Abstract
Background Context
As increasing numbers of elderly Americans undergo spinal surgery, it is important to identify which patients are at highest risk for poor cognitive and functional recovery. Frailty is a geriatric syndrome which has been closely linked to poor outcomes, and short form screening may be a helpful tool for preoperative identification of at risk patients.
Purpose
To conduct a pilot study on the usefulness of a short-form screening tool to identify elderly patients at increased risk for prolonged cognitive and functional recovery following elective spine surgery.
Study Design/Setting
Prospective, comparative cohort study.
Patient Sample
100 patients over age 65 undergoing elective spinal surgery (cervical or lumbar) at a single, large academic medical center from 2013–2014.
Outcome Measures
FRAIL scale, Quality of Recovery Scale (PQRS), and Instrumental Activities (IADLs) scores.
Methods
Included patients were given the FRAIL scale and stratified as robust, pre-frail, or frail. Post-operative Quality of Recovery Scale (PQRS) and Instrumental Activities (IADLs) scores were also obtained. Patients were re-examined at 1 day, 3 days, 1 month, and 3 months after surgery for cognitive recovery at 3-months, and secondarily, functional recovery at 3-months. This study was funded in part by grants from the National Institute on Aging (K23–17-015, National Institutes of Health, Bethesda, Maryland, USA) and the American Federation for Aging Research (New York City, NY, USA).
Results
At 3-months, only 50% of frail patients had recovered to their cognitive baseline compared to 60.7% of pre-frail and 69.2% of robust patients (trend). At 3-months, 66.7% of frail patients had recovered to their functional baseline compared to 57% of pre-frail and 76.9% of robust patients (trend). Using multivariate regression modelling, at 3 months, frail patients were less likely to have recovered to their cognitive baseline compared to pre-frail and robust patients (OR 0.39, CI 0.131–1.161).
Conclusions
This pilot study demonstrates a trend towards poorer cognitive recovery 3-months following elective spinal surgery for frail patients. Frailty screening can help pre-operatively identify patients who may experience protracted cognitive and functional recovery.
Keywords: Frailty, Elderly Spinal Surgery, Geriatric Spinal Surgery, Cognitive Recovery, Spinal Rehabilitation
INTRODUCTION
Elective spinal surgery on patients over 65 years of age has become increasingly common. There will be a continued increase in spine surgery in this age group due to the demographics of the U.S. population. From 1990– 2004 there has been a 28-fold increase in cervical spine fusion procedures, and in the United States in 2007, 1.6 billion dollars were spent on lumbar spine surgery in the elderly.[1,2] Older spine surgery patients are at increased risk for protracted and/or partial cognitive and functional recovery.[3] Postoperative cognitive decline is an insidious and common problem in the elderly, which affects approximately 13% of patients at 3 months after surgery.[4] In turn, a decline in cognition can be associated with falls and decreased ability to comply with medications and rehabilitation, even further impeding a patient’s recovery.[5–8]
Given the number of older adults undergoing spine surgery, identification of patients at high risk for protracted recovery is very important. Prior studies have shown that the American Society of Anesthesiology Physical Status can be a useful measure of co-morbidity to predict complications in a younger adult cohort.[8–10] The presence of medical co-morbidities, however, may not be the best way to identify which prospective elderly spine surgery patients are at risk for prolonged recovery.[11,12] After all, medical co-morbidities are present in greater than 75% of patients over the age of 65.[13] There is an emerging literature that suggests that frailty is a more holistic view of health status and might better describe the elderly patient’s physiologic reserve and ability to withstand the stress of surgery.[13–14]
Frailty is a geriatric syndrome characterized by weakness and fatigue.[15] It transcends age and comorbidity to describe the overall health status of the individual. The traditional screen for frailty is performed by a geriatrician and includes tests for physical strength, such as walking and grip strength. Given the constraints of the preoperative period, however, the ideal frailty screening tool must be brief and able to be administered by physician extenders and/or perioperative physicians. Recently, short form frailty screening tools amenable to use by non-geriatricians have been employed in the perioperative setting. The FRAIL scale is a simplified 5 point questionnaire that can be administered over the phone.[16] As a screening tool, this questionnaire has been shown to correlate strongly with cognitive and physical decline as well as mortality in a broad array of surgical groups.[17] To date, there has been no prospective study that has used the FRAIL scale in spine surgery patients.
Better risk stratification for elderly spine surgery patients has the potential to influence treatment planning, allocation of surgical resources, and thereby improve postoperative outcomes. To address whether short form frailty screening can predict recovery of postoperative cognition, ADLs, and IADLs, we conducted a prospective cohort study of 100 patients over 65 years of age undergoing elective cervical and lumbar surgery. Our hypothesis was that a patient’s frailty status before surgery is associated with worse cognitive recovery. Secondarily, we assessed whether baseline frailty is associated with worse functional recovery. If so, this information could be used for clinical decision making, patient counseling, and allocation of preoperative and postoperative resources to improve patient outcomes and to help minimize unnecessary morbidity associated with surgery.
METHODS
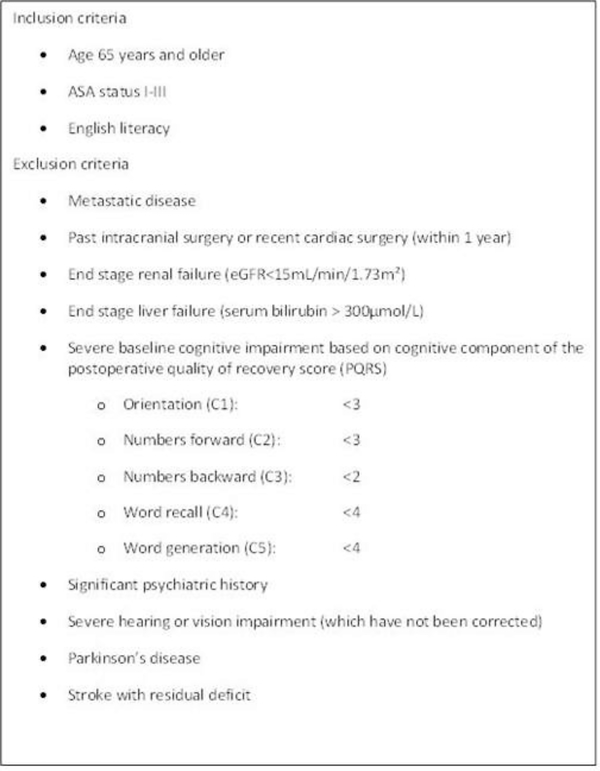
This prospective cohort study was approved by the Mount Sinai Institutional Review Board (HS#: 14–00429, GCO#:1:14–1224) from 2013–2014. Subjects were recruited from the Mount Sinai Electronic Scheduling system with the agreement of the patient’s surgeon. See Figure 1 for inclusion and exclusion criteria.
Figure 1:
Inclusion and exclusion criteria
Measurements:
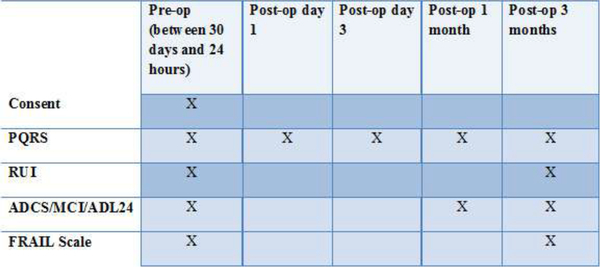
Data was collected at baseline and then at four time points after surgery: day 1, day 3, 1 month, and 3 months (Figure 2). In addition to demographic, medical, and surgical data, three instruments were used to assess recovery.
Figure 2:
Testing schedule for data collection
The FRAIL Scale is a 5 point frailty screening tool validated by Morley et al which operationalizes frailty as [1] fatigue over the past 4 months, [2] ability to climb a flight of stairs unassisted, [3] ability to walk 2 blocks unassisted, [4] medical co-morbidities and [5] loss of weight. Each component makes up one point and the total score is considered as follows: 0 = robust health status, 1–2 = pre-frail, and 3–5 = frail.
The Post-operative Quality of Recovery Scale (PQRS) measures recovery after surgery from baseline, and examines cognitive recovery and activities of daily living (ADL). ADL recovery is defined as return to baseline score or better. Cognitive recovery is defined as a return to the patient’s baseline score, allowing for a 2-point tolerance factor. The Alzheimer’s Disease Research Center (ADRC) Instrumental Activities (IADLs) are a more granular tool used to assess complex function.
Prior to surgery, included patients were contacted by telephone. Informed consent was obtained and demographic data was gathered in addition to baseline data with all three scales. The PQRS cognition and ADL questions were repeated at day 3, and 1 and 3 months after surgery. The ADRC IADL scale was repeated at 1 and 3 months after surgery. The data was stored and managed using REDCAP electronic data capture tools hosted at Mount Sinai.
Analysis:
We performed a univariate comparison at each of the three time points of the proportion of patients who were judged as cognitively “recovered,” and co-variates (demographics such as age, gender, education, and surgical procedure) in the frail, pre-frail, and robust groups. We then created a logistic regression model via backward selection with the outcome of cognitive recovery at 3 days and 3 months after surgery, using predictors that were identified a priori and at least moderately associated with the outcome (p<0.15). These were Age, Diabetes Mellitus, baseline ADL score, PQRS C1, PQRS C2, PQRS C3, PQRS C4, and PQRS C5.
We also performed a secondary analysis with a univariate comparison of the proportion of patients who recovered their ADLs and select IADLs. We then created a logistic regression model via backward selection with the outcome of ADL recovery at 3 days and 3 months after surgery, using predictors that were identified a priori and at least moderately associated with the outcome (p<0.15). These were age, gender, ASA level, BMI, diabetes status, hypertension status, cancer history, PQRS C2, C3, C4, C5, surgical procedure, frailty status, and education level.
RESULTS
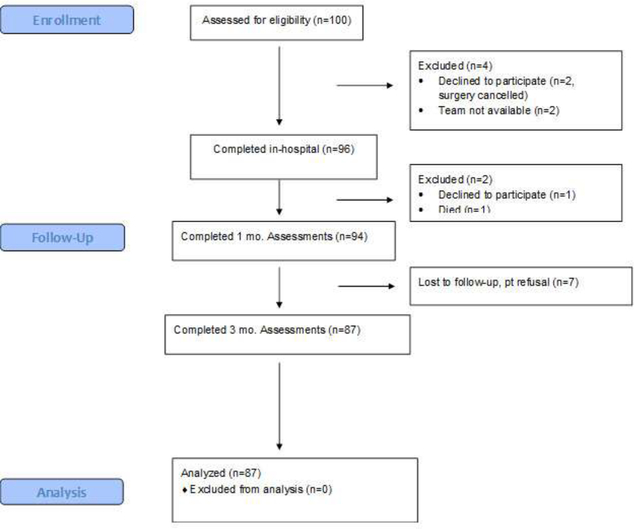
Among the 100 patients enrolled, there were 51 cervical and 49 lumbar surgeries. Of these, 87 patients completed 3 month testing with 7 patients dropping out due to postponement, change or cancellation of surgery, death, voluntary withdrawal, or loss to follow-up (see Figure 3). For the 87 patients who remained in the study, all required data points were completed.
Figure 3:
CONSORT Flow Diagram
Baseline demographic data are presented in Table 1. The median age was 71 years old (IQR 67–76), and 63% were male. Only 26% of patients were robust prior to surgery, 56% were prefrail, and 18% were frail. Our cohort was predominantly white and well educated (65% went to college). The vast majority had at least 1 medical co-morbidity, and were classified as ASA II (defined as a presence of systemic condition(s) which does not affect function, 58%), or ASA III (systemic condition(s) which do affect function, 39%).
Table 1:
Baseline Demographic Data
| Number | %/IQR | |
|---|---|---|
| Gender | ||
| Male | 63 | 63.00% |
| Female | 37 | 37.00% |
| Age | ||
| Median | 71 | 67,76 |
| Marital Status | ||
| Married | 61 | 61.00% |
| Divorced | 16 | 16.00% |
| Widowed | 11 | 11.00% |
| Never Married | 10 | 10.00% |
| Separated | 1 | 1.00% |
| Unknown | 1 | 1.00% |
| Race | ||
| Black or African American | 16 | 16.00% |
| White | 80 | 80.00% |
| Asian | 4 | 4.00% |
| American Indian/Alaska Native | 0 | 0.00% |
| Native Hawaiian or other Pacific Islander | 0 | 0.00% |
| Years of Education | ||
| Did not finish high school (<12 years) | 6 | 6.00% |
| Finished high school | 25 | 25.00% |
| Some college | 8 | 8.00% |
| Finished college | 16 | 16.00% |
| Graduate work | 41 | 41.00% |
| Unknown | 4 | 4.00% |
| Primary Language | ||
| English | 94 | 94.00% |
| Spanish | 2 | 2.00% |
| Other | 4 | 4.00% |
| BMI (Kg/m^2) | ||
| Underweight (<18.5) | 1 | 1.00% |
| Normal weight (18.5–24.9) | 19 | 19.00% |
| Overweight (25–29.9) | 53 | 53.00% |
| Obese (30–34.9) | 19 | 19.00% |
| Severely obese (35–39.9) | 6 | 6.00% |
| Unknown | 2 | 2.00% |
| ASA Status | ||
| 1 | 1 | 1.00% |
| 2 | 58 | 58.00% |
| 3 | 39 | 39.00% |
| Unknown | 2 | 2.00% |
| Frailty Status at Baseline | ||
| Robust Health | 26 | 26.00% |
| Pre-Frail | 56 | 56.00% |
| Frail | 18 | 18.00% |
Surgical data is presented in Table 2. Most operations performed were instrumented (76%) vs. laminectomy without instrumentation (24%). Median surgical and anesthetic duration were 168 minutes (116.75–253.25) and 263.5 minutes (207.75–367.25) respectively. The median number of levels for fusions was 2 (IQR 1–3.75) and for laminectomy was 3 (IQR 2–4). The majority of cervical procedures (76.5%) were via a posterior approach (posterior cervical laminectomy and fusion or cervical laminoplasty), which requires more extensive muscle dissection than anterior discectomy and fusion, and has resultant increased associated pain and in-hospital recovery time. Elderly patients typically have multilevel cervical pathology, which traditionally favors a posterior approach. All lumbar procedures were via a posterior approach (no anterior lumbar interbody fusions).
Table 2:
Surgical Data
| Number | %/IQR | |
|---|---|---|
| Duration of Surgery (median, minutes) | 168 | 116.8–253.3 |
| Duration of Anesthesia (median, minutes) | 263.5 | 207.8–367.3 |
| Cervical Surgery | 51 | 51% |
| Anterior Cervical Fusion | 12 | 23.5% |
| Posterior Cervical Fusion | 26 | 51.0% |
| Posterior Cervical Laminoplasty | 11 | 21.6% |
| Posterior Cervical, not instrumented | 2 | 3.9% |
| Lumbar Surgery | 49 | 49% |
| Posterior Lumbar, not instrumented | 22 | 44.9% |
| Posterior Lumbar Fusion | 27 | 55.1% |
| Extent of Surgery | ||
| Average n.of spinal levels, instrumented | 2 | 1–4 |
| Average n.of spinal levels, not instrumented | 3 | 2–4 |
Frailty and Cognitive Recovery:
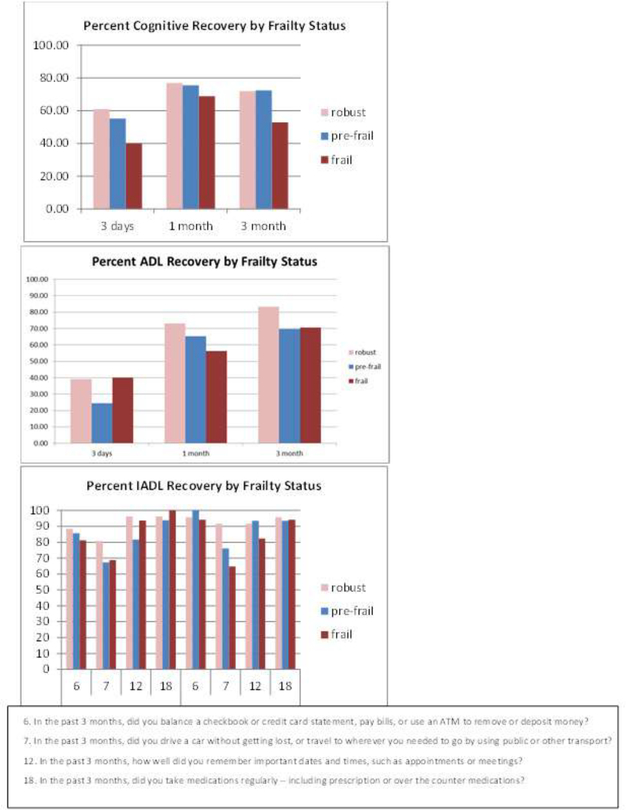
Figure 4 shows the percent that patients recovered in cognition, ADLs, and IADLs at 3 time points for robust, pre-frail, and frail patients. Upon univariate comparison, the robust and prefrail group had a larger proportion of patients who demonstrated cognitive recovery at 3 days after surgery (50.0%, 48.2%) vs. frail patients (33.3%). At 3 months, fewer frail patients were at their baseline (50.0%) vs. pre-frail and robust patients (60.7% and 69.2%, respectively). These differences, however, were trends and not statistically significant.
Figure 4:
Recovery of cognition, ADLS, IADLS by frailty status at 3 days, 1 month, and 3 months after surgery
Using a multivariate logistic regression model, several associations were identified as predictive of cognitive recovery at 3-days and 3-months. At 3-days, patients who had cervical surgery were less likely to be recovered compared to patients who had lumbar surgery (OR 0.23, CI 0.079–0.66), patients with higher BMI were less likely to have recovered (OR 0.88, CI 0.780.99), and patients with lower baseline performance on the C3 (numbers backwards) test from the PQRS (OR 0.53, CI 0.37–0.77) were associated with decreased odds of cognitive recovery. At 3-months, patients who were frail were less likely to have recovered to their cognitive baseline (compared to pre-frail and robust patients). However this was not statistically significant (OR 0.39, CI 0.131–1.161)
Frailty and Functional Recovery:
Functional recovery was measured using the ADL component of the PQRS with recovery defined as a return to the baseline score. Upon univariate comparison, the robust and frail group had a larger proportion of patients recovered at 3 days (34.6% and 33.3%, respectively) vs. prefrail patients (21.4%). The frail and robust group continued to improve at 3 months (66.7% and 76.9% respectively). The pre-frail (57.0%) group, however, had plateaued. These differences were not statistically significant.
Using a multivariate logistic regression model, at postoperative day 3, patients with higher baseline ADLs were less likely to have recovered (OR 0.65, CI 0.47–0.89). Several associations were identified as trends. Patients who were pre-frail were less likely to have recovered their ADLs compared to patients who were frail or robust (OR 0.43, CI 0.16–1.17), which was not statistically significant. Patients with higher educational level were more likely to have recovered their ADLS (OR 1.33, CI 0.95–1.88). At 3-months, odds of recovery of ADLs were lower with higher baseline ADLs (OR 0.55, CI 0.34–0.90), which was statistically significant. Patients who were frail and prefrail were less likely to have recovered compared to patients who were robust (OR 0.2, CI 0.37–1.10 and OR 0.32 CI 0.087–1.17, respectively).
In terms of IADLS, only some questions seemed to stratify by frailty status. Some examples are presented in Figure 4. For example, driving is a skill more quickly recovered by robust patients vs. frail. In contrast, the ability to self- administer medications and manage money was similar in all 3 groups at 1 and 3 months.
DISCUSSION
Our study found that frailty is related to cognitive and functional recovery (ADLs) after surgery. Frail patients recover more slowly, and a higher proportion of them do not recover as compared to robust patients. Regarding ADLs, a similar proportion of frail vs. robust patients were able to return to their baseline ADL status, but the frail patients had statistically lower ADL scores at baseline (i.e. easier to recover a low level of function). Notably, IADLs were different among frailty groups, with more cerebral functions self-reported as recovered (medications, money management), in contrast to more physical activities such as driving and transport.
An important consideration when examining return to functional baseline following elective spinal surgery stems from the goals of treatment and indication for surgery. Generally, the indications for elective spinal surgery are significant pain and weakness that impede functional ability and quality of life. Thus, the decision for spinal surgery often implies a baseline functional impairment. The goal of surgery should be to get the patient to return to or surpass his or her preoperative baseline. A failure to return to baseline is therefore particularly significant for spinal surgery, and indeed, perhaps a return to baseline alone may not represent true recovery. This study, however, did not specifically examine surgical indications, which would be worth examining in future work.
As with frailty’s relationship to many other disease states, there is overlap between the signs and symptoms of frailty and those of spine-disorder associated disability. This likely helps explain the high prevalence of pre-frail and frail patients in our study. Such overlap introduces a potential confounding effect, given that spine-disorder associated symptoms would hopefully improve after surgery, and may represent a modifiable rather than fixed characteristic. Even still, we think that the holistic approach of frailty, which has been demonstrated across many different disease states, is an effective and validated metric and useful in the setting of spine patients.
Our study is one of the most nuanced studies of the relationship of frailty to outcomes in spine surgery. To date, this is the first study to look at frailty, function, and cognition. In the spine literature, several studies have associated frailty with postoperative complications such as wound infection and need for reoperation. In a large cohort of 3,920 patients from the NSQIP database, Phan et al found that frailty is associated with increased surgical morbidity for patients undergoing anterior lumbar interbody fusion.[18,19] Miller et al found that frailty status is associated with increased risk for major intra- and post-operative complications in 417 patients undergoing adult spinal deformity surgery.[20] Ours is the first study, however, to prospectively investigate the role of frailty status in relationship to cognitive and post-surgical recovery in spine surgery.
With respect to the greater literature of frailty and outcomes, our findings are in agreement with Mitnitski et al, who found that frail patients were more likely to demonstrate cognitive decline over time.[21] Similarly, Armstrong et al found that frailty predicts a decline in cognition in a community dwelling cohort of older men.[22] These studies each used a different method to measure cognition, and had much longer follow up period (years) than our study. Compared to these studies, our patients had more co-morbidities and all underwent surgery, which may have made them more likely to experience cognitive decline over a shorter period of time. It is also important to note that in our study (and the previous literature), cognitive decline was a relatively subtle finding only discovered on neuropsychiatric testing. In all of these studies, the patient’s subjective experience of his or her cognition was not measured, and it may be interesting to investigate whether patients perceived a sense of impairment. Whether our frail patients’ cognition would improve or decline over a longer follow up period is unclear, and merits study in the future.
Frailty has also been shown to be a risk factor for further functional decline. Medina – Mirapeix et al found that frail patients who were hospitalized for acute COPD exacerbation were more likely than other groups to demonstrate functional decline 3 months after hospitalization.[23] In contrast, our frail patients appeared to be less vulnerable to functional decline from their baseline. This baseline function, however, was relatively low, and may have been subject to floor effects (i.e. difficult to decline when baseline scores are already low). This was still clinically relevant, however, since only two-thirds of frail patients were recovered to baseline function at 3 months after surgery.
Interestingly, our study demonstrated that the pre-frail patients took longer to recover, and fewer recovered overall in terms of ADLs relative to the robust and frail groups. Whether additional intervention such as prehabilitation or targeted physical therapy could help these individuals recover more quickly is unclear, but is an area for further investigation.
Our study demonstrates that a short form screening tool for frailty can identify spine surgery patients who are at risk for a decline in cognition and functional status after surgery. Short forms are important in the perioperative period, since most settings will not have a geriatrician to perform an in-depth exam of the large number of older patients who present for spine surgery. The FRAIL scale has been shown to perform well in community settings, and other medically complicated populations such as diabetics.[16,24] Although there is a fair amount of heterogeneity between frailty screening tools (components, sensitivity, and specificity), the FRAIL scale has generally compared favorably to other indices.[25,26] It may be worthwhile to undertake future studies to compare predictive ability of different frailty indices to predict specific outcomes in the spine population.
Limitations:
In longitudinal studies of the elderly, patients lost to follow up are often the sickest, which would tend to bias our findings towards the null. However, we did have a relatively high follow up rate. Tools like the PQRS may not capture subtle deficits, found on in-depth neuropsychiatric batteries. These batteries can be very difficult to perform in a normal clinical setting, however. Our findings are confounded by the effects and timing of pain medications, sleep quality, depression, and anxiety. We did not collect data on post-operative analgesia regimens, which may include benzodiazepines and other medications with known psychotropic effects that may impair recovery. In addition, we did not analyze our data according to the specific anesthesia agents that patients received during the perioperative and operative period. Both of these variables may have had a confounding effect.
There is the potential that self-reported functional measures may not be accurate in patients with cognitive impairment. Given that the data was collected via telephone interviews and was self-reported, it is difficult to verify the reliability of the information received from patients. Farias et al demonstrated, however, that even patients with cognitive impairment can generally report basic outcomes accurately.[27] Patients with severe cognitive impairment or psychiatric impairment were excluded from this study (see Figure 1). Future studies could utilize caregiver or physician assessments to maximize data accuracy.
One major limitation to our study was size. Post-hoc calculations demonstrated that our study was underpowered with only 100 patients; 80% power would require 364 patients. Our sample size, however, was limited by resources for follow up for greater than 100 patients. We aim to continue our investigation with both a larger sample as well as an extended follow up period (to 1 year).
Our small study may not have adjusted for all potential differences in spine surgery between patients. Future studies could look at more homogenous populations (i.e. cervical, thoracic, lumbar, fusion vs. laminectomy alone). Finally, our follow up period was relatively short and it is certainly possible that pre-frail and frail patients did continue to improve, albeit more slowly than robust patients.
CONCLUSIONS
The exponential growth in spine surgery in the elderly has produced a large and heterogeneous group of patients, some of whom are at higher risk for protracted or incomplete cognitive and/or functional recovery. Our study found that preoperative frailty status can identify patients who more often develop cognitive decline at 3 months, and that pre- frail and frail patients recover their functional status more slowly. Short form frailty screening is a promising avenue toward prospectively identifying which older patients may be at risk for prolonged or incomplete recovery after surgery. More studies are needed to evaluate which frailty screens are most useful, which outcomes are most meaningful to patients and providers, and where interventions prior to or immediately after surgery are possible. This information may also assist surgeons to preemptively identify the highest risk patients, and thus advise procedure choice, and counsel patients and families appropriately. Given the magnitude of the problem, better preoperative screening for elderly spine patients has the potential to ameliorate significant suffering, patient and caregiver burden, and healthcare cost.
ACKNOWLEDGMENTS
Funding:
This study was funded in part by grants from the National Institute on Aging (K23–17-015, National Institutes of Health, Bethesda, Maryland, USA) and the American Federation for Aging Research (New York City, NY, USA).
Footnotes
Competing Interests:
The authors declared no direct potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval:
This study was approved by the Mount Sinai Institutional Review Board (HS#: 14–00429, GCO#:1:14–1224) from 2013–2014.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Epstein NE. Spine surgery in geriatric patients: Sometimes unnecessary, too much, or too little. Surg Neurol Int 2011;2:188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wang MC, Chan L, Maiman DJ, Kreuter W, Deyo RA. Complications and Mortality Associated With Cervical Spine Surgery for Degenerative Disease in the United States. Spine 2007;32:342–7. [DOI] [PubMed] [Google Scholar]
- [3].Deyo RA, Mirza SK, Martin BI, Kreuter W, Goodman DC, Jarvik JG. Trends, major medical complications, and charges associated with surgery for lumbar spinal stenosis in older adults. JAMA 2010;303:1259–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Monk TG, Weldon BC, Garvan CW, Dede DE, van der Aa MT, Heilman KM, et al. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology 2008;108:18–30. [DOI] [PubMed] [Google Scholar]
- [5].Steinmetz J, Rasmussen LS. Choice Reaction Time and cognitive dysfunction following cardiac surgery. Acta Anaesthesiol Scand 2009;53:1230. [DOI] [PubMed] [Google Scholar]
- [6].Vu LN, Dean MJ, Mwamburi M, Au R, Qiu WQ. Executive function and mortality in homebound elderly adults. J Am Geriatr Soc 2013;61:2128–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gothe NP, Fanning J, Awick E, Chung D, Wójcicki TR, Olson EA, et al. Executive function processes predict mobility outcomes in older adults. J Am Geriatr Soc 2014;62:285–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lawrence VA, Hazuda HP, Cornell JE, Pederson T, Bradshaw PT, Mulrow CD, et al. Functional independence after major abdominal surgery in the elderly. J Am Coll Surg 2004;199:762–72. [DOI] [PubMed] [Google Scholar]
- [9].Di Capua J, Somani S, Kim JS, Phan K, Lee NJ, Kothari P, et al. Analysis of Risk Factors for Major Complications Following Elective Posterior Lumbar Fusion. Spine 2017;42:1347–54. [DOI] [PubMed] [Google Scholar]
- [10].Lee NJ, Guzman JZ, Kim J, Skovrlj B, Martin CT, Pugely AJ, et al. A Comparative Analysis Among the SRS M&M, NIS, and KID Databases for the Adolescent Idiopathic Scoliosis. Spine Deform 2016;4:420–4. [DOI] [PubMed] [Google Scholar]
- [11].Makary MA, Segev DL, Pronovost PJ, Syin D, Bandeen-Roche K, Patel P, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg 2010;210:901–8. [DOI] [PubMed] [Google Scholar]
- [12].Amrock LG, Deiner S. The implication of frailty on preoperative risk assessment. Curr Opin Anaesthesiol 2014;27:330–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Wolff JL, Starfield B, Anderson G. Prevalence, expenditures, and complications of multiple chronic conditions in the elderly. Arch Intern Med 2002;162:2269–76. [DOI] [PubMed] [Google Scholar]
- [14].Robinson TN, Wu DS, Stiegmann GV, Moss M. Frailty predicts increased hospital and six-month healthcare cost following colorectal surgery in older adults. Am J Surg 2011;202:511–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–56. [DOI] [PubMed] [Google Scholar]
- [16].Morley JE, Malmstrom TK, Miller DK. A simple frailty questionnaire (FRAIL) predicts outcomes in middle aged African Americans. J Nutr Health Aging 2012;16:601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Robinson TN, Wu DS, Pointer L, Dunn CL, Cleveland JC Jr, Moss M. Simple frailty score predicts postoperative complications across surgical specialties. Am J Surg 2013;206:544–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Phan K, Kim JS, Lee NJ, Somani S, Di Capua J, Kothari P, et al. Frailty is associated with morbidity in adults undergoing elective anterior lumbar interbody fusion (ALIF) surgery. Spine J 2017;17:538–44. [DOI] [PubMed] [Google Scholar]
- [19].Leven DM, Lee NJ, Kim JS, Kothari P, Steinberger J, Guzman J, et al. Frailty Is Predictive of Adverse Postoperative Events in Patients Undergoing Lumbar Fusion. Global Spine J 2017;7:529–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Miller EK, Neuman BJ, Jain A, Daniels AH, Ailon T, Sciubba DM, et al. An assessment of frailty as a tool for risk stratification in adult spinal deformity surgery. Neurosurg Focus 2017;43:E3. [DOI] [PubMed] [Google Scholar]
- [21].Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal 2001;1:323–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Armstrong JJ, Godin J, Launer LJ, White LR, Mitnitski A, Rockwood K, et al. Changes in Frailty Predict Changes in Cognition in Older Men: The Honolulu-Asia Aging Study. J Alzheimers Dis 2016;53:1003–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Medina-Mirapeix F, Bernabeu-Mora R, García-Guillamón G, Valera Novella E, Gacto-Sánchez M, García-Vidal JA. Patterns, Trajectories, and Predictors of Functional Decline after Hospitalization for Acute Exacerbations in Men with Moderate to Severe Chronic Obstructive Pulmonary Disease: A Longitudinal Study. PLoS One 2016;11:e0157377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Chode S, Malmstrom TK, Miller DK, Morley JE. Frailty, Diabetes, and Mortality in Middle-Aged African Americans. J Nutr Health Aging 2016;20:854–9. [DOI] [PubMed] [Google Scholar]
- [25].Theou O, Brothers TD, Mitnitski A, Rockwood K. Operationalization of frailty using eight commonly used scales and comparison of their ability to predict all-cause mortality. J Am Geriatr Soc 2013;61:1537–51. [DOI] [PubMed] [Google Scholar]
- [26].Malmstrom TK, Miller DK, Morley JE. A comparison of four frailty models. J Am Geriatr Soc 2014;62:721–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Farias ST, Mungas D, Jagust W. Degree of discrepancy between self and other-reported everyday functioning by cognitive status: dementia, mild cognitive impairment, and healthy elders. Int J Geriatr Psychiatry 2005;20:827–34. [DOI] [PMC free article] [PubMed] [Google Scholar]