Abstract
Background
Although periodic cardiac stress testing is commonly used to screen patients on the waiting list for kidney transplantation for ischemic heart disease, there is little evidence to support this practice. We hypothesized that cardiac stress testing in the 18 months prior to kidney transplantation would not reduce postoperative death, total myocardial infarction (MI) or fatal MI.
Methods
Using the United States Renal Data System, we identified ESRD patients ≥40 years old with primary Medicare insurance who received their first kidney transplant between 7/1/2006 and 11/31/2013. Propensity matching created a 1:1 matched sample of patients with and without stress testing in the 18 months prior to kidney transplantation. The outcomes of interest were death, total (fatal and nonfatal) MI or fatal MI within 30 days of kidney transplantation.
Results
In the propensity-matched cohort of 17,304 patients, death within 30 days occurred in 72 of 8,652 (0.83%) patients who underwent stress testing and in 65 of 8,652 (0.75%) patients who did not (OR 1.07; 95% CI: 0.79–1.45; P = 0.66). MI within 30 days occurred in 339 (3.9%) patients who had a stress test and in 333 (3.8%) patients who did not (OR 1.03; 95% CI: 0.89–1.21; P = 0.68). Fatal MI occurred in 17 (0.20%) patients who underwent stress testing and 15 (0.17%) patients who did not (OR 0.97; 95% CI: 0.71–1.32; P = 0.84).
Conclusion
Stress testing in the 18 months prior to kidney transplantation is not associated with a reduction in death, total MI or fatal MI within 30 days of kidney transplantation.
Introduction
Cardiovascular disease is a major cause of morbidity and mortality for patients with end stage renal disease (ESRD) on the waiting list for kidney transplantation and is the leading cause of death after kidney transplantation [1–5]. Thus, reducing cardiovascular mortality after kidney transplantation is critically important as donor kidneys are a limited resource that should not be allocated to patients who are at high risk of potentially fatal perioperative cardiovascular events.
Because patients with ESRD have higher rates of cardiovascular disease than the general population, especially ischemic heart disease, screening for ischemia prior to placing patients on the transplant waiting list is performed on most adult patients [6]. Patients without ischemia and other contraindications to transplant are placed on the waiting list. Patients with ischemia are commonly referred for coronary angiography and, if obstructive coronary artery disease is present, undergo revascularization with percutaneous coronary intervention (PCI) or coronary artery bypass graft surgery (CABG) after which they are placed on the waiting list. Patients with extensive disease that is not amenable to revascularization are usually excluded from consideration of transplant and never placed on the waiting list [7].
Since the timing of donor availability and transplant surgery is unpredictable, asymptomatic patients with ESRD who have already been screened for ischemia prior to placement on the waiting list commonly undergo stress testing [6] to identify and revascularize those who have developed ischemia since their initial screening for transplant candidacy. One common strategy is to perform annual stress tests while the patient is on the waiting list. However, the lack of clinical evidence supporting subsequent stress testing in patients already evaluated for ischemia prior to placement on the waiting list for kidney transplant has led to varying consensus recommendations and considerable practice variation across transplant centers [8]. Given that in other clinical settings there is no evidence that assessing for ischemia or revascularization in asymptomatic patients pre-operatively improves post-operative outcomes, we hypothesized that surveillance of patients with ESRD who are on the waiting list for kidney transplantation with cardiac stress testing in the 18 months prior to surgery would not be associated with a reduction in perioperative (30-day) death, total (fatal and nonfatal) myocardial infarction (MI) or fatal MI.
Methods
Data source
Data on patients undergoing renal transplantation were obtained from the USRDS (United States Renal Data System) database. The USRDS is a comprehensive data set that includes information on all patients in the United States who develop ESRD and who require renal replacement therapy, either dialysis or renal transplantation. The USRDS is funded by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). The USRDS includes International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) diagnosis and procedure codes, and Current Procedural Terminology, 4th Edition (CPT-4) codes for Medicare services and transplant information from the United Network for Organ Sharing (UNOS).
Study population
The study population included ESRD patients ≥40 years of age who underwent their first kidney transplant between 7/1/2006 and 11/31/2013. The study population was limited to patients who were insured by Medicare parts A and B for at least 18 months prior to kidney transplantation. Patients were excluded if no claims were made in the 18 months prior to transplant surgery. Stress tests were identified from ICD-9-CM or CPT-4 codes and included exercise and pharmacologic stress. To eliminate patients who may have undergone coronary angiography instead of stress testing as well as those with active ischemic heart disease, individuals were excluded if coronary angiography, PCI or CABG without stress testing or prior to stress testing were performed in the 18 months prior to transplantation.
Baseline characteristics including age, sex, height, weight, race and comorbidites were recorded for the entire study population. Comorbidities were recorded using the Elixhauser comorbidity classification which is a method of categorizing comorbidities based on ICD-9-CM codes found in administrative data [9]. Missing height and weight data (each <2%) were imputed using a sequential imputation algorithm. A regression predictive mean matching method was employed; all available data were used when developing regression models for height and weight imputation.
Primary outcomes of interest included death from any cause within 30 days following renal transplant surgery, total MI (fatal and nonfatal) within 30 days of surgery or fatal MI within 30 days of surgery. MI was defined by ICD-9-CM codes 410.xx. Fatal MI was defined by an MI following surgery and death within 30 days of the MI.
Statistics
Comparisons of patient characteristics between those with and without stress testing were performed using Student’s two sample t-test for continuous variables and chi-square test for categorical data. All non-normal and ordinal data were summarized by median (1st quartile, 3rd quartile) and compared using Mann-Whitney U-test. A logistic regression model was created to identify the propensity for stress testing using the following variables: age, sex, transplant year, race, body mass index (BMI), donor type, primary disease for ESRD, chronic heart failure, heart valve disease, pulmonary circulation disease, peripheral vascular disease, paralysis, other neurological disorders, chronic lung disease, diabetes, hypertension, hyperthyroidism, liver disease, peptic ulcer disease, acquired immune deficiency syndrome, lymphoma, metastatic cancer, solid tumor without metastasis, rheumatoid arthritis, coagulopathy, fluid and electrolyte disorders, chronic blood loss anemia, deficiency anemias, psychoses, depression, history of MI, CAD, pericardial disease, endocarditis, cardiomyopathy, cerebrovascular disease, drug abuse, alcohol abuse, weight loss, arrhythmia, cardiac arrest/ventricular fibrillation, tobacco use, lipid metabolism disorder, and transplant center volume. A Greedy matching algorithm was then used to create a 1:1 propensity score-matched sample. Standardized differences were calculated to examine covariate balance before and after matching.
Logistic regression analysis was performed to analyze the association between stress testing and the outcomes of MI, death and fatal MI in the 30-day postoperative period for the total sample and for the propensity score-matched sample. For the total sample, multivariable models were created that included all the same variables used to build the propensity score as independent adjustment variables. For the propensity score matched sample, a mixed model approach was used to account for the matching. Results from both the propensity score matching and multivariable regression analyses are provided separately. All models treated center as a random effect to account for practice variation across transplant facilities. All analyses were performed using SAS version 9.3. Two authors (MJS, EN) had full access to the data and assume responsibility for the integrity of the data and analyses performed.
Results
Study population
A total of 86,837 patients were eligible for analysis (Fig 1). 37,573 patients were excluded for having insurance other than Medicare parts A and B and 21,448 patients were excluded for not having Medicare parts A and B coverage for the entire 18 months prior to transplant. Twenty-five patients were excluded for not having any insurance claim in the 18 months prior to transplant. An additional 2,068 patients who did not undergo a stress test but who underwent angiography, PCI, or CABG in the 18 months prior to transplant were excluded, as were 905 patients who underwent angiography, PCI or CABG prior to their first stress test. After exclusions, the analytic sample included 24,818 patients of whom 14,811 (59.7%) underwent a stress test in the 18 months prior to kidney transplantation and 10,007 (39.3%) did not.
Fig 1. Flow diagram of study population.
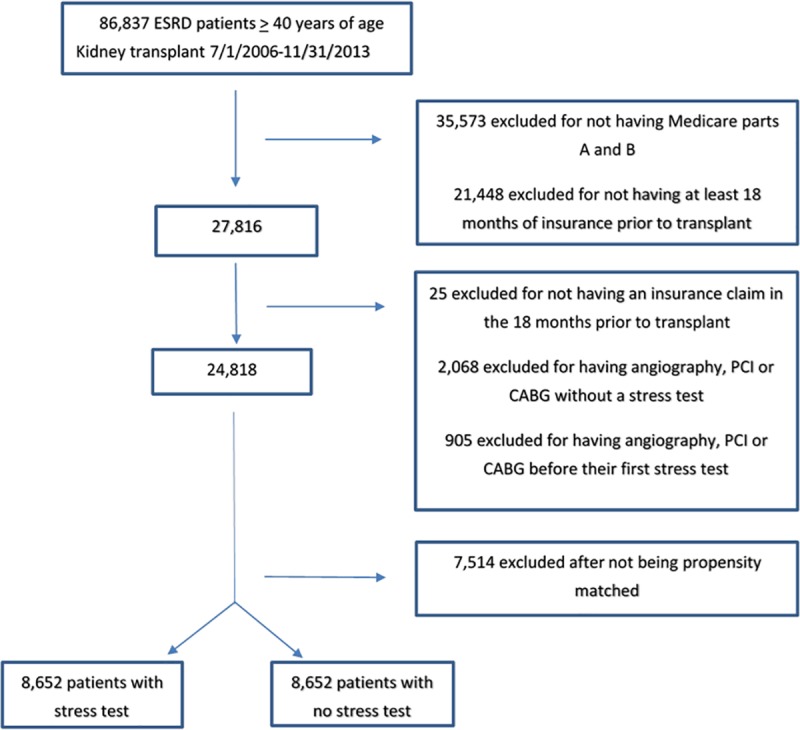
ESRD, end stage renal disease; PCI, percutaneous coronary intervention; CABG, coronary artery bypass graft surgery.
Baseline patient characteristics before matching are presented in Table 1. Patients who underwent stress testing were older (58.3. vs. 56 years, P<0.001), less often female (37.6% vs. 39%, P = 0.029), more often white (57.4% vs. 53.6%, P<0.001), and more often received their transplanted kidney from a living donor (12% vs. 9.5%, P<0.001). Patients who underwent stress testing had more risk factors for ischemic heart disease including diabetes (57.2% vs. 47.1%, P<0.001), hypertension (91.6% vs. 81.6%, P<0.001), tobacco use (19.9% vs. 16%, P<0.001) and dyslipidemia (64.5% vs. 52.2%, P<0.001). Cardiovascular disease was more common in patients who underwent stress testing including prior MI (7.2% vs. 2.5%, P<0.001), CAD (42.5% vs. 16.6%, P<0.001), chronic heart failure (24.9% vs. 15.2%, P<0.001), valvular disease (16.2% vs. 6.2%, P<0.001), arrhythmia (35.7% vs. 21.8%, P<0.001), peripheral vascular disease (25.8% vs. 16.2%, P<0.001) and cerebrovascular disease (11.2% vs. 6.6%, P<0.001).
Table 1. Patient characteristics before propensity matching.
| Overall (N = 24818) |
No Stress Test (N = 10007) |
Stress Test (N = 14811) |
P value | ||||
|---|---|---|---|---|---|---|---|
| Transplant year, No. (%) | < .001 | ||||||
| 2006 | 1577 | (6.4%) | 593 | (5.9%) | 984 | (6.6%) | |
| 2007 | 3125 | (12.6%) | 1200 | (12.0%) | 1925 | (13.0%) | |
| 2008 | 3183 | (12.8%) | 1237 | (12.4%) | 1946 | (13.1%) | |
| 2009 | 3335 | (13.4%) | 1294 | (12.9%) | 2041 | (13.8%) | |
| 2010 | 3346 | (13.5%) | 1354 | (13.5%) | 1992 | (13.4%) | |
| 2011 | 3496 | (14.1%) | 1454 | (14.5%) | 2042 | (13.8%) | |
| 2012 | 3391 | (13.7%) | 1411 | (14.1%) | 1980 | (13.4%) | |
| 2013 | 3365 | (13.6%) | 1464 | (14.6%) | 1901 | (12.8%) | |
| Recipient Age at Transplant Time | 57.34 | ± 9.7 | 56.00 | ± 9.7 | 58.25 | ± 9.6 | < .001 |
| Female, No. (%) | 9469 | (38.2%) | 3900 | (39.0%) | 5569 | (37.6%) | 0.029 |
| Race, No. (%) | < .001 | ||||||
| Black | 8929 | (36.0%) | 3833 | (38.3%) | 5096 | (34.4%) | |
| White | 13859 | (55.8%) | 5360 | (53.6%) | 8499 | (57.4%) | |
| Other/Multi-racial | 2030 | (8.2%) | 814 | (8.1%) | 1216 | (8.2%) | |
| Height (m) | 1.70 | ± 0.1 | 1.70 | ± 0.1 | 1.70 | ± 0.1 | 0.93 |
| Weight (kg) | 81.48 | ± 18.3 | 81.37 | ± 18.4 | 81.55 | ± 18.2 | 0.46 |
| Body mass index | 28.14 | ± 5.4 | 28.10 | ± 5.4 | 28.17 | ± 5.4 | 0.34 |
| Transplant hospitalization duration in days, median (1st quartile, 3rd quartile) | 6.0 | (0.0, 522.0) | 6.0 | (0.0, 522.0) | 6.0 | (0.0, 505.0) | < .001 |
| Donor type, No. (%) | < .001 | ||||||
| Cadaveric/Unknown | 22086 | (89.0%) | 9056 | (90.5%) | 13030 | (88.0%) | |
| Living | 2732 | (11.0%) | 951 | (9.5%) | 1781 | (12.0%) | |
| Number of transplants performed at facility, median (1st quartile, 3rd quartile) | 107.0 | (1.0, 385.0) | 100.0 | (1.0, 385.0) | 113.0 | (1.0, 385.0) | < .001 |
| Primary cause of end stage renal disease, No. (%) | < .001 | ||||||
| Diabetes | 8821 | (35.5%) | 3091 | (30.9%) | 5730 | (38.7%) | |
| Hypertension | 6772 | (27.3%) | 2893 | (28.9%) | 3879 | (26.2%) | |
| Glomerulonephritis | 4635 | (18.7%) | 2060 | (20.6%) | 2575 | (17.4%) | |
| Cystic kidney/Other urologic | 2053 | (8.3%) | 875 | (8.7%) | 1178 | (8.0%) | |
| Other | 2537 | (10.2%) | 1088 | (10.9%) | 1449 | (9.8%) | |
| Risk Factors | |||||||
| Diabetes, No. (%) | 13187 | (53.1%) | 4709 | (47.1%) | 8478 | (57.2%) | < .001 |
| Hypertension, No. (%) | 21634 | (87.2%) | 8062 | (80.6%) | 13572 | (91.6%) | < .001 |
| Disorders of lipid metabolism, No. (%) | 14779 | (59.5%) | 5228 | (52.2%) | 9551 | (64.5%) | < .001 |
| Tobacco use, No. (%) | 4552 | (18.3%) | 1600 | (16.0%) | 2952 | (19.9%) | < .001 |
| Cardiovascular History | |||||||
| Coronary artery disease, No. (%) | 7960 | (32.1%) | 1660 | (16.6%) | 6300 | (42.5%) | < .001 |
| History of myocardial infarction, No. (%) | 1318 | (5.3%) | 253 | (2.5%) | 1065 | (7.2%) | < .001 |
| Congestive heart failure, No. (%) | 5209 | (21.0%) | 1517 | (15.2%) | 3692 | (24.9%) | < .001 |
| Cardiomyopathy, No. (%) | 1682 | (6.8%) | 425 | (4.2%) | 1257 | (8.5%) | < .001 |
| Valvular disease, No. (%) | 3018 | (12.2%) | 621 | (6.2%) | 2397 | (16.2%) | < .001 |
| Arrhythmia/Conduction disease, No. (%) | 7479 | (30.1%) | 2186 | (21.8%) | 5293 | (35.7%) | < .001 |
| Cardiac arrest/Ventricular fibrillation, No. (%) | 243 | (1.0%) | 62 | (0.6%) | 181 | (1.2%) | < .001 |
| Pericardial disease, No. (%) | 475 | (1.9%) | 138 | (1.4%) | 337 | (2.3%) | < .001 |
| Peripheral vascular disease, No. (%) | 5437 | (21.9%) | 1618 | (16.2%) | 3819 | (25.8%) | < .001 |
| Cerebrovascular disease, No. (%) | 2311 | (9.3%) | 658 | (6.6%) | 1653 | (11.2%) | < .001 |
| Comorbidities | |||||||
| Chronic pulmonary disease, No. (%) | 3276 | (13.2%) | 1119 | (11.2%) | 2157 | (14.6%) | < .001 |
| Liver disease, No. (%) | 2012 | (8.1%) | 664 | (6.6%) | 1348 | (9.1%) | < .001 |
| Hypothyroidism, No. (%) | 3496 | (14.1%) | 1263 | (12.6%) | 2233 | (15.1%) | < .001 |
Outcomes
Of the 14,811 patients who underwent a stress test, death within 30 days after transplant occurred in 166 (1.1%) patients who underwent a stress test and in 74 (0.7%) of 10,007 patients who did not undergo a stress test (P = 0.003). MI within 30 days occurred in 812 (5.5%) of 14,811 patients who underwent stress testing and in 355 (3.5%) of the 10,007 patients who did not undergo a stress test (P<0.001). On multivariable analysis, stress testing prior to kidney transplantation was not associated with a reduced risk of death (OR, 1.21; 95% CI: 0.90–1.62; P = 0.21), MI (OR, 1.09; 95% CI: 0.94–1.26; P = 0.25) or total MI (OR, 1.10; 95% CI: 0.61–2.00; P = 0.74).
Propensity-matched patients
A total of 17,304 patients were propensity-matched in a 1:1 ratio. Baseline patient characteristics after matching are presented in Table 2. Propensity matching eliminated differences in demographics, risk factors and comorbidities between groups (Fig 2). Of propensity-matched patients who underwent stress testing, 1,149 (13.3%) subsequently underwent coronary angiography, 145 (1.7%) underwent PCI and 35 (0.4%) underwent CABG.
Table 2. Patient characteristics after propensity score matching.
| Overall (N = 17304) |
No Stress Test (N = 8652) |
Stress Test (N = 8652) |
P value | ||||
|---|---|---|---|---|---|---|---|
| Transplant year, No. (%) | 0.45 | ||||||
| 2006 | 1045 | (6.0%) | 534 | (6.2%) | 511 | (5.9%) | |
| 2007 | 2085 | (12.0%) | 1064 | (12.3%) | 1021 | (11.8%) | |
| 2008 | 2172 | (12.6%) | 1092 | (12.6%) | 1080 | (12.5%) | |
| 2009 | 2232 | (12.9%) | 1116 | (12.9%) | 1116 | (12.9%) | |
| 2010 | 2366 | (13.7%) | 1173 | (13.6%) | 1193 | (13.8%) | |
| 2011 | 2502 | (14.5%) | 1243 | (14.4%) | 1259 | (14.6%) | |
| 2012 | 2422 | (14.0%) | 1198 | (13.8%) | 1224 | (14.1%) | |
| 2013 | 2480 | (14.3%) | 1232 | (14.2%) | 1248 | (14.4%) | |
| Recipient Age at Transplant Time | 56.58 | ± 9.5 | 56.59 | ± 9.6 | 56.57 | ± 9.5 | 0.89 |
| Female, No. (%) | 6846 | (39.6%) | 3440 | (39.8%) | 3406 | (39.4%) | 0.59 |
| Race, No. (%) | 0.16 | ||||||
| Black | 6526 | (37.7%) | 3221 | (37.2%) | 3305 | (38.2%) | |
| White | 9384 | (54.2%) | 4711 | (54.4%) | 4673 | (54.0%) | |
| Other/Multi-racial | 1394 | (8.1%) | 720 | (8.3%) | 674 | (7.8%) | |
| Height (m) | 1.70 | ± 0.1 | 1.70 | ± 0.1 | 1.70 | ± 0.1 | 0.91 |
| Weight (kg) | 81.59 | ± 18.4 | 81.53 | ± 18.3 | 81.65 | ± 18.6 | 0.65 |
| Body mass index | 28.21 | ± 5.4 | 28.19 | ± 5.4 | 28.22 | ± 5.4 | 0.73 |
| Transplant hospitalization duration in days, median (1st quartile, 3rd quartile) | 6.0 | (0.0, 522.0) | 6.0 | (0.0, 522.0) | 6.0 | (0.0, 258.0) | 0.69 |
| Donor type, No. (%) | 0.49 | ||||||
| Cadaveric/Unknown | 15595 | (90.1%) | 7784 | (90.0%) | 7811 | (90.3%) | |
| Living | 1709 | (9.9%) | 868 | (10.0%) | 841 | (9.7%) | |
| Number of transplants performed at facility, median (1st quartile, 3rd quartile) | 106.0 | (1.0, 385.0) | 103.0 | (1.0, 385.0) | 108.0 | (1.0, 385.0) | 0.22 |
| Primary cause of end stage renal disease, No. (%) | 0.86 | ||||||
| Diabetes | 5767 | (33.3%) | 2864 | (33.1%) | 2903 | (33.6%) | |
| Hypertension | 4889 | (28.3%) | 2440 | (28.2%) | 2449 | (28.3%) | |
| Glomerulonephritis | 3333 | (19.3%) | 1679 | (19.4%) | 1654 | (19.1%) | |
| Cystic kidney/Other urologic | 1503 | (8.7%) | 763 | (8.8%) | 740 | (8.6%) | |
| Other | 1812 | (10.5%) | 906 | (10.5%) | 906 | (10.5%) | |
| Risk Factors | |||||||
| Diabetes, No. (%) | 8758 | (50.6%) | 4367 | (50.5%) | 4391 | (50.8%) | 0.70 |
| Hypertension, No. (%) | 15072 | (87.1%) | 7535 | (87.1%) | 7537 | (87.1%) | 0.95 |
| Disorders of lipid metabolism, No. (%) | 9739 | (56.3%) | 4847 | (56.0%) | 4892 | (56.5%) | 0.46 |
| Tobacco use, No. (%) | 3010 | (17.4%) | 1483 | (17.1%) | 1527 | (17.6%) | 0.37 |
| Cardiovascular History | |||||||
| Coronary artery disease, No. (%) | 3365 | (19.4%) | 1659 | (19.2%) | 1706 | (19.7%) | 0.07 |
| History of myocardial infarction, No. (%) | 534 | (3.1%) | 251 | (2.9%) | 283 | (3.3%) | 0.13 |
| Congestive heart failure, No. (%) | 2947 | (17.0%) | 1459 | (16.9%) | 1488 | (17.2%) | 0.54 |
| Cardiomyopathy, No. (%) | 834 | (4.8%) | 411 | (4.8%) | 423 | (4.9%) | 0.67 |
| Valvular disease, No. (%) | 1253 | (7.2%) | 618 | (7.1%) | 635 | (7.3%) | 0.56 |
| Arrhythmia/Conduction disease, No. (%) | 4266 | (24.7%) | 2142 | (24.8%) | 2124 | (24.5%) | 0.72 |
| Cardiac arrest/Ventricular fibrillation, No. (%) | 127 | (0.7%) | 60 | (0.7%) | 67 | (0.8%) | 0.53 |
| Pericardial disease, No. (%) | 271 | (1.6%) | 136 | (1.6%) | 135 | (1.6%) | 0.95 |
| Peripheral vascular disease, No. (%) | 3117 | (18.0%) | 1562 | (18.1%) | 1555 | (18.0%) | 0.88 |
| Cerebrovascular disease, No. (%) | 1265 | (7.3%) | 634 | (7.3%) | 631 | (7.3%) | 0.93 |
| Comorbidities | |||||||
| Chronic pulmonary disease, No. (%) | 2078 | (12.0%) | 1038 | (12.0%) | 1040 | (12.0%) | 0.96 |
| Liver disease, No. (%) | 1259 | (7.3%) | 627 | (7.2%) | 632 | (7.3%) | 0.88 |
| Hypothyroidism, No. (%) | 2325 | (13.4%) | 1166 | (13.5%) | 1159 | (13.4%) | 0.88 |
Fig 2. Covariate balance before and after propensity score matching.
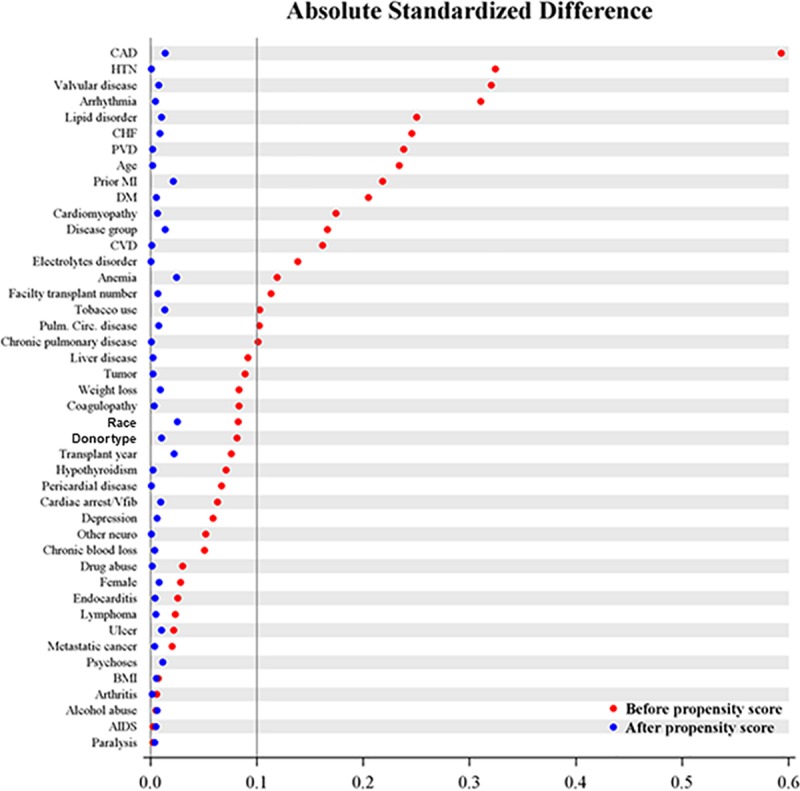
CAD, coronary artery disease; CHF, congestive heart failure; PVC, peripheral vascular disease; MI, myocardial infarction; DM, diabetes mellitus; CVD, cerebrovascular disease; Circ, circulation; VF, ventricular fibrillation; BMI, body mass index; AIDS, acquired immunodeficiency syndrome.
Outcomes for the propensity-matched cohort are presented in Table 3. Death within 30 days occurred in 72 (0.83%) patients who had a stress test and in 65 (0.75%) patients who did not have a stress test (OR 1.07; 95% CI: 0.79–1.45; P = 0.66). MI within 30 days occurred in 339 (3.9%) patients who had a stress test and in 333 (3.8%) patients who did not (OR 1.03; 95% CI: 0.89–1.21; P = 0.68). Fatal MI occurred in 17 patients who underwent a stress test (0.20%) and in 15 patients who did not (0.17%) (OR 0.968; 95% CI: 0.71–1.32; P = 0.84). In total, of the 672 patients who developed an MI, 32 (4.8%) subsequently died within 30 days. Of the 137 patients who died within 30 days of their transplant, only 32 (23%) had suffered an MI.
Table 3. Outcomes in propensity-matched cohort with and without stress testing.
| Outcome | Stress Test (N = 8652) |
No Stress Test (N = 8652) |
Odds Ratio (stress vs. no stress) | 95% Confidence Interval | P value |
|---|---|---|---|---|---|
| Death within 30 days after transplant | 72 (0.83%) | 65 (0.75%) | 1.07 | (0.79, 1.45) | 0.66 |
| Acute myocardial infarction within 30 days after transplant | 339 (3.9%) | 333 (3.8%) | 1.03 | (0.89, 1.21) | 0.68 |
| Fatal myocardial infarction | 17 (0.20%) | 15 (0.17%) | 0.97 | (0.71, 1.32) | 0.84 |
Discussion
The significant findings of this observational cohort study of cardiac stress testing in ESRD patients after placement on the waiting list but prior to kidney transplantation are two-fold. First, patients who are placed on the waiting list for kidney transplantation are at relatively low risk of perioperative death, MI or fatal MI. Second, and most importantly, cardiac stress testing in the 18 months prior to renal transplantation is not independently associated with a reduction in 30-day death, MI or fatal MI after adjustment for differences in demographics and comorbidities by logistic regression and propensity matching techniques.
The role of preoperative cardiovascular risk assessment is to assist the patient and health care providers in weighing the benefits and risks of surgery. Given the unpredictability of donor availability and the timing of transplant surgery, many patients on the kidney transplant waiting list undergo a version of preoperative risk assessment whereby they undergo periodic stress tests to monitor them for the development of ischemia. That the stress tests were intended for screening as opposed to investigation of ischemic symptoms is supported by the low rate of coronary angiography (13.3%) and revascularization (2.1%) in the propensity matched cohort. Screening stress tests are not recommended in other preoperative patient populations. The 2014 American College of Cardiology/American Heart Association (ACC/AHA) guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery recommends pre-operative cardiac evaluation, including stress testing, only if it would be indicated in the absence of the upcoming surgery [10]. Thus, in general, cardiac stress testing in anticipation of surgery should not be performed. However, ESRD is considered a significant risk factor for CAD and such patients undergoing kidney transplantation are considered at increased risk for perioperative adverse cardiac events. A 2016 study of United States hospital admissions from 2004 to 2013 found a 3% incidence of major adverse cardiovascular and cerebrovascular events (in-hospital, all-cause death, acute MI, or acute ischemic stroke) in the postoperative period [11]. These events were most common after vascular (7.7%), thoracic (6.5%), and transplant surgery (6.3%). In recognition of the increased risk of patients undergoing kidney transplant surgery, a 2012 AHA Consensus Statement, in the absence of any high-quality data, indicated that stress testing could be considered in kidney transplantation candidates with no active cardiac conditions based on the presence of multiple CAD risk factors regardless of functional status (Class IIb, Level of Evidence C) [2]. However, the current results suggest that patients with ESRD who have been screened for ischemia prior to placement on the waiting list and then undergo kidney transplantation are not at particularly high risk, perhaps because the highest risk patients are removed from consideration of transplant during the initial screening process. In the Coronary Artery Revascularization Prophylaxis (CARP) trial of patients who underwent surgery for peripheral arterial or aortic disease, 30-day mortality was 3.2% [12] as opposed to 0.8% in the propensity-matched cohort and 1.1% in the stress testing arm of the overall cohort in the current study. Furthermore, the fatal MI rate of 0.2% makes it highly unlikely that any screening strategy could be shown to reduce that rate further.
Many studies have demonstrated that stress testing can further stratify the risk of adverse perioperative events [10–21]. Although there is a clear relationship between the degree of myocardial ischemia detected and prognosis, there is no evidence that prophylactic revascularization prior to surgery improves outcomes [1, 22–30]. However, it is recommended that ESRD patients with ischemia on stress testing be referred for coronary angiography and revascularization, if technically feasible, to maintain their candidacy for transplant [31]. Data from CARP indicates that prophylactic revascularization does not improve outcomes in a vascular surgery population at 4-fold greater risk of death than ESRD patients undergoing kidney transplantation. In that randomized, controlled trial, PCI was performed in 59% and CABG was performed in 41% of patients assigned to prophylactic revascularization. At 2.7 years of follow-up, mortality in the revascularization group was 22% versus 23% in the no-revascularization group (relative risk, 0.98; 95% confidence interval, 0.70 to 1.37; P = 0.92). Within 30 days of the vascular operation, 3.1% of patients assigned to revascularization and 3.4% of patients not assigned to revascularization died (P = 0.87), whereas MI occurred in 12% of the revascularization group and 14% of the non-revascularization group (P = 0.37) [12].
The only prospective, randomized data on pre-operative revascularization in ESRD patients awaiting transplant comes from the 1992 study by Manske et al. who randomly assigned 31 transplantation candidates with insulin-dependent diabetes mellitus and CAD (>75% stenosis) to revascularization or medical therapy with a calcium channel blocker and aspirin [32]. Ultimately, 10 of 13 medically managed and 2 of 13 revascularized patients experienced the composite endpoint consisting of unstable angina, MI or cardiac death. Given the small sample size of exclusively diabetic patients, the high event rate among the medically managed group and advances that have occurred in the medical management of diabetes and ischemic heart disease, including the use of angiotensin-converting enzyme inhibitors and statins, the findings of this study are no longer applicable to contemporary practice.
There are several limitations of this study. First, although our list of confounders was extensive, propensity analyses cannot account for selection bias related to unmeasured characteristics. It should be noted however that a randomized clinical trial is unlikely to be performed to address the value of preoperative stress testing because the low event rates would require sample sizes too large to be feasible. Second, this study is based on administrative data including ICD codes that were designed for reimbursement, not clinical phenotyping. Third, administrative coding for MI does not distinguish between type I (plaque rupture) and type II (supply-demand mismatch) MI. These different types of MI are approached with different diagnostic and therapeutic strategies and have different prognostic implications that may influence the results of this study. In addition, there was no uniform practice to screen patients for the development of postoperative MI. Fourth, we cannot determine how many patients underwent stress testing during screening for placement on the waiting list and were never listed for transplant due to detection of extensive CAD. However, our intention was specifically to evaluate the role of stress testing after listing and not during the evaluation process for placement on the waiting list. In addition, since this data set does not provide specific reasons for removal from the waiting list, we cannot ascertain if the results of surveillance stress testing led to the removal of any patients from the waiting list. Finally, since follow-up was limited to 30 days, the conventionally defined perioperative period, the possibility of late benefit related to stress testing cannot be excluded.
In conclusion, the routine performance of cardiac stress tests on ESRD patients in the 18 months prior to kidney transplantation is not associated with a reduction in postoperative death, MI or fatal MI. The use of routine cardiac stress tests in this population should be reconsidered until a clinical benefit is demonstrated in randomized, controlled trials.
Data Availability
The third-party data underlying this study are owned by the United States Renal Data System and as such, cannot be made publicly available. Interested researchers can access this data by making a request to the United States Renal Data System. Information about obtaining data are available from: https://www.usrds.org/research.aspx. The authors of this study had no special access privileges that others would not have.
Funding Statement
The Center for Administrative Data Research is supported in part by the Washington University Institute of Clinical and Translational Sciences grant UL1 TR002345 from the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH), Grant Number R24 HS19455 through the Agency for Healthcare Research and Quality (AHRQ), and Grant Number KM1CA156708 through the National Cancer Institute (NCI) at the National Institutes of Health (NIH). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Pilmore H, Dent H, Chang S, McDonald SP, Chadban SJ. Reduction in cardiovascular death after kidney transplantation. Transplantation 2010; 89(7): 851–857. 10.1097/TP.0b013e3181caeead [DOI] [PubMed] [Google Scholar]
- 2.Lentine KL, Costa SP, Weir MR, Robb JF, Fleisher LA, Kasiske BL, et al. Cardiac disease evaluation and management among kidney and liver transplantation candidates: a scientific statement from the American Heart Association and the American College of Cardiology Foundation. J Am Coll Cardiol 2012; 60(5): 434–480. 10.1016/j.jacc.2012.05.008 [DOI] [PubMed] [Google Scholar]
- 3.Lentine KL, Brennan DC, Schnitzler MA. Incidence and predictors of myocardial infarction after kidney transplantation. J Am Soc Nephrol 2005; 16(2): 496–506. 10.1681/ASN.2004070580 [DOI] [PubMed] [Google Scholar]
- 4.Kasiske BL, Maclean JR, Snyder JJ. Acute myocardial infarction and kidney transplantation. J Am Soc Nephrol 2006; 17(3): 900–907. 10.1681/ASN.2005090984 [DOI] [PubMed] [Google Scholar]
- 5.Gill JS, Ma I, Landsberg D, Johnson N, Levin A. Cardiovascular events and investigation in patients who are awaiting cadaveric kidney transplantation. J Am Soc Nephrol 2005; 16(3): 808–816. 10.1681/ASN.2004090810 [DOI] [PubMed] [Google Scholar]
- 6.Hong E, Danovitch G. The kidney transplant waiting list in the United States. Brennan DC, Lam AQ, eds. UpToDate. Waltham, MA: UpToDate Inc. http://www.uptodate.com (Accessed on January 17, 2019.)
- 7.Rossi AP. Klein CL. Evaluation of the potential renal transplant recipient. Murphy B, Brennan DC, Lam AQ, eds. UpToDate. Waltham, MA: UpToDate Inc. http://www.uptodate.com (Accessed on January 17, 2019.)
- 8.Lentine KL, Hurst FP, Jindal RM, Villines TC, Kunz JS, Yuan CM, et al. Cardiovascular risk assessment among potential kidney transplant candidates: approaches and controversies. Am J Kidney Dis 2010; 55: 152–167. 10.1053/j.ajkd.2009.06.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care 1998; 36: 8–27. [DOI] [PubMed] [Google Scholar]
- 10.Fleisher LA, Fleischmann KE, Auerbach AD, Barnason SA, Beckman JA, Bozkurt B, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol 2014; 64: e77–e137. 10.1016/j.jacc.2014.07.944 [DOI] [PubMed] [Google Scholar]
- 11.Smilowitz NR, Gupta N, Ramakrishna H, Guo Y, Berger JS, Bangalore S. Perioperative Major Adverse Cardiovascular and Cerebrovascular Events Associated with Noncardiac Surgery. JAMA Cardiol. 2017;2(2):181–187. 10.1001/jamacardio.2016.4792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McFalls EO, Ward HB, Moritz TE, Goldman S, Krupski WC, Littooy F, et al. Coronary-artery revascularization before elective major vascular surgery. N Engl J Med 2004; 351: 2795–2804. 10.1056/NEJMoa041905 [DOI] [PubMed] [Google Scholar]
- 13.Eagle KA, Coley CM, Newell JB, Brewster DC, Darling RC, Strauss HW, et al. Combining clinical and thallium data optimizes preoperative assessment of cardiac risk before major vascular surgery. Ann Intern Med 1989; 110: 859–866. [DOI] [PubMed] [Google Scholar]
- 14.Vanzetto G, Machecourt J, Blendea D, Fagret D, Barrel E, Magne JL, et al. Additive value of thallium single-photon emission computed tomography myocardial imaging for prediction of perioperative events in clinically selected high cardiac risk patients having abdominal aortic surgery. Am J Cardiol 1996; 77: 143–148. [DOI] [PubMed] [Google Scholar]
- 15.Auerbach A, Goldman L. Assessing and reducing the cardiac risk of noncardiac surgery. Circulation 2006; 113: 1361–1376. 10.1161/CIRCULATIONAHA.105.573113 [DOI] [PubMed] [Google Scholar]
- 16.Kertai MD, Boersma E, Bax JJ, Heijenbrok-Kal MH, Hunink MG, L’talien GJ, et al. A meta-analysis comparing the prognostic accuracy of six diagnostic tests for predicting perioperative cardiac risk in patients undergoing major vascular surgery. Heart 2003; 89: 1327–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eagle KA, Singer DE, Brewster DC, et al. Dipyridamole-thallium scanning in patients undergoing vascular surgery. Optimizing preoperative evaluation of cardiac risk. JAMA 1987; 257: 2185–2189. [PubMed] [Google Scholar]
- 18.Boucher CA, Brewster DC, Darling RC, Darling RC, Mulley AG, Boucher CA. Determination of cardiac risk by dipyridamole-thallium imaging before peripheral vascular surgery. N Engl J Med 1985; 312: 389–394. 10.1056/NEJM198502143120701 [DOI] [PubMed] [Google Scholar]
- 19.Brown KA, Rowen M. Extent of jeopardized viable myocardium determined by myocardial perfusion imaging best predicts perioperative cardiac events in patients undergoing noncardiac surgery. J Am Coll Cardiol 1993; 21: 325–330. [DOI] [PubMed] [Google Scholar]
- 20.Younis L, Stratmann H, Takase B, Byers S, Chaitman BR, Miller DD. Preoperative clinical assessment and dipyridamole thallium-201 scintigraphy for prediction and prevention of cardiac events in patients having major noncardiovascular surgery and known or suspected coronary artery disease. Am J Cardiol 1994; 74: 311–317. [DOI] [PubMed] [Google Scholar]
- 21.Stratmann HG, Younis LT, Wittry MD, Amato M, Mark AL, Miller DD. Dipyridamole technetium 99m sestamibi myocardial tomography for preoperative cardiac risk stratification before major or minor nonvascular surgery. Am Heart J 1996; 132: 536–539. [DOI] [PubMed] [Google Scholar]
- 22.Das MK, Pellikka PA, Mahoney DW, Roger VL, Oh JK, McCully RB, et al. Assessment of cardiac risk before nonvascular surgery: dobutamine stress echocardiography in 530 patients. J Am Coll Cardiol 2000; 35: 1647–1653. [DOI] [PubMed] [Google Scholar]
- 23.Young EL, Karthikesalingam A, Huddart S, Pearse RM, Hinchliffe RJ, Loftus IM, et al. A systematic review of the role of cardiopulmonary exercise testing in vascular surgery. Eur J Vasc Endovasc Surg 2012; 44: 64–71. 10.1016/j.ejvs.2012.03.022 [DOI] [PubMed] [Google Scholar]
- 24.Landesberg G, Mosseri M, Shatz V, Akopnik I, Bocher M, Mayer M, et al. Cardiac troponin after major vascular surgery: the role of perioperative ischemia, preoperative thallium scanning, and coronary revascularization. J Am Coll Cardiol 2004; 44: 569–575. 10.1016/j.jacc.2004.03.073 [DOI] [PubMed] [Google Scholar]
- 25.Labib SB, Goldstein M, Kinnunen PM, Schick EC. Cardiac events in patients with negative maximal versus negative submaximal dobutamine echocardiograms undergoing noncardiac surgery: importance of resting wall motion abnormalities. J Am Coll Cardiol 2004; 44: 82–87. 10.1016/j.jacc.2004.03.049 [DOI] [PubMed] [Google Scholar]
- 26.Leppo J, Plaja J, Gionet M, Tumolo J, Paraskos JA, Cutler BS. Noninvasive evaluation of cardiac risk before elective vascular surgery. J Am Coll Cardiol 1987; 9: 269–276. [DOI] [PubMed] [Google Scholar]
- 27.Carliner NH, Fisher ML, Plotnick GD, Garbart H, Rapoport A, Kelemen MH, et al. Routine preoperative exercise testing in patients undergoing major noncardiac surgery. Am J Cardiol 1985; 56: 51–58. [DOI] [PubMed] [Google Scholar]
- 28.Sgura FA, Kopecky SL, Grill JP, Gibbons RJ. Supine exercise capacity identifies patients at low risk for perioperative cardiovascular events and predicts long-term survival. Am J Med 2000; 108: 334–336. [DOI] [PubMed] [Google Scholar]
- 29.Junejo MA, Mason JM, Sheen AJ, Moore J, Foster P, Atkinson D, et al. Cardiopulmonary exercise testing for preoperative risk assessment before hepatic resection. Br J Surg 2012; 99: 1097–1104. 10.1002/bjs.8773 [DOI] [PubMed] [Google Scholar]
- 30.Morgan PB, Panomitros GE, Nelson AC, Smith DF, Solanki DR, Zornow MH. Low utility of dobutamine stress echocardiograms in the preoperative evaluation of patients scheduled for noncardiac surgery. Anesth Analg 2002; 95: 512–516. [DOI] [PubMed] [Google Scholar]
- 31.Knoll G, Cockfield S, Blydt-Hansen T, Baran D, Kiberd B, Landsberg D, et al. Canadian Society of Transplantation consensus guidelines on eligibility for kidney transplantation. CMAJ 2005; 173: 1181–1184. 10.1503/cmaj.051291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manske CL, Wang Y, Rector T, Wilson RF, White CW. Coronary revascularization in insulin-dependent diabetes patients with chronic renal failure. Lancet 1992; 340: 998–1002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The third-party data underlying this study are owned by the United States Renal Data System and as such, cannot be made publicly available. Interested researchers can access this data by making a request to the United States Renal Data System. Information about obtaining data are available from: https://www.usrds.org/research.aspx. The authors of this study had no special access privileges that others would not have.


