Abstract
The Ca2+-calmodulin dependent protein kinase II (CaMKII) is an established central mediator of electrophysiological and contractile responses to cardiac stress, and its hyper-activation in cardiac diseases has been linked to heart failure (HF) and atrial and ventricular arrhythmias. Here we summarize the evidence supporting the role of CaMKII as a critical nodal point for therapeutic intervention against HF and atrial and ventricular tachyarrhythmias. Targeting of CaMKII in heart with inhibitors possessing appropriate selectivity might represent a novel therapeutic approach for HF and arrhythmias.
Keywords: CaMKII, heart failure, atrial fibrillation, arrhythmia, small molecules
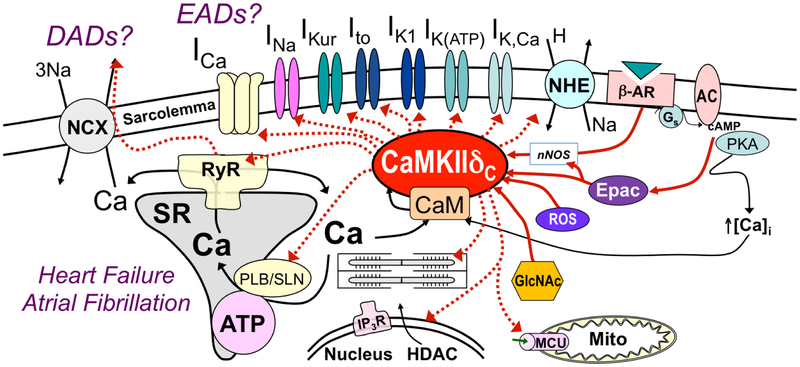
The multifunctional Ca2+-calmodulin dependent protein kinase II (CaMKII) is prominent for its central roles in the nervous system and heart [1], where it controls a diverse range of Ca2+-dependent processes, from learning and memory at the neuronal synapse to cellular growth and death in the myocardium. In cardiomyocytes, CaMKII directly regulates numerous ion channels and Ca2+-handling proteins [2], and controls the expression of an ever-increasing number of transcripts [3, 4] and their downstream products (Figure 1). Functionally, these actions are thought to orchestrate many of the electrophysiological and contractile adaptations to common cardiac stressors, such as fast atrial and ventricular activation rates, chronic adrenergic stimulation, and oxidative challenge [5], Indeed, besides canonical activation via Ca2+/calmodulin binding [6], CaMKII activity is regulated by a number of post-translation modifications including oxidation [5], S-nitrosylation [7], O-GlcNAcylation [8] and via a not fully resolved cAMP-Epac-NOS pathway [9].
Figure 1:
Mechanisms by which CaMKII regulates cardiomyocyte electrophysiology, Ca2+ handling, transcription and mitochondrial function. CaMKII is activated by Ca2/calmodulin binding, β-adrenergic activation (via Epac/NOS1), ROS, and GlcNAc. Epac: exchange protein activated by cAMP; NOS1: nitric oxide synthase 1; ROS: reactive oxygen species; GlcNAc: O-Linked β-N-Acetylglucosamine.
CaMKII Role in Heart Failure and Atrial Fibrillation
In the context of disease, CaMKII has been shown to contribute to a remarkably wide variety of cardiac pathologies [10], of which heart failure (HF) is the most conspicuous. Hyperactivity and chronic activation of CaMKII is an established contributor to pathological cardiac remodeling, and is widely thought to directly promote arrhythmia and contractile dysfunction during HF [11, 12] (see also reviews: [10, 13]). CaMKII upregulation in HF directly promotes increases in late Na+ current (INaL) [14] and diastolic sarcoplasmic reticulum (SR) Ca2+ leak [12] via the ryanodine receptor (RyR), which are the primary molecular defects in two genetically-linked arrhythmogenic syndromes, long QT type-3 and catecholaminergic polymorphic ventricular tachycardia. Indeed, several non-failing arrhythmia-susceptible phenotypes, which result from specific genetic channelopathies, functionally mimic constitutive channel phosphorylation by CaMKII [14-16],
While CaMKII may play a lesser arrhythmogenic role in paroxysmal forms of atrial fibrillation (AF) [17], this kinase is overexpressed and hyperactive in patients with persistent (chronic) AF (cAF) [18, 19] and many experimental AF models [20-25]. CaMKII upregulation in AF paradigms and AF patients and in electrical storm [26] promotes cardiac arrhythmogenesis likely through phosphorylation of the same targets that are relevant for HF ([17, 18, 27, 28]), although regulation of atrial-dominant ion channels and transport regulators has also been reported (e.g., IKur [29], IK,Ca [30] and sarcolipin [31]
Arrhythmogenic CaMKII-Na+-Ca2+ Positive Feedback
An arrhythmogenic synergistic interaction between upregulated CaMKII and perturbed Na+ and Ca2+ fluxes has been hypothesized in both failing ventricular and atrial cardiomyocytes, and computational models of cardiomyocyte electrophysiology, Ca2+- and Na+-handling, and signaling have begun to quantitatively confirm this notion [32, 33], Elevated INaL prolongs action potential duration (APD) and enhances the propensity for early afterdepolarizations, a well-known arrhythmia trigger. But longer APD and briefer diastole also cause Na+ and Ca2+ loading, which increases spontaneous SR Ca2+ releases and the likelihood of delayed afterdepolarizations. This Ca2+ loading also promotes CaMKII activation that reinforces INaL and RyR hyperactivation to further prolong APD, raise Na+ and SR Ca2+ load and leak. This generates a pathological positive feedback loop that promotes both mechanical cardiac dysfunction (systolic and diastolic) and arrhythmogenesis. High [Na+], also lowers mitochondrial [Ca2+] [34], thus limiting Ca2+-dependent dehydrogenases that help match energy supply with demand (and can increase reactive oxygen species to further activate CaMKII, RyR sensitivity and INaL). Breaking the CaMKII-Na+-Ca2+ positive feedback loop is therefore an attractive mean to normalize Ca2+, Na+ and membrane potential dynamics in HF (and AF), but should not be achieved at the expense of systolic function. CaMKII inhibition may be well suited to facilitate this outcome.
Need for Small Molecule Inhibitors Targeting Cardiac CaMKII
Because CaMKII contributes to both the acute and chronic manifestations of major cardiac diseases, but may be only minimally required for physiological homeostasis, it has come to be one of the most promising therapeutic drug targets in cardiac biology. However, currently available CaMKII inhibitors, including inhibitory peptides and the small molecule KN-93, have limited efficacy and imperfect selectivity and are not suitable for clinical use. Furthermore, while abundant in vitro data is available linking CaMKII to arrhythmias in hypertrophic or failing hearts, only a few studies have directly tested the antiarrhythmic effect of CaMKII inhibition in HF in vivo [35]. Thus, development of more specific and deliverable small molecule CaMKII antagonists remains a key priority for the field [36].
In the paper by Neef et al. [37] in this issue of the Journal of Molecular and Cellular Cardiology, a novel ATP-competitive CaMKII inhibitor AS105 was characterized and shown to potently (in vitro IC50 in the nanomolar range) inhibit both nonphosphorylated and phosphorylated CaMKII, despite competing with millimolar [ATP] in cardiomyocytes. When tested for its functional effects, AS105 reduced SR Ca2+ leak in atrial cardiomyocytes from human donors and ventricular cardiomyocytes from healthy and CaMKIIδc overexpressing mice with HF. In human atrial cells, AS105 significantly reduced the likelihood of arrhythmogenic spontaneous SR Ca2+-release events. In failing mouse ventricular cardiomyocytes, AS105 improved SR Ca2+ loading and release, and overall contractility.
While AS105 seems to provide a valuable tool for advancing CaMKII research, by overcoming many of the limitations inherent to the use of KN-93, including off-target effects, low potency (μM) and block of nonphosphorylated CaMKII only [36], this and other ATP-competitive or allosteric CaMKII inhibitors might not yet be ready for clinical application. Besides issues with cell penetration, particularly of substrate competitors with peptide structure like AIP and AC3-I, it is of critical importance for utilization in the clinic (and especially for chronic use) that CaMKII inhibitors exert no clinically relevant actions on α- and β-isoforms of CaMKII in brain, thereby preventing detrimental effects on memory and neuronal plasticity. Optimization of pharmacokinetic properties of small molecule CaMKII inhibitors will be required to minimize central nervous system penetration via the blood-brain barrier. Also, systemic CaMKII inhibition may have negative effects on fertility [38]. Therefore, the success of CaMKII-dependent therapeutic strategies will require the development of cardiac-specific CaMKII inhibitors. Of note, CaMKIIγ can substitute for CaMKIIδ in heart [39], rendering it likely that successful CaMKII inhibition in heart will require the inhibition of both CaMKIIδ and CaMKIIγ. Also, the existence of multiple CaMKII isoforms, each with various splice-variants, may provide opportunities to selectively target cardiac-specific pathological processes. Finally, a future approach to minimize CaMKII inhibition outside the heart might be gene therapy with viral vectors for localized expression of peptides or proteins to the heart. Advances in vector technology and delivery techniques now allow for efficient, safe and long-term gene transfer to the heart, although recent results from large clinical trials have provided mixed results [40]. Gene transfer of the SERCA2a cDNA by delivering a recombinant AAV1 (AAV1.SERCA2a) in patients with advanced HF pioneered the cardiac field. However, after the initial promise of the Calcium Up-regulation by Percutaneous Administration of Gene Therapy in Cardiac Disease (CUPID) clinical trial [41], CUPID2 study was neutral and failed to demonstrate the efficacy of AAV1.SERCA2a gene transfer in improving clinical outcomes of patients [42]. Suboptimal dosage might partially explain the neutral results, and thus future studies employing AAV1.SERCA2a gene transfer at appropriate dosages are needed to definitely disprove the viability of SERCA2a gene transfer for HF treatment.
Conclusions
After decades invested in the development of ion-channel blockers, CaMKII inhibition has emerged as a promising treatment strategy for control of HF and susceptibility to atrial and ventricular arrhythmias. Further refinement and development of small molecule ATP-competitive CaMKII inhibitors can pave the way to utilization of these drugs in the clinic. Although specificity is a major concern when using ATP-competitive CaMKII inhibitors, which might exert potential off-target effects on over 500 other kinases, successful employment of ATP-competitive inhibitors in oncology clearly demonstrates that appropriate selectivity is achievable. Local delivery of ATP-competitive CaMKII inhibitors with appropriate selectivity might represent a novel therapeutic approach against HF and arrhythmias.
Acknowledgements
The authors’ work is supported by the National Institutes of Health (R01-HL131517 to E.G. and D.D. and R01-HL136389 to D.D), the American Heart Association (15SDG24910015 to E.G.), the DZHK (German Center for Cardiovascular Research, grants 81X2800108, 81X2800161, and 81X2800136 to D.D.) and the German Research Foundation (DFG, Do 769/4-1 to D.D.).
Footnotes
Disclosures
Dr. Grandi has no conflicts of interest to disclose. Dr. Dobrev is on the Scientific Advisory Board of OMEICOS and Acesion and received speaker’s fees for educational lectures from Boston Scientific, Daiichi Sankyo and Servier. His laboratory executed a research contract for OMEICOS.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Grandi E, Edwards AG, Herren AW, Bers DM. CaMKII comes of age in cardiac health and disease. Front Pharmacol. 2014;5:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Bers DM, Grandi E. Calcium/calmodulin-dependent kinase II regulation of cardiac ion channels. J Cardiovasc Pharmacol. 2009;54:180–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Backs J, Backs T, Neef S, Kreusser MM, Lehmann LH, Patrick DM, Grueter CE, Qi X, Richardson Ja, Hill JA, Katus HA, Bassel-Duby R, Maier LS, Olson EN. The delta isoform of CaM kinase II is required for pathological cardiac hypertrophy and remodeling after pressure overload. Proc Natl Acad Sci U S A. 2009;106:2342–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Backs J, Song K, Bezprozvannaya S, Chang S, Olson EN. CaM kinase II selectively signals to histone deacetylase 4 during cardiomyocyte hypertrophy. J Clin Invest. 2006;116:1853–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Erickson JR, Joiner ML, Guan X, Kutschke W, Yang J, Oddis CV, Bartlett RK, Lowe JS, O'Donnell SE, Aykin-Burns N, Zimmerman MC, Zimmerman K, Ham AJ, Weiss RM, Spitz DR, Shea MA, Colbran RJ, Mohler PJ, Anderson ME. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008;133:462–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].De Koninck P, Schulman H. Sensitivity of CaM kinase II to the frequency of Ca2+ oscillations. Science. 1998;279:227–30. [DOI] [PubMed] [Google Scholar]
- [7].Erickson JR, Nichols CB, Uchinoumi H, Stein ML, Bossuyt J, Bers DM. S-Nitrosylation Induces Both Autonomous Activation and Inhibition of Calcium/Calmodulin-dependent Protein Kinase II delta. J Biol Chem. 2015;290:25646–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Erickson JR, Pereira L, Wang L, Han G, Ferguson A, Dao K, Copeland RJ, Despa F, Hart GW, Ripplinger CM, Bers DM. Diabetic hyperglycaemia activates CaMKII and arrhythmias by O-linked glycosylation. Nature. 2013;502:372–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Pereira L, Bare DJ, Galice S, Shannon TR, Bers DM. beta-Adrenergic induced SR Ca2+ leak is mediated by an Epac-NOS pathway. J Mol Cell Cardiol. 2017;108:8–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Anderson ME, Brown JH, Bers DM. CaMKII in myocardial hypertrophy and heart failure. J Mol Cell Cardiol. 2011;51:468–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Zhang T, Maier LS, Dalton ND, Miyamoto S, Ross J Jr., Bers DM, Brown JH. The deltaC isoform of CaMKII is activated in cardiac hypertrophy and induces dilated cardiomyopathy and heart failure. Circ Res. 2003;92:912–9. [DOI] [PubMed] [Google Scholar]
- [12].Maier LS, Zhang T, Chen L, DeSantiago J, Brown JH, Bers DM. Transgenic CaMKIIdeltaC overexpression uniquely alters cardiac myocyte Ca2+ handling: reduced SR Ca2+ load and activated SR Ca2+ release. Circ Res. 2003;92:904–11. [DOI] [PubMed] [Google Scholar]
- [13].Vincent KP, McCulloch AD, Edwards AG. Toward a hierarchy of mechanisms in CaMKII-mediated arrhythmia. Front Pharmacol. 2014;5:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Wagner S, Dybkova N, Rasenack EC, Jacobshagen C, Fabritz L, Kirchhof P, Maier SK, Zhang T, Hasenfuss G, Brown JH, Bers DM, Maier LS. Ca2+/calmodulin-dependent protein kinase II regulates cardiac Na+ channels. J Clin Invest. 2006;116:3127–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Grandi E, Puglisi JL, Wagner S, Maier LS, Severi S, Bers DM. Simulation of Ca-calmodulin-dependent protein kinase II on rabbit ventricular myocyte ion currents and action potentials. Biophys J. 2007;93:3835–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].van Oort RJ, McCauley MD, Dixit SS, Pereira L, Yang Y, Respress JL, Wang Q, De Almeida AC, Skapura DG, Anderson ME, Bers DM, Wehrens XH. Ryanodine receptor phosphorylation by calcium/calmodulin-dependent protein kinase II promotes life-threatening ventricular arrhythmias in mice with heart failure. Circulation. 2010;122:2669–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Voigt N, Heijman J, Wang Q, Chiang DY, Li N, Karck M, Wehrens XH, Nattel S, Dobrev D. Cellular and molecular mechanisms of atrial arrhythmogenesis in patients with paroxysmal atrial fibrillation. Circulation. 2014;129:145–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Voigt N, Li N, Wang Q, Wang W, Trafford AW, Abu-Taha I, Sun Q, Wieland T, Ravens U, Nattel S, Wehrens XH, Dobrev D. Enhanced sarcoplasmic reticulum Ca2+ leak and increased Na+-Ca2+ exchanger function underlie delayed afterdepolarizations in patients with chronic atrial fibrillation. Circulation. 2012;125:2059–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Neef S, Dybkova N, Sossalla S, Ort KR, Fluschnik N, Neumann K, Seipelt R, Schondube FA, Hasenfuss G, Maier LS. CaMKII-dependent diastolic SR Ca2+ leak and elevated diastolic Ca2+ levels in right atrial myocardium of patients with atrial fibrillation. Circ Res. 2010;106:1134–44. [DOI] [PubMed] [Google Scholar]
- [20].Chelu MG, Sarma S, Sood S, Wang S, van Oort RJ, Skapura DG, Li N, Santonastasi M, Muller FU, Schmitz W, Schotten U, Anderson ME, Valderrabano M, Dobrev D, Wehrens XH. Calmodulin kinase II-mediated sarcoplasmic reticulum Ca2+ leak promotes atrial fibrillation in mice. J Clin Invest. 2009;119:1940–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Greiser M, Neuberger HR, Harks E, El-Armouche A, Boknik P, de Haan S, Verheyen F, Verheule S, Schmitz W, Ravens U, Nattel S, Allessie MA, Dobrev D, Schotten U. Distinct contractile and molecular differences between two goat models of atrial dysfunction: AV block-induced atrial dilatation and atrial fibrillation. J Mol Cell Cardiol. 2009;46:385–94. [DOI] [PubMed] [Google Scholar]
- [22].Li N, Chiang DY, Wang S, Wang Q, Sun L, Voigt N, Respress JL, Ather S, Skapura DG, Jordan VK, Horrigan FT, Schmitz W, Muller FU, Valderrabano M, Nattel S, Dobrev D, Wehrens XHT. Ryanodine receptor-mediated calcium leak drives progressive development of an atrial fibrillation substrate in a transgenic mouse model. Circulation. 2014;129:1276–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Purohit A, Rokita AG, Guan X, Chen B, Koval OM, Voigt N, Neef S, Sowa T, Gao Z, Luczak ED, Stefansdottir H, Behunin AC, Li N, El-Accaoui RN, Yang B, Swaminathan PD, Weiss RM, Wehrens XH, Song LS, Dobrev D, Maier LS, Anderson ME. Oxidized Ca2+/calmodulin-dependent protein kinase II triggers atrial fibrillation. Circulation. 2013;128:1748–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wakili R, Yeh YH, Yan Qi X, Greiser M, Chartier D, Nishida K, Maguy A, Villeneuve LR, Boknik P, Voigt N, Krysiak J, Kaab S, Ravens U, Linke WA, Stienen GJ, Shi Y, Tardif JC, Schotten U, Dobrev D, Nattel S. Multiple potential molecular contributors to atrial hypocontractility caused by atrial tachycardia remodeling in dogs. Circ Arrhythm Electrophysiol. 2010;3:530–41. [DOI] [PubMed] [Google Scholar]
- [25].Yeh YH, Wakili R, Qi XY, Chartier D, Boknik P, Kaab S, Ravens U, Coutu P, Dobrev D, Nattel S. Calcium-handling abnormalities underlying atrial arrhythmogenesis and contractile dysfunction in dogs with congestive heart failure. Circ Arrhythm Electrophysiol. 2008;1:93–102. [DOI] [PubMed] [Google Scholar]
- [26].Tsuji Y, Hojo M, Voigt N, El-Armouche A, Inden Y, Murohara T, Dobrev D, Nattel S, Kodama I, Kamiya K. Ca2+-related signaling and protein phosphorylation abnormalities play central roles in a new experimental model of electrical storm. Circulation. 2011;123:2192–203. [DOI] [PubMed] [Google Scholar]
- [27].Heijman J, Voigt N, Wehrens XH, Dobrev D. Calcium dysregulation in atrial fibrillation: the role of CaMKII. Front Pharmacol. 2014;5:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Fischer TH, Herting J, Mason FE, Hartmann N, Watanabe S, Nikolaev VO, Sprenger JU, Fan P, Yiao L, Popov AF, Danner BC, Schondube F, Belardinelli L, Hasenfuss G, Maier LS, Sossalla S. Late INa increases diastolic SR-Ca2+-leak in atrial myocardium by activating PKA and CaMKII. Cardiovasc Res. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Tessier S, Karczewski P, Krause EG, Pansard Y, Acar C, Lang-Lazdunski M, Mercadier JJ, Hatem SN. Regulation of the transient outward K+ current by Ca2+/calmodulin-dependent protein kinases II in human atrial myocytes. Circ Res. 1999;85:810–9. [DOI] [PubMed] [Google Scholar]
- [30].Mizukami K, Yokoshiki H, Mitsuyama H, Watanabe M, Tenma T, Takada S, Tsutsui H. Small-conductance Ca2+-activated K+ current is upregulated via the phosphorylation of CaMKII in cardiac hypertrophy from spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol. 2015;309:H1066–74. [DOI] [PubMed] [Google Scholar]
- [31].Bhupathy P, Babu GJ, Ito M, Periasamy M. Threonine-5 at the N-terminus can modulate sarcolipin function in cardiac myocytes. J Mol Cell Cardiol. 2009;47:723–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Morotti S, Edwards AG, McCulloch AD, Bers DM, Grandi E. A novel computational model of mouse myocyte electrophysiology to assess the synergy between Na+ loading and CaMKII. J Physiol. 2014;592:1181–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Onal B, Gratz D, Hund TJ. Ca2+/calmodulin kinase II-dependent regulation of atrial myocyte late Na+ current, Ca2+ cycling and excitability: a mathematical modeling study. Am J Physiol Heart Circ Physiol. 2017:ajpheart 00185 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Maack C, Cortassa S, Aon MA, Ganesan AN, Liu T, O'Rourke B. Elevated cytosolic Na+ decreases mitochondrial Ca2+ uptake during excitation-contraction coupling and impairs energetic adaptation in cardiac myocytes. Circ Res. 2006;99:172–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hoeker GS, Hanafy MA, Oster RA, Bers DM, Pogwizd SM. Reduced Arrhythmia Inducibility With Calcium/Calmodulin-dependent Protein Kinase II Inhibition in Heart Failure Rabbits. J Cardiovasc Pharmacol. 2016;67:260–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Pellicena P, Schulman H. CaMKII inhibitors: from research tools to therapeutic agents. Front Pharmacol. 2014;5:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Neef S, Steffens A, Pellicena P, Mustroph J, Lebek S, Ort KR, Schulman H, Maier LS. Improvement of Cardiomyocyte Function by a Novel Pyrimidine-Based CaMKII-Inhibitor. J Mol Cell Cardiol. 2017;in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Backs J, Stein P, Backs T, Duncan FE, Grueter CE, McAnally J, Qi X, Schultz RM, Olson EN. The gamma isoform of CaM kinase II controls mouse egg activation by regulating cell cycle resumption. Proc Natl Acad Sci U S A. 2010;107:81–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kreusser MM, Lehmann LH, Keranov S, Hoting MO, Oehl U, Kohlhaas M, Reil JC, Neumann K, Schneider MD, Hill JA, Dobrev D, Maack C, Maier LS, Grone HJ, Katus HA, Olson EN, Backs J. Cardiac CaM Kinase II genes delta and gamma contribute to adverse remodeling but redundantly inhibit calcineurin-induced myocardial hypertrophy. Circulation. 2014;130:1262–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Hulot JS, Ishikawa K, Hajjar RJ. Gene therapy for the treatment of heart failure: promise postponed. Eur Heart J. 2016;37:1651–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Zsebo K, Yaroshinsky A, Rudy JJ, Wagner K, Greenberg B, Jessup M, Hajjar RJ. Long-term effects of AAV1/SERCA2a gene transfer in patients with severe heart failure: analysis of recurrent cardiovascular events and mortality. Circ Res. 2014;114:101–8. [DOI] [PubMed] [Google Scholar]
- [42].Greenberg B, Butler J, Felker GM, Ponikowski P, Voors AA, Desai AS, Barnard D, Bouchard A, Jaski B, Lyon AR, Pogoda JM, Rudy JJ, Zsebo KM. Calcium upregulation by percutaneous administration of gene therapy in patients with cardiac disease (CUPID 2): a randomised, multinational, double-blind, placebo-controlled, phase 2b trial. Lancet. 2016;387:1178–86. [DOI] [PubMed] [Google Scholar]