Version Changes
Revised. Amendments from Version 1
We have added an introductory paragraph where the paper transitions from describing the background to the findings in the stakeholders meeting, to ensure that the switch is clear to the readers. We have included some comment on relevance of the S. mansoni model to S. haematobium; on mitigation strategies to manage misinformation about the model; and on quality control for key assays. We have corrected our statement about the malaria challenge product, since this is not yet FDA approved. In Table 1 we have made some additions regarding the difficulties of testing schistosome vaccine efficacy in field trials which a CHI-S could help to overcome. In Table 3 we have expanded upon the ethical and community-based hurdles to be addressed.
Abstract
Controlled human infection (CHI) models are gaining recognition as an approach to accelerating vaccine development, for use in both non-endemic and endemic populations: they can facilitate identification of the most promising candidate vaccines for further trials and advance understanding of protective immunity. Helminths present a continuing health burden in sub-Saharan Africa. Vaccine development for these complex organisms is particularly challenging, partly because protective responses are akin to mechanisms of allergy. A CHI model for Schistosoma mansoni (CHI-S) has been developed at Leiden University Medical Centre, the Netherlands. However, responses to schistosome infections, and candidate vaccines, are likely to be different among people from endemic settings compared to schistosome-naïve Dutch volunteers. Furthermore, among volunteers from endemic regions who have acquired immune responses through prior exposure, schistosome challenge can be used to define responses associated with clinical protection, and thus to guide vaccine development. To explore the possibility of establishing the CHI-S in Uganda, a Stakeholders’ Meeting was held in Entebbe in 2017. Regulators, community members, researchers and policy-makers discussed implementation challenges and recommended preparatory steps: risk assessment; development of infrastructure and technical capacity to produce the infectious challenge material in Uganda; community engagement from Parliamentary to grass-roots level; pilot studies to establish approaches to assuring fully informed consent and true voluntariness, and strategies for selection of volunteers who can avoid natural infection during the 12-week CHI-S; the building of regulatory capacity; and the development of study protocols and a product dossier in close consultation with ethical and regulatory partners. It was recommended that, on completion, the protocol and product dossier be reviewed for approval in a joint meeting combining ethical, regulatory and environment management authorities. Most importantly, representatives of schistosomiasis-affected communities emphasised the urgent need for an effective vaccine and urged the research community not to delay in the development process.
Keywords: Controlled human infection model; Schistosoma mansoni; Uganda; The Netherlands
Introduction
Effective vaccines have proven extremely useful in the prevention of infectious diseases, but are still lacking for major poverty-related and neglected infections, including helminth infections. The conventional approach to vaccine development, testing efficacy in human subjects in large Phase III trials after safety and immunogenicity are confirmed through smaller Phase I and II trials, is lengthy and extremely costly. An alternative approach, to identify the most promising candidate vaccines through controlled human infection (CHI) models (typically referred to as Phase IIa), is gaining acceptance and application for infections including malaria, typhoid and others: to date about 22,000 volunteers have been infected, safely, with 23 different pathogens 1– 4.
Schistosomiasis is a major parasitic infectious disease, considered second only to malaria as a parasitic cause of morbidity and mortality 5. The current approach to control schistosomiasis is through mass drug administration (MDA) with praziquantel, but this is limited by high rates of re-infection and there are concerns about the possible emergence of drug resistance 6, 7. An effective vaccine would be an extremely valuable control tool but vaccine development for this complex organism is challenging. In a bid to accelerate this, Meta Roestenberg and colleagues at the Leiden University Medical Centre have developed a controlled human infection model for Schistosoma mansoni (CHI-S) and tested it among Dutch volunteers. However, the response to Schistosoma infection, and to candidate vaccines, is likely to differ markedly among people from endemic African populations (where vaccines are most needed and where people are exposed to an abundance of potentially immunomodulating infections) compared to European volunteers. Furthermore, individuals from endemic populations may display some resistance to CHI-S due to prior schistosome exposure. Vaccine development against several pathogens has been informed by studies in which naturally acquired immune responses are correlated with clinical protection, in order to inform vaccine developers on ideal antigens, epitopes and protective thresholds. Thus challenge studies among volunteers from endemic settings, who have naturally acquired immunity, have the potential also to accelerate the development of the next generation of vaccines by allowing desirable immune responses to be identified and prioritised. Implementation of the CHI-S model in an endemic setting would therefore provide critical additional information on markers of protective immunity and on immunogenicity, safety and efficacy of candidate vaccines.
As a first step towards establishing the CHI-S in an endemic setting, we held a stakeholders meeting in Entebbe, Uganda, in November 2017, to identify key challenges and to develop strategies to address them. Meeting participants included representatives of Uganda’s Ministry of Health (Vector Control Division), National Council for Science and Technology, National Drug Authority and National Environment Management Authority; researchers and clinicians who manage schistosomiasis and its complications; chairpersons, committee members and community representatives from various Ugandan ethics fora across the country (the Uganda Virus Research Institute, Makerere University and Mbarara University); representatives of potential volunteer communities (Makerere University students and community representatives from Koome Islands in Lake Victoria); colleagues with experience of implementing controlled human malaria infections (CHMI) from Kenya and with ethics expertise from Kenya and Malawi; and the team who developed the CHI-S from Leiden. Deliberations were informed by the earlier work on CHMI in Kenya, and by the proceedings of the meeting on CHI models held in Malawi in June 2017 8. We here report proceedings of the Uganda meeting.
Schistosomiasis
Schistosomiasis is estimated to affect 230 million people worldwide, the majority of them in sub-Saharan Africa 9. In Uganda, schistosomiasis was first described in the 1900s and was recognised as a serious public health problem in the 1950s 10. Mapping showed the distribution of infection around the major lakes and rivers, and peak intensity among children and adolescents in the five to 20 year old age range 11. The development of a control plan in the 1990s, provided a strong basis for the work of the Schistosomiasis Control Initiative, which launched its programme of control by Mass Drug Administration using praziquantel in Uganda in 2003. Initial results from MDA were promising 12 but recent data show that, despite enhanced coverage, both prevalence and intensity of infection remain high, especially among school-age children, in “hot spot” lakeshore, hard-to reach (such as island) communities. It is increasingly evident that MDA alone will not be adequate to achieve WHO’s target of elimination of schistosomiasis as a public health problem by 2030. Of Uganda’s population of 36 million, more than 4 million are estimated to be infected with schistosomiasis, and 55% of the present population is estimated to be at risk 13.
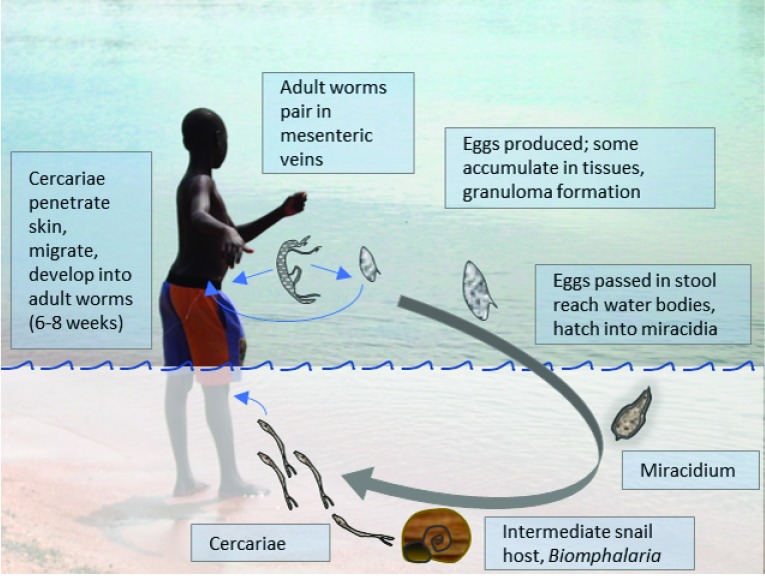
Adult Schistosoma worms reside in blood vessels around the gut ( S. mansoni, S. intercalatum and S. japonicum) or urinary bladder ( S. haematobium), where the female lays eggs which are excreted through the intestinal or bladder wall and voided in stool or urine. In water, each egg hatches producing a single miracidium. This enters the intermediate snail host where it multiplies asexually, producing identical cercariae. Cercariae are shed into the water where they again infect the human host by penetrating through the skin ( Figure 1) 9.
Figure 1. The life cycle of Schistosoma mansoni.
During natural infection, individuals are usually infected with multiple cercariae, both male and female, which mature into adults, pair in the mesenteric blood vessels, and produce eggs, the main cause of pathology. In the controlled human infection model, single sex (male) cercariae are used to avoid the development of eggs and consequent pathology.
Humans sometimes experience cercarial dermatitis in response to the penetrating parasites and a minority develop acute schistosomiasis syndrome (“Katayama Fever”) in reaction to an initial infection. However, most serious disease caused by schistosome infection is due to the eggs. Besides being excreted in stool or urine, many eggs also find their way into other tissues, notably the liver: progressive liver fibrosis results in portal hypertension, splenomegaly, and ascites; oesophageal varices develop which can lead to death through uncontrolled haemorrhage 9. Effective management, for example by repeated, endoscopic band ligation of the varices 14, is seldom available in the resource-limited settings where schistosomiasis is common: upper gastrointestinal bleeding, resulting from S. mansoni-induced periportal fibrosis is a common complaint in primary health care in Northern Uganda, along the course of the Nile 15, 16. Hepatosplenic schistosomiasis is also associated with stunted growth and anaemia, leucopenia and thrombocytopenia. Occasionally eggs can be found in the spinal cord or brain, causing neuropathology 9.
Vaccines for schistosomiasis
A vaccine for schistosomiasis has been ranked among the top 10 vaccines that need to be developed urgently 17. Schistosomes are large, multicellular animals that have evolved to co-exist with their human host. The immunoregulatory properties of schistosomes, which enable them to live in the portal vasculature without immune clearance, are likely to impede vaccine development 18. The complex interplay between T-helper (Th)1, Th2 and regulatory responses is still incompletely understood. Schistosome killing is mediated by antibody responses, and particularly by Immunoglobulin (Ig)E 19, presenting the risk that an effective IgE-inducing vaccine might induce allergic reactions, especially among previously-exposed individuals from endemic populations (as in the case of a candidate hookworm vaccine 20). T-helper (Th)2 response profiles are therefore undesirable. Th1 responses must be targeted. In animal models a Th1 response has been shown to be able to participate in immunity against schistosomes 21, but it is not yet certain which Th1 responses can induce protective immunity and correlates of protection have not been identified. An ideal anti-schistosome vaccine would be suitable for use among young children in endemic settings, given their high burden of infection, as well as adults in high-risk occupations; it would achieve 75% reduction in infection intensity (assessed by circulating antigen or egg production); it would require administration of, at most, two doses; it would induce protection lasting at least five to 10 years 22; it would not induce IgE; and it would be suitable to co-administer with MDA.
Attenuated whole organisms from some helminth species, including schistosome cercariae, have been shown to induce protective immunity in animals 23, 24, but production of attenuated cercariae for large-scale administration is not feasible, thus the current goal is to identify helminth antigens that induce protective responses, but not IgE. Approaches to this include the use of sera generated in animal studies using attenuated larvae, or from human population studies that determine resistance to re-infection after MDA, together with recombinant antigens developed from the investigation of the parasite transcriptome and proteome, to identify antigen- and stage-specific antibodies associated with protection 25, 26. To date, four antigens (SmTSP-2, Sm14, Smp80 and Sh28GST) have been identified and tested, and show promise of efficacy in animal models; three (SmTSP-2, Sm14, Smp80) are ready to enter Phase I trials and one (Sh28GST, Bilhvax) has undergone a Phase III trial ( NCT00870649: this has been completed but no data have yet been released on the outcome of the trial). However, transcriptomic-proteomic approaches suggest many more candidates that could be evaluated as vaccine antigens, either singly or in combinations 27– 29. Unfortunately, the limited resources available for schistosome vaccine development restrict the number of candidates that can be taken forward. As yet, the value of animal models for predicting efficacy of, and responses to, schistosome vaccine candidates in humans is unknown. Murine models may not be the optimal platform; baboons are considered the most suitable, and have been used to further the SmTSP-2 vaccine candidate to phase I testing in humans, but they are expensive and reagents for immunological studies are limited. In general, animal models have been of great utility in asking fundamental questions regarding immunology and demonstrating proof-of-principle of particular vaccination strategies, but do not recapitulate precisely the physiology of human infections, and therefore cannot be considered a substitute for human studies. Clinical testing of novel candidates in humans is needed to obtain true efficacy data.
The controlled human schistosome infection model
The CHI-S model addresses many of the roadblocks to development of an effective vaccine for schistosomiasis ( Table 1). The model alone will provide novel information on the evolution of immune responses following infection. Combined with a model “vaccine”, such as irradiated cercariae, it has the potential to identify correlates of protection, particularly protective Th1 responses that could be harnessed for vaccine development. Then, used as a challenge in phase I vaccine trials, CHI-S will allow efficient and timely selection of potential vaccine candidates, and hence could improve and accelerate the vaccine development pipeline. Implemented in the endemic setting, CHI-S will take into account the impact of prior and current exposure to schistosome infection (including pre-natal exposure) 30 and allow modelling of efficacy in target populations, and in association with praziquantel MDA. In addition, CHI-S offers potential for testing the efficacy of new drugs for treatment of schistosomiasis.
Table 1. Road blocks to schistosome vaccine development and how controlled human infection models for schistosomiasis can help.
(CHI-S) - Controlled human infection model for Schistosoma mansoni.
| Road blocks | How CHI-S can help | |
|---|---|---|
|
Vaccine candidates:
Several vaccine candidates are available; but • there are limited resources to take candidates forward • the focal and dynamic epidemiology of schistosomiasis may make efficacy studies difficult • the requirement for pretreatment of subjects before vaccine testing can influence epidemiology and complicate the efficacy evaluation |
|
✓ CHI-S quickly identifies candidates most likely to induce
protection ✓ CHI-S can be performed in populations with defined pre-exposure |
|
Animal models:
Suitability of various animals for predicting responses to, and efficacy of, vaccine candidates in humans not known |
|
✓ CHI-S provides direct evidence of responses in humans |
|
Immunological road-blocks:
• Schistosomes induce regulatory responses which could impair vaccine immunogenicity • Schistosomes induce Th2 responses and IgE with accompanying risk of allergic phenomena • Th1 responses involved in protection not known in humans • Correlates of protection not known |
|
✓ CHI-S describes evolution of immune responses
following infection ✓ Combined with a model “vaccine” (such as irradiated cercariae, predicted to be effective) CHI-S identifies protective Th1 responses and correlates of protection |
The Leiden CHI-S has been developed with detailed attention to safety in both production and administration of the infectious challenge product. Because eggs are the main source of morbidity and pathology, the CHI-S avoids permanent pathology by making use of single-sex infections. Using the laboratory lifecycle, individual snails are isolated and each snail is carefully infected with a single miracidium of Schistosoma mansoni. This undergoes asexual reproduction in the snail and after five weeks produces thousands of cercariae of a single clone, and hence single sex. Following several quality control steps and determination of male or female sex by PCR, male cercariae are used for the controlled infection of volunteers. This is done by taping a chamber of water containing a predetermined number of male cercariae onto the volunteer’s forearm for a 30-minute interval. Work towards an infection model using female cercariae is in progress, but male worms do better than females when not in pairs and the possibility of production of sterile eggs from females needs to be excluded. Successful infections can be detected (usually after six to 12 weeks) and quantified by measuring circulating anodic antigen (CAA) levels in the blood: this is a protein which is secreted into the blood in large quantities by adult worms 31. Volunteers are followed up for 12 weeks and then treated with praziquantel. The infected snails and preparation of cercariae are managed in customised, dedicated facilities following Good Manufacturing Practice guidelines. The volunteers are intentionally infected with the male cercariae, and followed up until after they are cured, under conditions analogous to a Phase I Clinical Trial.
The current CHI-S model from Leiden provides a blueprint for developing a model for S. haematobium (Sh) also. The need for a S. haematobium model, in addition to the existing S. mansoni model, will need to be evaluated from a vaccine pipeline perspective.
Considerations on implementation of the novel CHI-S model in the endemic setting in Uganda
Uganda is well-placed to host the first CHI-S in an endemic setting. Endemicity of S. mansoni is high. The schistosomiasis control programme, under the Vector Control Division of the Ministry of Health, has long been a key collaborator in world-leading schistosomiasis research and supports the CHI-S concept. There are good laboratory facilities and there is expertise in maintaining the S. mansoni laboratory life-cycle, as well as in molecular and immunological work. There is strong experience of community engagement, and expertise in clinical trials, complemented by an open and engaged ethical and regulatory environment.
At the Uganda Stakeholders’ Meeting reported here, key challenges were identified with regards to the technical procedures (around importation and, or, local production of cercariae for challenge and related risk assessment); community engagement and participant recruitment (around ensuring awareness and full understanding of study procedures and management of the potential for natural exposure during challenge experiments); and ethical as well as regulatory processes (around development of regulatory capacity, documentation and risk assessments). Details of discussions follow, and are summarised in Table 3.
Technical considerations for implementation of the CHI-S in Uganda
Technical considerations for implementation of the CHI-S in Uganda principally comprise the preparation of cercariae for the inoculum. Because the shelf-life of cercariae is just two hours, cercarial production for human challenge must be done locally. In Leiden, the S. mansoni life cycle is maintained in hamsters using a laboratory strain of S. mansoni which originated in Puerto Rico and Biomphalaria glabrata snails; this snail species is not endemic in Uganda. In Uganda, the laboratory life cycle has previously been maintained by the Vector Control Division of the Ministry of Health for another project, using mice and a range of endemic Biomphalaria species (including B. choanomphala, B. stanleyi, and B. sudanica) 32, but it is not actively maintained at present.
Options for preparing the inoculum in Uganda include (1) re-establishing the full S. mansoni laboratory life-cycle; (2) shipping cryopreserved miracidia or eggs from Leiden for snail infection and cercarial shedding in Entebbe (technologies for cryopreservation of miracidia or eggs still need to be developed); (3) shipping infected snails from Leiden for shedding in Entebbe.
The third option, of shipping infected snails, is currently the most feasible. Guidelines for shipping live snails (including infected snails), developed by the Danish Bilharzia Laboratory, are available. These will need to be combined with International Air Transport Association (IATA) requirements for shipping of infectious material. It will be important to work with customs officials and handling agents to ensure efficient release on arrival in Uganda. This process will need to be piloted. A risk assessment will need to be undertaken, in collaboration with the Uganda National Environment Management Authority (NEMA) regarding potential introduction of a new snail species and S. mansoni strain into Ugandan water bodies and risk management protocols will need to be implemented to ensure that this does not occur. Facilities for housing and shedding the snails, and preparing the inoculum in accordance with GMP guidelines, will need to be established.
Post-meeting, a fourth option for a truly-local Ugandan CHI-S was proposed. This would involve generating the inoculum by obtaining miracidia from stool samples of infected people in Uganda, and using these to infect snails of a local species in the laboratory. This would have advantages. The use of a non-endemic Schistosoma strain and of non-endemic snail species would be avoided, which would reduce the environmental risk involved with quarantined non-endemic snail and schistosome species. In addition, the model would be closer to field infections and thus might be considered more representative of endemic infections in Uganda. Additional capacity would be built in-country. However, this approach would also bring additional challenges. The full life-cycle (option (1) above) would need to be re-established in order to test the Ugandan schistosome strain obtained for praziquantel susceptibility and bioburden before use in controlled human infections. Also, the CHI-S model would need to be validated again, and a dose-finding study to identify the optimal balance between tolerability and attack rate would need to be performed. With this truly-local, Ugandan model, it would be more difficult to interpret any differences in responses to vaccines or to infection between studies in Uganda and studies in Leiden (or elsewhere). Work in CHI models of other pathogens has indicated substantial advantages to standardization of the CHI models across sites to ensure comparability of results. Nevertheless, this remains an important option for further discussion.
Good clinical laboratory practice (GCLP) accredited facilities and expertise for PCR (to confirm male sex of cercariae) are already available in Uganda, and plans are in place to provide equipment for high-sensitivity detection of infection by measurement of serum CAA in 2018. Because these assays represent a critical part of the quality control of the product and the primary endpoint of the trial, they will need to be validated extensively before the trial can start. Immunological expertise for the conduct of antibody ELISAs and cellular immune response assays is also available. However, training of the Ugandan team to undertake specific procedures, and to replicate quality control procedures that have been developed in Leiden, will be key.
Protocol development and participant recruitment considerations for CHI-S in Uganda
Ugandan researchers have substantial experience of community engagement and of conducting Phase I trials under Good Clinical Practice (GCP) conditions. However, the stakeholders’ meeting recognised that enhanced attention to aspects of these activities would be required for the CHI-S.
Full details of community engagement plans will be needed as part of the CHI-S protocol. There is need to involve opinion leaders, including members of Parliament such as the Parliamentary Committee on Health, and Resident District Commissioners and District Health Officers of the participating districts, as well as local council leaders, in order to prevent circulation of misinformation about the work. Appropriate information strategies as well as mitigation plans will be put in place to identify any miscommunication on platforms such as social media. Populations of interest for CHI-S will include Ugandans not previously exposed to schistosomiasis (perhaps from an urban setting) as well as those from schistosomiasis-endemic communities (prior exposure for inclusion or exclusion can be determined by measuring IgG antibody to schistosome egg antigen). Experience in Kenya with the controlled human malaria infection (CHMI) model showed that participants with different exposure profiles responded differently to the CHI: participants coming from areas with no active transmission (Nairobi residents) had low baseline responses to malaria, and a challenge response similar to Europeans 3, whereas those resident where active malaria transmission occurs had higher baseline responses and a distinct profile of response to challenge (Kapulu, Bejon personal communication).
Kenyan researchers involved university communities for their first CHMI studies 33, but a few attendees of the Uganda meeting expressed concern about specifically targeting students. Although adults, university students are often still dependents and parents might have objections. It was agreed that volunteers would be expected to inform their next of kin about their participation and that contact details for the next of kin must be provided in case of emergency. Critical to recruitment, and to obtaining informed consent, would be the inclusion of a test that clearly demonstrates a full understanding of the CHI-S model, and reassurance that participation is truly voluntary. Based on Leiden experience, the time taken by the team to know the potential volunteers, during the initial screening and recruitment procedures, is expected to be valuable in selecting those that will understand, and reliably comply with, the procedures.
Volunteers from endemic communities are likely to be actively infected with S. mansoni at the time of recruitment. Such infections will need to be treated with praziquantel before enrolment in the CHI-S. This may require more than one dose of treatment; cure can be determined using the highly-sensitive CAA assay. For individuals from endemic communities, there is also a substantial risk of re-exposure during the 12-week follow-up period between the CHI-S infection and cure; natural infection may be added to the CHI-S infection. While the resulting risk to the volunteer would be comparable to their usual lifestyle, this would invalidate the results of the study. Therefore, volunteers will need to be carefully selected to ensure that they are able and willing to avoid re-exposure. The 12-week duration of the CHI-S follow up means that admission to a facility (as practiced in some CHMI studies) would not be feasible. Follow up of a randomised, placebo group could be considered in order to assess whether there are substantial re-infection rates in a study group.
The Uganda CHI-S protocol will be expected to meet all the requirements of a phase I trial. A data and safety monitoring board (DSMB) will be needed, as well as internal and external monitoring. A realistic evaluation of risks to the volunteers must be included: the intensity of the risk is expected to be lower than for malaria (for example), such that hospital admission will not be necessary, but a 24-hour helpline will be needed. Treatability and methods of treatment of likely side effects and safety evaluations to be conducted must be mentioned. Insurance provision will be necessary: post-meeting information indicated that this should be provided by a local company or agent, but it was recognised that local insurance companies in Uganda are unfamiliar with clinical trials and education of these bodies is needed. Material transfer agreements will be required for protocols involving import of snails or miracidia, and for export of samples if assays are to be done outside Uganda; data sharing agreements, where necessary, would need to be developed and implemented.
Rates for compensation of lost time or income, and transport costs, to volunteers will need to be specified and it will be challenging to set amounts that recognise demands upon the volunteers but do not constitute an undue inducement, since almost any payment may be an inducement in Ugandan settings. Principles for setting the payments will include estimates of time and income loss from visits and reimbursement for transport costs and other inconveniences. Time compensation should be adjusted to the average income or wages in a particular community (for example the Kenyan CHMI studies offered higher rates of compensation in Nairobi than in Kilifi, based on the premise that Nairobi was an urban setting with higher income than in the coastal town of Kilifi which is in a rural setting). It is generally considered that participants should not be compensated for risk, since this could be interpreted as an undue inducement to take risks.
Among representatives from endemic communities, Mr. Asuman Muwumuza, Councillor for Koome sub-county which comprises island communities in Lake Victoria, expressed strong support for the development of the CHI-S in Uganda. He assured the Meeting that local communities would understand the purpose of the study and want to participate, would gladly volunteer and would do whatever would be needed to facilitate these complicated trials. He felt that the need for a vaccine for schistosomiasis was urgent and urged the research community not to delay.
Ethical and regulatory considerations for CHI-S in Uganda
The fundamental ethical issue of concern in relation to CHI models is the principle of non-maleficence, to do no harm: CHI models represent a new ethical challenge and dilemma – using harm with a view to achieving benefit. Historical atrocities involving deliberate infection of vulnerable populations have an important influence on thinking in this field. Guidelines governing the implementation of CHI models are not available in African countries. While guidelines would be desirable, these take a long time to be developed and approved. At the stakeholders’ meeting, it was recommended that the principles articulated by the World Health Organisation (2016) 34 and benchmarks developed at the Malawi meeting on Controlled Human Infection Models in Low Income Countries 8 be employed to govern the ethical and regulatory approval process. These are set out in Table 2, which also identifies ways in which the Uganda CHI-S will address them. Among the benchmarks outlined, critical elements discussed included the following. First, ethical and regulatory standards governing CHI studies in Africa must be equivalent to, or above, the minimum human protection standards applied internationally, as well as locally. When necessary, the capacity of ethical and regulatory bodies must be built, as well as the capacity of researchers. Second, risks must be examined and evaluated before considering possible benefits; there must be a favourable benefit: risk ratio. Arguably the risk associated with a controlled human infection may be more justifiable in an endemic population than in an unaffected population. Third, all stakeholders must be fully informed; in particular, as discussed above, volunteers must fully understand the study, its risks, and benefits, and must be shown to do so. Contributions of social science research to identifying ways of achieving this were desirable.
Table 2. Benchmarks identified in the Malawi framework, and approach to addressing them for the Uganda controlled human infection model for Schistosoma mansoni (CHI-S).
DSMB - data and safety monitoring board.
| Malawi framework benchmarks | Uganda CHI-S | ||
|---|---|---|---|
| 1 |
Issue of national importance,
within the research agenda |
|
✓ Over half of Uganda’s population estimated to be at risk from
schistosomiasis; vaccine development research supported by Vector Control Division (VCD), Ministry of Health |
| 2 | Safety already demonstrated |
|
✓ Safety data from Leiden trials
✓ Risk assessments to Uganda to be developed |
| 3 | Model
quality established by
published data |
|
✓ Publication of Leiden trials expected in 2018 |
| 4 |
Strong scientific case, without
alternative approach |
|
✓ Model has potential to fast-track selection of best vaccine candidates
accelerating development of safe, effective vaccines ✓ Available animal models may not determine correlates of protection and vaccine efficacy in humans ✓ Understanding and data needed regarding differences between endemic and non-endemic populations in response to candidate vaccines |
| 5 | Promotes
capacity development
in country |
|
✓ CHI-S preparatory activities already providing opportunities for learning
and debate for researchers, ethicists and regulators; continuing interaction between researchers and regulators is planned ✓ Further developments to include relevant infrastructure development and technical training of Ugandan researchers |
| 6 |
Ethical acceptability including
issues of understanding consent |
|
✓ Issues of understanding and voluntariness recognised and to be assured by
pilot work in target populations in preparation for CHI-S |
| 7 |
Governance structure in place
(DSMB, sponsor) |
|
✓ Protocol to be developed with due attention to these requirements |
Table 3. Establishing a controlled human schistosome infection model in Uganda: key recommendations and next steps.
(CHI-S) - Controlled human infection model for Schistosoma mansoni, (GCLP) - Good Clinical Laboratory Practice.
| Technical steps | |
|---|---|
| Managing and shedding
snails in Uganda |
• Establish GCLP level facility for housing and shedding snails in Uganda
• Obtain accreditation of facility |
| Identifying male cercariae
and preparing inoculum |
• Training Uganda team in technical and quality control and quality assurance
procedures |
| Detection and quantification
of schistosome infection in Uganda |
• Implementation of the highly sensitive CAA assay in Uganda |
| Shipping infected snails to
Uganda |
• Risk assessment regarding environmental contamination
• Implementation of risk management measures • Implementation of IATA shipping requirements • Ensuring Material Transfer Agreements are in place prior to shipment • Planning for efficient release by customs officials and handling agents on arrival |
| Role of endemic CHI-S | • Liaise with vaccine developers to position endemic CHI-S in the vaccine development pipeline |
| Community and participant recruitment steps | |
| Community engagement | • Raise awareness for CHI in local communities to ensure understanding and
support • Identify engaged communities who would be willing to participate • Include details of planned community engagement (from parliament to local council) in protocol; undertake further preparatory engagement activities |
| Informed consent | • With social science support, develop tools to ensure and document full
understanding by participants • Evaluate the informed consent process and assess the understanding of the study procedures |
| Management of natural
exposure |
• Determine feasibility for potential participants of avoiding natural exposure to
S. mansoni Infection during participation in the challenge model |
| Ethical and regulatory steps | |
| Regulatory capacity building | • Provide further information for ethicists and regulators and ethicists through
visits to the Leiden facilities • Liaise with African ethicists with previous CHI experience |
| CHI-S protocol for Uganda | • Draft protocol; pre-submission discussions with regulatory authorities |
| CHI-S product dossier | • Development of CHI-S product dossier and related documentation for Uganda |
In terms of the regulatory landscape, key stakeholders in Uganda include the Uganda National Council for Science and Technology (UNCST), the National Drug Authority (NDA) and National Environment Management Agency (NEMA) as well as Institutional Review Boards. The roles of these authorities were discussed and it was concluded that the UNCST would hold overall authority for approval of importation of snails (infected or otherwise) and for review and approval of a CHI-S protocol. A joint review meeting, with all regulatory authorities represented, was recommended, as well as engagement between the researchers and ethical and regulatory review bodies throughout the process of protocol development and implementation.
The nature of the human challenge product, the inoculum of infectious cercariae, was noted to present a particular dilemma. Whereas the product for malaria is being investigated under a FDA investigational new drug application 35, the CHI-S product must be generated locally for each infection. This requires local laboratory capacity for high-quality production on-site, and local regulatory capacity for approval of the facilities and processes. Under these circumstances, provision of documentation corresponding to the standard requirements of investigator’s brochure, certificate of good manufacturing practice, sample label, certificate of analysis and letter of authorisation from the product “owner” may be difficult, but a product dossier containing equivalent information will be needed. This would be considered alongside full documentation of procedures and results from Leiden by the regulatory bodies.
Conclusion and next steps
Researchers, community members and regulators participating in the stakeholders’ meeting expressed substantial support for establishing CHI-S in Uganda; this was considered both feasible and desirable.
Key next steps ( Table 3) include risk assessments for importation of infected snails, the development of facilities and expertise for production of the challenge product; community engagement and pilot studies to assess information and consent tools and comprehension by target communities, and to define appropriate populations (able to avoid re-infection, and to participate with full understanding and as true volunteers); provision of opportunities for regulators and ethicists to learn more about CHI-S through visits to Leiden and engagement with their Dutch counterparts; and development of a draft CHI-S protocol, product dossier and accompanying documentation for regulatory review.
Data availability
No data is associated with this article.
Acknowledgements
We thank especially Raymond Muganyizi, Moses Kizza, Keren Apio, Edward Lukyamuzi, Timothy Kimbowa, Joshua Mandre and Teopista Zansanze for their help and technical support in organising the meeting and, in addition, Suzanne van der Plas-Duivesteijn for help in arranging visits between Uganda and Leiden.
We thank all workshop participants for their valuable contributions to the discussions. In addition to the named authors of this article, the participants in the meeting were as follows: Agnes Ssali, Berna Kalanzi, Emmanuel Niwagaba, Gyaviira Nkurunungi, Jacent Nassuuna, Joy Kabagenyi, Mirriam Akello, Pamela Wairagala, Peter Hughes, Richard Sanya, Steve Cose (Medical Research Council/Uganda Virus Research Institute and London School of Hygiene & Tropical Medicine (MRC/UVRI and LSHTM) Uganda Research Unit); Patrice Mawa (Uganda Virus Research Institute); Isaac Ddamulira, Robinah Nakawunde (Medical Students, Makerere University, Uganda); James Kaweesa (Mukono District, Uganda); Edward Bukenya, Rebecca Akwi (community representatives for Schistosomiasis endemic areas, Uganda); Beth Mutumba, Hellen Opolot, Isaac Makhuwa (Uganda National Council for Science and Technology); Huldah Nassali, Agnes Kemigisha, Ismail Ntale, Rachel Kyeyune, Sheila Ampaire (Uganda National Drug Authority); Joseph Luwazo (Uganda National Environment Management Authority); Celia Nalwadda (Uganda National Academy of Sciences); John Bosco Barahika, Joseph Lutaakome (Research Ethics Committee members, UVRI); Francis Bajunirwe (Research Ethics Committee members, Mbarara University of Science & Technology); Erisa Mwaka, Joseph Ochieng, Paul Kutyabami (Research Ethics Committee members, Makerere University, Uganda); Eva Magambo (Community representative, Makerere University School of Health Sciences Research Ethics Committee, Uganda); Charles Obonyo (Kenya Medical Research Institute); Carola Feijt, Jacqueline Janse (Leiden University Medical Centre, The Netherlands).
Funding Statement
The Uganda Stakeholders’ Meeting and visits for Ugandan ethicists, regulators, and clinicians were funded by a discretionary award from the Wellcome Trust [209337]. Organisation of the meeting was also supported by the staff of the Makerere University/Uganda Virus Research Institute Centre of Excellence for Infection and Immunity Research and Training (MUII-plus). MUII-plus is supported through the DELTAS Africa Initiative [107743]. The DELTAS Africa Initiative is an independent funding scheme of the African Academy of Sciences (AAS), Alliance for Accelerating Excellence in Science in Africa (AESA), and supported by the New Partnership for Africa's Development Planning and Coordinating Agency (NEPAD Agency) with funding from the Wellcome Trust [107743] and the UK Government.
[version 2; peer review: 2 approved]
Disclaimer
The views expressed in this article are those of the authors. Publication in AAS Open Research does not imply endorsement by the African Academy of Sciences.
References
- 1. Waddington CS, Darton TC, Woodward WE, et al. : Advancing the management and control of typhoid fever: a review of the historical role of human challenge studies. J Infect. 2014;68(5):405–18. 10.1016/j.jinf.2014.01.006 [DOI] [PubMed] [Google Scholar]
- 2. Mordmüller B, Surat G, Lagler H, et al. : Sterile protection against human malaria by chemoattenuated PfSPZ vaccine. Nature. 2017;542(7642):445–449. 10.1038/nature21060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hodgson SH, Ewer KJ, Bliss CM, et al. : Evaluation of the efficacy of ChAd63-MVA vectored vaccines expressing circumsporozoite protein and ME-TRAP against controlled human malaria infection in malaria-naive individuals. J Infect Dis. 2015;211(7):1076–86. 10.1093/infdis/jiu579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Roestenberg M, et al. : Controlled human infections. Lancet Infect Dis. 2018; in press. [Google Scholar]
- 5. GBD 2015 DALYs and HALE Collaborators: Global, regional, and national disability-adjusted life-years (DALYs) for 315 diseases and injuries and healthy life expectancy (HALE), 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388(10053):1603–1658. 10.1016/S0140-6736(16)31460-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gurarie D, Yoon N, Li E, et al. : Modelling control of Schistosoma haematobium infection: predictions of the long-term impact of mass drug administration in Africa. Parasit Vectors. 2015;8:529. 10.1186/s13071-015-1144-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Crellen T, Walker M, Lamberton PH, et al. : Reduced Efficacy of Praziquantel Against Schistosoma mansoni Is Associated With Multiple Rounds of Mass Drug Administration. Clin Infect Dis. 2016;63(9):1151–1159. 10.1093/cid/ciw506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gordon SB, Rylance J, Luck A, et al. : A framework for Controlled Human Infection Model (CHIM) studies in Malawi: Report of a Wellcome Trust workshop on CHIM in Low Income Countries held in Blantyre, Malawi [version 1; referees: 2 approved]. Wellcome Open Res. 2017;2:70. 10.12688/wellcomeopenres.12256.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Colley DG, Bustinduy AL, Secor WE, et al. : Human schistosomiasis. Lancet. 2014;383(9936):2253–64. 10.1016/S0140-6736(13)61949-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Odongo-Aginya EI, Grigull L, Schweigmann U, et al. : High prevalence and morbidity of Schistosoma mansoni along the Albert Nile in Uganda. Afr Health Sci. 2002;2(3):99–106. [PMC free article] [PubMed] [Google Scholar]
- 11. Kabatereine NB, Brooker S, Tukahebwa EM, et al. : Epidemiology and geography of Schistosoma mansoni in Uganda: implications for planning control. Trop Med Int Health. 2004;9(3):372–80. 10.1046/j.1365-3156.2003.01176.x [DOI] [PubMed] [Google Scholar]
- 12. Zhang Y, Koukounari A, Kabatereine N, et al. : Parasitological impact of 2-year preventive chemotherapy on schistosomiasis and soil-transmitted helminthiasis in Uganda. BMC Med. 2007;5:27. 10.1186/1741-7015-5-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Loewenberg S: Uganda's struggle with schistosomiasis. Lancet. 2014;383(9930):1707–8. 10.1016/S0140-6736(14)60817-5 [DOI] [PubMed] [Google Scholar]
- 14. Siqueira ES, Rohr MR, Libera ED, et al. : Band ligation or sclerotherapy as endoscopic treatment for oesophageal varices in schistosomotic patients: results of a randomized study. HPB Surg. 1998;11(1):27–32. 10.1155/1998/68394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Opio CK, Kazibwe F, Ocama P, et al. : Profiling lifetime episodes of upper gastrointestinal bleeding among patients from rural Sub-Saharan Africa where schistosoma mansoni is endemic. Pan Afr Med J. 2016;24:296. 10.11604/pamj.2016.24.296.9755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Ocama P, Opio KC, Seremba E, et al. : The burden, pattern and factors that contribute to periportal fibrosis in HIV-infected patients in an S. mansoni endemic rural Uganda. Afr Health Sci. 2017;17(2):301–307. 10.4314/ahs.v17i2.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cohen J: Unfilled Vials. Science. 2016;351(6268):16–9. 10.1126/science.351.6268.16 [DOI] [PubMed] [Google Scholar]
- 18. Maizels RM, McSorley HJ: Regulation of the host immune system by helminth parasites. J Allergy Clin Immunol. 2016;138(3):666–75. 10.1016/j.jaci.2016.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Colley DG, Secor WE: Immunology of human schistosomiasis. Parasite Immunol. 2014;36(8):347–57. 10.1111/pim.12087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Diemert DJ, Pinto AG, Freire J, et al. : Generalized urticaria induced by the Na-ASP-2 hookworm vaccine: implications for the development of vaccines against helminths. J Allergy Clin Immunol. 2012;130(1):169–76.e6. 10.1016/j.jaci.2012.04.027 [DOI] [PubMed] [Google Scholar]
- 21. Smythies LE, Coulson PS, Wilson RA: Monoclonal antibody to IFN-gamma modifies pulmonary inflammatory responses and abrogates immunity to Schistosoma mansoni in mice vaccinated with attenuated cercariae. J Immunol. 1992;149(11):3654–8. [PubMed] [Google Scholar]
- 22. Alsallaq RA, Gurarie D, Ndeffo Mbah M, et al. : Quantitative assessment of the impact of partially protective anti-schistosomiasis vaccines. PLoS Negl Trop Dis. 2017;11(4):e0005544. 10.1371/journal.pntd.0005544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fukushige M, Mitchell KM, Bourke CD, et al. : A Meta-Analysis of Experimental Studies of Attenuated Schistosoma mansoni Vaccines in the Mouse Model. Front Immunol. 2015;6:85. 10.3389/fimmu.2015.00085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lin D, Tian F, Wu H, et al. : Multiple vaccinations with UV- attenuated cercariae in pig enhance protective immunity against Schistosoma japonicum infection as compared to single vaccination. Parasit Vectors. 2011;4:103. 10.1186/1756-3305-4-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Hewitson JP, Maizels RM: Vaccination against helminth parasite infections. Expert Rev Vaccines. 2014;13(4):473–87. 10.1586/14760584.2014.893195 [DOI] [PubMed] [Google Scholar]
- 26. Hotez PJ, Bethony JM, Diemert DJ, et al. : Developing vaccines to combat hookworm infection and intestinal schistosomiasis. Nat Rev Microbiol. 2010;8(11):814–26. 10.1038/nrmicro2438 [DOI] [PubMed] [Google Scholar]
- 27. Hokke CH, Yazdanbakhsh M: Final Report Summary - THESCHISTOVAC (The targeted development of a new generation Vaccine for Schistosomiasis). 2015. Reference Source [Google Scholar]
- 28. Merrifield M, Hotez PJ, Beaumier CM, et al. : Advancing a vaccine to prevent human schistosomiasis. Vaccine. 2016;34(26):2988–2991. 10.1016/j.vaccine.2016.03.079 [DOI] [PubMed] [Google Scholar]
- 29. Dewalick S, Bexkens ML, van Balkom BW, et al. : The proteome of the insoluble Schistosoma mansoni eggshell skeleton. Int J Parasitol. 2011;41(5):523–32. 10.1016/j.ijpara.2010.12.005 [DOI] [PubMed] [Google Scholar]
- 30. Novato-Silva E, Gazzinelli G, Colley DG: Immune responses during human schistosomiasis mansoni. XVIII. Immunologic status of pregnant women and their neonates. Scand J Immunol. 1992;35(4):429–37. 10.1111/j.1365-3083.1992.tb02878.x [DOI] [PubMed] [Google Scholar]
- 31. Corstjens PL, De Dood CJ, Kornelis D, et al. : Tools for diagnosis, monitoring and screening of Schistosoma infections utilizing lateral-flow based assays and upconverting phosphor labels. Parasitology. 2014;141(14):1841–55. 10.1017/S0031182014000626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Adriko M, Standley CJ, Tinkitina B, et al. : Compatibility of Ugandan Schistosoma mansoni isolates with Biomphalaria snail species from Lake Albert and Lake Victoria. Acta Trop. 2013;128(2):303–8. 10.1016/j.actatropica.2013.02.014 [DOI] [PubMed] [Google Scholar]
- 33. Hodgson SH, Juma E, Salim A, et al. : Lessons learnt from the first controlled human malaria infection study conducted in Nairobi, Kenya. Malar J. 2015;14:182. 10.1186/s12936-015-0671-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. WHO Expert Committee on Biological Standardisation: Human challenge trials for vaccine development: regulatory considerations. 2016. Reference Source [Google Scholar]
- 35. Roestenberg M, Mordmüller B, Ockenhouse C, et al. : The frontline of controlled human malaria infections: A report from the controlled human infection models Workshop in Leiden University Medical Centre 5 May 2016. Vaccine. 2017;35(51):7065–7069. 10.1016/j.vaccine.2017.10.093 [DOI] [PubMed] [Google Scholar]