Abstract
A lipid profile resistant to oxidative damage is an inherent trait associated with animal lifespan. However, there is a lack of lipidomic studies on human longevity. Here we use mass spectrometry based technologies to detect and quantify 137 ether lipids to define a phenotype of healthy humans with exceptional lifespan. Ether lipids were chosen because of their antioxidant properties and ability to modulate oxidative stress. Our results demonstrate that a specific ether lipid signature can be obtained to define the centenarian state. This profile comprises higher level of alkyl forms derived from phosphatidylcholine with shorter number of carbon atoms and double bonds; and decreased content in alkenyl forms from phosphatidylethanolamine with longer chain length and higher double bonds. This compositional pattern suggests that ether lipids from centenarians are more resistant to lipid peroxidation, and that ether lipid signature expresses an optimized feature associated with exceptional human longevity. These results are in keeping with the free radical theory of aging.
Keywords: Alkenyl phospholipids, Alkyl phospholipids, Centenarians, Fatty acid unsaturation, Mass spectrometry, Phosphatidylcholine, Phosphatidylethanolamine, Plasmalogens
Graphical abstract
Highlights
-
•
Centenarians have higher level of alkyl forms derived from phosphatidylcholine.
-
•
Centenarians show decreased alkenyl forms from phosphatidylethanolamine.
-
•
Ether lipids from centenarians are more resistant to lipid peroxidation.
-
•
Ether lipid signature expresses an optimized trait associated with exceptional human longevity.
1. Introduction
Maximum lifespan (MLSP) is a species-specific feature that may differ more than 5000-fold among animal species being about 120 years in humans [1], [2]. Centenarians are considered an exceptional human model of healthy aging and extreme longevity [3], [4]. Available evidences reveal the existence of a link between MLSP and lipids [5]. Thus, the findings from several studies demonstrate that the membrane fatty acid profile differs between animal species (including vertebrates, invertebrates, and exceptionally long-lived animal species) and that cell membrane susceptibility to lipid peroxidation is inversely related to MLSP [5], [6], [7]. Furthermore, a recent phylogenomic approach showed that genes involved in lipid metabolism have undergone an increased selective pressure in long-lived species [8], reinforcing the idea that cell membrane lipid profile has been an optimized evolutionary adaption [5]. As an extension of these findings, recent lipidomics studies confirm that the lipidome is also species-specific and an improved feature associated with animal lifespan [9], [10]. In addition, there are observations of genetic changes in regulatory genes of lipid metabolism to play a role in human lifespan [11], [12], [13]. All these observations point to lipids as a key target to study the molecular adaptive mechanisms underlying differences in lifespan among animal species and within a giving species, even as humans.
Ether lipids are subclass of glycerophospholipids (GP) that have an alkyl chain attached by an ether bond at the sn-1 position of the glycerol backbone [14], [15]. The sn-2 position of ether lipids has an ester-linked acyl chain, as in diacyl phospholipids. Some ether-linked phospholipids, called alkenyl-acylphospholipids, contain a cis or Z double bond adjacent to the ether linkage and are commonly referred to as plasmalogens. The terms "plasmanyl-" and "plasmenyl-" lipids for alkyl and alkenyl ethers, respectively, can also be used. The alkyl ether linkage is represented by the "O-" prefix, and the (1Z)-alkenyl ether (plasmalogen) species by the "P-" prefix. Ether lipids are mostly present as phosphatidylcholine (PC) and phosphatidylethanolamine (PE) species [14]. Ether lipids constitute around 20% of the total glycerophospholipid pool in mammalian species and have a heterogeneous distribution depending on the tissue [16]. For instance, plasmalogens levels are particularly high in cardiac and neural tissues. At a cellular level, the ether lipid biosynthesis begins in the peroxisome and is completed in the endoplasmic reticulum [14], [15], [16].
The physiological role of ether lipids, and specially plasmalogens, is essentially linked to their function as membrane components. Thus, plasmalogens seem to play a key role in specific properties of cell membrane such as membrane fluidity, formation and stability of lipid raft microdomains, and as a source of second messengers [14], [15], [16]. Other functions where plasmalogens are involved are transmembrane protein function, cholesterol transport, vesicular function, membrane fusion events, and G-protein mediated signal transduction [14], [15], [16]. Interestingly, an antioxidant effect has also been ascribed to plasmalogens [14], [15], [16], [17]. Effectively, the vinyl-ether linkage of the plasmalogens is particularly susceptible to oxidation by reactive species such as reactive oxygen species and hypochlorous acid, and thus, like a scavenger, could protect unsaturated membrane lipids (as well as lipoproteins) against oxidation. Consequently, plasmalogens could have a modulatory effect on oxidative stress, lipid-derived inflammation and cell signalling mechanisms. Lipidomic studies reveal that ether lipids are inversely associated with genetic peroxisomal disorders, and also suggest that they are negatively associated with prevalent disease states such as obesity, prediabetes, type 2 diabetes mellitus, cardiovascular disease, cancer and Alzheimer disease, among others [14], [15], [16], [18], [19]. Notably, these pathological states share as common trait an increased oxidative stress, and a potential mechanistic role for plasmalogens.
As a consequence of these multiple observations, it is plausible to postulate that deciphering why centenarians markedly delay or in several cases even avoid age-associated diseases can help us in better understanding the aging process and the pathogenesis of age-related diseases, and that in these processes ether lipids and particularly plasmalogens can be involved.
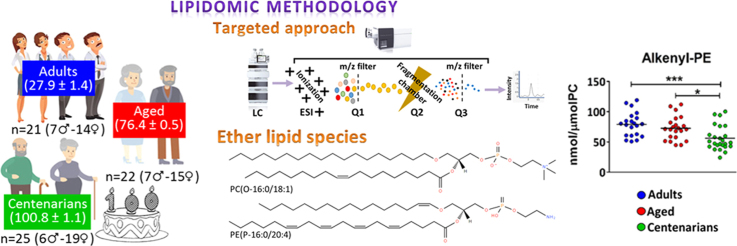
Although the fact that systems biology-based approaches allow a comprehensive molecular characterization of complex biological systems, up to date no targeted lipidomic studies investigating differences in plasma of exceptionally long-lived humans have been reported. To this end, we have designed a study that represents the most detailed lipidomic analysis of plasma ether lipids associated with human longevity to detect and quantify a panel of ether lipids including 137 molecular species: 14 PE(O-), 4 LPE(P-), 55 PE(P-), 22 PC(O-), 10 LPC(O-), 6 LPC(P-), and 26 PC(P-). The plasma ether lipid profile was determined using a LC-QQQ-MS/MS platform to systematically define specific phenotypic patterns associated with genotypes of human extreme longevity. We discovered a particular ether lipid signature related to the condition of extreme longevity, allowing the identification of potential mechanisms and biomarkers of healthy aging.
2. Results
In the present study 137 ether lipid molecular species in human plasma were measured. This broad panel of ether lipids includes: 46 alkyl-phospholipids, and 91 alkenyl-phospholipids or plasmalogens. From alkyl-phospholipids, 14 molecular species were PE(O-), 22 PC(O-), and 10 LPC(O-); from alkenyl-phospholipids, 55 molecular species were PE(P-), 4 LPE(P-), 26 PC(P-), and 6 LPC(P-). Hence, all the ether lipids detected are present as phosphatidylethanolamine (PE(O-) and PE(P-), n = 73) or phosphatidylcholine (PC(O-) and PC(P-), n = 64).
The baseline characteristics of the study groups including basic lipid biochemical determinations are shown in Table 1. No significant differences were observed for HDL-cholesterol, VLDL-cholesterol, free cholesterol and plasma triacylglycerides among groups; in contrast, total-cholesterol and LDL-cholesterol showed significantly increased levels in the aged group compared to adults and centenarians.
Table 1.
Participant characteristics. Number of subjects, age and sex of the participants and basic lipid biochemistry.
| Adults (n = 21) | Aged (n = 22) | Centenarians (n = 25) | p value | Post hoc | |
|---|---|---|---|---|---|
| Age (years) | 27.9 ± 1.4 | 76.4 ± 0.5 | 100.8 ± 1.1 | < 0.0001 | Ad vs Ag*** |
| Ag vs C*** | |||||
| Ad vs C*** | |||||
| n (n of females) | 21 (14) | 22 (15) | 25 (19) | 0.7038 | ns |
| Total cholesterol (mg/dl) | 177.0 ± 8.3 | 204.3 ± 6.9 | 174.2 ± 7.6 | 0.0081 | Ad vs Ag* |
| Ag vs C* | |||||
| HDL-cholesterol (mg/dl) | 56.7 ± 4.0 | 54.9 ± 2.2 | 55.1 ± 2.3 | 0.8867 | ns |
| LDL -cholesterol (mg/dl) | 92.9 ± 6.1 | 120.4 ± 5.7 | 98.0 ± 6.0 | 0.0024 | Ad vs Ag** |
| Ag vs C* | |||||
| VLDL-cholesterol (mg/dl) | 27.4 ± 3.8 | 29.0 ± 2.6 | 21.1 ± 1.6 | 0.0796 | ns |
| Free cholesterol (mg/dL) | 41.7 ± 1.5 | 41.4 ± 1.5 | 43.3 ± 1.4 | 0.5458 | ns |
| Triacylglycerides (mg/dL) | 70.3 ± 2.8 | 66.7 ± 3.7 | 67.9 ± 2.8 | 0.7113 | ns |
Values shown are mean ± standard error (SEM).
ns, not significant differences.
p < 0.05.
p < 0.01.
p < 0.001.
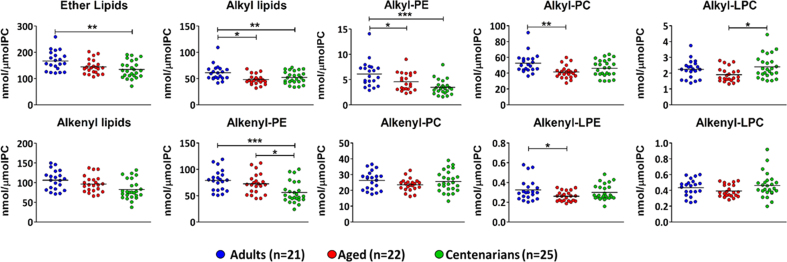
In a first approach, we would like to test whether global inter-group (adults, aged and centenarians) differences exist (Fig. 1). The results show that total ether lipids are statistically lower in centenarians with respect to adults (by 20%; p < 0.01), while no differences are detected for aged subjects (Fig. 1). This difference can be ascribed to alkyl lipids, which are decreased in the aged (by 15%; p < 0.05) and centenarian (by 21%; p < 0.01) groups with respect to adults; and alkenyl lipids, only decreased in the centenarian group (by 22%; p < 0.01) respect to adults. More specifically, for alkyl lipids, we observe a decreased content for alkyl-PE (by 25%; p < 0.05) and alkyl-PC (by 21%; p < 0.01) in the aged group compared to adults, while a decreased content in alkyl-PE is shown in centenarians compared to adults (by 43%; p < 0.001). Alkyl-LPCs were significantly higher in centenarians with respect to aged (by 26%; p < 0.05). For alkenyl lipids, a decreased content of plasmalogens in centenarians with respect to the adults (by 26%; p < 0.001) and aged subjects (by 9%; p < 0.05) was observed.
Fig. 1.
Graphical representation of the amounts of ether lipids in each individual of each experimental group in total or cluster by the type of glycerophospholipid and ether bond. The expressed concentrations are the experimental group mean ± standard error of the sum of the all the molecular species within that class or subclass of ether lipids. p-values obtained by one-way ANOVA post hoc Tukey's * p˂0.05; * *p ˂0.01 and * **p ˂0.001.
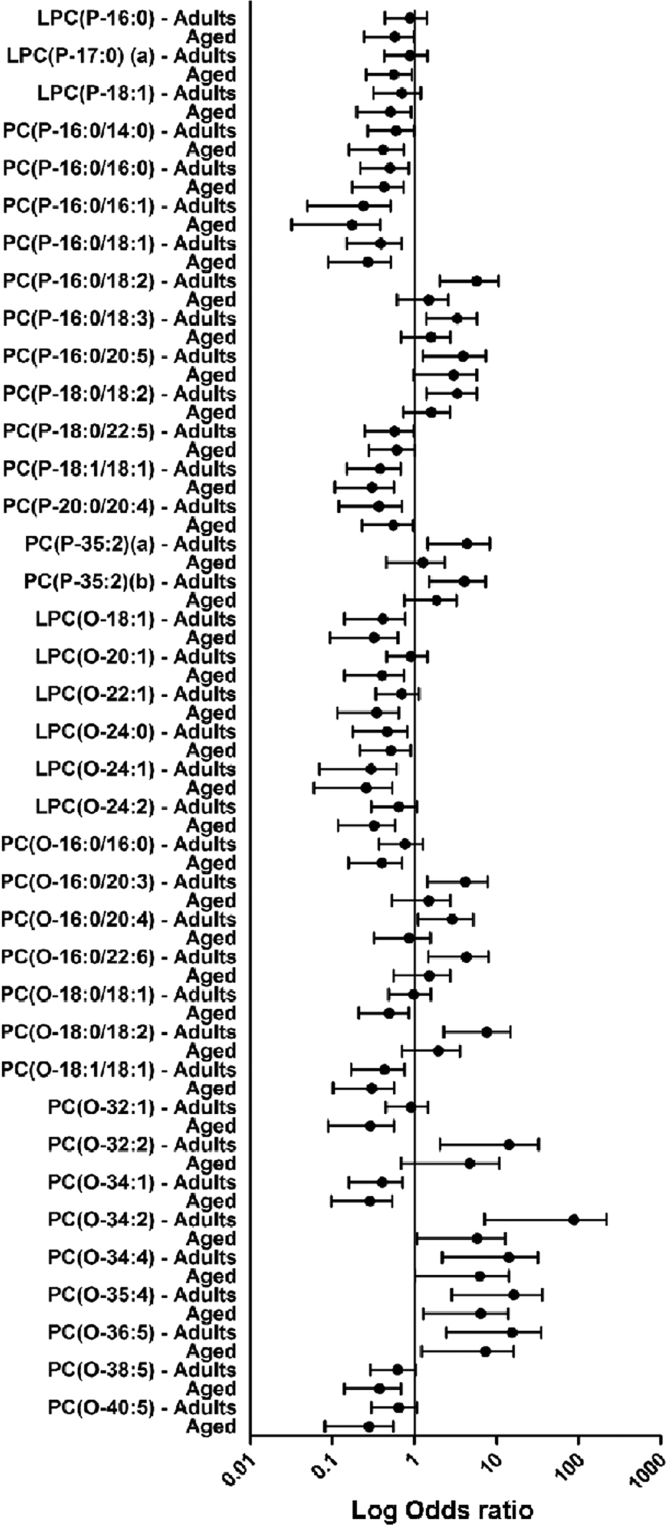
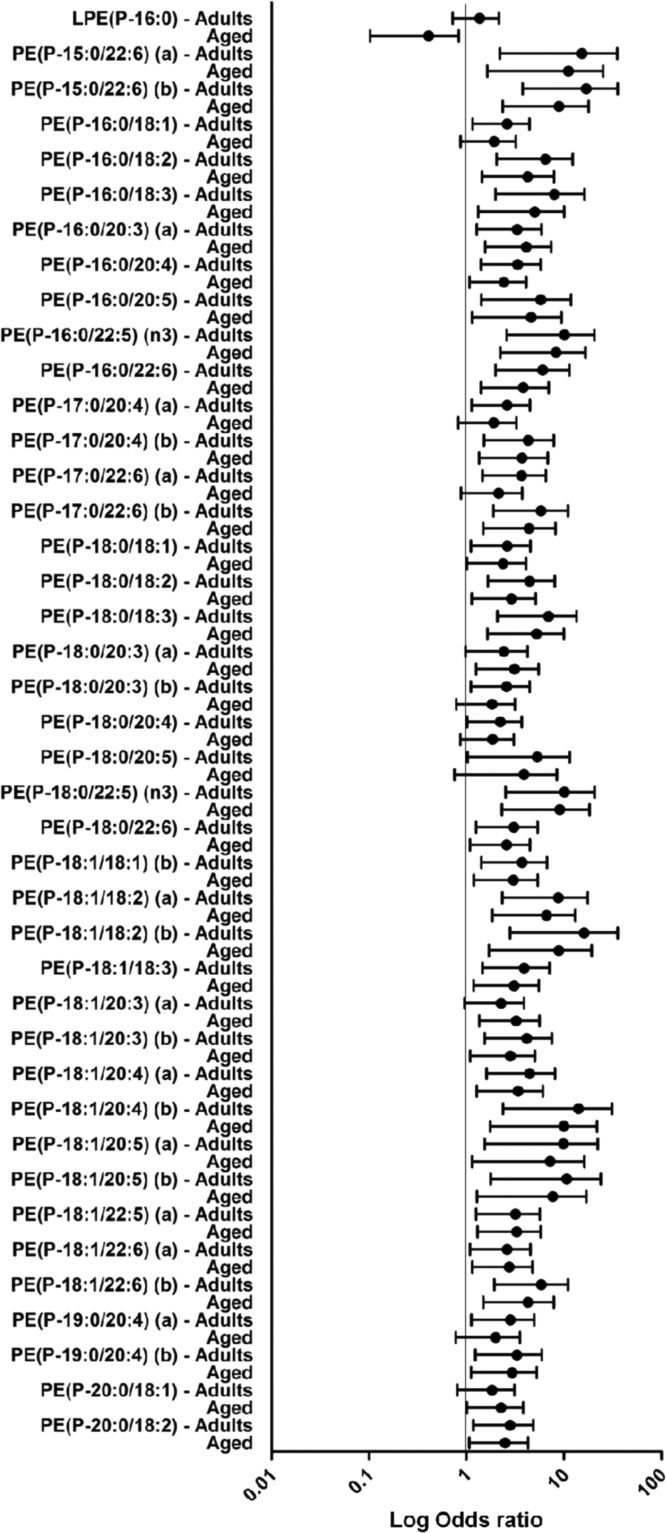
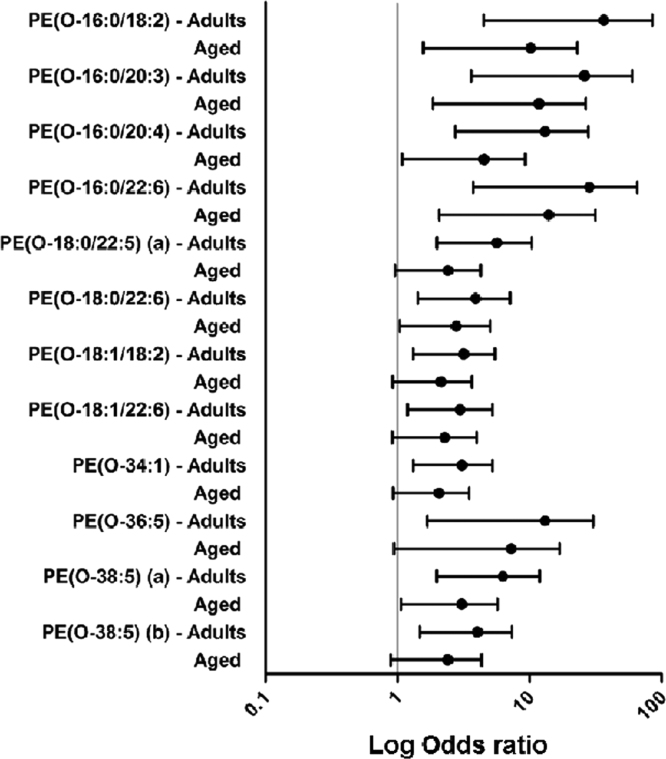
Multinomial regression models have been performed with the centenarian group as reference in order to find associations and differential ether lipid species. Results are represented as the logarithmic value of odds ratio (log OR) and their confidence intervals, lack of association between the concentration of the ether lipids species and the fact of being a centenarian will show a log OR equal to 1. On the other hand, values under or above the unit will represent an increase or a decrease, respectively, of the ether lipid in the centenarian group. Thus, 91 of the 137 detected ether lipid species (66.4%) were statistically different in adults or aged compared to centenarians. Among them, 50 (36.5%) ether lipid species were different between centenarians and the other two groups, 16 (11.7%) just between centenarians and aged, and 25 (18.2%) just between centenarians and adults (Fig. 2, Fig. 3, Fig. 4). In particular, when adults are compared to centenarians, the differential molecular species belong 47% to PE(P-) and 19% of PC(O-); and when aged group is compared to centenarians, the affected molecules were 52% PE(P-) and 26% PC(O-). Therefore both groups (adults and aged) present a similar proportion among the differential lipid species compared to centenarians.
Fig. 2.
Multinomial logistic regression model examining the association between PC ether lipid species and the experimental groups (adults, aged and centenarians). Odds ratio in logarithmic scale represent the estimate differences in the concentration of the ether lipid species in adults and aged with respect to centenarians which is the reference group.
Fig. 3.
Multinomial logistic regression model examining the association between PE(P-) ether lipid species and the experimental groups (adults, aged and centenarians). Odds ratio in logarithmic scale represent the estimate changes in the concentration of the ether lipid species in adults and aged with respect to centenarians which is the reference group.
Fig. 4.
Multinomial logistic regression model examining the association between PE(O-) ether lipid species and the experimental groups (adults, aged and centenarians). Odds ratio in logarithmic scale represent the estimate changes in the concentration of the ether lipid species in adults and aged with respect to centenarians which is the reference group.
To evaluate the potential influence of the variable ‘age’ in the observed differences among groups, we performed a linear regression analysis with the Benjamini-Hochberg correction. The outcome of this analysis shows that the human plasma concentration of 72 (52.5% of total ether lipids measured) molecular species are significantly correlated with age (see Table S2); 64 are negatively correlated with age, while 8 are positively correlated with age. Among the negatively correlated species with age, 34% are alkyl-phospholipids (9 PC(O-) and 13 PE(O-)), and 63% plasmalogens (6 PC(P-) and 36 PE(P-)). In contrast, all the ether lipids which correlate positively with age are derived exclusively from phosphatidylcholine, being 50% of molecular species alkyl-PC (2 LPC(O-) and 2 PC(O-)), and 50% alkenyl-PC or plasmalogens (4 PC(P-)).
To know whether exist a specific ether lipid signature which defines the centenarian condition in an age-independent way we evaluated which differential lipids did not correlate with age. Comparing the multinomial regression models with age-associated results, there were 21 ether lipid species significantly different that did not show age correlation. Among them, 16 lipid species could be used as biomarkers of healthy aging being 13 of them increased in both adults and centenarians but not in aged individuals and the other 3 ether lipids, PE(P-) species, decreased in adults and centenarians but not in aged. On the other hand, 4 ether lipid species (LPC(O-24:0), PC(P-16:0/16:0), PE(P-18:1/22:6) (a) and PE(P-18:1/22:5) (a)) are significantly different in centenarians compared to both groups, adults and aged. Therefore, they could be possible biomarkers for the centenarian condition being both PC species increased and both PE(P-) decreased. Finally, the ether lipid PC(P-18:0/22:5) is significantly different only between adults and centenarians.
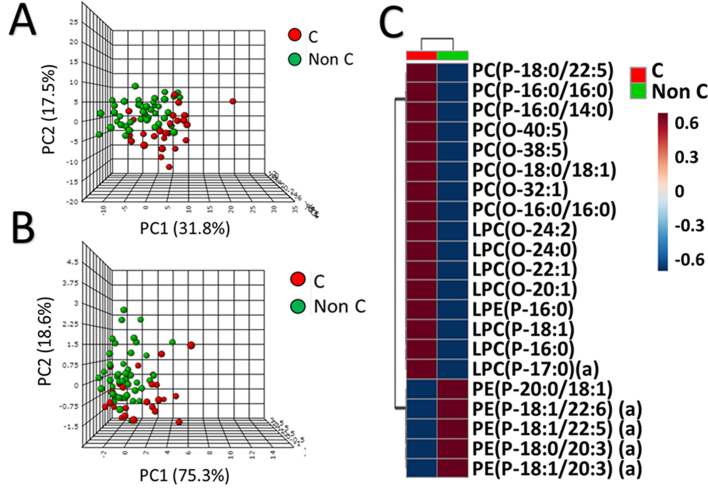
In order to evaluate the weight of these ether lipid species in plasma variability across samples we have analyzed plasma ether lipid profile from healthy human grouped as centenarians or non-centenarians (grouping adults and aged subjects). Principal Component Analysis (PCA) (Fig. 5A) shows a good clusterization of non-centenarians and centenarians subjects. Interestingly, some of non-centenarians subjects (all of them aged subjects) cluster together with centenarians individuals suggesting a higher probability to become centenarians. When a PCA of the centenarians and non-centenarians is performed with the 21 ether lipid species found differential between the experimental groups in an age-independent way the separation between the centenarians and non-centenarians is better (40% higher in component 1) (Fig. 5B). Likewise, performing a heat map with these 21 ether lipid species allows us to visualize possible clustering (Fig. 5C) and a pattern emerges suggesting an ether lipid signature that defines the centenarian condition. In this centenarian signature, 15 ether lipids derived from PC are significantly increased, while 6 ether lipids derived from PE are significantly decreased, except for LPE(P-16:0) increased in aged population compared to centenarians.
Fig. 5.
Defining the centenarian profile by ether lipids A) Principal component analysis (PCA) of the centenarian (red) and non-centenarian subjects (green) based on the 137 ether lipid species detected. B) PCA of the centenarians and non-centenarians based on the twenty-one ether lipid species significantly different in centenarians in an age independent manner. C) Heat map of the twenty one ether lipid species significantly different in centenarians in an age independent manner.
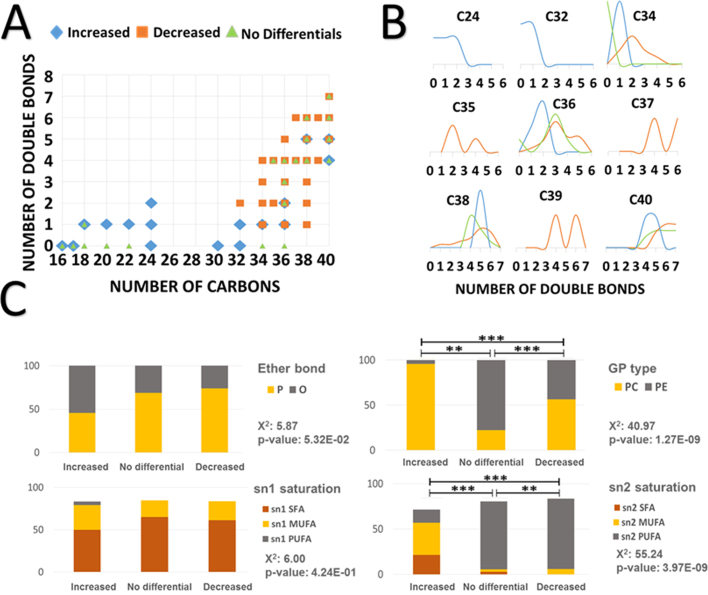
We have also analyzed the differential ether lipids which define the centenarian condition to unravel potential structural and/or compositional traits. Thus, the distribution of the differential ether lipids according to the number of carbons and the number of double bonds shows that increased ether lipids are almost all of them PC species with shorter number of carbon atoms and lower number of double bonds, presenting significantly differences in the saturation of the sn2 chain. On the other hand, the decreased ether lipids are longer in carbon atoms and higher in double bonds, especially in the sn2 chain being almost all them PUFA. As for other fatty acids chains the number of carbon and unsaturations is independent of the centenarian condition being able to be increased, decreased or not to be modified as (40:5). (Fig. 6A-D).
Fig. 6.
Structural characteristics of the ether lipids. A, B) Distribution of the lipids according to the number of carbons and the number of double bonds. Data was obtain by a multinomial regression model with centenarians as a reference group. C) Distribution of the lipids according to the type of ether bond, glycerophospholipid type and unsaturation of the fatty acid present at sn1 and sn2 position. Statistical values were obtained from Pearson chi-squared test. * <p.0.05; * *<p.0.01; * ** <p.0.001.
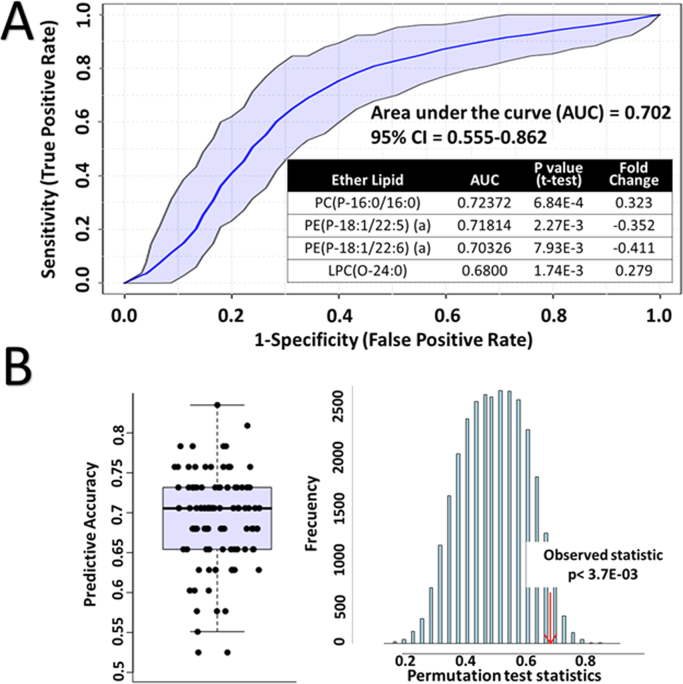
Finally, a model based on a ROC curve biomarker analysis was performed (Fig. 7A). A random forest algorithm was chosen for the ROC analysis and the chosen ether lipid species to generate the model were the possible biomarkers of the centenarian condition. All of the ether lipids chosen were significantly different in centenarians compared with adult and aged individual but did not show correlation with the variable “age”. AUC, p-value and fold change of these 4 lipid species is represented in Fig. 7A. Half of them were PE(P-) with polyunsaturated fatty acids at the sn-2 position, as we have seen they are decreased in centenarians and the other half, saturated PC species increased in centenarians, one LPC(O-) and other PC(P-). As a result, the predictive accuracy test of the ROC curve generated (Fig. 7B, left) indicates an average accuracy of 0.702 based on 100 cross validations. Likewise, the permutation test with predictive accuracy as performance measure and 1000 permutations showed a p value < 3.7E-03 (Fig. 7B, right).
Fig. 7.
A) ROC curve based biomarker analysis performed by random forest algorithm on four chosen ether lipids. The species chosen for the model were the possible biomarkers of the centenarian condition: two sn2 PUFA alkenyl-PE decreased in centenarians, and two SFA PC species increased in centenarians. Table inside represents the area under the curve, p value obtained by t-test and fold change values for each ether lipid in the model. B) Predicted accuracy test for the validation of the model. On the right, the average accuracy based on 100 cross validations is 0.712. On the left the permutation test with predictive accuracy as performance measure and 1000 permutation times. The observed statistic p value was ˂ 3.7E-03.
3. Discussion
Although their wide presence and abundance in living organisms and their relevance to human health, the detection and quantification of ether lipids represents a technical challenge. The reduced number of the studied ether lipids species and the indirect determination of the plasmalogen content (for instance, measuring the content of fatty acid dimethyl acetals) are common restrictions and, consequently, no conclusive results have been obtained yet. This fact represents a constraint for studying their structural and compositional diversity and their cellular functions in animal species, as well as their association with complex processes such as aging and longevity. The findings obtained from lipidomic studies in animal models of exceptional longevity such as the worm C. elegans [20], the mud clam Arctica islandica [21], and the naked mole-rat (Heterocephalus glaber) [22] suggest an association between their content and animal longevity. For instance, the naked mole-rat had no docosahexaenoic acid-containing plasmenyl phospholipids, but they had much higher levels of total plasmenylcholines [22]. For bivalves, no correlation between plasmalogens content and longevity comparing five different bivalves species differing in their lifespan was found [21]. For worms, C. elegans strains carrying loss-of-function mutations in genes encoding protein required for ether lipid biosynthesis demonstrated a shorter lifespan, and a decreased resistance to oxidative stress [20]. Lipidomic studies on human longevity describe different plasma concentration of “plasmanyl” and “plasmenyl” lipid species in centenarian subjects in respect to aged and adult subjects [13], and in middle-aged offspring of nonagenarians as compared to their controls [23], [24]. Further, for aging studies, the few works performed in relation to human aging suggest decreased plasmalogen content with age [25], [26].
In accordance with previous works, changes in total cholesterol and LDL-cholesterol were found in the elderly compared with adults and centenarians [27], [28], [29]. Focusing on ether lipid species, our results demonstrate that total ether lipids are significant lower in centenarians. This difference can globally be ascribed to both alkyl and alkenyl lipids, which are decreased in centenarian group. More specifically, for alkyl lipids, we observed decreased content in alkyl-PE, while alkyl-LPC was significantly and surprisingly higher in centenarians. For alkenyl lipids, a decreased content in plasmalogens in centenarians with respect to the adults and aged subjects was observed. In this context, it is possible to postulate that the described ether lipid profile in centenarians could positively affect cognition performance and accordingly the survival as previously proposed [30].
Our study also demonstrates that 72 molecular species are significantly correlated with age; 64 of them are negatively correlated, while 8 are positively correlated with age. Globally, the decreased ether lipids with age, mostly PE, lead to a profile where there is a decreased content of polyunsaturated fatty acids in sn-2, which is the typical fatty acid present in sn-2 position of these ether lipids. In contrast, the ether lipids that correlate positively with age are all of them derived exclusively from phosphatidylcholine. Interestingly, all these molecular species share in their structural composition the exclusive presence of saturated and monounsaturated lipids.
A specific ether lipid signature can be obtained in plasma of centenarians. In this centenarian signature, 15 ether lipids derived from PC are significantly increased, 5 ether lipids derived from PE are significantly decreased while LPE(P-16:0) lipid species is only increased in aged. Notably, this signature responds to specific traits in the ether lipids. Thus, the increased ether lipids are preferentially from molecular species with shorter number of carbon atoms and lower number of double bonds, predominates the presence of the alkyl form, and are ascribed to PC molecular species. In contrast, the decreased ether lipids are longer in carbon atoms and higher in double bonds, the alkenyl form is predominant, and is preferentially ascribed to PE molecular species. These differences are not due to the fatty alcohol present at position sn-1 of the glycerol backbone, but to the fatty acid esterified at sn-2 position. This new compositional pattern determines that the density of double bonds and susceptibility to peroxidation, which takes into account that the sensitivity to peroxidation increases as a function of the number of double bonds per fatty acid [31], were significantly lower in centenarians compared to non-centenarians, suggesting that ether lipids from centenarians are more resistant to lipid peroxidation. Reinforcing this concept of resistance to oxidation, the increased PC molecular species in centenarians are predominantly alkyl forms, which are not susceptible to oxidation in the way that vinyl-ether species are. So, PC(O-) species with few double bonds should be very stable. Thus, the ether lipid profile predominant in centenarians suggest a greater resistance to oxidation based on the increased alkyl forms and the decreased content in PUFAs and increased for monounsaturated fatty acid, analogously to the resistance to lipid peroxidation of membrane fatty acid composition described for long-lived animal species, animals maintained on caloric restriction, and offspring of long-lived individuals [5]. It is still unknown if this fatty acid profile observed in ether lipids species is a specific trait of these species in centenarians or is a conserved pattern among other lipid categories in centenarians. In this scenario, we propose that low levels of whole ether lipids and specific increase in alkyl PC forms in centenarians express a physiological adaption to an inherently low oxidative stress present in centenarians. In fact, plasma levels of oxidative markers such as malondialdehyde and protein carbonylation are lower in centenarians than aged [32].
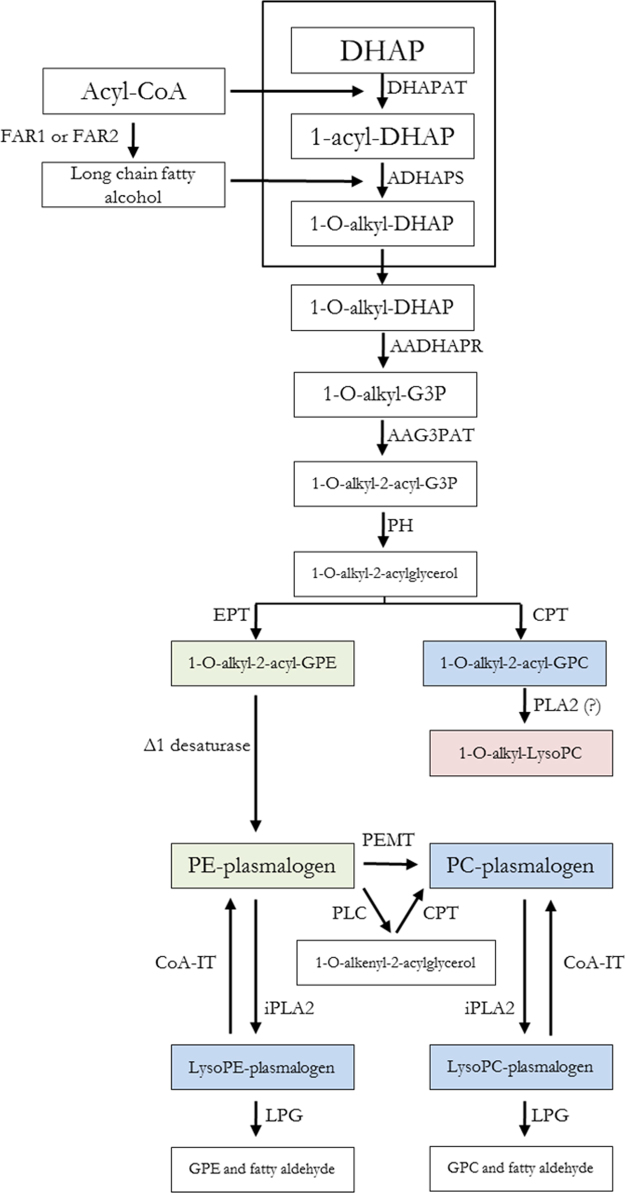
The molecular mechanisms underlying this adaption in centenarians could be related to a different regulation in the biosynthesis pathways of ether lipids [19] (see Fig. 8). In this scenario, and in order to explain the specific ether lipid profile observed in centenarians, it is proposed that: 1) centenarians have a greater FAR1/2 activity to maintain a higher pool of saturated and monounsaturated fatty alcohols; 2) centenarians present lower ethanolamine phosphotransferase and delta1-desaturase activities to maintain decreased levels of PE plasmalogens; 3) centenarians have increased phosphatidylethanolamine methyltransferase activity and/or increased hydrolysis of PE plasmalogens and choline phosphotransferase activity to maintain a normal level of PC plasmalogens; and 4) it is postulated the presence of a specific human phospholipase A2 for the synthesis of 1-O-alkyl-LysoPC whose activity is increased in centenarians (Fig. 8).
Fig. 8.
Physiological adaptions in the biosynthesis pathway of plasmalogens in centenarians. Abbreviations: For metabolites: DHAP, dihydroxyacetone phosphate; G3P, glycerol-3-phosphate; GPC, glycerophosphocholine; GPE, glycerophosphoethanolamine; PC, phosphatidylcholine; PE, phosphatidylethanolamine; for enzymes: FAR1/2, fatty acyl-CoA reductase 1 or 2; DHAPAT, DHAP acyltransferase; ADHAPS, alkyl-DHAP synthase; AADHAPR, acyl/alkyl DHAP reductase; AAG3PAT; acyl/alkyl G3P acyltransferase; PH, phosphohydrolase; CPT, choline phosphotransferase; EPT, ethanolamine phosphotransferase; PLA2, phospholipase A2; PLC, phospholipase C; CoA-IT, coenzyme A-independent transacylase; iPLA2, calcium independent phospholipase A2; LPG, lysoplasmalogenase. Blue boxes: not changes; Red boxes: High levels; Green boxes: Low levels.
A limitation of the present study is that we are far from understanding the biological significance of this compositional complexity. In addition, although we controlled the time of sampling to minimize the dietary influence in lipidome, we cannot completely reject that some compounds resulting from nutrients metabolism could affect plasma lipidome in general, and ether lipid profile in particular. Further, our whole cohort represents a population from a restricted geographic area in Spain and as such can be considered relatively homogeneous regarding lifestyle, and dietary habits. However, several studies confirm that the influence of diet on lipid composition is limited, reinforcing the validity of our findings. Although our findings must be compared and validated in much bigger cohorts to provide future predictive utility, they show the power of lipidomics to better understand the phenotype of extreme longevity in the human population. Finally, a crucial step is to integrate our results with transcriptomic and genomic studies in order to define the genetics of longevity based on the centenarian phenotype. In conclusion, our findings suggest that i) centenarians are resistant to particular age-related changes in ether lipids; and ii) ether lipid species and their metabolism are linked to the human longevity determination, emerging specific ether lipids as potential biomarkers of longevity.
4. Conclusions
Overall, our findings provide robust information about the existence of an ether lipid signature in long-lived humans. This signature is expressed through higher level of alkyl forms derived from phosphatidylcholine; and decreased content in alkenyl forms from phosphatidylethanolamine. In addition, in these lipid species there is a specific compositional pattern in carbon chain with an increased relative abundance for chains with shorter number of carbon atoms and double bonds, and decreased content for longer chain length and higher number of double bonds. As a result, the ether lipids from centenarians are more resistant to lipid peroxidation. So, it is proposed that the ether lipid signature expresses an optimized molecular feature associated with exceptional human longevity.
5. Methods
5.1. Chemicals
Lipid internal standards (ISTD) included species within the classes of phosphatidylcholine (PC(13:0/13:0)), and phosphatidylethanolamine (PE(17:0/17:0)) and were obtained from Avanti (Alabaster AL, USA). The solvents 1-butanol, methanol and chloroform were HPLC-grade and purchased from Merck KGaA (Darmstadt, Germany).
5.2. Study Population
Potential healthy subjects were selected from the population data system of the 11th Health Department of the Valencian Community (Valencia, Spain), which is composed of 29 towns (240,000 inhabitants). The inclusion criteria were to live in the 11th Health Department for at least the last 6 years and to sign the informed consent. The exclusion criterion was to be under statin-therapy or any pharmacological treatment affecting lipid metabolism or to be terminally ill for any reason. We found 25 (6 males/19 females) centenarians; 22 (7 males/15 females) randomly recruited aged subjects, and 21 (7 males/14 females) adult individuals. All experimental procedures were approved by the Committee for Ethics in Clinical Research of the Hospital de la Ribera (Alzira, Valencia, Spain). All subjects or their relatives were fully informed of the aims and scope of the research and signed an informed consent.
5.3. Blood collection and plasma isolation
Blood samples were obtained by venipunction in the morning (between 7 and 8 a.m.) after fasting overnight (8–10 h) and collected in one VACUTAINER CPT (Cell Preparation Tube; BD, Franklin Lakes, NJ) containing sodium heparin as the anticoagulant. Plasma fraction were collected after blood sample centrifugation, and immediately frozen in liquid nitrogen, and transferred before 4 h to a − 80 °C freezer for storage, to be used later for lipidomic analyses.
5.4. Biochemical determinations
Total cholesterol was measured by means of enzymatic assays, and HDL-cholesterol concentration was recorded with a Beckman LX-20 autoanalyzer (Beckman Coulter, La Brea, CA, USA) employing a direct method. LDL-cholesterol and VLDL-cholesterol concentrations were calculated from the Friedewald method. Free cholesterol and triacylglycerides were determined by targeted lipidomics.
5.5. Targeted lipidomics
5.5.1. Preparation of lipid standards
Lipid standards consisting of non-physiological lipid species were used for external standardization (i.e., lipid family assignment) and internal standardization (i.e., for adjustment of potential inter- and intra-assay variances) (Table S1). Stock solutions were prepared by dissolving lipid standards in chloroform: methanol (1:1 v/v), and working solutions were at 10 µg/ml in chloroform: methanol.
5.5.2. Sample preparation and lipid extraction
Plasma samples from the subjects were randomized prior to lipid extraction. Quality control plasma samples were included at a ratio of 1:10. Samples were thawed and 1 µl of the antioxidant butylhydroxytoluene (BHT) (100 mM in ethanol) per 1 ml of plasma was added. To each plasma sample (10 µl) a mixture of internal standards in chloroform: methanol (1:1, 15 µl) was added. Lipids were extracted in a single phase chloroform: methanol (2:1) procedure as described previously [33].
5.5.3. Lipid analysis
Lipid analysis was performed by an LC ESI-QQQ MS/MS model 6490 from Agilent Technologies (Melbourne, Australia). 1 µl of lipid extract was applied onto ZORBAX eclipse plus C18 column, 2.1 × 100 mm 1.8 µm, (Agilent Technologies) heated to 60 °C and the auto-sampler regulated to 25 °C. Flow rate was 400 µl/min with solvent A composed of 10 mM ammonium formate in acetonitrile-water-isopropanol (50:30:20, v/v) and solvent B composed of 10 mM ammonium formate in acetonitrile-water-isopropanol (9:1:90, v/v). The gradient started at 10% of mobile phase B, reached 100% B in 11 min and held for 1 min. Finally, the system was switched back to 10% of mobile phase B and was equilibrated for 3 min. Data was collected in the multiple reaction monitoring scan type and the capillary voltage was set at 3500 V. Positive polarity of electrospray ionization was set using N2 at 20 psi as nebulizer gas (17 L/min, 150 °C) and the sheath gas parameters were flow at 10 L/min and temperature at 200 °C. For all the standard lipid species the cell accelerator voltage was 5 V, fragmentor was 380 V (Table S1). Ether lipid molecular species concentrations were normalized to levels of total PC to reduce technical variance associated with protein determination as previously reported [34].
5.5.4. Data analysis
The MassHunter Data Analysis Software (Agilent Technologies, Melbourne, Australia) was used to collect the results and the software MassHunter Quantitative Analysis (Agilent Technologies, Melbourne, Australia) was used to quantify every lipid specie in the samples. Concentrations were obtained first in pmol/ml and then normalize to micrograms of total of phosphatidylcholine. Multivariate statistics (hierarchical clustering and principal component analysis) were done using Metaboanalyst software and the rest of the statistical analysis was performed with R statistical software, version 3.3.1.
Acknowledgements
We thank the following members of the Spanish Centenarian Study group for their help in this study: Dr F.J. Tarazona; Dr J.R. Domenech, Dr Carmen Valldecabres, Dr. David Cuesta, Dr Ricardo Bou (Hospital de la Ribera, Alzira). Special thanks go to the nurses: Rosa Carrasco, Maria Jesús Martínez, Miriam Hoya and Alicia Pérez for their excellent care of the people involved in this study. Finally, we also thank Rosa Gomez and David Argiles by the technical support.
Acknowledgments
Conflict of interest
The authors declare no conflict of interest
Funding
We acknowledge funding from the Spanish Ministry of Education and Science (ref. SAF2013–44663-R), and from the ‘Red Tematica de Investigación Cooperativa en Envejecimiento y Fragilidad’ (RETICEF) (ref. ISCIII2012-RED-43-029) to J.V.; and from the Spanish Ministry of Economy and Competitiveness/Institute of Health Carlos III (ref. PI14/00328), and the Autonomous Government of Catalonia, Department of Health (ref. SLT002/16/00250) and Department of Business and Knowledge (ref. 2017SGR696) to R.P. This study has been co-financed by FEDER funds from the European Union (“Una manera de hacer Europa”). I.P. was supported by a University of Lleida Predoctoral Fellowship. K.H. was supported by a Dementia Australia Research Foundation Scholarship.
Author contributions
P.J.M., J.V. and R.P. designed the study. I.P., M.J., K.H., J.P., M.I., and C.B. performed experimental work. I.P., M.J., K.H. and R.P. analysed the data. R.P. supervised the design and data interpretation. The manuscript was written by I.P., M.J., J.V., P.J.M., and R.P. and edited by R.P. All authors discussed the results and commented on the manuscript.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.redox.2019.101127.
Contributor Information
I. Pradas, Email: ipradas@mex.udl.cat.
M. Jové, Email: mariona.jove@udl.cat.
K. Huynh, Email: kevin.huynh@baker.edu.au.
J. Puig, Email: jpuigmd@gmail.com.
M. Ingles, Email: marta.ingles@uv.es.
C. Borras, Email: consuelo.borras@uv.es.
J. Viña, Email: jose.vina@uv.es.
PJ. Meikle, Email: Peter.Meikle@baker.edu.au.
R. Pamplona, Email: reinald.pamplona@mex.udl.cat.
Appendix A. Supplementary material
Supplementary material
Supplementary material
References
- 1.Oeppen J., Vaupel J.W. Demography: enhanced: broken limits to life expectancy. Sci. (80-.) 2002;296:1029–1031. doi: 10.1126/science.1069675. [DOI] [PubMed] [Google Scholar]
- 2.Marck A., Antero J., Berthelot G., Saulière G., Jancovici J.-M., Masson-Delmotte V., Boeuf G., Spedding M., Le Bourg É., Toussaint J.-F. Are we reaching the limits of homo sapiens? Front. Physiol. 2017;8:812. doi: 10.3389/fphys.2017.00812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Perls T.T., Wilmoth J., Levenson R., Drinkwater M., Cohen M., Bogan H., Joyce E., Brewster S., Kunkel L., Puca A. Life-long sustained mortality advantage of siblings of centenarians. Proc. Natl. Acad. Sci. 2002;99:8442–8447. doi: 10.1073/pnas.122587599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franceschi C., Passarino G., Mari D., Monti D. Centenarians as a 21st century healthy aging model: a legacy of humanity and the need for a world-wide consortium (WWC100+) Mech. Ageing Dev. 2017;165:55–58. doi: 10.1016/j.mad.2017.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Naudí A., Jové M., Ayala V., Portero-Otín M., Barja G., Pamplona R. Membrane lipid unsaturation as physiological adaptation to animal longevity. Front. Physiol. 2013;4:372. doi: 10.3389/fphys.2013.00372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hulbert A.J., Pamplona R., Buffenstein R., Buttemer W.A. Life and death: metabolic rate, membrane composition, and life span of animals. Physiol. Rev. 2007;87:1175–1213. doi: 10.1152/physrev.00047.2006. [DOI] [PubMed] [Google Scholar]
- 7.Pamplona R. Membrane phospholipids, lipoxidative damage and molecular integrity: a causal role in aging and longevity. Biochim. Biophys. Acta. 2008:1249–1262. doi: 10.1016/j.bbabio.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Jobson R.W., Nabholz B., Galtier N. An evolutionary genome scan for longevity-related natural selection in mammals. Mol. Biol. Evol. 2010;27:840–847. doi: 10.1093/molbev/msp293. [DOI] [PubMed] [Google Scholar]
- 9.Jové M., Naudí A., Aledo J.C., Cabré R., Ayala V., Portero-Otin M., Barja G., Pamplona R. Plasma long-chain free fatty acids predict mammalian longevity. Sci. Rep. 2013;3:3346. doi: 10.1038/srep03346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bozek K., Khrameeva E.E., Reznick J., Omerbašić D., Bennett N.C., Lewin G.R., Azpurua J., Gorbunova V., Seluanov A., Regnard P., Wanert F., Marchal J., Pifferi F., Aujard F., Liu Z., Shi P., Pääbo S., Schroeder F., Willmitzer L., Giavalisco P., Khaitovich P. Lipidome determinants of maximal lifespan in mammals. Sci. Rep. 2017;7:5. doi: 10.1038/s41598-017-00037-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barzilai N., Gabriely I., Gabriely M., Iankowitz N., Sorkin J.D. Offspring of centenarians have a favorable lipid profile. J. Am. Geriatr. Soc. 2001;49:76–79. doi: 10.1046/j.1532-5415.2001.49013.x. 〈http://www.ncbi.nlm.nih.gov/pubmed/11207846〉 (accessed 18 June 2018) [DOI] [PubMed] [Google Scholar]
- 12.Vaarhorst A.A.M., Beekman M., Suchiman E.H.D., van Heemst D., Houwing-Duistermaat J.J., Westendorp R.G.J., Slagboom P.E., Heijmans B.T. Leiden Longevity Study (LLS) Group, lipid metabolism in long-lived families: the leiden longevity study. Age (Omaha). 2011;33:219–227. doi: 10.1007/s11357-010-9172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jové M., Naudí A., Gambini J., Borras C., Cabré R., Portero-Otín M., Viña J., Pamplona R. A stress-resistant lipidomic signature confers extreme longevity to humans. J. Gerontol. A. Biol. Sci. Med. Sci. 2017;72:30–37. doi: 10.1093/gerona/glw048. [DOI] [PubMed] [Google Scholar]
- 14.Dean J.M., Lodhi I.J. Structural and functional roles of ether lipids. Protein Cell. 2018;9:196. doi: 10.1007/s13238-017-0423-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wallner S., Schmitz G. Plasmalogens the neglected regulatory and scavenging lipid species. Chem. Phys. Lipids. 2011;164:573–589. doi: 10.1016/j.chemphyslip.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 16.Braverman N.E., Moser A.B. Functions of plasmalogen lipids in health and disease. Biochim. Biophys. Acta - Mol. Basis Dis. 2012;1822:1442–1452. doi: 10.1016/j.bbadis.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 17.Goldfine H. The appearance, disappearance and reappearance of plasmalogens in evolution. Prog. Lipid Res. 2010;49:493–498. doi: 10.1016/j.plipres.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 18.Huynh K., Martins R.N., Meikle P.J. Lipidomic profiles in diabetes and dementia. J. Alzheimers Dis. 2017;59:433–444. doi: 10.3233/JAD-161215. [DOI] [PubMed] [Google Scholar]
- 19.Meikle P.J., Summers S.A. Sphingolipids and phospholipids in insulin resistance and related metabolic disorders. Nat. Rev. Endocrinol. 2017;13:79–91. doi: 10.1038/nrendo.2016.169. [DOI] [PubMed] [Google Scholar]
- 20.Shi X., Tarazona P., Brock T.J., Browse J., Feussner I., Watts J.L. A Caenorhabditis elegans model for ether lipid biosynthesis and function. J. Lipid Res. 2016;57:265–275. doi: 10.1194/jlr.M064808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munro D., Blier P.U. The extreme longevity of Arctica islandica is associated with increased peroxidation resistance in mitochondrial membranes. Aging Cell. 2012;11:845–855. doi: 10.1111/j.1474-9726.2012.00847.x. [DOI] [PubMed] [Google Scholar]
- 22.Mitchell T.W., Buffenstein R., Hulbert A.J. Membrane phospholipid composition may contribute to exceptional longevity of the naked mole-rat (Heterocephalus glaber): a comparative study using shotgun lipidomics. Exp. Gerontol. 2007;42:1053–1062. doi: 10.1016/j.exger.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Gonzalez-Covarrubias V. Lipidomics in longevity and healthy aging. Biogerontology. 2013;14:663–672. doi: 10.1007/s10522-013-9450-7. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez-Covarrubias V., Beekman M., Uh H.-W., Dane A., Troost J., Paliukhovich I., Van Der Kloet F.M., Houwing-Duistermaat J., Vreeken R.J., Hankemeier T., Slagboom E.P. Lipidomics of familial longevity. Aging Cell. 2013;12:426–434. doi: 10.1111/acel.12064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maeba R., Maeda T., Kinoshita M., Takao K., Takenaka H., Kusano J., Yoshimura N., Takeoka Y., Yasuda D., Okazaki T., Teramoto T. Plasmalogens in human serum positively correlate with high- density lipoprotein and decrease with aging. J. Atheroscler. Thromb. 2007;14:12–18. doi: 10.5551/jat.14.12. 〈http://www.ncbi.nlm.nih.gov/pubmed/17332687〉 (accessed 18 June 2018) [DOI] [PubMed] [Google Scholar]
- 26.Maeba R., Hara H., Ishikawa H., Hayashi S., Yoshimura N., Kusano J., Takeoka Y., Yasuda D., Okazaki T., Kinoshita M., Teramoto T. Myo-inositol treatment increases serum plasmalogens and decreases small dense LDL, particularly in hyperlipidemic subjects with metabolic syndrome. J. Nutr. Sci. Vitaminol. (Tokyo) 2008;54:196–202. doi: 10.3177/jnsv.54.196. 〈http://www.ncbi.nlm.nih.gov/pubmed/18635905〉 (accessed 18 June 2018) [DOI] [PubMed] [Google Scholar]
- 27.Barbagallo C.M., Averna M.R., Fradà G., Noto D., Cavera G., Notarbartolo A. Lipoprotein profile and high-density lipoproteins: subfractions distribution in centenarians. Gerontology. 1998;44:106–110. doi: 10.1159/000021992. [DOI] [PubMed] [Google Scholar]
- 28.Kolovou G., Kolovou V., Vasiliadis I., Wierzbicki A.S., Mikhailidis D.P. Ideal lipid profile and genes for an extended life span. Curr. Opin. Cardiol. 2011;26:348–355. doi: 10.1097/HCO.0b013e32834659d4. [DOI] [PubMed] [Google Scholar]
- 29.Rose G., Crocco P., De Rango F., Corsonello A., Lattanzio F., De Luca M., Passarino G. Metabolism and successful aging: polymorphic variation of syndecan-4 (SDC4) gene associate with longevity and lipid profile in healthy elderly Italian subjects. Mech. Ageing Dev. 2015;150:27–33. doi: 10.1016/j.mad.2015.08.003. [DOI] [PubMed] [Google Scholar]
- 30.Mossakowska M., Broczek K., Wieczorowska-Tobis K., Klich-Rączka A., Jonas M., Pawlik-Pachucka E., Safranow K., Kuznicki J., Puzianowska-Kuznicka M. Cognitive performance and functional status are the major factors predicting survival of centenarians in Poland. J. Gerontol. A. Biol. Sci. Med. Sci. 2014;69:1269–1275. doi: 10.1093/gerona/glu003. [DOI] [PubMed] [Google Scholar]
- 31.Yin H., Xu L., Porter N.A. Free radical lipid peroxidation: mechanisms and analysis. Chem. Rev. 2011;111:5944–5972. doi: 10.1021/cr200084z. [DOI] [PubMed] [Google Scholar]
- 32.Borras C., Abdelaziz K.M., Gambini J., Serna E., Inglés M., de la Fuente M., Garcia I., Matheu A., Sanchís P., Belenguer A., Errigo A., Avellana J.-A., Barettino A., Lloret-Fernández C., Flames N., Pes G., Rodriguez-Mañas L., Viña J. Human exceptional longevity: transcriptome from centenarians is distinct from septuagenarians and reveals a role of Bcl-xL in successful aging. Aging (Albany NY). 2016;8:3185–3208. doi: 10.18632/aging.101078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meikle P.J., Wong G., Tsorotes D., Barlow C.K., Weir J.M., Christopher M.J., Macintosh G.L., Goudey B., Stern L., Kowalczyk A., Haviv I., White A.J., Dart A.M., Duffy S.J., Jennings G.L., Kingwell B.A. Plasma lipidomic analysis of stable and unstable coronary artery disease. Alrterioscle Thromb. Vasc. Biol. 2011;31:2723–2732. doi: 10.1161/ATVBAHA.111.234096. [DOI] [PubMed] [Google Scholar]
- 34.Y.K. Tham, B.C. Bernardo, K. Huynh, C. Giles, P.J. Meikle, J.R. Mcmullen Correspondence, Lipidomic Profiles of the Heart and Circulation in Response to Exercise versus Cardiac Pathology: A Resource of Potential Biomarkers and Drug Targets, 2018. doi: 10.1016/j.celrep.2018.08.017. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material