Supplemental Digital Content is available in the text.
Keywords: angioplasty, carotid stenosis, coronary artery disease, endarterectomy, risk
Abstract
Background and Purpose—
We investigated whether procedural stroke or death risk of carotid artery stenting (CAS) compared with carotid endarterectomy (CEA) is different in patients with and without history of coronary heart disease (CHD) and whether the treatment-specific impact of age differs.
Methods—
We combined individual patient data of 4754 patients with symptomatic carotid stenosis from 4 randomized trials (EVA-3S [Endarterectomy Versus Angioplasty in Patients With Symptomatic Severe Carotid Stenosis], SPACE [Stent-Protected Angioplasty Versus Carotid Endarterectomy], ICSS [International Carotid Stenting Study], and CREST [Carotid Revascularization Endarterectomy Versus Stenting Trial]). Procedural risk was defined as any stroke or death ≤30 days after treatment. We compared procedural risk between both treatments with Cox regression analysis, stratified by history of CHD and age (<70, 70–74, ≥75 years). History of CHD included myocardial infarction, angina, or coronary revascularization.
Results—
One thousand two hundred ninety-three (28%) patients had history of CHD. Procedural stroke or death risk was higher in patients with history of CHD. Procedural risk in patients treated with CAS compared with CEA was consistent in patients with history of CHD (8.3% versus 4.6%; hazard ratio [HR], 1.96; 95% CI, 0.67–5.73) and in those without (6.9% versus 3.6%; HR, 1.93; 95% CI, 1.40–2.65; Pinteraction=0.89). In patients with history of CHD, procedural risk was significantly higher after CAS compared with CEA in patients aged ≥75 (CAS-to-CEA HR, 2.78; 95% CI, 1.32–5.85), but not in patients aged <70 (HR, 1.71; 95% CI, 0.79–3.71) and 70 to 74 years (HR, 1.09; 95% CI, 0.45–2.65). In contrast, in patients without history of CHD, procedural risk after CAS was higher in patients aged 70 to 74 (HR, 3.62; 95% CI, 1.80–7.29) and ≥75 years (HR, 2.64; 95% CI, 1.52–4.59), but equal in patients aged <70 years (HR, 1.05; 95% CI, 0.63–1.73; 3-way Pinteraction=0.09).
Conclusions—
History of CHD does not modify procedural stroke or death risk of CAS compared with CEA. CAS might be as safe as CEA in patients with history of CHD aged <75 years, whereas for patients without history of CHD, risk after CAS compared with CEA was only equal in those aged <70 years.
About 10% to 15% of ischemic strokes are caused by atherosclerotic stenosis of the internal carotid artery.1 Carotid endarterectomy (CEA) and carotid artery stenting (CAS) both reduce the long-term risk of ipsilateral stroke in patients with symptomatic internal carotid artery stenosis, but the 30-day stroke or death risk is higher after CAS than after CEA.2
To improve the balance between risk and benefit of treatment, it is important to identify patient characteristics that are associated with higher periprocedural risks of CAS or CEA. The risk of CAS depends strongly on age, with increasing periprocedural risk at older ages for patients assigned to CAS and the absence of an effect of age on risk for patients assigned to CEA.3 Furthermore, clinicians may prefer CAS instead of CEA in patients with a history of coronary heart disease (CHD) because the periprocedural risk of myocardial infarction (MI) is lower after CAS compared with CEA in randomized trials.2 A recent meta-analysis showed that history of CHD increased 30-day risk of MI in patients who underwent CEA, but not in those who underwent CAS.4 Another study showed a trend toward increased periprocedural stroke or death risk of CAS compared with CEA in patients without history of CHD, but more similar risks in the 2 treatment groups in patients with history of CHD.5
The influence of age on procedural risks in patients with history of CHD is unknown. Therefore, we studied the association between age and procedural risk stratified by history of CHD. We hypothesized that if CAS is as safe as CEA in patients with history of CHD, the previously reported age cutoff of ≥70 years at which CEA is clearly superior to CAS3 might shift to a higher age in patients with history of CHD.
Methods
Study Population and Design
We used individual patient data from 4 trials that randomly assigned patients with symptomatic carotid stenosis to undergo CAS or CEA and that are pooled by the Carotid Stenosis Trialists’ Collaboration: the EVA-3S trial (Endarterectomy Versus Angioplasty in Patients With Symptomatic Severe Carotid Stenosis),6 the SPACE trial (Stent-Protected Angioplasty Versus Carotid Endarterectomy),7 the ICSS (International Carotid Stenting Study),8 and the CREST (Carotid Revascularization Endarterectomy Versus Stenting Trial).9 In total, these 4 trials included 4754 patients with symptomatic moderate to severe internal carotid artery stenosis (≥50% lumen narrowing on imaging according to the NASCET [North American Symptomatic Carotid Endarterectomy Trial] method10). Detailed methods were described previously.6–9 Requests for anonymized pooled data will be considered by the Carotid Stenosis Trialists’ Collaboration Steering Committee (Leo.Bonati@usb.ch).
For the purposes of this post hoc subgroup analysis, we used trial-specific definitions of history of CHD (Table I in the online-only Data Supplement). History of CHD included any of the following: CHD (not further specified), MI, angina, or any type of coronary revascularization.
Outcome Measures
The primary outcome measure was any stroke or death during the procedural period (within 30 days after the procedure). The secondary outcome measure was any stroke, MI, or death during the procedural period.
Stroke was defined as the occurrence of acute symptoms of focal neurological dysfunction that lasted >24 hours and resulted from intracranial vascular disturbance (ischemic or hemorrhagic). Diagnosis of MI required presence of at least 2 of 3 criteria: history of typical chest pain, development of specific abnormalities on an ECG, or rise of specific cardiac enzyme levels. MI was not a prespecified end point in SPACE; nevertheless, adverse event reports from this trial were adjudicated post hoc using the criteria for MI stated above. In CREST, MI was diagnosed only if patients had elevated cardiac enzyme levels and either history of typical chest pain or specific abnormalities on an ECG. Because of the different definition in CREST, we did not include MI in the primary outcome measure and analyzed the secondary outcome measure in patients from EVA-3S, SPACE, and ICSS only.
Statistical Analysis
The primary analysis for the primary and secondary outcome measures was on a per-protocol (PP) basis including patients who received their randomly allocated treatment and who did not suffer a stroke before the procedure. Analysis was based on the first occurrence of a primary outcome event within 30 days after the procedure. Cox proportional hazards analysis was performed to estimate hazard ratios (HRs) and 95% CIs adjusted for trial. The proportional hazards assumption was evaluated visually with log-minus-log plots. Potential effect modification by history of CHD was analyzed by including an interaction term in the model. A priori, we considered P <0.10 suggestive of effect modification.
We assessed if the previously reported treatment-by-age effect modification3 was consistent by history of CHD, by adding a 3-way interaction term (treatment×age×history of CHD) to the model. We classified age into 3 groups (<70, 70–74, and ≥75 years) and assessed the change in procedural risk with increasing age stratified by treatment and history of CHD. We used this classification of age because we sought to examine whether CAS is a safe alternative to CEA until a higher age in patients with history of CHD than the previously reported cutoff of 70 years in the overall group.
Additional analyses for the primary outcome measure included an intention-to-treat (ITT) analysis including events that occurred within 120 days after randomization in all patients who were randomized, irrespective of their compliance with the study protocol.
All analyses were done with R version 3.4.0.
Results
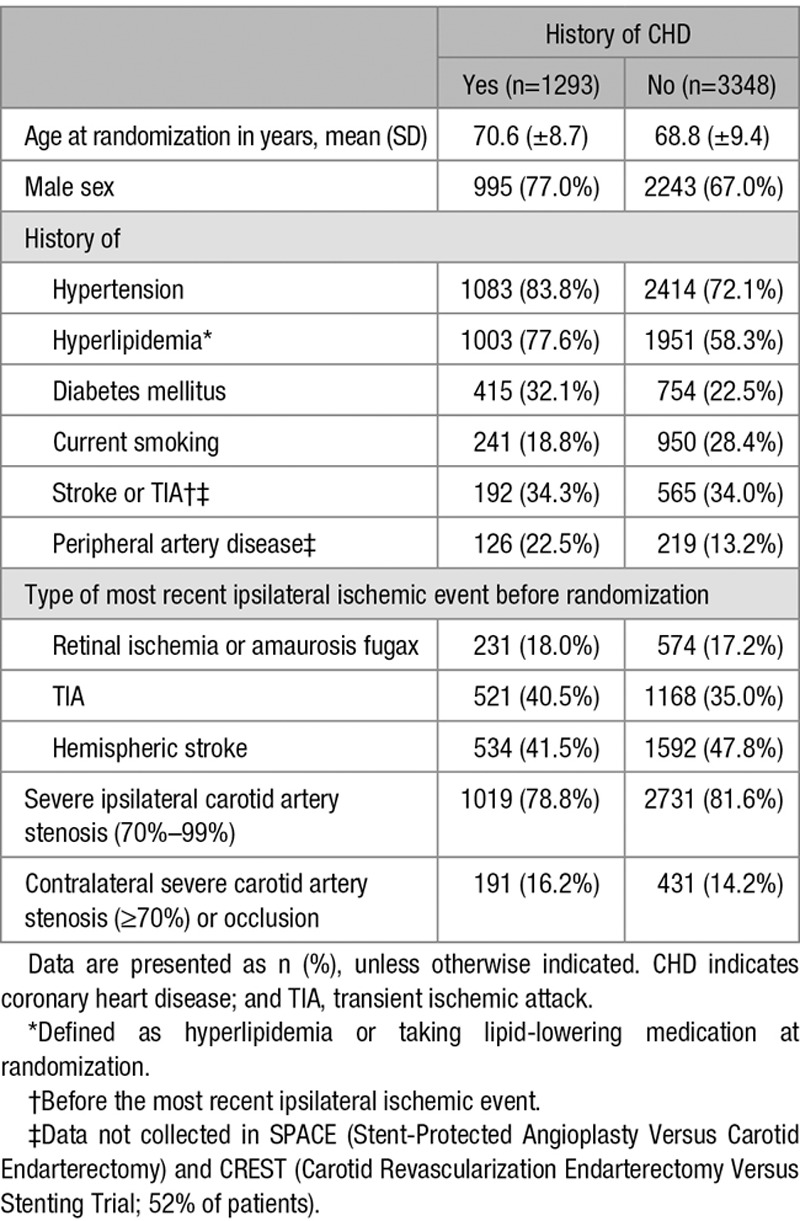
Of the 4754 patients, 113 (2%) patients had missing data on history of CHD and these patients were excluded from the analyses. Of the remaining 4641 patients, 1293 (28%) had history of CHD; this proportion varied across the 4 trials (range 18%–38%; Table I in the online-only Data Supplement). Patients with history of CHD were more often male and more often had a history of hypertension, hyperlipidemia, diabetes mellitus, and peripheral artery disease than patients without history of CHD. Proportion of current smokers was higher in patients without history of CHD (Table 1).
Table 1.
Baseline Characteristics in Intention-to-Treat Population With and Without History of CHD
Of the 4641 patients, 4486 remained for the PP analyses. In this group, stroke or death within 30 days after the procedure occurred in 251 (5.6%) patients (220 nonfatal strokes and 31 deaths). This risk was consistently higher in patients treated with CAS compared with CEA, both in patients with (8.3% CAS versus 4.6% CEA; HR, 1.96; 95% CI, 0.67–5.73) and in those without history of CHD (6.9% CAS versus 3.6% CEA; HR, 1.93; 95% CI, 1.40–2.65), and the CAS-to-CEA ratio was consistent between the subgroups (Pinteraction=0.89; Figure 1). Results were essentially the same for the ITT analysis of the primary outcome measure (Pinteraction=0.80; Figure I in the online-only Data Supplement). The results for the secondary outcome were virtually the same, with stroke, MI, or death within 30 days occurring in 128 (7.7%) patients after CAS and in 78 (4.8%) patients after CEA from EVA-3S, SPACE, and ICSS. The CAS-to-CEA ratio was consistent between patients with and without history of CHD (HR, 1.44 versus 1.74; Pinteraction=0.57; Figure II in the online-only Data Supplement).
Figure 1.
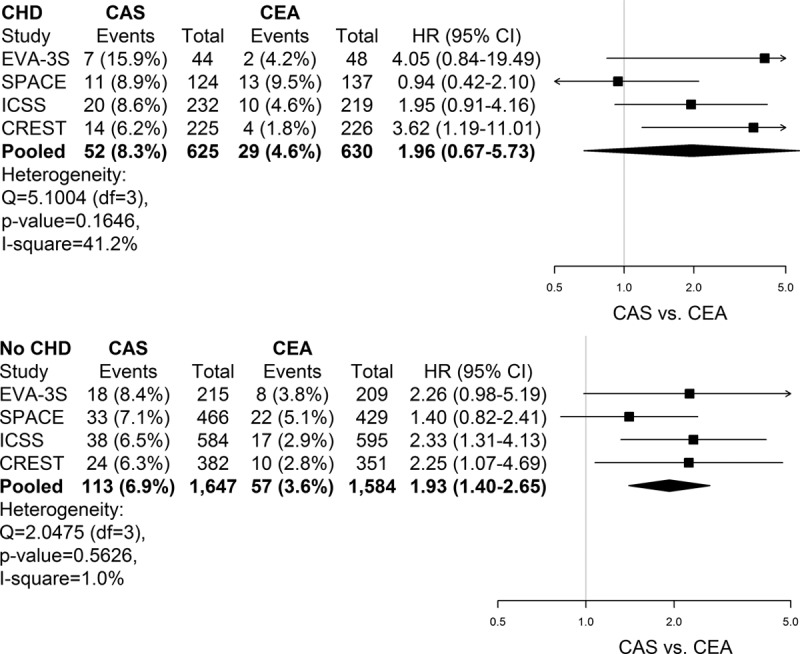
Treatment hazard ratios (HRs) of stroke or death within 30 days after treatment according to history of coronary heart disease (CHD). Forest plots of carotid artery stenting (CAS) vs carotid endarterectomy (CEA) HRs in individual trials and in pooled analysis of patients with and without a history of CHD. Data are presented as n (%), unless otherwise indicated. Analysis was performed on a per-protocol basis. Interaction P value (stratified across trials): 0.89. CREST indicates Carotid Revascularization Endarterectomy Versus Stenting Trial; EVA-3S, Endarterectomy Versus Angioplasty in Patients With Symptomatic Severe Carotid Stenosis; ICSS, International Carotid Stenting Study; and SPACE, Stent-Protected Angioplasty Versus Carotid Endarterectomy.
Numbers and risks of stroke or death within 30 days of CAS and CEA, as well as CAS-to-CEA ratios for each age group, stratified by history of CHD, are provided in Table 2 and Figure 2. In patients without history of CHD, the CAS-to-CEA HR increased with advancing age, from equal risk for patients aged <70 years (HR, 1.05; 95% CI, 0.63–1.73) to increased risk for those aged 70 to 74 years (HR, 3.62; 95% CI, 1.80–7.29) and for those aged ≥75 years (HR, 2.64; 95% CI, 1.52–4.59). This increasing ratio was caused by an increasing event risk in CAS-treated patients, while risk was relatively stable in the CEA-treated patients across the age spectrum. The increasing risk after CAS was primarily driven by an increase in strokes, with a higher CAS-to-CEA HR across age strata (Table II in the online-only Data Supplement). In contrast, in patients with history of CHD, the CAS-to-CEA HR was equal in those aged <70 years (HR, 1.71; 95% CI, 0.79–3.71) and those aged 70 to 74 years (HR, 1.09; 95% CI, 0.45–2.65), whereas it was increased in those aged ≥75 years (HR, 2.78; 95% CI, 1.32–5.85). As previously reported, there was evidence of a treatment-by-age interaction for patients without history of CHD. Introduction of a 3-way interaction term (treatment×age×history of CHD) did suggest that the age modification of treatment effect was influenced by history of CHD status in the PP analysis (P=0.09).
Table 2.
Treatment HRs of Stroke or Death Within 30 Days After Treatment Stratified by Age and History of CHD
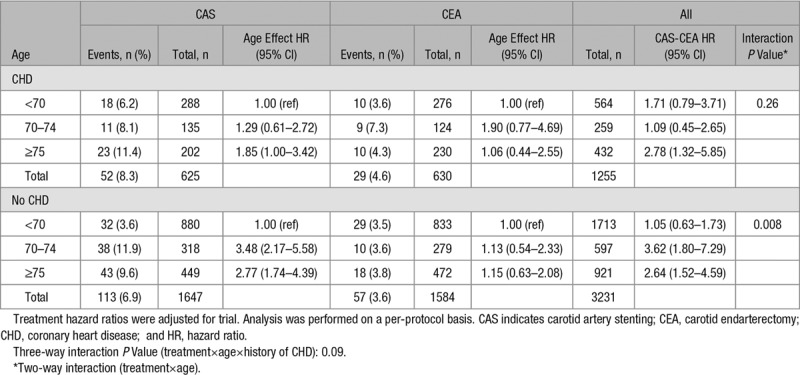
Figure 2.
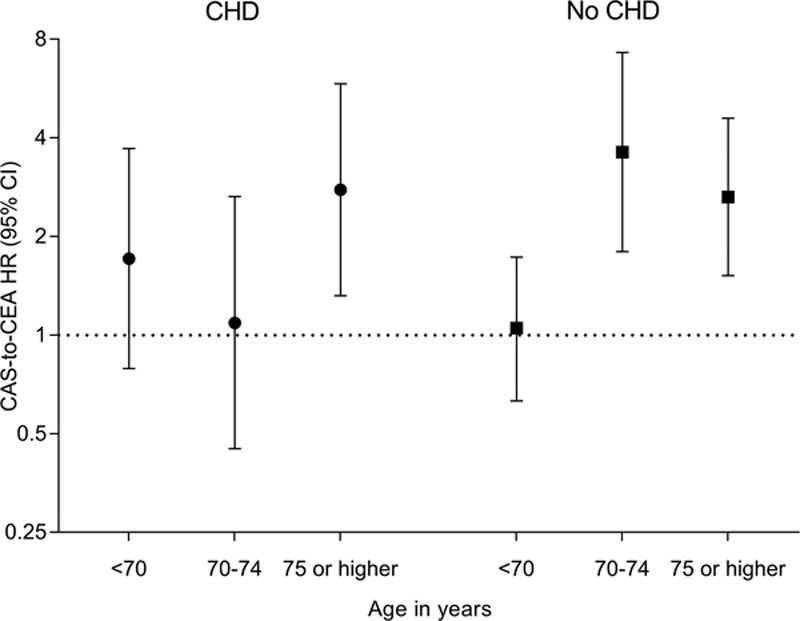
Treatment hazard ratios (HRs) of stroke or death within 30 days after treatment stratified by age and history of coronary heart disease (CHD). Treatment HRs were adjusted for trial. Analysis was performed on a per-protocol basis. CAS indicates carotid artery stenting; CEA, carotid endarterectomy; CHD, coronary heart disease; and HR, hazard ratio.
Results were essentially similar for the ITT analysis of the primary outcome measure and the PP analysis of the secondary outcome measure, with the exception of the tests for a 3-way interaction (treatment×age×history of CHD) which were not statistically significant (P=0.25 and P=0.11; Figures III and IV in the online-only Data Supplement; Tables III and IV in the online-only Data Supplement).
Discussion
Our study showed that patients with symptomatic carotid stenosis and history of CHD had a higher procedural risk of stroke or death after CAS and CEA compared with patients without history of CHD, but CAS-to-CEA HRs were similar between the 2 groups. Therefore, CEA should not be avoided in patients with history of CHD. In patients with history of CHD, procedural stroke or death risk was almost equal after CAS and CEA for those younger than age 75 years, but higher after CAS relative to CEA for those aged 75 and older, whereas in patients without history of CHD, risk was only equal for those younger than age 70 years. However, because we did not find consistent evidence for modification of treatment effect by age and history of CHD in both the PP and the ITT analyses and for both the primary and secondary outcome measures, these results must be interpreted with caution.
The previous pooled analysis of EVA-3S, SPACE, and ICSS showed a trend toward a lower CAS-to-CEA risk ratio in patients with history of CHD compared with patients without history of CHD (relative risk, 1.26 versus 1.66).5 With the addition of patients with symptomatic carotid stenosis enrolled in CREST, the HRs in the group with and without history of CHD became more similar (HR, 1.51 versus 1.61; Figure I in the online-only Data Supplement). Hence, our results did not confirm our hypothesis that CAS is as safe as CEA in patients with history of CHD. In CREST, the CAS-to-CEA HR in patients with history of CHD was higher than in the other 3 trials (HR, 2.54; 95% CI, 1.11–5.80; Figure I in the online-only Data Supplement). This may be caused by differences in the study population characteristics of patients with history of CHD between the 4 trials because of differences in the definition of history of CHD. However, because the confidence intervals of the CAS-to-CEA HRs in the 4 trials are wide and overlap, this may also be caused by chance alone.
The randomized SAPPHIRE trial (Stenting and Angioplasty With Protection in Patients at High Risk for Endarterectomy) investigated whether the presence of certain coexisting conditions that potentially increase the risk posed by CEA, among which clinically significant cardiac disease, influenced the safety of CAS compared with CEA. Stroke, MI, or death within 30 days after the procedure occurred in fewer patients who underwent CAS (4.8%) compared with those who underwent CEA (9.8%).11 These results are different from our results, which is most likely explained by differences between study populations: the SAPPHIRE trial included both patients with symptomatic and asymptomatic carotid stenosis, who were considered at high risk of complications after CEA but of whom only a part (16.2%) had clinically significant CHD as coexisting condition. The trials contributing data to our pooled analysis enrolled symptomatic patients at standard surgical risk who were equally suited for both procedures.
Strokes contributed most to the composite primary and secondary outcome measure in our study, irrespective of history of CHD, whereas the absolute risk of MI was low. Indeed, CEA was associated with higher absolute risk of MI in patients with history of CHD versus those without history of CHD (0.7% versus 0.3%), whereas this was not the case for CAS (0.2% versus 0.2%), which is consistent with the findings of a recent meta-analysis.4 However, the number of MIs was too small to examine effect modification by history of CHD for this outcome alone.
In 2 previous large cohort studies of patients who underwent different types of noncardiac surgery (among which vascular surgery) of whom some had history of CHD, the overall absolute risk of MI was much higher than in our study, 5.0% and 8.0%.12,13 In both studies, the risk of myocardial injury was higher in patients with than in those without history of CHD (5.9% versus 4.3% and 18.2% versus 6.6%).12,13 Only few studies have specifically compared the risk of MI after noncardiac surgery between patients with and without history of CHD. One study examined risk of MI in 377 CHD patients who underwent noncardiac vascular surgery and found an absolute risk of MI of 26.5%.14 Several reasons may explain why the absolute risk of MI was lower in our study than in the aforementioned studies; no regular screening for procedural MI was performed in EVA-3S, SPACE, and ICSS, and elevation in cardiac enzymes without specific abnormalities on an ECG or typical chest discomfort was not counted as MI. Also, certain types of surgery other than carotid revascularization may involve more hemodynamic stress during the procedure and therefore may be associated with a higher risk of cardiac complications.
With regards to procedural stroke or death risk, our results suggest that CAS may be as safe as CEA in patients with history of CHD until the age of 75 years instead of the age of 70 years which is reported for the total group of patients with symptomatic carotid stenosis.3,15 One explanation for this trend may be that patients who were assigned to CAS received more aggressive antiplatelet treatment than those assigned to CEA (mostly acetylsalicylic acid in combination with clopidogrel), which may have prevented more procedural vascular events in patients with history of CHD who underwent CAS compared with those who underwent CEA. However, because the number of outcome events was low for patients with history of CHD in some age and treatment groups, and results were not consistent for both the primary and secondary outcome measures, no definite conclusions can be drawn.
A strength of our study is that we pooled individual patient data from 4 randomized trials resulting in a large sample size. In addition, the proportion of patients with missing data on history of CHD was low (2%). Our study also has limitations. First, the definition of history of CHD was not consistent across all trials, which could have caused differences in treatment effect. However, we found little evidence of heterogeneity between trials in our analysis. Second, CREST patients could not be included in the analysis of the secondary outcome measure because the definition of MI in this trial was different from the definitions used in the other 3 trials. Consequently, the absolute number of clinical MIs was small. Third, we could not examine the treatment-by-age effect stratified by history of CHD in more detail because we did not have sufficient power to classify patients with history of CHD into smaller age groups. Fourth, findings of our analysis represent results for CAS and CEA from about 10 to 15 years ago and may not represent that of a contemporary setting.
The current American Heart Association/American Stroke Association Guidelines for the Prevention of Stroke and Transient Ischemic Attack adopted the results of the previous pooled analysis on the age-treatment effect of CAS relative to CEA3 and suggested that CEA may give improved outcomes compared with CAS for patients aged 70 years or older.15 Although our results suggest that CAS may be as safe as CEA up to the age of 75 years in patients with history of CHD, we did not find consistent statistical evidence for modification of treatment effect by age and history of CHD in both the PP and the ITT analyses and for both outcome measures. Therefore, further studies are needed to assess whether the treatment effect by age is truly different for patients with and without history of CHD.
Acknowledgments
Drs Volkers, Greving, Algra, and Kappelle designed the study plan. Drs Volkers, Greving, and Algra performed the statistical analysis and interpreted the results together with Dr Kappelle. Dr Mas, Dr Ringleb, Dr Bonati, and G. Howard extracted individual patient data from the contributing trials. Dr Volkers wrote the first version of the article. All authors contributed to data interpretation and critical revision of the article and approved the final version. All authors gave final approval to submit for publication. Involvement of the authors in the Carotid Stenosis Trialists’ Collaboration (CSTC) Steering Committee is as follows: Dr Algra (independent chair); EVA-3S (Endarterectomy Versus Angioplasty in Patients With Symptomatic Severe Carotid Stenosis): Drs Becquemin, Calvet, and Mas; ICSS (International Carotid Stenting Study): Dr Bonati (coordinator), Drs de Borst, Brown, and Hendrikse; SPACE (Stent-Protected Angioplasty Versus Carotid Endarterectomy) and SPACE-2: Drs Eckstein, Fraedrich, Jansen, and Ringleb; CREST (Carotid Revascularization Endarterectomy Versus Stenting Trial) and CREST-2: Dr Brott, G. Howard, and Dr Roubin; ACST-1 (Asymptomatic Carotid Surgery Trial) and ACST-2: Dr Bulbulia and Dr Halliday; and trial statistician: Dr Gregson. The members of the Steering Committees and a list of Investigators contributing data to the trials including those in this pooled analysis can be found in earlier publications.
Sources of Funding
Drs Greving and Volkers are supported by the Dutch Heart Foundation (grant number 2013T128). Dr Halliday’s research is funded by the National Institute for Health Research, Oxford Biomedical Research Center. Dr Bonati received grants from the Swiss National Science Foundation (PBBSB-116873), the University of Basel, Switzerland, and The Stroke Association, United Kingdom. G. Howard and Dr Brott are funded by the National Institutes of Health/National Institute of Neurological Disorders and Stroke (NIH/NINDS).
Disclosures
Dr de Borst has received an advisory board fee from Bayer. Dr Bonati has received an unrestricted research grant from AstraZeneca; consultancy or advisory board fees or speaker’s honoraria from Amgen, Bayer, Bristol-Myers Squibb, and Claret Medical; and travel grants from Amgen and Bayer. Dr Ringleb has received advisory board fees or speaker’s honoraria from Bayer, Boehringer Ingelheim, Daiichi Sankyo, and Pfizer. Dr Gregson has received consultancy fees from Edwards Lifesciences. The other authors report no conflicts.
Supplementary Material
Footnotes
Guest Editor for this article was Seemant Chaturvedi, MD.
Drs Bonati, Brott, Mas, Ringleb, and Greving contributed equally.
The online-only Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/STROKEAHA.118.023085.
References
- 1.Flaherty ML, Kissela B, Khoury JC, Alwell K, Moomaw CJ, Woo D, et al. Carotid artery stenosis as a cause of stroke. Neuroepidemiology. 2013;40:36–41. doi: 10.1159/000341410. doi: 10.1159/000341410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonati LH, Lyrer P, Ederle J, Featherstone R, Brown MM. Percutaneous transluminal balloon angioplasty and stenting for carotid artery stenosis. Cochrane Database Syst Rev. 2012:CD000515. doi: 10.1002/14651858.CD000515.pub4. [DOI] [PubMed] [Google Scholar]
- 3.Howard G, Roubin GS, Jansen O, Hendrikse J, Halliday A, Fraedrich G, et al. Carotid Stenting Trialists’ Collaboration. Association between age and risk of stroke or death from carotid endarterectomy and carotid stenting: a meta-analysis of pooled patient data from four randomised trials. Lancet. 2016;387:1305–1311. doi: 10.1016/S0140-6736(15)01309-4. doi: 10.1016/S0140-6736(15)01309-4. [DOI] [PubMed] [Google Scholar]
- 4.Boulanger M, Camelière L, Felgueiras R, Berger L, Rerkasem K, Rothwell PM, et al. Periprocedural myocardial infarction after carotid endarterectomy and stenting: systematic review and meta-analysis. Stroke. 2015;46:2843–2848. doi: 10.1161/STROKEAHA.115.010052. doi: 10.1161/STROKEAHA.115.010052. [DOI] [PubMed] [Google Scholar]
- 5.Bonati LH, Dobson J, Algra A, Branchereau A, Chatellier G, Fraedrich G, et al. Carotid Stenting Trialists’ Collaboration. Short-term outcome after stenting versus endarterectomy for symptomatic carotid stenosis: a preplanned meta-analysis of individual patient data. Lancet. 2010;376:1062–1073. doi: 10.1016/S0140-6736(10)61009-4. doi: 10.1016/S0140-6736(10)61009-4. [DOI] [PubMed] [Google Scholar]
- 6.Mas JL, Chatellier G, Beyssen B, Branchereau A, Moulin T, Becquemin JP, et al. EVA-3S Investigators. Endarterectomy versus stenting in patients with symptomatic severe carotid stenosis. N Engl J Med. 2006;355:1660–1671. doi: 10.1056/NEJMoa061752. doi: 10.1056/NEJMoa061752. [DOI] [PubMed] [Google Scholar]
- 7.Ringleb PA, Allenberg J, Brückmann H, Eckstein HH, Fraedrich G, Hartmann M SPACE Collaborative Group. 30 day results from the SPACE trial of stent-protected angioplasty versus carotid endarterectomy in symptomatic patients: a randomised non-inferiority trial. Lancet. 2006;368:1239–1247. doi: 10.1016/S0140-6736(06)69122-8. doi: 10.1016/S0140-6736(06)69122-8. [DOI] [PubMed] [Google Scholar]
- 8.Ederle J, Dobson J, Featherstone RL, Bonati LH, van der Worp HB, de Borst GJ International Carotid Stenting Study Investigators. Carotid artery stenting compared with endarterectomy in patients with symptomatic carotid stenosis (International Carotid Stenting Study): an interim analysis of a randomised controlled trial. Lancet. 2010;375:985–997. doi: 10.1016/S0140-6736(10)60239-5. doi: 10.1016/S0140-6736(10)60239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brott TG, Hobson RW, II, Howard G, Roubin GS, Clark WM, Brooks W, et al. CREST Investigators. Stenting versus endarterectomy for treatment of carotid-artery stenosis. N Engl J Med. 2010;363:11–23. doi: 10.1056/NEJMoa0912321. doi: 10.1056/NEJMoa0912321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.North American Symptomatic Carotid Endarterectomy Trial (NASCET) Steering Committee. North American Symptomatic Carotid Endarterectomy Trial. Methods, patient characteristics, and progress. Stroke. 1991;22:711–720. doi: 10.1161/01.str.22.6.711. [DOI] [PubMed] [Google Scholar]
- 11.Yadav JS, Wholey MH, Kuntz RE, Fayad P, Katzen BT, Mishkel GJ, et al. Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy Investigators. Protected carotid-artery stenting versus endarterectomy in high-risk patients. N Engl J Med. 2004;351:1493–1501. doi: 10.1056/NEJMoa040127. doi: 10.1056/NEJMoa040127. [DOI] [PubMed] [Google Scholar]
- 12.Devereaux PJ, Xavier D, Pogue J, Guyatt G, Sigamani A, Garutti I, et al. POISE (PeriOperative ISchemic Evaluation) Investigators. Characteristics and short-term prognosis of perioperative myocardial infarction in patients undergoing noncardiac surgery: a cohort study. Ann Intern Med. 2011;154:523–528. doi: 10.7326/0003-4819-154-8-201104190-00003. doi: 10.7326/0003-4819-154-8-201104190-00003. [DOI] [PubMed] [Google Scholar]
- 13.Botto F, Alonso-Coello P, Chan MT, Villar JC, Xavier D, Srinathan S, et al. Vascular events In noncardiac Surgery patIents cOhort evaluatioN (VISION) Writing Group, on behalf of The Vascular events In noncardiac Surgery patIents cOhort evaluatioN (VISION) Investigators; Appendix 1. The Vascular events In noncardiac Surgery patIents cOhort evaluatioN (VISION) Study Investigators Writing Group; Appendix 2. The Vascular events In noncardiac Surgery patIents cOhort evaluatioN Operations Committee; Vascular events In noncardiac Surgery patIents cOhort evaluatioN VISION Study Investigators. Myocardial injury after noncardiac surgery: a large, international, prospective cohort study establishing diagnostic criteria, characteristics, predictors, and 30-day outcomes. Anesthesiology. 2014;120:564–578. doi: 10.1097/ALN.0000000000000113. doi: 10.1097/ALN.0000000000000113. [DOI] [PubMed] [Google Scholar]
- 14.McFalls EO, Ward HB, Moritz TE, Apple FS, Goldman S, Pierpont G, et al. Predictors and outcomes of a perioperative myocardial infarction following elective vascular surgery in patients with documented coronary artery disease: results of the CARP trial. Eur Heart J. 2008;29:394–401. doi: 10.1093/eurheartj/ehm620. doi: 10.1093/eurheartj/ehm620. [DOI] [PubMed] [Google Scholar]
- 15.Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, et al. American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Peripheral Vascular Disease. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45:2160–2236. doi: 10.1161/STR.0000000000000024. doi: 10.1161/STR.0000000000000024. [DOI] [PubMed] [Google Scholar]


