Abstract
BDNF Val66Met polymorphism may be important source of heterogeneity seen in cognitive aging, although the specific relationship between this polymorphism and cognition remains controversial and may depend on the sex of participants. We assessed 2668 older black and white adults and fit linear mixed models to Digit Symbol Substitution Test (DSST) performance assessed in years 0 (baseline), 4, 7 and 9 to examine the interaction between sex and BDNF genotype on the intercept (i.e., estimated baseline DSST) and change in DSST over 9-years, adjusted for covariates. Sex interacted with BDNF genotype to predict DSST intercept (F[1,1599]=7.4, p<0.01) and 9-year change (F[1,1183]=4.1, p=0.04) in white participants only. Initially, white male Val/Val carriers had lower DSST scores (37.6, SE = 0.8) in comparison to male Met carriers (difference, −1.7; 95% CI, −3.2 to −0.3) and female Val/Val carriers (difference, −5.6; 95% CI, −6.8 to −4.3). White female Met carriers showed a slower rate of change (annual rate of change=−0.6, SE=0.1) in comparison to Female Val/Val carriers (difference, −0.2; 95% CI, −0.4 to −0.02) and Male Met carriers (difference, −0.3; 95% CI, −0.5 to −0.02). Our findings suggest that BDNF Val66Met and sex should be considered in future endeavours aimed at treating or preventing neurodegenerative disorders.
Keywords: Cognitive aging, Epidemiology, Brain Derived Neurotrophic Factor
1. Introduction
Aging is characterized by multifaceted changes within several cognitive domains and the brain regions that subserve them (Salthouse, 2011). Notably, aging negatively impacts executive functions and processing speed, cognitive processes that are significantly associated with the ability to perform activities of daily living and all-cause mortality (Pavlik et al., 2003; Royall et al., 2004). However, the deleterious effects of aging are not equally seen across individuals, and a significant proportion of the population maintain cognitive function even into older age (Hayden et al., 2011; Wilson et al., 2002). Understanding the sources of this variation in cognitive aging will be crucial for future endeavours aiming to treat or prevent neurodegenerative disorders such as Alzheimer’s disease (AD). Importantly, genetic studies suggest that allelic variation within the neuroplasticity-related gene encoding for brain-derived neurotrophic factor (BDNF) may be a potential source of the heterogeneity seen in cognitive aging, although the exact relationship between polymorphisms within this gene and cognitive decline remain controversial.
BDNF is a neurotrophin critically involved in neuronal proliferation, differentiation and survival, synaptic plasticity and the cellular mechanisms required for learning and memory (Cowansage et al., 2010). A common single nucleotide polymorphism (SNP) is found within the pro-domain region of the human BDNF gene, resulting in an amino acid substitution of valine (Val) to methionine (Met) at position 66, termed the Val66Met substitution. Frequency of the variant Met allele within the general population ranges from ~4% in African Americans to ~30% in Caucasians and ~50% in Asians (Petryshen et al., 2010; Pivac et al., 2009; Shimizu et al., 2004). The Met allele alters intracellular trafficking of the precursor form of BDNF (proBDNF), reducing the activity-dependent neuronal secretion of the mature form of BDNF by approximately 30% while total central BDNF levels remain normal compared with Val/Val carriers (Chen et al., 2008; Egan et al., 2003). Functionally, the direction of the relationship between this SNP and cognitive decline remains controversial.
Although it has been suggested that the Val allele may be neuroprotective and related to higher cognition and the Met allele is related to impaired cognitive function (Chen et al., 2008), the evidence regarding the association between the BDNF Val66Met polymorphism and cognitive performance is not straighforward. Earlier cross-sectional studies found that compared to homozygous Val carriers, Met carriers presented with reduced episodic memory and smaller hippocampal volume in younger adults (Egan et al., 2003; Hariri et al., 2003), as well as reduced verbal learning and memory and processing speed in older adults (Kennedy et al., 2015; Miyajima et al., 2008). Longitudinal declines in verbal learning and memory were greater in middle-aged Met allele carriers at risk for AD (Boots et al., 2017). However, in contrast to these findings, other studies have failed to find a significant association between the Met allele and cognitive decline in participants at risk for AD (Honea et al., 2013; Lim et al., 2013; Weinstein et al., 2014). Interestingly, studies have even found the opposite, with greater cognitive performance in Met carriers compared to Val/Val carriers in different populations, including patients with Parkinson’s Disease, AD, and MCI as well as in cognitively healthy older adults (Erickson et al., 2008; Feher et al., 2009; Gajewski et al., 2011, 2012; Harris et al., 2006; Nagata et al., 2012; van der Kolk et al., 2015).
These conflicting results may be due to several reasons, including the specific cognitive domain under investigation as many of the original studies have focused on memory-based tasks and there is suggestion that executive functions may show a greater association with this polymorphism (Mandelman and Grigorenko, 2012), the age of participants, and differences in cognitive status between populations. Importantly, a recent meta-analysis indicated that performance on memory based tasks was greater in Val/Val carriers whereas performance on tasks assessing executive functions was greater in Met carriers (Toh et al., 2018). Further, the biological sex of participants may help explain discrepancies in the relationship between the BDNF Val66Met polymorphism and cognition, as sex differences exist in the effect of the Met allele on several outcomes, including hippocampal blood flow, volume of the dorsolateral prefrontal cortex, and on AD risk (Fukumoto et al., 2010; Nemoto et al., 2006; Wei et al., 2012). And, importantly, older women may show less age-associated decline across certain cognitive domains, including executive functions and processing speed (Gerstorf et al., 2011; McCarrey et al., 2016; McDowell et al., 2004).
We sought to determine whether BDNF Val66Met polymorphism interacts with sex to influence rate of change in the Digit Symbol Substitution Test (DSST), a test of executive functioning and processing speed, in older adults. To that aim, we conducted a secondary analysis of data from the Health, Aging and Body Composition (Health ABC) Study – a 10-year longitudinal, cohort study of cognitively health older. Importantly, peripheral BDNF levels decline with increasing chronological age and this decline is related to cognitive impairment, which may be more pronounced in women than men (Komulainen et al., 2008; Weinstein et al., 2014). Previously, a cross-sectional analysis of a subsample of the parent Health ABC cohort showed a strong sex related difference in serum BDNF levels as well as a trend for a main effect of BDNF polymorphism that was not explored in relationship to biological sex (Nettiksimmons et al., 2014). Therefore, we conducted an exploratory analysis to determine whether BDNF genotype differences in peripheral BDNF levels differed by sex.
2. Methods
2.1. Study Design and Participants
The Health ABC study is a 10-year prospective, epidemiological, biracial cohort study of older adults 70–79 years of age at baseline. The present study involves 2668 of the 3,075 community-dwelling black and white participants without dementia who were recruited in 1997 from Memphis, TN or Pittsburgh, PA, with complete baseline data on the main measures of interest (i.e., cognition, sex, BDNF genotyping). All eligible participants gave written informed consent and institutional review boards at the University of Tennessee Memphis, the University of Pittsburgh, and the University of California San Francisco gave study approval.
2.2. Measurements
Descriptive variables and covariates.
Baseline demographics and health characteristics were assessed at baseline in 1997/1998. Demographics included age, race (white or black), sex, and educational attainment (< or > high school). Health related characteristics included depressive symptoms as assessed with the 20-item Center for Epidemiologic Studies-Depression Scale (CES-D) (Radloff, 1977) with scores above 16 indicative of depression. Diabetes status was determined by self-report and confirmed by medication use. Gait speed (m/s) was assessed over a 6-meter walkway. Self-reported time-spent walking was assessed annually from year 0 to year 9 using a standardized questionnaire developed for the Health ABC study and modeled on a previous questionnaire (Taylor et al., 1978). Based on previous work suggesting a link between physical activity and the BDNF Val66Met polymorphism (Erickson et al., 2013), we used the average time spent walking across all time points for the current analyses.
Cognitive functioning.
The Digit Symbol Substitution Test (DSST) was administered to participants in years 0, 4, 7, and 9 after baseline to measure executive functioning and information processing. The test consists 9 digit-symbol pairs, and participants must fill in as many corresponding symbols for the given digits within 90-seconds. The DSST score is the total number of items correctly coded, with higher scores indicating better executive functioning. Global cognitive performance was measured with the modified mini-mental status examination (3MS) (Teng and Chui, 1987), with scores ranging from 0 to 100 and scores lower than 80 indicating cognitive impairment in years 1, 5, 8 and 10.
DNA collection and BDNF genotyping.
DNA was extracted from whole-blood samples (Gentra Systems, Minneapolis, MN) and the Val66Met polymorphism (rs6265) was determined using standard methods (Nettiksimmons et al., 2014).
BDNF serum levels.
BDNF was measured in serum from fasting blood samples obtained from a random subset of 1000 participants without possible dementia (score on the 3MS < 80) in year 2 of the Health ABC study (Nettiksimmons et al., 2014). Serum was stored at −70°C and shipped to R&D Systems’ Analytical Testing Service (Minneapolis, MN) for measurement of BDNF using an enzyme-linked immunosorbent assay (ELISA) with a detection limit of 1250 pg/mL. Average intra- and inter-assay coefficients of variation were <10%. Platelet count was measured in samples obtained in year 3 as previously reported (Nettiksimmons et al., 2014). Data from 77 participants was excluded for technical reasons and 166 participants were further excluded because of missing DSST scores or important covariates.
2.3. Statistical Analyses
Linear mixed models were fitted using the lme4 package (Version 1.14) (Bates et al., 2015) in R version 3.4.3 (Team, 2017). Analyses used restricted maximum likelihood estimation (REML) using all available data from each participant with at least baseline data. REML is an implicit imputation procedure under the assumption that data are missing at random (Enders, 2013). This approach has been shown to provide less biased estimates and to increase generalizability in comparison to deletion approaches that remove individuals with missing follow-up data (Elobeid et al., 2009). The intercepts were specified as a random effect. Time was entered as a within-subjects repeated measure (year 0[baseline], 4, 7, 9). Sex (females, males) and BDNF genotype (Met carrier, Val/Val carrier) and their interactions with time (i.e., sex*time, BDNF genotype*time, sex*BDNF genotype*time) were entered as between-subjects fixed effects. Based on previous findings, the following covariates and their interactions with time were also included as fixed effects: depression score (CES-D score), average self-reported time spent walking (minutes per week), age, education, diabetes status, and study site (Memphis, Pittsburgh) (Hosang et al., 2014; Kennedy et al., 2015; Zhou et al., 2013). Additionally, slope score was specified as a random effect, and due to potential racial differences (Petryshen et al., 2010; Pivac et al., 2009; Rosano and Lopez, 2015; Shimizu et al., 2004), we stratified based on participant race (white versus black). We report only the highest-order interaction that was significant (e.g., 3-way interaction). Post-hoc pairwise comparisons were conducted with z-score contrasts. Statistical significance was set at a two-tailed alpha = 0.05 for each analysis.
3. Results
3.1. Genotype frequency
The sample consisted of 1,618 white and 1,050 black participants. In white participants, the genotype distribution was 66.4% Val/Val, 30.2% Val/Met and 3.4% Met/Met, and this distribution fit the Hardy-Weinberg equilibrium (χ2 = 0.005, p = 0.94). In black participants, the genotype distribution was 91.0% Val/Val, 8.9% Val/Met and 0.2% Met/Met, and this was in conformity with Hardy-Weinberg equilibrium (χ2 = 0.023, p = 0.88). The genotype frequency significantly differed between whites and blacks (χ2 = 214.50, p < 0.001), as previously reported (Petryshen et al., 2010; Shimizu et al., 2004). Following conventions established in the field, Met/Met participants and Val/Met participants were combined into a single group because of the low frequency of the Met/Met genotype (less than 5%). The frequency of the two genotypes did not differ between males and females in either whites (χ2 = 0.009, p = 0.93) or blacks (χ2 = 1.62, p = 0.20). See Table 1 for final group numbers in white and black participants.
Table 1.
Demographics and baseline characteristics across BDNF Val66Met genotype in male and female white and black participants in the Health ABC study.
| White participants | |||||
|---|---|---|---|---|---|
| Val/Val carriers | Met carriers | ||||
| Males | Females | Males | Females | p value of interaction^ | |
| N (%) | 564 (34.9%) | 510 (31.5%) | 287 (17.7%) | 257 (15.9%) | 0.93 |
| Age, mean (SD) | 73.9 (2.9) | 73.6 (2.8) | 73.7 (2.9) | 73.4 (2.7) | 0.89 |
| Education < high school, % | 224 (39.7%) | 275 (53.9%) | 118 (41.1%) | 135 (52.5%) | 0.60 |
| Weight kg, mean (SD) | 81.3 (12.9) | 67.0 (12.6) | 81.4 (11.5) | 65.0 (11.8) | 0.12 |
| Diabetes, % | 76 (13.5%) | 40 (7.8%) | 37 (12.9%) | 18 (7.0%) | 0.85 |
| CES-D, mean (SD) | 3.8 (4.5) | 5.5 (5.9) | 4.3 (4.9) | 5.3 (6.0) | 0.17 |
| Baseline 3MS, mean (SD) | 92.3 (5.9) | 93.5 (5.4) | 92.7 (5.4) | 93.9 (5.4) | 0.98 |
| Gait speed (m/s), mean (SD) | 1.3 (0.2) | 1.2 (0.2) | 1.3 (0.2) | 1.2 (0.2) | 0.61 |
| Average self-reported time spent walking (min/week), mean (SD) | 136.4 (288.6) | 84.7 (108.3) | 129.9 (163.9) | 96.2 (125.4) | 0.99 |
| Black participants | |||||
| N (%) | 388 (37.0) | 567 (54.0) | 45 (4.3) | 50 (4.8) | 0.20 |
| Age, mean (SD) | 73.5 (2.8) | 73.2 (2.9) | 73.5 (2.7) | 73.2 (3.0) | 0.86 |
| Education < high school, % | 272 (70.1%) | 405 (71.4%) | 35 (77.8%) | 38 (76.0%) | 0.88 |
| Weight kg, mean (SD) | 81.7 (15.1) | 76.0 (15.7) | 80.2 (12.8) | 74.5 (17.6) | 0.99 |
| Diabetes, % | 91 (23.5%) | 117 (20.6%) | 6 (13.3%) | 7 (14.0%) | 0.72 |
| CES-D, mean (SD) | 4.4 (4.9) | 4.9 (5.2) | 3.0 (3.8) | 5.0 (5.6) | 0.20 |
| Baseline 3MS, mean (SD) | 86.3 (8.3) | 88.1 (7.8) | 88.0 (6.7) | 88.5 (7.8) | 0.50 |
| Baseline Gait Speed (m/s), mean (SD) | 1.1 (0.2) | 1.0 (0.2) | 1.1 (0.2) | 1.1 (0.1) | 0.26 |
| Average self-reported time spent walking (min/week), mean (SD) | 103.0 (158.1) | 54.4 (91.5) | 105.2 (201.1) | 63.3 (95.9) | 0.85 |
p values testing sex by BDNF Val66Met genotype interaction from 2-way analysis of variance for continuous variables and logistic regression for categorical variables.
3.2. Demographics and baseline characteristics by BDNF genotype and sex
Table 1 provides baseline characteristics of the Health ABC sample, stratified by race, genotype, and sex. Among white participants, groups did not significantly differ at baseline in age, education level, diabetes status, depression, or global cognition (all p’s > 0.17). Among black participants, groups did not significantly differ at baseline on any variable examined (all p’s > 0.20).
3.3. Sex difference in the association between BDNF genotype and change in executive functioning.
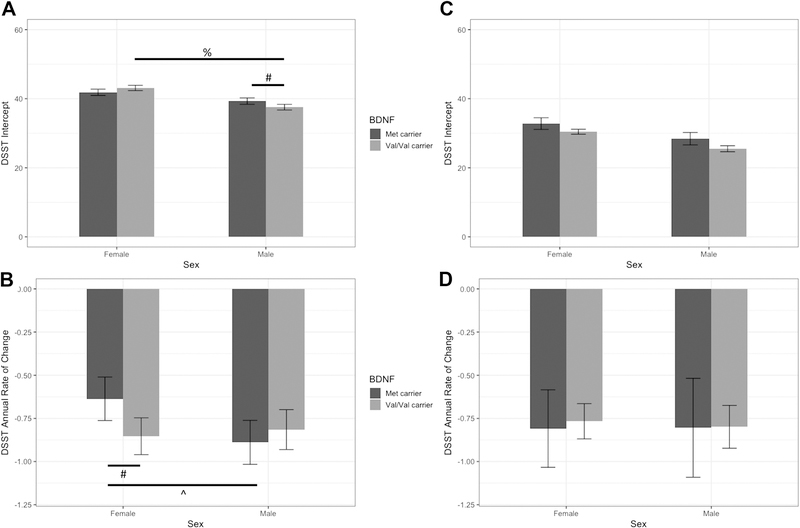
Table 2 summarizes the main effects of, and interactions between, sex and BDNF genotype on the DSST intercept and slope, stratified by participant race. Among white participants, sex interacted with BDNF genotype to predict the DSST intercept (F[1,1599] = 7.4, p < 0.01) and slope (F[1,1183] = 4.1, p = 0.04). Figure 1 depicts differences in the DSST intercept in white participants (panel A) and black participants (panel C), as well as annual rate of change in white participants (panel B) and black participants (panel D) as a function of sex and BDNF genotype. In white participants, initially, male Val/Val carriers had a lower DSST scores (37.6, SE = 0.8) in comparison to male Met carriers (difference, −1.7; 95% CI, −3.2 to −0.3) and female Val/Val carriers (difference, −5.6; 95% CI, −6.8 to −4.3). Among females, there was no significant difference in DSST intercept between Met and Val/Val carriers (difference, −1.3; 95% CI, −2.8 to 0.3). The effect of BDNF genotype on the DSST interecept in males was significantly different from its effect in females (difference, 3.0, 95% CI, 0.8 to 5.2).
Table 2.
Summary of main effects of sex and BDNF Val66Met genotype, and their interaction on DSST intercept and slope, stratified by participant race.
| Predicting DSST in white participants | |||
|---|---|---|---|
| Predicting intercept | F value | degrees of freedom | P value |
| Sex | 51.1 | 1, 1598 | <.001 |
| BDNF genotype | 0.2 | 1, 1599 | .66 |
| Sex X BDNF genotype | 7.4 | 1, 1599 | <.01 |
| Predicting change over time | |||
| Time | 61.9 | 1, 1260 | <.001 |
| Sex X Time | 2.2 | 1, 1184 | .14 |
| BDNF genotype X Time | 1.0 | 1, 1184 | .32 |
| Sex X BDNF genotype X Time | 4.1 | 1, 1183 | .04 |
| Predicting DSST in black participants | |||
| Predicting intercept | F value | degrees of freedom | P value |
| Sex | 14.4 | 1, 1034 | <.001 |
| BDNF genotype | 4.7 | 1, 1034 | .03 |
| Sex X BDNF genotype | 0.1 | 1, 1034 | .82 |
| Predicting change over time | |||
| Time | 39.5 | 1, 736 | <.001 |
| Sex X Time | 0.01 | 1, 787 | .94 |
| BDNF genotype X Time | 0.02 | 1, 789 | .89 |
| Sex X BDNF genotype X Time | 0.01 | 1, 792 | .92 |
Note: Not shown are the effects of the covariates: age, CES-D, education, clinical site, average amount of self-reported time spent walking, and diabetes.
Figure 1.
Estimated Digit Symbol Substitution Test (DSST) intercepts in white participants (panel A) and black participants (panel C), as well as annual rate of change (slope) in white participants (panel B) and black participants (panel D) separated by sex and BDNF genotype. Values are adjusted for clinical site, depression, age, education, self-reported time spent walking, and prevalent diabetes. Error bars represent standard errors. A significant interaction was found between sex and BDNF genotype in white participants (panels A and B) but not black participants (panels C and D). #: indicates significant difference between Met carriers and Val/Val carriers; %: indicates significant difference between female and male Val/Val carriers; ^: indicates significant difference between female and male Met carriers.
With regard to slope scores, in white participants all groups showed significant decreases in DSST scores over time (all p’s < 0.001); however, Female Met carriers showed a slower rate of change in DSST (annual rate of change, −0.6, SE = 0.1) in comparison to Female Val/Val carriers (difference, −0.2; 95% CI, −0.4 to −0.02) and Male Met carriers (difference, −0.3; 95% CI, −0.5 to −0.02). The effect of BDNF genotype on the DSST rate of change in males was significantly different from its effect in females (difference, 0.3, 95% CI, 0.01 to 0.6). No other between group differences were observed (all p’s > 0.08).
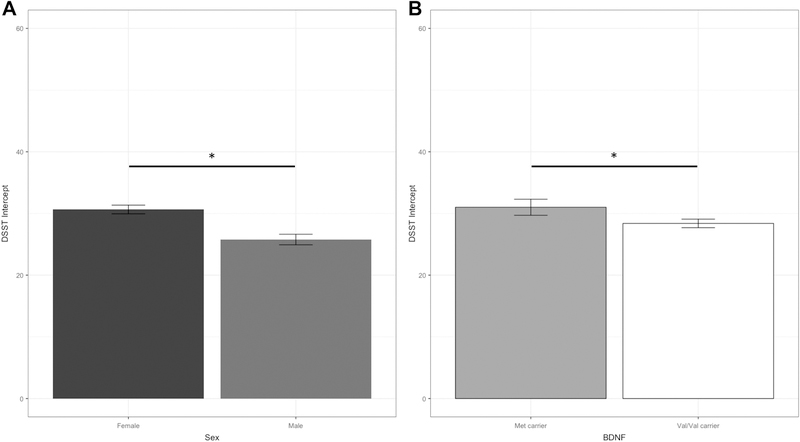
Among black participants, there was no significant interaction between sex and BDNF genotype on either DSST intercept (p = 0.82; Figure 1 panel C) or slope (p = 0.92; Figure 1 panel D); however, there was a main effect of sex (F[1,1034] = 14.4, p < .001) and BDNF genotype (F[1,1034] = 4.7, p = .03) on the DSST intercept (see Table 2). As depicted in Figure 2, females had higher intial DSST scores than males (panel A; difference, 4.9; 95% CI, 3.5 to 6.3), and Met carriers had higher intercepts than Val/Val carriers (panel B; difference, 2.6; 95% CI, 0.2 to 5.0). No differences by sex or genotype were observed on slope scores (all p’s > 0.89).
Figure 2.
Estimated Digit Symbol Substitution Test (DSST) intercepts separated by sex (panel A) and BDNF genotype (panel B) in black participants. Values are adjusted for clinical site, depression, age, education, self-reported time spent walking, and prevalent diabetes. Error bars represent standard errors. * indicates significant difference between groups.
3.4. Sex difference in the association between BDNF genotype and change in global cognitive funcitoning.
Table 3 summarizes the main effects of, and interactions between, sex and BDNF genotype on the 3MS intercept and slope, stratified by participant race. In black and white participants, there was a main effect of sex on the 3MS intercept (p < .001) and significant decrease in 3MS scores over time (p < .001), but no main effect of BDNF genotype or interaction of BDNF genotype and sex. As shown in Figure 3, black women had higher initial 3MS scores than black men (difference, 1.7, 95% CI, 0.8 to 2.6) and white women had higher 3MS scores than white men (difference, 1.5, 95% CI, 1.0 to 2.0).
Table 3.
Summary of main effects of sex and BDNF Val66Met genotype, and their interaction on 3MS intercept and slope, stratified by participant race.
| Predicting 3MS in white participants | |||
|---|---|---|---|
| Predicting intercept | F value | degrees of freedom | P value |
| Sex | 26.5 | 1, 1546 | <.001 |
| BDNF genotype | 3.3 | 1, 1560 | .07 |
| Sex X BDNF genotype | 0.7 | 1, 1547 | .42 |
| Predicting change over time | |||
| Time | 15.1 | 1, 1140 | <.001 |
| Sex X Time | 0.001 | 1, 1056 | .98 |
| BDNF genotype X Time | 0.5 | 1, 1070 | .46 |
| Sex X BDNF genotype X Time | 0.1 | 1, 1045 | .72 |
| Predicting 3MS in black participants | |||
| Predicting intercept | F value | degrees of freedom | P value |
| Sex | 15.3 | 1, 1008 | <.001 |
| BDNF genotype | 3.2 | 1, 1043 | .08 |
| Sex X BDNF genotype | 0.9 | 1, 1023 | .34 |
| Predicting change over time | |||
| Time | 30.0 | 1, 645 | <.001 |
| Sex X Time | 0.3 | 1, 619 | .58 |
| BDNF genotype X Time | 0.2 | 1, 888 | .65 |
| Sex X BDNF genotype X Time | 0.2 | 1, 770 | .67 |
Note: Not shown are tde effects of tde covariates: age, CES-D, education, clinical site, average amount of self-reported time spent walking, and diabetes.
Figure 3.

Estimated Modified Mini-mental State Examation (3MS) intercepts separated by sex and BDNF genotype in white participants (panel A) and black participants (panel B). Values are adjusted for clinical site, depression, age, education, self-reported time spent walking, and prevalent diabetes. Error bars represent standard errors. * indicates significant difference between females and males, regardless of BDNF genotype.
3.5. Sex difference in the association between BDNF genotype and BDNF levels
Serum BDNF levels were measured in a random subset of participants. Limitations in sample size precluded our ability to stratify analyses by race. Therefore, race was entered into the model as a covariate. A limited number of extreme values were found, therefore as previously done (Nettiksimmons et al., 2014), the data was trimmed to exclude the top and bottom 1% to reduce the possibility of undue influence of extreme values (final n = 742). An analysis of covariance, adjusting for age, CES-D, education, clinical site, diabetes, race and platelet count, found a significant interaction between sex and BDNF genotype (F[1,731] = 3.9, p = 0.049, partial ƞ2 = 0.005; see Figure 4). Male Met carriers (n = 74) showed significantly lower levels of BDNF compared to male Val/Val carriers (n = 256) (difference, −2.059ng/mL; 95% CI, −4.090 to −0.028) and female Met carriers (n = 84) (difference, −3.330ng/mL; 95% CI, −5.782 to −0.878).
Figure 4.
Circulating serum levels of BDNF (ng/mL) in year 2 by sex and BDNF Val66Met genotype in white and black participants in the Health ABC study. Values are adjusted for age, CES-D, education, clinical site, race, diabetes and platelet count. #: indicates significant difference between Met carriers and Val/Val carriers; ^: indicates significant difference between female and male Met carriers.
4. Discussion
We found evidence that there is an interaction with sex and BDNF Val66Met polymorphism to influence rate of decline over time on a test of executive functioning and processing speed but not on a test of global cognition in older, cognitively healthy older adults. Specifically, female Met carriers showed the least amount of decline in performance on the DSST compared to male Met carriers and Val/Val carriers of both sexes over 10 years. This is one of few longitudinal studies to explore the association between the BDNF Val66Met polymorphism and declines in executive functioning over time, and is unique in examining the role of sex. Our finding adds to the growing body of literature showing that sex differences exist in the effect of the BDNF Val66Met polymorphism on brain and behaviour (Foltynie et al., 2005; Fukumoto et al., 2010; Jiang et al., 2017; Kim et al., 2016; Marrocco et al., 2017; Nemoto et al., 2006; Wei et al., 2012), and highlights the importance of incorporating sex-based analyses into studies examining the role of genetic variability in cognitive aging.
Findings from previous studies in humans have been equivocal, with the Met allele associated with negative, positive or null effects on cognitive performance and decline over time (Boots et al., 2017; Egan et al., 2003; Erickson et al., 2008; Feher et al., 2009; Gajewski et al., 2011, 2012; Hariri et al., 2003; Harris et al., 2006; Honea et al., 2013; Kennedy et al., 2015; Lim et al., 2013; Miyajima et al., 2008; Nagata et al., 2012; van der Kolk et al., 2015; Weinstein et al., 2014). Our finding that the Met allele is associated with less decline in females lends further support for the protective effect of the Met allele on executive functioning in older adults that are cognitively healthy. Van der Kolk et al. (2015) also found this beneficial effect of the Met allele on executive function in older adults with Parkinson’s disease. The Met variant may also be protective against declines in executive functions and processing speed in participants with multiple sclerosis (Zivadinov et al., 2007) and systemic lupus erythematosus (Oroszi et al., 2006), suggesting that the positive effect of the Met allele is not disease specific. Interestingly, the effect of the Met allele may not generalize to all races and ethnicities. In our analysis of the Health ABC study, in black participants, Met allele carriers had higher intercepts (initial performance on the executive functioning task) than Val/Val carriers. However, the black Met and Val/Val carriers did not differ in slope, indicating that all participants declined similarly over time. Racial and ethnic differences are found in the frequency of the Met allele, ranging from 0% in Sub-Saharan African populations to over 50% in Asian populations (Petryshen et al., 2010; Pivac et al., 2009; Shimizu et al., 2004). These substantial differences place the BDNF Val66Met polymorphism within the top 1% of genes in terms of allelic variability between populations across the world (Vulturar et al., 2016). Relevant to the current study is the significantly lower frequency of the Met allele in people of African decent, with frequencies ranging from 0% to 15% (Petryshen et al., 2010). Thus the large differences in allelic frequency coupled with the possible racial differences in functional effects of the Met allele, highlight the need to take into account population diversity in the BDNF Val66Met polymorphism in future studies.
The effects of the Met allele on the brain are highly complex and a number of explanations have been proposed to address the discrepancies found in the literature in addition to ethnicity, including age differences between subjects. Specifically, it has been suggested that the Met allele is detrimental to cognitive and brain structure and function at younger ages but confers some neuroprotection at more advanced ages (Erickson et al., 2008; Feher et al., 2009; Gajewski et al., 2011, 2012; Harris et al., 2006; Honea et al., 2013; Nagata et al., 2012; van der Kolk et al., 2015; Voineskos et al., 2011). Indeed, our findings support this claim as the Met allele was associated with less decline in executive processing over time in older adults, and also extend previous findings by showing that this protective effect was exclusive to females. The protective effects with aging may be related to differential effects of the two proBDNF variants resulting from the Met66 allele versus the Val66 allele on synaptic plasticity. ProBDNF is the precursor form of BDNF, and is itself biologically active, regulating neuronal morphology and physiology in a manner that is opposite of the mature BDNF form (Hempstead, 2015). Specifically, proBDNF, through activation of the p75 receptor, induces apoptosis, reduces spine density and dendritic complexity, and facilitates long-term depression in the hippocampus (Buhusi et al., 2017; Koshimizu et al., 2009; Woo et al., 2005; Yang et al., 2014). The proBDNF molecule consists of 2 regions: the N-terminal pro-domain region that is cleaved from the C-terminal mature domain region. The pro-domain contains the Val66Met SNP. Recently, the Val66 variant, but not the Met66 variant, was shown to be responsible for the proBDNF depression of synaptic activity (Kailainathan et al., 2016), suggesting a possible explanation for the protective effects of the Met allele in cognitive aging. However, the role of biological sex in these relationships has yet to be investigated.
Interestingly, the BDNF Val66Met polymorphism has been associated with risk for major depression (Zhao et al., 2018) although the relationship may be more pronounced in males than females as seen in a meta-analysis of 14 studies (Verhagen et al., 2010). Further, inflammation has been proposed to play a role in the pathogenesis of major depression, potentially by modulating BDNF levels. Specifically, systemic immune challenges and increased pro-inflammatory cytokines such as IL-1β lead to reduced BDNF expression (Calabrese et al., 2014). A recent study examined for the first time, the association between the Val66Met polymorphism and inflammation in depressed patients and found that in a mainly female sample (84.9% female) that carriers of the polymorphism presented with higher circulating BDNF and lower levels of TNF-α, a pro-inflammatory cytokine (Caldieraro et al., 2018). Thus, taken together, these results suggest that the BDNF Val66Met polymorphism has complex effects on the brain and body, which may be dependent on participant characteristics including sex, psychiatric health, and inflammatory status.
Support for a sex difference in the effect of the BDNF Val66Met polymorphism on cognition was recently shown using a knock-in mouse model in which young adult female carriers of the Met allele but not males showed impaired hippocampus-dependent spatial memory (Marrocco et al., 2017). Importantly, ovariectomy negated the cognitive impairment seen in female Met carriers, suggesting that circulating ovarian hormones such as estradiol interact with the Met allele to induce cognitive deficits (Marrocco et al., 2017). At first glance, this may seem contradictory with our finding that the Met allele was beneficial for executive functioning. However, our female participants were well past the onset of menopause and were thus most likely in an estrogen-deficient state similar to ovariectomized female mice. The possible modulation of Met allele effects by estradiol was also recently demonstrated in humans as aberrant hippocampal recruitment during an executive functioning task was seen in female Met carriers only after pharmacological treatment with estradiol (Wei et al., 2017). Thus, the neuromodulatory role of estradiol may interact with the Met allele to help further explain discrepancies within the literature.
Mechanistically, we provide preliminary evidence that circulating serum levels of BDNF were reduced in Met allele carriers, although somewhat surprisingly this effect was only seen in males. BDNF is released from neurons via two possible pathways: regulated secretory pathway or the constitutive pathway (Thomas and Davies, 2005). The Met variant is believed to retard intracellular trafficking of proBDNF, which leads to reduced regulated activity-dependent secretion of the mature form of BDNF (Egan et al., 2003) but not influencing constitutive (non-regulated) secretion (Chen et al., 2005). Thus, it may be the case that in female Met carriers there is a compensatory upregulation of the constitutive BDNF pathway. Interestingly some studies find greater levels of BDNF in various brain regions such as the hippocampus, amygdala and prefrontal cortex (Bakos et al., 2009; Bland et al., 2005; Hayley et al., 2015; Snigdha et al., 2011), as well as in the peripheral circulation (Bus et al., 2012; Driscoll et al., 2012; Golden et al., 2010), in females than males, though our findings suggest that this may only be the case for Met carriers. Future studies are required that specifically examine the activity-dependent and constitutive secretory BDNF pathways in female and male Met allele carriers.
The present study is limited by the number of homozygous Met carriers was very small, preventing a dose-response exploration of the Met allele. In the Health ABC study, BDNF levels were measured cross-sectionally in year 2 of the study when cognitive testing was not conducted. This, in conjunction with the small samples size, preclude our ability to conducted mediation analyses to determine whether serum BDNF levels at the time of cognitive testing underlie the effect of the interaction between sex and the BDNF Val66Met polymorphism on DSST performance. Although we were able to assess the effect of the BDNF polymorphism and sex on the 3MS, we were unable to specifically examine performance on a memory-based task. Further, the DSST assesses performance across several domains, including processing speed, sustained attention and working memory. Thus, is it unclear which specific domain the BDNF Val66Met polymorphism is influencing. The BDNF gene contains several functionally important SNPs (Huang et al., 2007; Miyajima et al., 2008). We were only able to conduct analyses using one BDNF SNP (i.e., rs6265). Future studies should explore sex-dependent effects of other BDNF SNPs in older populations.
5. Conclusions
The present findings suggest the Met allele is associated with less decline in executive functioning and processing speed over time in older white females but not white males. Interestingly, although in black participants the Met allele was associated with greater performance on the test of executive functioning but not with less decline over time, this was irrespective of sex. Further, we found that circulating serum levels were lower in male Met carriers compared to all other groups. Our findings that sex and possibly race are important moderators of the relationship between the BDNF Val66Met polymorphism and executive functioning, highlight the complexity of this polymorphism’s effects on the brain and suggests new avenues of inquiry for future studies.
Highlights:
BDNF Val66Met polymorphism effect on cognitive decline is controversial and may be due to an interaction with biological sex.
There was an interaction such that the Met allele was protective against declines in executive functioning for females, but in white participants only.
Interaction between sex and BDNF Val66Met polymorphism not seen in black participants.
6. Acknowledgements
Teresa Liu-Ambrose is a Canada Research Chair (Tier 2) in Physical Activity, Mobility and Cognitive Neuroscience. Cindy Barha is an Alzheimer’s Association Research Fellow.
7. Funding
This research was supported by National Institute on Aging (NIA) Contracts N01-AG-6-2101; N01-AG-6-2103; N01-AG-6-2106; NIA grant R01-AG028050, and NINR grant R01-NR012459. This research was funded in part by the Intramural Research Program of the NIH, National Institute on Aging.
This manuscript and associated analyses have not been published or submitted elsewhere. All authors approve of the procedures and the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors Statement:
Dr. Barha reports no disclosures.
Dr. Liu-Ambrose reports no disclosures.
Dr. Best reports no disclosures.
Dr. Yaffe reports no disclosures.
Dr. Rosano reports no disclosures.
References:
- Bakos J, Hlavacova N, Rajman M, Ondicova K, Koros C, Kitraki E, Steinbusch HW, Jezova D, 2009. Enriched environment influences hormonal status and hippocampal brain derived neurotrophic factor in a sex dependent manner. Neuroscience 164(2), 788–797. [DOI] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B, Walker S, 2015. Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67(1), 1–48. [Google Scholar]
- Bland ST, Schmid MJ, Der-Avakian A, Watkins LR, Spencer RL, Maier SF, 2005. Expression of c-fos and BDNF mRNA in subregions of the prefrontal cortex of male and female rats after acute uncontrollable stress. Brain research 1051(1–2), 90–99. [DOI] [PubMed] [Google Scholar]
- Boots EA, Schultz SA, Clark LR, Racine AM, Darst BF, Koscik RL, Carlsson CM, Gallagher CL, Hogan KJ, Bendlin BB, Asthana S, Sager MA, Hermann BP, Christian BT, Dubal DB, Engelman CD, Johnson SC, Okonkwo OC, 2017. BDNF Val66Met predicts cognitive decline in the Wisconsin Registry for Alzheimer’s Prevention. Neurology 88(22), 2098–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buhusi M, Etheredge C, Granholm AC, Buhusi CV, 2017. Increased Hippocampal ProBDNF Contributes to Memory Impairments in Aged Mice. Frontiers in aging neuroscience 9, 284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bus BA, Tendolkar I, Franke B, de Graaf J, den Heijer M., Buitelaar JK, Oude Voshaar RC, 2012. Serum brain-derived neurotrophic factor: determinants and relationship with depressive symptoms in a community population of middle-aged and elderly people. World J Biol Psychiatry 13(1), 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese F, Rossetti AC, Racagni G, Gass P, Riva MA, Molteni R, 2014. Brain-derived neurotrophic factor: a bridge between inflammation and neuroplasticity. Frontiers in cellular neuroscience 8, 430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldieraro MA, McKee M, Leistner-Segal S, Vares EA, Kubaski F, Spanemberg L, Brusius-Facchin AC, Fleck MP, Mischoulon D, 2018. Val66Met polymorphism association with serum BDNF and inflammatory biomarkers in major depression. World J Biol Psychiatry 19(5), 402–409. [DOI] [PubMed] [Google Scholar]
- Chen ZY, Bath K, McEwen B, Hempstead B, Lee F, 2008. Impact of genetic variant BDNF (Val66Met) on brain structure and function. Novartis Found Symp 289, 180–188; discussion 188–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZY, Ieraci A, Teng H, Dall H, Meng CX, Herrera DG, Nykjaer A, Hempstead BL, Lee FS, 2005. Sortilin controls intracellular sorting of brain-derived neurotrophic factor to the regulated secretory pathway. The Journal of neuroscience : the official journal of the Society for Neuroscience 25(26), 6156–6166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowansage KK, LeDoux JE, Monfils MH, 2010. Brain-derived neurotrophic factor: a dynamic gatekeeper of neural plasticity. Curr Mol Pharmacol 3(1), 12–29. [DOI] [PubMed] [Google Scholar]
- Driscoll I, Martin B, An Y, Maudsley S, Ferrucci L, Mattson MP, Resnick SM, 2012. Plasma BDNF is associated with age-related white matter atrophy but not with cognitive function in older, non-demented adults. PloS one 7(4), e35217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, Zaitsev E, Gold B, Goldman D, Dean M, Lu B, Weinberger DR, 2003. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 112(2), 257–269. [DOI] [PubMed] [Google Scholar]
- Elobeid MA, Padilla MA, McVie T, Thomas O, Brock DW, Musser B, Lu K, Coffey CS, Desmond RA, St-Onge MP, Gadde KM, Heymsfield SB, Allison DB, 2009. Missing data in randomized clinical trials for weight loss: scope of the problem, state of the field, and performance of statistical methods. PloS One 4, e6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enders CK, 2013. Dealing With Missing Data in Developmental Research. Child Development Perspectives 7(1), 27–31. [Google Scholar]
- Erickson KI, Banducci SE, Weinstein AM, Macdonald AW 3rd, Ferrell RE, Halder I, Flory JD, Manuck SB, 2013. The brain-derived neurotrophic factor Val66Met polymorphism moderates an effect of physical activity on working memory performance. Psychological science 24(9), 1770–1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson KI, Kim JS, Suever BL, Voss MW, Francis BM, Kramer AF, 2008. Genetic contributions to age-related decline in executive function: a 10-year longitudinal study of COMT and BDNF polymorphisms. Front Hum Neurosci 2, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feher A, Juhasz A, Rimanoczy A, Kalman J, Janka Z, 2009. Association between BDNF Val66Met polymorphism and Alzheimer disease, dementia with Lewy bodies, and Pick disease. Alzheimer Dis Assoc Disord 23(3), 224–228. [DOI] [PubMed] [Google Scholar]
- Foltynie T, Lewis SG, Goldberg TE, Blackwell AD, Kolachana BS, Weinberger DR, Robbins TW, Barker RA, 2005. The BDNF Val66Met polymorphism has a gender specific influence on planning ability in Parkinson’s disease. J Neurol 252(7), 833–838. [DOI] [PubMed] [Google Scholar]
- Fukumoto N, Fujii T, Combarros O, Kamboh MI, Tsai SJ, Matsushita S, Nacmias B, Comings DE, Arboleda H, Ingelsson M, Hyman BT, Akatsu H, Grupe A, Nishimura AL, Zatz M, Mattila KM, Rinne J, Goto Y, Asada T, Nakamura S, Kunugi H, 2010. Sexually dimorphic effect of the Val66Met polymorphism of BDNF on susceptibility to Alzheimer’s disease: New data and meta-analysis. American journal of medical genetics. Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics 153B(1), 235–242. [DOI] [PubMed] [Google Scholar]
- Gajewski PD, Hengstler JG, Golka K, Falkenstein M, Beste C, 2011. The Metallele of the BDNF Val66Met polymorphism enhances task switching in elderly. Neurobiology of aging 32(12), 2327 e2327–2319. [DOI] [PubMed] [Google Scholar]
- Gajewski PD, Hengstler JG, Golka K, Falkenstein M, Beste C, 2012. The Met-genotype of the BDNF Val66Met polymorphism is associated with reduced Stroop interference in elderly. Neuropsychologia 50(14), 3554–3563. [DOI] [PubMed] [Google Scholar]
- Gerstorf D, Ram N, Hoppmann C, Willis SL, Schaie KW, 2011. Cohort differences in cognitive aging and terminal decline in the Seattle Longitudinal Study. Dev Psychol 47(4), 1026–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden E, Emiliano A, Maudsley S, Windham BG, Carlson OD, Egan JM, Driscoll I, Ferrucci L, Martin B, Mattson MP, 2010. Circulating brain-derived neurotrophic factor and indices of metabolic and cardiovascular health: data from the Baltimore Longitudinal Study of Aging. PloS one 5(4), e10099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri AR, Goldberg TE, Mattay VS, Kolachana BS, Callicott JH, Egan MF, Weinberger DR, 2003. Brain-derived neurotrophic factor val66met polymorphism affects human memory-related hippocampal activity and predicts memory performance. The Journal of neuroscience : the official journal of the Society for Neuroscience 23(17), 6690–6694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris SE, Fox H, Wright AF, Hayward C, Starr JM, Whalley LJ, Deary IJ, 2006. The brain-derived neurotrophic factor Val66Met polymorphism is associated with age-related change in reasoning skills. Mol Psychiatry 11(5), 505–513. [DOI] [PubMed] [Google Scholar]
- Hayden KM, Reed BR, Manly JJ, Tommet D, Pietrzak RH, Chelune GJ, Yang FM, Revell AJ, Bennett DA, Jones RN, 2011. Cognitive decline in the elderly: an analysis of population heterogeneity. Age Ageing 40(6), 684–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayley S, Du L, Litteljohn D, Palkovits M, Faludi G, Merali Z, Poulter MO, Anisman H, 2015. Gender and brain regions specific differences in brain derived neurotrophic factor protein levels of depressed individuals who died through suicide. Neuroscience letters 600, 12–16. [DOI] [PubMed] [Google Scholar]
- Hempstead BL, 2015. Brain-Derived Neurotrophic Factor: Three Ligands, Many Actions. Transactions of the American Clinical and Climatological Association 126, 9–19. [PMC free article] [PubMed] [Google Scholar]
- Honea RA, Cruchaga C, Perea RD, Saykin AJ, Burns JM, Weinberger DR, Goate AM, Alzheimer’s Disease Neuroimaging, I., 2013. Characterizing the role of brain derived neurotrophic factor genetic variation in Alzheimer’s disease neurodegeneration. PloS one 8(9), e76001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosang GM, Shiles C, Tansey KE, McGuffin P, Uher R, 2014. Interaction between stress and the BDNF Val66Met polymorphism in depression: a systematic review and meta-analysis. BMC Med 12, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R, Huang J, Cathcart H, Smith S, Poduslo SE, 2007. Genetic variants in brain-derived neurotrophic factor associated with Alzheimer’s disease. J Med Genet 44(2), e66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang R, Babyak MA, Brummett BH, Siegler IC, Kuhn CM, Williams RB, 2017. Brain-derived neurotrophic factor (BDNF) Val66Met polymorphism interacts with gender to influence cortisol responses to mental stress. Psychoneuroendocrinology 79, 13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kailainathan S, Piers TM, Yi JH, Choi S, Fahey MS, Borger E, Gunn-Moore FJ, O’Neill L, Lever M, Whitcomb DJ, Cho K, Allen SJ, 2016. Activation of a synapse weakening pathway by human Val66 but not Met66 pro-brain-derived neurotrophic factor (proBDNF). Pharmacol Res 104, 97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy KM, Reese ED, Horn MM, Sizemore AN, Unni AK, Meerbrey ME, Kalich AG Jr., Rodrigue KM, 2015. BDNF val66met polymorphism affects aging of multiple types of memory. Brain research 1612, 104–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SW, Lee JY, Kang HJ, Kim SY, Bae KY, Kim JM, Shin IS, Yoon JS, 2016. Gender-specific Associations of the Brain-derived Neurotrophic Factor Val66Met Polymorphism with Neurocognitive and Clinical Features in Schizophrenia. Clin Psychopharmacol Neurosci 14(3), 270–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komulainen P, Pedersen M, Hanninen T, Bruunsgaard H, Lakka TA, Kivipelto M, Hassinen M, Rauramaa TH, Pedersen BK, Rauramaa R, 2008. BDNF is a novel marker of cognitive function in ageing women: the DR’s EXTRA Study. Neurobiology of learning and memory 90(4), 596–603. [DOI] [PubMed] [Google Scholar]
- Koshimizu H, Kiyosue K, Hara T, Hazama S, Suzuki S, Uegaki K, Nagappan G, Zaitsev E, Hirokawa T, Tatsu Y, Ogura A, Lu B, Kojima M, 2009. Multiple functions of precursor BDNF to CNS neurons: negative regulation of neurite growth, spine formation and cell survival. Mol Brain 2, 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim YY, Villemagne VL, Laws SM, Ames D, Pietrzak RH, Ellis KA, Harrington KD, Bourgeat P, Salvado O, Darby D, Snyder PJ, Bush AI, Martins RN, Masters CL, Rowe CC, Nathan PJ, Maruff P, Australian Imaging B., Lifestyle Research G., 2013. BDNF Val66Met, Abeta amyloid, and cognitive decline in preclinical Alzheimer’s disease. Neurobiology of aging 34(11), 2457–2464. [DOI] [PubMed] [Google Scholar]
- Mandelman SD, Grigorenko EL, 2012. BDNF Val66Met and cognition: all, none, or some? A meta-analysis of the genetic association. Genes Brain Behav 11(2), 127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrocco J, Petty GH, Rios MB, Gray JD, Kogan JF, Waters EM, Schmidt EF, Lee FS, McEwen BS, 2017. A sexually dimorphic pre-stressed translational signature in CA3 pyramidal neurons of BDNF Val66Met mice. Nat Commun 8(1), 808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarrey AC, An Y, Kitner-Triolo MH, Ferrucci L, Resnick SM, 2016. Sex differences in cognitive trajectories in clinically normal older adults. Psychol Aging 31(2), 166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell I, Xi G, Lindsay J, Tuokko H, 2004. Canadian Study of Health and Aging: Study description and patterns of early cognitive decline. Aging, Neuropsychology and Cognition 11(2–3), 149–168. [Google Scholar]
- Miyajima F, Ollier W, Mayes A, Jackson A, Thacker N, Rabbitt P, Pendleton N, Horan M, Payton A, 2008. Brain-derived neurotrophic factor polymorphism Val66Met influences cognitive abilities in the elderly. Genes Brain Behav 7(4), 411–417. [DOI] [PubMed] [Google Scholar]
- Nagata T, Shinagawa S, Nukariya K, Yamada H, Nakayama K, 2012. Association between BDNF polymorphism (Val66Met) and executive function in patients with amnestic mild cognitive impairment or mild Alzheimer disease. Dementia and geriatric cognitive disorders 33(4), 266–272. [DOI] [PubMed] [Google Scholar]
- Nemoto K, Ohnishi T, Mori T, Moriguchi Y, Hashimoto R, Asada T, Kunugi H, 2006. The Val66Met polymorphism of the brain-derived neurotrophic factor gene affects age-related brain morphology. Neuroscience letters 397(1–2), 25–29. [DOI] [PubMed] [Google Scholar]
- Nettiksimmons J, Simonsick EM, Harris T, Satterfield S, Rosano C, Yaffe K, Health ABCS, 2014. The associations between serum brain-derived neurotrophic factor, potential confounders, and cognitive decline: a longitudinal study. PloS one 9(3), e91339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oroszi G, Lapteva L, Davis E, Yarboro CH, Weickert T, Roebuck-Spencer T, Bleiberg J, Rosenstein D, Pao M, Lipsky PE, Goldman D, Lipsky RH, Illei GG, 2006. The Met66 allele of the functional Val66Met polymorphism in the brain-derived neurotrophic factor gene confers protection against neurocognitive dysfunction in systemic lupus erythematosus. Ann Rheum Dis 65(10), 1330–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlik VN, de Moraes SA, Szklo M, Knopman DS, Mosley TH Jr., Hyman DJ, 2003. Relation between cognitive function and mortality in middle-aged adults: the atherosclerosis risk in communities study. American journal of epidemiology 157(4), 327–334. [DOI] [PubMed] [Google Scholar]
- Petryshen TL, Sabeti PC, Aldinger KA, Fry B, Fan JB, Schaffner SF, Waggoner SG, Tahl AR, Sklar P, 2010. Population genetic study of the brain-derived neurotrophic factor (BDNF) gene. Mol Psychiatry 15(8), 810–815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pivac N, Kim B, Nedic G, Joo YH, Kozaric-Kovacic D, Hong JP, Muck-Seler D, 2009. Ethnic differences in brain-derived neurotrophic factor Val66Met polymorphism in Croatian and Korean healthy participants. Croat Med J 50(1), 43–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff L, 1977. The CES-D scale: a self report depression scale for research in the general population. Appl Psychol Meas 1, 385–401. [Google Scholar]
- Rosano C, Lopez OL, 2015. Editorial: Impact of Racial Differences on Brain Health among the Oldest Old. Curr Alzheimer Res 12(7), 606. [DOI] [PubMed] [Google Scholar]
- Royall DR, Palmer R, Chiodo LK, Polk MJ, 2004. Declining executive control in normal aging predicts change in functional status: the Freedom House Study. Journal of the American Geriatrics Society 52(3), 346–352. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, 2011. Neuroanatomical substrates of age-related cognitive decline. Psychological bulletin 137(5), 753–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu E, Hashimoto K, Iyo M, 2004. Ethnic difference of the BDNF 196G/A (val66met) polymorphism frequencies: the possibility to explain ethnic mental traits. American journal of medical genetics. Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics 126B(1), 122–123. [DOI] [PubMed] [Google Scholar]
- Snigdha S, Neill JC, McLean SL, Shemar GK, Cruise L, Shahid M, Henry B, 2011. Phencyclidine (PCP)-induced disruption in cognitive performance is gender-specific and associated with a reduction in brain-derived neurotrophic factor (BDNF) in specific regions of the female rat brain. J Mol Neurosci 43(3), 337–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor HL, Jacobs DR Jr., Schucker B, Knudsen J, Leon AS, Debacker G, 1978. A questionnaire for the assessment of leisure time physical activities. J Chronic Dis 31(12), 741–755. [DOI] [PubMed] [Google Scholar]
- Team, R.C., 2017. R: A language and environment for statistical computing https://www.R-project.org/.
- Teng EL, Chui HC, 1987. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry 48(8), 314–318. [PubMed] [Google Scholar]
- Thomas K, Davies A, 2005. Neurotrophins: a ticket to ride for BDNF. Curr Biol 15(7), R262–264. [DOI] [PubMed] [Google Scholar]
- Toh YL, Ng T, Tan M, Tan A, Chan A, 2018. Impact of brain-derived neurotrophic factor genetic polymorphism on cognition: A systematic review. Brain Behav 8(7), e01009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Kolk NM, Speelman AD, van Nimwegen M, Kessels RP, IntHout J, Hakobjan M, Munneke M, Bloem BR, van de Warrenburg BP, 2015. BDNF polymorphism associates with decline in set shifting in Parkinson’s disease. Neurobiology of aging 36(3), 1605 e1601–1606. [DOI] [PubMed] [Google Scholar]
- Verhagen M, van der Meij A, van Deurzen PA, Janzing JG, Arias-Vasquez A, Buitelaar JK, Franke B, 2010. Meta-analysis of the BDNF Val66Met polymorphism in major depressive disorder: effects of gender and ethnicity. Mol Psychiatry 15(3), 260–271. [DOI] [PubMed] [Google Scholar]
- Voineskos AN, Lerch JP, Felsky D, Shaikh S, Rajji TK, Miranda D, Lobaugh NJ, Mulsant BH, Pollock BG, Kennedy JL, 2011. The brain-derived neurotrophic factor Val66Met polymorphism and prediction of neural risk for Alzheimer disease. Archives of general psychiatry 68(2), 198–206. [DOI] [PubMed] [Google Scholar]
- Vulturar R, Chis A, Hambrich M, Kelemen B, Ungureanu L, Miu AC, 2016. Allelic distribution of BDNF Val66Met polymorphism in healthy Romanian volunteers. Transl Neurosci 7(1), 31–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei SM, Baller EB, Kohn PD, Kippenhan JS, Kolachana B, Soldin SJ, Rubinow DR, Schmidt PJ, Berman KF, 2017. Brain-derived neurotrophic factor Val(66)Met genotype and ovarian steroids interactively modulate working memory-related hippocampal function in women: a multimodal neuroimaging study. Mol Psychiatry [DOI] [PMC free article] [PubMed]
- Wei SM, Eisenberg DP, Kohn PD, Kippenhan JS, Kolachana BS, Weinberger DR, Berman KF, 2012. Brain-derived neurotrophic factor Val(6)(6)Met polymorphism affects resting regional cerebral blood flow and functional connectivity differentially in women versus men. The Journal of neuroscience : the official journal of the Society for Neuroscience 32(20), 7074–7081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein G, Beiser AS, Choi SH, Preis SR, Chen TC, Vorgas D, Au R, Pikula A, Wolf PA, DeStefano AL, Vasan RS, Seshadri S, 2014. Serum brain-derived neurotrophic factor and the risk for dementia: the Framingham Heart Study. JAMA Neurol 71(1), 55–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Beckett LA, Barnes LL, Schneider JA, Bach J, Evans DA, Bennett DA, 2002. Individual differences in rates of change in cognitive abilities of older persons. Psychol Aging 17(2), 179–193. [PubMed] [Google Scholar]
- Woo NH, Teng HK, Siao CJ, Chiaruttini C, Pang PT, Milner TA, Hempstead BL, Lu B, 2005. Activation of p75NTR by proBDNF facilitates hippocampal long-term depression. Nature neuroscience 8(8), 1069–1077. [DOI] [PubMed] [Google Scholar]
- Yang J, Harte-Hargrove LC, Siao CJ, Marinic T, Clarke R, Ma Q, Jing D, Lafrancois JJ, Bath KG, Mark W, Ballon D, Lee FS, Scharfman HE, Hempstead BL, 2014. proBDNF negatively regulates neuronal remodeling, synaptic transmission, and synaptic plasticity in hippocampus. Cell Rep 7(3), 796–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, Chen L, Yang J, Han D, Fang D, Qiu X, Yang X, Qiao Z, Ma J, Wang L, Jiang S, Song X, Zhou J, Zhang J, Chen M, Qi D, Yang Y, Pan H, 2018. BDNF Val66Met polymorphism, life stress and depression: A meta-analysis of gene-environment interaction. J Affect Disord 227, 226–235. [DOI] [PubMed] [Google Scholar]
- Zhou JX, Li HC, Bai XJ, Chang BC, Li CJ, Sun P, Chen LM, 2013. Functional Val66Met polymorphism of Brain-derived neurotrophic factor in type 2 diabetes with depression in Han Chinese subjects. Behav Brain Funct 9, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivadinov R, Weinstock-Guttman B, Benedict R, Tamano-Blanco M, Hussein S, Abdelrahman N, Durfee J, Ramanathan M, 2007. Preservation of gray matter volume in multiple sclerosis patients with the Met allele of the rs6265 (Val66Met) SNP of brain-derived neurotrophic factor. Hum Mol Genet 16(22), 2659–2668. [DOI] [PubMed] [Google Scholar]