Abstract
Background:
Exposure to inorganic arsenic (As) from drinking water is associated with modest deficits in intellectual function in young children; it is unclear whether deficits occur during adolescence, when key brain functions are more fully developed.
Objectives:
We sought to determine the degree to which As exposure is associated with adolescent intelligence, and the contributory roles of lead, cadmium, manganese and selenium.
Methods:
We recruited a cross-section of 726 14–16 year olds (mean age = 14.8 years) whose mothers are participants in the Bangladesh Health Effects of Arsenic Longitudinal Study (HEALS), and whose household well water As levels, which varied widely, were well characterized. Using a culturally modified version of the WISC-IV, we examined raw Full Scale scores, and Verbal Comprehension, Perceptual Reasoning, Working Memory and Processing Speed Indices. Blood levels of As (BAs), Mn, Pb, Cd and Se were assessed at the time of the visit, as was creatinine-adjusted urinary As (UAs/Cr).
Results:
Linear regression analyses revealed that BAs was significantly negatively associated with all WISC-IV scores except for Perceptual Reasoning. With UAs/Cr as the exposure variable, we observed significantly negative associations for all WISC-IV scores. Except for Se, blood levels of other metals, were also associated with lower WISC-IV scores. Controlling for covariates, doubling BAs, or UAs/Cr, was associated with a mean decrement (95% CI) of 3.3 (1.1, 5.5), or 3.0 (1.2, 4.5) points, respectively, in raw Full scale scores with a sample mean of 177.6 (SD=36.8). Confirmatory analyses using Bayesian Kernel Machine Regression, which identifies important mixture members, supported these findings; the primary contributor of the mixture was BAs, followed by BCd.
Conclusions:
Our data indicate that the adverse consequences of As exposure on neurodevelopment observed in other cross-sectional studies of younger children are also apparent during adolescence. They also implicate Cd as a neurotoxic element that deserves more attention.
Keywords: Arsenic exposure, Metal mixtures, Cognitive test scores, Intellectual Function, Adolescence
The World Health Organization (WHO) has estimated that more than 200 million people across 70 countries are chronically exposed to levels of inorganic arsenic (As) in drinking water (WAs) that exceed the WHO guideline and the U.S. Environmental Protection Agency (EPA) maximum contaminant level (MCL) of 10 μg/L (WHO, 2008). The National Research Council (NRC), in a recent review, created a hierarchy of the many adverse health outcomes resulting from this exposure. The top tier for concern, with strong evidence of a causal association, included lung, skin and bladder cancer, ischemic heart disease, and skin lesions. The second tier of concern (of three), with weaker support for causality, included six additional adverse health outcomes, including neurodevelopmental deficits (U.S. National Research Council, 2013), the topic of this investigation. Not surprisingly, the U.S. Agency for Toxic Substances and Disease Registry (ATSDR) gives As its highest ranking among chemicals that pose public health hazards (ATSDR, 2015).
Over the past two decades, As has shown consistent associations with neurodevelopmental deficits in children (reviewed in (Rodriguez-Barranco et al., 2013; Tolins, Ruchirawat, & Landrigan, 2014; Tyler & Allan, 2014)), although different studies show associations with different developmental domains, reflecting in part the variety of assessment methods. Some studies highlight associations with verbal domains (Calderon et al., 2001), some with memory and attention (Rosado et al., 2007) and others utilize a range of subtests (von Ehrenstein et al., 2007). Most studies are based on populations outside the United States and have made use of a wide range of tests of intellectual function with varying cultural adaptations. In one US study (Wright, Woolf, Jim, & Bellinger, 2006), levels of As in hair were related primarily to verbal intelligence scores.
In Bangladesh, in 10-year old children drinking from wells with widely ranging WAs concentrations (0.1–790 μg/L), and using an earlier version of the WISC (WISC-III), we found negative associations between WAs and Performance scales (Wasserman et al., 2004). Adverse associations with Performance scores on the WPPSI-III were also observed, but of smaller size in a younger sample of Bangladeshi 6-year olds (Wasserman et al., 2007).
In the re-standardization that created the WISC-IV, certain WISC-III Performance subtests were reorganized into Working Memory, Processing Speed and Perceptual Reasoning domains (and other subtests revised, added or eliminated). The Digit Span subtest is common to both WISC-IV Working Memory and WISC-III Performance Scale. More recently, we reported negative associations between As measured in blood (BAs) and WISC-IV Working Memory in 8–11 year olds; with adjustment for blood manganese (BMn), and for other covariates, this association was of borderline statistical significance (Wasserman et al., 2011). In a follow-up study of these same children, two years after installation of deep wells with low WAs concentrations, 12 year old children’s WISC-IV Working Memory scores showed significant improvement, but other test indices were unchanged (Wasserman et al., 2015). Finally, in a parallel US investigation among elementary school children in Maine (Wasserman et al., 2014), after adjusting for other sociodemographic covariates, exposure to well WAs >5 μg/L was adversely associated with most WISC-IV Indices, most strongly with Working Memory and Verbal Comprehension. Average WISC-IV Full Scale IQ and Working Memory scores were roughly 5 points lower in children with WAs ≥ 5 μg/L compared to those with WAs < 5 μg/L.
Recent research has begun to focus on the impact of exposures to mixtures of metals on children’s health. A recent systematic review reported on nine studies that evaluated the association of metal mixtures with child cognitive and motor development (Claus Henn, Coull, & Wright, 2014), studies that collectively focused on exposures to Pb, Mn, Cd, As and Hg. In the current study, we assess the impact of the first four of these elements in a setting where dietary fish intake (i.e., Hg) is minimal. In addition, we explored potential beneficial effects of selenium (Se), an element known to lessen the overt toxicity of As by facilitating its elimination via bile (Levander, 1977). In the current study, we explore the contributions of these additional elements and utilize a relatively new statistical method to delineate their impacts in a mixture exposure scenario. In addition, we explore a developmental period - adolescence - not typically evaluated in studies of developmental neurotoxicology, but one that is important for our understanding of the long-term impact of As on neurodevelopment. Key structures and processing capacities that support certain domains of intellectual function do not emerge until later childhood or early adolescence. Assessments at younger ages may not readily discriminate the impact of exposure, especially for frontal and prefrontal functions that are not fully operational until adolescence (Sander, Lindenberger, & Werkle-Bergner, 2012). The current study adds to our understanding of the relation of As to neurodevelopment by extending observations to a sample of adolescents.
Materials and Methods
Study overview and timeline.
Between October 2000 and May 2002, 11,746 men and women 18–75 years of age were recruited into the Health Effects of Arsenic Longitudinal Study (HEALS) in Araihazar, Bangladesh (Ahsan et al., 2005). To be eligible for inclusion into HEALS, individuals – including the mothers of the current adolescent study participants – had to have been drinking from their household well for at least three years. The current adolescent study participants were born during that three year period. Thus, their maternal HEALS baseline urinary As concentration adjusted for creatinine (mUAscr) is an indicator of in utero As exposure.
Since women bring water into the household, mothers’ WAs levels over time offer a useful proxy for their child’s exposure. As evidence for this proxy relationship, we note that for 502 HEALS mother-child pairs in two of our earlier As studies (Wasserman et al., 2007; Wasserman et al., 2004), the correlation between children’s urinary arsenic (UAs) and mother’s UAs (measured most closely to the child’s test date) was high (r(500)=0.75, p<0.0001), corroborating that both the mother and the young child largely consume water from the same source.
In order to assure a range of levels of early WAs exposure, we recruited adolescent offspring of these HEALS female participants from four strata, based on maternal WAs history. We began with 927 households (in 51 villages) for whom mother’s WAs history was complete from baseline through her fourth HEALS follow-up visit, and whose households included a child aged 14 years up to (but not including) 17 years, at the time of recruitment. Four strata of these 927 HEALS women included: (1) 264 with WAs <10 μg /L at HEALS baseline (representing the child’s prenatal exposure) and <10 μg /L at follow-up visits corresponding to child ages 3, 6, and 9 years; (2) 304 with WAs between 10 and 50 μg /L at HEALS baseline and all relevant follow-up visits; (3) 252 with WAs >50 μg /L at all visits; and (4) 107 with WAs > 50 μg /L at baseline and then < 50 μg /L on all subsequent assessments. The recruitment of adolescent participants took place between December, 2012 and December, 2016. This study was approved by the Columbia University Medical Center IRB and the Bangladesh Medical Research Council IRB.
Participants.
Of the 927-targeted households, 12 home visits found no child in the anticipated age range, and in 12 additional homes, the child had died. Of the remaining 903 households with potentially eligible children, 29 were excluded because they had moved away (20 migrating to another region in Bangladesh, one residing in a foreign country, and eight who had married and moved away). Of the remaining 874 households, 25 were excluded for other reasons (14 with a child with a severe deficit noted at the home visit (e.g., autism), one with a pair of twins, and nine whose children aged out before they could be assessed). Of the 849 households with existing, resident, and not excluded children, 111 refused participation, nine had incomplete assessments, and three had been in one of our prior studies. This resulted in a final sample size of 726. In comparison to those included in the study, those who were excluded did not differ on HEALS baseline WAs (mWAs0: 74.4 vs 68.0 μg/L, respectively, P = 0.10); WMn was slightly but significantly higher (1296.0 vs 1150.1 μg/L, respectively, P = 0.01). Mothers of those included in the study were younger (27.3 vs 29.4 years, P < 0.001) and had higher education (3.65 vs 3.2 years, P = 0.02). Fathers of those included were also younger (35.1 vs 36.7 years, P = 0.01) but did not differ in education (3.9 vs 3.7 years, P = 0.42).
Procedure.
During initial home visits, once parental informed consent and child assent were obtained, the field team collected household well water samples and sociodemographic information, and appointments were made for mother and child to visit the field clinic. At field clinic visits, physical exams and neurodevelopmental assessments were performed; concurrent urine and blood samples for As analyses were collected and employed as biomarkers of exposure in this cross-sectional study. Adolescents received a small age-appropriate gift in appreciation for participation.
Measures.
Information about mother’s well use was collected when these families were recruited into the HEALS cohort. Field sample collection and laboratory analysis procedures for water arsenic (WAs) and water manganese (WMn) are described elsewhere in detail (Cheng, Zheng, Mortlock, & van Geen, 2004; van Geen, Ahmed, Seddique, & Shamsudduha, 2003; van Geen et al., 2005).
Urinary Measurements.
Total UAs concentrations were assayed by graphite furnace atomic absorption spectrophotometry (GFAA), using a Perkin-Elmer Analyst 800 system as described (Nixon, Mussmann, Ecktahdahl, & Moyer, 1991). The detection limit for total urinary arsenic (UAs) was 2 μg/L. None of the samples were below the LOD. The interprecision and intraprecision coefficients of variation (CVs) were 4.2% and 4.1%, respectively. UAs levels were also adjusted for urinary creatinine (UCr) concentrations, which were analyzed by a colorimetric method based on Jaffe’s reaction (Heinegard & Tiderstrom, 1973).
ICP-MS Blood Measurements.
The concurrent venous whole blood samples were analyzed for BAs, BMn, BPb, BCd and BSe using a Perkin-Elmer NexION 350S equipped with an Elemental Scientific autosampler 4DX. ICP-MS- with dynamic reaction cell (ICP-MS-DRC) methods for metals in whole blood were developed according to published procedures (Pruszkowski, Neubauer, & Thomas, 1998; Stroh, 1988). The limits of detection for blood measurements of Pb, Cd, Mn, Se and As were 0.51, 0.08, 0.45, 0.58 and 0.06 μg/L, respectively. All measurements of BPb, BMn, BSe and BAs were above the LOD and only one sample was lower than the LOD for Cd and was excluded. The intraprecision CVs for BMn, BAs, BSe, BPb, and BCd were 3.2%, 3.7%, 2.0%, 1.6%, and 7.8% respectively, and the interprecision CVs were 5.9%, 7.3%, 5.3%, 3.9%, and 16.0%, respectively.
Arsenic Metabolites.
UAs metabolites were measured using HPLC separation of arsenobetaine, arsenocholine, AsIII, AsV, MMA, and DMA, and detection by ICP-MS-DRC as described by Reuter (Reuter, Davidowski, & Neubauer, 2003) (intra- and inter-assay CVs for arsenobetaine + arsenocholine; 6.4%, 7.8%; AsIII + AsV: 1.8%, 2.9%; MMA: 2.9%, 2.9%; DMA: 0.6%, 1.1%). AsIII can oxidize to AsV during sample storage and analysis, and therefore total InAs (AsIII + AsV) is reported. The detection limit for arsenocholine + arsenobetaine was 0.2 μg/L. Nineteen samples fell below this LOD. For arsenite, arsenate, DMA and MMA, the detection limit was 0.5 μg/L. None of the samples fell below the LOD for DMA. For arsenite + arsenate (i.e., inorganic As), 2 samples were below the LOD, while for MMA, 10 fell below the LOD.
Child and maternal intelligence.
The Wechsler Intelligence Scale for Children-Fourth Edition (WISC-IV: (Wechsler, 2003) is an individually administered assessment of intellectual function, suitable for children 6 through 17 years old. This revised version of the WISC-III (Wechsler, 1991) has excellent psychometrics (e.g., standard errors of measurement for 15-year-olds for the subtests used average 1.13), and provides measures of general intellectual ability (Full Scale IQ) and specific cognitive domains (Verbal Comprehension, Perceptual Reasoning, Working Memory, and Processing Speed Indices). We administered a battery of the following subtests (listed with their respective Composites): Similarities, Comprehension, and Information (Verbal Comprehension); Block Design, Matrix Reasoning, and Picture Completion (Perceptual Reasoning); Digit Span and Letter-Number Sequencing (Working Memory); Coding and Symbol Search (Processing Speed). As described in our earlier work (Wasserman et al., 2011), we modified certain test items that were thought to be culturally inappropriate (e.g., we did not administer Comprehension items referring to seatbelts or libraries, as villagers rarely ride in automobiles, and there are no local public libraries).
We measured maternal intelligence on the Wechsler Abbreviated Scale of Intelligence (WASI: (The Psychological Corporation, 1999), a short and reliable measure of intelligence, across the age span. It consists of two Performance subtests (Block Design and Matrix Reasoning) and two Verbal (Vocabulary and Similarities) subtests. Our battery included Vocabulary and Matrix Reasoning subtests. Cultural considerations prompted slight changes to the Vocabulary subtest (e.g., we eliminated the definition of a “cart”, a vehicle not used in this setting).
Because neither these tests nor any other widely used assessments of intellectual function have been standardized for Bangladesh, we used Raw scores in all analyses.
Sociodemographic characteristics.
We assessed sociodemographic characteristics during a structured interview with a parent during the home visit, including maternal and paternal age, education and occupation, and the quality of home construction [concrete walls vs other (e.g., mud, thatched)] as a marker of socioeconomic status (SES). We asked about the presence of child disability that might impact our evaluations. We inquired about whether the child continued to reside in his or her childhood village, the child’s number of years of schooling completed, and whether the child was currently attending school or working. Child’s height, weight and head circumference were also measured.
Home environment.
During the home visit, we obtained information about nine items that described enriching materials and opportunities available to the child at home (see (Wasserman et al., 2011), as an analogue for a measure of the home environment found to contribute to child intelligence (HOME: (Caldwell & Bradley, 1984, 2003). The items included presence of a dictionary/reference book in the home; availability of sports gear; permission for the child to express opinions at home; daily participation in family meals; participation in regular household chores; the presence of family rules about peer contact; a visit to a science or art museum in the past year; visits with relatives or friends; and whether the child had made a trip away from the region in the preceding six months. Collectively these items had a Cronbach’s alpha of 0.65.
Translation and training.
All tests and interviews were translated (and back-translated) between Bangla (Bengali) and English with the incorporation of culturally appropriate adaptations (Wasserman et al., 2011). Two testers were trained (by GW) and continued with supervised practice sessions for two weeks. All written test responses were sent to Columbia University for entry and checked for valid ranges and missing data, prior to analysis.
Data analysis.
We calculated summary statistics to describe the sample characteristics for the whole sample and by quartile of current adolescent BAs. We used Chi-square and Kruskal-Wallis tests to detect group differences in categorical and continuous variables, respectively. Spearman correlation coefficient was used to examine bivariate association between continuous variables. Linear regression analysis was employed to examine associations between BAs and each of the five measures of intellectual function (Verbal Comprehension, Working Memory, Processing Speed, Perceptual Reasoning and Full Scale raw scores), with and without adjusting for potential confounding variables. We selected potential covariates, including sociodemographic and parental factors that were associated with WISC outcomes (p < 0.10). We used linear regression models to identify covariates with independent associations with at least one WISC-IV scale (p < 0.05). The final set of covariates included child’s sex, years of education, head circumference, maternal intelligence, paternal education and home construction wall type. Paternal education (no education, 1–5 years, above 5 years, missing) and house construction type (concrete vs. other) were defined categorically; the other variables were defined as continuous measures. We fit two models for WISC-IV outcomes. In Model 1, we examined contributions of BAs, mUAscr, and the six above-mentioned covariates. In Model 2, we added blood measures of other metals. The variables mUAscr, creatinine adjusted urinary As (UAs/Cr), BAs, BPb, BMn, BSe and BCd (all of which had right skewed distributions) were all transformed by natural logarithmic function before being included in the models to reduce the impact of extreme values and improve model fitting. Next, all analyses were repeated using UAs/Cr rather than BAs as the biomarker of As exposure.
To explore the interaction of BAs with other metals on WISC-IV outcomes we extended Model 2 by adding a term for BAs by another metal interaction. We used Chi-square and Wilcoxon rank sum tests to look for sex differences in all variables, and conducted regression analysis for the association between As exposure and WISC-IV outcomes using Model 2, separately for males and females. We used the Wald test to assess sex differences in the regression coefficients relating As exposure to the outcomes, and for relating other metals to the outcomes.
Confirmatory Mixtures Analyses.
To confirm the results from the linear regression analyses and further explore other possibly informative patterns, we implemented Bayesian Kernel Machine Regression (BKMR) to investigate the joint effects of co-exposure to BAs, BPb, BMn, BCd, BSe, and mWAs0 on Full Scale raw scores. Briefly, BKMR identifies important mixture members and accounts for the correlated structure of the mixture, estimating complex and potentially non-linear and non-additive exposure-response functions and evaluating high-order effects, i.e. interactions (Bobb et al., 2014; Coull et al., 2015). Blood metal concentrations were also log-transformed in the BKMR model to preserve consistency with the main analysis. BKMR assesses exposure to the mixture in the model using a flexible function of the metals in the mixture specified by a kernel function, while incorporating a component-wise variable selection process. The same covariates included in the main analysis with linear models were included in the BKMR model for confounding adjustment.
Results
Sample characteristics.
Table 1 presents information on test scores, As exposure and control variables, for the 697 adolescents with complete data by quartile of concurrent BAs. Beyond the expected differences in measures of As exposure, several sociodemographic features differed significantly across levels of current BAs, based on Chi-square and Kruskal-Wallis tests. Those with higher BAs were significantly more likely to be male (p < 0.01), to be currently employed (p < 0.0001), were less likely to be attending school (p < 0.01), and had marginally smaller head circumference (p < 0.07). Fathers of those at higher exposure had fewer years of schooling (p < 0.001); conversely, mothers of those with higher BAs exposure had more years of school (p < 0.05) but received lower WASI scores (p < 0.01); HOME scores for those at higher exposures were significantly lower (p < 0.001), and home construction was less likely to be based on concrete walls (p < 0.0001).
Table 1.
Sample Characteristics by Quartile of Current BAs
| Total n = 697 | Quartile 1 BAs≤2.20 μg /L (n=17 4) | Quartile 2 2.20<BAs≤3.48 μg /L (n=17 5) | Quartile 3 3.48<BAs≤5.81 μg /L (n=17 4) | Quartile 4 BAs >5.81 μg /L (n=17 4) | ||
|---|---|---|---|---|---|---|
| Measures | Mean ± SD or % (n) | Mean ± SD or % (n) | Mean ± SD or % (n) | Mean ± SD or % (n) | Mean ± SD or % (n) | p-value |
| Child characteristics | ||||||
| Male | 46.48 (324) | 37.93 (66) | 46.29 (81) | 45.40 (79) | 56.32 (98) | 0.0075 |
| Age (years) | 14.75 ± 0.69 | 14.73 ± 0.71 | 14.70 ± 0.65 | 14.77 ± 0.66 | 14.82 ± 0.72 | 0.2905 |
| Years in school | 6.86 ± 2.18 | 7.38 ± 1.80 | 6.93 ± 2.21 | 6.91 ± 2.13 | 6.24 ± 2.39 | <0.0001 |
| Currently attending school | 83.86 (582) | 91.28 (157) | 86.21 (150) | 81.61 (142) | 76.44 (133) | 0.0015 |
| Currently employed | 11.19 (78) | 4.02 (7) | 9.14 (16) | 12.64 22) | 18.97 (33) | <0.0001 |
| Head circumference (cm) | 51.67 ± 1.72 | 51.80 ± 1.80 | 51.75 ± 1.75 | 51.42 ± 1.65 | 51.72 ± 1.65 | 0.0676 |
| BMI | 18.28 ± 2.83 | 18.30 ± 3.03 | 18.55 ± 3.08 | 18.26 ± 2.77 | 18.01 ± 2.42 | 0.6497 |
| Family characteristics | ||||||
| Paternal age at baseline | 47.24 ± 7.15 (n=664) | 47.36 ± 7.12 (n=167) | 47.43 ± 7.62 (n=168) | 46.60 ± 6.84 (n=163) | 47.54 ± 7.01 (n=166) | 0.5445 |
| Paternal education (years) | 4.27 ± 4.14 (n=684) | 5.17 ± 4.20 (n=171) | 4.45 ± 4.19 (n=173) | 4.31 ± 4.23 (n=172) | 3.15 ± 3.72 (n=168) | 0.0001 |
| Paternal education 6–16 years 1–5 years None | 33.33 (228) 28.51(195) 38.16 (261) | 38.60 (66) 33.33 (57) 28.07 (48) | 36.99 (64) 25.43 (44) 37.57 (65) | 37.21 (64) 21.51 (37) 41.28 (71) | 20.24 (34) 33.93 (57) 45.83 (77) | 0.0002 |
| Maternal age (years) | 40.41 ± 5.17 | 40.09 ± 5.05 | 40.69 ± 5.50 | 40.10 ± 5.10 | 40.75 ± 5.01 | 0.3675 |
| Maternal education (years) | 3.62 ± 3.57 | 4.41 ± 3.81 | 3.58 ± 3.55 | 3.72 ± 3.61 | 2.78 ± 3.12 | 0.0008 |
| Maternal education 6–13 years 1–5 years None | 27.40 (191) 31.42 (219) 41.18 (287) | 34.48 (60) 31.03 (54) 34.48 (60) | 27.43 (48) 31.43 (55) 41.14 (72) | 29.31 (51) 29.31 (51) 41.38 (72) | 18.39 (32) 33.91 (59) 47.7 0 (83) | 0.0453 |
| Maternal IQ (WASI) | 33.36 ± 9.01 | 35.09 ± 9.38 | 33.43 ± 8.98 | 33.24 ± 9.38 | 31.69 ± 7.96 | .0039 |
| HOME Total | 3.71 ± 1.82 | 4.33 ± 1.63 | 3.58 ± 1.83 | 3.62 ± 1.84 | 3.31 ± 1.81 | <0.0001 |
| Home construction (concrete wall) | 28.12 (196) | 29.7.93 (66) | 32.57 (57) | 25.29 (44) | 16.67 (29) | <0.0001 |
| Exposure characteristics | ||||||
| Baseline well water As: WAs (μg /L) | 73.87 ± 103.97 | 40.20 ± 82.34 | 62.83 ± 100.68 | 76.78 ± 106.96 | 115.75 ± 109.36 | <0.0001 |
| Baseline well water Mn: WMn (μg /L) | 1284.18±882.04 (n=695) | 1030.98±965.82 | 1214.74±864.14 | 1411.19±895.85(n=173) | 1482.09±719.40(n=173) | <0.00 01 |
| Baseline maternal urinary As UAs/Cr (μg/gCr) | 283.22 ± 308.41 | 182.52 ± 225.96 | 235.63 ± 236.37 | 292.09 ± 357.04 | 422.89 ± 342.20 | <0.0001 |
| Current well water As: WAs (μg /L) | 37.57± 65.95 (n=674) | 4.06 ± 9.01 (n=162) | 13.46 ± 22.49 (n=169) | 31.19 ± 36.37 (n=171) | 99.16 ± 98.67 (n=172) | <0.0001 |
| Current well water Mn: WMn (μg /L) | 1141.65±1014.34 (n=682) | 742.01±886.70 (n=168) | 822.09±752.98 (n=171) | 1292.59±1002.78 (n=171) | 1699.63±1082.29 (n=172) | <0.0001 |
| Biological measures | ||||||
| UAs/Cr (μg /gCr) | 157.79 ± 208.87 (n=694) | 53.28 ± 50.23 | 90.05 ± 92.19 (n=174) | 153.43 ± 191.38 | 336.47 ± 284.05 (n=172) | <0.0001 |
| Urine %DMA | 74.32 ± 8.78 (n=693) | 74.16 ± 11.37 | 75.30 ± 8.21 (n=174) | 74.79 ± 7.76 (n=173) | 73.00 ± 7.03 (n=172) | 0.0004 |
| Urine %MMA | 11.17 ± 3.54 (n=693) | 10.62 ± 3.51 | 10.96 ± 3.40 (n=174) | 10.90 ± 3.43 (n=173) | 12.21± 3.65 (n=172) | 0.0003 |
| Urine %InAs | 14.51 ± 8.27 (n=693) | 15.22 ± 11.90 | 13.74 ± 7.50 (n=174) | 14.31 ± 6.88 (n=173) | 14.79 ± 5.26 (n=172) | 0.0090 |
| Blood Mn: BMn (μg /L) | 11.38 ± 3.70 | 11.55 ± 3.59 | 11.44 ± 3.99 | 11.32 ± 3.56 | 11.21 ± 3.65 | 0.6489 |
| Blood As: BAs (μg /L) | 4.84 ± 4.62 | 1.55 ± 0.35 | 2.78 ± 0.37 | 4.51 ± 0.66 | 10.54 ± 6.09 | <0.0001 |
| Blood Pb: BPb (μg /L) | 98.65 ± 43.02 | 97.28 ± 46.48 | 101.09 ± 41.03 | 97.29 ± 42.78 | 98.95 ± 41.84 | 0.4638 |
| Blood Cd: BCd (μg /L) | 0.61 ± 0.31 | 0.65 ± 0.33 | 0.65 ± 0.36 | 0.59 ± 0.30 | 0.55 ± 0.25 | 0.0312 |
| Blood Se: BSe (μg /L) | 132.29 ± 18.11 | 132.19 ± 17.47 | 134.01 ± 16.45 | 131.97 ± 19.61 | 130.99 ± 18.76 | 0.2964 |
| Hematocrit | 39.57 ± 5.57 (n=658) | 39.60 ± 5.57 (n=166) | 39.63 ± 5.92 (n=169) | 39.45 ± 5.70 (n=163) | 39.59 ± 5.09 (n=160) | 0.9617 |
| Child Intelligence (raw scores) | ||||||
| Full Scale | 177.63 ± 36.82 | 187.04 ± 37.13 | 181.91 ± 37.70 | 176.28 ± 33.36 | 165.25 ± 35.64 | <0.0001 |
| Working Memory | 27.39 ± 6.09 | 28.93 ± 6.15 | 27.66 ± 5.76 | 27.54± 5.73 | 25.43 ± 6.24 | <0.0001 |
| Verbal Comprehensi on | 39.28 ± 9.53 | 41.40 ± 9.47 | 40.44 ± 9.69 | 38.53 ± 9.01 | 36.73 ± 9.32 | <0.0001 |
| Perceptual Reasoning | 54.64 ± 15.18 | 57.20 ± 15.75 | 55.93 ± 16.26 | 53.87 ±13.85 | 51.54 ± 14.26 | 0.0032 |
| Processing Speed | 56.32 ± 14.16 | 59.51 ± 14.02 | 57.88 ± 14.55 | 56.33± 13.17 | 51.55 ± 13.74 | <0.0001 |
p-values are from testing group differences in categorical variables (Chi-square test) and in quantitative variables (Kruskal-Wallis test).
WMn measured both at HEALS baseline and in the current study were significantly higher across BAs quartiles (both p’s < 0.0001), although BMn was not, likely because BMn levels are relatively tightly regulated by physiologic mechanisms. Among other metals, only BCd differed significantly across BAs quartiles (p < 0.05). WISC-IV Full Scale and all Index scores were significantly lower at the higher range of current BAs (for Full Scale, Verbal Comprehension, Working Memory and Processing Speed raw scores, all p’s < 0.0001; for Perceptual Reasoning, p < 0.01).
Relationships among biomarkers.
Table 2 presents correlations among baseline maternal As exposures and adolescent UAs and blood concentrations of As, Mn, Pb, Cd and Se. As hypothesized, current participants’ BAs was significantly positively correlated with baseline maternal water (mWAs0) or urine As (r(695) =0.47, p <0.0001), although not substantially so, likely reflecting both our long-term As mitigation efforts (e.g., (Chen et al., 2007) and adolescents’ mobility relative to their home wells. BCd was significantly related to mWAs0 and to all other blood metal measures except for BSe: negatively for mWAs0 (r(695) =−0.16, p < 0.0001 ) and current BAs (r(695)= −0.11, p < 0.01), and positively for BPb ( r(695)=0.26, p < 0.0001) and BMn (r(695)= 0.20, p < 0.0001). Beyond its association with BCd, BPb was weakly correlated with BSe (r(695)= 0.12, p < 0.01).
Table 2.
Spearman correlation coefficient for inter-correlations among adolescents’ concurrent biomarkers and maternal baseline urinary and water arsenic (n = 697)
| mUAscr | cWAs | UAscr | BAs | BMn | BPb | BCd | BSe | |
|---|---|---|---|---|---|---|---|---|
| Baseline maternal water As (mWAs0) | 0.76*** | 0.35*** | 0.42*** | 0.47*** | −0.03 | −0.03 | −0.16*** | 0.03 |
| Baseline maternal urinary As (mUAscr) | 0.36*** | 0.44*** | 0.47*** | −0.03 | −0.04 | −0.20*** | 0.03 | |
| Concurrent water As (cWAs) n=674 | 0.63*** | 0.65*** | −0.05 | −0.08* | −0.16*** | 0.03 | ||
| Concurrent urinary As (UAscr) n=694 | 0.86*** | −0.04 | −0.06 | −0.16*** | −0.10** | |||
| BAs | −0.05 | 0.01 | −0.11** | −0.05 | ||||
| BMn | 0.06 | 0.20*** | 0.01 | |||||
| BPb | 0.26*** | 0.12** | ||||||
| BCd | 0.06 |
p < 0.05
p < 0.01
p < 0.0001
Associations between As exposure and child intelligence.
Table 3 shows associations between BAs and WISC-IV raw Full Scale and Index scores. Associations with sociodemographic features were all in the expected directions. Children with more schooling, those whose fathers had more schooling, and those whose mothers scored higher on the WASI had higher average WISC-IV scores. In addition, living in better-constructed homes and increasing head circumference were associated with higher WISC-IV scores. After adjusting for these features, and mUAscr, BAs was significantly negatively associated with all WISC-IV scores, except for Perceptual Reasoning (where the association approached significance). Altogether, these models explained between 28.7% and 49.8% of the variance in intellectual function outcomes, with BAs contributing between 0.33% and 0.62% of the explained variance. Derived from the estimated model parameters, the covariate-adjusted raw score loss (95% CI) for doubling BAs were −3.3 (−5.5, −1.1) on Full scale, −1.0 (−1.9, −0.2) on Processing Speed, −0.8 (−1.4, −0.1) on Verbal Comprehension, and −0.5 (−0.9, −0.1) on Working Memory.
Table 3.
Estimated regression coefficient B (standard error se) for the association between BAs and WISC-IV raw scores, with adjustment for socio-demographic features (n=697)
| Full Scale B (se) | Working Memory B (se) | Processing Speed B (se) | Verbal Compre-hension B (se) | Perceptual Reasoning B (se) | |
|---|---|---|---|---|---|
| House construction | 5.29 (2.40) * | 0.48 (0.44) | 0.61 (0.96) | 1.82 (0.72) * | 2.38 (1.18) * |
| Paternal Education (years, compared to None) | |||||
| Beyond grade school | 4.68 (2.72) | 1.47 (0.50) ** | 0.84 (1.08) | 1.95 (0.81) * | 0.42 (1.34) |
| Grade school only | 4.19 (2.57) | 0.77 (0.47) | 1.59 (1.03) | 1.44 (0.77) m | 0.40 (1.27) |
| Maternal Intelligence | 0.46 (0.12)*** | 0.07 (0.02) ** | 0.07 (0.05) | 0.14 (0.04) *** | 0.19 (0.06) ** |
| Child Education (years) | 9.87 (0.52) **** | 1.41 (0.10)**** | 3.82 (0.21) **** | 1.80 (0.16) **** | 2.84 (0.26) **** |
| Head circumference | 1.56 (0.60) ** | 0.07 (0.11) | 0.18 (0.24) | 0.42 (0.18) * | 0.88 (0.29) ** |
| Boy (compared to girl) | 3.69 (2.06) m | 0.90 (0.38) * | -3.93 (0.82) **** | 1.39 (0.62) * | 5.33 (1.01) **** |
| log(mUAscr) | 0.82 (1.38) | 0.07 (0.11) | 0.37 (0.55) | 0.37 (0.41) | 0.05 (0.68) |
| log(BAs) | −4.69 (1.62)** | −0.70 (0.30) * | −1.51 (0.65) * | −1.08 (0.48)* | −1.41 (0.79)m |
| ΣR2 | 49.83% | 38.41% | 45.90% | 32.99% | 28.68% |
| R2 attributable to log BAs | 0.62% | 0.50% | 0.47% | 0.48% | 0.33% |
p < 0.077
p < 0.05
p < 0.01
p < 0.001
p < 0.0001.
Next, we considered the further contributions of blood levels of Mn, Pb, Cd and Se (Supplemental Table 1). The pattern of associations was diverse, although for each WISC-IV outcome, associations with current BAs remained significant except for Perceptual Reasoning, where the estimate remained marginally significant. Adjusting for all other features, BCd was significantly negatively associated with Full Scale [B (se)=−5.1 (2.2), p < 0.05] and Verbal Comprehension scores [B (se)=−2.2 (0.7), p < 0.01], and BPb was significantly negatively associated with Working Memory scores [B (se)=−1.6 (0.5), p < 0.001] and with Verbal Comprehension scores [B (se)=−1.7 (0.8), p < 0.05]. BSe was associated with Full scale and some subscale scores in an inverse U-shape pattern, reflecting its effect as a nutrient while becoming toxic at high levels. There was no evidence of interaction of BAs with BMn or BCd on WISC scores. The interaction of BAs with BPb was only significant for Verbal Comprehension such that the negative impact of BAs became more profound with higher BPb.
Sex differences.
Because many effects of As exposure on health vary by sex (U.S. National Research Council, 2013), we next considered males and females separately. There were no significant sex differences in associations between BAs and outcomes. BAs remained significantly negatively associated with Full Scale and Verbal Comprehension scores for both sexes (p < 0.05), and associations for Working Memory were of marginal significance for both sexes (data not shown).
UAs/Cr.
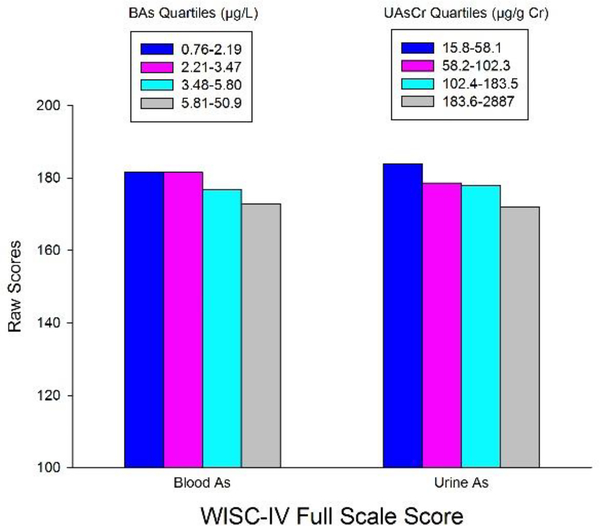
When we applied the same models using UAs/Cr as the predictor, associations with all outcome scores remained (and the association for Perceptual Reasoning now achieved statistical significance), as did the pattern of associations for other metals (BMn, BPb, BCd, BSe) (Supplemental Table 2). Based on Model 1, doubling UAs/Cr may result in a covariatea-djusted decrement of 3.0 (95% CI: 1.2–4.9) on raw full scale scores. The covariate-adjusted relationships between quartiles of BAs and UAs and WISC IV scores are illustrated in Figure 1. The mean difference (95%CI) in Full Scale scores between the highest and lowest quartiles of BAs was -corresponding difference of −1.5 (−2.7, −0.4) was smaller but still statistically significant.
Figure 1.
The relationships between quartiles of WAs and UAs and adjusted WISC-IV full scale raw scores. Scores were adjusted for child’s years 8.2 (−14.5, of education, head circumference, maternal intelligence, paternal education and home construction wall type. The mean difference in Full Scale scores between the highest and lowest quartiles of BAs was −8.2 (−14.5, −1.8); for WM the corresponding difference was smaller but still significant [ −1.5 (−2.7, −0.4)].
Confirmatory Mixtures Analyses.
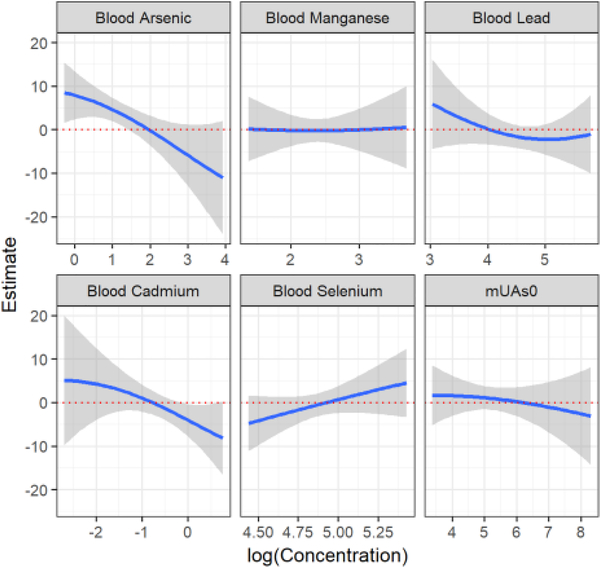
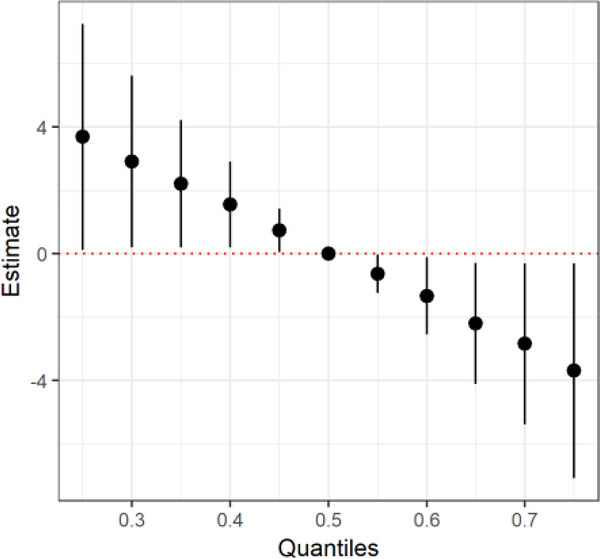
Analysis with BKMR largely confirms Model 2 for Full Scale scores. The posterior inclusion probability of BAs was 95.2%, followed by BCd (83.2%), BPb (78.9%), BSe (73.5%), BMn (56.9%), and finally mUAscr (50.9%). This means that BAs, followed by BCd, are the two most important components in this mixture. Figure 2 shows that the association between log-transformed metals and Full Scale score remains approximately linear while allowing for flexibility of associations and accounting for covariates. We observed significantly negative associations between both BAs and BCd and Full Scale raw scores. No other metals reached significance in the BKMR analysis, and there was no evidence of interaction among metals (results not shown). The overall effect of the mixture on Full Scale scores showed a consistent significant decrease in Full Scale raw score with increased exposure to the metals mixture (Figure 3).
Figure 2.
Metal-specific effect estimates of the mixture on Full Scale raw score of adolescents in Araihazar, Bangladesh estimated by Bayesian Kernel Machine Regression (BKMR). Model adjusted for child’s sex, years of education, head circumference, maternal intelligence, paternal education, and type of home construction (wall materials). Single predictor associations and 95% confidence bands for each metal with other metals fixed at the median. Estimate represents the predicted standardized Full Scale raw score. All metal concentrations (μg /L) were log transformed. mUAscr = mother’s urinary arsenic at HEALS baseline.
Figure 3.
Joint effect of the mixture on Full Scale raw score of adolescents in Araihazar, Bangladesh estimated by Bayesian Kernel Machine Regression (BKMR). Model adjusted for child’s sex, years of education, head circumference, maternal intelligence, paternal education, and type of home construction (wall materials). Overall effect of the mixture (estimates and 95% credible intervals), comparing Full Scale raw score when all exposures are at a particular quantile to the median. Estimate represents the predicted standardized Full Scale raw score. All metal concentrations (BAs, BMn, BPb, BCd, BSe, mUAscr (μg /g)) were log transformed.
Discussion.
In this relatively large study of adolescents with well-documented exposure data from the prenatal period to adolescence, current blood and urine As concentrations were consistently associated with deficits in intellectual function. These associations held with adjustment for an array of sociodemographic features and for blood concentrations of other neurotoxic metals and a beneficial element (BSe). In contrast, prenatal and childhood exposure, estimated from mUAscr, were unrelated to adolescents’ intellectual function. Associations with concurrent biomarkers of As exposure demonstrated dose-dependent relationships (Figure 1), and were similar in boys and girls.
Continuing neurodevelopmental risk.
The work presented here documents that exposure to As in childhood has an adverse impact on a wide range of intellectual functions, with implications for diminished performance likely to continue into adulthood. We found that, for these adolescents, concurrent BAs was more closely related to intellectual outcomes than was early life exposure (as reflected by maternal urinary As during pregnancy and early childhood). However, the current cross-sectional study design does not allow us to definitively tease apart the relative impacts of early life and concurrent As exposure.
Most previous investigations of arsenic exposure have focused on young children, with findings generally more robust for school-aged children (e.g., 6–12 years old) than for younger ones. Of the 12 well-controlled, confounder-adjusted, investigations of associations between As exposure and child intelligence (Rodriguez-Barranco et al., 2013), associations are weaker and more scattered for those examining infants and children younger than age six (Hamadani et al., 2010; Hamadani et al., 2011; Tofail et al., 2009). In comparison to our work with 10-year olds (Wasserman et al, 2004), our study of 6-year olds (Wasserman et al, 2007) reported that the magnitude of the association between water As and intellectual function was weaker, and the dose–response relationship was less stable. Of the above studies, some include adolescents within their samples, but none has examined adolescents in particular. This is important, since there is evidence that key structures and processing capacities that support working memory do not emerge until later childhood or early adolescence (Sander et al., 2012). Assessments at younger ages may not readily discriminate cognitive impact of exposure, since many frontal and prefrontal cortex functions are not fully operational until adolescence.
Metal Mixtures Analyses.
Research concerning the impact of exposures to mixtures of environmental chemicals on human health outcomes has become a priority because human populations are virtually never exposed to environmental chemicals one at a time. For example, two recent reports have described adverse associations of mixed metal exposures (umbilical cord concentrations of As, Mn and Pb) in a birth cohort of Bangladeshi children living in two districts with differing levels of exposures, and evaluated with scores on the Bayley Scales of Infant Development at 20–40 months of age (Rodrigues et al., 2016; Valeri et al., 2017). When blood lead levels were low, adverse effects of arsenic and manganese on neurodevelopment were observed. It is difficult to make direct comparisons of those findings with ours because of the differences in ages, the neuropsychological tests employed and their reliance largely on cord blood measurements. A prior systematic review of the literature (Claus Henn et al., 2014) concerning metal mixtures and children’s health identified 14 studies, nine of which dealt with neuropsychological outcomes in populations of various ages around the world. Among these are two of our own cross-sectional studies, which failed to observe As-Mn interactions on either intellectual function (Wasserman et al, 2011) or teacher-reported behavior (Khan et al., 2011) in 8- to 11-year old Bangladeshi children. The review article notes that exposure levels for both As and Mn in our two previous studies may have been so high that interactions were less likely, in that thresholds for toxicity of each element may have already been crossed (Claus Henn et al., 2014). A prospective cohort study of BPb and BMn levels in two year-old children in Thailand observed significantly lower scores on the Comprehensive Developmental Inventory for Infants and Toddlers (CDIIT) among those with high (>75th percentile) cord BPb and BMn as compared to those with lower BPb and BMn levels (Lin et al., 2013). In Mexican children 1–3 years of age, evaluated using the Bayley Scales of Infant Development II (Spanish version), an effect of increased Pb toxicity on mental and motor development was observed in those in the highest quintile of BMn, as compared to those with lower BMn levels (Claus Henn et al., 2012). A prospective study of prenatal exposure to Pb and Cd in Korea reported an adverse interaction between maternal pre-natal BPb and BCd on Bayley Scale mental development scores in 6-month olds (Kim et al., 2013).
In the current cross-sectional study of Bangladeshi adolescents, for whom we had measures of BAs, BCd, BPb, BMn and BSe, BKMR analyses revealed that BAs, followed by BCd, were the most important independent components of this mixture, with BPb and BSe close behind. The advantage of BKMR is that it allows the estimation of the exposure-response function for each metal, while also taking into account all the other metals included in the model, without any constraints on the shape of this exposure-response function. This means that this approach can estimate both positive and negative, as well as linear and non-linear, associations between each metal and the outcome (here, WISC score), while accounting for linear or non-linear co-metal confounding. Moreover, it also allows for all higher-order interactions (linear or non-linear) across the metals.
Mechanisms of As toxicity.
The molecular mechanisms of arsenic toxicity have been studied for decades, and include the inhibition of thiol-containing enzymes, reduced DNA repair, uncoupling of oxidative phosphorylation, and increased oxidative damage to important macromolecules (reviewed in (Hughes, Beck, Chen, Lewis, & Thomas, 2011; Watanabe & Hirano, 2013), as well as interruption of the expression and functioning of zinc-finger proteins (Zhou et al., 2011). A substantial body of evidence now indicates that arsenic also alters the epigenome in peripheral blood in humans (Howe et al., 2017; Pilsner et al., 2007, 2009) and in the brain cortex and hippocampus in mice (Cronican et al., 2013). The latter is notable in that the hippocampus plays roles in spatial memory and in the consolidation of short-term working memory into long-term memory.
A recent review (Tyler & Allan, 2014) summarized the numerous experimental findings indicating that As alters learning and memory in rodent models. Most studies have focused on the hippocampus, a brain region that is critically important for learning and memory. Working memory is frequently found to be vulnerable to As exposure, in both the present and in prior investigations (Wasserman et al., 2014; Wasserman et al., 2015). Rodent arsenic exposure paradigms and concentrations vary widely, and many studies exposed animals to As in drinking water with concentrations in the ppm range rather than the more relevant ppb range to which human populations are exposed. An exception (Martinez-Finley, Ali, & Allan, 2009) examined the consequences of pre- and perinatal exposure to 50 ppb sodium arsenate in drinking water to mice on biochemical and behavioral outcomes later in life. The investigators found that their exposed mice had reduced levels of corticosterone receptors in the hippocampus, where they are essential for functioning, and that the mice had experienced adverse effects on learning and memory. The latter finding is remarkably consistent with those of the current study of adolescents in Bangladesh.
Exposure to cadmium.
BCd was negatively associated with Full Scale and Verbal Comprehension scores, findings that are largely consistent with prior epidemiologic investigations. In two studies in China, cord blood serum Cd was negatively associated with WPPSI Verbal intelligence scores in 5-year olds (Liu et al., 2015) and with WPPSI-R Full Scale and Performance scores at age four (Tian et al., 2009), after adjustment for potential confounders. In Korea, early pregnancy maternal blood Cd concentration was inversely associated with Performance IQ in children ages 3–7 (Jeong et al., 2015). Similarly, in Bangladesh, after covariate adjustment, peri-conceptual, early childhood and concurrent Cd exposure measured via Cd concentrations in 5-year-olds’ urine have also been negatively associated with Verbal IQ and Full-Scale IQ (Kippler et al., 2012). In contrast, in a study in Spain, higher UCd was associated with lower Full Scale IQ in boys but not girls (Rodríguez-Barranco et al., 2014). Other investigations have reported a lack of association between maternal prenatal UCd and early childhood intelligence (Forns et al., 2014), or between early childhood BCd (in Pb-exposed children) and neurocognitive outcomes in the U.S. (Cao et al., 2009). Overall, the existing findings on Cd and IQ are somewhat mixed, and are often complicated by exposures to other metals.
Investigations in animals offer some insight regarding mechanisms. In comparison to controls, Cd-treated embryos show unclear morphologically distinct boundaries between brain regions, particularly in the mid- to hindbrain regions, changes in commitment of neural progenitor cells, as well as fewer differentiated neurons and glial cells, suggesting that Cd exposure may impair neurogenesis and neuronal differentiation (Chow, Hui, Lin, & Cheng, 2008). Other work in rats reports deficits in memory associated with Cd exposure (Haider et al., 2015).
Limitations.
As in our earlier work in 6 and 10 year-old children in Bangladesh (Wasserman et al., 2007; Wasserman et al., 2004), we cannot estimate the number of IQ points lost in relation to As exposure, because the culturally adapted version of the WISC-IV has not been standardized in Bangladesh. We note, however, that our study of 10 year-old children in Maine (Wasserman et al., 2014) found that in comparison to children drinking from household wells with < 5μg /L As, exposure to ≥ 5μg /L was associated with reductions of 5–6 points in both Full Scale IQ and most Index scores. In addition, because only a relatively small number of the current study participants (N = 107) were exposed to high levels of WAs during only the prenatal and early neonatal periods, we were unable to discern whether the consequences of As exposure on neurodevelopment may be due to exposure very early in life. Finally, because this study was conducted in one specific setting, it is not clear how generalizable these findings are to other places in the world.
Conclusions.
This cross-sectional study examines the consequences of exposure to As in drinking water in adolescence, a period when many brain functions achieve or approach full operational status. This relatively large study also carefully controlled for biomarkers of other potentially neurotoxic metals that are present in the environment. The findings are consistent with other studies of As exposure in younger children around the world (reviewed in (Rodriguez-Barranco et al., 2013), in that concurrent blood and urinary levels of As were adversely associated with Full Scale scores as well as sub-scales of the WISC-IV. We cannot rule out, however, that the observed findings may in fact be a consequence of lifetime As exposure in this study population. Nevertheless, collectively, we believe that this body of work, coupled with the mechanistic studies described above, strongly points to a causal link between inorganic As exposure and poorer intellectual functioning across a range of developmental periods, and that neurodevelopmental toxicity belongs in the top tier of concern, along with cardiovascular disease and various cancers.
Supplementary Material
HIGHLIGHTS.
Arsenic exposure is associated with deficits in intelligence in adolescents
Exposure to a mixture of 5 elements was associated with decreased intelligence
Study findings implicate cadmium as a neurotoxic element that deserves more attention
Arsenic exposure in childhood has an impact on a wide range of intellectual functions
Acknowledgements:
We acknowledge our Bangladeshi field staff and the people of Araihazar.
This work was supported by National Institute of Environmental Health Sciences grants: P42 ES010349, P30 ES009089, R01 ES028805, T32 ES007322 and S10 OD016384
Footnotes
The authors declare they have no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Ahsan H, Chen Y, Parvez F, Argos M, Hussain AI, Momotaj H,… Graziano JH (2005). Health Effects of Arsenic Longitudinal Study (HEALS): Description of a multidisciplinary epidemiologic investigation. Journal of Exposure Analysis and Environmental Epidemiology, 1–15. [DOI] [PubMed] [Google Scholar]
- ATSDR. (2015). Substance priority list. Retrieved from https://www.atsdr.cdc.gov//SPL/index.html
- Bobb JF, Valeri L, Claus HB, Christiani DC, Wright RO, Mazumdar M,… Coull BA (2014). Bayesian kernel machine regression for estimating the health effects of multi-pollutant mixtures.. Biostatistics, 16(3), 493–508. doi: 10.1093/biostatistics/kxu058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderon J, Navarro ME, Jimenez-Capdeville ME, Santos-Diaz MA, Golden A, Rodriguez-Leyva I,… Diaz-Barriga F (2001). Exposure to arsenic and lead and neuropsychological development in Mexican children. Environmental Research, 85(2), 69–76. [DOI] [PubMed] [Google Scholar]
- Caldwell BM, & Bradley RH (1984). Home Observation for Measurement of the Environment. Little Rock, AR: University of Arkansas at Little Rock. [Google Scholar]
- Caldwell BM, & Bradley RH (2003). Home Observation for Measurement of the Environment: Administration Manual. Tempe, AZ:: Family & Human Dynamics Research Institute, Arizona State University. [Google Scholar]
- Cao Y, Chen A, Radcliffe J, Dietrich KN, Jones RL, Caldwell K, & Rogan WJ (2009). Postnatal cadmium exposure, neurodevelopment, and blood pressure in children at 2, 5, and 7 years of age.. Environmental Health Perspectives, 117(10), 1580–1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, van Geen A, Graziano JH, Pfaff A, Madajewics M, Parvez F,… Ahsan H (2007). Reduction in urinary arsenic levels in response to arsenic mitigation efforts in Araihazar, Bangladesh. Environmental Health Perspectives, 115, 917–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng ZY, Zheng Y, Mortlock R, & van Geen A (2004). Rapid multi-element analysis of groundwater by high-resolution inductively coupled plasma mass spectrometry. Analytical and Bioanalytical Chemistry, 379, 513–518. [DOI] [PubMed] [Google Scholar]
- Chow ES, Hui MN, Lin CC, & Cheng SH (2008). Cadmium inhibits neurogenesis in zebrafish embryonic brain development. Aquatic Toxicology, 87(3), 157–169. [DOI] [PubMed] [Google Scholar]
- Claus Henn B, Coull BA, & Wright RO (2014). Chemical mixtures and children’s health. Curr Opin Pediatr, 26(2), 223–229. doi: 10.1097/mop.0000000000000067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus Henn B, Schnaas L, Ettinger AS, Schwartz J, Lamadrid-Figueroa H, HernandezAvila M,… Tellez-Rojo MM (2012). Associations of early childhood manganese and lead coexposure with neurodevelopment. Environ Health Perspect, 120(1), 126–131. doi: 10.1289/ehp.1003300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull BA, Bobb JF, Wellenius GA, Kioumourtzoglou MA, Mittleman MA, Koutrakis P, & Godleski JJ (2015). Part 1. Statistical Learning Methods for the Effects of Multiple Air Pollution Constituents.. Retrieved from [PubMed]
- Cronican AA, Fitz NF, Carter A, Saleem M, Shiva S, Barchowsky A,… Lefterov I (2013). Genome-wide alteration of histone H3K9 acetylation pattern in mouse offspring prenatally exposed to arsenic. 8,. PLOS One 8(2), e53478. doi: 10.1371/journal.pone.0053478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forns J, Fort M, Casas M, Caceres A, Guxens M, Gascon M,… Sunyer J (2014). Exposure to metals during pregnancy and neuropsychological development at the age of 4 years. NeuroToxicology, 40, 16–22. [DOI] [PubMed] [Google Scholar]
- Haider S, Anis L, Batool Z, Sajid I, Naqvi F, Khaliq S, & Ahmed S (2015). Short term cadmium administration dose dependently elicits immediate biochemical, neurochemical and neurobehavioral dysfunction in male rats.. Metabolic brain disease, 30(1):83–92. [DOI] [PubMed] [Google Scholar]
- Hamadani JD, Grantham-McGregor S, Tofail F, Nermell B, Fangstrom B, Huda SN,… Vahter M (2010). Pre- and postnatal aresenic exposure and child development at 18 months of age: a cohort study in rural Bangladesh. International Journal of Epidemiology, 39(4), 1206–1216. [DOI] [PubMed] [Google Scholar]
- Hamadani JD, Tofail F, Nermell B, Gardner R, Shiraji S, Bottai M,… Vahter M (2011). Critical windows of exposure for arsenic-associated impairment of cognitive function in pre-school girls and boys: a population-based cohort study. International Journal of Epidemiology, 40, 1593–1604. [DOI] [PubMed] [Google Scholar]
- Heinegard D, & Tiderstrom G (1973). Determination of serum creatinine by a direct colorimetric method. Clinica Chimica Acta, 43(3), 305–310. [DOI] [PubMed] [Google Scholar]
- Howe CG, Liu X, Hall MN, Ilievski V, Caudill MA, O Malysheva,… Gamble MV (2017). Sex specific associations between one-carbon metabolism indices and posttranslational histone modifications in arsenic exposed Bangladeshi adults. Cancer Epidemiology, Biomarkers & Prevention, 26(2), 261–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes MF, Beck BD, Chen Y, Lewis AS, & Thomas DJ (2011). Arsenic exposure and toxicology: A historical perspective. Toxicologic Science, 123, 305–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong KS, Park H, Ha E, Hong YC, Ha M, H P,… Kim Y (2015). Performance IQ in children is associated with blood cadmium concentration in early pregnancy. Journal of trace elements in medicine and biology, 30, 107–111. [DOI] [PubMed] [Google Scholar]
- Khan K, Factor-Litvak P, Wasserman GA, Liu X, Ahmed E, Parvez F,… Graziano JH (2011). Manganese exposure from drinking water and children’s classroom behavior in Bangladesh. Environ Health Perspect, 119(10), 1501–1506. doi: 10.1289/ehp.1003397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y, Ha EH, Park H, Ha M, Kim Y, Hong YC,… Kim BN (2013). Prenatal lead and cadmium co-exposure and infant neurodevelopment at 6 months of age: the Mothers and Children’s Environmental Health (MOCEH) study. Neurotoxicology, 35, 15–22. doi: 10.1016/j.neuro.2012.11.006 [DOI] [PubMed] [Google Scholar]
- Kippler M, F. T, Hamadani JD, Gardner RM, Grantham-McGregor SM, Bottai M, & Vahter M (2012). Early-life cadmium exposure and child development in 5-year-old girls and boys: a cohort study in rural Bangladesh. Environmental Health Perspectives, 120(10), 1462–1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levander O (1977). Metabolic relationships between arsenic and selenium. Environmental Health Perspectives, 19, 159–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CC, Chen YC, Su FC, Lin CM, Liao HF, Hwang YH,… Chen PC (2013). In utero exposure to environmental lead and manganese and neurodevelopment at 2 years of age. Environ Res, 123, 52–57. doi: 10.1016/j.envres.2013.03.003 [DOI] [PubMed] [Google Scholar]
- Liu Y, Chen Q, Wei X, Chen L, Zhang X, Chen K,… Li T (2015). Relationship between perinatal antioxidant vitamin and heavy metal levels and the growth and cognitive development of children at 5 years of age.. Asia Pacific journal of clinical nutrition, 24(4), :650–658. [DOI] [PubMed] [Google Scholar]
- Martinez-Finley EJ, Ali AM, & Allan AM (2009). Learning deficits in C57BL/6J mice following perinatal arsenic exposure: consequence of lower corticosterone receptor levels?. Pharmacology Biochemistry & Behavior, 94, 271–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nixon DE, Mussmann GV, Ecktahdahl SJ, & Moyer TP (1991). Total arsenic in urine: Palladium-persulfate vs nickel as a matrix modifier for graphite furnace atomic absorption spectrophotometry. Clinical Chemistry, 37, 1575–1579. [PubMed] [Google Scholar]
- Pilsner JR, Liu X, Ahsan H, Ilievski V, Slavkovich V, Levy D,… Gamble MV (2007). Genomic methylation of peripheral blood leukocyte DNA: influences of arsenic and folate in Bangladeshi adults. American Journal of Clinical Nutrition, 86, 1179–1186. [DOI] [PubMed] [Google Scholar]
- Pilsner JR, Liu X, Ahsan H, Ilievski V, Slavkovich V, Levy D,… Gamble MV (2009). Folate deficiency, hyperhomocysteinemia, low urinary creatinine, and hypomethylation of leukocyte DNA are risk factors for arsenic-induced skin lesions. Environmental Health Perspectives, 117(2), 254–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruszkowski E, Neubauer K, & Thomas R (1998). An overview of clinical applications by Inductively Coupled Plasma Mass Spectrometry. Atomic Spectroscopy, 19(4), 111–115. [Google Scholar]
- Reuter W, Davidowski L, & Neubauer K (2003). Speciation of Five Arsenic Compounds in Urine by HPLC/ICP-MS PerkinElmerSCIEX
- Rodrigues EG, Bellinger DC, Valeri L, Hasan MO, Quamruzzaman Q, Golam M,… Mazumdar M (2016). Neurodevelopmental outcomes among 2- to 3-year-old children in Bangladesh with elevated blood lead and exposure to arsenic and manganese in drinking water. Environ Health, 15, 44. doi: 10.1186/s12940-016-0127-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Barranco M, Lacasana M, Aguilar-Garduno C, Alguacil J, Gil F, Gonzalez-Alzaga B, & Rojas-Garcia A (2013). Association of arsenic, cadmium and manganese exposure with neurodevelopment and behavioural disorders in children: A systematic review and meta-analysis. Science of the Total Environment, 454–455, 562–577. doi: 10.1016/j.scitoenv.2013.03.047 [DOI] [PubMed] [Google Scholar]
- Rodríguez-Barranco M, Lacasaña M, Gil F, Lorca A, Alguacil J, Rohlman DS,… Aguilar-Garduño C (2014). Cadmium exposure and neuropsychological development in school children in southwestern Spain. Environmental Research, 134, 66–73. [DOI] [PubMed] [Google Scholar]
- Rosado JL, Ronquillo D, Kordas K, Rojas O, Alatorre J, Lopez P,… Stoltzfus R (2007). Arsenic exposure and cognitive performance in Mexican schoolchildren. Environmental Health Perspectives, 115(9), 1371–1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sander MC, Lindenberger U, & Werkle-Bergner M (2012). Lifespan age differences in working memory: A two-component framework. Neuroscience and Biobehavioral Reviews, 36, 2007–2033. [DOI] [PubMed] [Google Scholar]
- Stroh A (1988). Determination of Pb and Cd in whole blood using isotope dilution ICP-MS. Atomic Spectroscopy, 14(5), 141–143. [Google Scholar]
- The Psychological Corporation. (1999). Wechsler Abbreviated Scale of Intelligence manual. San Antonio, TX: The Psychological Corporation. [Google Scholar]
- Tian LL, Zhao YC, Wang XC, Gu JL, Sun ZJ, Zhang YL, & Wang JX (2009). Effects of Gestational Cadmium Exposure on Pregnancy Outcome and Development in the Offspring at Age 4.5 Years. Biological Trace Element Research, 132(1–3), 51–59. [DOI] [PubMed] [Google Scholar]
- Tofail F, Vahter M, Hamadani JD, Nermell B, Huda SN, Yunus M,… Grantham-McGregor SM (2009). Effect of arsenic exposure during pregnancy on infant development at 7 months in rural Matlab, Bangladesh. Environmental Health Perspectives, 117(2), 288–293. doi: 10.1289/ehp.11670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tolins M, Ruchirawat M, & Landrigan PJ (2014). The developmental neurotoxicity of arsenic: Cognitive and behavioral consequnces of early life exposure. Annals of Global Health, 80, 303–314. doi:dx.doi.org/ 10.1016/j.aogh.2014.09.005 [DOI] [PubMed] [Google Scholar]
- Tyler CR, & Allan AM (2014). The effects of arsenic exposure on neurological and cognitive dysfunction in human and rodent studies: A review. Current Environmental Health Report, 1, 132–147. doi: 10.1007/s40572-014-0012-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. National Research Council. (2013). Critical aspects of the EPA’s IRIS assessment of inorganic arsenic: Interim report Retrieved from Washington, D.C.: [Google Scholar]
- Valeri L, Mazumdar MM, Bobb JF, Claus Henn B, Rodrigues E, Sharif OIA,… Wright RO (2017). The Joint Effect of Prenatal Exposure to Metal Mixtures on Neurodevelopmental Outcomes at 20–40 Months of Age: Evidence from Rural Bangladesh. Environ Health Perspect, 125(6), 067015. doi: 10.1289/ehp614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Geen A, Ahmed KM, Seddique AA, & Shamsudduha M (2003). Community wells to mitigate the current arsenic crisis in Bangladesh. Bulletin of the World Health Organization, 82, 632–638. [PMC free article] [PubMed] [Google Scholar]
- van Geen A, Cheng ZY, Seddique AA, Hoque A, Gelman A, Graziano JH,… Ahmed KM (2005). Reliability of a commercial kit to test groundwater for arsenic in Bangladesh. Environmental Science and Technology, 39(1), 299–303. [PubMed] [Google Scholar]
- von Ehrenstein OS, Poddar S, Yuan Y, Mazumder DG, Eskenazi B, Basu A,… Smith AH (2007). Children’s intellectual function in relation to arsenic exposure. Epidemiology, 18(1), 44–51. [DOI] [PubMed] [Google Scholar]
- Wasserman GA, Liu X, LoIacono NJ, Kline JK, Factor-Litvak P, van Geen A,… Graziano JH (2014). A cross-sectional study of well water arsenic and child IQ in Maine schoolchildren. Environmental Health, 13(1), 13–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman GA, Liu X, Parvez F, Ahsan H, Factor-Litvak P, Kline JK,… Graziano JH (2007). Water arsenic exposure and intellectual function in 6-year-old children in Araihazar, Bangladesh. Environmental Health Perspectives, 115(1), 285–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman GA, Liu X, Parvez F, Ahsan H, Factor-Litvak P, van Geen A,… Graziano JH (2004). Water arsenic exposure and children’s intellectual function in Araihazar, Bangladesh. Environmental Health Perspectives, 112(13), 1329–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman GA, Liu X, Parvez F, Factor-Litvak P, Ahsan H, Levy D,… Graziano JH (2011). Arsenic and manganese exposure and children’s intellectual function. Neurotoxicology, 32, 450–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasserman GA, Liu X, Parvez F, Factor-Litvak P, Kline JK, Siddique AB,… Graziano JH (2015). Child intelligence and reductions in water arsenic and manganese: A two-year follow-up study in Bangladesh. Environmental Health Perspectives, 123(7), 1114–1120. doi:dx.doi.org/ 10.1289/ehp.1509974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe T, & Hirano S (2013). Metabolism of arsenic and its toxicological relevance. Archives of Toxicology, 87, 969–979. [DOI] [PubMed] [Google Scholar]
- Wechsler D (1991). Manual for the WISC-III. San Antonio, TX: Psychological Corporation. [Google Scholar]
- Wechsler D (2003). WISC-IV Administration and Scoring manual. San Antonio, TX: Harcourt Assessment. [Google Scholar]
- WHO. (2008). Guidelines for drinking-water quality: Incorporating first and second addenda Geneva: WHO Press; Retrieved from http.who.int/water_sanitation_health/dwq/gdwq3/index.html. [PubMed] [Google Scholar]
- Wright RO, Woolf AD, Jim R, & Bellinger DC (2006). Neuropsychological correlates of hair arsenic, manganese, and cadmium levels in school-age children residing near a hazardous waste site. Neurotoxicology, 27, 210–216. [DOI] [PubMed] [Google Scholar]
- Zhou X, Sun X, Cooper KL, Wang F, Liu KJ, & Hudson LG (2011). Arsenite interacts selectively with zinc finger proteins containing C3H1 or C4 motifs Journal of Biological Chemistry, 286, 22855–22863. doi: 10.1074/jbc.M111.232926 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.