Supplemental Digital Content is available in the text.
Keywords: middle cerebral artery, reperfusion, stroke, thrombectomy, treatment outcome
Abstract
Background and Purpose—
It is unclear whether endovascular treatment (EVT) is beneficial for patients with acute ischemic stroke with occlusion of the M2 segment of the middle cerebral artery. We aimed to compare functional outcomes, technical aspects, and complications of EVT between patients with acute ischemic stroke because of M2 and M1 occlusions in clinical practice. Furthermore, outcome and complications after EVT in dominant and nondominant caliber M2 division occlusions were studied.
Methods—
Data were obtained from the MR CLEAN Registry (Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands) which is an ongoing observational study in 16 Dutch centers performing EVT in the Netherlands. Functional outcome was measured with the modified Rankin Scale score at 90 days. Neurological recovery (delta National Institutes of Health Stroke Scale), successful reperfusion rates (extended Thrombolysis in Cerebral Infarction ≥2B), and safety outcomes were also investigated. Associations between occlusion location and outcome were analyzed with ordinal logistic regression models, with adjustment for other prognostic factors.
Results—
In total, 244 (24%) patients with an M2 and 759 (76%) patients with an M1 occlusion who underwent EVT were analyzed. Functional outcomes were not significantly different between patients with M2 versus M1 occlusions (adjusted common odds ratio, 1.24; 95% CI, 0.87–1.73). Occurrence of symptomatic intracerebral hemorrhage was also similar for M2 and M1 occlusions (6.6% versus 5.9%; P=0.84). Further analysis about dominance of an M2 branch was performed in 175 (72%) patients. Neurological recovery was comparable (mean delta National Institutes of Health Stroke Scale, −2±10 for dominant M2, −5±5 for nondominant M2, and −4±9 [P=0.24] for M1 occlusions). Furthermore, the effect of reperfusion status on functional outcome was comparable between occlusion divisions (common odds ratio, 1.27; 95% CI, 1.06–1.53 for dominant M2; common odds ratio, 1.32; 95% CI, 0.93–1.87 for nondominant M2; and common odds ratio, 1.35; 95% CI, 1.24–1.46 for M1 occlusions).
Conclusions—
Outcomes and complication rates after EVT were similar in patients with M2 and M1 occlusions. Although based on observational data and a limited sample size, a similar association of reperfusion status with functional outcome for all subgroups provides no evidence that patients with either a dominant or a nondominant M2 occlusion should be routinely excluded from EVT.
Acute endovascular treatment (EVT) has become part of the usual care for appropriately selected patients with ischemic stroke caused by an occlusion in the proximal intracranial anterior circulation. However, the role of EVT for patients with more distal occlusions in the second-order branches (M2) of the middle cerebral artery (MCA) is less certain given that this population was underrepresented in randomized controlled trials.1–3
Considering the distal location, smaller diameter and thinner walls of the M2 arterial segment, an M2 occlusion may result in more challenging endovascular procedures and an increased risk of periprocedural complications.1 These drawbacks potentially counterbalance the benefit of reperfusion. Furthermore, intravenous thrombolysis alone is more effective for recanalizing M2 occlusions relative to more proximal occlusions and therefore might limit the additional benefit of EVT.4,5
A recent meta-analysis reported that patients with M2 occlusions achieved similar reperfusion and mortality rates as patients with M1 occlusions.6 Although symptomatic intracerebral hemorrhages were observed more frequently with M2 occlusions, rates of functional independence were higher among patients with an M2 occlusion. A lower baseline National Institutes of Health Stroke Scale (NIHSS) score in M2 occlusion compared with M1 occlusion patients might explain this observation.6
The wide anatomic variation about the branching pattern of the MCA strongly influences the arterial territory at risk in case of an M2 occlusion. Several MCA branching patterns have been described in the anatomic and neuroradiological literature.7,8 The most important variation is in the blood supply to the parietal lobe, which can be fed by the inferior or superior M2 division or both. The M2 division that supplies the majority of the parietal lobe has a larger caliber, the dominant M2 division. In some patients, a dominant M2 division can even approximate the diameter of the M1 segment. Owing to their larger territory at risk, dominant M2 occlusions typically lead to worse clinical outcomes than nondominant M2 occlusions.3,8
Previous studies of the effect of EVT in patients with ischemic stroke did not distinguish between occlusion of dominant and nondominant M2 divisions. It is probable that an occlusion of a dominant M2 may yield similar outcomes and treatment benefit as an M1 occlusion, whereas nondominant M2 branch occlusions may have a better natural history and reduced treatment effect.
Current stroke guidelines concluded that it may be reasonable to treat patients experiencing an M2 occlusion with EVT, but further evidence is warranted.9 The aim of this study is to investigate 3-month functional outcome and the technical and safety aspects of EVT in patients with ischemic stroke because of M2 versus M1 occlusions. In addition, differences in outcome between dominant and nondominant M2 division occlusions are studied.
Methods
Data were obtained from the MR CLEAN Registry (Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands), which is an ongoing prospective observational study in all centers performing EVT in the Netherlands. Detailed study design and methods have been described previously.10 The MR CLEAN Registry was approved by the ethics committee of the Erasmus MC, Rotterdam, the Netherlands (MEC-2014-235). With this approval, it was approved by the research board of each participating center. At University Medical Center Utrecht, approval to participate in the study has been obtained from their own research board and ethics committee. Source data are not be made available because of legislative issues on patient privacy, but detailed analytic methods and study materials, including log files of statistical analyses, will be made available to other researchers on request (email: mrclean@erasmusmc.nl).
Patients
All patients aged 18 years and older with a clinical diagnosis of acute ischemic stroke because of a proximal arterial occlusion in the anterior circulation demonstrated by computed tomography angiography (CTA) and in whom EVT was initiated (defined as arterial puncture) within 6.5 hours after stroke onset were studied.10 For the present study, only patients with a documented M1 or M2 target occlusion on baseline digital subtraction angiography (DSA) were included. EVT consisted of mechanical thrombectomy or aspiration whether or not combined with arterial delivery of a thrombolytic agent. Method of EVT was left to the discretion of the interventionist. Patients included in this analysis were treated between March 16, 2014, and June 15, 2016.
Image Analysis
Noncontrast CT, CTA, and DSA were scored by a core laboratory, except for the anatomic classification of the M2 branch which was assessed by 1 experienced neurointerventionist (A.C.G.M. van Es). Imaging analyses were performed blinded to clinical characteristics and outcomes. Alberta Stroke Program Early CT Score was measured on baseline noncontrast CT, collateral score on baseline CTA, and both occlusion segment, and extended Thrombolysis in Cerebral Infarction score was measured on DSA.11–13 DSA-only procedures were cases in which the target occlusion resolved or migrated too distally (M3 or M4 branches) caused by contrast flushing or manipulation with the catheter, without actual performing of intended EVT.
In accordance with previously used definitions, the M1 segment and M2 branches were defined as follows on DSA: the M1 (horizontal or sphenoidal) segment extends from the bifurcation of the internal carotid artery below the anterior perforated substance to the limen insulae.1 At the limen insulae, the M1 makes a posterosuperior turn (genu) into the insula. The anterior temporal artery is identified as the artery that supplies the anterior temporal pole regardless of its size and whether it continues into the Sylvian fissure. All anatomic variations of the anterior temporal branch are considered M1 branches in this study. The M2 branches are considered those branches that are distal to the main bifurcation at the distal end of the horizontal M1 segment. M2 branches extend from the posterosuperior turn (genu) of M1 to the circular sulcus of the insula.
For the purpose of this study, caliber dominance was considered present if 1 M2 branch had a larger diameter than the other on DSA or if the perfusion defect associated with the occluded M2 branch was larger than 50% of MCA territory (Figure 1). Only when the diameters of both the inferior and superior branches were equal and the associated perfusion defect was ≈50% of MCA territory, the branches were considered codominant.
Figure 1.
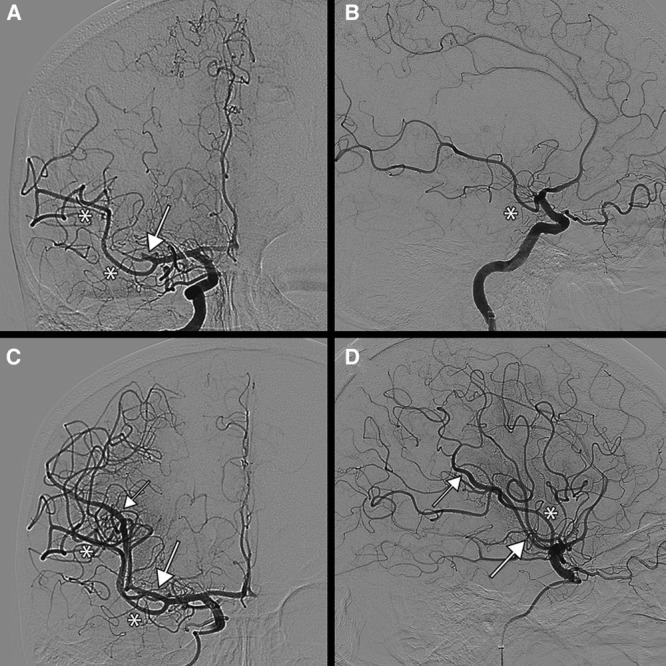
Case example of a dominant M2 superior division on digital subtraction angiography (DSA). Arrows indicate the superior M2 division. Asterisks indicate the inferior M2 division. A, Anteroposterior view on DSA pre-endovascular treatment (EVT), where a large part of the parietal lobe not perfused. B, Lateral view on DSA pre-EVT. C, Anteroposterior view on DSA after EVT. The parietal lobe is reperfused. D, Lateral view on DSA after EVT.
Outcomes
Primary outcome was functional outcome assessed with the modified Rankin Scale score at 90 days ranging from 0 (no symptoms or disability) to 6 (death).14 Secondary outcomes were functional independence (modified Rankin Scale score of 0–2), stroke severity, and change in stroke severity. Stroke severity was measured using the NIHSS which ranges from 0 to 42 points (higher score indicating a more severe stroke). Follow-up NIHSS was assessed at 24 to 48 hours after treatment. Missing follow-up NIHSS scores in patients who died within 48 hours were assigned 42 points. Change in stroke severity was assessed with delta-NIHSS which was calculated as the absolute difference between baseline and follow-up NIHSS: a negative value indicates a neurological improvement, whereas a positive value indicates a neurological decline. Evaluation of technical aspects included post-EVT reperfusion status (extended Thrombolysis in Cerebral Infarction score), procedural duration, number of stent retriever passes, and procedural complications (defined as vasospasm, vessel dissection or perforation, presence of distal thrombi, and new clots in a different vascular territory). Complete DSA runs including anteroposterior and lateral views were required to reach an extended Thrombolysis in Cerebral Infarction score of 2B or higher. A missing lateral view resulted in a maximum possible score of 2A. Successful reperfusion was considered when extended Thrombolysis in Cerebral Infarction 2B or higher was achieved. Safety aspects included symptomatic intracranial hemorrhage15 and stroke progression (defined as a neurological deterioration by at least 4 points on the NIHSS).
Statistical Analyses
Patients who underwent EVT for acute ischemic stroke because of an M2 occlusion were compared with those treated with an M1 occlusion. Baseline characteristics of both patient groups were compared by means of the Wilcoxon rank-sum test or Student t test where appropriate and a χ2 test for categorical variables.
The difference in functional outcome between M2 and M1 occlusions was expressed as a common odds ratio obtained from multivariable ordinal logistic regression.16 Adjustments were made for age, sex, NIHSS at baseline, time from stroke onset to groin puncture, intravenous thrombolysis, prestroke modified Rankin Scale, and CTA collateral status. For regression analysis, missing values were imputed by multiple imputations.17 To compare the association between functional outcome and reperfusion grade in each subgroup of occluded division, unadjusted ordinal logistic regression was performed. Unadjusted and adjusted common odds ratios were reported with 95% CI. All analyses were performed with R (version 3.4.2) and packages rms, ggplot2, Hmisc, readxl, tableone, and haven. Probability values of <0.05 were considered significant for all tests.
Results
Baseline Patient Characteristics
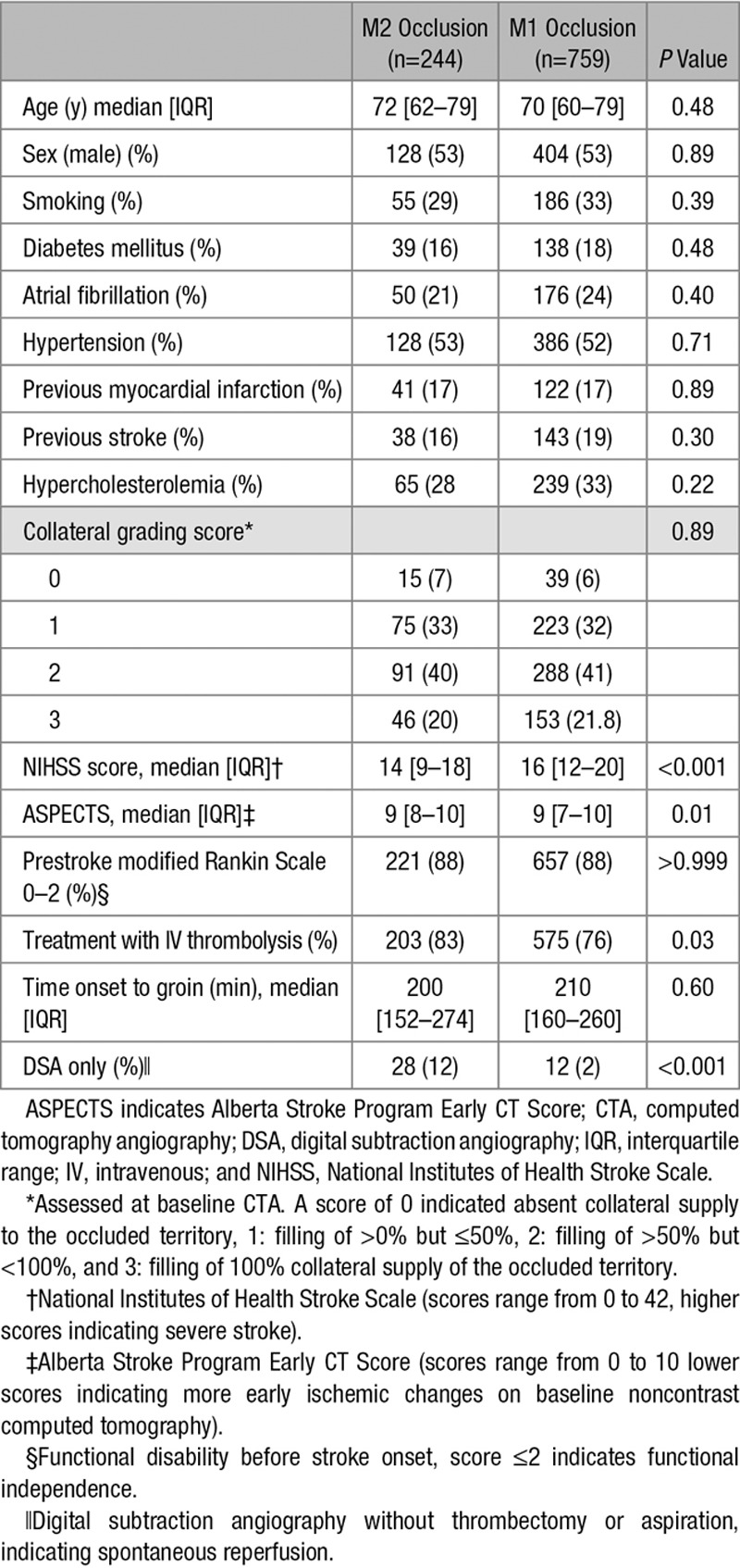
Of the 1003 included patients, 244 (24%) patients had an M2 occlusion and 759 (76%) had an M1 occlusion (Figure I in the online-only Data Supplement). No significant differences were observed between patients with M2 and M1 occlusions regarding age, sex and clinical risk factors for ischemic stroke, collateral grading score and time from stroke onset to groin puncture (Table 1). Compared with patients with M1 occlusions, those with M2 occlusions had significantly lower median NIHSS scores (14 versus 16; P<0.001), higher ASPECT scores (median, 9; interquartile range [IQR], 8–10 versus median, 9; IQR, 7–10; P=0.01), and greater intravenous thrombolysis treatment (83% versus 76%; P=0.03). DSA-only procedures because of reperfusion before EVT occurred more often in the M2 occlusion group (12% versus 2%; P<0.001).
Table 1.
Baseline Characteristics of Analyzed Patients
In 69 patients (28%) with an M2 occlusion, dominance of the M2 branch could not be reliably assessed because of poor quality or insufficient DSA imaging. This left 175 (72%) patients for further analysis. Dominance of either superior or inferior division was observed in 137 (78%) patients, and codominance of M2 branches was observed in 38 (22%) patients with M2 occlusion. Dominance of a branch was equally often observed in the superior or inferior division (respectively, 49% versus 51%; P>0.99). In patients with a dominant M2 branch, the occlusion was located in the dominant division in 124 of the cases and in 51 of the cases in the nondominant branch. Baseline NIHSS was lower in patients with a dominant M2 occlusion than in patients with an M1 occlusion (median, 14; IQR, 9–17 versus median, 16; IQR, 12–20; P<0.001; Table I in the online-only Data Supplement).
Patients with an occlusion in a co- or nondominant division had a lower NIHSS at baseline than patients with an M1 occlusion (median, 14; IQR, 9–17 versus median, 16; IQR, 12–20; P=0.002), and they had more DSA-only procedures (25% versus 2%; P<0.001; Table I in the online-only Data Supplement).
Outcomes
There was no significant difference in functional outcome between patients with an M2 occlusion and patients with an M1 occlusion (adjusted common odds ratio, 1.24; 95% CI, 0.92–1.68; Figure 2). Likewise, the proportion of patients who achieved functional independence (modified Rankin Scale score of 0–2) at follow-up did not differ (101 [46%] for M2 occlusions versus 270 [39%]; P=0.10). Patients with an M2 occlusion had lower follow-up NIHSS scores (median, 8; IQR, 3–16 versus median, 11; IQR, 4–18; P=0.01). However, the difference in delta-NIHSS between the groups was not statistically significant (mean [SD], −3±9 versus −4±9; P=0.49; Table 2).
Figure 2.
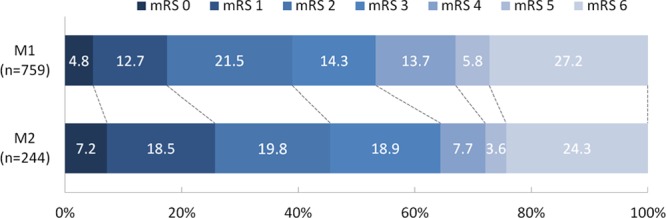
Modified Rankin Scale (mRS) scores at 90 days: M1 vs M2 occlusion. Functional outcomes were statically significant difference between patients with M2 and M1 occlusions (common odds ratio, 1.40; 95% CI, 1.07–1.83). However, after adjustment for age, sex, National Institutes of Health Stroke Scale (NIHSS) baseline, time from stroke onset to groin puncture, intravenous thrombolysis (IVT), prestroke mRS, and collateral status, functional outcome was no longer statistically different (adjusted common odds ratio, 1.24; 95% CI, 0.92–1.68).
Table 2.
Clinical, Technical, and Safety Outcomes in Patients With an M2 Occlusion on DSA Compared With Patients With an M1 Occlusion
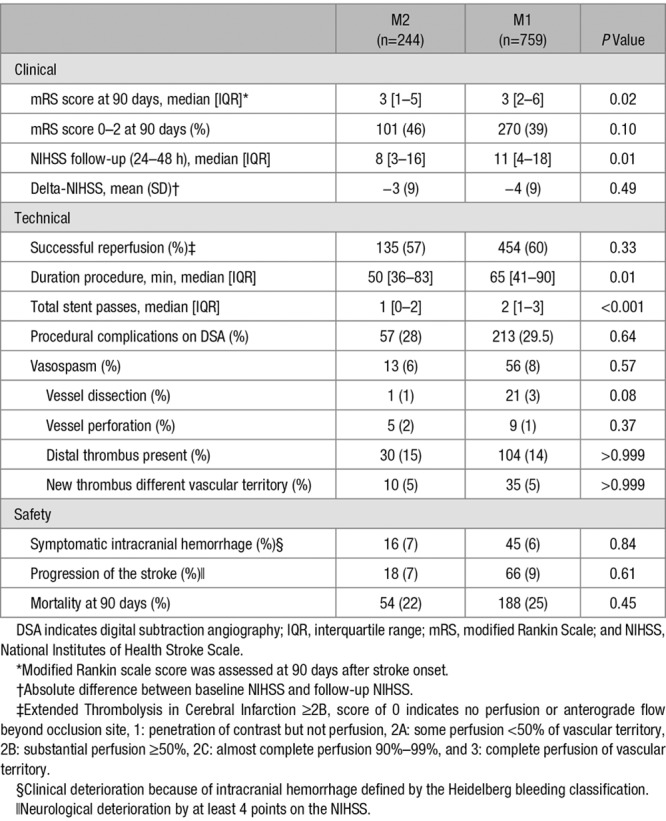
Successful reperfusion was similar in patients with M2 or M1 occlusion (454 [60%] versus 135 [57%]; P=0.33), although duration of procedure was shorter in patients with an M2 occlusion (median, 50 minutes; IQR, 36–83 minutes versus median, 65 minutes; IQR, 41–90 minutes). There were no significant differences about procedural complications or symptomatic intracranial hemorrhage in both groups (28% versus 30%; P=0.64).
In patients with dominant M2 occlusions, EVT resulted in a similar functional outcomes compared with M1 occlusions (adjusted common odds ratio, 1.19; 95% CI, 0.79–1.80) and the proportion of patients with functional independence was not significantly different (42 [39%] versus 270 [39%]; P>0.99; Figure II in the online-only Data Supplement). Compared with M1 occlusions, the proportion of functional independence stratified by reperfusion grade was similar to patients with a dominant M2 branch (Table 3). In addition, NIHSS at follow-up and delta-NIHSS did not differ, and technical and safety aspects were either equivalent or favorable in patients with a dominant M2 division occlusion (Table 4).
Table 3.
Proportions of Functional Independence Stratified by Reperfusion Grades *
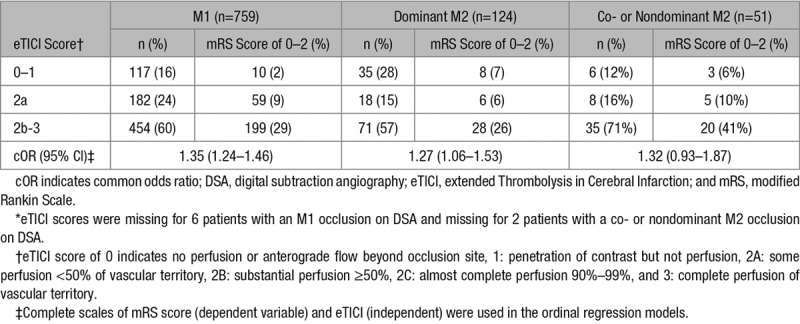
Table 4.
Clinical, Technical, and Safety Outcomes in Patients With an M1, or Dominant M2 Division, or Co- or Nondominant M2 Division Occlusion
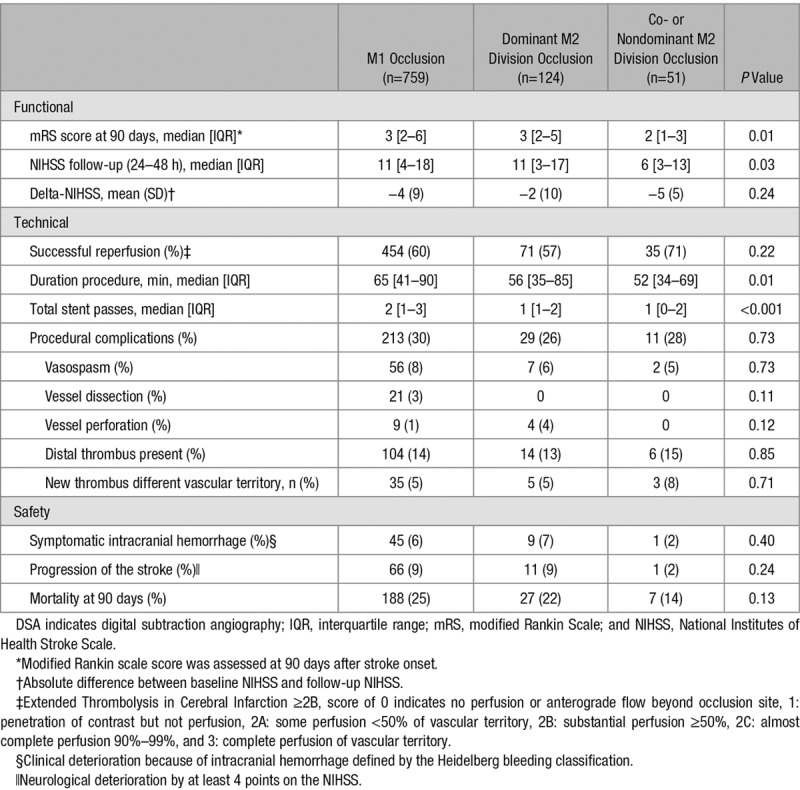
Patients with co- or nondominant division occlusions of the M2 branch had better functional outcomes than patients with an M1 occlusion (adjusted common odds ratio, 2.22; 95% CI, 1.27–3.87; Figure III in the online-only Data Supplement). Also, patients with a co- or nondominant M2 branch occlusion became functionally independent more often than patients with an M1 occlusion (29 [59%] versus 270 [39%]; P<0.01). NIHSS at follow-up was lower in the co- or nondominant division occlusions, but delta-NIHSS was equal between both groups. Safety aspects did not differ between both groups (Table 4).
Discussion
In this study, we found that patients with ischemic stroke because of an M2 occlusion had similar functional outcomes compared with stroke patients with an M1 occlusion. In addition, about neurological recovery (delta-NIHSS), no significant differences were observed between both groups. Furthermore, no significant differences in occurrence of symptomatic intracerebral hemorrhage or other safety aspects were observed. The majority of the M2 occlusions in our study were occlusions of a caliber dominant M2 branch. These occlusions may be often responsible for perfusion of the main part of the MCA territory.18 This can explain the similarity in clinical outcome between M1 and M2 occlusions. Furthermore, the association of reperfusion with functional outcome was similar between M1 and caliber dominant M2 occlusions. Compared with these occlusions, patients with occlusions of the nondominant M2 branch had better functional outcomes, likely owing to the smaller territory at risk and hence better natural history.
In accordance with a recent meta-analysis, we observed similar clinical outcomes in patients with M2 and M1 occlusions.6 Interestingly, we found lower complication rates which might be explained by advances in endovascular devices and increasing experience of neurointerventionists. Similar to previous studies, we also found that NIHSS scores at baseline were lower in patients with M2 occlusions than in patients with M1 occlusions, indicating that M2 occlusion strokes are on average less severe.1,19 A previous population-based study showed that M2 occlusions were as often located in the superior as in the inferior division.20 However, other studies reported no differences in NIHSS scores at baseline which might be explained by differences in M2 segment definition, patient selection, or both.3 Furthermore, as in our study, no differences in technical and safety aspects between M2 and M1 occlusion segments were observed in previous studies.18,21 Distal occlusions can still cause functional dependence, indicating that reperfusion of small brain regions is important, as they may involve eloquent areas.22,23 A recent study of 212 patients compared outcomes after thrombectomy for M1 or M2 occlusions. Dominancy of caliber was not taken into account.24 M2 occlusions were more often located in the superior branch. However, occlusion in a specific branch was not a predictor for clinical outcome.
Limitations
This study has several limitations beyond the common limitations of an observational study. First, we used DSA to identify thrombus location, although decision making in clinical practice is based on CTA findings. Therefore, results cannot be directly generalized to clinical decision making in the emergency room. Our objective was to assess technical and safety aspects of EVT in patients with an M2 occlusion.25 Second, ischemic stroke patients with a large-vessel occlusion who did not undergo EVT are not registered in the MR CLEAN Registry. Therefore, patients with distal M2 occlusions may have been excluded, which could have resulted in selection bias. Nevertheless, patients with prestroke functional dependence, poor collateral grading scores, and poor vascular status were not excluded from the MR CLEAN Registry, and our results represent current routine practice. Third, anatomic variation of the M2 division branching pattern could not be assessed in all patients because of insufficient imaging of M2 branches in our retrospective analysis. Although we did not use advanced imaging such as perfusion scans to determine dominance, the anatomic distribution of dominant M2 branches as was described in the present study is in line with previously published anatomic studies. Also, our simple definition of dominant division observation during intervention on DSA makes it applicable during interventional procedures. However, adequate imaging of M2 branches is important in these patients from our experience. Fourth, in line with our third limitation, the precision of our estimates is limited, and results must be interpreted with care. Fifth, our study was based on an observational multicenter registry. Although covariable-adjusted regression analyses were performed, there might still be residual confounding in our study. Finally, follow-up imaging was not required in our registry, which precludes the analysis of infarct volume and asymptomatic hemorrhage as a secondary outcome.
In conclusion, outcomes and complication rates after EVT were similar in patients with M2 and M1 occlusions. Although based on observational data and a limited sample size, a similar association of reperfusion status with functional outcome for all subgroups provides no evidence that patients with either a dominant or nondominant M2 occlusion should be routinely excluded from EVT.
Sources of Funding
The MR CLEAN Registry was partly funded by Toegepast Wetenschappelijk Instituut voor Neuromodulatie (TWIN) Foundation, Erasmus MC University Medical Center, Maastricht University Medical Center, and Amsterdam University Medical Center Utrecht.
Disclosures
Erasmus MC received funds from Dutch Heart Foundation, Brain Foundation Netherlands, the Netherlands Organisation for Health Research and Development, Health Holland Top Sector Life Sciences & Health, AngioCare BV, Medtronic/Covidien/EV3, AC Gmbh/LAMEPRO, Penumbra Inc, Top Medical/Concentric, Stryker, Stryker European Operations BV, Thrombolytic Science, LLC, for research by Drs Dippel, van der Lugt, and Emmer. Amsterdam Medical Center received funds from Stryker for consultations by Dr Majoie, Maastricht University Medical Center received funds from Stryker and Cerenovus for consultations by Dr van Zwam. Dr Yoo reports research grants form Penumbra Inc and Neuravi Inc, received consultant fees from Cerenovus/Johnson & Johnson, and has equity ownership in Insera Therapeutics Inc. Dr Majoie received research grants European Commission (paid to institution) and is a shareholder at Nico.lab B.V. (company that focuses on use of artificial intelligence for medical image analysis). The other authors report no conflicts.
Supplementary Material
Appendix
MR CLEAN Registry investigators are as follows: Executive committee: Diederik W.J. Dippel; Aad van der Lugt; Charles B.L.M. Majoie; Yvo B.W.E.M. Roos; Robert J. van Oostenbrugge; Wim H. van Zwam; Jelis Boiten; Jan Albert Vos. Study coordinators: Ivo G.H. Jansen; Maxim J.H.L. Mulder; Robert-Jan B. Goldhoorn.
Local principal investigators: Wouter J. Schonewille; Jan Albert Vos; Charles B.L.M. Majoie; Jonathan M. Coutinho; Marieke J.H. Wermer; Marianne A.A. van Walderveen; Julie Staals; Wim H. van Zwam; Jeannette Hofmeijer; Jasper M. Martens; Geert J. Lycklama à Nijeholt; Jelis Boiten; Bob Roozenbeek; Bart J. Emmer; Sebastiaan F. de Bruijn; Lukas C. van Dijk; H. Bart van der Worp; Rob H. Lo; Ewoud J. van Dijk; Hieronymus D. Boogaarts; Paul L.M. de Kort; Jo J.P. Peluso; Jan S.P. van den Berg; Boudewijn A.A.M. van Hasselt; Leo A.M. Aerden; René J. Dallinga; Maarten Uyttenboogaart; Omid Eshghi; Tobien H.C.M.L. Schreuder; Roel J.J. Heijboer; Koos Keizer; Lonneke S.F. Yo; Heleen M. den Hertog; Emiel J.C. Sturm
Imaging assessment committee: Charles B.L.M. Majoie (chair); Wim H. van Zwam; Aad van der Lugt; Geert J. Lycklama à Nijeholt; Marianne A.A. van Walderveen; Marieke E.S. Sprengers; Sjoerd F.M. Jenniskens; René van den Berg; Albert J. Yoo; Ludo F.M. Beenen; Alida A. Postma; Stefan D. Roosendaal; Bas F.W. van der Kallen; Ido R. van den Wijngaard; Adriaan C.G.M. van Es; Bart J. Emmer; Jasper M. Martens; Lonneke S.F. Yo; Jan Albert Vos; Joost Bot, Pieter-Jan van Doormaal.
Writing committee: Diederik W.J. Dippel (chair); Aad van der Lugt; Charles B.L.M. Majoie; Yvo B.W.E.M. Roos; Robert J. van Oostenbrugge; Wim H. van Zwam; Geert J. Lycklama à Nijeholt; Jelis Boiten; Jan Albert Vos; Wouter J. Schonewille; Jeannette Hofmeijer; Jasper M. Martens; H. Bart van der Worp; Rob H. Lo
Adverse event committee: Robert J. van Oostenbrugge (chair); Jeannette Hofmeijer; H. Zwenneke Flach
Trial methodologist: Hester F. Lingsma
Research nurses/local trial coordinators: Naziha el Ghannouti; Martin Sterrenberg; Corina Puppels; Wilma Pellikaan; Rita Sprengers; Marjan Elfrink; Joke de Meris; Tamara Vermeulen; Annet Geerlings; Gina van Vemde; Tiny Simons; Cathelijn van Rijswijk; Gert Messchendorp; Hester Bongenaar; Karin Bodde; Sandra Kleijn; Jasmijn Lodico; Hanneke Droste; M. Wollaert; D. Jeurrissen; Ernas Bos; Yvonne Drabbe; Marjan Elfrink; Berber Zweedijk; Mostafa Khalilzada.
PhD/Medical students: Esmee Venema; Vicky Chalos; Kars C.J. Compagne; Ralph R. Geuskens; Tim van Straaten; Saliha Ergezen; Roger R.M. Harmsma; Daan Muijres; Anouk de Jong; Wouter Hinseveld; Olvert A. Berkhemer; Anna M.M. Boers; J. Huguet; P.F.C. Groot; Marieke A. Mens; Katinka R. van Kranendonk; Kilian M. Treurniet; Manon Kappelhof; Manon L. Tolhuijsen; Heitor Alves.
Affiliations: Department of Neurology (D.W.J.D., M.J.H.L.M., B.R., D.W.J.D., N.e.G., M.S., V.C., S.E., R.R.M.H., D.M., A.d.J., O.A.B.), Department of Radiology (A.v.d.L., M.J.H.L.M., B.J.E., A.C.G.M.v.E., P.-J.v.D., K.C.J.C.), and Department of Public Health (H.F.L., E.V., V.C.), Erasmus MC University Medical Center, Rotterdam, the Netherlands; Department of Radiology and Nuclear Medicine (C.B.L.M.M., I.G.H.J., M.E.S.S., R.v.d.B., L.F.M.B., S.D.R., B.J.E., R.R.G., O.A.B., A.M.M.B., J.H., P.F.C.G., M.A.M., K.R.v.K., K.M.T., M.K., M.L.T., H.A.), Department of Neurology (Y.B.W.E.M.R., J.M.C., R.S.), and Department of Biomedical Engineering & Physics (A.M.M.B.), Amsterdam University Medical Center Utrecht, University of Amsterdam, the Netherlands; Department of Neurology (R.J.v.O., R.-J.B.G., J.S., M.W., D.J.) and Department of Radiology (W.H.v.Z., R.-J.B.G., A.A.P., O.A.B.), Maastricht University Medical Center and Cardiovascular Research Institute Maastricht (CARIM), the Netherlands; Department of Neurology (W.J.S., C.P., W.P., W.H.) and Department of Radiology (J.A.V.), Sint Antonius Hospital, Nieuwegein, the Netherlands; Department of Neurology (M.J.H.W., E.B.) and Department of Radiology (M.A.A.v.W.), Leiden University Medical Center, the Netherlands; Department of Neurology (J.H., M.E.) and Department of Radiology (J.M.M.), Rijnstate Hospital, Arnhem, the Netherlands; Department of Radiology (G.J.L.à.N., B.F.W.v.d.K., I.R.v.d.W.) and Department of Neurology (J.B., J.d.M., T.V.), Haaglanden MC, The Hague, the Netherlands; Department of Neurology (S.F.d.B., Y.D., M.K.) and Department of Radiology (L.C.v.D.), HAGA Hospital, The Hague, the Netherlands; Department of Neurology (H.B.v.d.W., B.Z.) and Department of Radiology (R.H.L.), University Medical Center Utrecht, the Netherlands; Department of Neurology (E.J.v.D., A.G., T.v.S.), Department of Neurosurgery (H.D.B.), and Department of Radiology (S.F.M.J.), Radboud University Medical Center, Nijmegen, the Netherlands; Department of Neurology (P.L.M.d.K., C.v.R.) and Department of Radiology (J.J.P.P.), Sint Elisabeth Hospital, Tilburg, the Netherlands; Department of Neurology (J.S.P.v.d.B., H.M.d.H., G.v.V.) and Department of Radiology (B.A.A.M.v.H., H.Z.F.), Isala Klinieken, Zwolle, the Netherlands; Department of Neurology (L.A.M.A., K.B.) and Department of Radiology (R.J.D.), Reinier de Graaf Gasthuis, Delft, the Netherlands; Department of Neurology (M.U., G.M.) and Department of Radiology (O.E.), University Medical Center Groningen, the Netherlands; Department of Neurology (T.H.C.M.L.S., T.S.) and Department of Radiology (R.J.J.H.), Atrium Medical Center, Heerlen, the Netherlands; Department of Neurology (K.K., H.B.) and Department of Radiology (L.S.F.Y.),Catharina Hospital, Eindhoven, the Netherlands; Department of Neurology (S.K., J.L., H.D.) and Department of Radiology (E.J.C.S.), Medical Spectrum Twente, Enschede, the Netherlands; Department of Radiology, Amsterdam University Medical Center Utrecht, Vrije Universiteit van Amsterdam, the Netherlands (J.B.); and Department of Radiology, Texas Stroke Institute, Plano (A.J.Y.).
Footnotes
Guest Editor for this article was Gregory W. Albers, MD.
A list of MR CLEAN Registry investigators is listed in the Appendix.
The online-only Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/STROKEAHA.118.023117.
References
- 1.Coutinho JM, Liebeskind DS, Slater LA, Nogueira RG, Baxter BW, Levy EI, et al. Mechanical thrombectomy for isolated M2 occlusions: a post hoc analysis of the STAR, SWIFT, and SWIFT PRIME studies. AJNR Am J Neuroradiol. 2016;37:667–672. doi: 10.3174/ajnr.A4591. doi: 10.3174/ajnr.A4591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al. HERMES C; ollaborators. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387:1723–1731. doi: 10.1016/S0140-6736(16)00163-X. doi: 10.1016/S0140-6736(16)00163-X. [DOI] [PubMed] [Google Scholar]
- 3.Tomsick TA, Carrozzella J, Foster L, Hill MD, von Kummer R, Goyal M, et al. IMS III Investigators. Endovascular therapy of M2 occlusion in IMS III: role of M2 segment definition and location on clinical and revascularization outcomes. AJNR Am J Neuroradiol. 2017;38:84–89. doi: 10.3174/ajnr.A4979. doi: 10.3174/ajnr.A4979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.del Zoppo GJ, Poeck K, Pessin MS, Wolpert SM, Furlan AJ, Ferbert A, et al. Recombinant tissue plasminogen activator in acute thrombotic and embolic stroke. Ann Neurol. 1992;32:78–86. doi: 10.1002/ana.410320113. doi: 10.1002/ana.410320113. [DOI] [PubMed] [Google Scholar]
- 5.Sims JR, Rordorf G, Smith EE, Koroshetz WJ, Lev MH, Buonanno F, et al. Arterial occlusion revealed by CT angiography predicts NIH stroke score and acute outcomes after IV tPA treatment. AJNR Am J Neuroradiol. 2005;26:246–251. [PMC free article] [PubMed] [Google Scholar]
- 6.Saber H, Narayanan S, Palla M, Saver JL, Nogueira RG, Yoo AJ, et al. Mechanical thrombectomy for acute ischemic stroke with occlusion of the M2 segment of the middle cerebral artery: a meta-analysis. J Neurointerv Surg. 2018;10:620–624. doi: 10.1136/neurintsurg-2017-013515. doi: 10.1136/neurintsurg-2017-013515. [DOI] [PubMed] [Google Scholar]
- 7.Gibo H, Carver CC, Rhoton AL, Jr, Lenkey C, Mitchell RJ. Microsurgical anatomy of the middle cerebral artery. J Neurosurg. 1981;54:151–169. doi: 10.3171/jns.1981.54.2.0151. doi: 10.3171/jns.1981.54.2.0151. [DOI] [PubMed] [Google Scholar]
- 8.Goyal M, Menon BK, Krings T, Patil S, Qazi E, McTaggart RA, et al. What constitutes the M1 segment of the middle cerebral artery? J Neurointerv Surg. 2016;8:1273–1277. doi: 10.1136/neurintsurg-2015-012191. doi: 10.1136/neurintsurg-2015-012191. [DOI] [PubMed] [Google Scholar]
- 9.Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. American Heart Association Stroke Council. 2018 guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49:e46–e110. doi: 10.1161/STR.0000000000000158. doi: 10.1161/STR.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 10.Jansen IGH, Mulder MJHL, Goldhoorn RB MR CLEAN Registry Investigators. Endovascular treatment for acute ischaemic stroke in routine clinical practice: prospective, observational cohort study (MR CLEAN Registry). BMJ. 2018;360:k949. doi: 10.1136/bmj.k949. doi: 10.1136/bmj.k949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a quantitative computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. ASPECTS Study Group. Alberta Stroke Programme Early CT Score. Lancet. 2000;355:1670–1674. doi: 10.1016/s0140-6736(00)02237-6. [DOI] [PubMed] [Google Scholar]
- 12.Tan IY, Demchuk AM, Hopyan J, Zhang L, Gladstone D, Wong K, et al. CT angiography clot burden score and collateral score: correlation with clinical and radiologic outcomes in acute middle cerebral artery infarct. AJNR Am J Neuroradiol. 2009;30:525–531. doi: 10.3174/ajnr.A1408. doi: 10.3174/ajnr.A1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goyal M, Fargen KM, Turk AS, Mocco J, Liebeskind DS, Frei D, et al. 2C or not 2C: defining an improved revascularization grading scale and the need for standardization of angiography outcomes in stroke trials. J Neurointerv Surg. 2014;6:83–86. doi: 10.1136/neurintsurg-2013-010665. doi: 10.1136/neurintsurg-2013-010665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, van Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke. 1988;19:604–607. doi: 10.1161/01.str.19.5.604. [DOI] [PubMed] [Google Scholar]
- 15.von Kummer R, Broderick JP, Campbell BC, Demchuk A, Goyal M, Hill MD, et al. The Heidelberg Bleeding Classification: classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke. 2015;46:2981–2986. doi: 10.1161/STROKEAHA.115.010049. doi: 10.1161/STROKEAHA.115.010049. [DOI] [PubMed] [Google Scholar]
- 16.Saver JL. Novel end point analytic techniques and interpreting shifts across the entire range of outcome scales in acute stroke trials. Stroke. 2007;38:3055–3062. doi: 10.1161/STROKEAHA.107.488536. doi: 10.1161/STROKEAHA.107.488536. [DOI] [PubMed] [Google Scholar]
- 17.Moons KG, Donders RA, Stijnen T, Harrell FE., Jr. Using the outcome for imputation of missing predictor values was preferred. J Clin Epidemiol. 2006;59:1092–1101. doi: 10.1016/j.jclinepi.2006.01.009. doi: 10.1016/j.jclinepi.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 18.Sheth SA, Yoo B, Saver JL, Starkman S, Ali LK, Kim D, et al. UCLA Comprehensive Stroke Center. M2 occlusions as targets for endovascular therapy: comprehensive analysis of diffusion/perfusion MRI, angiography, and clinical outcomes. J Neurointerv Surg. 2015;7:478–483. doi: 10.1136/neurintsurg-2014-011232. doi: 10.1136/neurintsurg-2014-011232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhogal P, Bücke P, AlMatter M, Ganslandt O, Bäzner H, Henkes H, et al. A comparison of mechanical thrombectomy in the M1 and M2 segments of the middle cerebral artery: a review of 585 consecutive patients. Interv Neurol. 2017;6:191–198. doi: 10.1159/000475535. doi: 10.1159/000475535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rai AT, Domico JR, Buseman C, Tarabishy AR, Fulks D, Lucke-Wold N, et al. A population-based incidence of M2 strokes indicates potential expansion of large vessel occlusions amenable to endovascular therapy. J Neurointerv Surg. 2018;10:510–515. doi: 10.1136/neurintsurg-2017-013371. doi: 10.1136/neurintsurg-2017-013371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dorn F, Lockau H, Stetefeld H, Kabbasch C, Kraus B, Dohmen C, et al. Mechanical thrombectomy of M2-occlusion. J Stroke Cerebrovasc Dis. 2015;24:1465–1470. doi: 10.1016/j.jstrokecerebrovasdis.2015.04.013. doi: 10.1016/j.jstrokecerebrovasdis.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 22.Hernández-Pérez M, Pérez de la Ossa N, Aleu A, Millán M, Gomis M, Dorado L, et al. Natural history of acute stroke due to occlusion of the middle cerebral artery and intracranial internal carotid artery. J Neuroimaging. 2014;24:354–358. doi: 10.1111/jon.12062. doi: 10.1111/jon.12062. [DOI] [PubMed] [Google Scholar]
- 23.Lima FO, Furie KL, Silva GS, Lev MH, Camargo EC, Singhal AB, et al. Prognosis of untreated strokes due to anterior circulation proximal intracranial arterial occlusions detected by use of computed tomography angiography. JAMA Neurol. 2014;71:151–157. doi: 10.1001/jamaneurol.2013.5007. doi: 10.1001/jamaneurol.2013.5007. [DOI] [PubMed] [Google Scholar]
- 24.Salahuddin H, Ramaiah G, Slawski DE, Shawver J, Buehler M, Zaidi SF, et al. Mechanical thrombectomy of M1 and M2 middle cerebral artery occlusions. J Neurointerv Surg. 2018;10:330–334. doi: 10.1136/neurintsurg-2017-013159. doi: 10.1136/neurintsurg-2017-013159. [DOI] [PubMed] [Google Scholar]
- 25.Kaesmacher J, Maegerlein C, Kaesmacher M, Zimmer C, Poppert H, Friedrich B, et al. Thrombus migration in the middle cerebral artery: incidence, imaging signs, and impact on success of endovascular thrombectomy. J Am Heart Assoc. 2017;6:e005149. doi: 10.1161/JAHA.116.005149. doi: 10.1161/JAHA.116.005149. [DOI] [PMC free article] [PubMed] [Google Scholar]


