Abstract
BACKGROUND
Acute gastroenteritis develops in millions of children in the United States every year, and treatment with probiotics is common. However, data to support the use of probiotics in this population are limited.
METHODS
We conducted a prospective, randomized, double-blind trial involving children 3 months to 4 years of age with acute gastroenteritis who presented to one of 10 U.S. pediatric emergency departments. Participants received a 5-day course of Lactobacillus rhamnosus GG at a dose of 1×1010 colony-forming units twice daily or matching placebo. Follow-up surveys were conducted daily for 5 days and again 14 days after enrollment and 1 month after enrollment. The primary outcome was moderate-to-severe gastroenteritis, which was defined as an illness episode with a total score on the modified Vesikari scale of 9 or higher (scores range from 0 to 20, with higher scores indicating more severe disease), within 14 days after enrollment. Secondary outcomes included the duration and frequency of diarrhea and vomiting, the duration of day-care absenteeism, and the rate of household transmission (defined as the development of symptoms of gastroenteritis in previously asymptomatic household contacts).
RESULTS
Among the 971 participants, 943 (97.1%) completed the trial. The median age was 1.4 years (interquartile range, 0.9 to 2.3), and 513 participants (52.9%) were male. The modified Vesikari scale score for the 14-day period after enrollment was 9 or higher in 55 of 468 participants (11.8%) in the L. rhamnosus GG group and in 60 of 475 participants (12.6%) in the placebo group (relative risk, 0.96; 95% confidence interval, 0.68 to 1.35; P = 0.83). There were no significant differences between the L. rhamnosus GG group and the placebo group in the duration of diarrhea (median, 49.7 hours in the L. rhamnosus GG group and 50.9 hours in the placebo group; P = 0.26), duration of vomiting (median, 0 hours in both groups; P = 0.17), or day-care absenteeism (median, 2 days in both groups; P = 0.67) or in the rate of household transmission (10.6% and 14.1% in the two groups, respectively; P = 0.16).
CONCLUSIONS
Among preschool children with acute gastroenteritis, those who received a 5-day course of L. rhamnosus GG did not have better outcomes than those who received placebo. (Funded by the Eunice Kennedy Shriver National Institute of Child Health and Human Development and others; ClinicalTrials.gov number, NCT01773967.)
Acute Gastroenteritis Causes Substantial complications and is the second leading cause of death worldwide in children younger than 5 years of age.1 Although rarely lethal in the United States, acute gastroenteritis in children is burdensome, accounting for approximately 1.7 million visits to the emergency department (ED) and more than 70,000 hospitalizations per year.2 In addition, acute gastroenteritis in children is associated with considerable nonmedical costs, including lost earnings for caregivers.2 Current treatment options are limited to controlling symptoms, preventing dehydration, and preventing secondary infections among contacts.3
Meta-analyses have suggested that probiotics improve outcomes in children with acute gastroenteritis4–6 through multiple mechanisms, including host immune response modulation.7,8 These trials have prompted recommendations for the use of probiotics in the treatment of acute gastroenteritis in children.9–12 However, the trials included in these meta-analyses had methodologic limitations, including small sample sizes, a lack of quality control of the probiotics, outcomes of questionable relevance, attrition biases, unclear randomization strategies, and inadequate concealment of treatment assignments.5,13 Moreover, few trials evaluated ambulatory children, and one trial that was conducted at a U.S. ED showed no benefit associated with probiotic use, even though an exploratory analysis identified a benefit in a subgroup of patients who had symptoms that lasted for 48 hours or longer.14
Despite the paucity of adequate evidence of the efficacy of probiotics for the treatment of gastroenteritis and for other indications, probiotic use is increasing in the United States15 and in other regions of the world. The global market for probiotics is predicted to expand from $37 billion in U.S dollars in 2015 to $64 billion in U.S. dollars by 2023.16 Hence, there is a need for high-quality, sufficiently powered, randomized controlled trials that evaluate clinically useful and validated outcomes in relevant patient populations to provide guidance to consumers and clinicians.6,13,17 The Pediatric Emergency Care Applied Research Network (PECARN) probiotic trial was designed to test the hypothesis that among children presenting to an ED with acute gastroenteritis, treatment with Lactobacillus rhamnosus GG, a commonly recommended and used probiotic,5,9,13,18 administered twice daily for 5 days, would result in a smaller proportion of children having moderate-to-severe acute gastroenteritis in the 2 weeks after the ED visit than placebo.
Methods
TRIAL DESIGN AND OVERSIGHT
This prospective, randomized, double-blind trial was conducted at 10 geographically diverse university-affiliated pediatric EDs in the United States that participate in PECARN19 (Table S1 in the Supplementary Appendix, available with the full text of this article at NEJM.org). Children 3 months to 4 years of age who presented with acute gastroenteritis were randomly assigned to receive L. rhamnosus GG (Chr. Hansen), at a dose of 1×1010 colony-forming units twice daily for 5 days, or matching placebo. The product and placebo were provided in kind by iHealth, the distributors of Culturelle in the United States; however, iHealth did not contribute financially to the trial or to the investigators, and their employees did not have access to the trial data. Personnel at iHealth had no role in the design or conduct of the trial; in the collection, management, analysis, or interpretation of the data; in the preparation of the manuscript; or in the decision to submit the manuscript for publication. Parents or guardians provided written informed consent for their children to participate, and the institutional review board at each participating institution approved the trial protocol, available at NEJM.org. At multiple time points, a data and safety monitoring board reviewed participant enrollment, trial procedures, completion of the case-report forms, data quality, the rate of loss to follow-up and the drop-in rate, and interim safety and efficacy results.17 The authors vouch for the completeness and accuracy of the data and for the fidelity of the trial to the protocol. Complete details of the trial can be found in the protocol and the statistical analysis plan.
TRIAL POPULATION
Children 3 months to 4 years of age were eligible for participation if an ED provider made a diagnosis of acute gastroenteritis, which was defined as three or more episodes of watery stools per day, with or without vomiting, for fewer than 7 days. Children were excluded if they or their direct caregivers had risk factors for bacteremia (i.e., immunocompromised status, use of systemic glucocorticoids in the previous 6 months, presence of an indwelling catheter, known structural heart disease, or history of prematurity among children who were younger than 6 months of age at enrollment) or if they had a chronic gastrointestinal disorder (e.g., inflammatory bowel disease). Children were also excluded if they had pancreatitis, bilious emesis, or hematochezia; if they had a known allergy to L. rhamnosus GG or to microcrystalline cellulose or a known allergy to erythromycin, clindamycin, and beta-lactam antibiotic agents (since these agents might be needed to treat an invasive infection caused by L. rhamnosus GG); or if their caregiver did not speak English or Spanish. Children who had been receiving antibiotics were not excluded because probiotics may remain viable and effective in the presence of antibiotics.20
RANDOMIZATION AND INTERVENTION
Randomization was performed through a Web based system (www.randomize.net) with the use of permuted blocks with random block sizes. Randomization was stratified according to trial site and duration of symptoms (<48 hours vs. ≥48 hours). After assignment to a trial group, participants received the first dose of L. rhamnosus GG or placebo orally in the ED; ED personnel prepared the dose by emptying the contents of the assigned capsule into 20 ml of liquid maintained at room temperature. Caregivers received oral and written instructions for administering subsequent doses. The L. rhamnosus GG and placebo were identical in appearance, texture, and flavor. If vomiting occurred within 15 minutes after administration of the probiotic or placebo, the dose was repeated once. Participants and their caregivers, physicians, and personnel who assessed the trial outcomes were unaware of the trial-group assignments.
FOLLOW-UP PROCEDURES
Caregivers were instructed to complete a daily diary to record symptoms. Follow-up data were collected through email or by telephone on a daily basis for 5 days or until symptoms resolved (if they had not resolved by 5 days) and again at 14 days and at 1 month (at 1 month, only information on adverse events was collected). Chart reviews were performed at the end of the follow up period to assess whether any adverse events had been missed. All follow-up telephone calls were made by research coordinators at the lead site who were fluent in both English and Spanish.
TESTING OF STOOL SAMPLES AND L. RHAMNOSUS GG TESTING
Stool specimens for the testing of enteric pathogens were obtained by rectal swab (FecalSwab, Copan Diagnostics) or by bulk stool sampling, as available,21,22 in the ED. A multiplex polymerase chain-reaction assay was performed on the Luminex xTag Gastrointestinal Pathogen Panel platform, which identifies 15 microorganisms.23,24 Each batch of L. rhamnosus GG capsules was independently tested every 6 to 9 months before the expiration date to ensure the absence of contaminants and the maintenance of viability.
OUTCOMES MEASURES
The primary outcome was the presence of moderate-to-severe gastroenteritis, which was defined as an illness episode with a total score on the modified Vesikari scale of 9 or higher (scores range from 0 to 20, with higher scores indicating more severe disease) during the 14-day follow-up period after enrollment. The Vesikari scale scoring system is used to assess the severity of gastroenteritis and is validated for use in pediatric patients treated at EDs in North America25,26 (Table 1). The post-enrollment Vesikari scale score (i.e., the score for the primary outcome) was based only on symptoms or events that occurred between randomization and day 14 while daily symptoms of gastroenteritis persisted (i.e., if both vomiting and diarrhea ceased for 24 hours, subsequent symptoms were not included in the score). The highest scores assigned to each of the seven component variables were summed on day 14 to determine a total score (further details are provided in the protocol). Secondary outcomes included the frequency and duration of diarrhea and vomiting, the incidence of un-scheduled health care visits for symptoms of gastroenteritis within 2 weeks after the index visit, the number of days of day care missed by participants, the number of hours of work missed by caregivers, and the rate of household transmission (defined as the development of symptoms of gastroenteritis in previously asymptomatic household contacts). Safety outcomes included extraintestinal infection by L. rhamnosus GG (e.g., bacteremia), side effects (i.e., anticipated symptoms, as specified in the protocol and the statistical analysis plan), and adverse events (i.e., untoward medical occurrences).
Table 1.
Modified Vesikari Scale.*
| Scale component | Score on the Vesikari Scale | |||
|---|---|---|---|---|
| 0 Points | 1 Point | 2 Points | 3 Points | |
| Duration of diarrhea (hr) | 0 | 1–96 | 97–120 | ≥121 |
| Maximum no. of watery stools per 24 hr | 0 | 1–3 | 4–5 | ≥6 |
| Duration of vomiting (hr) | 0 | 1–24 | 25–48 | ≥49 |
| Maximum no. of vomiting episodes per 24 hr | 0 | 1 | 2–4 | ≥5 |
| Maximum recorded rectal temperature (°C)† | <37.0 | 37.1–38.4 | 38.5–38.9 | ≥39.0 |
| Unscheduled health care visit | None | NA | Primary care | Emergency department |
| Treatment | None | Rehydration with intravenous fluids | Hospitalization | NA |
In the modified Vesikari scale score, one variable (percent dehydration) in the original score was replaced with the variable of unscheduled health care visits to better measure the effect of acute gastroenteritis in outpatients, given that the ability to perform frequent in-person assessments in an outpatient cohort of children can be challenging. Scores range from 0 to 20, with higher scores indicating more severe disease. Children with a score of 9 or more were considered to have moderate-to-severe gastroenteritis.25,26 NA denotes not applicable.
Temperatures were adjusted for the location of measurement: 1.1°C was added to axillary temperatures and 0.6°C was added to oral temperatures.27
STATISTICAL ANALYSIS
In estimating the sample size, we assumed that 25% of participants who received placebo would have moderate-to-severe gastroenteritis in the 14 days after presenting to the ED.25,26 Ten content experts in the field of emergency medicine and gastroenterology determined that a 10 percentage-point difference between the two trial groups in the proportion of participants having moderate-to-severe gastroenteritis would represent a minimal clinically meaningful difference. We estimated that enrollment of 670 participants would provide the trial with 90% power to detect a treatment effect at a two-sided alpha level of 0.05. We increased the target recruitment number to 900 participants to account for the following assumptions: a loss-to-follow-up rate of 10%, a drop-in rate of 3%, and a dropout rate of 5%. Furthermore, during the fall of 2015 (15 months after initiation of the trial), 36 participants were potentially exposed to a batch of L. rhamnosus GG capsules that were later found to contain insufficient colony-forming units of L. rhamnosus GG. To maintain the statistical power of the trial under a worst-case scenario (while maintaining blinding to assigned trial regimen and outcome), we assumed that exposure to the lower-count capsules would have the same effect as dropping out of the trial. Thus, the required sample size was increased to 970 participants.17
Because we based our trial design and analyses of statistical power on the assumption of a homogeneous treatment effect, and taking into consideration previous data that showed a trend toward benefit in patients who had symptoms for at least 48 hours before treatment with probiotics was initiated,14 we incorporated an enrichment design to restore statistical power in the event that a subpopulation with a substantially lower treatment effect was identified.28 Analyses were performed according to the intention-to-treat principle, with the exception of side effects, which were performed in the as-treated population. We also performed a separate as-treated analysis to provide additional insight in the event that nonadherence would result in an underestimation of the treatment effect.29,30
In cases in which information needed to derive the primary outcome was incomplete, we applied multiple imputation methods using a sequence of regression models31 as well as standard methods.32 The primary outcome was analyzed with the use of a Mantel–Haenszel test, stratified according to clinical site and duration of symptoms. We also analyzed the primary outcome using van Elteren’s modification of the Mann–Whitney test to evaluate the post-enrollment modified Vesikari scale score as a continuous variable. We analyzed secondary outcomes using the Mantel–Haenszel test for dichotomous outcomes and van Elteren’s modification of the Mann–Whitney test for continuous outcome measures. Significance levels were adjusted for multiple comparisons with the use of the Holm procedure.33 We assessed the consistency of the main trial results in prespecified subgroups defined according to age (<1 year vs. ≥1 year), duration of symptoms (<48 hours vs. ≥48 hours), use or nonuse of antibiotics in the 14 days before enrollment, and type of enteric pathogen (virus, bacteria, or undetected). Significance levels were adjusted for multiple subgroups. No post hoc subgroups were analyzed. We used IVEware software (University of Michigan) for imputation and SAS software, version 9.4 (SAS Institute), for all other analyses. Our findings are reported in accordance with 2010 CONSORT (Consolidated Standards of Reporting Trials) guidelines.34
RESULTS
PARTICIPANTS AND ADHERENCE TO TRIAL INTERVENTION
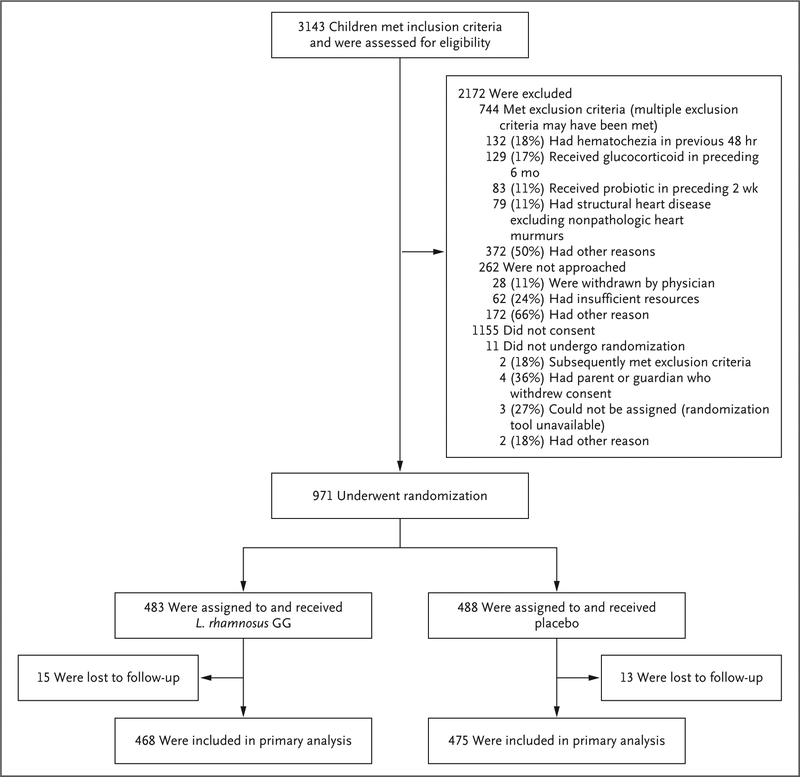
From July 2014 through June 2017, a total of 971 participants underwent randomization, of whom 483 (49.7%) were assigned to the L. rhamnosus GG group and 488 (50.3%) to the placebo group (Fig. 1). A total of 15 participants in the L. rhamnosus GG group and 13 in the placebo group were lost to follow-up; among these participants, 5 in the L. rhamnosus GG group and 8 in the placebo group withdrew from the trial for various reasons (Table S2 in the Supplementary Appendix). Disease severity at the time of enrollment was similar in the two groups, as evidenced by similar pre-enrollment modified Vesikari scale scores and similar percentages of participants who received intravenous fluids and who were admitted to the hospital (Table 2). Stool samples were obtained from 761 participants. A total of 347 of the samples (45.6%) were positive for viruses, including 6 viral coinfections; 116 (15.2%) were positive for bacteria that are probable or possible pathogens, including 34 viral–bacterial coinfections; and 9 (1.2%) were positive for a parasite, including 2 parasitic–viral coinfections and 1 parasitic–bacterial coinfection. No pathogenic organisms were detected in 326 participants (42.8%). The percentage of participants who received antibiotics or ondansetron after randomization was similar in the two groups (Table S3 in the Supplementary Appendix). The rate of adherence to the trial regimen (with adherence defined as having received at least 7 of 10 doses) was 86.5% in the L. rhamnosus GG group and 87.8% in the placebo group. The rates of completion of the follow-up surveys were 96.0% (932 of 971 participants) for the daily surveys (the f 5 days) and 95.3% (925 of 971 participants) for the 14-day survey. For most of the participants (644 of 971 participants [66.3%]), caregivers chose to complete follow-up by telephone (Table S4 in the Supplementary Appendix).
Figure 1.
Enrollment and Randomization.
Table 2.
Baseline Characteristics of Enrolled Participants, According to Assigned Trial Group.*
| Characteristic |
L. rhamnosus GG (N=483) |
Placebo (N=488) |
Overall (N = 971) |
|---|---|---|---|
| Age — yr | |||
| No. of participants assessed | 482 | 488 | 970 |
| Median (IQR) | 1.4 (0.9–2.4) | 1.4 (0.8–2.3) | 1.4 (0.9–2.3) |
| Male sex — no./total no. (%) | 247/482 (51.2) | 266/488 (54.5) | 513/970 (52.9) |
| Racial or ethnic category — no. (%)† | |||
| No. of participants assessed | 362 | 350 | 712 |
| American Indian or Alaskan Native | 9 (2.5) | 0 | 9 (1.3) |
| Asian | 8 (2.2) | 6 (1.7) | 14 (2.0) |
| Black | 176 (48.6) | 162 (46.3) | 338 (47.5) |
| Native Hawaiian or Other Pacific Islander | 1 (0.3) | 3 (0.9) | 4 (0.6) |
| White | 144 (39.8) | 163 (46.6) | 307 (43.1) |
| Multiracial | 24 (6.6) | 16 (4.6) | 40 (5.6) |
| Hispanic or Latino ethnic group — no./total no. (%)† | 168/471 (35.7) | 189/476 (39.7) | 357/947 (37.7) |
| Spanish reported as preferred language — no./total no. (%) | 91/481 (18.9) | 94/484 (19.4) | 185/965 (19.2) |
| Breast-fed exclusively for <6 mo — no./total no. (%) | 2/29 (6.9) | 3/39 (7.7) | 5/68 (7.4) |
| Modified Vesikari scale score‡ | |||
| No. of participants assessed | 469 | 480 | 949 |
| Median (IQR) | 12 (10–14) | 12 (9–14) | 12 (10–14) |
| Distribution — no. (%) | |||
| 0 to 8 | 82 (17.5) | 74 (15.4) | 156 (16.4) |
| 9 or higher | 387 (82.5) | 406 (84.6) | 793 (83.6) |
| Duration of diarrhea — hr | |||
| No. of participants assessed | 472 | 480 | 952 |
| Median (IQR) | 54.3 (29.5–85.3) | 52.4 (28.2–79.1) | 53.2 (29.0–81.3) |
| Maximum no. of diarrhea stools in 24 hr | |||
| No. of participants assessed | 482 | 485 | 967 |
| Median (IQR) | 7 (5–10) | 6 (4–10) | 6 (5–10) |
| Duration of vomiting — hr | |||
| No. of participants assessed | 474 | 482 | 956 |
| Median (IQR) | 26.6 (0.0–66.3) | 27.9 (3.9–59.1) | 27.5 (2.2–61.9) |
| Maximum no. of vomiting episodes in 24 hr | |||
| No. of participants assessed | 482 | 485 | 967 |
| Median (IQR) | 3 (0–6) | 4 (1–6) | 3 (1–6) |
| Treatment for acute gastroenteritis — no. (%) | |||
| None | 401 (83.0) | 397 (81.4) | 798 (82.2) |
| Intravenous fluids | 59 (12.2) | 69 (14.1) | 128 (13.2) |
| Hospital admission | 23 (4.8) | 22 (4.5) | 45 (4.6) |
| Maximum recorded temperature — no. (%) | |||
| No. of participants assessed | 482 | 485 | 967 |
| <37°C | 286 (59.3) | 287 (59.2) | 573 (59.3) |
| 37.1 to 38.4°C | 31 (6.4) | 24 (4.9) | 55 (5.7) |
| 38.5 to 38.9°C | 35 (7.3) | 37 (7.6) | 72 (7.4) |
| ≥39°C | 130 (27.0) | 137 (28.2) | 267 (27.6) |
| Dehydration score§ | |||
| No. of participants assessed | 480 | 481 | 961 |
| Median (IQR) | 0 (0–1) | 0 (0–1) | 0 (0–1) |
| Distribution — no. (%) | |||
| 0 | 347 (72.3) | 348 (72.3) | 695 (72.3) |
| 1 to 4 | 124 (25.8) | 124 (25.8) | 248 (25.8) |
| 5 to 8 | 9 (1.9) | 9 (1.9) | 18 (1.9) |
| Duration of symptoms <48 hr — no. (%) | 175 (36.2) | 197 (40.4) | 372 (38.3) |
| Receipt of antibiotics in previous 14 days — no./total no. (%) | 39/478 (8.2) | 36/480 (7.5) | 75/958 (7.8) |
| Receipt of any dose of rotavirus vaccine — no./total no. (%) | 210/313 (67.1) | 206/319 (64.6) | 416/632 (65.8) |
| Stool sample for multiplex polymerase-chain-reaction assay | |||
| No. of participants from whom stool sample was obtained (%) | 379 (78.5) | 382 (78.3) | 761 (78.4) |
| Result — no. (%) | |||
| Negative | 153 (40.4) | 173 (45.3) | 326 (42.8) |
| Norovirus Gl or GII | 79 (20.8) | 70 (18.3) | 149 (19.6) |
| Rotavirus A | 75 (19.8) | 60 (15.7) | 135 (17.7) |
| Adenovirus 40 or 41 | 28 (7.4) | 41 (10.7) | 69 (9.1) |
| Clostridium difficile toxin A or B | 25 (6.6) | 31 (8.1) | 56 (7.4) |
| Shigella | 23 (6.1) | 15 (3.9) | 38 (5.0) |
| Campylobacter | 5 (1.3) | 4 (1.0) | 9 (1.2) |
| Salmonella | 4 (1.1) | 3 (0.8) | 7 (0.9) |
| Escherichia coli O157 | 2 (0.5) | 4 (1.0) | 6 (0.8) |
| Enterotoxigenic E. coli LT or ST | 2 (0.5) | 3 (0.8) | 5 (0.7) |
| Shiga toxin-producing E. coli stx1 or stx2 | 2 (0.5) | 3 (0.8) | 5 (0.7) |
| Giardia | 2 (0.5) | 2 (0.5) | 4 (0.5) |
| Cryptosporidium | 2 (0.5) | 1 (0.3) | 3 (0.4) |
| Entamoeba histolytica | 1 (0.3) | 1 (0.3) | 2 (0.3) |
| Vibrio cholerae | 0 | 1 (0.3) | 1 (0.1) |
| Yersinia enterocolitica | 0 | 0 | 0 |
No significant differences were observed between the two groups in the above characteristics, with the exception of racial or ethnic category (P = 0.02). We used Kruskal-Wallis tests for between-group comparisons of continuous variables (age, modified Vesikari scale score, duration of diarrhea and vomiting, maximum number of episodes of diarrhea and vomiting, and dehydration scores) and chi-square tests for comparisons of all other variables. IQR denotes interquartile range.
Race and ethnic group were reported by the caregiver.
Moderate-to-severe gastroenteritis was defined as gastroenteritis with a modified Vesikari scale score of 9 or higher; scores on the Vesikari scale range from 0 to 20, with higher scores indicating more severe disease.
A score of 0 indicates no dehydration, scores of 1 to 4 indicate some dehydration, and scores of 5 to 8 indicate moderate to severe dehydration.
PRIMARY OUTCOME
The post-enrollment modified Vesikari scale score (i.e., the score for the 14-day period after enrollment) was 9 or higher in 55 of 468 participants (11.8%) in the L. rhamnosus GG group and in 60 of 475 participants (12.6%) in the placebo group. The relative risk of a moderate-to-severe episode of acute gastroenteritis with L. rhamnosus GG was 0.96 (95% confidence interval, 0.68 to 1.35; P = 0.83) (Table 3).
Table 3.
Trial Outcomes, According to Assigned Trial Group.*
| Outcome |
L. rhamnosus GG (N=468) |
Placebo (N=475) |
Relative Risk (95% Cl) |
P Value† |
|---|---|---|---|---|
| Primary outcome‡ | ||||
| Modified Vesikari scale score of 0 to 8 — no. (%) | 413 (88.2) | 415 (87.4) | ||
| Modified Vesikari scale score ≥9 — no. (%) | 55 (11.8) | 60 (12.6) | 0.96 (0.68–1.35) | 0.83 |
| Secondary outcomes and individual components of 14-day modified Vesikari scale score | ||||
| Median modified Vesikari scale score (IQR) | 4 (2–6) | 4 (2–6) | 0.85 | |
| Diarrhea | ||||
| Median time to the last watery stool (IQR) — hr | 49.7 (18.8–86.4) | 50.9 (25.0–88.2) | 0.26 | |
| Median total no. of diarrheal episodes (IQR) | 7 (3–12) | 7 (3–14) | 0.43 | |
| Vomiting | ||||
| Median time to the last vomiting episode (IQR) — hr§ | 0.0 (0.0–21.9) | 0.0 (0.0–17.8) | 0.17 | |
| Median total no. of vomiting episodes (IQR)§ | 0 (0–2) | 0(0–2) | 0.20 | |
| Receipt of intravenous fluids — no. (%) | 19 (4.1) | 22 (4.6) | 0.59 | |
| Hospital admission — no. (%) | 15 (3.2) | 15 (3.2) | 0.93 | |
| Repeat health care visit within 2 weeks after the index visit — no. (%) | 57 (12.2) | 80 (16.8) | 0.03¶ | |
| Repeat emergency department visit within 2 weeks after the index visit — no. (%) | 32 (6.8) | 37 (7.8) | 0.55 | |
| Median no. of days of day care missed (IQR)‖ | 2 (1–3) | 2 (1–3) | 0.67 | |
| Median no. of hours of work missed by parent or guardian (IQR) | 0 (0–8) | 0(0–8) | 0.52 | |
| Household transmission — no. (%)** | 43 (10.6) | 59 (14.1) | 0.16 |
Outcomes were evaluated from randomization through the time that symptoms lasted, up to 2 weeks after the index visit (i.e., up to the time of the daily survey that recorded that vomiting and diarrhea had ceased or at the time of the day 14 survey), except for repeat health care visit and repeat emergency department visit. Outcomes of receipt of intravenous fluids and of hospital admission do not include receipt of fluids at the enrollment health care visit or hospital admissions that were associated with the enrollment health care visit unless the hospitalization lasted longer than 48 hours. Participants who had no follow-up were excluded; data for participants who had partial follow-up were multiply imputed.
P values were calculated with the use of Cochran-Mantel-Haenszel tests (outcomes for which the number and percentage of participants are reported) or Van Elteren tests (outcomes for which median and interquartile range are reported), stratified according to trial site and duration of symptoms at enrollment (<48 hours vs. ≥48 hours).
A modified Vesikari scale score of 9 or higher indicates moderate-to-severe gastroenteritis.
The denominator for this variable was 457 participants (218 in the L. rhamnosus GG group and 239 in the placebo group) who had had at least three vomiting episodes within 24 hours before enrollment.
The difference between the two groups was not significant after significance levels were adjusted for multiple comparisons with the use of the Holm procedure (a P value of 0.008 was considered to indicate significance).
The denominator for this variable was 353 participants (175 in the L. rhamnosus GG group and 178 in the placebo group) who attended day care.
Household transmission was defined as the development of symptoms of gastroenteritis in at least one previously asymptomatic household contact after enrollment of the participant. The denominator for this variable was 826 participants (407 in the L. rhamnosus GG group and 419 in the placebo group) who were surveyed about sickness among members of the household.
SECONDARY OUTCOMES
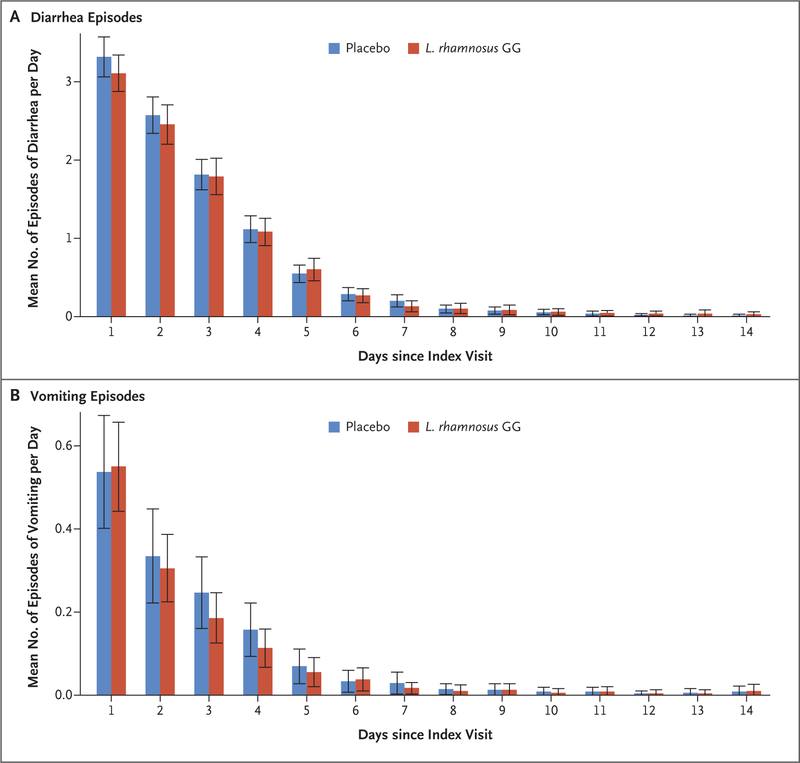
No significant differences between the groups were observed with respect to the frequency or duration of diarrhea or vomiting, the proportion of participants who had unscheduled health care visits for symptoms of gastroenteritis or complications associated with gastroenteritis within 2 weeks after the index visit, the number of days of day care missed by participants, the number of hours of work missed by caregivers, or the rate of household transmission (Table 3 and Fig. 2). The median post-enrollment modified Vesikari scale score and the interquartile range were similar in the two groups (median, 4 [interquartile range, 2 to 6]; P = 0.85). Prespecified subgroup analyses of the primary and secondary outcomes showed no significant differences between the two trial groups according to age, duration of symptoms, use of antibiotics in the 14 days preceding enrollment, and type of enteric pathogen identified (Fig. S1 in the Supplementary Appendix). The results of the as-treated analyses were similar to the results of the intention-to-treat analyses (Table S5 in the Supplementary Appendix).
Figure 2. Mean Number of Episodes of Diarrhea or Vomiting per Day, According to Assigned Trial Group.
Data from all participants who completed any follow-up were included in the analyses. Daily surveys that reported no diarrhea or vomiting episodes and daily surveys that were not completed because of previous resolution of symptoms contributed a value of zero when each daily mean was calculated. I bars denote 95% confidence intervals.
ADVERSE EVENTS AND SIDE EFFECTS
No participant had extraintestinal L. rhamnosus GG infection. No significant differences between the trial groups were observed in the rates of adverse events or in the rates of side effects, with the exception of wheezing, which was reported in five participants in the L. rhamnosus group and in no participants in the placebo group (P = 0.03) (Tables S6 and S7 in the Supplementary Appendix).
L. RHAMNOSUS GG TESTING
Analyses of batches of L. rhamnosus GG capsules identified no contaminants. A sample from one batch contained 1.96×109 colony-forming units per capsule 5 months before the expiration date, and a sample from another batch contained 1.98×109 colony-forming units per capsule 17 months before the expiration date; both batches were discarded. A total of 36 participants potentially received a dose that was lower than intended (17 of these participants were in the L. rhamnosus GG group). All 36 participants were evaluated in the group to which they were randomly assigned. The results of sensitivity analyses of the primary and secondary outcomes in which the 36 participants were excluded were similar to those of the main analyses (Table S8 in the Supplementary Appendix).
DISCUSSION
This double-blind, randomized, placebo-controlled trial involving 971 children showed that a 5-day course of L. rhamnosus GG, administered twice daily at a dose of 1×1010 colony-forming units, did not result in a smaller proportion of participants having moderate-to-severe gastroenteritis after an ED visit than placebo. The results were also similar in the L. rhamnosus GG group and the placebo group in analyses of secondary outcomes and in subgroup analyses of the primary and secondary outcomes.
Our pragmatic trial, conducted in a geographically diverse population, confirms and extends the findings of a previous smaller trial that was performed at an ED in the United States.14 However, our results differ from those of smaller trials included in meta-analyses of trials of probiotics in general6 and of L. rhamnosus GG in particular.5 A potential explanation stems from our use of a validated composite outcome measure that incorporates multiple aspects of gastroenteritis severity25,26 rather than relying on individual symptoms. However, even when we analyzed individual symptoms, there were no significant differences between the two trial groups. To confirm that our findings were not a result of inadequacy of the trial product,35,36 we performed a product analysis of the recommended dose of the probiotic5,9 and adjusted the sample size when inadequate batches were identified.17 Furthermore, we used an enrichment design to ensure that patients who were most likely to benefit (such as those who had a longer duration of symptoms) were well represented in our cohort.37,38 Thus, the rigor of our research design calls into question recommendations to use L. rhamnosus GG in the treatment of children with acute gastroenteritis.
It is not uncommon for large, randomized, controlled trials to contradict results of previous meta-analyses,39 because even carefully designed meta-analyses are subject to the limitations inherent to the nature of included trials. Examples in addition to our trial include large trials that failed to show any benefit of probiotics to prevent antibiotic-associated diarrhea and Clostridium difficile infection in adults40 and necrotizing enterocolitis in preterm infants41 or to reduce pharyngitis symptoms in children and adults.42 Furthermore, recent studies show that responsiveness to probiotics may follow highly individualized patterns and that their effect, including negative outcomes, may vary according to indigenous microbiota and gene-expression profiles.43,44 These examples highlight the importance of conducting high-quality studies to systematically assess the efficacy and safety of probiotics.45
Although previous trials have suggested that approximately 25% of children with acute gastroenteritis would have moderate-to-severe courses after an ED visit,25,26 we observed a lower percentage of such events in both groups. This finding may reflect a smaller percentage of children with rotavirus infection in our cohort than in earlier cohorts46 and clinical trials,6 many of which were restricted to children with rotavirus infection or were conducted before rota-virus vaccine use had become widespread. Furthermore, our follow-up procedures were more detailed than those in previous trials involving a similar population,25,26 in which outcomes were based on a single interview conducted 14 days after enrollment and thus might have been subject to greater recall bias. Hence, our data probably present a more accurate portrayal of acute gastroenteritis in children in the United States. The possibility exists that we tested the intervention in healthier populations, but 82% of the participants in our trial had moderate-to-severe disease at presentation, and 5% in each group were hospitalized, findings that are similar to those reported in previous trials.25,26
Despite these attributes, our trial has several limitations. First, we enrolled participants during days and evenings when research staff were available, but we did not collect data on eligible children who were missed because they presented after hours or on children whose caregivers chose not to participate. Second, we relied on parental reports of adherence and symptoms, and inaccurate recall cannot be excluded. Given the large sample size across many centers and the fact that the trial was blinded, systematic enrollment biases or systematic differences in recall between groups would not be expected. To reduce the effect of the latter, we used a composite validated outcome measure, provided care-givers with diaries to record daily events, and used a standardized data collection survey, and we achieved excellent follow-up rates. Third, although we conducted chart reviews at each site to assess potentially missed health care visits that occurred after randomization, families may have sought care elsewhere. Fourth, although care-givers were instructed to keep the trial medication refrigerated, it is possible that the medication was exposed to temperature extremes in the home or during transport, which could have affected bacterial viability. This was a pragmatic trial, however, and commercial probiotic products would be prone to the same limitations.
In this randomized, placebo-controlled trial involving 971 preschool children with acute gastroenteritis, those who received a 5-day course of L. rhamnosus GG did not have better outcomes than those who received placebo. Treatment with L. rhamnosus GG did not result in a smaller proportion of participants having moderate-to-severe gastroenteritis and failed to show benefit with respect to the duration or frequency of vomiting or diarrhea, the rate of household transmission, or the duration of day-care or work absenteeism.
Supplementary Material
CLINICAL TRIAL REGISTRATION.
The Journal requires investigators to register their clinical trials in a public trials registry. The members of the International Committee of Medical Journal Editors (ICMJE) will consider most reports of clinical trials for publication only if the trials have been registered. Current information on requirements and appropriate registries is available at www.icmje.org/about-icmje/faqs/.
Acknowledgments
Supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development (grant R01HD071915); the Emergency Medical Services for Children Program of the Maternal and Child Health Bureau, Health Resources and Services Administration, under cooperative agreement (awards U03MC00001, U03MC00003, U03MC00006, U03MC00007, U03MC00008, U03MC22684, and U03MC22685); the Washington University Biobank Core, supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (grant P30DK052574); and iHealth, which provided L. rhamnosus GG and placebo capsules in kind. Dr. Freedman is supported by the Alberta Children’s Hospital Foundation Professorship in Child Health and Wellness.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
We thank the staff at the data coordinating center, the research coordinators and clinicians at all participating sites, and the investigators and subcommittees of the Pediatric Emergency Care Applied Research Network, without whose support this trial could not have been conducted; Ms. Sheila Mason and Dr. Richard Buller for stool testing; Dr. Carey-Ann Burnham, Dr. Erik Dubberke, and Ms. Tiffany Hink for L. rhamnosus GG sample testing; and the members of the data and safety monitoring board (Dr. David Reboussin [chair], Dr. Anupam Kharbanda, Dr. Oscar Gomez, Dr. George Fuchs, and Dr. Robert Shulman).
References
- 1.World Health Organization. Diarrhoeal disease fact sheet. May 2017. (http://www.who.int/mediacentre/factsheets/fs330/en/).
- 2.Freedman SB, Steiner MJ, Chan KJ. Oral ondansetron administration in emergency departments to children with gastroenteritis: an economic analysis. PLoS Med 2010; 7(10): e1000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freedman SB, Pasichnyk D, Black KJ, et al. Gastroenteritis therapies in developed countries: systematic review and meta-analysis. PLoS One 2015; 10(6): e0128754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feizizadeh S, Salehi-Abargouei A, Akbari V. Efficacy and safety of Saccharomyces boulardii for acute diarrhea. Pediatrics 2014; 134(1): e176–e191. [DOI] [PubMed] [Google Scholar]
- 5.Szajewska H, Skórka A, Ruszczyński M, Gieruszczak-Białek D. Meta-analysis: Lactobacillus GG for treating acute gastroenteritis in children — updated analysis of randomised controlled trials. Aliment Pharmacol Ther 2013; 38: 467–76. [DOI] [PubMed] [Google Scholar]
- 6.Allen SJ, Martinez EG, Gregorio GV, Dans LF. Probiotics for treating acute infectious diarrhoea. Cochrane Database Syst Rev 2010; 11: CD003048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Reid G Probiotics: definition, scope and mechanisms of action. Best Pract Res Clin Gastroenterol 2016; 30: 17–25. [DOI] [PubMed] [Google Scholar]
- 8.Lomax AR, Calder PC. Probiotics, immune function, infection and inflammation: a review of the evidence from studies conducted in humans. Curr Pharm Des 2009; 15: 1428–518. [DOI] [PubMed] [Google Scholar]
- 9.Szajewska H, Guarino A, Hojsak I, et al. Use of probiotics for management of acute gastroenteritis: a position paper by the ESPGHAN Working Group for Probiotics and Prebiotics. J Pediatr Gastroenterol Nutr 2014; 58: 531–9. [DOI] [PubMed] [Google Scholar]
- 10.Guarino A, Ashkenazi S, Gendrel D, Lo Vecchio A, Shamir R, Szajewska H. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition/European Society for Pediatric Infectious Diseases evidence-based guidelines for the management of acute gastroenteritis in children in Europe: update 2014. J Pediatr Gastroenterol Nutr 2014; 59: 132–52. [DOI] [PubMed] [Google Scholar]
- 11.Vandenplas Y, De Greef E, Hauser B, Devreker T, Veereman-Wauters G. Probiotics and prebiotics in pediatric diarrheal disorders. Expert Opin Pharmacother 2013; 14: 397–409. [DOI] [PubMed] [Google Scholar]
- 12.Guarino A, Lo Vecchio A, Canani RB. Probiotics as prevention and treatment for diarrhea. Curr Opin Gastroenterol 2009; 25: 18–23. [DOI] [PubMed] [Google Scholar]
- 13.Schnadower D, Finkelstein Y, Freedman SB. Ondansetron and probiotics in the management of pediatric acute gastroenteritis in developed countries. Curr Opin Gastroenterol 2015; 31: 1–6. [DOI] [PubMed] [Google Scholar]
- 14.Nixon AF, Cunningham SJ, Cohen HW, Crain EF. The effect of Lactobacillus GG on acute diarrheal illness in the pediatric emergency department. Pediatr Emerg Care 2012; 28: 1048–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MarketsandMarkets. U.S. digestive health enzymes, prebiotics and probiotics market (2010–2015). 2017. (http://www.marketsandmarkets.com/Market-Reports/digestive-health-225.html).
- 16.Global Market Insights. Probiotics market size to exceed USD 64 billion by 2023. May 10, 2016. (http://www.prnewswire.com/news-releases/probiotics-market-size-to-exceed-usd-64-billion-by-2023-global-market-insights-inc-578769201.html).
- 17.Schnadower D, Tarr PI, Casper TC, et al. Randomised controlled trial of Lactobacillus rhamnosus (LGG) versus placebo in children presenting to the emergency department with acute gastroenteritis: the PECARN probiotic study protocol. BMJ Open 2017; 7(9): e018115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lo Vecchio A, Vandenplas Y, Benninga M, et al. An international consensus report on a new algorithm for the management of infant diarrhoea. Acta Paediatr 2016; 105(8): e384–e389. [DOI] [PubMed] [Google Scholar]
- 19.Pediatric Emergency Care Applied Research Network home page. 2017. (http://pecarn.org/index.html).
- 20.Goldenberg JZ, Lytvyn L, Steurich J, Parkin P, Mahant S, Johnston BC. Probiotics for the prevention of pediatric antibiotic-associated diarrhea. Cochrane Database Syst Rev 2015; 12: CD004827. [DOI] [PubMed] [Google Scholar]
- 21.Goldfarb DM, Steenhoff AP, Pernica JM, et al. Evaluation of anatomically designed flocked rectal swabs for molecular detection of enteric pathogens in children admitted to hospital with severe gastroenteritis in Botswana. J Clin Microbiol 2014; 52: 3922–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Freedman SB, Xie J, Nettel-Aguirre A, et al. Enteropathogen detection in children with diarrhoea, or vomiting, or both, comparing rectal flocked swabs with stool specimens: an outpatient cohort study. Lancet Gastroenterol Hepatol 2017; 2: 662–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mengelle C, Mansuy JM, Prere MF, et al. Simultaneous detection of gastrointestinal pathogens with a multiplex Luminex-based molecular assay in stool samples from diarrhoeic patients. Clin Microbiol Infect 2013; 19: E458–E465. [DOI] [PubMed] [Google Scholar]
- 24.Claas EC, Burnham CA, Mazzulli T, Templeton K, Topin F. Performance of the xTAG gastrointestinal pathogen panel, a multiplex molecular assay for simultaneous detection of bacterial, viral, and parasitic causes of infectious gastroenteritis. J Microbiol Biotechnol 2013; 23: 1041–5. [DOI] [PubMed] [Google Scholar]
- 25.Schnadower D, Tarr PI, Gorelick MH, et al. Validation of the modified Vesikari score in children with gastroenteritis in 5 US emergency departments. J Pediatr Gastroenterol Nutr 2013; 57: 514–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Freedman SB, Eltorky M, Gorelick M. Evaluation of a gastroenteritis severity score for use in outpatient settings. Pediatrics 2010; 125(6): e1278–e1285. [DOI] [PubMed] [Google Scholar]
- 27.Alpern ER, Henretig FM. Fever In: Fleisher GR, Ludwig S, eds. Textbook of pediatric emergency medicine. 6th ed Philadelphia: Lippincott Williams & Wilkins, 2010:266–75. [Google Scholar]
- 28.Bhatt DL, Mehta C. Adaptive designs for clinical trials. N Engl J Med 2016; 375: 65–74. [DOI] [PubMed] [Google Scholar]
- 29.Nagelkerke N, Fidler V, Bernsen R, Borgdorff M. Estimating treatment effects in randomized clinical trials in the presence of non-compliance. Stat Med 2000; 19: 1849–64. [DOI] [PubMed] [Google Scholar]
- 30.Lewis JA, Machin D. Intention to treat — who should use ITT? Br J Cancer 1993; 68: 647–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raghunathan TE, Lepkowski JM, Van Hoewyk J, Solenberger P. A multivariate technique for multiply imputing missing values using a sequence of regression models. Surv Methodol 2001; 27: 85–95. [Google Scholar]
- 32.Rubin DB. Multiple imputation for nonresponse in surveys. New York: John Wiley, 1987. [Google Scholar]
- 33.Holm S A simple sequentially rejective multiple test procedure. Scand J Stat 1979; 6: 65–70. [Google Scholar]
- 34.Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010; 340: c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hill C, Scott K, Klaenhammer TR, Quigley E, Sanders ME. Probiotic nomenclature matters. Gut Microbes 2016; 7: 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanders MML, Bush S. “Lactobacillus sporogenes” is not a Lactobacillus probiotic. ASM News 2001; 68: 385–6. [Google Scholar]
- 37.Meurer WJ, Lewis RJ, Berry DA. Adaptive clinical trials: a partial remedy for the therapeutic misconception? JAMA 2012; 307: 2377–8. [DOI] [PubMed] [Google Scholar]
- 38.Rosenblum M, Van der Laan MJ. Optimizing randomized trial designs to distinguish which subpopulations benefit from treatment. Biometrika 2011; 98: 845–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.LeLorier J, Grégoire G, Benhaddad A, Lapierre J, Derderian F. Discrepancies between meta-analyses and subsequent large randomized, controlled trials. N Engl J Med 1997; 337: 536–42. [DOI] [PubMed] [Google Scholar]
- 40.Allen SJ, Wareham K, Wang D, et al. Lactobacilli and bifidobacteria in the prevention of antibiotic-associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): a randomised, double-blind, placebo-controlled, multi-centre trial. Lancet 2013; 382: 1249–57. [DOI] [PubMed] [Google Scholar]
- 41.Costeloe K, Hardy P, Juszczak E, Wilks M, Millar MR. Bifidobacterium breve BBG-001 in very preterm infants: a randomised controlled phase 3 trial. Lancet 2016; 387: 649–60. [DOI] [PubMed] [Google Scholar]
- 42.Little P, Stuart B, Wingrove Z, et al. Probiotic capsules and xylitol chewing gum to manage symptoms of pharyngitis: a randomized controlled factorial trial. CMAJ 2017; 189: E1543–E1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Suez J, Zmora N, Zilberman-Schapira G, et al. Post-antibiotic gut mucosal micro-biome reconstitution is impaired by probiotics and improved by autologous FMT. Cell 2018; 174: 1406–23. [DOI] [PubMed] [Google Scholar]
- 44.Zmora N, Zilberman-Schapira G, Suez J, et al. Personalized gut mucosal colonization resistance to empiric probiotics is associated with unique host and micro-biome features. Cell 2018;174:1388–405. [DOI] [PubMed] [Google Scholar]
- 45.Cohen PA. Probiotic safety — no guarantees. JAMA Intern Med 2018. September 17 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 46.Denno DM, Shaikh N, Stapp JR, et al. Diarrhea etiology in a pediatric emergency department: a case control study. Clin Infect Dis 2012; 55: 897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.