ABSTRACT
Background
Air pollution exposures are novel contributors to the growing childhood obesity epidemic. One possible mechanism linking air pollution exposures and obesity is through changes in food consumption patterns.
Objective
The aim of this study was to examine the longitudinal association between childhood exposure to air pollutants and changes in diet among adolescents.
Design
School-age children were enrolled in the Southern California Children's Health Study during 1993–1994 (n = 3100) and were followed for 4–8 y. Community-level regional air pollutants [e.g., nitrogen dioxide (NO2), elemental carbon (EC), and fine particles with aerodynamic diameter <2.5 µm (PM2.5)] were measured at central monitoring stations. Line dispersion modeling was used to estimate concentrations of traffic-related air pollutants based on nitrogen oxides (NOx) at participants’ residential addresses. In addition, self-reported diet information was collected annually using a structured youth/adolescent food-frequency questionnaire during 1997–2001. Generalized linear mixed-effects models were used in the association analyses.
Results
Higher exposures to regional and traffic-related air pollutants were associated with intake of a high-trans-fat diet, after adjusting for confounders including socioeconomic status and access to fast food in the community. A 2-SD (12.2 parts per billion) increase in regional NO2 exposure was associated with a 34% increased risk of consuming a high-trans-fat diet compared with a low-trans-fat diet (OR: 1.34; 95% CI: 1.05, 1.72). In addition, higher exposures to acid vapor, EC, PM2.5, and non-freeway NOx were all associated with higher consumption of dietary trans fat (all P < 0.04). Notably, higher exposures to regional NO2, acid vapor, and EC were also associated with a higher consumption of fast food (all P < 0.05).
Conclusions
Childhood exposures to regional and traffic-related air pollutants were associated with increased consumption by adolescents of trans fat and fast foods. Our results indicate that air pollution exposures may contribute to obesogenic behaviors. This study was registered at clinicaltrials.gov as NCT03379298.
Keywords: air pollution, traffic pollution, diet, fat intake, trans fat, fast food and obesity
Introduction
Emergent evidence suggests that childhood exposures to regional and traffic-related air pollutants can be significant contributors to the increasing rates of childhood obesity (1–3) and metabolic dysfunction (4–11). However, the mechanisms linking air pollution to obesity are unknown but may include increased oxidative stress (12–14) and inflammation (15, 16). In addition, a recent study in mice documented that the increase in weight gain from prenatal diesel exhaust exposure was paralleled by changes in neuroinflammation and neuronal structure in cognitive and emotional brain areas (17). These brain-behavioral systems are thought to be involved in food-seeking and dietary decision-making behavior (18, 19). Thus, these findings suggest that air pollution may increase the risk of obesity by altering food intake and dietary behaviors.
To date, it is unknown whether chronic air pollution exposures affect dietary behaviors, especially among children and adolescents. Given the dynamic pattern of brain maturation across childhood and adolescence, this time frame of development is thought to be a sensitive period for environmental factors to impart potentially long-lasting effects on brain and behavior (20, 21). Therefore, the aim of this study was to examine the associations between childhood air pollution exposures and dietary patterns in adolescents from the longitudinal Children’s Health Study (CHS) in southern California. We hypothesized that adolescents with higher childhood exposures to regional and traffic-related air pollutants would be more likely to consume an unhealthy diet.
Methods
Study design
The CHS design has been described in detail previously (22–24). The flow of participants’ enrollment is described in Supplemental Figure 1. Briefly, children from 12 Southern California communities were enrolled into the study in the fourth and seventh grades (1993–1994) and were followed annually until high school graduation (1998–2001). The original enrollment involved 5321 children who were recruited in 3 waves (i.e., cohorts B, C, and D). Annual dietary information was collected from 3142 participants during the years 1997–2001. Among them, 34 children with extreme mean caloric intake (>5000 or <500 kcal/d) were excluded in order to be consistent with previous studies using the youth/adolescent food-frequency questionnaire (YAQ) (25). In addition, 8 children without demographic information were excluded, resulting in a total of 3100 participants for the final analysis. The study protocol was approved by the Institutional Review Board for Human Studies at the University of Southern California. Written parental informed consent and youth assent were obtained for all participants.
Dietary assessment
Annual dietary intake was assessed by the 131-item YAQ (25, 26). The YAQ consists of a list of foods with a standardized serving size and asks participants to report the frequency of consumption during the past year on a scale ranging from “less than once per month” to “2 or more servings per day.” Average daily calorie and nutrient intake were calculated at the Harvard Channing Laboratory by multiplying the frequency of consumption of each unit of food from the YAQ by the nutrient content of the specified portion. The YAQ has been shown to have acceptable reproducibility and validity for measuring nutrient and food consumption in multiethnic populations (25, 26). In addition, frequencies of having dinner prepared away from home during the past year were assessed by YAQs using 5 ordinal choices ranging from “never/less than once per month” to “5 or more times per week.”
Regional and traffic-related air pollutant exposures
Air pollution monitoring stations were established in each of the 12 communities as part of the study design. All stations monitored hourly concentrations of nitrogen dioxide (NO2), nitrogen oxide (NO), particles with an aerodynamic diameter <10 µm (PM10), and ozone (O3) throughout the CHS follow-up. Two-week integrated samplers were used to measure fine particles with an aerodynamic diameter <2.5 µm (PM2.5) and acid vapor. In addition, the amounts of elemental carbon (EC) and organic carbon (OC) were determined using method 5040 of the National Institute for Occupational Safety and Health (27). Additional details have been previously published (28, 29).
Residential addresses were geocoded using the TeleAtlas database and software (Tele Atlas, Inc.). Nitrogen oxides (NOx), a surrogate of the near-roadway air pollutant mixture, were estimated using the CALINE-4 line-source dispersion model (30) at each participant's residential address. We estimated traffic exposure separately for freeways, all non-freeway roads (i.e., highways and community streets), and community streets only, in order to differentiate the potentially distinct chemical compositions represented by NOx near these different types of roadways (31–33).
Confounders and effect modifiers
At study entry, a parent or legal guardian completed baseline questionnaires including questions on race, Hispanic ethnic origin, parental income and education, in utero exposure to maternal smoking, second-hand smoke, participation in team sports during the 12 mo before the study visit, and indoor use of a gas stove for cooking. Yearly questionnaires were used to update information on personal smoking and second-hand smoke. Children's height and weight were measured at each study visit. BMI was calculated as kg/m2. Overweight and obese categories were determined using the 85th and 95th BMI percentile thresholds based on the age- and sex-specific CDC 2000 BMI growth curves (34). Basal metabolic rates were estimated using age- and sex-specific Schofield equations (35, 36) based on individual weight measures. In addition, the number of fast-food restaurants near schools and homes and variables representative of community-level social economic position such as median household income, proportion of respondents with low education (i.e., no high school diploma), and percentage living in poverty were examined as covariates in a subset of 2385 participants where data were available. More details of contextual variables have been published previously (37). Briefly, contextual characteristics were aggregated from census blocks of residential addresses using data from the 1990 US census (38).
Statistical analysis
Childhood exposures to air pollutants were represented by the annual mean concentrations of air pollutants from the years 1994–1996. For dietary outcomes, we first conducted a K-means model to identify 3 disjoint dietary patterns based on proportion of daily intake of carbohydrates, fat, and protein (39). Second, we sought to identify specific macronutrient intake that was influenced by air pollution exposures. Therefore, we analyzed the percentage of calorie intake from each macronutrient (40). Dietary fat intake was further analyzed by subtypes of fat including saturated, monounsaturated, polyunsaturated, and trans fat (41, 42). Finally, we aimed to investigate which food items contributed to the air pollution–induced changes in macronutrient intake. Thus, principal components analysis with varimax orthogonal rotation was used to distinguish various food patterns (43, 44). Factors with eigenvalues ≥1.0 were retained. Consumption frequencies of food items with a factor loading ≥0.3 were used to define food consumption patterns.
Multinomial logistic regression with a random intercept for communities was used to analyze the associations between childhood exposures to air pollutants and adolescent dietary macronutrient patterns. Next, mixed-effects models were used to assess the associations of air pollution exposures with total calorie intake, the percentage calorie intake from each macronutrient, and food pattern factor scores during the longitudinal follow-up. Additionally, macronutrient data were further dichotomized based on USDA guidelines (45, 46) for children aged 14–18 y including <2200 kcal/d for males and <1800 kcal/d for females, with <30% total fat and <10% saturated fat as part of the total calorie intake, and minimum trans fat consumption. Age, sex, ethnicity, parental educational levels, household income, in utero exposure to maternal smoking, and indoor use of a gas stove for cooking (yes or no) were included in each model as baseline covariates. Time-dependent variables included prior-year history of participation in team sports, having health insurance, second-hand smoke and personal smoking, frequencies of having dinner prepared away from home, and seasons of dietary assessments. All covariates were selected using a directed acyclic graph (47) (Supplemental Figure 2). The ratio of self-reported daily caloric intake to the basal metabolic rate was categorized into quartiles and was included in the model as an additional covariate adjustment for potential underreporting and overreporting in the self-reported YAQs (48, 49). Participants with missing covariate information were included in the analysis using the missing indicator method (50).
In sensitivity analyses, potential geospatial clustering was accounted for by adjusting for spatial autocorrelations of residential addresses using an exponential semivariance model of latitude and longitude. Community-level contextual variables were adjusted for in a subset of adolescents where data were available. Last, a consistent mediation model (51) with the Sobel test (52, 53) was used for path analysis of childhood air pollutant exposure, with food pattern factor scores as the mediation variable and obesity and overweight status at the end of the dietary assessments as the outcome variable. The effect estimates of associations were scaled to 2 SDs of 3-y-averaged air pollutant concentrations estimated from the entire sample. Finally, effect modifications by sex, race/ethnicity, parental education, in utero exposure to maternal smoking, second-hand smoke, and obesity were examined. All statistical tests were considered significant with a 2-sided P value <0.05. SAS version 9.4 (SAS Institute, Inc.) was used for data analysis.
Results
At the time of the first dietary assessment, the mean ages of participants were 17.9, 16.1, and 13.2 y in cohorts B (n = 489), C (n = 1186), and D (n = 1425), respectively. The child and home characteristics are presented in Table 1. Repeated dietary questionnaires were collected among 67.7% of participants at 2–3 follow-up visits. From cohorts B–D combined, 44.8%, 61.7%, and 69.7% of adolescents had total calorie, total fat, and saturated fat intakes over current USDA guidelines as described in Methods, respectively. Half of adolescents had ≥2% calorie intake from trans fats, whereas only 12 participants were below the current mean level of trans fat consumption in the US population (0.6% calorie intake from trans fats) (54).
TABLE 1.
Child and home characteristics among 3100 adolescents when the first dietary assessment was collected in the Children's Health Study1
| Mean ± SD or n(%)2 | |
|---|---|
| Age, y | 15.0 ± 1.9 |
| Female sex | 1671 (53.9) |
| Ethnicity | |
| Non-Hispanic white | 1740 (56.1) |
| Hispanic white | 883 (28.5) |
| Others | 477 (15.4) |
| Parental education | |
| Less than or completed grade 12 (high school) | 932 (30.1) |
| Some college or technical school | 1340 (43.2) |
| Completed 4 y of college or higher | 708 (22.8) |
| Household annual income | |
| <$15,000 | 387 (12.5) |
| $15,000–$49,999 | 1123 (36.2) |
| ≥$50,000 | 1117 (36.0) |
| Number of team sports participated in the previous year3 | |
| 0 | 1273 (41.1) |
| 1 | 987 (31.8) |
| >1 | 743 (24.0) |
| Health insurance | |
| No | 443 (14.3) |
| Yes | 2575 (83.1) |
| BMI categories4 | |
| Normal weight | 2135 (70.7) |
| Overweight | 489 (16.2) |
| Obesity | 396 (13.1) |
| Maternal smoke exposure in utero | |
| No | 2534 (81.7) |
| Yes | 458 (14.8) |
| Lifetime cigarette smoke | |
| No (<100 cigarettes during lifetime) | 2842 (94.2) |
| Yes (≥100 cigarettes during lifetime) | 176 (5.8) |
| Second-hand cigarette smoke exposure | |
| No | 2063 (67.2) |
| Yes | 1007 (32.8) |
| Indoor use of a gas stove for cooking | |
| No | 687 (22.2) |
| Yes | 2326 (75.0) |
| Dietary intake during the prior year | |
| Total calorie intake,5 kcal/d | 1790 ± 757 |
| Total carbohydrate, % | 55.8 ± 6.3 |
| Protein, % | 14.1 ± 2.5 |
| Total fat, % | 31.2 ± 5.1 |
| Saturated fat, % | 11.2 ± 2.4 |
| Monounsaturated fat, % | 11.6 ± 2.0 |
| Polyunsaturated fat, % | 5.9 ± 1.4 |
| trans Fat, % | 2.1 ± 0.6 |
Children in the Children's Health Study (as described in Methods) were enrolled in 3 waves (cohorts B, C, and D) of recruitment starting in the year 1993 and were followed up from a mean age of 6.6 to 15.2 y.
Continuous data are presented as means ± SDs. All the other categorical variables are presented as the number of children in each category and the percentage each category constitutes of the entire cohort, i.e., n (%). The total numbers of subjects may differ due to missing values of different categorical variables.
Team sports include baseball/softball, basketball, football, soccer, swimming, tennis, volleyball, and other self-reported sports team.
Overweight and obese children were defined as children having a BMI ≥85th percentile and ≥95th percentile, respectively, compared with the applicable sex-specific CDC growth curve (34).
Total daily calorie intake was log-transformed in the analysis. Therefore, the geometric mean and SD calculated using the delta method are presented for daily calorie intake.
From 1994 to 1996, there was substantial variation in the mean concentrations of regional and traffic-related air pollutants across the 12 study communities (Supplemental Figure 3, Supplemental Table 1). However, annual concentrations of regional air pollutants had little year-to-year variation within each community. Concentrations of NO2, PM10, PM2.5, EC, and acid vapor were positively correlated with each other (all Spearman rs > 0.8) (Supplemental Table 2).
Based on the percentage calorie intakes from macronutrients, 3 distinct dietary patterns were identified—low fat and high carbohydrate (LFHC): 25.2% fat, 63.5% carbohydrate, and 13.0% protein (n = 551); moderate fat and moderate carbohydrate (MFMC): 30.7% fat, 56.6% carbohydrate, and 14.0% protein (n = 1436); and high fat and low carbohydrate (HFLC): 35.3% fat, 50.5% carbohydrate, and 15.2% protein (n = 1113). Child and home characteristics were compared across the 3 dietary pattern groups (Supplemental Table 3). Compared with the LFHC group, the MFMC and HFLC groups had more males and non-Hispanic whites. The mean total calorie intake was lowest in the LFHC group and was similar in the other 2 groups.
Association of childhood exposure to regional and traffic-related air pollutants with dietary trans fat intake
Higher childhood exposure to regional air pollutants was associated with the consumption of a higher dietary proportion of fat (Table 2). For example, a 2-SD (12.2 parts per billion) increase in NO2 exposure was significantly associated with a 27% increased risk of consuming the MFMC diet compared with the LFHC diet (OR: 1.27; 95% CI: 1.01, 1.61), after adjusting for confounders. Significantly positive associations were also observed between exposures to NO, acid vapor, PM2.5, and EC and increased likelihood to consume the MFMC diet rather than the LFHC diet. However, no significant associations were observed between air pollutant exposures and odds of consuming the HFLC diet compared with the LFHC diet. In addition, no significant associations were observed between exposures to air pollutants and total calorie intake (Supplemental Table 4).
TABLE 2.
Multinomial logistic regression associations of childhood exposures to regional and traffic-related air pollutants with dietary macronutrient patterns among 3100 adolescents aged 13–19 y1
| MFMC diet vs. LFHC diet | HFLC diet vs. LFHC diet | |||
|---|---|---|---|---|
| OR | (95% CI) | OR | (95% CI) | |
| Regional air pollutants | ||||
| NO2 (2 SDs = 12.2 ppb) | 1.27 | (1.01, 1.61) | 1.18 | (0.92, 1.50) |
| NO (2 SDs = 12.4 ppb) | 1.25 | (1.01, 1.55) | 1.19 | (0.95, 1.49) |
| 8-h O3 (2 SDs = 12.1 ppb) | 1.08 | (0.87, 1.33) | 1.06 | (0.85, 1.32) |
| Acid vapor (2 SDs = 3.5 ppb) | 1.31 | (1.04, 1.65) | 1.22 | (0.97, 1.55) |
| PM10 (2 SDs = 14.2 μg/m3) | 1.22 | (0.98, 1.53) | 1.17 | (0.93, 1.48) |
| PM2.5 (2 SDs = 9.6 μg/m3) | 1.28 | (1.02, 1.61) | 1.17 | (0.92, 1.48) |
| EC (2 SDs = 0.45 μg/m3) | 1.28 | (1.02, 1.61) | 1.22 | (0.96, 1.55) |
| OC (2 SDs = 2.7 μg/m3) | 1.17 | (0.94, 1.47) | 1.11 | (0.88, 1.41) |
| Traffic-related air pollutants | ||||
| Freeway NOx (2 SDs = 18.9 ppb) | 1.12 | (0.88, 1.44) | 1.12 | (0.87, 1.46) |
| Non-freeway NOx (2 SDs = 8.8 ppb) | 1.20 | (0.93, 1.55) | 1.16 | (0.89, 1.52) |
| Local street NOx (2 SDs = 8.2 ppb) | 1.29 | (0.99, 1.67) | 1.26 | (0.96, 1.64) |
| Total NOx (2 SDs = 24.2 ppb) | 1.17 | (0.91, 1.51) | 1.16 | (0.89, 1.51) |
Multinomial logistic regression models were used to analyze the association between childhood exposures to air pollutants and the likelihood to consume MFMC and HFLC diets compared with an LFHC diet. The association was adjusted for age, sex, ethnicity, parental educational levels, household income, having health insurance (yes or no), number of team sports attended in the last 12 mo, in utero exposure to maternal smoking, second-hand smoke, personal smoking, indoor use of a gas stove at home, frequencies of having dinner prepared away from home, seasons of dietary assessments, and the indicator variable for study cohorts. Dietary patterns of macronutrient intake were identified using K-means clustering methods. Three distinct patterns were represented by the mean daily intake of macronutrients for each cluster: LFHC (25.2% fat, 63.5% carbohydrate, and 13.0% protein, n = 551), MFMC (30.7% fat, 56.6% carbohydrate, and 14.0% protein, n = 1436), and HFLC (35.3% fat, 50.5% carbohydrate, and 15.2% protein, n = 1113). This dietary pattern was treated as the ordinal outcome in the association analysis (i.e., 1 = LFHC, 2 = MFMC, and 3 = HFLC). Association effect sizes are presented as ORs (95% CIs). The OR was scaled to a 2-SD range of each air pollutant exposure. EC, elemental carbon; HFLC, high fat and low carbohydrate; LFHC, low fat and high carbohydrate; MFMC, moderate fat and moderate carbohydrate; NO, nitrogen oxide; Non-freeway NOx, the sum of local street NOx and highway-related NOx; NOx, nitrogen oxides; NO2, nitrogen dioxide; OC, organic carbon; O3, ozone; PM2.5, particulate matter with aerodynamic diameter <2.5 µm; PM10, particulate matter with aerodynamic diameter <10 µm; ppb, parts per billion; Total NOx, the sum of non-freeway NOx and freeway NOx.
Analyses of individual macronutrients showed that childhood exposures to regional and traffic-related air pollutants were associated with higher percentage calorie intakes from subtypes of fat, particularly trans fat, over time after adjusting for potential confounders (Table 3). Increases of 2 SDs of NO2, NO, acid vapor, EC, non-freeway–, and local street-related NOx were all associated with an 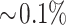 increase in total calorie intake from trans fat (all P < 0.04). This effect size was
increase in total calorie intake from trans fat (all P < 0.04). This effect size was 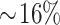 of the mean amount of trans fat intake for the entire US population in the year 2012 (54). After categorizing participants by low- and high-trans-fat diet (<2% and ≥2% calories from trans fat, respectively), we found that children with a 2-SD increase in NO2, EC, and acid vapor exposure had 30–40% increased risk of consuming a high-trans-fat diet compared with a low-trans-fat diet (OR: 1.34; 95% CI: 1.05, 1.72 for NO2; OR: 1.37; 95% CI: 1.08, 1.74 for EC; and OR: 1.32; 95% CI: 1.02, 1.69 for acid vapor) (Figure 1). Notably, significant associations were only observed between air pollutant exposures and a high-trans-fat diet. None of the air pollutant exposures were significantly associated with a high-fat diet.
of the mean amount of trans fat intake for the entire US population in the year 2012 (54). After categorizing participants by low- and high-trans-fat diet (<2% and ≥2% calories from trans fat, respectively), we found that children with a 2-SD increase in NO2, EC, and acid vapor exposure had 30–40% increased risk of consuming a high-trans-fat diet compared with a low-trans-fat diet (OR: 1.34; 95% CI: 1.05, 1.72 for NO2; OR: 1.37; 95% CI: 1.08, 1.74 for EC; and OR: 1.32; 95% CI: 1.02, 1.69 for acid vapor) (Figure 1). Notably, significant associations were only observed between air pollutant exposures and a high-trans-fat diet. None of the air pollutant exposures were significantly associated with a high-fat diet.
TABLE 3.
Regression coefficients (SEs) for linear associations of childhood exposures to regional and traffic-related air pollutants with longitudinal assessments of percentage calorie intake from total fat and subtypes of fat among 3100 adolescents1
| Percentage of | |||||
|---|---|---|---|---|---|
| Total fat | Saturated fat | Monounsaturated fat | Polyunsaturated fat | trans Fat | |
| Regional air pollutants | |||||
| NO2 (2 SDs = 12.2 ppb) | 0.20 (0.24) | −0.05 (0.10) | 0.12 (0.10) | 0.12 (0.07) | 0.10 (0.03)** |
| NO (2 SDs = 12.4 ppb) | 0.21 (0.22) | −0.03 (0.09) | 0.12 (0.10) | 0.12 (0.06) | 0.08 (0.03)* |
| 8-h O3 (2 SDs = 12.1 ppb) | 0.05 (0.23) | 0.01 (0.09) | −0.02 (0.11) | 0.06 (0.07) | −0.03 (0.04) |
| Acid vapor (2 SDs = 3.5 ppb) | 0.26 (0.23) | −0.002 (0.10) | 0.13 (0.10) | 0.13 (0.07) | 0.08 (0.04)* |
| PM10 (2 SDs = 14.2 μg/m3) | 0.33 (0.21) | 0.08 (0.09) | 0.15 (0.10) | 0.10 (0.07) | 0.06 (0.04) |
| PM2.5 (2 SDs = 9.6 μg/m3) | 0.28 (0.23) | 0.03 (0.10) | 0.15 (0.10) | 0.10 (0.07) | 0.07 (0.04) |
| EC (2 SDs = 0.45 μg/m3) | 0.31 (0.23) | −0.005 (0.10) | 0.15 (0.10) | 0.15 (0.07)* | 0.09 (0.03)* |
| OC (2 SDs = 2.7 μg/m3) | 0.27 (0.23) | 0.06 (0.09) | 0.12 (0.10) | 0.08 (0.08) | 0.06 (0.04) |
| Traffic-related air pollutants | |||||
| Freeway NOx (2 SDs = 18.9 ppb) | 0.25 (0.21) | 0.08 (0.09) | 0.11 (0.09) | 0.01 (0.06) | 0.01 (0.03) |
| Non-freeway NOx (2 SDs = 8.8 ppb) | 0.29 (0.22) | 0.12 (0.10) | 0.15 (0.09) | −0.03 (0.07) | 0.07 (0.03)* |
| Local street NOx (2 SDs = 8.2 ppb) | 0.40 (0.22) | 0.17 (0.10) | 0.19 (0.09)* | 0.02 (0.07) | 0.08 (0.03)** |
| Total NOx (2 SDs = 24.2 ppb) | 0.31 (0.22) | 0.11 (0.10) | 0.15 (0.09) | 0.002 (0.07) | 0.04 (0.03) |
Mixed-effects models were used to assess the associations of childhood exposures to regional and traffic-related air pollutants with longitudinal assessments of percentage calorie intakes from total fat and subtypes of fat. A random intercept for communities and individuals was used to adjust for the design clusters of communities and internal correlations of repeated dietary assessments from each individual. In addition, age, sex, ethnicity, parental educational levels, household income, number of team sports attended in the last 12 mo, in utero exposure to maternal smoking, second-hand smoke and personal smoking, indoor use of a gas stove at home, frequencies of having dinner prepared away from home, seasons of dietary assessments, and the indicator variable for study cohorts were adjusted for as potential confounders. Wald's test was used to estimate P values for significance based on regression coefficients and SEs (*P < 0.05, **P < 0.01). Association effect sizes are presented as regression coefficients (SEs). Regression coefficients were scaled to 2-SD ranges of each air pollutant exposure. EC, elemental carbon; NO, nitrogen oxide; Non-freeway NOx, the sum of local street NOx and highway-related NOx; NOx, nitrogen oxides; NO2, nitrogen dioxide; OC, organic carbon; O3, ozone; PM2.5, particulate matter with aerodynamic diameter <2.5 µm; PM10, particulate matter with aerodynamic diameter <10 µm; ppb, parts per billion; Total NOx, the sum of non-freeway NOx and freeway NOx.
FIGURE 1.
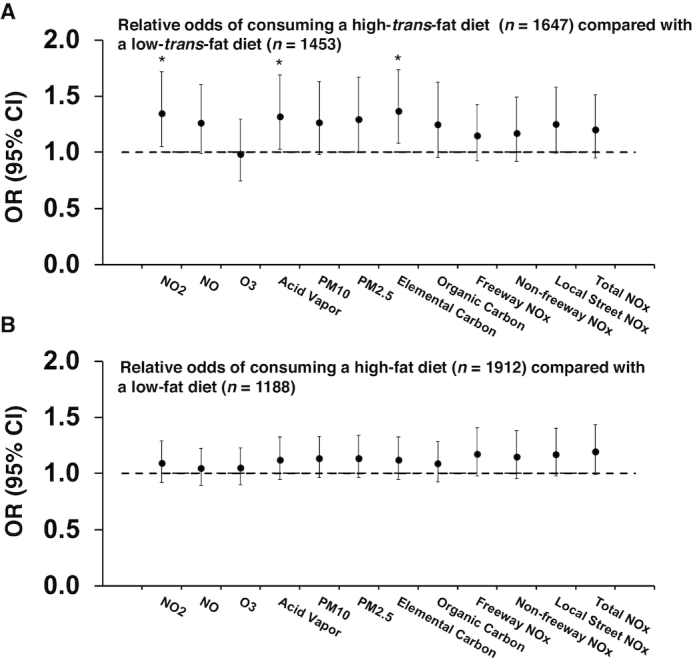
Associations between childhood air pollution exposures and the relative odds of consuming a high-trans-fat diet (≥2% calories from trans fat) compared with a low-trans-fat diet (<2% calories from trans fat) (A) and of consuming a high-fat diet (≥30% calories from total fat) compared with a low-fat diet (<30% calories from total fat) (B) among 3100 adolescents. A generalized mixed-effects model with logit link function was used to estimate the associations of childhood exposures to regional and traffic-related air pollutants with the relative odds of consuming a high-trans-fat or high-fat diet compared with a low-trans-fat or low-fat diet classified from longitudinal assessments. A random intercept for communities and individuals was used to adjust for the study design clusters of communities and internal correlations of repeated dietary assessments from each individual. In addition, age, sex, ethnicity, parental educational levels, household income, number of team sports attended in the last 12 mo, in utero exposure to maternal smoking, second-hand smoke and personal smoking, indoor use of a gas stove, frequencies of having dinner prepared away from home, season of dietary assessment, and the indicator variable for study cohorts were adjusted for as potential confounders. The effect sizes were scaled to 2-SD ranges of each air pollutant exposure (see more details on the SDs of each air pollutant in Table 2). *P < 0.05. NO, nitrogen oxide; Non-freeway NOx, the sum of local street NOx and highway-related NOx; NOx, nitrogen oxides; NO2, nitrogen dioxide; O3, ozone; PM2.5, particulate matter with aerodynamic diameter <2.5 µm; PM10, particulate matter with aerodynamic diameter <10 µm; Total NOx, the sum of non-freeway NOx and freeway NOx.
Association of childhood exposure to regional and traffic-related air pollutants with fast-food and sweet-food consumption patterns
The principal components analysis identified 31 orthogonal factors with eigenvalues ≥1.0. We analyzed the top 5 factors, which explained 18.2% of the variance in the consumption frequencies of all food items (Supplemental Figure 4). Childhood exposures to regional and traffic-related air pollutants were significantly associated with 2 factor scores after adjusting for potential confounders (Table 4). These factors included the following: 1) “fast food,” which included consumption of soda, cheeseburger, hamburger, pizza, tacos and burritos, chicken nuggets, and hotdogs, and 2) “sweet food,” which included consumption of cakes, snack cakes, sweet rolls, pastries, donuts, cookies, brownies, pies, chocolate, and candy bars. Higher exposures to regional air pollutants (e.g., NO2, NO, acid vapor, PM10, PM2.5, EC, and OC) were associated with increased consumption of fast food (all P < 0.05). Notably, a 1-unit increase in the “fast food” factor score was significantly associated with a 25% increased risk of being obese or overweight compared with being normal weight among adolescents at the time of last dietary assessment (mean age: 16 y) after adjusting for confounders (OR: 1.25; 95% CI: 1.13, 1.38). In addition, higher total traffic-related NOx exposure was associated with greater sweet-food consumption (P = 0.03). However, because there was no significant association between childhood air pollutant exposure and obesity or overweight in this study sample, the mediation effect of food pattern factor scores in the association between air pollutant exposure and obesity could not be examined under a consistent mediation model (51).
TABLE 4.
Linear associations of childhood exposures to regional and traffic-related air pollutants with diet food patterns identified by principal components analysis of 131 food items included in the food-frequency questionnaires1
| “Fast food” factor score2 | “Sweet food” factor score3 | |||
|---|---|---|---|---|
| β (SE) | P | β (SE) | P | |
| Regional air pollutants | ||||
| NO2 (2 SDs = 12.2 ppb) | 0.16 (0.07) | 0.023 | 0.004 (0.05) | 0.94 |
| NO (2 SDs = 12.4 ppb) | 0.16 (0.06) | 0.012 | −0.03 (0.05) | 0.57 |
| 8-h O3 (2 SDs = 12.1 ppb) | −0.01 (0.07) | 0.93 | 0.03 (0.05) | 0.50 |
| Acid vapor (2 SDs = 3.5 ppb) | 0.15 (0.07) | 0.025 | 0.02 (0.05) | 0.65 |
| PM10 (2 SDs = 14.2 μg/m3) | 0.18 (0.06) | 0.003 | 0.04 (0.05) | 0.34 |
| PM2.5 (2 SDs = 9.6 μg/m3) | 0.20 (0.05) | <0.001 | 0.05 (0.05) | 0.34 |
| EC (2 SDs = 0.45 μg/m3) | 0.17 (0.07) | 0.009 | 0.02 (0.05) | 0.75 |
| OC (2 SDs = 2.7 μg/m3) | 0.17 (0.07) | 0.010 | 0.03 (0.05) | 0.52 |
| Traffic-related air pollutants | ||||
| Freeway NOx (2 SDs = 18.9 ppb) | 0.06 (0.05) | 0.22 | 0.09 (0.05) | 0.09 |
| Non-freeway NOx (2 SDs = 8.8 ppb) | 0.02 (0.06) | 0.77 | 0.08 (0.05) | 0.10 |
| Local street NOx (2 SDs = 8.2 ppb) | 0.05 (0.06) | 0.34 | 0.09 (0.05) | 0.09 |
| Total NOx (2 SDs = 24.2 ppb) | 0.06 (0.05) | 0.24 | 0.11 (0.05) | 0.03 |
Mixed-effects models were used to assess the associations of childhood exposures to regional and traffic-related air pollutants with factor scores of diet food patterns. A random intercept for communities was used to adjust for the design clusters of communities. In addition, age, sex, ethnicity, parental educational levels, household income, number of team sports attended in the last 12 mo, in utero exposure to maternal smoking, second-hand smoke and personal smoking, indoor use of a gas stove at home, frequencies of having dinner prepared away from home, seasons of dietary assessments, and the indicator variable for study cohorts were adjusted for as potential confounders. Wald's test was used to estimate P values for significance based on regression coefficients and SEs. Association effect sizes are presented as regression coefficients: β (SE). Regression coefficients were scaled to 2-SD ranges of each air pollutant exposure. EC, elemental carbon; NO, nitrogen oxide; Non-freeway NOx, the sum of local street NOx and highway-related NOx; NOx, nitrogen oxides; NO2, nitrogen dioxide; OC, organic carbon; O3, ozone; PM2.5, particulate matter with aerodynamic diameter <2.5 µm; PM10, particulate matter with aerodynamic diameter <10 µm; ppb, parts per billion; Total NOx, the sum of non-freeway NOx and freeway NOx.
The “Fast food” factor is represented by frequent consumption of soda, cheeseburger, hamburger, pizza, tacos and burritos, chicken nuggets, and hotdogs.
The “Sweet food” factor is represented by frequent consumption of cakes, snack cakes, sweet rolls, pastries, donuts, cookies, brownies, pies, chocolate, and candy bars.
Next, sensitivity analyses were performed to determine whether residential clustering and latent community-level confounders altered the associations between air pollution exposures and dietary patterns. Results from this analysis showed that higher regional air pollutant (NO2, NO, acid vapor, PM2.5, EC, and OC) exposures remained significantly associated with greater consumption of fast food after adjusting for spatial autocorrelations of residential addresses (Supplemental Table 5). Furthermore, higher exposures to freeway and total traffic-related NOx were significantly associated with an increase in the “fast food” factor score after adjusting for spatial autocorrelations (both P = 0.03). In addition, contextual variables including community-level social economic position and accessibility to fast-food restaurants within a 500-m buffer from homes and schools were examined in a subset of 2385 participants (Supplemental Table 6). Childhood exposures to air pollutants (NO, PM10, PM2.5, EC, and OC) remained significantly associated with increased fast-food and sweet-food consumption after adjusting for contextual covariates (Supplemental Table 7). Moreover, after excluding children with only 1 dietary assessment, we found that the associations between increased air pollution exposures and more consumption of trans fat remained with similar effect sizes among 2099 children who completed 2 or 3 dietary assessments over time, although no associations were significant in this subset of the sample (Supplemental Figure 5). No significant effect modification was observed for sex, parental educational levels and income, race/ethnicity, in utero and second-hand smoke exposures, and obesity status (all interaction P > 0.05) (Supplemental Tables 8and9).
Discussion
In this prospective study, we examined longitudinal associations between childhood air pollution exposures and dietary patterns in adolescence. We found that higher childhood exposures to air pollutants, particularly regional air pollutants (e.g., NO2 and EC) with the main source being traffic and traffic-related NOx emissions, were associated with greater intake of dietary fat, specifically trans fat, in adolescents. These findings were consistent with the observation that increased air pollution exposures were associated with greater fast-food consumption. All of these significant findings were conditioned on socioeconomic status, geospatial autocorrelations, and community-level confounders including the accessibility of fast-food restaurants. Also, associations between air pollutants and the consumption of trans fat and a fast-food diet did not vary significantly by sociodemographic characteristics. Overall, these results suggest that air pollution exposures were associated with adolescents’ dietary behaviors. This novel finding has important public health consequences because dietary behaviors formed in adolescence track into adulthood (55).
One of our main findings suggested that higher childhood exposures to regional and traffic-related air pollutants were associated with higher consumption of fast food, which is known to contain a high amount of trans fat due to wide use of partially hydrogenated oils. This finding was further verified by consistent findings of positive associations between air pollutant exposure and trans fat intake in this cohort. Increased evidence suggests that trans fat is harmful for human health including increased risk of obesity (56, 57), therefore, the US Food and Drug Administration banned the use of trans fat in food by 2018 (58). Supporting the detrimental effect of trans fat, we also observed that higher consumption of fast food was associated with increased odds of being obese or overweight in this study. However, because we did not observe significant associations between childhood air pollutant exposure and obesity in this study sample, we could not draw a conclusion about the mediation effect of fast-food consumption linking air pollutant exposure and obesity in adolescents. Future research should be conducted to clarify the role of diet in the association between air pollutant exposure and obesity and other cardiometabolic disorders.
Although this is the first study, to our knowledge, to examine air pollution and dietary behaviors in humans, supporting evidence for our findings comes from experimental studies using mouse models and a human study of polycyclic aromatic hydrocarbon exposure. In one mouse study (17), in utero exposure to diesel exhaust resulted in mice consuming 14% more calories than offspring of pregnant mice exposed to filtered air. Interestingly, diesel exhaust offspring also had significantly higher levels of microglial cell surface antigen expression in the hypothalamus and hippocampus—brain regions previously linked to the regulation of food intake (59, 60). Another birth cohort study suggested that higher maternal exposure to polycyclic aromatic hydrocarbons during pregnancy was associated with delayed development of self-regulatory capacity in their children (61). Although our study was not designed to examine the effect of prenatal air pollution exposure, our results suggest that childhood air pollution exposures were associated with dietary patterns in adolescents. Future studies with lifetime measurement of air pollutant exposure and longitudinal dietary assessments will be needed to dissect the effect of air pollution on dietary behavior across sensitive time windows during development from the prenatal period to adolescence. In addition, although our study did not measure endogenous concentrations of air pollutant chemicals, our main results suggested the air pollutants that were most significantly associated with increased consumption of fast food and a high-trans-fat diet were traffic-source pollutants (NO2, NOx, and EC). Future studies are warranted to identify specific air pollutant chemicals that could have a causal effect on altering children's dietary behavior.
The strengths of the current study include individual assessment of air pollution exposure and dietary information that was collected in a large prospective cohort of children. To our knowledge, this is the first study in children to examine the association between air pollution exposures and dietary behaviors while also adjusting for key confounders (e.g., individual socioeconomic status, geospatial clustering, and community characteristics). Importantly, the observed associations between increased air pollution exposures and greater consumption of a high-trans-fat and fast-food diet were not significantly confounded by the accessibility of fast-food restaurants in residential areas.
Despite the strengths, this study is limited in that dietary information was self-reported. However, the YAQ used in this study has been previously validated in other youth and adolescent studies (25, 26) and has acceptable reproducibility in the current study with intraindividual correlation coefficients for total calorie and percentage calorie intake from fat of 0.5 and 0.4, respectively. In addition, macronutrient intake and food patterns were calculated by averaging repeated dietary assessments, helping to reduce the measurement error in the self-reported questionnaire. We also adjusted for the potential overreporting and underreporting in the self-reported questionnaires in this analysis to maximally eliminate bias in our results. Although we considered the important confounders such as geospatial autocorrelations, community characteristics, and physical activity, there may have also been other unmeasured community- or individual-level confounders that could have influenced our observed associations between air pollution exposures and dietary patterns, such as the built environment and microbiota. Another limitation of this study was that no biological measure was assessed to examine biological pathways linking air pollution exposure and dietary behavior. Future human studies and animal models will be needed to examine whether neuroinflammation (62) and emotional and cognitive neurocircuitry (63) could play a role in the detrimental effect of air pollution on unhealthy dietary behavior. Notably, multiple testing was not adjusted for in this study because dietary outcomes are highly correlated and Bonferroni or similar correction methods for multiple testing would be overly conservative (64). Importantly, our main findings were mostly related with 1 subtype of fat (trans fat) and a broad spectrum of air pollutant exposure was consistently associated with higher consumption of dietary trans fat. Finally, we could not draw conclusions that an unhealthy diet mediates the association between air pollutant exposure and obesity and cardiometabolic disorders in this study. However, traffic-related air pollutant exposures have been associated with increased risk of childhood obesity in previous studies (1–3); therefore, future studies with ample sample size and longitudinal assessments of air pollutant exposure, diet, and adiposity and cardiometabolic outcomes are warranted to further investigate the role of diet in the relation between air pollution exposure and obesity and cardiometabolic diseases.
In conclusion, higher exposures to regional and traffic-related air pollutants during childhood were associated with increased consumption of a high-trans-fat diet that is commonly observed in fast-food items among adolescents. Future animal models and human studies are warranted to better understand the possible mechanisms linking air pollution exposures and high-fast-food dietary behaviors across childhood and adolescence. This study addressed a significant gap in the current research, namely the lack of longitudinal studies that investigate how air pollution exposure may be associated with dietary behaviors from childhood to adolescence (65).
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—ZC: conducted the analyses, wrote the manuscript, contributed to study design, had full access to all the data in the study, and takes responsibility for the integrity of the data and the accuracy of the data analysis; FDG: contributed to the study design and data collection; MMH: contributed to the manuscript writing and editing; LC, BRB, TLA, RSM, and FDG: edited the manuscript and contributed to discussion; and all authors: read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
Notes
Supported by Southern California Environmental Health Sciences Center grant 5P30ES007048 funded by the National Institute of Environmental Health Sciences; National Institute of Environmental Health Sciences supported grants 5P01ES011627, K99ES027870, and K99ES027853; Southern California Children's Environmental Health Center grants funded by the National Institute of Environmental Health Sciences (5P01ES022845-03); the US Environmental Protection Agency (RD83544101); the University of Southern California's Diabetes and Obesity Research Institute (DORI) Pilot Project; the Southern California Clinical and Translational Science Institute(SC CTSI) Pilot Funding Program grant UL1TR001855; and the Hastings Foundation.
Supplemental Figures 1–5 and Supplemental Tables 1–9 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: CHS, Children's Health Study; EC, elemental carbon; HFLC, high fat and low carbohydrate; LFHC, low fat and high carbohydrate; MFMC, moderate fat and moderate carbohydrate; NO, nitrogen oxide; NOx, nitrogen oxides; OC, organic carbon; PM2.5, fine particles with aerodynamic diameter <2.5 µm; PM10, particles with aerodynamic diameter <10 µm; YAQ, youth/adolescent food-frequency questionnaire.
References
- 1. McConnell R, Shen E, Gilliland FD, Jerrett M, Wolch J, Chang CC, Lurmann F, Berhane K. A longitudinal cohort study of body mass index and childhood exposure to secondhand tobacco smoke and air pollution: the Southern California Children's Health Study. Environ Health Perspect. 2015;123(4):360–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jerrett M, McConnell R, Wolch J, Chang R, Lam C, Dunton G, Gilliland F, Lurmann F, Islam T, Berhane K. Traffic-related air pollution and obesity formation in children: a longitudinal, multilevel analysis. Environ Health. 2014;13(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. La Merrill M, Birnbaum LS. Childhood obesity and environmental chemicals. Mt Sinai J Med. 2011;78(1):22–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Teichert T, Vossoughi M, Vierkötter A, Sugiri D, Schikowski T, Schulte T, Roden M, Luckhaus C, Herder C, Krämer U. Association between traffic-related air pollution, subclinical inflammation and impaired glucose metabolism: results from the SALIA study. PLoS One. 2013;8(12):e83042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chuang KJ, Yan YH, Cheng TJ. Effect of air pollution on blood pressure, blood lipids, and blood sugar: a population-based approach. J Occup Environ Med. 2010;52(3):258–62. [DOI] [PubMed] [Google Scholar]
- 6. Chen Z, Salam MT, Toledo-Corral C, Watanabe RM, Xiang AH, Buchanan TA, Habre R, Bastain TM, Lurmann F, Wilson JP et al.. Ambient air pollutants have adverse effects on insulin and glucose homeostasis in Mexican Americans. Diabetes Care. 2016;39(4):547–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Toledo-Corral CM, Alderete TL, Habre R, Berhane K, Lurmann FW, Weigensberg MJ, Goran MI, Gilliland FD. Effects of air pollution exposure on glucose metabolism in Los Angeles minority children. Pediatr Obes. 2018;13(1):54–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Thiering E, Cyrys J, Kratzsch J, Meisinger C, Hoffmann B, Berdel D, von Berg A, Koletzko S, Bauer CP, Heinrich J. Long-term exposure to traffic-related air pollution and insulin resistance in children: results from the GINIplus and LISAplus birth cohorts. Diabetologia. 2013;56(8):1696–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Brook RD, Xu X, Bard RL, Dvonch JT, Morishita M, Kaciroti N, Sun Q, Harkema J, Rajagopalan S. Reduced metabolic insulin sensitivity following sub-acute exposures to low levels of ambient fine particulate matter air pollution. Sci Total Environ. 2013;448:66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kelishadi R, Mirghaffari N, Poursafa P, Gidding SS. Lifestyle and environmental factors associated with inflammation, oxidative stress and insulin resistance in children. Atherosclerosis. 2009;203(1):311–19. [DOI] [PubMed] [Google Scholar]
- 11. Kim JH, Hong YC. GSTM1, GSTT1, and GSTP1 polymorphisms and associations between air pollutants and markers of insulin resistance in elderly Koreans. Environ Health Perspect. 2012;120(10):1378–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Delfino RJ, Staimer N, Vaziri ND. Air pollution and circulating biomarkers of oxidative stress. Air Qual Atmos Health. 2011;4(1):37–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ghio AJ, Carraway MS, Madden MC. Composition of air pollution particles and oxidative stress in cells, tissues, and living systems. J Toxicol Environ Health B Crit Rev. 2012;15(1):1–21. [DOI] [PubMed] [Google Scholar]
- 14. Kelly FJ. Oxidative stress: its role in air pollution and adverse health effects. Occup Environ Med. 2003;60(8):612–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rajagopalan S, Brook RD. Air pollution and type 2 diabetes: mechanistic insights. Diabetes. 2012;61(12):3037–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rich DQ, Kipen HM, Huang W, Wang G, Wang Y, Zhu P, Ohman-Strickland P, Hu M, Philipp C, Diehl SR et al.. Association between changes in air pollution levels during the Beijing Olympics and biomarkers of inflammation and thrombosis in healthy young adults. JAMA. 2012;307(19):2068–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bolton JL, Smith SH, Huff NC, Gilmour MI, Foster WM, Auten RL, Bilbo SD. Prenatal air pollution exposure induces neuroinflammation and predisposes offspring to weight gain in adulthood in a sex-specific manner. FASEB J. 2012;26(11):4743–54. [DOI] [PubMed] [Google Scholar]
- 18. Kenny PJ. Reward mechanisms in obesity: new insights and future directions. Neuron. 2011;69(4):664–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tzameli I. Appetite and the brain: you are what you eat. Trends Endocrinol Metab. 2013;24(2):59–60. [DOI] [PubMed] [Google Scholar]
- 20. Marco EM, Macri S, Laviola G. Critical age windows for neurodevelopmental psychiatric disorders: evidence from animal models. Neurotox Res. 2011;19(2):286–307. [DOI] [PubMed] [Google Scholar]
- 21. Masten AS. Regulatory processes, risk, and resilience in adolescent development. Ann N Y Acad Sci. 2004;1021:310–19. [DOI] [PubMed] [Google Scholar]
- 22. Chen Z, Salam MT, Eckel SP, Breton CV, Gilliland FD. Chronic effects of air pollution on respiratory health in Southern California children: findings from the Southern California Children's Health Study. J Thorac Dis. 2015;7(1):46–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gauderman WJ, Urman R, Avol E, Berhane K, McConnell R, Rappaport E, Chang R, Lurmann F, Gilliland F. Association of improved air quality with lung development in children. N Engl J Med. 2015;372(10):905–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Urman R, McConnell R, Islam T, Avol EL, Lurmann FW, Vora H, Linn WS, Rappaport EB, Gilliland FD, Gauderman WJ. Associations of children's lung function with ambient air pollution: joint effects of regional and near-roadway pollutants. Thorax. 2014;69(6):540–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rockett HRH, Wolf AM, Colditz GA. Development and reproducibility of a food frequency questionnaire to assess diets of older children and adolescents. J Am Diet Assoc. 1995;95(3):336–40. [DOI] [PubMed] [Google Scholar]
- 26. Rockett HR, Breitenbach M, Frazier AL, Witschi J, Wolf AM, Field AE, Colditz GA. Validation of a youth/adolescent food frequency questionnaire. Prev Med. 1997;26(6):808–16. [DOI] [PubMed] [Google Scholar]
- 27. National Institute for Occupational Safety and Health. Elemental carbon (diesel exhaust). In: NIOSH manual of analytical methods. No. 5040. Issue 3 (interim report) Cincinnati (OH): NIOSH; 1999. [Google Scholar]
- 28. Gauderman WJ, Avol E, Gilliland F, Vora H, Thomas D, Berhane K, McConnell R, Kuenzli N, Lurmann F, Rappaport E et al.. The effect of air pollution on lung development from 10 to 18 years of age. N Engl J Med. 2004;351(11):1057–67. [DOI] [PubMed] [Google Scholar]
- 29. Gauderman WJ, Avol E, Lurmann F, Kuenzli N, Gilliland F, Peters J, McConnell R. Childhood asthma and exposure to traffic and nitrogen dioxide. Epidemiology. 2005;16(6):737–43. [DOI] [PubMed] [Google Scholar]
- 30. Benson P. CALINE4—a dispersion model for predicting air pollution concentrations near roadways [Internet]. 1989. [cited 2016 Jun 9]. Available from: http://www.dot.ca.gov/env/air/docs/c4-manual-searchable.pdf. [Google Scholar]
- 31. Fujita EM, Zielinska B, Campbell DE, Arnott WP, Sagebiel JC, Mazzoleni L, Chow JC, Gabele PA, Crews W, Snow R et al.. Variations in speciated emissions from spark-ignition and compression-ignition motor vehicles in California's south coast air basin. J Air Waste Manag Assoc. 2007;57(6):705–20. [DOI] [PubMed] [Google Scholar]
- 32. Clements AL, Jia Y, Denbleyker A, McDonald-Buller E, Fraser MP, Allen DT, Collins DR, Michel E, Pudota J, Sullivan D et al.. Air pollutant concentrations near three Texas roadways, part II: chemical characterization and transformation of pollutants. Atmos Environ. 2009;43(30):4523–34. [Google Scholar]
- 33. Zhu Y, Pudota J, Collins D, Allen D, Clements A, DenBleyker A, Fraser M, Jia Y, McDonald-Buller E, Michel E. Air pollutant concentrations near three Texas roadways, part I: ultrafine particles. Atmos Environ. 2009;43(30):4513–22. [Google Scholar]
- 34. Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, Wei R, Curtin LR, Roche AF, Johnson CL. 2000 CDC growth charts for the United States: methods and development. Vital Health Stat 11. 2002;May(246):1–190. [PubMed] [Google Scholar]
- 35. UN University; WHO; FAO of the United Nations [Internet]. Human energy requirements: report of a Joint FAO/WHO/UNU Expert Consultation, 2001Available from: http://www.fao.org/3/a-y5686e.pdf. [Google Scholar]
- 36. Schofield WN. Predicting basal metabolic rate, new standards and review of previous work. Hum Nutr Clin Nutr. 1985;39(Suppl 1):5–41. [PubMed] [Google Scholar]
- 37. Jerrett M, Shankardass K, Berhane K, Gauderman WJ, Kunzli N, Avol E, Gilliland F, Lurmann F, Molitor JN, Molitor JT et al.. Traffic-related air pollution and asthma onset in children: a prospective cohort study with individual exposure measurement. Environ Health Perspect. 2008;116(10):1433–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tatalovich Z, Wilson JP, Milam JE, Jerrett ML, McConnell R. Competing definitions of contextual environments. Int J Health Geogr. 2006;5:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Spath H. Cluster analysis algorithms. Chichester (United Kingdom): Ellis Horwood; 1989. [Google Scholar]
- 40. The Merck Manuals. Overview of nutrition. [Internet] 2016. [cited 2017 Jul 24]. Available from: http://www.merckmanuals.com/professional/nutritional-disorders/nutrition-general-considerations/overview-of-nutrition. [Google Scholar]
- 41. Litin L, Sacks F. Trans-fatty-acid content of common foods. N Engl J Med. 1993;329(26):1969–70. [DOI] [PubMed] [Google Scholar]
- 42. London SJ, Sacks FM, Caesar J, Stampfer MJ, Siguel E, Willett WC. Fatty acid composition of subcutaneous adipose tissue and diet in postmenopausal US women. Am J Clin Nutr. 1991;54(2):340–5. [DOI] [PubMed] [Google Scholar]
- 43. Varraso R, Garcia-Aymerich J, Monier F, Le Moual N, De Batlle J, Miranda G, Pison C, Romieu I, Kauffmann F, Maccario J. Assessment of dietary patterns in nutritional epidemiology: principal component analysis compared with confirmatory factor analysis. Am J Clin Nutr. 2012;96(5):1079–92. [DOI] [PubMed] [Google Scholar]
- 44. Reedy J, Wirfalt E, Flood A, Mitrou PN, Krebs-Smith SM, Kipnis V, Midthune D, Leitzmann M, Hollenbeck A, Schatzkin A et al.. Comparing 3 dietary pattern methods—cluster analysis, factor analysis, and index analysis—with colorectal cancer risk: the NIH-AARP Diet and Health Study. Am J Epidemiol. 2010;171(4):479–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. USDA. Dietary guidelines for Americans 2015–2020. [Internet]. 8th ed. USDA; 2015. [cited 2016 Sep 5]. Available from: https://www.cnpp.usda.gov/2015-2020-dietary-guidelines-americans. [Google Scholar]
- 46. US Department of Health and Human Services. Expert Panel on integrated guidelines for cardiovascular health and risk reduction in children and adolescents. Bethesda (MD): National Heart, Lung, and Blood Institute; 2011. [Google Scholar]
- 47. Textor J, Hardt J, Knuppel S. DAGitty: a graphical tool for analyzing causal diagrams. Epidemiology. 2011;22(5):745. [DOI] [PubMed] [Google Scholar]
- 48. Yamaguchi M, Steeves EA, Shipley C, Hopkins LC, Cheskin LJ, Gittelsohn J. Inconsistency between self-reported energy intake and body mass index among urban African-American children. PLoS One. 2016;11(12):e0168303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Mendez MA, Popkin BM, Buckland G, Schroder H, Amiano P, Barricarte A, Huerta JM, Quiros JR, Sanchez MJ, Gonzalez CA. Alternative methods of accounting for underreporting and overreporting when measuring dietary intake-obesity relations. Am J Epidemiol. 2011;173(4):448–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Chen Z, Salam MT, Alderete TL, Habre R, Bastain TM, Berhane K, Gilliland FD. Effects of childhood asthma on the development of obesity among school-aged children. Am J Respir Crit Care Med. 2017;195(9):1181–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. MacKinnon DP, Krull JL, Lockwood CM. Equivalence of the mediation, confounding and suppression effect. Prev Sci. 2000;1(4):173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sobel ME. Some new results on indirect effects and their standard errors in covariance structure models. Sociol Methodol. 1986;16:159–86. [Google Scholar]
- 53. Sobel ME. Asymptotic confidence intervals for indirect effects in structural equation models. Sociol Methodol. 1982;13:290–312. [Google Scholar]
- 54. Doell D, Folmer D, Lee H, Honigfort M, Carberry S. Updated estimate of trans fat intake by the US population. Food Addit Contam Part A Chem Anal Control Expo Risk Assess. 2012;29(6):861–74. [DOI] [PubMed] [Google Scholar]
- 55. Craigie AM, Lake AA, Kelly SA, Adamson AJ, Mathers JC. Tracking of obesity-related behaviours from childhood to adulthood: a systematic review. Maturitas. 2011;70(3):266–84. [DOI] [PubMed] [Google Scholar]
- 56. Centers for Disease Control and Prevention (CDC). Trans fat: the facts. [Internet] CDC; 2010; [cited 2017 Sep 5]. Available from: https://www.cdc.gov/nutrition/downloads/trans_fat_final.pdf. [Google Scholar]
- 57. Micha R, Mozaffarian D. Trans fatty acids: effects on metabolic syndrome, heart disease and diabetes. Nat Rev Endocrinol. 2009;5(6):335–44. [DOI] [PubMed] [Google Scholar]
- 58. US Food and Drug Administration [Internet]. Final determination regarding partially hydrogenated oils (removing trans fat). Available from: https://www.fda.gov/Food/IngredientsPackagingLabeling/FoodAdditivesIngredients/ucm449162.htm. [Google Scholar]
- 59. Stevenson RJ, Francis HM. The hippocampus and the regulation of human food intake. Psychol Bull. 2017;143(10):1011–32. [DOI] [PubMed] [Google Scholar]
- 60. Woods SC, Seeley RJ, Cota D. Regulation of food intake through hypothalamic signaling networks involving mTOR. Annu Rev Nutr. 2008;28:295–311. [DOI] [PubMed] [Google Scholar]
- 61. Margolis AE, Herbstman JB, Davis KS, Thomas VK, Tang D, Wang Y, Wang S, Perera FP, Peterson BS, Rauh VA. Longitudinal effects of prenatal exposure to air pollutants on self-regulatory capacities and social competence. J Child Psychol Psychiatry. 2016;57(7):851–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kodavanti UP. Stretching the stress boundary: linking air pollution health effects to a neurohormonal stress response. Biochim Biophys Acta. 2016;1860(12):2880–90. [DOI] [PubMed] [Google Scholar]
- 63. Dallman MF, Pecoraro N, Akana SF, La Fleur SE, Gomez F, Houshyar H, Bell ME, Bhatnagar S, Laugero KD, Manalo S. Chronic stress and obesity: a new view of “comfort food”. Proc Natl Acad Sci USA. 2003;100(20):11696–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1(1):43–6. [PubMed] [Google Scholar]
- 65. Winpenny EM, Penney TL, Corder K, White M, van Sluijs EMF. Change in diet in the period from adolescence to early adulthood: a systematic scoping review of longitudinal studies. Int J Behav Nutr Phys Act. 2017;14(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


