and does not result in the killing of the tumor [12]. Given that most tumor antigens are self- or mutated self-antigens and that the pancreatic tumor microenvironment is immunosuppressive, this is not surprising. Interestingly, both the prevalence of Tregs in peripheral blood and tumor, and the expression level of PD-L1 in tumor independently predict a poor survival in pancreatic cancer. Tregs, which constitute 5–10 % of CD4 + T cells and induce immune tolerance by suppressing host immune responses against both self- and non-self-antigens, play a crucial role in tolerance and the immune response to cancer. These findings strengthen the notion that pancreatic cancers induce antitumor immune responses. Therefore, attempts to improve the clinical efficacy of immunotherapy should involve strategies to neutralize or overcome immune suppression [13].
HERV-H LTR-associating 2 (HHLA2, also known as B7H7/B7-H5) is a new identified member of B7 family, analogous to PD-L1, PD-L2, B7x, and B7-H3. HHLA2 can bind to its putative receptor(s) on a variety of immune cells, including B cells, T cells, and antigen-presenting cells [14, 15]. Currently, TMIGD2 (Transmembrane and Immunoglobulin Domain Containing 2, also known as IGPR-1 and CD28H) is the only receptor that has been identified for HHLA2 to date. However, the exact physiologic function of HHLA2 is not yet clear, as both co-stimulatory and co-inhibitory properties have been described. Zhao et al. published that HHLA2 serves as a co-inhibitory molecule member with negative effects on T cell proliferation and cytokine production in CD 4+ and CD8+ T cells [16]. In contrast, Zhu et al. reported that the HHLA2/CD28H interaction co-stimulates human T cell growth and cytokine production via an AKT-dependent signaling cascade [17]. Importantly, TMIGD2 is reportedly only expressed on naïve T cells and expression has not been found on other kinds of immune cells. Furthermore, TMIGD2 expression has been shown to rapidly disappear when the naïve T cells are activated and begin maturation phase [17]. As regional tumor-infiltrating immune cells are not naïve cells, the interactions between HHLA2 and TMIGD2 are unlikely to explain the inhibition of the anticancer immune response, efforts should be made in identifying new receptor(s) for HHLA2 in the future.
The expression of HHLA2 has been reported in a majority of tumor specimens, including breast, lung, thyroid, melanoma, ovary, and pancreas [18-20]. The localization of the protein is both membranous and cytoplasmic in tumor cells. As HHLA2 is a transmembrane protein, this type of distribution is common and may be attributed to shuttling of the protein between the cytoplasm and the membrane [16]. HHLA2 protein has also been detected in a lower percentage of other cancers such as liver, bladder, colon, prostate, kidney, and esophagus [19]. According to a previous report by Janakiram et al., half of PDAC tissues examined (5/10 or 50%) displayed increased HHLA2 expression [19]. However, Byers et al. report that HHLA2 was not upregulated in PDAC samples (n=15) when compared with normal pancreas, but was significantly elevated in IPMN (n=4) and the expression level of HHLA2 varied with the degree of dysplasia [20]. To resolve these discrepancies, we analyzed HHLA2 expression by immunohistochemistry in a large cohort of PDAC with matched peritumoral tissue, as well as in IPMNs. The expression level of HHLA2 with respect to clinical outcome in PDAC was analyzed. HHLA2 transcriptional levels in 30 different cancer types accessible from The Cancer Genome Atlas (TCGA) public database were also evaluated in this study.
2. Materials and Methods
2.1. Human samples
Human PDAC tissue microarrays (TMA) were constructed from 92 cases of surgically resected PDAC tumor tissues (2013-2014), along with 91 cases of matched peritumoral tissues from the Pancreas Center & Department of General Surgery, The First Affiliated Hospital of Nanjing Medical University, China. For accurate assessment, the cohort excluded samples with insufficient tumor tissue. All tissue specimens were reviewed by H&E staining and representative areas free from necrosis and hemorrhage were selected in the paraffin blocks. 1 mm diameter cylinders were taken from intratumoral or peritumoral tissues (at distances of 1-2 cm from the tumor edge) and transferred to the TMA by the Pathology Department (The First Affiliated Hospital of Nanjing Medical University, China). The relevant clinical data was collected through retrospective clinical chart reviews. Survival information was collected every three months, with the last update for this study performed on 11/22/2017. All protocols were reviewed and approved by the Academic Ethics Committee.
The demographic information and post-surgical follow-up of the 92 PDAC cases are shown in Table 1. The majority of patients were diagnosed (post-surgically) as stage Ⅱ according to the American Joint Committee on Cancer (AJCC) staging 7th edition (78/92, 84.78%), 3 cases were diagnosed as AJCC stage Ⅰ, and 11 cases were AJCC stage III or Ⅳ. Serum levels of tumor-associated antigens such as CA19-9 and CEA were documented prior to the surgery. 81.52% (75/92) patients presented with elevated CA19-9 and 64.13% (59/92) patients had high CEA level before surgery. Pathological evaluation showed that most PDAC cases were moderately differentiated (81/92, 88.04%), while 7 cases were poorly differentiated, and 4 cases were well-differentiated. The presence of perineural invasion was detected in 66.3% cases (61/92) and vascular invasion was found in 17.39% cases (16/92). 81.52% (75/92) of cases PDAC were located in head of pancreas with the minority (17/92, 18.48%) located in the body or tail of pancreas.
Table 1.
Clinicopathologic characteristics of patients with surgically resected PDAC with respect to HHLA2 expression.
| Parameter | HHLA2 Negative | HHLA2 Positive | P Value | |
|---|---|---|---|---|
| Age, year | 62.05 | 61.32 | 0.733 | |
| Gender | 0.833 | |||
| 0 Female(n=28) | 6(21.43%) | 22(78.57%) | ||
| 1 Male(n=64) | 15(23.44%) | 49(76.56%) | ||
| AJCC staging | 0.233 | |||
| 1 I(n=3) | 1(33.33%) | 2(66.67%) | ||
| 2 II A(n=38) | 6(15.79%) | 32(84.21%) | ||
| II B(n=40) | 11(27.5%) | 29(72.5%) | ||
| III ≦(n=11) | 2(18.18%) | 9(81.82%) | ||
| TNM staging | 0.374 | |||
| T0-2(n=5) | 3(60%) | 2(40%) | ||
| T3(n=76) | 16(21.05%) | 60(78.95%) | ||
| T4(n=11) | 2(18.18%) | 9(81.82%) | ||
| N0(n=37) | 8(21.62%) | 29(78.38%) | ||
| N1(n=55) | 13(23.64%) | 42(76.36%) | ||
| Differentiation level | 0.157 | |||
| low(n=7) | 3(42.86%) | 4(57.14%) | ||
| medium(n=81) | 16(19.75%) | 65(80.25%) | ||
| high(n=4) | 2(50%) | 2(50%) | ||
| CA19-9 | 0.377 | |||
| ≦ 37(n=17) | 2(11.76%) | 15(88.24%) | ||
| 37 <(n=75) | 19(25.33%) | 56(74.67%) | ||
| CEA | 0.809 | |||
| ≦ 3(n=33) | 8(24.24%) | 25(75.76%) | ||
| 3 <(n=59) | 13(22.03%) | 46(77.97%) | ||
| Neural invasion | 0.572 | |||
| yes(n=61) | 15(24.59%) | 46(75.41%) | ||
| no(n=31) | 6(19.35%) | 25(80.65%) | ||
| Vascular invasion | 0.578 | |||
| yes(n=16) | 5(31.25%) | 11(68.75%) | ||
| no(n=76) | 16(21.05%) | 60(78.95%) | ||
| Tumor location | 1 | |||
| head(n=75) | 17(22.67%) | 58(77.33%) | ||
| body & tail(n=17) | 4(23.53%) | 13(76.47%) | ||
| Total | 21(22.83%) | 71(77.17%) | ||
Human IPMN tissue microarrays were constructed as above from archival formalin fixed, paraffin embedded surgical resection tissue at the Columbia University Medical Center Department of Pathology. The 41 cases were chosen to create a representative sampling of low- and high-grade IPMNs as well as the gastric (n=23), intestinal (n=12), and pancreaticobiliary subtypes (n=6). The cases were selected from pancreatic resections performed from January, 1995- June, 2008. The acquisition of the tissue specimens was approved by the Institutional Review Board at Columbia University Medical Center.
2.2. Immunohistochemistry and scoring
For immunohistochemistry (IHC), TMA sections derived from the formalin-fixed and paraffin-embedded blocks were deparaffinized and hydrated by routine procedures. Sodium citrate buffer (pH 6.0) was used as the antigen retrieval (100°C, 20 minutes). The primary antibody, anti-HHLA2 (HPA055478, Sigma-Aldrich) at 1:1000 dilution was incubated overnight at room temperature. Envision+system-HRP (anti-rabbit polymer-HRP, Dako, Code #: K4011) was used as the secondary antibody. The TMA sections were developed with Dako DAB+ Chromogen and Dako Substrate Buffer and then subsequently counterstained with Harris Modified Hematoxylin (Fisher Chemical, Catalog #: SH30-500D) and Scott’s Bluing Reagent (RICCA, Catalog #: 6697-1). After dehydrating sections were mounted with permanent mounting medium (VectaMount, Catalog #: H-5000).
The quantitation of HHLA2 immunostaining was based on the percentage of positively staining cells. TMA slides was evaluated by two independent pathologists (Koehne de Gonzalez and Remotti) who were blinded to patient information. When discrepancies were observed, results were jointly assessed by both investigators and the final score was formed by consensus. HHLA2 immunostaining was quantified by the percentage of positive cells and scored according to the following metric: score 0 (0%), score 1 (1%~5%), score 2 (6%~30%), score 3 (31%~60%), and score 4 (61%~ 100%). Scores of 0 and 1 were considered negative for HHLA2 expression while scores of 2, 3, and 4 were considered positive.
Automated staining of TMA slides was performed for the primary antibody, CD8 (4B11), (Leica Bond prediluted antibody, Catalog# PA0183) utilizing the Leica Bond III system for slide preparation and staining. TMA slides stained for CD8 were scanned in the Leica Aperio AT2 digital slide scanner and quantitated using Aperio area quantification and nuclear algorithms. CD8 quantification was expressed as the total of CD8-positive cells per unit area (mm2).
2.3. Statistical analysis
For each variable parameter, χ2 or Fisher’s exact test were used to analyze the distribution of each categorical variable between HHLA2 negative and positive groups by SPSS. For survival analysis, comparison of the Kaplan-Meier survival curves between different thresholds of HHLA2 expression were performed by Log-rank test using GraphPad Prism 7. We also performed an additional comparison between the relative high and low expression groups (higher 46 samples vs. lower 46 samples).
3. Results
3.1. HHLA2 expression was absent in normal pancreas but abundant in PanIN lesions.
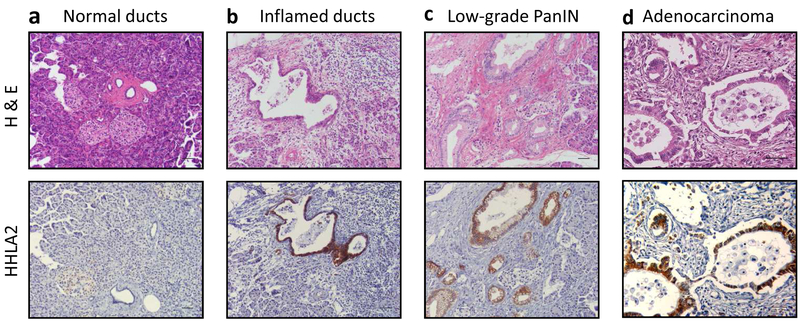
To evaluate the expression level of HHLA2 in primary PDAC, we performed immunohistochemical staining on the TMA slides of both adenocarcinoma and peritumoral tissues and analyzed both independently (Table 2). In the peritumoral tissues, we identified normal pancreas, chronic pancreatitis, and various stages of PanIN lesions. Overall, normal duct cells, islets, and acinar cells were negative for HHLA2 IHC labeling (Fig. 1a). However, we observed HHLA2 positive labeling in some inflamed duct cells. The overall positive labeling of HHLA2 in normal duct cells including inflamed duct cells was 9.89% (9/91) (Fig. 1b). Interestingly, 20 cases of peritumoral tissues contained low grade PanIN lesions (20/91) and most of these PanIN lesions displayed HHLA2 positive labeling (19/20, 95%) (Fig. 1c).
Table 2.
HHLA2 IHC labeling analysis of peritumoral and adenocarcinoma tissues.
| PDAC cohort | |
|---|---|
| Peritumoral tissues (n=91) | HHLA2 positivity rate |
| Normal and inflammed duct cells | 9/91(9.89%) |
| Acinar cells | 1/91(1.09%) |
| Islet cells | 3/91(3.29%) |
| PanIN (n=20) | 19/20(95%) |
| Adenocarcinoma (n=92) | 71/92(77.17%) |
Fig. 1. Representative specimens showing HHLA2 IHC labeling pattern in normal pancreatic ducts, precancerous lesions, and adenocarcinoma.
H&E staining and HHLA2 IHC from the same section of the TMA core are presented. (a) Normal ducts. (b) Inflamed ducts. (c) Low-grade PanIN. (d) Adenocarcinoma. Scale bar, 50um. Magnification, 200x.
3.2. HHLA2 expression was upregulated in PDAC.
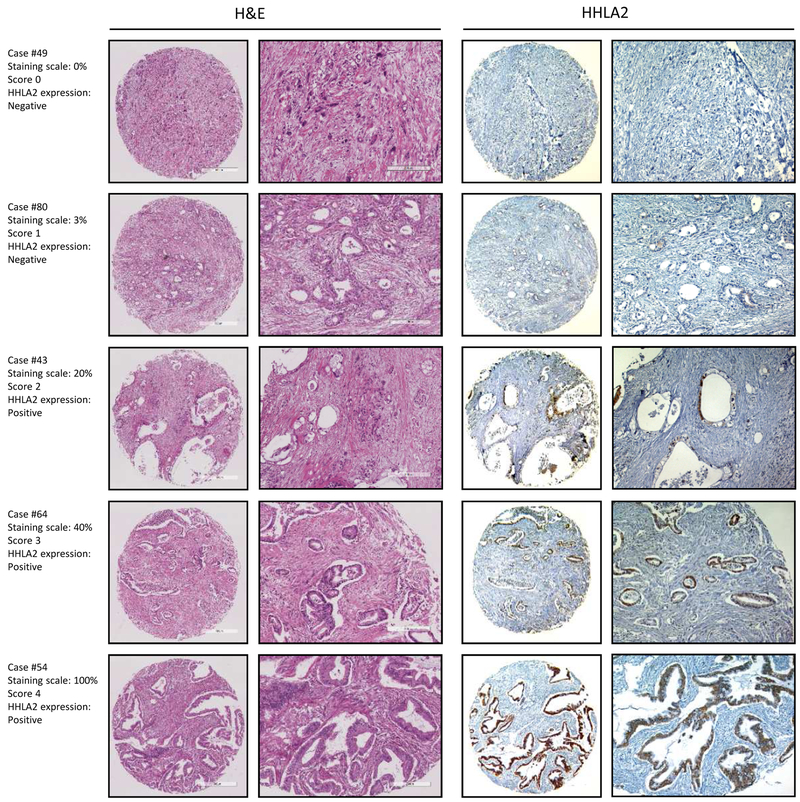
Consistent with the high ratio of HHLA2-positive expression found in PanIN lesions, the majority of the adenocarcinoma tissue exhibited positive HHLA2 expression as well (77.17%, 71/92, Fig. 1d). However, the expression level of HHLA2 among PDAC varied from scores 0-4: 8.7% with score 0 (n=8), 14.13% with score 1 (n=13), 19.57% with score 2 (n=18), 18.48% with score 3 (n=17), and 39.13% in score 4 (n=36). Scores of 0 and 1 were considered negative for HHLA2 expression while scores of 2, 3, and 4 were considered positive (For more details, please refer to the Materials and Methods section). A representative case of HHLA2 IHC labeling for each score is shown in Fig. 2. These results show that HHLA2 expression was limited in normal pancreas but occasionally upregulated in inflammatory foci. A high level of HHLA2 expression was observed in both early and late stages of tumor progression, including low-grade PanIN lesions and invasive PDAC.
Fig. 2. HHLA2 is overexpressed in adenocarcinoma.
Representative sample of each score showing variable HHLA2 IHC staining. HHLA2 IHC expression scoring: score 0 (0%), score 1 (1%~5%), score 2 (6%~30%), score 3 (31%~60%), score 4 (61%~100%). H&E staining and HHLA2 IHC from the same section of the TMA core are presented. Scale bar, 500um. Magnification, 200x.
We investigated the relationship between tumor HHLA2 expression status (negative/positive) and the clinicopathologic variables in PDAC cohort (Table 1); no significant correlation was found between these clinicopathologic parameters and tumoral HHLA2 expression.
3.3. HHLA2 was widely overexpressed in IPMN.
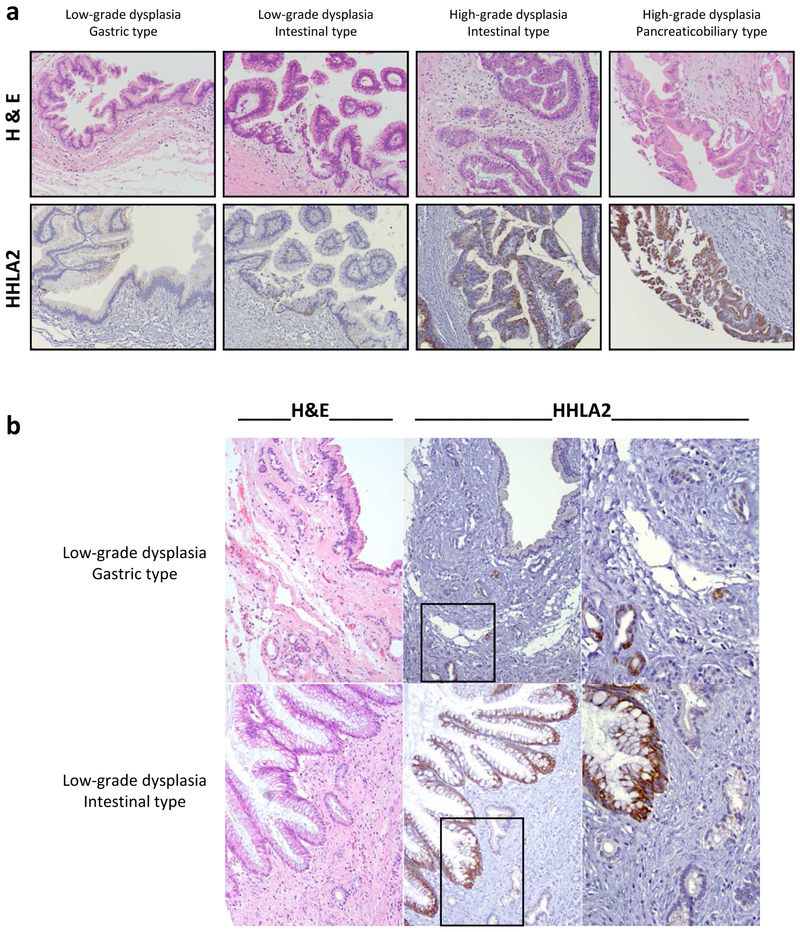
In order to broaden our understanding of HHLA2 expression in pancreatic precancerous lesions, we extended our analysis of HHLA2 expression to include an independent IPMN cohort (n=41). The overall HHLA2 positivity in the IPMN cohort was 70.73% (29/41, Table 3), with low-grade dysplasia at 67.65% (23/34) and high-grade dysplasia at 85.71% (6/7). The ascending trend of HHLA2 expression is not statistically significantly associated with IPMN grade (p=0.65). Among the different morphological subtypes, HHLA2 positive labeling rates of the intestinal type (100%, 12/12) and pancreaticobiliary type (83.33%, 5/6) were relatively higher than the gastric type (47.83%, 11/23, p=0.002). Representative HHLA2 IHC labeling of the different morphological subtypes of IPMN is shown in Fig. 3a. The normal adjacent pancreatic tissue was negative for HHLA2, except for rare proliferative ducts associated with chronic pancreatitis adjacent to IPMN (Fig. 3b).
Table 3.
HHLA2 IHC labeling analysis of IPMN cohorts
| IPMN cohort, n=41 | |
|---|---|
| Grade of dysplasia | HHLA2 positivity rate |
| Low-grade | 23/34(67.65%) |
| High-grade | 6/7(85.71%) |
| Histological subtype | |
| Gastric | 12/23(52.17%) |
| Intestinal | 12/12(100.00%) |
| Pancreatobiliary | 5/6(83.33%) |
| Overall | 29/41(70.73%) |
Fig. 3. Prevalent upregulation of HHLA2 expression in IPMN but not in adjacent tissue.
(a) Representative specimens showing HHLA2 experssion pattern in IPMN lesions with low-grade or high-grade dysplasia and different morphological subtypes. H&E staining and HHLA2 IHC from the same section of the TMA cores are presented. Magnification: 200x. (b) Representative IPMN specimens showing variable HHLA2 IHC staining, with only rare scattered proliferating HHLA2 positive ductules within adjacent chronic pancreatitis. H&E staining and HHLA2 IHC from the same section of the TMA cores are presented. The low grade gastric type IPMN is negative for HHLA2; within the adjacent chronic pancreatitis, scattered proliferating ducts are HHLA2 positive. The low grade intestinal type IPMN is positive for HHLA2; within the adjacent chronic pancreatitis, pancreatic ducts are negative for HHLA2. Magnification: 200x, 400x (inset).
3.4. HHLA2 overexpression was associated with better prognosis in the PDAC cohort.
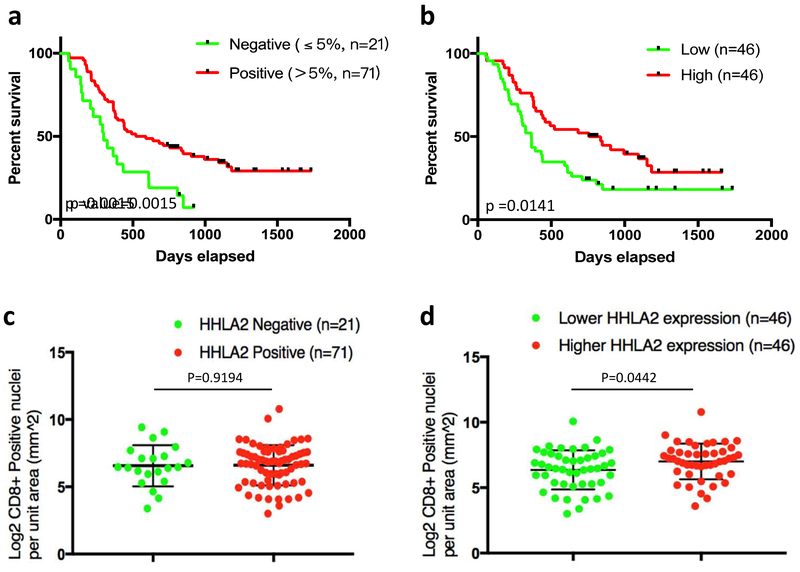
Given the prevalence of HHLA2 overexpression in pancreatic precancerous lesions and adenocarcinoma, the prognostic relevance of the HHLA2 expression in the PDAC cohort was then analyzed. Kaplan-Meier survival curves were generated to investigate potential correlation at multiple HHLA2 expression thresholds as well as separation by median HHLA2 level. Grouping the HHLA2 expression thresholds into the negative (HHLA2 labeling percentage ≤ 5%) and positive (HHLA2 labeling percentage ≤ 5%) groups, the positive group (n=71) was significantly associated with better survival rate than the negative group (n=21, log-rank p=0.0015, Fig. 4a). By separating the PDAC cohort into a relatively high HHLA2 expression group (n=46) and a relatively low HHLA2 expression group (n=46), the Kaplan Meier curve also demonstrated a significant positive correlation between HHLA2 expression and long-term survival rate (log-rank p=0.0141, Fig. 4b). Both of these analyses support the finding that HHLA2 expression is significantly associated with better survival.
Fig. 4. Overexpression of HHLA2 is associated with better prognosis and augmented CD8+ T cells infiltration in PDAC.
(a) The HHLA2 positive PDAC group (>5% positivity, n=71) exhibited a better survival rate than the negative group (n=21, log-rank p-value=0.0015). (b) Median separation of the PDAC cohort by HHLA2 expression levels (higher 46 vs. lower 46): the relative high HHLA2 expression group also demonstrated a longer survival (log-rank p-value=0.0141). (c and d) CD8+ T cells quantitation was analyzed in the PDAC cohort in relation to HHLA2 expression. The association between HHLA2 expression and CD8+ T cell density was analyzed. Overall, there was augmented CD8+ T cell infiltration observed with higher HHLA2 expression. Although the increasing trend of CD8+ T cells infiltration was not statistically significantly associated with HHLA2 positivity when comparing the HHLA2-negative group vs. HHLA2-positive group (unparalleled t test, p=0.9194) (c), there was a statistically significant correlation when comparing the median separation of the PDAC cohort by HHLA2 expression levels (higher 46 vs. lower 46, unparalleled t test, p=0.0442) (d).
Higher levels of CD8+ T cells have been demonstrated to contribute to prolonged survival of PDAC patients [21-23]; therefore, we performed IHC in our TMAs to investigate if CD8+ T cell infiltration contributed to the overall survival of patient cohort in relation to HHLA2 expression level. We observed that there was an overall trend of augmented CD8+ T cell infiltration with increasing HHLA2 expression. Although CD8+ T cell density was not statistically significantly associated with HHLA2 positivity when comparing the HHLA2 negative group vs. positive group (unparalleled t test, p=0.9194) (Fig. 4c), there was a statistically significant correlation when comparing the median separation of the PDAC cohort by HHLA2 expression levels (higher 46 vs. lower 46, unparalleled t test, p=0.0442) (Fig. 4d).
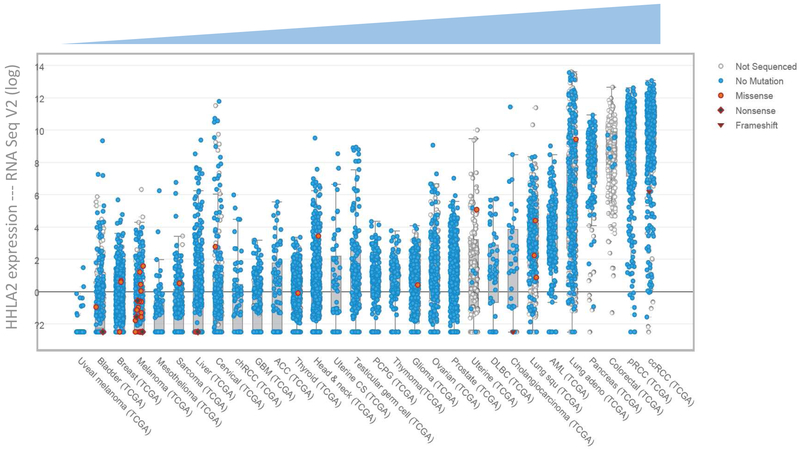
3.5. Upregulation of HHLA2 RNA expression was detected in the majority types of cancers.
To assess the spectrum of HHLA2 expression on a larger scale, we examined the TCGA public database, and scatter plots of HHLA2 RNA expression across 30 different types of human cancer were generated via the cBioPortal [24, 25]. The RNA expression level of HHLA2 in each human cancer compared to respective normal tissue is presented in the log scale in Fig. 5, sorted in ascending order. The majority of the cancer types examined in the database show an upregulated HHLA2 transcription level, including pancreatic cancer, which is consistent with our findings with respect to HHLA2 protein level, and further validate HHLA2 overexpression in pancreatic cancer.
Fig. 5. HHLA2 RNA is overexpressed in the majority types of cancers.
Ascending scatter plots of HHLA2 RNA in different cancer types were sorted by median HHLA2 transcriptional level compared with normal tissues.
4. Discussion
In this study, we focused on examining the expression of HHLA2 in pancreatic tumorigenesis by IHC labeling of human pancreatic precancerous lesions and surgically resected PDAC samples. By independently analyzing the peritumoral tissues and adenocarcinoma tissues, we found that HHLA2 was widely expressed in precancerous lesions of PDAC (PanINs and IPMNs) and PDAC, while essentially negative by IHC in benign peritumoral tissue (Fig. 1, 2). Our findings are consistent with previous reports that HHLA2 expression was not detected in most organs, but was widely expressed in human cancers of the breast, lung, thyroid, melanoma, pancreas, ovary, liver, liver, bladder, colon, prostate, kidney, and esophagus [19]. However, a previous study by Byers et al. reported an expression pattern of HHLA2 opposite to ours, describing high expression of HHLA2 in the normal ducts with loss of HHLA2 expression in PDAC [20]. We are unable to reconcile the discrepancy due to the lack of information on the primary antibody utilized in that study (PDAC cohort, n=15; IPMN cohort, n=4) [20]. Our study was performed with larger cohorts of PDAC (n=92, matched peritumoral tissue n=91) and IPMN (n=41) from two academic institutions, and all the tissues examined showed consistent expression of HHLA2 from precancerous lesions (PanINs and IPMNs) to invasive PDAC (Fig. 1-3). Importantly, the absence of HHLA2 expression in multiple non-neoplastic cell types highlights the association of elevated HHLA2 with pathological changes during tumorigenesis and clarifies the HHLA2 expression pattern in the pancreas. The upregulated HHLA2 RNA transcripts documented for numerous cancer types in the TCGA database, including pancreatic cancer, further support our findings.
Our study is the first to report the prognostic significance of HHLA2 overexpression in PDAC. In our Kaplan Meier survival analysis, higher HHLA2 expression was consistently and significantly associated with better survival rates (Fig. 4 a-b). Given that HHLA2 expression was not associated with any other clinicopathologic parameter (Table 1), HHLA2 acted as an independent prognostic factor in overall survival in this PDAC cohort. Since overexpression of immune checkpoint inhibitors is often associated with poor prognosis while upregulation of costimulators favors better patient survival, our findings suggest that HHLA2 appears to function as a co-stimulating ligand in pancreatic cancer. Infiltration of cytotoxic CD8+ T cells and their spatial proximity to cancer cells have been demonstrated to correlate with increased overall survival for PDAC patients [21-23]. Here we have observed a statistically significant correlation between CD8+ T cell density with HHLA2 expression levels (higher 46 vs. lower 46, unparalleled t test, p=0.0442) (Fig. 4d). Although an increasing trend of CD8+ T cell infiltration was observed with HHLA2 positivity (negative group vs. positive group), the association was not statistically significant (unparalleled t test, p=0.9194, Fig. 4c). The lack of statistical power may be attributed to the low case number in the negative group (n= 21). Interestingly CD8-density alone did not correlate with patient survival in our cohort, suggesting that while CD8+ T cells may contribute to the better patient prognosis associated with HHLA2 expression, other factors are involved.
HHLA2 overexpression is not always a favorable predictor for patient survival in other cancer types. The prognostic significance of HHLA2 expression in osteosarcoma was found to be correlated with metastasis and worse survival [26]. Similarity, in triple-negative breast cancer (TNBC), overexpressed HHLA2 was associated with lymph node positivity and advanced stage of the disease at the time of diagnosis, and also associated with an increased risk of recurrence [19]. These findings imply that HHLA2 overexpression is associated with cancer progression in osteosarcoma and TNBC. It is not uncommon for members of the B7 family to have dual functions depending on the immune milieu, receptor engagement or blockade, or interaction with different receptors. HHLA2 belongs to group III of the B7 family, which also include B7-H3 and B7x. As with HHLA2, the prognostic significance of B7-H3 and B7x also remain to be further delineated [14, 15]. Xie et al. found that soluble B7-H3 promotes invasion and metastasis through the TLR4/NF-κB pathway in pancreatic carcinoma [27]. Xu et al. also identified B7-H3 and B7x as independent predictors of poor prognosis in pancreatic cancer [28]. However, results demonstrating B7-H3 as a co-stimulatory molecule associated with prolonged survival in pancreatic cancer [29] and gastric cancer [30] have also been reported. Given the conflicting results presented thus far on group III of the B7 family, continued research and an improved understanding of these pathways are needed to reconcile these discrepancies and to elucidate their function(s) in the tumor immune microenvironment. Our findings offer an alternative hypothesis: that HHLA2 may behave as a costimulatory ligand in pancreatic cancer, in contrast to other B7 family members which are predominantly characterized as checkpoint inhibitors.
Although our data suggest HHLA2 is a promising line of inquiry in pancreatic cancer, our study has limitations. Due to the small area sampled, the use of TMAs inherently incurs a certain risk for misrepresenting the whole tumor due to the possibility of heterogeneous expression of immune markers, including HHLA2 [31]. Also, moderately differentiated, resectable tumors are overrepresented in our PDAC cohort, likely owning to the fact that only15% of cases are resectable at the time of PDAC diagnosis [32]. Future studies, involving whole tissue sections that would allow more thorough investigations of the immune compartment and functional assays that would explore the role of HHLA2 in pancreatic cancer tumorigenesis, are clearly warranted before successful immunotherapeutic applications can be fully realized. Our study is the first to demonstrate prognostic implications of HHLA2 expression in PDAC and underscores the potential therapeutic relevance of these future interrogations.
Highlights of this study:
We investigated the potential role of a novel B7 immune checkpoint protein, HHLA2, in pancreatic tumorigenesis.
HHLA2 was largely absent in the normal pancreas tissues, but widely expressed from early pancreatic precancerous lesions (both PanINs and IPMNs) to invasive PDAC.
HHLA2 expression was associated with better survival in post-surgical PDAC patients.
Augmented CD8+ T cell infiltration may partially contribute to the improved survival associated with higher HHLA2 expression.
Interrogating the TCGA database, HHLA2 was found transcriptionally upregulated in most of human cancers, including pancreatic cancer.
Our findings suggest that HHLA2 may behave as a costimulatory ligand in pancreatic cancer.
Acknowledgments
Han Yan was supported by the China Scholarship Council (CSC) for research at Columbia University Medical Center. This study was supported by NIH/NCI R01 CA178445 and NIH/NCI R01 CA217207.
Grant Support: This study was supported by NIH/NCI R01 CA178445, NIH/NCI R01 CA217207.
Footnotes
Conflicts of Interest Statement
There is no conflict of interest pertaining to this publication to be disclosed by any of the authors.
Disclosure of Potential Conflicts of Interest: No potential conflict of interest was reported by the authors.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference
- [1].Martinez-Bosch N, Vinaixa J, Navarro P, Immune Evasion in Pancreatic Cancer: From Mechanisms to Therapy, Cancers (Basel), 10 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Vanella V, Festino L, Strudel M, Simeone E, Grimaldi AM, Ascierto PA, PD-L1 inhibitors in the pipeline: Promise and progress, Oncoimmunology, 7 (2017) e1365209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Andrews LP, Marciscano AE, Drake CG, Vignali DA, LAG3 (CD223) as a cancer immunotherapy target, Immunol Rev, 276 (2017) 80–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zhu H, Gu S, Yin M, Shi M, Xin C, Zhu J, Wang J, Huang S, Xie C, Ma J, Pan C, Tang J, Xu M, Bai XF, Analysis of infantile fibrosarcoma reveals extensive T-cell responses within tumors: Implications for immunotherapy, Pediatr Blood Cancer, 65 (2018). [DOI] [PubMed] [Google Scholar]
- [5].Buisseret L, Pommey S, Allard B, Garaud S, Bergeron M, Cousineau I, Ameye L, Bareche Y, Paesmans M, Crown JPA, Di Leo A, Loi S, Piccart-Gebhart M, Willard-Gallo K, Sotiriou C, Stagg J, Clinical significance of CD73 in triple-negative breast cancer: multiplex analysis of a phase III clinical trial, Ann Oncol, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Thind K, Padrnos LJ, Ramanathan RK, Borad MJ, Immunotherapy in pancreatic cancer treatment: a new frontier, Therap Adv Gastroenterol, 10 (2017) 168–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].von Bernstorff W, Voss M, Freichel S, Schmid A, Vogel I, Johnk C, Henne-Bruns D, Kremer B, Kalthoff H, Systemic and local immunosuppression in pancreatic cancer patients, Clin Cancer Res, 7 (2001) 925s–932s. [PubMed] [Google Scholar]
- [8].Foley K, Kim V, Jaffee E, Zheng L, Current progress in immunotherapy for pancreatic cancer, Cancer Lett, 381 (2016) 244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Clark CE, Beatty GL, Vonderheide RH, Immunosurveillance of pancreatic adenocarcinoma: insights from genetically engineered mouse models of cancer, Cancer Lett, 279 (2009) 1–7. [DOI] [PubMed] [Google Scholar]
- [10].Padron A, Hurez V, Gupta HB, Clark CA, Pandeswara SL, Yuan B, Svatek RS, Turk MJ, Drerup JM, Li R, Curiel TJ, Age effects of distinct immune checkpoint blockade treatments in a mouse melanoma model, Exp Gerontol, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Derre L, Rivals JP, Jandus C, Pastor S, Rimoldi D, Romero P, Michielin O, Olive D, Speiser DE, BTLA mediates inhibition of human tumor-specific CD8+ T cells that can be partially reversed by vaccination, J Clin Invest, 120 (2010) 157–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Aroldi F, Zaniboni A, Immunotherapy for pancreatic cancer: present and future, Immunotherapy, 9 (2017) 607–616. [DOI] [PubMed] [Google Scholar]
- [13].Mayor S, Immunotherapy improves overall survival in pancreatic cancer, Lancet Oncol, 16 (2015) e58. [DOI] [PubMed] [Google Scholar]
- [14].Janakiram M, Shah UA, Liu W, Zhao A, Schoenberg MP, Zang X, The third group of the B7-CD28 immune checkpoint family: HHLA2, TMIGD2, B7x, and B7-H3, Immunol Rev, 276 (2017) 26–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Xiao Y, Freeman GJ, A New B7:CD28 Family Checkpoint Target for Cancer Immunotherapy: HHLA2, Clin Cancer Res, 21 (2015) 2201–2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Zhao R, Chinai JM, Buhl S, Scandiuzzi L, Ray A, Jeon H, Ohaegbulam KC, Ghosh K, Zhao A, Scharff MD, Zang X, HHLA2 is a member of the B7 family and inhibits human CD4 and CD8 T-cell function, Proc Natl Acad Sci U S A, 110 (2013) 9879–9884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Zhu Y, Yao S, Iliopoulou BP, Han X, Augustine MM, Xu H, Phennicie RT, Flies SJ, Broadwater M, Ruff W, Taube JM, Zheng L, Luo L, Zhu G, Chen J, Chen L, B7-H5 costimulates human T cells via CD28H, Nat Commun, 4 (2013) 2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Cheng H, Janakiram M, Borczuk A, Lin J, Qiu W, Liu H, Chinai JM, Halmos B, Perez-Soler R, Zang X, HHLA2, a New Immune Checkpoint Member of the B7 Family, Is Widely Expressed in Human Lung Cancer and Associated with EGFR Mutational Status, Clin Cancer Res, 23 (2017) 825–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Janakiram M, Chinai JM, Fineberg S, Fiser A, Montagna C, Medavarapu R, Castano E, Jeon H, Ohaegbulam KC, Zhao R, Zhao A, Almo SC, Sparano JA, Zang X, Expression, Clinical Significance, and Receptor Identification of the Newest B7 Family Member HHLA2 Protein, Clin Cancer Res, 21 (2015) 2359–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Byers JT, Paniccia A, Kaplan J, Koenig M, Kahn N, Wilson L, Chen L, Schulick RD, Edil BH, Zhu Y, Expression of the Novel Costimulatory Molecule B7-H5 in Pancreatic Cancer, Ann Surg Oncol, 22 Suppl 3 (2015) S1574–1579. [DOI] [PubMed] [Google Scholar]
- [21].Fukunaga A, Miyamoto M, Cho Y, Murakami S, Kawarada Y, Oshikiri T, Kato K, Kurokawa T, Suzuoki M, Nakakubo Y, Hiraoka K, Itoh T, Morikawa T, Okushiba S, Kondo S, Katoh H, CD8+ tumor-infiltrating lymphocytes together with CD4+ tumor-infiltrating lymphocytes and dendritic cells improve the prognosis of patients with pancreatic adenocarcinoma, Pancreas, 28 (2004) e26–31. [DOI] [PubMed] [Google Scholar]
- [22].Ino Y, Yamazaki-Itoh R, Shimada K, Iwasaki M, Kosuge T, Kanai Y, Hiraoka N, Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer, Br J Cancer, 108 (2013) 914–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Carstens JL, Correa de Sampaio P, Yang D, Barua S, Wang H, Rao A, Allison JP, LeBleu VS, Kalluri R, Spatial computation of intratumoral T cells correlates with survival of patients with pancreatic cancer, Nat Commun, 8 (2017) 15095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Cerami E, Gao J, Dogrusoz U, Gross BE, Sumer SO, Aksoy BA, Jacobsen A, Byrne CJ, Heuer ML, Larsson E, Antipin Y, Reva B, Goldberg AP, Sander C, Schultz N, The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data, Cancer discovery, 2 (2012) 401–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, Cerami E, Sander C, Schultz N, Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal, Sci Signal, 6 (2013) pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Koirala P, Roth ME, Gill J, Chinai JM, Ewart MR, Piperdi S, Geller DS, Hoang BH, Fatakhova YV, Ghorpade M, Zang X, Gorlick R, HHLA2, a member of the B7 family, is expressed in human osteosarcoma and is associated with metastases and worse survival, Sci Rep, 6 (2016) 31154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Xie C, Liu D, Chen Q, Yang C, Wang B, Wu H, Soluble B7-H3 promotes the invasion and metastasis of pancreatic carcinoma cells through the TLR4/NF-kappaB pathway, Sci Rep, 6 (2016) 27528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Xu H, Chen X, Tao M, Chen K, Chen C, Xu G, Li W, Yuan S, Mao Y, B7-H3 and B7-H4 are independent predictors of a poor prognosis in patients with pancreatic cancer, Oncol Lett, 11 (2016) 1841–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Loos M, Hedderich DM, Ottenhausen M, Giese NA, Laschinger M, Esposito I, Kleeff J, Friess H, Expression of the costimulatory molecule B7-H3 is associated with prolonged survival in human pancreatic cancer, BMC Cancer, 9 (2009) 463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Wu CP, Jiang JT, Tan M, Zhu YB, Ji M, Xu KF, Zhao JM, Zhang GB, Zhang XG, Relationship between co-stimulatory molecule B7-H3 expression and gastric carcinoma histology and prognosis, World J Gastroenterol, 12 (2006) 457–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Botti G, Scognamiglio G, Cantile M, PD-L1 Immunohistochemical Detection in Tumor Cells and Tumor Microenvironment: Main Considerations on the Use of Tissue Micro Arrays, Int J Mol Sci, 17 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Ansari D, Gustafsson A, Andersson R, Update on the management of pancreatic cancer: surgery is not enough, World J Gastroenterol, 21 (2015) 3157–3165. [DOI] [PMC free article] [PubMed] [Google Scholar]