Abstract
Background
The heart is a metabolically active organ, and plasma acylcarnitines are associated with long-term risk for myocardial infarction. We hypothesized that myocardial ischemia from cardiac stress testing will produce dynamic changes in acylcarnitine and amino acid levels compared to levels seen in matched control patients with normal stress tests.
Methods
We analyzed targeted metabolomic profiles in a pilot study of 20 case patients with inducible ischemia on stress testing from an existing prospectively collected repository of 357 consecutive patients presenting with symptoms of Acute Coronary Syndrome (ACS) in an Emergency Department (ED) observation unit between November 2012 and September 2014. We selected 20 controls matched on age, sex, and body-mass index (BMI). A peripheral blood sample was drawn <1 hour before stress testing and 2 hours after stress testing on each patient. We assayed 60 select acylcarnitines and amino acids by tandem mass spectrometry (MS/MS) using a Quattro Micro instrument (Waters Corporation, Milford, MA). Metabolite values were log transformed for skew. We then performed bivariable analysis for stress test outcome and both individual timepoint metabolite concentrations and stress-delta metabolite ratios (T2/T0). False discovery rates (FDR) were calculated for 60 metabolites while controlling for age, sex, and BMI. We built multivariable regularized linear models to predict stress test outcome from metabolomics data at times 0, 2 hours, and log ratio between these two. We used leave-one-out cross-validation to estimate the performance characteristics of the model.
Results
Nine of our 20 case subjects were male. Cases’ average age was 55.8, with an average BMI 29.5. Bivariable analysis identified 5 metabolites associated with positive stress tests (FDR < 0.2): alanine, C14:1-OH, C16:1, C18:2, C20:4. The multivariable regularized linear models built on T0 and T2 had Area Under the ROC Curve (AUC-ROC) between 0.5 and 0.55, however, the log(T2/T0) model yielded 0.625 AUC, with 65% sensitivity and 60% specificity. The top metabolites selected by the model were: Ala, Arg, C12-OH/C10-DC, C14:1-OH, C16:1, C18:2, C18:1, C20:4 and C18:1-DC.
Conclusions
Stress-delta metabolite analysis of patients undergoing stress testing is feasible. Future studies with a larger sample size are warranted.
Introduction
Acute Coronary Syndrome (ACS) remains the leading cause of morbidity and mortality for Americans, accounting for 1 out of every 3 deaths [1]. Each year, over 6 million people are rushed to emergency departments (ED) with ACS symptoms and many more receive testing as outpatients [2]. Cardiac biomarkers and provocative stress testing are the mainstays of ACS risk assessment but have limitations [3–6].
Because the heart is a highly metabolically active organ, a variety of humoral metabolic assessments have been suggested to assist in risk stratification for ACS [7–9]. Metabolite factors such as medium and long chain acylcarnitines have been found to be associated with long-term risk for AMI or death in outpatient populations [10, 11]. Acylcarnitines are able to enter the mitochondria and are thus surrogate markers of fatty acid Beta-oxidation. As another example, angina patients have been demonstrated to have higher branched-chain amino acids and phenylalanine concentrations [12]. Sabatine et al. [13] compared a range of metabolic factors in 18 patients with inducible myocardial ischemia compared to matched controls who were undergoing stress testing, finding differential changes in 6 factors, including a number of amino acids. Studies of other forms of myocardial stress such as a planned ablation or bypass surgery indicate that metabolomic profiling can similarly identify early myocardial injury and ischemia [14, 15]. However, few studies have examined dynamic changes in metabolites and no prior studies have examined symptomatic patients undergoing stress testing in an emergency department.
Cardiac stress testing presents an ideal method to investigate early changes in the plasma associated with myocardial ischemia. Given that prior work has demonstrated that single-timepoint acylcarnitine and amino acid concentrations can risk stratify patients for subsequent cardiac events, we sought to demonstrate the feasibility of testing whether recently symptomatic ED patients with inducible myocardial ischemia on cardiac stress echocardiogram testing have distinct changes in blood acylcarnitine and amino acid levels compared to matched control patients who have normal stress tests. By obtaining multiple timepoints, we allow each patient to functionally act as their own control.
Methods
Study setting and population
We analyzed the baseline and 2-hour post-stress blood samples of 20 case patients with inducible ischemia on stress testing and 20 controls with normal stress tests who were matched for age, sex, and body-mass index (BMI). These patients were selected from a repository created from a prospective cohort study [16]. We briefly describe these methods here for reference. Samples were collected from ED patients at a single academic medical center with an approximate annual census of 75,000 visits. We recruited 357 patients from November 2012 to September 2014 from among consecutive patients presenting on weekdays (and intermittently on weekends) with symptoms suggestive of ACS. Subjects had ruled out for myocardial infarction by serial troponins and were assigned to an adjoining ED-based observation unit. Out of this repository, 27 patients had inducible ischemia on stress testing, from which our 20 cases were selected. Our sample size was thus limited by the number of patients with ischemia on stress testing.
Patients were older than 30 years of age and all underwent an exercise echocardiogram cardiac stress test testing. Prior to stress testing all patients had serial troponin assays performed (Roche Elecsys 4th generation Troponin T (TnT) and were below institutional cutoff (<0.1 ng/mL) over 8 hours. We excluded patients with acute ischemic changes on ECG, significant arrhythmias, unstable vital signs, or other acute conditions that would preclude stress testing. All patients provided written informed consent to participate and the study protocol was approved by the Institutional Review Board of Duke University Medical Center.
We prospectively collected demographics and other relevant medical history, including laboratory testing, radiographic testing, and physical exam findings. We recorded any subsequent MI (ICD9 code from subsequent hospitalization); abnormal stress testing; significant coronary disease by angiography (lesions >50% in a major epicardial coronary artery), percutaneous coronary intervention, or coronary artery bypass graft surgery; death (cardiovascular and all-cause). Study data were collected and managed using REDCap (Research Electronic Data Capture) [17] electronic data capture tools hosted at Duke University.
Sample collection and metabolomic analysis
A peripheral blood sample was drawn before stress testing, and at 2 hours after stress testing (2-hour Post-Stress Value). Blood was centrifuged within 1 hour for collection of plasma. This plasma was transferred into 0.5 mL aliquots and were frozen at -80°C within 8 hours of collection. Samples were drawn from existing intravenous catheters when possible.
Samples were processed at the Duke Molecular Physiology Institute. Given that prior work by one of the authors demonstrated the predictive value of acylcarnitines for acute coronary syndrome in outpatients, we utilized the same methods to study acylcarnitines in the current subjects. Mass spectrometry was used to assay select acylcarnitines and amino acids, as previously described in detail [18]. The complete list of metabolites that we assayed are shown in S1 Table. Liquid-handling steps were routinely performed on a Genesis RSP 150/4 robotic sample processor (Tecan AG, Maennedorf, Switzerland). Quantitative measurement of targeted analytes was achieved by spiking of plasma samples with cocktails of stable isotope-labeled standards specific to each assay module prior to sample extraction or other manipulations [18]. For the preparation of plasma acylcarnitines and amino acids, the proteins were first removed by precipitation with methanol. Supernatant was dried and then esterified with hot, acidic methanol (acylcarnitines) or n-butanol (amino acids). Acylcarnitines and amino acids were then analyzed by tandem mass spectrometry (MS/MS) using a Quattro Micro instrument (Waters Corporation, Milford, MA). The amino acid (AA) lower level of quantitation (LOQ) was 0.5 μM and the acylcarnitine (AC) LOQ was 0.015 μM.
Exercise stress echocardiography
Patients underwent standard exercise testing on a treadmill with echocardiography evaluation. We used the Bruce protocol with mandatory termination for angina, ≥3 mm ST-segment depression, decrease of ≥20 mmHg in systolic blood pressure, serious arrhythmias, fatigue, or severe dyspnea. Inducible ischemia was defined as the occurrence of new segmental wall motion abnormalities during stress. The stress echocardiograms were interpreted as per usual care by board-certified cardiologists who were blinded to the results of study-specific metabolomic testing.
Two reviewers further reviewed stress test reports of all patients with abnormal tests. Disagreements or remaining indeterminate reports were adjudicated by a third reviewer, a board-certified cardiologist, using the entire stress test report and clinical records.
Primary data analysis
Our goal was to determine the feasibility of this analysis and some of the parameters that would impact future study of this stress-delta metabolomics paradigm. We assessed bivariable associations between stress test outcome (case vs. control) and clinical variables. For continuous variables we used Student’s t test and for categorical variables Chi-square test. We also performed bivariable associations between stress test outcome and individual metabolite concentrations from pre- and post-test timepoint, and between outcomes and metabolite ratios (metabolite at T2/metabolite at T0) using generalized linear models. False discovery rates (FDR) were calculated for each of 60 metabolites. We controlled for clinical variables relevant to the outcome, namely, aspirin use, history of smoking, hypertension, and hyperlipidemia. The following confounded variables were not considered in the analyses: history of coronary artery disease, revascularization, and angiography.
We developed multivariable regularized linear models to predict stress test outcome from metabolomics data at times 0, 2 hours, and log ratio between these two. We used nested Leave-One-Out Cross-Validation (LOOCV) to estimate the performance characteristics of the model. LOOCV allows us to obtain an estimation of the performance metric (AUC-ROC) that is unbiased and independent of the estimation of the parameters (regression coefficients) and hyperparameters of the model (regularization coefficients). This is done by estimating the parameters and hyperparameters of the model on the N (sample size) subsets of size N-1, then estimating performance on the predictions made on the left-out samples (one on each iteration). Confidence intervals for AUC-ROC, sensitivity and specificity were estimated using bias corrected and accelerated percentile bootstrapping. For further exploratory analyses, we examined whether classification of cases as only those patients with angiographically proven coronary artery stenosis >50% or those patients requiring coronary artery bypass grafting (CABG) impacted results, or whether categorizing abnormal stress tests in ordinal fashion by severity of ischemia induced (as opposed to dichotomously) would change our analysis. Based on a prior study from this repository (submitted, under consideration), we also secondarily analyzed whether pro-B-type natriuretic peptide (pro-BNP) levels could distinguish cases from controls. All analyses were done in R core stats package [19].
For exploratory purposes, based on the effect sizes we observed, we performed a sample size calculation for a future study that would have 90% power to detect at alpha <0.05 an effect size d of 0.5 of delta-metabolite levels between groups using an unmatched t test. We calculated sample sizes required to study only one candidate of interest, or 5 metabolites with a Bonferroni correction.
Results
Table 1 shows the demographic and clinical characteristics of chosen patients, and each individual’s characteristics are shown in S2 Table. The prevalence of known coronary artery disease and of coronary artery disease risk factors was low. Nine of our 20 case subjects were male. Cases’ median age was 55.5 and median BMI 30.1.
Table 1. Demographics and characteristics of enrolled patients.
| Characteristic | Cases N (%) | Controls N (%) | p values |
|---|---|---|---|
| Age (Years), Median (Range) | 55.5 (42–79) | 55.0 (44–71) | 0.876 |
| Sex | 0.999 | ||
| Male | 9 (45%) | 9 (45%) | |
| Female | 11 (55%) | 11 (55%) | |
| Race | 0.538 | ||
| Asian | 0 (0%) | 1 (5%) | |
| Black or African American | 7 (35%) | 7 (35%) | |
| White / Caucasian | 13 (65%) | 12 (60%) | |
| Other | 0 (0%) | 1 (5%) | |
| Hypertension | 14 (70%) | 5 (25%) | 0.004 |
| Diabetes | 5 (25%) | 2 (10%) | 0.21 |
| History of Tobacco Use | 13 (65%) | 8 (40%) | 0.11 |
| Hyperlipidemia | 13 (65%) | 2 (10%) | 0.0003 |
| Past Myocardial Infarction | 3 (15%) | 1 (5%) | 0.29 |
| Coronary Artery Disease | 6 (30%) | 0 (0%) | 0.008 |
Individual metabolite levels for each timepoint in each subject are shown in S3 Table and boxplots are shown in S1 and S2 Figs Among clinical variables tested, hypertension and hyperlipidemia were associated with stress test outcome (p < 0.05). Coronary artery disease, history of revascularization, and history of angiography were not used in the model due to confounding in cases.
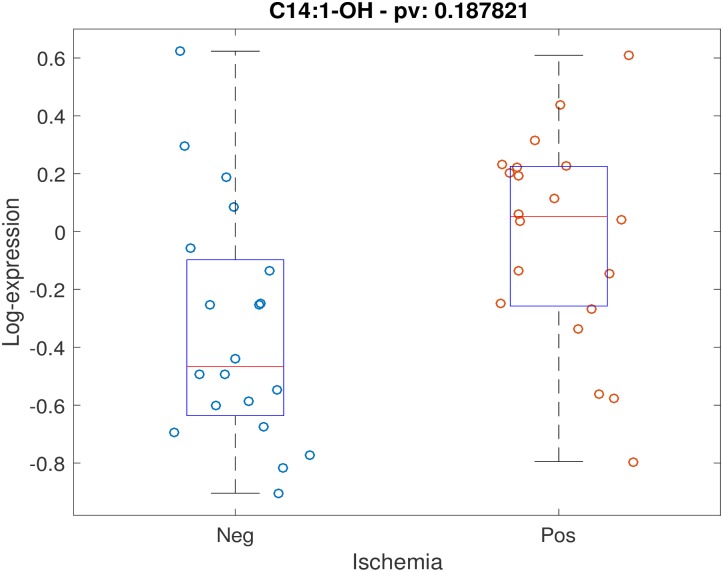
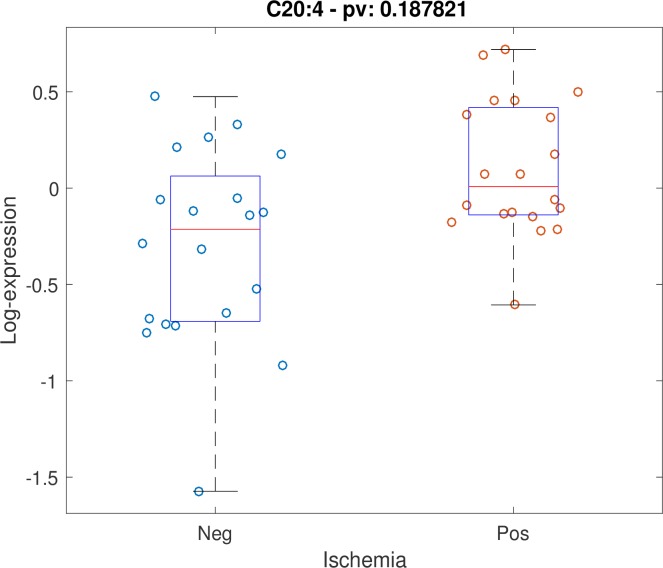
Positive stress test was not associated with individual timepoint metabolite concentrations at either timepoint with FDR < 0.2. Testing for association between stress test outcome and metabolite timepoint ratios (changes in levels across timepoints) while controlling for age, gender and BMI, demonstrated 5 metabolites with FDR < 0.2: alanine, C14:1-OH, C16:1, C18:2, C20:4. Scatter plots for these 5 metabolites are shown (Figs 1–5). After controlling for aspirin, history of smoking, hypertension, and hyperlipidemia, there were no metabolites with FDR < 0.2. However, raw p-values were still correlated (Fig 6).
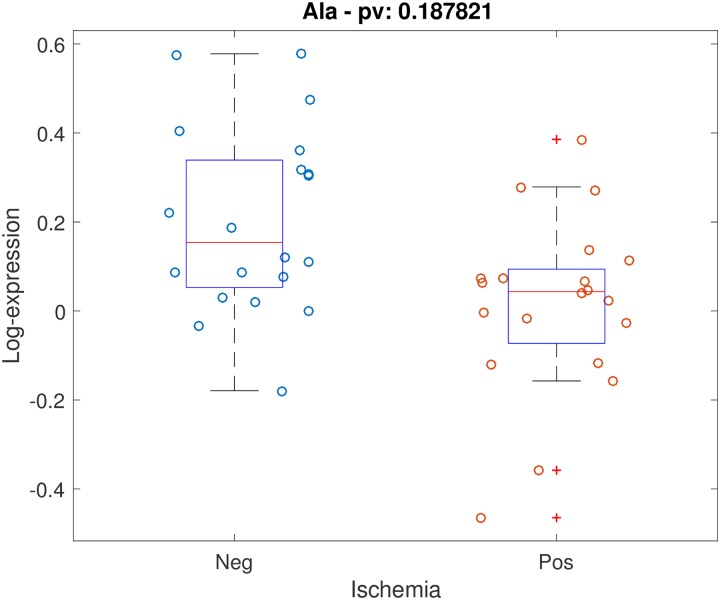
Fig 1. Scatter plot of stress-delta in alanine for patients who were positive versus negative for ischemia.
When assessing changes in metabolite levels across timepoints while controlling for age, gender and BMI, 5 metabolites had FDR < 0.2: alanine, C14:1-OH, C16:1, C18:2, C20:4.
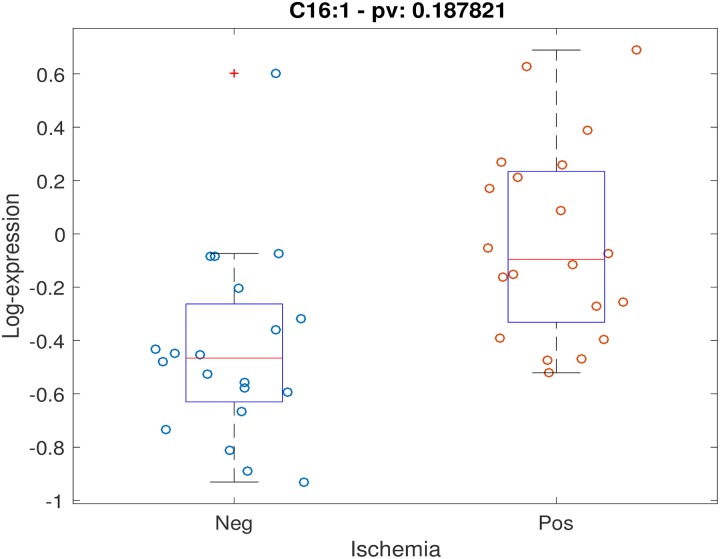
Fig 5. Scatter plot of stress-delta in C16:1 for patients who were positive versus negative for ischemia.
When assessing changes in metabolite levels across timepoints while controlling for age, gender and BMI, 5 metabolites had FDR < 0.2: alanine, C14:1-OH, C16:1, C18:2, C20:4.
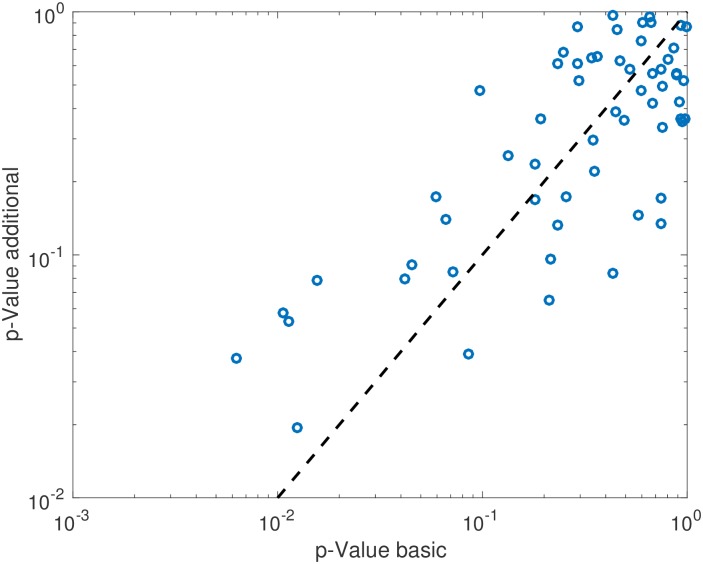
Fig 6. Raw p-values of metabolites after controlling for clinical variables.
After controlling for aspirin, history of smoking, hypertension and hyperlipidemia, raw p-values correlated.
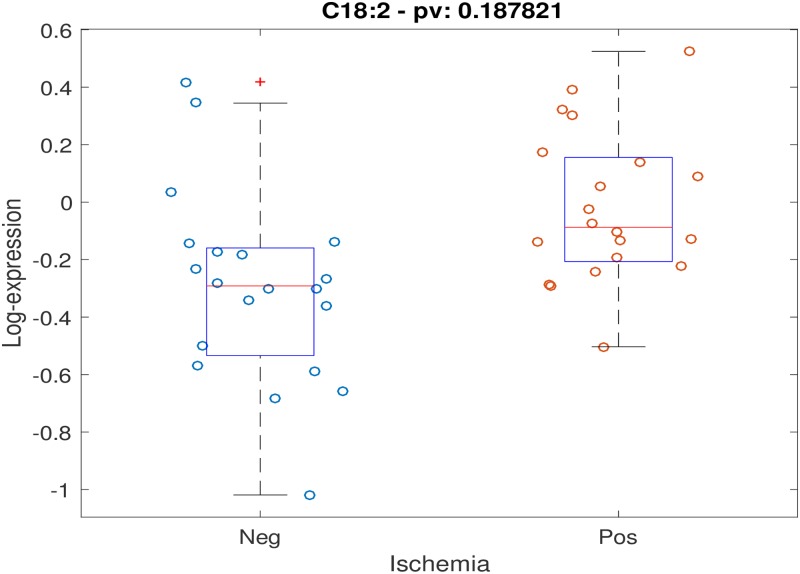
Fig 2. Scatter plot of stress-delta in C18:2 for patients who were positive versus negative for ischemia.
When assessing changes in metabolite levels across timepoints while controlling for age, gender and BMI, 5 metabolites had FDR < 0.2: alanine, C14:1-OH, C16:1, C18:2, C20:4.
Fig 3. Scatter plot of stress-delta in C14:1-OH for patients who were positive versus negative for ischemia.
When assessing changes in metabolite levels across timepoints while controlling for age, gender and BMI, 5 metabolites had FDR < 0.2: alanine, C14:1-OH, C16:1, C18:2, C20:4.
Fig 4. Scatter plot of stress-delta in C20:4 for patients who were positive versus negative for ischemia.
When assessing changes in metabolite levels across timepoints while controlling for age, gender and BMI, 5 metabolites had FDR < 0.2: alanine, C14:1-OH, C16:1, C18:2, C20:4.
The multivariable regularized linear models built on T0 and T2 had Area Under the ROC Curve (AUC-ROC) values between 0.5 and 0.55, however, the log(T2/T0) model yielded 0.625 (IQR 0.558 0.682) AUC, with 65% (IQR 0.570 0.720) sensitivity and 60% (IQR 0.667 0.524) specificity. The top metabolites selected by the model were: Alanine, Arginine, C12-OH/C10-DC, C14:1-OH, C16:1, C18:2, C18:1, C20:4 and C18:1-DC. Coefficients of the model and associated statistics are shown in Table 2.
Table 2. Multivariable regularized linear model coefficients.
| Metabolite | Coefficient | Standard Error | t Statistic | p-value |
|---|---|---|---|---|
| Alanine | -3.708 | 4.218 | -0.879 | 0.379 |
| Arginine | -8.131 | 6.001 | -1.355 | 0.175 |
| C12-OH/C10-DC | 3.461 | 2.090 | 1.656 | 0.098 |
| C14:1-OH | -1.904 | 2.267 | -0.840 | 0.401 |
| C16:1 | 9.018 | 4.252 | 2.121 | 0.034 |
| C18:2 | 6.445 | 4.032 | 1.599 | 0.110 |
| C18:1 | -11.738 | 5.793 | -2.026 | 0.043 |
| C20:4 | 2.181 | 1.936 | 1.127 | 0.260 |
| C18:1-DC | -4.299 | 2.412 | -1.782 | 0.075 |
In our further exploratory analyses, defining cases as only those patients with angiographically proven stenosis or CABG did not directionally change comparisons of metabolite levels or changes in metabolite levels. When we considered categorizing stress tests ordinally, we found that it impacted only 5 patients’ categorization and thus did not impact results. Finally, an analysis of pro-BNP levels demonstrated significant differences between baseline and 2-hour levels but not the change in levels based on stress test results. A similar analysis of pro-BNP using angiographically proven stenosis or CABG as the cases demonstrated no significant differences between groups in pro-BNP levels. Furthermore, our sample size calculation for a future study demonstrated that for a future study analyzing one metabolite, the largest sample size required (based on the differences observed for C20:4) would be 178 total patients. If a future study analyzed 5 metabolites of interest, using Bonferroni correction for 5 metabolites, a total of 220 patients would be required.
Discussion
There is considerable interest in blood-borne markers of myocardial ischemia and early myocardial injury. Metabolomic profiles have been proposed as one approach for identifying these entities, both through systems biology approaches and as a means to identify traditional biomarker candidates. In this study, we matched patients with ischemia on exercise stress testing to patients without ischemia to compare metabolomic profile changes from baseline to 2 hours after their stress test. We have preliminarily found 5 metabolites that appear to change in concentration differently in patients with myocardial ischemia compared to those without on standard stress testing. We consider this to be hypothesis-generating work for further exploration and validation, but demonstrates the feasibility of the data collection and statistical analysis.
Others have attempted to identify metabolomic signatures of early myocardial injury with different models. In patients undergoing a planned myocardial infarction for treatment of hypertrophic cardiomyopathy, a metabolic signature consisting of aconitic acid, hypoxanthine, trimethylamine N-oxide, and threonine differentiated patients with myocardial injury from those undergoing diagnostic coronary angiography [14, 20]. Turer et al. studied patients undergoing cardiac surgery and found that the patient’s baseline ventricular health predicted metabolic responses to reperfusion and ischemia [15]. In their study, 2 specific acylcarnitine species were released following reperfusion: acetylcarnitine and 3-hydroxybutyryl-carnitine. They further found global limit of uptake of metabolic fuel in patients with underlying dysfunctional ventricles.
Numerous efforts have previously examined systems biology approaches to differentiating ST elevation myocardial infarction (STEMI) patients from normal controls. Some have used untargeted approaches to determine a metabolomic signature or transcriptomic approaches to differentiate STEMI from unstable angina or healthy controls [21, 22]. Previous investigators have demonstrated significant metabolic dysfunction in fatty acid metabolism in patients with coronary artery stenosis [23]. DeFilippis et al. demonstrated 19 metabolites with an intra-subject fold change from time of acute thrombotic MI presentation to the quiescent state, including lipids 2-hydroxybutyrate, and amino acids [24]. In other analyses of STEMI patients, lysophosphatidylcholines, caffeine, glycolysis, tryptophan and sphingomyelin metabolism and minerals were found to be disrupted compared to healthy controls [25]. We were interested in determining whether myocardial ischemia, which occurs earlier in the infarction process, could be detected through metabolomic signatures.
Prior work examining metabolic profiles in patients undergoing exercise generally found that exercise-induced increases in glycerol were strongly related to fitness levels in normal individuals and were attenuated in subjects with myocardial ischemia [26]. Sabatine et al. published the earliest report on metabolomic analysis of patients undergoing cardiac stress testing [13]. They identified several amino acids that allowed differentiation of patients with ischemia on their stress test. In this study, we add deeper analysis of acylcarnitines to this model and examined recently symptomatic patients in our emergency department observation unit.
Our work, though preliminary, is in alignment with other research that indicates resting metabolomic profiles can discriminate over and above traditional ACS risk stratification systems, even in asymptomatic patients [11, 27]. In particular, medium and long chain acylcarnitines have been associated with increased risk. This may reflect an underlying defect in mitochondrial function and Beta-oxidation, which have been shown to be a mechanism underlying increased insulin resistance [18, 28]. These processes may also increase inflammation within the cardiovascular system, leading to increased likelihood of atherosclerotic plaque rupture.
Based on this prior work, we studied the same metabolites in our patients undergoing provocative cardiac stress testing to determine whether the changes identified in the prior studies occur early in the ischemia process. Prior work has examined patients with acute irreversible infarction and/or patients undergoing elective coronary intervention [21]. They likewise discovered that palmitic acid, linoleic acid, stearic acid and oleic acid, (all 16 or 18-carbon chain fatty acids) were relatively elevated in the serum of STEMI patients. This further confirms that fatty acid oxidation, as evidenced by elevated levels of acylcarnitines, is dysfunctional in the ischemic myocardial cell.
We propose that one reason such work was not able to identify differences between healthy control and elective coronary intervention (“unstable angina”) patients may be that the unstable angina patients’ blood samples were obtained long after their symptomatic episodes. In our model, we examined two timepoints in close proximity to a cardiac stress test as opposed to single resting baseline measurements. This allowed us to look for dynamic changes that occur early in the ischemia process.
There may be serum metabolite changes that are identifiable early in the process of acute myocardial ischemia. Further investigation into the metabolomic changes that occur early in the ischemia process may shed light on the changes that can be expected over longer periods of time. If so, this would not only inform our understanding of myocardial ischemia, but suggest an alternative clinical method for detecting inducible ischemia. Such work may produce new biomarkers of myocardial ischemia and/or panels of markers that can identify patients at short and long-term risk of adverse cardiac events. Our sample size calculations indicate that only a modest sized study of 220 patients would be required to validate these findings under the current conditions of age, sex, and BMI-matched cases and controls. A larger validation study that does not match cases with controls might require a larger study sample, since there would likely be larger variation between non-matched group comparisons.
Limitations
This study is limited by small sample size. While we are underpowered to make definitive statements about the changes observed in metabolites following ischemia on stress testing, we conducted this pilot to prove feasibility of obtaining multiple samples before and after stress testing and assaying and analyzing multiple metabolites. A further limitation is that samples were acquired 2 hours following stress testing, which may be too late after an event to detect some metabolic changes. This timing was dictated by the repository’s parent study protocol. Patients were classified as either case or control on the basis of standard of care stress echocardiography readings. There is some variation in intra-observer agreement for this test. Because patients were enrolled from an observational registry, we were unable to control for workup bias downstream from the stress test. Therefore, some case patients had confirmatory cardiac angiography whereas others did not. Furthermore, even among those patients who are found to have significant coronary artery stenosis, there is some question over how important of a patient-oriented outcome this represents.
Conclusion
We studied whether there were dynamic changes in a large number of selected metabolites between patients with myocardial ischemia on stress testing compared to matched normal controls, preliminarily identifying 5 metabolites that appear to change differentially. It is unclear whether these results can contribute additional information above standard clinical evaluation for myocardial ischemia. Delta-metabolite concentrations may offer more discriminative value than static single-timepoint metabolite measurement. This model of metabolomic exploration warrants further investigation.
Supporting information
Sixty amino acids and acylcarnitine concentrations were measured in each plasma sample.
(XLSX)
Each patient’s individual demographic and clinical history data are shown.
(XLSX)
Individual metabolite levels for each timepoint in each subject are shown. All units are μM. T0 = baseline and T2 = 2 hours after stress test.
(XLSB)
Boxplots show mean, standard deviation, and range, with outliers. Pre = baseline levels. Post = 2 hours post-stress testing. All units are μM. Con = controls.
(PDF)
Pre = baseline levels. Post = 2 hours post-stress testing. All units are μM. Con = controls.
(PDF)
Acknowledgments
We would like to acknowledge manuscript preparation assistance of Ms. Ashley Phillips.
Data Availability
All the data is included in the paper or its Supporting Information files, and is available without restrictions.
Funding Statement
This study was made possible by an ENABLE grant from the Duke Private Diagnostic Clinics and Duke University. The study authors retained rights to data and decision of whether to publish. Dr. Tsalik works at the Durham VA Hospital but is not a government employee. Dr. Shah and Dr. Ginsburg report having an unrelated unlicensed patent on a metabolomic finding, “Predicting coronary artery disease and risk of cardiovascular events”, patent application number #20160195543. Dr. Ginsburg also serves on the Scientific Advisory Board for CardioDx and has received research funding from Abbott Laboratories but is not an employee. Dr. Limkakeng has received research grants from Roche International, Abbott Laboratories, and Siemens Healthcare Diagnostics but is not an employee. None of these entities provided funding for this study. They provided support in the form of salaries for authors GG and ATL, but did not have any role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. The specific roles of these authors are articulated in the ‘author contributions’ section.
References
- 1.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al. Heart Disease and Stroke Statistics—2014 Update: A Report From the American Heart Association. Circulation. 2014;129(3):e28–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Niska R, Bhuiya F, Xu J. National Hospital Ambulatory Medical Care Survey: 2007 Emergency Department Summary. Hyattsville,MD: National Center for Health Statistics; 2010. [PubMed] [Google Scholar]
- 3.ACCF/ASE/AHA/ASNC/HFSA/HRS/SCAI/SCCM/SCCT/SCMR 2011 Appropriate Use Criteria for Echocardiography. J Am Soc Echocardiography. 2011;24(3):229–67. 10.1016/j.echo.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 4.Ely S, Chandra A, Mani G, Drake W, Freeman D, Limkakeng AT Jr,. Utility of observation units for young emergency department chest pain patients. J Emerg Med. 2013;44(2):306–12. Epub 2012/09/15. 10.1016/j.jemermed.2012.07.048 . [DOI] [PubMed] [Google Scholar]
- 5.Foy AJ, Liu G, Davidson WR Jr, Sciamanna C, Leslie DL. Comparative effectiveness of diagnostic testing strategies in emergency department patients with chest pain: An analysis of downstream testing, interventions, and outcomes. JAMA Intern Med. 2015;175(3):428–36. 10.1001/jamainternmed.2014.7657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Makam AN, Nguyen OK. Use of Cardiac Biomarker Testing in the Emergency Department. JAMA Intern Med. 2014. Epub 2014/11/18. 10.1001/jamainternmed.2014.5830 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barderas MG, Laborde CM, Posada M, de la Cuesta F, Zubiri I, Vivanco F, et al. Metabolomic profiling for identification of novel potential biomarkers in cardiovascular diseases. J Biomed Biotechnol. 2011;2011:790132 Epub 2011/01/29. 10.1155/2011/790132 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bodi V, Marrachelli VG, Husser O, Chorro FJ, Vina JR, Monleon D. Metabolomics in the diagnosis of acute myocardial ischemia. J Cardiovasc Transl Res. 2013;6(5):808–15. Epub 2013/08/31. 10.1007/s12265-013-9505-9 . [DOI] [PubMed] [Google Scholar]
- 9.Heather LC, Wang X, West JA, Griffin JL. A practical guide to metabolomic profiling as a discovery tool for human heart disease. J Mol Cell Cardiol. 2013;55:2–11. Epub 2012/12/13. 10.1016/j.yjmcc.2012.12.001 . [DOI] [PubMed] [Google Scholar]
- 10.Shah AA, Craig DM, Sebek JK, Haynes C, Stevens RC, Muehlbauer MJ, et al. Metabolic profiles predict adverse events after coronary artery bypass grafting. J Thorac Cardiovasc Surg. 2012;143(4):873–8. Epub 2012/02/07. 10.1016/j.jtcvs.2011.09.070 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah SH, Sun JL, Stevens RD, Bain JR, Muehlbauer MJ, Pieper KS, et al. Baseline metabolomic profiles predict cardiovascular events in patients at risk for coronary artery disease. Am Heart J. 2012;163(5):844–50 e1. Epub 2012/05/23. 10.1016/j.ahj.2012.02.005 . [DOI] [PubMed] [Google Scholar]
- 12.DeRatt BN, Ralat MA, Lysne V, Tayyari F, Dhar I, Edison AS, et al. Metabolomic Evaluation of the Consequences of Plasma Cystathionine Elevation in Adults with Stable Angina Pectoris. J Nutrition 2017;147(9):1658–68. Epub 2017/08/11. 10.3945/jn.117.254029 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sabatine MS, Liu E, Morrow DA, Heller E, McCarroll R, Wiegand R, et al. Metabolomic identification of novel biomarkers of myocardial ischemia. Circulation. 2005;112(25):3868–75. Epub 2005/12/14. 10.1161/CIRCULATIONAHA.105.569137 . [DOI] [PubMed] [Google Scholar]
- 14.Lewis GD, Wei R, Liu E, Yang E, Shi X, Martinovic M, et al. Metabolite profiling of blood from individuals undergoing planned myocardial infarction reveals early markers of myocardial injury. J Clin Inv. 2008;118(10):3503–12. Epub 2008/09/05. 10.1172/jci35111 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turer AT, Stevens RD, Bain JR, Muehlbauer MJ, van der Westhuizen J, Mathew JP, et al. Metabolomic profiling reveals distinct patterns of myocardial substrate use in humans with coronary artery disease or left ventricular dysfunction during surgical ischemia/reperfusion. Circulation. 2009;119(13):1736–46. Epub 2009/03/25. 10.1161/CIRCULATIONAHA.108.816116 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Limkakeng A Jr., Drake W, Lokhnygina Y, Meyers H, Shogilev D, Christenson R, et al. Myocardial Ischemia on Cardiac Stress Testing Is Not Associated with Changes in Troponin T Levels. J of Appl Lab Med. 2017;1(5):532–43. [DOI] [PubMed] [Google Scholar]
- 17.Harris P, Taylor R, Thielke R, Payne J, Gonzalez N, Conde J. Research electronic datacapture (REDCap) ‐ A metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab. 2009;9(4):311–26. Epub 2009/04/10. 10.1016/j.cmet.2009.02.002 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Team RC R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2015. [Google Scholar]
- 20.Baumgartner C, Lewis GD, Netzer M, Pfeifer B, Gerszten RE. A new data mining approach for profiling and categorizing kinetic patterns of metabolic biomarkers after myocardial injury. Bioinformatics (Oxford, England). 2010;26(14):1745–51. Epub 2010/05/21. 10.1093/bioinformatics/btq254 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ali SE, Farag MA, Holvoet P, Hanafi RS, Gad MZ. A Comparative Metabolomics Approach Reveals Early Biomarkers for Metabolic Response to Acute Myocardial Infarction. Scientific Reports. 2016;6:36359 Epub 2016/11/09. 10.1038/srep36359 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park HJ, Noh JH, Eun JW, Koh YS, Seo SM, Park WS, et al. Assessment and diagnostic relevance of novel serum biomarkers for early decision of ST-elevation myocardial infarction. Oncotarget. 2015;6(15):12970–83. Epub 2015/05/31. 10.18632/oncotarget.4001 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gao X, Ke C, Liu H, Liu W, Li K, Yu B, et al. Large-scale Metabolomic Analysis Reveals Potential Biomarkers for Early Stage Coronary Atherosclerosis. Scientific Reports. 2017;7(1):11817 Epub 2017/09/20. 10.1038/s41598-017-12254-1 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeFilippis AP, Trainor PJ, Hill BG, Amraotkar AR, Rai SN, Hirsch GA, et al. Identification of a plasma metabolomic signature of thrombotic myocardial infarction that is distinct from non-thrombotic myocardial infarction and stable coronary artery disease. PloS one. 2017;12(4):e0175591 Epub 2017/04/18. 10.1371/journal.pone.0175591 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yin X, de Carvalho LP, Chan MY, Li SFY. Integrated metabolomics and metallomics analyses in acute coronary syndrome patients. Metallomics: integrated biometal science. 2017;9(6):734–43. Epub 2017/05/19. 10.1039/c7mt00071e . [DOI] [PubMed] [Google Scholar]
- 26.Lewis GD, Farrell L, Wood MJ, Martinovic M, Arany Z, Rowe GC, et al. Metabolic signatures of exercise in human plasma. Science translational medicine. 2010;2(33):33ra7 Epub 2010/05/28. 10.1126/scitranslmed.3001006 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rizza S, Copetti M, Rossi C, Cianfarani MA, Zucchelli M, Luzi A, et al. Metabolomics signature improves the prediction of cardiovascular events in elderly subjects. Atherosclerosis. 2014;232(2):260–4. Epub 2014/01/29. 10.1016/j.atherosclerosis.2013.10.029 . [DOI] [PubMed] [Google Scholar]
- 28.Shah SH, Bain JR, Muehlbauer MJ, Stevens RD, Crosslin DR, Haynes C, et al. Association of a peripheral blood metabolic profile with coronary artery disease and risk of subsequent cardiovascular events. Circulation Cardiovascular genetics. 2010;3(2):207–14. Epub 2010/02/23. 10.1161/CIRCGENETICS.109.852814 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Sixty amino acids and acylcarnitine concentrations were measured in each plasma sample.
(XLSX)
Each patient’s individual demographic and clinical history data are shown.
(XLSX)
Individual metabolite levels for each timepoint in each subject are shown. All units are μM. T0 = baseline and T2 = 2 hours after stress test.
(XLSB)
Boxplots show mean, standard deviation, and range, with outliers. Pre = baseline levels. Post = 2 hours post-stress testing. All units are μM. Con = controls.
(PDF)
Pre = baseline levels. Post = 2 hours post-stress testing. All units are μM. Con = controls.
(PDF)
Data Availability Statement
All the data is included in the paper or its Supporting Information files, and is available without restrictions.