ABSTRACT
Background
Evidence on the effect of small-quantity lipid-based nutrient supplements (SQ-LNSs) on early child growth and development is mixed.
Objective
This study assessed the effect of daily consumption of 2 different SQ-LNS formulations on linear growth (primary outcome), psychomotor development, iron status (secondary outcomes), and morbidity in infants from age 6 to 12 mo within the context of a maize-based complementary diet.
Methods
Infants (n = 750) were randomly assigned to receive SQ-LNS, SQ-LNS-plus, or no supplement. Both SQ-LNS products contained micronutrients and essential fatty acids. SQ-LNS-plus contained, in addition, docosahexaenoic acid, arachidonic acid (important for brain and eye development), lysine (limiting amino acid in maize), phytase (enhances iron absorption), and other nutrients. Infants’ weight and length were measured bimonthly. At age 6 and 12 mo, psychomotor development using the Kilifi Developmental Inventory and South African Parent Rating Scale and hemoglobin, plasma ferritin, C-reactive protein, and α1-acid glycoprotein were assessed. WHO Motor Milestone outcomes, adherence, and morbidity were monitored weekly through home visits. Primary analysis was by intention-to-treat, comparing each SQ-LNS group with the control.
Results
SQ-LNS-plus had a positive effect on length-for-age zscore at age 8 mo (mean difference: 0.11; 95% CI: 0.01, 0.22; P = 0.032) and 10 mo (0.16; 95% CI: 0.04, 0.27; P = 0.008) but not at 12 mo (0.09; 95% CI: −0.02, 0.21; P = 0.115), locomotor development score (2.05; 95% CI: 0.72, 3.38; P = 0.003), and Parent Rating Score (1.10; 95% CI: 0.14, 2.07; P = 0.025), but no effect for weight-for-age zscore. Both SQ-LNS (P = 0.027) and SQ-LNS-plus (P = 0.005) improved hemoglobin concentration and reduced the risk of anemia, iron deficiency, and iron-deficiency anemia. Both SQ-LNS products reduced longitudinal prevalence of fever, coughing, and wheezing but increased incidence and longitudinal prevalence of diarrhea, vomiting, and rash/sores.
Conclusions
Point-of-use fortification with SQ-LNS-plus showed an early transient effect on linear growth and improved locomotor development. Both SQ-LNS products had positive impacts on anemia and iron status. This trial was registered at clinicaltrials.gov as NCT01845610.
Keywords: infants and young children, stunting, lipid-based nutrient supplements, iron-deficiency anemia, psychomotor development, morbidity, point-of-use fortification, South Africa
Introduction
Growth faltering and associated stunting mainly occur within the 1000-d window after conception (1) and are a strong predictor of poor child development (2). In 2016, stunting affected 155 million children aged <5 y globally, with most of the stunted children living in Asia and Africa (3). The complementary diets in Asia and Africa are predominantly plant-based, with high levels of antinutritional factors, and of low energy and nutrient density (4), which contributes to growth faltering and undernutrition during early childhood. Stunted children are known to experience delayed cognitive development and increased morbidity and mortality (5). Children under age 5 y are also the most vulnerable age group affected by anemia and its associated complications (6).
Small-quantity lipid-based nutrient supplements (SQ-LNSs) were designed to be used as point-of-use fortificants to provide key nutrients and cover nutrient gaps of complementary foods (4). Several studies evaluated SQ-LNSs for efficacy and effectiveness (7–27), including growth, psychomotor development, iron status, and morbidity as outcomes, with mixed results. Although some studies showed positive effects of SQ-LNS provision on linear growth (8, 10), motor development (8, 17), and walking (18) in infants and young children (from 6 to 18 mo), others showed limited (9, 15) or no (16, 19, 20) effect. When providing SQ-LNSs during both the prenatal and postnatal period, earlier achievement of certain developmental milestones (21) and improvement in motor and language development (22) were observed. Lipid-based nutrient supplement trials (20- to 54-g rations) that investigated morbidity outcomes in infants and children showed an increase in diarrhea or no effect or could not show any evidence for noninferiority (7, 8, 10, 23, 24), whereas 2 studies found a beneficial effect on diarrhea incidence (25, 26).
The impact of SQ-LNSs in the context of a predominantly maize-based diet has not been well investigated. Compared with rice or wheat, maize has higher levels of phytates, which bind trace elements such as iron and zinc and inhibit their absorption (28). Two SQ-LNS products were developed especially for this setting. Both SQ-LNS products contained essential fatty acids and 50% of the Recommended Nutrient Intake (RNI) for most of the micronutrients, instead of 100% like most of the other SQ-LNSs. One of the products, SQ-LNS-plus, contained additional nutrients. These were, among others, the long-chain PUFAs, arachidonic acid and DHA, which accumulate in the brain and eye during infant development (29); lysine, the amino acid that is limiting for growth when children predominantly consume their protein from maize-based complementary foods; and phytase to improve iron and zinc bioavailability (30). The aim of this study was to evaluate the efficacy of these novel SQ-LNS products on linear growth (primary outcome) and psychomotor development, iron status, and anemia (secondary outcomes) in children from 6 to 12 mo of age residing in a peri-urban setting in South Africa in the context of a predominantly maize-based diet. Morbidity symptoms were monitored and recorded for the duration of the study.
Methods
Study setting, design, and participants
The study was a 6-mo follow-up, partially blind, randomized controlled trial and was carried out in the peri-urban Jouberton area of the Matlosana municipality, North West province of South Africa, from September 2013 to July 2015. Infants (n = 750) were enrolled in the study at the age of 6 mo and excluded if they had never received any breast milk previously, had severe obvious congenital abnormalities, had a hemoglobin concentration <70 g/L, had a weight-for-length z score (WLZ) < –3, or had other diseases and were referred for hospitalization by clinic staff; the mothers planned to move out of the study area within the next 7 mo; they were receiving special nutritional supplements as part of feeding programs; they were known to be HIV positive (no screening for infant's HIV status was done in this study); they were known to be allergic/intolerant to peanuts, soy, cow-milk protein, or fish; and they had not been born as a singleton. The mother–infant pairs were recruited from the study area by fieldworkers speaking the local language who explained the expectations of the study to the mothers, after which willing mothers signed informed consent. Block randomization of sizes of 3, 6, and 9 was used to randomly allocate the infants to 1 of 3 groups—namely SQ-LNS (n = 250), SQ-LNS-plus (n = 250), and a no-supplement control group (n = 250). Each of the 3 groups was assigned a color code to mask the treatment to the statistician, anthropometry assessors, and laboratory staff and to mask the recipients to which version of SQ-LNS they received. Group randomization lists were prepared by the study statistician using NQuery version 7 (Statistical Solutions Ltd.). During the baseline visit (at age 6 mo), the study nurse allocated the next sequential participant and group code to the next infant enrolled. The study was approved by the ethics committees of North-West University (NWU-00001–11-A1) and the South African Medical Research Council (EC-01-03/2012), adhered to the principles of the Declaration of Helsinki, and followed Good Clinical Practice guidelines.
Infants who were severely anemic (hemoglobin <70 g/L) or malnourished (WLZ < –3) during screening were referred to the primary health care clinic for medical attention and excluded from the study. Symptoms collected with the morbidity questionnaire were used to register adverse events (AEs), and administration of AEs was carried out according to Good Clinical Practice guidelines. If a product-related AE (e.g., allergy) was suspected, the infant was assessed clinically, a skin-prick test was performed, and after a food-challenge test with the SQ-LNS the infant was withdrawn or continued in the study accordingly. AEs were classified as serious (SAE) if an infant was hospitalized or in the case of a death. The intensity was classified as mild if discomfort was noted, but there was no disruption to normal daily activities; moderate if the discomfort was sufficient to reduce or affect normal daily activities; and severe if there was an inability to perform normal daily activities. All blinded AEs and SAEs were reviewed quarterly by an independent Data Safety Monitoring Board consisting of a pediatrician/ethics expert, nutrition researcher, and biostatistician. The randomized controlled trial was registered at clinicaltrials.gov/registry as NCT01845610.
Sample-size calculation
The sample size for the study was based on the expected difference in growth achieved during the 6 mo of active intervention. Using linear growth as the primary outcome, sample size was calculated and based on an expected 1-sided difference of 0.15 length-for-age zscores (LAZs) at age 12 mo with a pooled SD of 0.54 (i.e., effect size of 0.27) between intervention groups and the control group with 80% power and a type 1 error of 5% to detect differences between the intervention groups and the control group equal to or higher than a “medium” effect size of 0.25 (31). The expected differences and pooled SDs were based on a study done in Ghana (8). The required sample size of 186 infants/group was adapted to allow for an expected dropout rate of 25%. Therefore, 250 infants/group (750 in total) were enrolled.
Study products
Infants in the SQ-LNS group received a fortified lipid-based paste containing protein, linoleic and α-linolenic acid, and vitamins and minerals. Infants in the SQ-LNS-plus group received a similar fortified lipid-based paste that contained 4 additional micronutrients (phosphorus, potassium, magnesium, manganese), DHA, arachidonic acid, l-lysine, and phytase, and additional vitamin C and E, protein (from skim-milk powder) and choline (from lecithin). Both products contained soy and dairy protein, but in different ratios, and neither of the products contained peanuts. The products were flavored with vanilla and contained 50% of the RNIs for children aged 1–3 y for most of the micronutrients. The products contained no added sugar. The detailed nutritional profiles of SQ-LNS and SQ-LNS-plus are presented in Table 1. The participants and study staff involved in distribution of the 2 SQ-LNS products were blinded for the version of SQ-LNS that participants received, but it was not possible to blind the control group because they did not receive SQ-LNSs for the duration of the trial (they received SQ-LNSs after completion of the trial from age 12 to 18 mo as a delayed intervention).
TABLE 1.
Energy and nutrient content of the 2 SQ-LNS products used in the study1
| SQ-LNS | SQ-LNS-plus | |
|---|---|---|
| Amount (1 portion), g | 20 | 20 |
| Energy, kcal | 114 | 113 |
| Energy density, kcal/g | 5.7 | 5.7 |
| Protein, g | 3.0 | 3.7 |
| Percentage of calories from protein | 10 | 13 |
| Fat, g | 8.0 | 8.8 |
| Percentage of calories from fat | 63 | 70 |
| Essential fatty acids | ||
| Linoleic acid, g | 1.5 | 1.8 |
| α-Linolenic acid, mg | 265 | 348 |
| Linoleic acid:α-linolenic acid | 5.7 | 5.0 |
| Long-chain PUFAs, mg | ||
| DHA2 | — | 75 |
| Arachidonic acid | — | 75 |
| Micronutrients3 | ||
| Vitamin A, µg | 200 | 200 |
| Vitamin D, µg | 2.5 | 2.5 |
| Vitamin E, mg | 2.5 | 3.8 |
| Vitamin K, µg | 7.5 | 7.5 |
| Thiamine, mg | 0.25 | 0.25 |
| Riboflavin, mg | 0.25 | 0.25 |
| Niacin, mg | 3 | 3 |
| Pantothenate, mg | 1.0 | 1.0 |
| Vitamin B-6, mg | 0.25 | 0.25 |
| Biotin, µg | 4.0 | 4.0 |
| Folate (vitamin B-9), µg | 80 | 80 |
| Vitamin B-12, µg | 0.45 | 0.45 |
| Vitamin C, mg | 23.3 | 103 |
| Calcium, mg | 250 | 396 |
| Iodine, µg | 45 | 45 |
| Iron, mg | 5.8 | 5.8 |
| Zinc, mg | 6.2 | 6.2 |
| Copper, mg | 0.28 | 0.28 |
| Selenium, µg | 8.5 | 8.5 |
| Magnesium, mg | — | 30 |
| Manganese, mg | — | 0.6 |
| Phosphorus, mg | — | 230 |
| Potassium, mg | — | 257 |
| Choline (from lecithin), mg | 2.0 | 7.8 |
| l-Lysine, mg | — | 160 |
| Phytase, FTUs | — | 200 |
The SQ-LNS and SQ-LNS-plus products were provided by Unilever R&D and DSM Nutritional Products Ltd., respectively. The SQ-LNS was manufactured by Unilever R&D Vlaardingen BV and packed by Budelpack BV; SQ-LNS-plus was manufactured by GC Rieber Compact India Pvt. Limited. The SQ-LNS product was packed in sachets containing a 20-g daily ration and did not require any preparation before consumption. SQ-LNS and SQ-LNS-plus contained 1.6 and 4.6 g skimmed-milk powder, respectively. FTU, phytase activity unit; SQ-LNS, small-quantity lipid-based nutrient supplement.
Fish oil also contained 17 mg EPA.
From added micronutrient mix, excluding micronutrients from other raw material sources.
TABLE 2.
Baseline characteristics of the 750 participants at enrollment1
| Characteristics | SQ-LNS (n = 250) | SQ-LNS-plus (n = 250) | Control (n = 250) |
|---|---|---|---|
| Infant characteristics | |||
| Male, n (%) | 113 (45.2) | 143 (57.2) | 131 (52.4) |
| Female, n (%) | 137 (54.8) | 107 (42.8) | 119 (47.6) |
| Age, mo | 6.22 ± 0.262 | 6.22 ± 0.24 | 6.22 ± 0.25 |
| Breastfeeding at age 6 mo, n (%) | 182 (72.8) | 178 (71.2) | 165 (66.3) |
| Birth weight,3 kg | 2.97 ± 0.54 | 2.93 ± 0.52 | 3.04 ± 0.47 |
| Low birth weight (<2.5 kg), n (%) | 38 (15.7) | 40 (16.9) | 24 (9.9) |
| Anthropometric status | |||
| Length, cm | 63.7 ± 2.5 | 63.8 ± 2.5 | 64.1 ± 2.5 |
| Weight, kg | 7.2 ± 1.1 | 7.3 ± 1.0 | 7.3 ± 1.1 |
| LAZ | −1.47 ± 1.07 | −1.54 ± 1.06 | −1.37 ± 1.09 |
| Stunted (< −2 LAZ), n (%) | 71 (28.4) | 78 (31.2) | 72 (28.8) |
| WAZ | −0.63 ± 1.12 | −0.54 ± 1.18 | −0.55 ± 1.27 |
| Underweight (< −2 WAZ), n (%) | 26 (10.4) | 27 (10.8) | 32 (12.8) |
| WLZ | 0.48 ± 1.15 | 0.65 ± 1.15 | 0.49 ± 1.16 |
| Wasted (< −2 WLZ), n (%) | 4 (1.6) | 3 (1.2) | 4 (1.6) |
| Overweight (> +2 WLZ), n (%) | 18 (7.2) | 30 (12.0) | 27 (10.8) |
| Midupper arm circumference, cm | 14.3 ± 1.25 | 14.4 ± 1.25 | 14.3 ± 1.26 |
| Head circumference, cm | 42.8 ± 1.36 | 43.1 ± 1.34 | 43.0 ± 1.39 |
| Kilifi Developmental Inventory | |||
| Locomotor development score | 16 (15, 18)4 | 16 (15, 18) | 16 (15, 18) |
| Eye–hand coordination score | 17 (15, 19) | 17 (15, 19) | 18 (16, 20) |
| Parent rating | 21 (19, 22) | 20 (18, 22) | 20 (18, 22) |
| Iron status | |||
| Hemoglobin, g/L | 112 (105, 121) | 115 (105, 123) | 113 (105, 121) |
| Anemic (hemoglobin <110 g/L), n (%) | 98 (39.2) | 86 (34.4) | 90 (36.0) |
| PF5,6 | 24.9 (16.0, 40.6) | 25.3 (16.4, 40.2) | 25.4 (15.3, 39.8) |
| Iron deficient (PF <12 µg/L),5,6n (%) | 23 (13.9) | 31 (19.2) | 23 (14.9) |
| IDA (hemoglobin <110 g/L + PF <12 µg/L),5,6n (%) | 18 (10.9) | 17 (10.6) | 15 (9.7) |
| Elevated CRP (>5 mg/L),6n (%) | 29 (17.6) | 24 (14.9) | 20 (13.0) |
| Elevated AGP (>1 g/L),6n (%) | 49 (29.7) | 50 (31.1) | 52 (33.8) |
| Caregiver characteristics | |||
| Age, y | 28.1 ± 8.4 | 27.9 ± 8.0 | 29.3 ± 9.2 |
| Education, grade 10 or higher, n (%) | 209 (83.6) | 197 (78.8) | 195 (78.0) |
| Married, n (%) | 29 (11.6) | 20 (8.0) | 31 (12.4) |
| Household characteristics | |||
| Electricity at home, n (%) | 229 (91.6) | 230 (92.0) | 233 (93.2) |
| Tap water at home, n (%) | 237 (94.8) | 241 (96.4) | 241 (96.4) |
| Flush toilet at home, n (%) | 237 (94.8) | 239 (95.6) | 237 (94.8) |
| Number of people in household | 6 (4, 7) | 5 (4, 7) | 6 (4, 7) |
| Number of child grants per household | 2 (2, 3) | 2 (1, 3) | 2 (1, 3) |
AGP, α1-glycoprotein; CRP, C-reactive protein; IDA, iron-deficiency anemia; LAZ, length-for-age zscore; PF, plasma ferritin; SQ-LNS, small-quantity lipid-based nutrient supplement; WAZ, weight-for-age z score; WLZ, weight-for-length z-score.
Means ± SDs (all such values).
Twenty-nine (3.9%) infants had missing information on birth weight.
Median; 25th and 75th percentile in parentheses (all such values).
Adjusted for inflammation considering both CRP and AGP (43).
n = 480.
The efficacy trial was preceded by acceptability trials of the 2 SQ-LNS products in 6- to 12-mo-old infants in the same community. Based on the mother's own acceptance and her perception of the infant's acceptance, the products were considered acceptable by >80% of the mothers (32).
Intervention and follow-up
At age 6 and 12 mo, anthropometric measurements and blood samples were taken, psychomotor development was assessed, and information on feeding practices and infant morbidity was collected. Sociodemographic information on the household was collected at age 6 mo. Acceptability of the study product was assessed at the end of the 6-mo study period. In addition, the infants’ weight and length measurements were taken bimonthly (at age 8 and 10 mo). The fieldworkers performed weekly home visits to all participants to deliver supplements, monitor adherence, collect forms on which mothers/caregivers recorded the daily amount of supplement consumed by the infant, and for morbidity surveillance.
The 2 SQ-LNS groups and the control group were treated and monitored similarly, with the exception that the control group did not receive supplements. Parents were advised to feed infants 1 sachet/d (20 g) of the SQ-LNS for 6 mo (26 wk). During the first week, only half of the sachet was given to the infant. Mothers were advised to give SQ-LNS paste as part of the first meal and to mix it with usual complementary foods.
In accordance with Good Clinical Practice principles, site-monitoring visits were conducted biweekly by an independent clinical research organization (OnQ Research Pty Ltd.) where the monitor visited the study site and reviewed participant files for completeness. Monthly refresher trainings were conducted for the fieldworkers, nurses, and students who were involved in the study execution.
Data were recorded on paper forms and transcribed to paper case report files. The socioeconomic, anthropometric, psychomotor development, and blood results data were captured principally by an independent clinical research organization (ClinTec, International Pvt. Ltd.) using Pharmaceutical Applications Release version 4.6.6. Morbidity and feeding practices data were captured into EpiData version 3.1 (EpiData Association).
Measurement of outcome variables
All measurements and data collection took place at a central study site except for data on developmental milestone outcomes using the WHO Motor Milestone Chart, adherence, and morbidities that were collected weekly at household level. Structured questionnaires were used to assess breastfeeding and complementary feeding practices, collect information on sociodemographics of the household and infant morbidities, and assess the acceptability of the study product, which included aspects of the infant's and mother's acceptability toward the supplement, use of the supplement, and possible future use of the supplement.
Anthropometric status was assessed using the WHO Child Growth Standards (33). Wasting was defined as WLZ less than –2 SDs, stunting as LAZ less than −2 SDs, and underweight and overweight as weight-for-age zscore (WAZ) less than −2 SDs or >2 SDs, respectively. Weight and recumbent length were taken according to WHO standardized techniques (34). The anthropometry assessors were trained according to the WHO Training Course on Child Growth Assessment for the infants (35). During fieldwork, 5% of infants were randomly selected for repeated duplicate measurements by the same assessor and checked by the team leader. Another 2% of participants were selected for a repeated measurement by the team leader at each measurement stage (age 6, 8, 10, and 12 mo). The measurement by the team leader was used as the standard to compare the correctness of the measurement by the assessor. Only 5 persons, including the supervisor, team leader, and 3 assessors, performed the measurements. The same equipment and room were used throughout the study. Infants were undressed and weighed to the nearest 0.01 kg using a digital infant scale (Seca model 354; maximum weight: 20 kg). The primary outcome, recumbent length, was measured to the nearest 0.1 cm using an infantometer (Seca model 416). Midupper arm circumference (MUAC) and head circumference (HC) were measured using a measuring tape (Seca 201 and Seca 212, respectively). All measurements were done in duplicate, and if the first 2 measurements differed by >0.05 kg for weight or by >0.3 cm for length or by >0.2 cm for MUAC or HC, a third measurement was done, and the 2 closest values were recorded. Anthropometric indexes were generated using WHO Anthro 2005 software.
Psychomotor development as a secondary outcome was assessed by direct observation and parental report using the Kilifi Developmental Inventory (KDI) and the South African Parent Rating Scale, respectively. The KDI assesses locomotor development, which relates to the infant's ability to select and modify his or her ongoing movement appropriately (36), and eye–hand coordination, which relates to the ability to reach or contact objects (37). The KDI includes a culturally relevant 69-item inventory and is designed specifically for use in resource-poor settings by assessors with little experience in child development. The KDI has been evaluated for reliability and validity in normal and disease-exposed populations in Kenya (38, 39). For the Parent Rating Scale, the mother/principal caregiver provides a rating of the child on gross motor developmental milestones. The Parent Rating Scale has previously been validated in a study that included children aged 6–36 mo from rural and urban settings in South Africa (40). Both the KDI and the Parent Rating Scale were translated into the local language; the translations were verified and corrected where needed. Two selected individuals were trained as cognitive assessors under the guidance of the study psychologist (JDK), and for the duration of the study all assessments were performed by these 2 assessors, thereby reducing interassessor variability. The assessments were always performed in a separate room to avoid distracting the infants. The caregiver was asked to assist where needed—for example, to get the attention of the infant. KDI scores were calculated by adding up the scores for each individual KDI item, which were coded as 0 = unable to perform task, 1 = partially able to perform task, and 2 = able to perform task; some activities were scored on a scale of 0–4. Scoring the question and observation items of the Parent Rating Scales were 0 = infant was not able and 1 = infant was able.
During the weekly home visits, fieldworkers filled in a pictorial Gross Motor Milestones Chart according to the WHO standards. A description for each pictorial milestone was given. The date on which a particular milestone was observed by the parent/caregiver was recorded. This information was used to determine the age at which each child attained the 10th milestone or above.
Iron status as a secondary outcome was determined by blood sample analysis. A 4-mL blood sample was taken into EDTA-coated trace-element-free evacuated tubes (Becton Dickinson) via antecubital venipuncture at the age of 6 and 12 mo by a professional nurse. Blood was successfully collected from 480 of 750 (64.0%) of the infants at baseline and 350 of 514 (68.1%) at endpoint (those who completed the study). If blood drawing failed, a finger-prick blood sample was taken. Hemoglobin concentration was measured on all samples (baseline, n = 750; endpoint, n = 514) immediately after the blood sample was taken using the HemoCue Hb 201+ System. At the field laboratory, the blood was processed to obtain plasma aliquots and stored at 4°C. The samples were transported daily from the study site in cooler bags with refrigerant gel ice packs to North-West University (32 miles) for storage at −80°C. The samples were later shipped for analysis to VitMin Lab Willstaett, Germany, in accordance with the standard shipment procedures of Department of Health in South Africa.
Plasma ferritin (PF) concentration was measured using a sensitive sandwich ELISA (Ramco Laboratories) technique (41). The acute and chronic inflammation markers, high-sensitivity C-reactive protein (CRP), and α1-glycoprotein (AGP) were measured with a sandwich ELISA method at the VitMin Laboratory in Munich, Germany (41, 42). Anemia was defined as hemoglobin <110 g/L, iron deficiency (ID) as PF <12 μg/L, and iron-deficiency anemia (IDA) as both PF <12 μg/L and hemoglobin <110 g/L (43, 44). The inflammatory markers AGP and CRP were used to detect the presence of inflammation as AGP >1 g/L and CRP >5 mg/L (45). Individual PF concentrations were adjusted to each subject's inflammatory status, namely incubation (CRP >5 mg/L, AGP ≤1 g/L), early convalescence (CRP >5 mg/L, AGP >1 g/L), and late convalescence (CRP ≤5 mg/L, AGP >1 g/L), using correction factors (0.77, 0.53, and 0.75) (45).
Morbidity measures were a prespecified outcome. During the weekly home visits, fieldworkers obtained morbidity data with a structured questionnaire. To improve the accuracy of the morbidity data, the symptoms of the infant were also recorded daily by the caregiver using a pictorial wellness/illness calendar (46). Morbidity symptoms were defined in local language terms and comprised maternal-reported fever without the specified use of a thermometer (“hot body”), diarrhea (“liquid stools,” defined as ≥3 loose or watery stools within 24 h), vomiting (“to give up,” but not spitting or “to let down”), runny nose (“if discharge comes from the nose”), coughing (“severe coughing from the chest”), wheezing (“suffer from poor breathing”), and rash/sores (“sores/rash on the skin”). Incidences of morbidity symptoms were separated by 2 d without the symptom. To calculate the total child-days of SQ-LNS exposure for each group, the time in the study up to 26 wk or dropout, based on the weekly contact tracking log of each child irrespective of any morbidities, was used.
Statistical analysis
A statistical analysis plan to assess intervention effects was developed, and the data set was locked before data analysis. For data analysis, each of the 2 SQ-LNS groups was compared with the control; the control group was therefore not blinded for data analysis. Unblinding of group assignment for the 2 SQ-LNS groups was carried out after data analysis was completed. Data analysis was performed using SAS version 9.4 (SAS Institute, Inc.), STATA version 14 (StataCorp Ltd.), and SPSS software version 23 (IBM Corporation).
All analyses were carried out based on intention-to-treat, and differences between each of the 2 SQ-LNS groups compared with the control were assessed. In general, linear mixed-effect models were used for the continuous outcomes measured bimonthly (LAZ and WAZ), whereas linear regression models were used for MUAC and HC measured at age 6 and 12 mo. Quantile regression models were used for the child development outcomes and the continuous blood parameters measured at age 6 and 12 mo. Logistic regression was used for the binary outcomes (anemia, ID, and IDA). Time to reach the 10th WHO motor milestone outcome was evaluated using a Cox proportional hazard model. Morbidity was evaluated using Poisson regression. Missing values were dealt with using full maximum likelihood for outcomes with bimonthly measurements and multiple imputations for outcomes for which there was only 1 measurement postrandomization at 12 mo. For outcomes relying on blood drawing, we did not impute due to the low success rates of drawing blood (and therefore a high number of missing values)
Growth outcomes
For the primary outcome, LAZ, a mixed-effects (piecewise) linear spline model with 2 knots (at 8.3 and 10.4 mo) was fitted via maximum likelihood (47) to all participants in the trial (n = 750). The baseline value of the measurement (at age 6 mo) was included in the set of repeated outcome measures for each participant, and the test for interaction between a specific SQ-LNS group indicator and age was used to test for the hypothesis of differential LAZ profiles over age compared with the control arm. The fixed effects for the mixed-effects model contained the indicators for the SQ-LNS arms, the spline terms for age, and the interaction effects, whereas the random effect was the participant; and the model included an intercept and spline terms for age. The covariance matrix for the random-effects model was specified as unstructured, and the degrees of freedom for the inference used the Kenward–Roger approach. Because the baseline LAZ was included in the outcome, the age-specific effects were estimated using a difference in differences approach, and full maximum likelihood estimation was the imputation strategy implemented. Baseline factors associated with dropout at 8, 10, or 12 mo were assessed via an ordinal regression model; and the factors sex, age of the mother/caregiver, and number of people in the household were included as fixed effects in an adjusted linear spline model for LAZ as a sensitivity analysis. The SQ-LNS arms were also associated with dropout, but they are, by design, part of the intention-to-treat model. The same linear spline model was used for WAZ. An independent covariance structure for the random effects was, however, specified to secure proper convergence of the model. Linear regression models with baseline values as covariate and adjusted for sex were used for MUAC and HC.
Psychomotor development outcomes
In the intention-to-treat analysis for psychomotor development outcomes KDI and Parent Rating Scale, multiple imputations were carried out to cater to the participants who had a single postrandomization evaluation at 12 mo. Imputations were carried out using multivariate normal regression analysis with the baseline value of the outcomes, sex, age of mother, number of people in the household, LAZ, WAZ, MUAC, and HC at baseline as covariates. Imputations were carried out separately by study group; 10 imputations were carried out. Psychomotor development outcomes were analyzed using imputation-based quantile regression; in general, an ANCOVA was used with the baseline measurement as a covariate. The 10th WHO motor milestone (able to take a few staggering walking steps without support) or higher was taken as the overall milestone event for the time-to-event analysis, because it provides a good number of events. The time to reach the 10th WHO motor milestone was analyzed using a Cox proportional hazard model, adjusting for baseline LAZ, with participants not reaching this endpoint censored at the age of their last assessment or end of the study.
Anemia and iron status outcomes
Imputed quantile regression was done to assess intervention effects on hemoglobin concentrations adjusted for baseline values, whereas quantile regression was done to assess intervention effects on PF concentrations adjusted for baseline hemoglobin values. To test the effect of SQ-LNSs on the likelihood of anemia, ID, and IDA, logistic regression models were used to estimate ORs and 95% CIs, adjusting for the baseline measurement of hemoglobin to improve the precision.
Morbidity outcomes
The intervention effects of the SQ-LNS products on morbidity symptoms were analyzed using Poisson regression with a log linear link adjusted for sex and correcting for exposure time in the study, using a modified intention-to-treat analysis that excluded participants who had no follow-up after enrollment. The intervention effect estimates from this model were reported as incidence and prevalence rate ratios of exposed (SQ-LNS) compared with unexposed (control) participants with 95% CIs.
Results
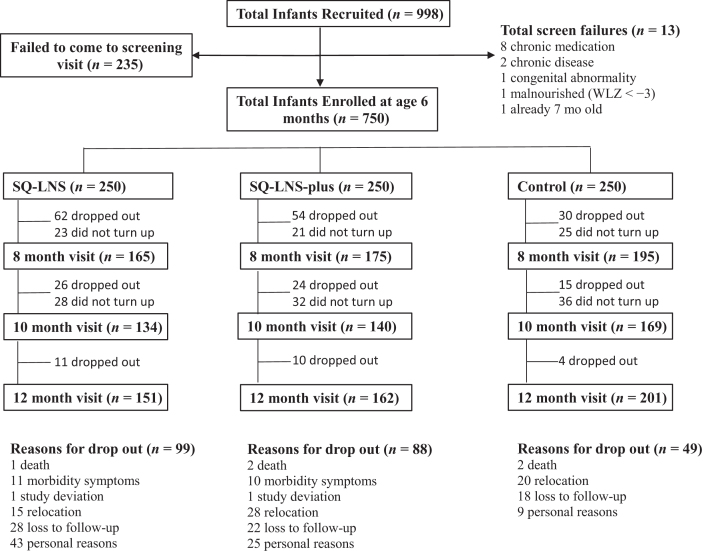
A total of 998 caretaker-infant pairs were recruited, of whom 750 were enrolled in the study; 235 failed to come for a baseline visit, and 13 were excluded because they were not eligible (Figure 1). In total, 514 (68.5%) infants completed the trial, of whom 388 completed all visits. There were 146 (19.5%) children with no follow-up postrandomization. The reasons for loss to follow-up were as follows: mothers relocating out of the study area or changing address without notifying study staff, infants refusing the SQ-LNS, AE/SAE-related concerns, and personal reasons. Five infants died due to causes unrelated to the study; this is lower than the expected number of deaths (n = 9), which was calculated before the study based on the reported South African infant mortality rate. The mean age of the infants was 6.22 ± 0.25 mo, and 51.6% were boys (Table 2). Low birth weight (<2.5 kg) was observed in 14.1% of the infants. At age 6 mo, 70.1% of infants were still being breastfed; of those no longer being breastfed, 90.8% frequently (≥4 d/wk) received formula milk feeds. In total, 41 SAEs (either death or hospitalization) were reported for the duration of the study (SQ-LNS, n = 13; SQ-LNS-plus, n = 16; and control, n = 12); children who were hospitalized did not necessarily drop out of the study. Less than 1% of the AEs were classified as SAEs, with 0.7%, 0.9%, and 0.9% in the SQ-LNS, SQ-LNS-plus, and control group, respectively. The intensities of all individual AEs (diarrhea, vomiting, coughing, wheezing, fever, rash/sores, and runny nose) were mild for ≥98% of cases (Supplemental Table 1).
FIGURE 1.
Flow diagram of participant progression through the intervention study. A total of 998 infants were recruited, and 750 were randomly assigned to 1 of the 3 groups: SQ-LNS, SQ-LNS-plus, and control. In total, 514 (68.5%) infants completed the trial. The infants were followed weekly by fieldworkers to monitor morbidity, adherence, and achievement of motor milestones. SQ-LNS, small-quantity lipid-based supplement; WLZ, weight-for-length z score.
Based on the duration of the trial (182 d), the number of supplements issued, and the number of empty sachets collected during the weekly home visits for accountability monitoring, the estimated mean adherence to the intervention (proportion of days when the supplements were consumed) was 94.1% and 94.4% in the SQ-LNS and SQ-LNS-plus groups, respectively. The reported mean weekly consumption (based on recorded daily consumption using a 4-point pictorial scale) showed that 78.8% and 78.2% of infants from the SQ-LNS and SQ-LNS-plus groups, respectively (P > 0.05), consumed the full amount of the weekly supply (20 g/d).
Effect on growth
Prevalences of stunting, wasting, underweight, and overweight were 29.5%, 1.5%, 11.3%, and 10.0% at baseline (age 6 mo) and 38.3%, 1.6%, 12.5%, and 5.1% at the end of the study (age 12 mo), respectively. The 2 SQ-LNS groups (pooled) had a significantly lower LAZ when compared with the control group at baseline. The mean LAZ profile of the SQ-LNS-plus group was significantly different from the control overall (P = 0.036) but not for the SQ-LNS group (P = 0.457) based on the results from a mixed-effects regression model using linear splines. There were significant age-specific differences in LAZ means at age 8 mo (P = 0.032) and 10 mo (P = 0.008) but not at age 12 mo between the SQ-LNS-plus group and the control group (Table 3, Supplemental Figure 1). The results from the intention-to-treat LAZ model adjusted for baseline factors associated with dropout were not different from the unadjusted intention-to-treat analysis. There was no significant intervention effect for either SQ-LNS or SQ-LNS-plus for WAZ (P = 0.559 and 0.186, respectively), MUAC (P = 0.680 and 0.843, respectively), and HC (P = 0.428 and 0.346, respectively).
TABLE 3.
Outcomes at follow-up and estimated intervention effects for growth, psychomotor development scores, and anemia and iron status indicators for active treatments compared with control1
| SQ-LNS vs. control | SQ-LNS-plus vs. control | ||||||
|---|---|---|---|---|---|---|---|
| SQ-LNS | SQ-LNS-plus | Control | Effect (95% CI) | P | Effect (95% CI) | P | |
| Anthropometric measures2 | |||||||
| Age 8 mo: height, cm | 66.9 ± 2.73 | 67.1 ± 2.6 | 67.3 ± 2.7 | ||||
| LAZ | −1.37 ± 1.12 | −1.37 ± 1.04 | −1.32 ± 1.13 | 0.06 (−0.05, 0.16) | 0.279 | 0.11 (0.01, 0.22) | 0.032 |
| Weight, kg | 7.8 ± 1.2 | 8.0 ± 1.1 | 8.0 ± 1.2 | ||||
| WAZ | −0.68 ± 1.25 | −0.50 ± 1.20 | −0.58 ± 1.27 | 0.01 (−0.07, 0.09) | 0.783 | 0.08 (−0.001, 0.15) | 0.054 |
| Age 10 mo: height, cm | 69.4 ± 3.0 | 69.9 ± 2.5 | 69.9 ± 2.8 | ||||
| LAZ | −1.39 ± 1.20 | −1.34 ± 0.95 | −1.34 ± 1.15 | 0.07 (−0.05, 0.19) | 0.246 | 0.16 (0.04, 0.27) | 0.008 |
| Weight, kg | 8.4 ± 1.2 | 8.7 ± 1.1 | 8.5 ± 1.2 | ||||
| WAZ | −0.57 ± 1.22 | −0.40 ± 1.08 | −0.55 ± 1.22 | 0.07 (−0.04, 0.18) | 0.228 | 0.08 (−0.03, 0.19) | 0.161 |
| Age 12 mo: height, cm | 71.0 ± 2.9 | 71.4 ± 2.6 | 71.5 ± 3.2 | ||||
| LAZ | −1.71 ± 1.18 | −1.71 ± 1.01 | −1.58 ± 1.22 | 0.02 (−0.10, 0.13) | 0.774 | 0.09 (−0.02, 0.21) | 0.115 |
| Weight, kg | 8.7 ± 1.3 | 9.0 ± 1.6 | 8.9 ± 1.3 | ||||
| WAZ | −0.75 ± 1.22 | −0.55 ± 1.12 | −0.61 ± 1.22 | 0.02 (−0.09, 0.12) | 0.764 | 0.05 (−0.06, 0.15) | 0.388 |
| Overall: LAZ | — | — | — | 0.457 | 0.036 | ||
| WAZ | — | — | — | 0.559 | 0.186 | ||
| Midupper arm circumference, cm | 14.5 ± 1.30 | 14.7 ± 1.24 | 14.6 ± 1.31 | −0.02 (−0.09, 0.06) | 0.680 | −0.01 (−0.08, 0.07) | 0.843 |
| Head circumference, cm | 45.1 ± 1.42 | 45.6 ± 1.29 | 45.3 ± 1.46 | −0.03 (−0.09, 0.04) | 0.428 | 0.03 (−0.03, 0.10) | 0.346 |
| Kilifi Developmental Inventory4 | |||||||
| Locomotor development score | 24 (22, 28)5 | 26 (22, 30) | 24 (22, 29) | 0.51 (−1.27, 2.29) | 0.565 | 2.05 (0.72, 3.38) | 0.003 |
| Eye–hand coordination score | 24 (18, 27) | 25 (20, 28) | 24 (19, 27) | 0.32 (−1.47, 2.10) | 0.725 | 1.19 (−0.36, 2.75) | 0.132 |
| Parent rating6 | |||||||
| Adjusted | 33 (30, 36) | 33 (30, 35) | 32 (30, 35) | 0.65 (−0.34, 1.64) | 0.195 | 1.10 (0.14, 2.07) | 0.025 |
| Adjusted and imputed | — | — | — | 0.74 (−0.25, 1.73) | 0.144 | 0.87 (−0.18, 1.91) | 0.102 |
| Time reaching 10th milestone7 | 43.9 | 49.6 | 52.9 | 1.15 (0.88, 1.50) | 0.296 | 1.25 (0.97. 1.62) | 0.079 |
| Hemoglobin and iron status8 | |||||||
| Hemoglobin,9 g/L | 118 (111, 127) | 120 (109, 129) | 115 (106, 125) | 3.94 (0.68, 7.19) | 0.018 | 4.81 (1.40, 8.22) | 0.006 |
| Anemia10 | 23.8 | 25.5 | 34.8 | 0.56 (0.34, 0.92) | 0.021 | 0.61 (0.38, 0.99) | 0.044 |
| Plasma ferritin,11 µg/L | 21.1 (13.7, 32.0) | 23.8 (15.4, 33.1) | 17.3 (9.4, 27.9) | 3.23 (−1.16, 7.61) | 0.149 | 5.37 (1.13, 9.61) | 0.013 |
| Iron deficiency12 | 19.6 | 13.8 | 36.8 | 0.41 (0.22, 0.76) | 0.004 | 0.26 (0.14, 0.50) | <0.001 |
| Iron-deficiency anemia13 | 3.1 | 5.5 | 20.8 | 0.11 (0.03, 0.37) | <0.001 | 0.19 (0.07, 0.48) | 0.001 |
LAZ, length-for-age zscore; SQ-LNS, small-quantity lipid-based nutrient supplement; WAZ, weight-for-age zscore.
Intervention effects were estimated with a mixed-effects (piecewise) linear spline model, fitted via maximum likelihood to all participants in the trial. Infant anthropometric measures of length and weight were taken at bimonthly visits to the study site during the intervention period (6- to 12-mo-old); number of participants seen at specific visits: baseline (n = 750), age 8 mo (n = 535), age 10 mo (n = 443), age 12 mo (n = 514), resulting in 604 (80.5%) participants with ≥1 visit after baseline; the prevalence of stunting at age 12 mo (endpoint) was 41.1% for SQ-LNS, 38.3% for SQ-LNS-plus, and 36.3% for the control group.
Means ± SDs (all such values)
Intervention effects were estimated with imputed quantile regression adjusted for baseline; imputations (n = 10) were carried out using multivariate normal regression analysis with baseline values, sex, age of mother, number of people in the household, LAZ, WAZ, midupper arm circumference, and head circumference at baseline as covariates.
Median; 25th and 75th percentile in parentheses (all such values).
Intervention effects were estimated with quantile regression and imputed quantile regression, both adjusted for baseline measurement.
Values are percentages of participants who had reached the 10th WHO milestone outcome or higher at age 12 mo; intervention effects were estimated using a Cox proportional hazard model, adjusting for baseline LAZ, with participants not reaching this endpoint censored at the age of their last assessment or end of the study.
Intervention effect on hemoglobin concentration was assessed with imputed quantile regression adjusted for baseline values and on plasma ferritin concentration with quantile regression adjusted for baseline hemoglobin. To test the effect of SQ-LNS on the likelihood of anemia, iron deficiency, and iron-deficiency anemia, logistic regression models adjusted for baseline hemoglobin were used.
Baseline (n = 750), age 12 mo (n = 513).
Hemoglobin <110 g/L; values are percentages of participants; intervention effects are ORs (95% CIs), adjusted for baseline hemoglobin.
Adjusted for inflammation considering both C-reactive protein and α1-glycoprotein (43); baseline (n = 480), age 12 mo (n = 350).
Plasma ferritin <12 µg/L; values are percentages of participants; intervention effects are ORs (95% CIs); the prevalence of iron deficiency at age 12 mo (endpoint) was 19.6% for SQ-LNS, 13.8% for SQ-LNS-plus, and 36.8% for the control group.
Hemoglobin <110 g/L and plasma ferritin <12 µg/L; values are percentages of participants; intervention effects are ORs (95% CIs).
Effect on psychomotor development
Locomotor skills assessed using the KDI included movement in space, static and dynamic balance, and motor coordination. The adjusted and imputed median regression analysis (Table 3) showed that the median locomotor score was significantly higher in the SQ-LNS-plus group with a median difference at age 12 mo of 2.05 points (95% CI: 0.72, 3.38; P = 0.003). This translates to a 25% better improvement compared with the control group who improved by 8 points due to normal motor development during the 6-mo intervention. Eye–hand coordination was assessed using the KDI based on the infant's ability to manipulate objects and engage in activities requiring fine-motor coordination. The adjusted and imputed median regression analysis (Table 3) showed that the SQ-LNS and SQ-LNS-plus groups were not significantly different from the control group (P = 0.725 and P = 0.132, respectively). Results of the adjusted and imputed median regression analyses showed no intervention effect for the Parent Rating Scale (gross motor development) for either of the SQ-LNS groups, whereas SQ-LNS-plus showed an intervention effect in the adjusted model (P = 0.025) compared with the control (Table 3).
In terms of time reaching the 10th WHO motor milestone (able to take a few staggering walking steps without support), findings from the Cox regression analysis adjusted for baseline LAZ showed that there was no significant intervention effect for either SQ-LNS (P = 0.296) or SQ-LNS-plus (P = 0.079). Because children with higher baseline LAZ status were more likely to reach the target motor milestone and because the control group had a higher mean LAZ, adjusting for baseline LAZ provides a more precise estimate of the intervention effect in the 2 SQ-LNS arms.
Effect on anemia and iron status
Table 3 shows the intervention effect on median hemoglobin and PF concentrations. PF was adjusted for CRP and AGP using correction factors (43). For infants who completed the study (n = 514), there was a significant increase in median hemoglobin concentrations in the intervention groups compared with the control group (SQ-LNS, P = 0.027; SQ-LNS-plus, P = 0.005; data not shown in table). The baseline hemoglobin concentration was positively associated with the endpoint hemoglobin concentration. Imputed quantile regression with hemoglobin adjusted for baseline showed a positive intervention effect on hemoglobin concentration for both intervention groups compared with the control group (SQ-LNS, P = 0.018; SQ-LNS-plus, P = 0.006). Logistic regression analysis, adjusting for baseline hemoglobin concentrations, showed a significant decrease in the likelihood of anemia for both SQ-LNS (P = 0.021) and SQ-LNS-plus (P = 0.044).
Because blood sampling was unsuccessful for a large proportion of the infants, the number of infants with values for PF was low, only 480 at baseline (thus only 64%). Imputation was therefore not done, and these iron status indicators are considered exploratory. At exit, the SQ-LNS-plus group had a significantly higher median PF (P = 0.013) than the control group, when adjusted for inflammation based on CRP and AGP. Logistic regression analysis, adjusting for baseline hemoglobin concentrations, showed a significant decrease in the likelihood of ID and IDA for both SQ-LNS and SQ-LNS-plus.
Effect on morbidity symptoms
Both SQ-LNS and SQ-LNS-plus (Table 4) reduced the longitudinal prevalence of fever (P < 0.001 and P = 0.006), coughing (P < 0.001 and P = 0.001), and wheezing (both P < 0.001) by 18% and 10%, 9% and 6%, and 78% and 53%, respectively, whereas SQ-LNS reduced the incidence of wheezing by 57% (P = 0.006). SQ-LNS also reduced the longitudinal prevalence of runny nose by 7% (P = 0.002), and SQ-LNS-plus increased it by 5% (P = 0.035). Both SQ-LNS and SQ-LNS-plus increased the longitudinal prevalence and incidence of diarrhea by 30% and 68% (P = 0.002 and P = 0.001) and 25% and 26% (P = 0.005 and P < 0.001), respectively. The SQ-LNS group had 4.8 times and the SQ-LNS-plus group 2.4 times more total sick days with vomiting compared with the control group, and both SQ-LNS groups had a higher incidence of vomiting (3.3 times for SQ-LNS and 2.4 times for SQ-LNS-plus) compared with the control group (all P < 0.001). SQ-LNS and SQ-LNS-plus increased the longitudinal prevalence of rash/sores by 25% (P = 0.002) and 28% (P < 0.001), respectively, and the incidence of rash/sores by 36% and 42% (P = 0.031 and P = 0.013), respectively. A sensitivity analysis (n >710) showed that none of the symptoms was affected by outliers. For this analysis, a reasonable cutoff for the duration of each symptom was selected (fever, 30 d; coughing, 100 d; wheezing, 30 d, runny nose, 100 d; diarrhea, 43 d; vomiting, 40 d; and rash, 60 d). These cutoffs were also selected to keep the exclusion of incidences to a minimal; and as such for all sensitivity analyses, the number of cases was >710, whereas the total number of cases was 730.
TABLE 4.
Effect of SQ-LNS and SQ-LNS-plus on caretaker-reported incidence and longitudinal prevalence of infant morbidity symptoms during the total study period1
| Group2 | ||||||||
|---|---|---|---|---|---|---|---|---|
| SQ-LNS (n = 244) (child-days = 35,005) | SQ-LNS-plus (n = 242) (child-days = 36,459) | Control (n = 244) (child-days = 40,489) | Intervention effects | |||||
| Median (no. of times / days)3 | Rate or % | Median (no. of times / days)3 | Rate or % | Median (no. of times / days)3 | Rate or % | SQ-LNS | SQ-LNS plus | |
| Fever | ||||||||
| Incidence | 2 (1, 3) | 2.04 | 2 (1, 4) | 2.24 | 2 (1, 3) | 2.04 | 1.00 (0.88, 1.150)5 | 1.08 (0.94, 1.23)5 |
| Longitudinal prevalence | 4 (2, 8) | 3.26 | 5 (2, 10) | 3.56 | 5 (2, 11) | 3.96 | 0.82 (0.76, 0.88)7** | 0.90 (0.84, 0.97)7* |
| Duration per episode | 1 (1, 3) | — | 1 (1, 3) | — | 1 (1, 4) | — | — | — |
| n | 232 | 237 | 236 | — | — | |||
| Coughing | ||||||||
| Incidence | 3 (2, 5) | 3.64 | 3 (2, 5) | 3.74 | 3 (2, 5) | 3.44 | 1.05 (0.95, 1.17)5 | 1.08 (0.98, 1.20)5 |
| Longitudinal prevalence | 17 (7, 30) | 14.66 | 19 (8, 38) | 15.16 | 20 (9, 42) | 16.16 | 0.91 (0.88, 0.94)7** | 0.94 (0.91, 0.98)7* |
| Duration per episode | 3 (1, 8) | — | 5 (2, 9) | — | 5 (2, 11) | — | — | — |
| n | 203 | 197 | 214 | — | — | |||
| Wheezing | ||||||||
| Incidence | 1 (1, 1) | 0.14 | 1 (1, 1) | 0.14 | 1 (1, 2) | 0.24 | 0.43 (0.23, 0.79)5* | 0.67 (0.40, 1.13)5 |
| Longitudinal prevalence | 4 (1, 7) | 0.16 | 4 (2, 7) | 0.36 | 7 (4, 10) | 0.76 | 0.22 (0.16, 0.30)7** | 0.47 (0.38, 0.59)7** |
| Duration per episode | 3.5 (1, 5) | — | 3 (1, 7) | — | 5 (2, 7) | — | — | — |
| n | 144 | 161 | 169 | — | — | |||
| Runny nose | ||||||||
| Incidence | 2 (1, 4) | 2.64 | 2 (1, 5) | 2.74 | 3 (1, 5) | 2.74 | 0.97 (0.86, 1.09)5 | 1.00 (0.89, 1.13)5 |
| Longitudinal prevalence | 14 (6, 32) | 11.06 | 17 (5.5, 35) | 12.36 | 15 (6, 40) | 11.86 | 0.93 (0.90, 0.98)7* | 1.05 (1.00, 1.09)7* |
| Duration per episode | 4 (11, 10) | — | 5 (2, 11) | 6 (2, 9) | — | — | — | |
| n | 161 | 172 | 180 | — | — | |||
| Diarrhea | ||||||||
| Incidence | 2 (1, 3) | 2.14 | 2 (1, 4) | 2.14 | 2 (1, 3) | 1.74 | 1.25 (1.08, 1.43)5* | 1.26 (1.09, 1.44)5** |
| Longitudinal prevalence | 6 (3, 12) | 4.26 | 7 (3, 14) | 5.46 | 6 (2, 12) | 3.26 | 1.30 (1.20, 1.40)7** | 1.68 (1.57, 1.81)7** |
| Duration per episode | 2 (1, 5) | — | 2 (1, 4) | — | 2 (1, 4) | — | — | — |
| n | 154 | 149 | 148 | — | — | |||
| Vomiting | ||||||||
| Incidence | 1 (1, 2) | 0.84 | 1 (1, 2) | 0.64 | 1 (1, 2) | 0.24 | 3.26 (2.40, 4.43)5** | 2.44 (1.78, 3.36)5* |
| Longitudinal prevalence | 3 (2, 10) | 1.86 | 2 (1, 5) | 0.96 | 2 (1, 6) | 0.46 | 4.81 (4.03, 5.74)7** | 2.38 (1.94, 2.89)7** |
| Duration per episode | 2 (1, 4) | — | 1 (1, 3) | — | 1 (1, 4) | — | — | — |
| n | 86 | 76 | 39 | — | — | |||
| Rash/sores | ||||||||
| Incidence | 1 (1, 2) | 0.64 | 1 (1, 2) | 0.64 | 1 (1, 2) | 0.44 | 1.36 (1.03, 1.80)5* | 1.42 (1.08, 1.87)5* |
| Longitudinal prevalence | 6 (3, 13) | 2.46 | 7 (4, 13) | 4.26 | 8 (3, 13) | 2.16 | 1.25 (1.11, 1.40)7* | 1.28 (1.14, 1.43)7** |
| Duration per episode | 5 (2, 8) | — | 6 (3, 9) | — | 6 (2, 10) | — | — | |
| n | 77 | 77 | 61 | — | — | |||
Intervention effects for incidence and longitudinal prevalence of symptoms were estimated using Poisson regression with a log linear link, with a modified intent-to-treat analysis, excluding children with no data after enrollment and taking exposure (days in study) into account. The number of children who were ill with each symptom is indicated below each section.*P < 0.05, **P < 0.001. SQ-LNS, small-quantity lipid-based nutrient supplement.
Twenty participants with no follow-up were excluded from analysis: 6, 8, and 6 in SQ-LNS, SQ-LNS-plus, and control groups, respectively.
Values are medians and 25th and 75th percentile in parentheses (all such values).
Values are incidence rates over the 182 d of the study = (number of total episodes of a symptom during the study/child-days) × 182.
Values are incidence rate ratios; 95% CIs in parentheses (all such values).
Values are percentages: (total days with a symptom/child-days in study) × 100.
Values are prevalence rate ratios; 95% CI in parentheses (all such values).
Durations of fever, wheezing, runny nose, and diarrhea (Supplemental Figure 2A, B, D, and E) were stable with similar patterns across groups throughout the period of the study. The durations of coughing (C) episodes seemed to decrease toward the end of the study, regardless of intervention group. Vomiting (F) clustered slightly in both intervention groups at the start of the study. All 3 groups had stable durations of rash (G) over time.
Acceptability of SQ-LNSs
For mothers who completed the study, information collected by questionnaire during the exit interview showed that acceptability of the 2 SQ-LNS products did not differ. A lack of difference in acceptability was also observed in a separate acceptability study performed before the study (32). For the 2 products combined, the SQ-LNS was acceptable to 99.3% of mothers and 97.4% of infants (as perceived by the mother); 86.3% of mothers stated that the product was easy to mix with the infant's food, 6.9% had problems feeding the infant the SQ-LNS, 61.7% sometimes mixed the SQ-LNS with foods other than porridge, and 5.6% sometimes gave the daily dosage in small portions throughout the day.
Discussion
Provision of SQ-LNS-plus showed a transient positive effect on linear growth and improved locomotor development, whereas both SQ-LNS treatments reduced the likelihood of anemia, ID, and IDA. Both SQ-LNS treatments resulted in a lower longitudinal prevalence of fever, coughing, and wheezing but higher incidences and longitudinal prevalence of diarrhea, vomiting, and rash/sores.
A review of studies that evaluated the effect of provision of SQ-LNSs on child growth showed that results across studies were varied (48); most of these studies measured the change in linear growth for a longer duration (up to 18 or 24 mo of age) but less frequently (1–2 times). The different formulations of SQ-LNS may have contributed toward varying results between studies. Similar to our study, in Malawi, Mangani et al. (9) showed that provision of milk medium-quantity (MQ)-LNS, but not soy MQ-LNS, promoted linear growth in infants between 9 and 12 mo of age but not thereafter. It should be noted that SQ-LNS-plus contained 3 times more milk powder than SQ-LNS in addition to several other additional nutrients known to be important for child growth (Table 1). On the contrary, Maleta et al. (16) reported no effect of SQ-LNS on linear growth in Malawian infants from 6 to 18 mo of age, regardless of whether the SQ-LNS contained milk. Because the process of stunting may start during early development, intervening both during pregnancy and during the postnatal stage may be a more prudent approach to improve linear growth (27).
The finding that an intervention effect for SQ-LNS-plus was observed at ages 8 and 10 mo, but not at age 12 mo, is difficult to explain. It may be that the dose of the supplement (50% RNI for minerals and vitamins) was not sufficient to sustain the improved growth when children's absolute energy and nutrient requirements increased. The high prevalence of stunting at baseline could also have contributed to the limited effect observed on linear growth, particularly as SQ-LNSs are developed to prevent, rather than treat, undernutrition (49). To affect linear growth, intervention may be needed before age 6 mo. Furthermore, baseline data showed that, at age 6 mo, stunting was associated with maternal height and infant birth weight (50), indicating possible intergenerational nutritional deficits and challenges. Many closely linked and multifaceted nondietary factors affect child growth (51).
The prevalence of stunting increased in all 3 groups. Irrespective of treatment group, LAZ deteriorated steeply after 10 mo of age and may indicate a critical period in growth faltering. The 2 SQ-LNS groups had a lower baseline LAZ than the control group. It is possible that the same conditions that were associated with this lower baseline LAZ prevailed and contributed to the sharp drop in LAZ after 10 mo of age in the 2 SQ-LNS groups. The nutrients provided by the SQ-LNS could initially have counteracted a decline in LAZ, but it appears that the relatively small quantity of nutrients (50% of the RNIs) was not sufficient to sustain linear growth over the 6-mo intervention period. The Mangani et al. (9) study in Malawi, using MQ-LNSs, also showed a decline in LAZ after age 9 mo, although the decline was smaller and over a 3-mo period. Furthermore, the 2016 South African Demographic and Health Survey (52), although cross-sectional, showed a higher prevalence of stunting after age 8 mo than among infants younger than 6 mo. The nutrition needed to support optimal growth in the last quarter of the first year of life may be more challenging to achieve. Also, infections need to be prevented and managed to support optimal growth.
The positive effect of SQ-LNS-plus for locomotor development agrees with the findings of previous studies, which showed positive effects of MQ-LNS and SQ-LNS on motor development by intervening during pregnancy, lactation, and/or infancy from 6 to 18 mo or sometimes 24 mo postnatally (16, 21, 22). SQ-LNS-plus contained choline, as well as DHA and arachidonic acid, which have been linked to improved neurodevelopment in infants (53). Prado et al. (54) assessed the effect of lipid-based nutrient supplements in 4 prospective cohorts and showed that both linear growth and hemoglobin/iron status were significant predictors of language and motor development at age 18 mo.
The effects of SQ-LNSs on morbidity were in the same direction for both treatment groups compared with the control group, and <1% of AEs were classified as SAEs or as being of severe intensity. The decrease in fever and respiratory symptoms agrees with an MQ-LNS study in Chad (25). The inclusion of n–3 PUFAs in the formulation could have contributed to this positive effect in our study. Respiratory symptom improvement by n–3 PUFAs is mainly attributed to their anti-inflammatory effect through the production of anti-inflammatory lipid mediators (55).
The finding of higher incidence and longitudinal prevalence of diarrhea with both SQ-LNS treatments requires further attention. Although, in comparison with other SQ-LNS intervention studies, our study found the most pronounced increase in the incidence of diarrhea, the longitudinal prevalence (3.2%, 4.2%, and 5.4% among the 3 groups) was similar to that (between 2% and 6%) reported in other studies (8, 10, 22–25). Introduction of a new food, overreporting (study not double blind), or the increase in pathogenic bacteria in the gut due to unabsorbed iron (∼80% or ∼4 mg/d) (56) may have contributed toward the observed increased incidence of diarrhea. The incidences for diarrhea and rash/sores were similar, and it is therefore possible that the increase in rash/sores may have been related to the diarrhea, because we did not specifically assess diaper rash. Other similar or related SQ-LNS studies showed no effect on diarrhea prevalence (8, 13, 24) or could not prove noninferiority, set at 20% to the control (23). However, lipid-based nutrient supplement provision for a longer term did reduce diarrhea in older children (26). Many SQ-LNS studies in healthy children did not measure morbidity, but other iron-containing interventions, including multiple micronutrient supplement studies, have shown increases in diarrhea and respiratory infection incidence (57). Despite the increased incidence and longitudinal prevalence of vomiting in our study, there was a very low longitudinal prevalence of vomiting (0.4–1.8%), which was still lower than the 2.2–2.6% seen in Chad (25). Because fever was not also increased (mostly eliminating infectious disease), a possible reason for the observed increase in vomiting could be that the additional fat bolus of ∼8 g in infants with a fat intake of already >40% of total energy (58) may have caused gastroesophageal reflux (59, 60). Because there was no obvious morbidity symptom clustering or clusters with a longer duration between 10 and 12 mo of age (Supplemental Figure 2), it is unlikely that morbidity had a direct link with the decline in growth over this time period.
The products were well accepted by mothers, with an adherence rate of 94% in the infants. Dietary data indicated that energy and nutrient intake from the complementary diet (excluding SQ-LNSs, breast milk, and formula milk feeds) did not differ significantly across the 3 groups, at both age 6 and 12 mo, suggesting that provision of SQ-LNSs did not affect dietary intake (M Faber, unpublished data, 2018).
Strengths of the study include weekly home visits to ensure compliance and adequate stock, as well as to assess morbidity symptoms; bimonthly anthropometric measurements; all anthropometric measurements and psychomotor development assessments taken at a central site by the same set of assessors for the duration of the study, thereby reducing the interassessor variability; and strict quality assurance measures during data collection. The study made use of a combination of tools to assess child development with some overlapping in domains tested. The KDI is a culturally appropriate tool used to good effect in Africa (18, 19, 61). The initial intervention effect of growth at age 8 and 10 mo would have been missed if only baseline and endpoint measurements were taken. Frequent anthropometric measurements may therefore help us to better understand changes over time in response to different interventions and to identify the most critical age for intervening and recommend age-appropriate SQ-LNS dosage.
The 31.5% dropout rate was higher than the expected 25%, which was used for sample-size calculations based on previous studies conducted in rural areas (62, 63). Major reasons for dropping out were loss to follow-up (n = 68) and relocation (n = 63), suggesting that a peri-urban area is prone to high population mobility. Limitations in the study were that the biggest dropout occurred between 6 and 8 mo and the higher dropout rate for the 2 SQ-LNS groups compared with the control, which could have introduced bias. Also, the study was not fully blinded because of the no-supplement control group. However, cognitive assessors and laboratory staff were blinded to group assignment, and the statistician was blinded to the 2 SQ-LNS groups. Although comprehensive morbidity data were collected and supported by a daily calendar, the self-reported nature of the data was a limitation.
In conclusion, provision of SQ-LNS-plus had an overall positive effect on LAZ that was driven by improved linear growth at 8 and 10 mo (not 12 mo) and on locomotor development. Both SQ-LNS treatments reduced the likelihood for anemia, ID, and IDA and lowered the longitudinal prevalence of fever, coughing, and wheezing. Even though there was an increase in the incidence and longitudinal prevalence of vomiting, diarrhea, and rash/sores in both groups, these symptoms were mostly mild, and <1% were classified as SAEs. The early intervention effect observed (≤2 mo) in linear growth for SQ-LNS-plus highlights the advantage of regular growth monitoring, including length measurements, during this critical period of child growth. Although our results support the potential use of SQ-LNSs as a point-of-use fortificant to improve early child development and nutritional status, more context-specific evidence is needed on the optimal cost-effectiveness, composition, and the most-responsive age group and context/setting.
Supplementary Material
ACKNOWLEDGEMENTS
We thank the entire Tswaka team for executing the study, Cristiana Berti for her contribution in the planning and early phase of the study, and the administrative units of Matlosana municipality and the Department of Health and local clinics for their collaboration and support. We thank Klaus Kraemer (Sight and Life Foundation) for critically reviewing the manuscript. We also thank Manon Zeelenberg (Unilever R&D) for formulating, Sjef Dickhoff (Unilever R&D) for producing, and Budelpack BV for packing the SQ-LNS study product; and Nicolle Goetz (DSM Nutritional Products) and GC Rieber Compact India for formulating and producing the SQ-LNS-plus study product.
The authors’ responsibilities were as follows—CMS: was the principal investigator of the study, developed the study design, was overall responsible for data collection and data analysis, and had primary responsibility for the final content of the manuscript; CMS, MF, and LM: drafted the manuscript; MF: was the co-principal investigator of the study and contributed to the study design; CJL: was the study statistician and analyzed the data and interpreted statistical analysis; LM and HSK: contributed to study protocols; HSK: edited the draft manuscript; MR and TMM: were the study coordinators; MR, TMM, and KJ: helped to execute the study and edited the manuscript; JDK: was the study psychologist, contributed to study protocols, and edited the manuscript; NC: contributed to study protocols; SJMO, MJB, and LGJF: contributed to the design of the study, provided advice during study execution, and edited the manuscript; and all authors: read and approved the final manuscript. Interpretation of data was jointly discussed among the principal investigator of the study (CMS), sponsor (SJMO), and cofunders (MJB and LJGF); however, the final decisions on interpretation and dissemination of results were with the principal investigator of the study (CMS). CMS received traveling support from Unilever, DSM, and Sight and Life; TMM received speaking honorarium from DSM. SJMO is a consultant to the Global Alliance for Improved Nutrition(GAIN). GAIN was the sponsor of the study and is a nonprofit organization working in micronutrient nutrition. MJB is employed by DSM Nutritional Products, a supplier of vitamins, carotenoids, and n–3 and n–6 nutritional lipids. LGJF is employed by Unilever R&D Vlaardingen. DSM and Unilever were cofunders of the study and provided the test products free of charge. None of the other researchers had any conflicts of interest.
Notes
This study was funded by the Global Alliance for Improved Nutrition (GAIN), Geneva, Switzerland, with DSM and Unilever as cofunders. The SQ-LNS product was provided by Unilever R&D Vlaardingen BV, and the SQ-LNS-plus product was supplied by DSM Nutritional Products Ltd.
Supplemental Table 1 and Supplemental Figures 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/ajcn/.
Abbreviations used: AE, adverse event; AGP, α1-glycoprotein; CRP, C-reactive protein; HC, head circumference; ID, iron deficiency; IDA, iron-deficiency anemia; KDI, Kilifi Developmental Inventory; LAZ, length-for-age zscore; MQ, medium-quantity; MUAC, midupper arm circumference; PF, plasma ferritin; RNI, Recommended Nutrient Intake; SAE, serious adverse event; SQ-LNS, small-quantity lipid-based nutrient supplement; WAZ, weight-for-age zscore; WLZ, weight-for-length zscore.
References
- 1. Rieger M, Trommlerová SK. Age-specific correlates of child growth. Demography. 2016;53:241–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, De Onis M, Ezzati M, Grantham-McGregor S, Katz J, Martorell R. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382:427–51. [DOI] [PubMed] [Google Scholar]
- 3. Development Initiatives. Global nutrition report 2017: nourishing the SDGs. Bristol (United Kingdom): Development Initiatives; 2017. [Google Scholar]
- 4. Dewey KG. The challenge of meeting nutrient needs of infants and young children during the period of complementary feeding: an evolutionary perspective. J Nutr. 2013;143:2050–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Victora CG, de Onis M, Hallal PC, Blössner M, Shrimpton R. Worldwide timing of growth faltering: revisiting implications for interventions. Pediatrics. 2010;125:e473–80. [DOI] [PubMed] [Google Scholar]
- 6. Kassebaum NJ, Jasrasaria R, Naghavi M, Wulf SK, Johns N, Lozano R, Regan M, Weatherall D, Chou DP, Eisele TP. A systematic analysis of global anemia burden from 1990 to 2010. Blood. 2014;123:615–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Unger SA, Drammeh S, Hasan J, Ceesay K, Sinjanka E, Beyai S, Sonko B, Dondeh BL, Fulford AJ, Moore SE et al.. Impact of fortified versus unfortified lipid-based supplements on morbidity and nutritional status: a randomised double-blind placebo-controlled trial in ill Gambian children. PLoS Med. 2017;14(8):e1002377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Adu-Afarwuah S, Lartey A, Brown KH, Zlotkin S, Briend A, Dewey KG. Randomized comparison of 3 types of micronutrient supplements for home fortification of complementary foods in Ghana: effects on growth and motor development. Am J Clin Nutr. 2007;86:412–20. [DOI] [PubMed] [Google Scholar]
- 9. Mangani C, Maleta K, Phuka J, Cheung YB, Thakwalakwa C, Dewey K, Manary M, Puumalainen T, Ashorn P. Effect of complementary feeding with lipid-based nutrient supplements and corn-soy blend on the incidence of stunting and linear growth among 6- to 18-month-old infants and children in rural Malawi. Matern Child Nutr. 2013;11(S4):132–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Iannotti LL, Dulience SJ, Green J, Joseph S, Francois J, Antenor ML, Lesorogol C, Mounce J, Nickerson NM. Linear growth increased in young children in an urban slum of Haiti: a randomized controlled trial of a lipid-based nutrient supplement. Am J Clin Nutr. 2014;99:198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Christian P, Shaikh S, Shamim AA, Mehra S, Wu L, Mitra M, Ali H, Merrill RD, Choudhury N, Parveen M et al.. Effect of fortified complementary food supplementation on child growth in rural Bangladesh: A cluster-randomized trial. Int J Epidemiol. 2015;44:1862–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dewey KG, Mridha MK, Matias SL, Arnold CD, Cummins JR, Khan MSA, Maalouf-Manasseh Z, Siddiqui Z, Ullah MB, Vosti SA. Lipid-based nutrient supplementation in the first 1000 d improves child growth in Bangladesh: a cluster-randomized effectiveness trial. Am J Clin Nutr. 2017;105:944–57. [DOI] [PubMed] [Google Scholar]
- 13. Null N, Stewart CP, Pickering AJ, Dentz HN, Arnold BF, Arnold CD, Benjamin-Chung J, Clasen T, Dewey KG, Fernald LCH et al.. Effects of water quality, sanitation, handwashing, and nutritional interventions on diarrhoea and child growth in rural Kenya: a cluster-randomised controlled trial. Lancet Glob Health. 2018; 6: e316–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hess SY, Abbeddou S, Jimenez EY, Somé JW, Vosti SA. Small-quantity lipid-based nutrient supplements, regardless of their zinc content, increase growth and reduce the prevalence of stunting and wasting in young Burkinabe children: a cluster-randomized trial. PLoS ONE. 2015;10:e0122242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Phuka JC, Maleta K, Thakwalakwa C, Cheung YB, Briend A, Manary MJ, Ashorn P. Complementary feeding with fortified spread and incidence of severe stunting in 6-to 18-month-old rural Malawians. Arch Pediatr Adolesc Med. 2008;162:619–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Maleta KM, Phuka J, Alho L, Cheung YB, Dewey KG, Ashorn U, Phiri N, Phiri TE, Vosti SA, Zeilani M et al.. Provision of 10–40 g/d lipid-based nutrient supplements from 6 to 18 months of age does not prevent linear growth faltering in Malawi. J Nutr. 2015;145:1909–15. [DOI] [PubMed] [Google Scholar]
- 17. Prado EL, Abbeddou S, Yakes Jimenez E, Somé JW, Ouédraogo ZP, Vosti SA, Dewey KG, Brown KH, Hess SY, Ouedraogo JB. Lipid-based nutrient supplements plus malaria and diarrhea treatment increase infant development scores in a randomized trial in Burkina Faso. J Nutr. 2016;146:814–22. [DOI] [PubMed] [Google Scholar]
- 18. Prado EL, Adu-Afarwuah S, Lartey A, Ocansey M, Ashorn P, Vosti SA, Dewey KG. Effects of pre- and post-natal lipid-based nutrient supplements on infant development in a randomized trial in Ghana. Early Hum Dev. 2016;99:43–51. [DOI] [PubMed] [Google Scholar]
- 19. Prado EL, Phuka J, Maleta K, Ashorn P, Ashorn U, Vosti SA, Dewey KG. Provision of lipid-based nutrient supplements from age 6 to 18 months does not affect infant development scores in a randomized trial in Malawi. Matern Child Health J. 2016;10:2199–08. [DOI] [PubMed] [Google Scholar]
- 20. Mangani C, Cheung YB, Maleta K, Phuka J, Thakwalakwa C, Dewey K, Manary M, Puumalainen T, Ashorn P. Providing lipid-based nutrient supplements does not affect developmental milestones among Malawian children. Acta Paediatr. 2014;103:e17–26. [DOI] [PubMed] [Google Scholar]
- 21. Prado EL, Maleta K, Ashorn P, Ashorn U, Vosti SA, Sadalaki J, Dewey KG. Effects of maternal and child lipid-based nutrient supplements on infant development: a randomized trial in Malawi. Am J Clin Nutr. 2016;103:784–93. [DOI] [PubMed] [Google Scholar]
- 22. Matias SL, Mridha MK, Tofail F, Arnold CD, Khan MS, Siddiqui Z, Ullah MB, Dewey KG. Home fortification during the first 1000 d improves child development in Bangladesh: A cluster-randomized effectiveness trial. Am J Clin Nutr. 2017;105:958–69. [DOI] [PubMed] [Google Scholar]
- 23. Mangani C, Ashorn P, Maleta K, Phuka J, Thakwalakwa C, Dewey K, Manary M, Puumalainen T, Cheung YB. Lipid-based nutrient supplements do not affect the risk of malaria or respiratory morbidity in 6-to 18-month-old Malawian children in a randomized controlled trial. Am J Clin Nutr. 2014;144:1835–42. [DOI] [PubMed] [Google Scholar]
- 24. Bendabenda J, Alho L, Ashorn U, Cheung Y, Dewey K, Vosti S, Phuka J, Maleta K, Ashorn P. The effect of providing lipid-based nutrient supplements on morbidity in rural Malawian infants and young children: a randomized controlled trial. Public Health Nutr. 2016;19:1893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huybregts L, Houngbé F, Salpéteur C, Brown R, Roberfroid D, Ait-Aissa M, Kolsteren P. The effect of adding ready-to-use supplementary food to a general food distribution on child nutritional status and morbidity: a cluster-randomized controlled trial. PLoS Med. 2012;9:e1001313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Luby SP, Rahman M, Arnold BF, Unicomb L, Ashraf S, Winch PJ, Stewart CP, Begum F, Hussain F, Benjamin-Chung J et al.. Effects of water quality, sanitation, handwashing, and nutritional interventions on diarrhoea and child growth in rural Bangladesh: a cluster randomised controlled trial. Lancet Glob Health. 2018;6:e302–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Adu-Afarwuah S, Lartey A, Okronipa H, Ashorn P, Peerson JM, Arimond M, Ashorn U, Zeilani M, Vosti S, Dewey KG. Small-quantity, lipid-based nutrient supplements provided to women during pregnancy and 6 mo postpartum and to their infants from 6 mo of age increase the mean attained length of 18-mo-old children in semi-urban Ghana: a randomized controlled trial, 2. Am J Clin Nutr. 2016;104:797–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Troesch B, Jing H, Laillou A, Fowler A. Absorption studies show that phytase from Aspergillus niger significantly increases iron and zinc bioavailability from phytate-rich foods. Food Nutr Bull. 2013;34(2 Suppl):S90–101. [DOI] [PubMed] [Google Scholar]
- 29. Makrides M, Neumann MA, Byard RW, Simmer K, Gibson RA. Fatty acid composition of brain, retina, and erythrocytes in breast- and formula-fed infants. Am J Clin Nutr. 1994;60:189–94. [DOI] [PubMed] [Google Scholar]
- 30. Troesch B, van Stuijvenberg ME, Smuts CM, Kruger HS, Biebinger R, Hurrell RF, Baumgartner J, Zimmermann MB. A micronutrient powder with low doses of highly absorbable iron and zinc reduces iron and zinc deficiency and improves weight-for-age Z-scores in South African children. J Nutr. 2011;141(2):237–42. Erratum in: J Nutr 2011;141(4):718. [DOI] [PubMed] [Google Scholar]
- 31. Cohen J. Statistical power analysis in the behavioral sciences. 2nd ed Hillsdale (NJ): Lawrence Erlbaum; 1988. [Google Scholar]
- 32. Rothman M, Berti C, Smuts CM, Faber M, Covic N. Acceptability of novel small-quantity lipid-based nutrient supplements for complementary feeding in a peri-urban South African community. Food Nutr Bull. 2015;36:455–66. [DOI] [PubMed] [Google Scholar]
- 33. World Health Organization Multicenter Growth Reference Study Group. WHO child growth standards based on length/height, weight and age. Geneva (Switzerland): World Health Organization; 2006. [Google Scholar]
- 34. World Health Organization. Physical status: the use of and interpretation of anthropometry, report of a WHO Expert Committee. Geneva (Switzerland): World Health Organization; 1995. [PubMed] [Google Scholar]
- 35. World Health Organization. Training course on child growth assessment. Geneva (Switzerland): World Health Organization; 2008. [Google Scholar]
- 36. Berger SE, Adolph KE. Learning and development in infant locomotion. Prog Brain Res. 2007;164:237–55. [DOI] [PubMed] [Google Scholar]
- 37. Neggers Y. Maternal prepregnancy body mass index and psychomotor development in children. Acta Obstet. 2003;82:235–40. [DOI] [PubMed] [Google Scholar]
- 38. Abubakar A, Holding P, Van Baar A, Newton CRJC, Van de Vijver FJR. Monitoring psychomotor development in a resource-limited setting: an evaluation of the Kilifi Developmental Inventory. Ann Trop Paediatr. 2008;28:217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kitsao-Wekulo P, Holding P, Abubakar A, Kvalsvig J, Taylor HG, King CL. Describing normal development in an African setting: the utility of the Kilifi Developmental Inventory among young children at the Kenyan coast. Learn Individ Differ. 2016;46:3–10. [Google Scholar]
- 40. Kvalsvig JD, Govender K, Taylor M. Research on the age validation of NELDS related to the cognitive development of children between 0 and 4 years of ages. Report to UNICEF and the Department of Education; 2009. [cited 2017 Jan]. Available from: http://www.education.gpg.gov.za/Documents/NELDS guidelines 8 Jan.pdf. [Google Scholar]
- 41. Erhardt JG, Estes JE, Pfeiffer CM, Biesalski HK, Craft NE. Combined measurement of ferritin, soluble transferrin receptor, retinol binding protein, and C-reactive protein by an inexpensive, sensitive, and simple sandwich enzyme-linked immunosorbent assay technique. J Nutr. 2004;134:3127–32. [DOI] [PubMed] [Google Scholar]
- 42. Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. New Engl J Med. 1999;340:448–54. [DOI] [PubMed] [Google Scholar]
- 43. World Health Organization. Serum ferritin concentrations for the assessment of iron status and iron deficiency in populations. Geneva (Switzerland): World Health Organization; 2011. [Google Scholar]
- 44. World Health Organization. Haemoglobin concentrations for the diagnosis of anaemia and assessment of severity. Vitamin and mineral nutrition information system, 2011. [cited 2017 Jan]. Available from: http://www.who.int/vmnis/indicators/haemoglobin.pdf. [Google Scholar]
- 45. Thurnham DI, Northrop-Clewes CA, Knowles J. The use of adjustment factors to address the impact of inflammation on vitamin A and iron status in humans. J Nutr. 2015;145:1137S–43S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Goldman N, Vaughan B, Pebley AR. The use of calendars to measure child illness in health interview surveys. Int J Epidemiol. 1998;27: 505–12. [DOI] [PubMed] [Google Scholar]
- 47. Tilling K, Macdonald-Wallis C, Lawlor DA, Hughes RA, Howe LD. Modelling childhood growth using fractional polynomials and linear splines. Ann Nutr Metab. 2014;65:129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Matsungo TM, Smuts CM, Kruger HS, Faber M. Lipid-based nutrient supplements and linear growth in children under 2 years: a review. Proc Nutr Soc. 2017;76(4):580–8. [DOI] [PubMed] [Google Scholar]
- 49. Dewey KG, Arimond M. Lipid-based nutrient supplements: how can they combat child malnutrition?. PLoS Med. 2012;9:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Matsungo TM, Kruger HS, Faber M, Rothman M, Smuts CM. The prevalence and factors associated with stunting among infants aged 6 months in a peri-urban South African community. Public Health Nutr. 2017;20:3209–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. United Nations Children's Fund. Improving child nutrition. The achievable imperative for global process. New York: UNICEF; 2013. [Google Scholar]
- 52. Stats SA; South African Medical Research Council (SAMRC); ICF. South Africa Demographic and Health Survey 2016: key indicators. Pretoria (South Africa): NDoH, Stats SA, SAMRC, and ICF; 2017. [Google Scholar]
- 53. Colombo J, Carlson SE, Cheatham CL, Fitzgerald-Gustafson KM, Kepler A, Doty T. Long-chain polyunsaturated fatty acid supplementation in infancy reduces heart rate and positively affects distribution of attention. Pediatr Res. 2011;70:406–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Prado EL, Abbeddou S, Adu-Afarwuah S, Arimond M, Ashorn P, Ashorn U, Bendabenda J, Brown KH, Hess SY, Kortekangas E et al.. Predictors and pathways of language and motor development in four prospective cohorts of young children in Ghana, Malawi, and Burkina Faso. J Child Psychol Psychiatry. 2017;58(11):1264–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Calder PC, Willemsen LE. Immunopharmacology of fatty acids. Eur J Pharmacol. 2016;785:1. [DOI] [PubMed] [Google Scholar]
- 56. Paganini D, Uyoga MA, Cercamondi CI, Moretti D, Mwasi E, Schwab C, Bechtler S, Mutuku FM, Galetti V, Lacroix C et al.. Consumption of galacto-oligosaccharides increases iron absorption from a micronutrient powder containing ferrous fumarate and sodium iron EDTA: a stable-isotope study in Kenyan infants. Am J Clin Nutr. 2017;106:1020–31. [DOI] [PubMed] [Google Scholar]
- 57. Soofi S, Cousens S, Iqbal SP, Akhund T, Khan J, Ahmed I, Zaidi AK, Bhutta ZA. Effect of provision of daily zinc and iron with several micronutrients on growth and morbidity among young children in Pakistan: a cluster-randomised trial. Lancet. 2013;382:29–40. [DOI] [PubMed] [Google Scholar]
- 58. Rothman AMP. Nutritional status, feeding practices and motor development of 6-month-old infants [dissertation]. Potchefstroom (South Africa): North-West University; 2015. [Google Scholar]
- 59. Mahan KL, Escott-Stump S. Krause's food nutrition and diet therapy. 13th ed Philadelphia: Saunders Elsevier; 2012. [Google Scholar]
- 60. Czinn SJ, Blanchard S. Gastroesophageal reflux disease in neonates and infants. Pediatr Drugs. 2013;15:19–27. [DOI] [PubMed] [Google Scholar]
- 61. Kvalsvig J, Holding P, Taylor M, Chhagan M, Kitsao-Wekulo P, Zulu S, Mwangome F, Jaeggi T, Kauchali S, Brouwer I. Instapa WP6: the effects of micronutrient supplementation with reduced iron content on motor development in infants in a malarial area. Ann Nutr Metab Conference: 431; 2013:doi:http://dx.doi.org/10.1159/000354245. [Google Scholar]
- 62. Smuts CM, Dhansay MA, Faber M, van Stuijvenberg ME, Swanevelder S, Gross R, Benadé AJS. Efficacy of multiple micronutrient supplementation for improving anemia, micronutrient status, and growth in South African infants. J Nutr. 2005;135:653S–9S. [DOI] [PubMed] [Google Scholar]
- 63. Faber M, Kvalsvig JD, Lombard CJ, Benadé AJS. Effect of a fortified maize-meal porridge on anemia, micronutrient status, and motor development of infants. Am J Clin Nutr. 2005;82:1032–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.