Abstract
Background:
Concentrations of 6-thioguanine (6TG) nucleotides and 6-methylmercaptopurine (6MMP) nucleotides in RBCs were measured using liquid chromatography-tandem mass spectrometry (LC-MS/MS). This assay was validated for clinical use and was applied to blood samples from patients taking mercaptopurine (6MP).
Methods:
RBCs were hemolyzed and deproteinized using perchloric acid, followed by heating for the hydrolysis of nucleotides, and the resultant base was measured using LC-MS/MS. Precision, recovery, linearity, matrix effect, and limit of quantification was validated for clinical application. Our results were compared with another institution's established LC-MS/MS assay. We measured the concentrations of 6TG and 6MMP in RBCs of pediatric patients with acute lymphoblastic leukemia (ALL), and the clinical impact of those metabolites was investigated.
Results:
The imprecision coefficient of variations of 6TG and 6MMP were 5.7%–8.1%, and the bias was within 5%. Lower limits of quantification were set at 54 ng/mL for 6TG and 1036 ng/mL for 6MMP. Correlation coefficients for 6TG and 6MMP were 0.997 and 1.0 in a comparison study. For clinical proof-of-concept, 74 blood samples were collected from 37 pediatric ALL patients receiving maintenance therapy. Concentration of 6TG ranged from 16.1 to 880 pmol/8 × 108 RBCs and that of 6MMP from 55 to 20,937 pmol/8 × 108 RBCs. The 6MP metabolites were not correlated with WBC or absolute neutrophil count. On the other hand, the higher 6MMP level was associated with elevated alanine aminotransferase and aspartate aminotransferase.
Conclusions:
In this study, an assay for the quantification of 6TG and 6MMP in RBCs was established and applied to pediatric ALL patients. Interindividual variability in 6MP metabolite concentrations was considerable and associated with elevation of liver enzymes, which may be useful in the clinical monitoring of 6MP maintenance therapy in pediatric ALL patients.
Key Words: RBC, 6-thioguanine (6TG), 6-methylmercaptopurine (6MMP), LC-MS/MS, pediatric ALL
INTRODUCTION
Mercaptopurine (6MP) is a prodrug with a structure similar to purines. Its metabolic active form is involved in DNA replication, DNA repair, and purine biosynthesis.1 On entering the cell, 6MP enters the purine salvage pathway, which starts to form the nucleotide, followed by base modification and phosphorylation to yield 6-thioguanosine nucleotide (6TGN), the active metabolite. The deoxy form of 6TGN, thiodeoxyguanosine triphosphate is structurally similar to deoxyguanosine triphosphate, which is an essential substrate in DNA replication. This nucleotide analogue is incorporated into the double-stranded DNA, activating the mismatch repair system and causing DNA strands to break, although the exact mechanism remains unclear.2
Two other major pathways competing with the purine salvage pathway are oxidation and thiol-methylation. Oxidation is a major catabolic route of 6MP if absorbed through the intestinal mucosa and liver, and is mediated by xanthine oxidase to form the inactive metabolite thiouric acid. The second pathway is thiol-methylation, mediated by intracellular thiopurine methyltransferase (TPMT), which is responsible for the methylation of several nucleotides in the purine salvage pathway. TPMT converts 6MP to 6-methylmercaptopurine (6MMP), thereby reducing reactive 6TGN production. TPMT activity is inherited in a codominant pattern: individuals who are homozygotes of the variant genotype have reduced activity of the enzyme, although allele frequency of the variant is lower in far-east Asians than in whites.3 Standard dose of 6MP in patients with the homozygote variant of TPMT potentially produces extensive 6TGN, which may cause life-threatening myelosuppression.4,5 In addition, heterozygous patients with 2 different single-nucleotide polymorphisms on the 2 TPMT alleles exhibit similar patterns to homozygous patients, and they are exposed to a substantial risk of developing myelosuppression at standard doses of thiopurine drugs.6
6MP is used as an immunosuppressant in the treatment of inflammatory bowel disease and as an antineoplastic agent in maintenance therapy for acute lymphoblastic leukemia (ALL). Most treatment protocols of ALL require 2–3 years of daily oral 6MP and weekly oral methotrexate (MTX) ingestion to suppress relapse after the induction of remission.7 During this long period, maintaining a proper level of myelosuppression is important for a favorable prognosis.8 By contrast, overdose of 6MP is related to severe neutropenia, resulting in the cessation of maintenance therapy and an increased risk of secondary malignancies.9 Most physicians evaluate myelosuppression based on WBC and absolute neutrophil count (ANC), and adjust the dose of 6MP or MTX to achieve clinically acceptable myelosuppression. However, identical WBCs and ANCs do not reflect identical level of myelosuppression between different patients.10 Moreover, in some patients, side effects such as hepatotoxicity occurs before myelosuppression. WBC and ANC may also be affected by intake of other drugs during maintenance therapy. In addition, to determine compliance of the patients to the prescribed drugs, more reliable clinical markers are needed.
Therefore, proper drug delivery to the target cell is determined by measuring 6MP and its metabolites. The half-life of plasma concentrations of the 6MP parent drug is very short (1–2 hours), and its bioavailability varies widely even intraindividually.11 Thus, as a surrogate marker, 6TGN, the final metabolite of the purine salvage pathway accumulating in RBC, was measured to determine its association with the abovementioned clinical parameters and prognosis. After several days of oral administration of 6MP, concentration of 6TGN in RBC reaches a steady state. This concentration was reported to be associated with myelotoxicity, duration of remission, and risk of disease relapse.12,13 Obviously, lower 6TGN concentrations were found in patients with low compliance to therapy,14 higher concentrations in patients with the variant TPMT genotypes/low activity of TPMT.15 6TGN concentrations were also found correlated with the concentration of thioguanine incorporated into leukocyte DNA.16 In addition to 6TGN, 6-methylmercaptopurine nucleotide (6MMPN) in RBC, the metabolite representing the activity of methylation pathway, was found to be related to increased activity of liver enzymes.17 For an overall evaluation of 6MP metabolism, both 6TGN and 6MMPN should be measured in RBC samples from patients.
Procedures for measuring 6TGN and 6MMPN in RBC were already developed decades ago. To quantify concentrations of intracellular metabolites, samples should be hemolysed and the nucleotides should be hydrolyzed with heat and acid into bases and phosphoribosyl groups. After proper extraction, concentrations of the cleaved 6TG and 6MMP were quantified using high-performance liquid chromatography (HPLC) with ultraviolet detection, which was first introduced 30 years ago.18 Recently, instrumentation for the quantification of said metabolites has increasingly been shifted to the more accurate and sensitive liquid chromatography-tandem mass spectrometry (LC-MS/MS) systems.14 Moreover, differences in extraction protocols could result in about 2.7-fold interassay differences in 6TG,19 and instability of metabolites at room temperature was found to reduce the relevance of the results.20 Consequently, analytical aspects of the quantification method require careful development, validation, and standardization.
In this study, we developed an assay for the quantification of the 2 key 6MP metabolites in RBC using LC-MS/MS. After validation of the analytical performance, as proof-of-concept, 6TG and 6MMP in RBC were measured in samples from pediatric ALL patients receiving 6MP maintenance therapy. These results were compared with the aforementioned conventional clinical biomarkers for monitoring 6MP treatment and dose adjustments, such as drug dosage, WBC, ANC, aspartate aminotransferase (AST), and alanine aminotransferase (ALT).
MATERIALS AND METHODS
Chemicals and Materials
Stock solutions of 6TG and 6MMP were prepared (>98% purity; Sigma Aldrich, St. Louis, MO), and isotope-labeled derivatives (6TG-13C215N and 6MMP-D3) were used as internal standards (IS) (Toronto Research Chemicals, North York, ON, Canada). The stock solutions were prepared as follows: 6TG 2 mg/mL in 1M sodium hydroxide, 6MMP 2 mg/mL in 1M sodium hydroxide, 6TG IS 1 mg/mL in DMSO, and 6MMP IS 2.5 mg/mL in methanol. Perchloric acid (70%) and dithiothreitol used in the hydrolysis process were purchased from Sigma Aldrich.
Working solutions were prepared by diluting the stock solutions with distilled water (DW). Seven concentrations were prepared as calibrators as follows: 125, 250, 500, 1000, 2000, 4000, and 8000 ng/mL for 6TG; 2500, 5000, 10,000, 20,000, 40,000, 80,000, and 160,000 ng/mL for 6MMP. The concentrations of quality control (QC) materials were prepared at 1250 and 2500 ng/mL for 6TG; 25,000 and 50,000 ng/mL for 6MMP. Each working solution of IS was prepared at a concentration of 1 mcg/mL for 6TG IS and 2.5 mcg/mL for 6MMP IS.
Sample Preparation
Samples were prepared as described by Dervieux and Boulieu21 and Shipkova et al19 with slight modification. One milliliter of EDTA-anticoagulated whole blood was centrifuged at 1000g for 10 minutes at room temperature. After removing plasma and the upper layer of packed RBCs, the remaining RBCs were washed with saline and centrifuged twice at 1000g for 10 minutes. Packed RBCs were diluted 10-fold with saline, and 250 µL of the solution was dispensed into a tube and stored at −80°C until further use. Cryopreserved RBCs from healthy individuals were used as matrices for calibration, QC, and method validation. EDTA blood samples from patients included in this study were pretreated on the day received.
The hydrolysis and extraction process were performed as follows: diluted RBC solution (250 µL) was mixed with 20 µL of IS, 20 µL of 1.1 M dithiothreitol, and 50 µL of DW, vortexed for 30 seconds, and spun down. To prepare the samples for calibration, QC, and method evaluation, 20 µL of calibrator, QC working solution, or method evaluation material, instead of 20 µL of DW, were added to the mixture. Then, 34 µL of 70% perchloric acid was added, vortexed for 30 seconds, and centrifuged at 3000g for 15 minutes at room temperature. The supernatant (220 µL) was transferred to another polypropylene tube and hydrolyzed at 100°C for 1 hour. After cooling at room temperature, the acidic solution was neutralized with 220 µL of sodium hydroxide. Then, 50 µL of this solution was transferred to 96-well polypropylene plates (300 µL) for analysis.
LC-MS/MS Analysis
The HPLC instrument was a 1200 series Infinity system (Agilent Technologies, Santa Clara, CA), equipped with HiP autosampler, binary pump, and column thermostat. Chromatographic separation was performed using an Eclipse plus C18 column (3.5 µm, 4.6 × 100 mm; Agilent Technologies). Mobile phase A was 0.1% formic acid in DW and mobile phase B was 0.1% formic acid in acetonitrile. The flow rate was 1 mL/min. The gradient was 90% A for the first minute, 50% A from 2 to 2.6 minutes, followed by 100% A from 2.7 minutes until the end of the gradient. The injection volume was 5 µL, and the total run-time per sample was 5 minutes. The retention time of 6TG and 6TG IS was approximately 2.39 minutes, and that of 6MMP and 6MMP IS was approximately 2.85 minutes.
The LC was connected to a 6490 triple quadrupole MS (Agilent Technologies). Sample analysis was performed using electrospray ionization in the positive ion mode. The m/z transitions were as follows: 168>150.9 for 6TG, 171.1>154 for 6TG IS, 167>125.1 for 6MMP, and 170.1>125.1 for 6MMP IS. Nitrogen was used as nebulization and collision gas. The collision energy used for 6TG and 6TG IS was 26 V, and 30 V for 6MMP and 6MMP IS. Other key MS/MS parameters were: capillary voltage 3.5 kV, dwell time 50 ms, sheath gas flow 11 L/min at 200°C, and desolvation gas flow 16 L/min at 200°C. The ratio of the peak area of analyte to that of its corresponding IS was used for quantification using the MassHunter Workstation (Agilent Technologies). Calibration curve was fitted using linear regression after 1/x-weighting. To compensate for the difference from RBC counts, RBC counts and hematocrit measured before sample preparation were used to transform concentrations to pmol/8 × 108 RBC units according to the equations described below. RBC was mainly analyzed by using XE 2100 hematology analyzer (Sysmex Corporation, Kobe, Japan) during each patient's visit. For RBC, a blood volume of 25 µL was used, and molecular weights of the respective analytes were 167 and 166 g/mol. Dividing the value in ng/mL by 6 gives the approximate value of pmol/8 × 108 RBC.
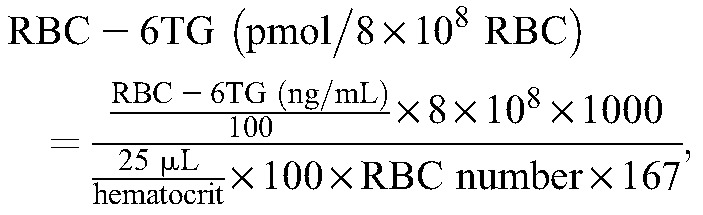 |
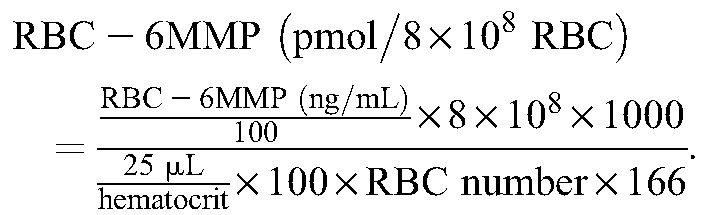 |
Validation Procedure
The analytical performance of the developed method was validated in terms of precision, recovery, linearity, lower limit of quantification (LOQ), and matrix effects. To evaluate precision, 2 concentrations of the QC working solutions were spiked into the diluted RBC from healthy donors. The 2 samples were analyzed in triplicate for 7 consecutive days. As there was no reference method for the analysis of 6TG and 6MMP, accuracy was evaluated by recovery fraction of the spiked concentrations following the same procedure for precision analysis. In addition, we performed experiments to assess precision using patient samples. Due to limited stability of the analytes in RBC,20,22 only within-day imprecision was evaluated through 10 replicates of RBC samples from 2 patients.
Linearity was evaluated by linear regression analysis of results from 7 calibrators during the validation period. For determination of the LOQ, 4 concentrations of 6TG and 6MMP were prepared by 2-fold serial dilution of the second lowest calibrator. After the pretreatment process, each diluted sample was measured in triplicate for 4 consecutive days. For each validation batch and all 15 batches of patients' samples, linearity of the calibration curve built with 7 levels of calibrators was verified with regression coefficient, slope, and bias.
Matrix effect, extraction recovery, and process efficiency was evaluated according to previously described postextraction method23 using samples from 4 different healthy persons. The same concentrations of the analytes and IS were prepared in DW (set 1), extracted hemolysate (set 2), and RBCs spiked before extraction (set 3). Percent ratio of set 2 to set 1 represents matrix effect, and that of set 3 to set 2 represents the recovery of analytes after extraction, and that of set 3 to set 1 represents efficiency of the whole process.
Results of our method were compared with those of another institution's using fresh whole blood specimens collected from patients taking 6MP. There was one Korean institution that currently measures 6TG and 6MMP in RBC using an established assay.22,24
Clinical Application
Seventy-seven EDTA venous blood samples were collected from 38 ALL patients receiving 6MP maintenance therapy. All patients were younger than 18 years at the time of blood sampling and were in their first remission. All samples were collected after more than 4 weeks of maintenance therapy consisting of daily oral 6MP and weekly oral MTX. Therefore, the drug concentrations were believed to have reached a steady state when enrolled in this study except for 3 excluded cases: 2 samples were excluded because the prescription of oral 6MP and MTX was stopped for 7 and 16 days due to neutropenic fever. In these patients, 6TG concentrations were the lowest, whereas 6MMP concentrations were not; one sample was also excluded because of suspicion of noncompliance, probably due to high fever after first administration of 6MP: the concentrations of 6TG and 6MMP measured after 2 weeks of prescription were still under the LOQ despite being prescribed the highest dosage of 6MP.
The method for adjusting 6MP dose was similar: an initial dose of 6MP at the start of maintenance therapy was determined to be 50 mg/m2 per day; subsequent doses were adjusted based on complete blood count to obtain adequate myelosuppression and based on liver function tests to prevent possible hepatotoxicity. Goal of myelosuppression was slightly differed from patient to patient, but WBC 1500–2000/μL and/or ANC 500–1000/μL was the rough range of acceptance. The protocol was approved by the institutional review board of Seoul National University Hospital, and written informed assent and/or consent was obtained from the patients/guardians, whatever was appropriate.
Statistical Analysis
EP evaluator 11 (Data Innovations, Burlington, VT) was used for processing and interpreting data from method validation studies, and IBM SPSS statistics 23 (IBM, Armonk, NY) and MedCalc 12 (MedCalc Software, Ostend, Belgium) was used for processing clinical data. In EP evaluator 11, complex precision module compatible for EP15-A2 and LOQ module compatible for EP17-A2 were used.25,26 The correlation values between 6MP metabolites in RBC and various clinical parameters were expressed as Spearman's rho, when comparing 2 consecutive variables. To determine diagnostic usefulness of 6TG and 6MMP for detecting clinical outcome, receiver operating curve (ROC) was used and areas under the curve were calculated with Analyse-it version 4.96 (Analyse-it Software, Leeds, United Kingdom). Cutoff for dividing the patients into 2 groups was established by Youden's index. Multiple regression analysis with enter and stepwise methods was performed to predict myelosuppression and hepatotoxicity by adjusting the effects of other factors related to drug administration. Appropriate myelosuppression and hepatotoxicity were classified under a certain threshold, and the risk was analyzed by logistic regression analysis using forward methods.
RESULTS
Assay Validation
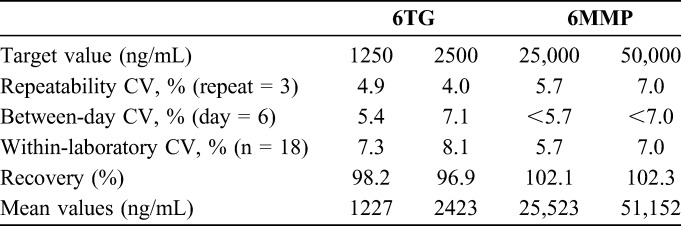
Results of the precision experiments are shown in Table 1. These experiments were performed by adding 6TG and 6MMP to RBC from a healthy person, and pretreating the blood samples, in ways similar to the RBC from study patients, as described in the Materials and Methods section. As the samples were analyzed in triplicate for 7 days, a total of 21 data points were obtained per concentration. However, on one day, one of the triplicate measurements was less than half of the expected concentration; hence, the data from this day were regarded as outlier and excluded from data analysis, which results in 18 data points per concentration. The repeatability imprecision coefficients of variation (CVs) were 4.0%–4.9% for 6TG and 5.7%–7.0% for 6MMP. The within-laboratory imprecision CVs were 7.3%–8.1% for 6TG and 5.7%–7.0% for 6MMP. The recovery rates in the same experiment as precision analysis ranged from 96.9% to 102.3%. The R2 values from linear regression during the validation period were above 0.99 for both 6TG and 6MMP. The highest calibrator concentrations of 8000 ng/mL for 6TG and 160,000 ng/mL, which can be arbitrarily converted to 1400 pmol/8 × 108 RBC and 28,000 pmol/8 × 108 RBC, for 6MMP were high enough to cover the concentration range of most of the patients' samples.
TABLE 1.
Imprecision and Recovery Rate of 6TG and 6MMP
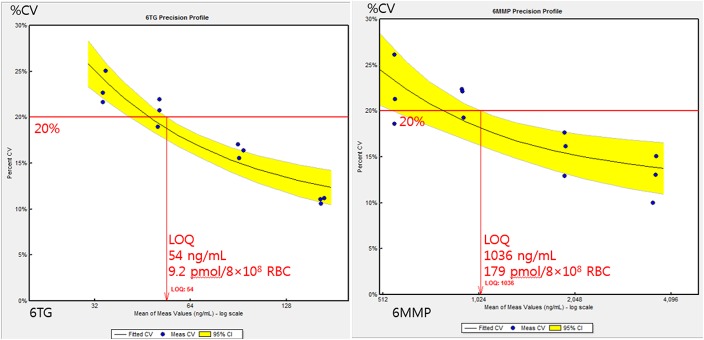
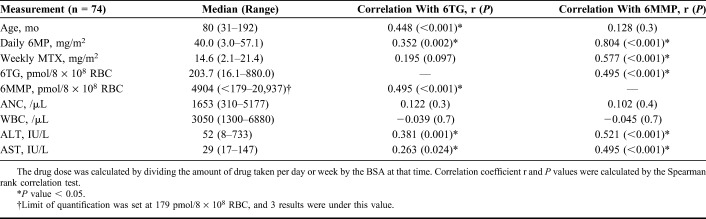
To determine the LOQ, 4 serially diluted solutions were measured in triplicate for 4 days, and the imprecision of each concentration was calculated (Fig. 1). The mean concentrations and CVs were as follows: 165 (10.0%), 92 (15.0%), 51 (18.9%), and 34 ng/mL (21.0%) for 6TG, and 3655 (11.7%), 1907 (14.2%), 911 (19.2%), and 557 ng/mL (20.1%) for 6MMP. The LOQ values were set as 54 ng/mL for 6TG and 1036 ng/mL for 6MMP using the EP evaluator imprecision default acceptance limit of 20%. When converted to the pmol/8 × 108 RBC unit using hematocrit and RBC number, the LOQ values for 6TG and 6MMP were estimated to be 9.2 pmol/8 × 108 RBC and 179 pmol/8 × 108 RBC, respectively. Analysis of clinical samples showed that 3 of 74 samples had concentrations below the LOQ for 6MMP, and one sample had a concentration below the LOQ for 6TG.
FIGURE 1.
LOQ for the measurement of 6TG (left) and 6MMP (right). Triplicate measurements were performed at 4 concentrations on 4 days by 2-fold dilution of the second lowest calibrator. In each analyte, acceptable imprecision level for determining the limit of quantification was 20% of CV.
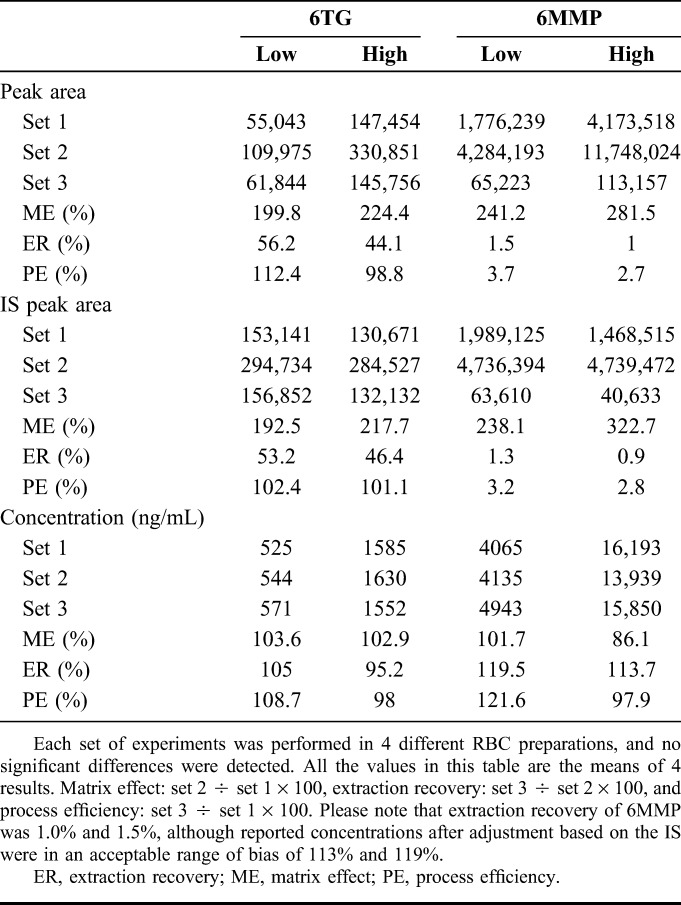
The matrix effects for 6TG were around 200% and extraction recovery was about 50% resulting in a process efficiency of around 100% when calculated by peak area. As IS was affected to a similar degree, final concentrations were not affected by matrix effect or extraction recovery. The degree of ionization enhancement and low extraction recovery was more severe for 6MMP. The matrix effect was more than 200% and extraction recovery around 1%, which produced a process efficiency of about 3%. However, like 6TG, the effects were also true for the IS and final concentrations were not significantly affected (Table 2).
TABLE 2.
Matrix Effect, Extraction Recovery, and Process Efficiency of 6TG and 6MMP
Our 6TG and 6MMP results in RBC from 10 different patients taking 6MP were compared with another institution that had an established assay. The range of measured 6TG was from 340 to 1090 ng/mL, and that of measured 6MMP was from 900 to 114,960 ng/mL. By correlation analysis, 6TG showed r = 0.997 and 6MMP showed r = 1. A scatterplot with linear regression analysis for both analytes is shown in Figure 2.
FIGURE 2.
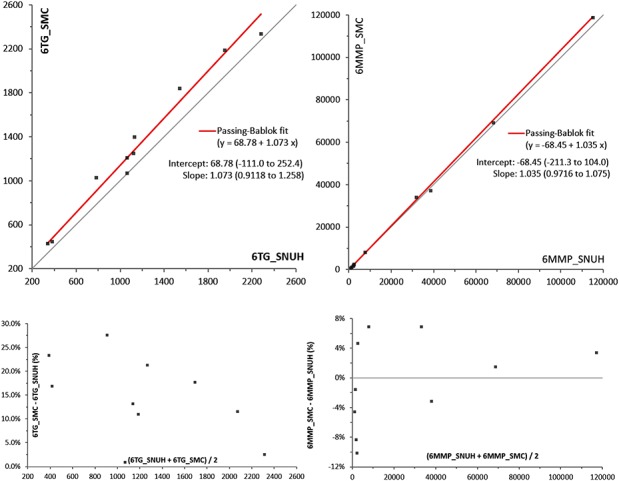
Results of the method comparison study with an established LC-MS/MS assay at another institution. Whole blood samples from 10 patients taking 6MP were analyzed and send to the other institution. Our results were on the X axis, and other institution's results on the Y axis. Results of Passing–Bablok linear regression analysis were marked on the right side of the graph. Percent bias plots were also proposed in the bottom of scatterplots.
Clinical Application
In total, 74 blood samples from 37 patients were collected during the proof-of-concept study to test the clinical utility of the present assay. Twenty-eight of these patients were male. There were 17, 7, and 2 patients whose blood samples were collected 2, 3, and 4 times in the study period, respectively. The average age at the time of study was 7.4 (range, 2–16) years. Time intervals from the start of maintenance to time of study (duration of therapy) averaged 10.1 months and ranged from 1 to 27 months. The drug dose, concentration of 6MP metabolites as analyzed in the 74 samples, other clinical parameters, and their correlations are summarized in Table 3. The drug dose was calculated by dividing the amount of drug taken per day or week by the body surface area (BSA). Concentrations of both 6MP metabolites in RBC were positively correlated with dose of drugs per BSA (Figs. 3A, B). The 6MMP concentrations were better associated with 6MP than with 6TG concentrations. The concentrations of both metabolites were positively correlated with each other.
TABLE 3.
6MP Metabolite Concentrations in RBC and Correlations With Clinical Parameters
FIGURE 3.
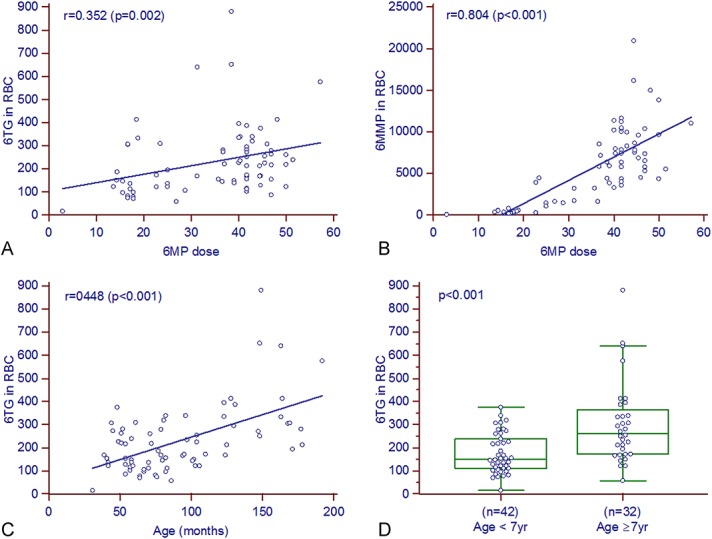
Factors related to concentrations of 6MP metabolites in RBC (n = 74). A and B, scatterplot of 6MP dose and 6MP metabolites accumulated in RBC. C and D, scatterplot of age and 6TG in RBC, and comparison according to the age group. 6TG in RBC was increased in older children. Please note that 6 cases >400 pmol/8 × 108 RBC were all older than 12 years. Units: 6TG and 6MMP in pmol/8 × 108 RBC; dose of daily oral 6MP per BSA, and weekly oral MTX per BSA in mg/m2. The lines in each graph indicate the regression line, and the r and P values were calculated by the Spearman correlation test or the Mann–Whitney test.
In addition, we examined the effects of other clinical parameters on the concentrations of 6TG and 6MMP. We found that the 6TG concentrations increased significantly with the age of the patients (Figs. 3C, D). Six samples with 6TG above 400 pmol/8 × 108 RBC, which is known to be associated with cytopenia, were all collected from patients older than 10 years. However, 6MP dose and 6MMP concentrations were not correlated with age (r = −0.064, P = 0.58 and r = 0.128, P = 0.28, respectively). This age-related increase could be an independent characteristic of 6TG in RBC. Other factors such as sex, and the duration of maintenance therapy did not affect concentrations of 6MP metabolites.
There was no statistically significant correlation between 6MP metabolites and ANC or WBC values (Table 3). In addition, there was no significant correlation between the 6MP metabolites and ANC or WBC values obtained after 14 and 28 days of 6MP metabolite measurements. Similar results were observed when comparing mean values of 6TG and 6MMP with those of ANC and WBC in patients whose blood was collected multiple times. Instead, duration of maintenance therapy was better correlated with WBC (r = 0.352, P = 0.002) and ANC (r = 0.448, P < 0.001). In a multiple regression analysis, age, dose of 6MP or MTX, or 6MP metabolites did not have a significant effect on ANC and WBC. The previous treatment period was the only significant variable correlating with WBC (β = 49.8, P = 0.01) and ANC (β = 56.2, P < 0.001). When low counts of WBC and ANC were defined as under 2500/µL and 1000/µL, 18 cases and 15 cases were classified as the low WBC group and low ANC group, respectively. Logistic regression analysis adjusted to other factors revealed that MTX dose and duration of maintenance showed significant odds ratio (OR) of 1.176 (95% CI, 1.022–1.354) and 0.893 (0.812–0.982) for low WBC, and OR of 1.226 (1.032–1.457) and 0.826 (0.708–0.964) for low ANC. Thus, longer duration of maintenance before therapy seemed to be the only factor affecting higher neutrophils and leukocytes.
The concentrations of 6MMP were positively correlated with ALT (r = 0.521, P < 0.001), and that of 6TG was also positively correlated with ALT (r = 0.381, P = 0.001). In addition, AST also showed a relatively weak correlation with the 2 6MP metabolites (Table 3). ALT was increased over 80 U/L in 28 cases, and AST was increased over 40 U/L in 21 cases. ROC for detecting ALT elevation showed area under the curve (AUC) 0.657 (95% CI, 0.530–0.783) for 6TG, 0.755 (0.635–0.875) for 6MMP, but the difference between them was not significant [P = 0.161, Fig. 4 (top)]. Similarly, ROC analysis of AST elevation over 40 U/L was performed and 6MMP (0.729, 0.587–0.870) showed a better AUC value than 6TG (0.586, 0.450–0.722). Cutoff values for dividing patients with or without ALT elevation were determined as 235 pmol/8 × 108 RBC for 6TG and 10,000 pmol/8 × 108 RBC for 6MMP according to Youden's index. The comparison of 6TG and 6MMP results in relationship to hepatotoxicity is shown in Figure 4 (bottom).
FIGURE 4.
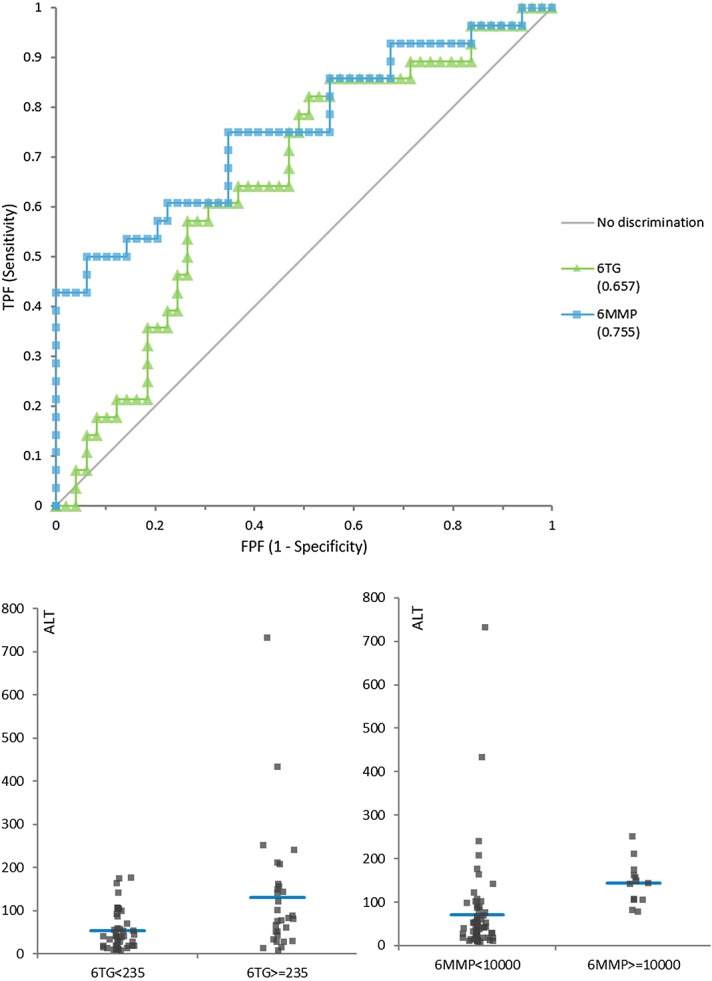
Diagnostic utility of 6TG and 6MMP for detection of hepatotoxicity (ALT >80 U/L). Top: ROC analysis of 6TG and 6MMP for detecting hepatotoxicity defined as elevated ALT over 80 U/L. AUC for 6TG and 6MMP was 0.657 (0.530–0.783) and 0.755 (0.635–0.875), respectively, and the 2 curves were not significantly different (P = 0.16). Bottom: Comparison of groups divided by the cutoff value offered by the ROC curve analysis. Cutoff was decided according to Youden's index to minimize false positive and false negative, which was 235 pmol/8 × 108 RBC for 6TG and 10,000 pmol/8 × 108 RBC for 6MMP. Mean results of the 2 groups were different with P = 0.001 for 6TG and P = 0.02 for 6MMP. FPF, false positive fraction; TPF, true positive fraction.
In addition, ALT showed a negative correlation with duration of 6MP maintenance therapy (r = −0.269, P = 0.02), whereas ALT and AST showed the highest correlation coefficients with doses of MTX (r = 0.628, P < 0.001 and r = 0.509, P < 0.001). The association between 6MP metabolites and liver enzymes was not significant in a multivariate analysis. Using the stepwise method, the MTX dose was the only significant variable for both ALT (β = 6.53, P = 0.007) and AST (β = 1.5, P = 0.004). Using threshold values of 120 IU/L for ALT and 40 IU/L for AST, 16 and 20 cases were grouped into increased ALT and AST categories, respectively. Logistic regression analysis using the forward method showed that MTX dose (OR 1.363, 95% CI 1.114–1.668) and duration of maintenance therapy (OR 0.91, 0.829–1.0) remained significant risk factors for an increase in ALT. Duration of maintenance therapy was a significant risk factor for elevation of AST (OR 0.913, 0.84–0.992). The OR for 6MMP concentrations was 1.0002 (1.0001–1.0004).
DISCUSSION
In this study, a reliable assay for measuring 6TG and 6MMP in RBC was developed and validated using LC-MS/MS, and applied to patient samples. The imprecision of the method ranged from 4.0% to 8.1%, which was satisfactory for clinical use. In the absence of a comparison study, analyte concentrations were compared with previous reports of pediatric ALL patients regarding 6MP dosage.12,17,27 In the familiarization and validation period, some QC materials showed reduced concentrations, which was attributed to errors in preparing the samples. The hydrolysis and extraction methods used in this experiment were based on the method described by Dervieux and Boulieu21 and modified from that described by Shipkova et al.19 This deproteinization method using perchloric acid showed a better recovery rate while being a less labor-intensive procedure, as compared to the liquid–liquid extraction methods described by Lennard and Singleton28 and modified by Cuffari et al.29 However, careful pipetting and adequate mixing was essential for reducing variation between samples, as the spun down RBC layer may precipitate even after centrifugation, thus resulting in variation in the number of RBC when pipetting.
After the first description of the LC-MS/MS method for the quantification of 6TG and 6MMP in RBC,15 significant progress was made by numerous research studies. Kirchherr et al30 changed the sample from washed RBC to whole blood and used stable isotope-labeled analytes as IS such as ours. Their procedure reduced the redundant centrifuge-washing step but required standardization to minimize variation of hemoglobin in different samples. Hofmann et al31 developed a LC-MS/MS method for the simultaneous quantification of 11 6MP metabolites including monophosphate, diphosphate, triphosphate nucleotides of 6TG and 6MMP. Our conventional acid hydrolysis step converted all the nucleotides regardless of the number of phosphates, but their method showed individual fraction of each nucleotide, which could need further research to find out clinical importance. Two more articles described application of solid-phase extraction for sample preparation, in which 6MP metabolites in plasma (not in RBC) were measured and a limited number of patients' samples were analyzed without sufficient information for clinical application.32,33
In this study, 54 and 1036 ng/mL as the LOQ for the measurement of 6TG and 6MMP, respectively. Assessment of assay sensitivity in previous studies mostly used certain signal-to-noise ratios of the chromatograms, rather than the evaluation of imprecision as recommended by CLSI guideline EP17-A2.26 The LOQ for 6MMP, of 179 pmol/8 × 108 RBC, was higher than those reported in previous studies: 110 pmol/8 × 108 RBC14 and 120 pmol/8 × 108 RBC.34 This difference may be due to between-day imprecision and instability of 6MMP at room temperature, which necessitates special care for the evaluation of the LOQ.20,22 According to the CLSI EP17-A2, LOQ experiments should be designed to reflect variation between different days, the LOQ value from this study may be more realistic than those reported in previous studies. Among the clinical samples, 3 samples were below the LOQ for 6MMP and several samples only slightly above the LOQ. As there may be delays during the collection, transport, and analysis process in clinical settings, the effect of short-term storage at different temperatures should be verified.
In addition to instability, evaluation of the matrix effect and extraction recovery of 6MMP showed a significant decline over time during the extraction process. Hemolysate showed approximately doubled the analytical response for both 6TG and 6MMP. The extraction process did not recover about 50% of 6TG and 99% of 6MMP, resulting, in combination with the observed ion enhancement during electrospray ionization, in 2%–3% total process efficiency in 6MMP and 100% in 6TG. This reduction in 6MMP recovery could be explained by the influence of the acidic conditions required for the hydrolysis of nucleotides.21 Dervieux and Boulieu35 suggested to measure the derivatized 6MMP molecule as generated during the hydrolysis step, but variable recovery rate due to pH and acidic damage to the autosampler instrument remained a problem. As in our assay, ionization enhancement and extraction efficiency for the isotope-labeled 6MMP IS was found equivalent to that of the unlabeled analyte, the IS was considered adequate to compensate for said effects. The low extraction efficiency may cause higher variability during extraction procedures, leading to increased imprecisions. In our method, isotope-labeled 6MMP was used to adjust for such variations. Moreover, acidic solutions were neutralized to protect the components of the HPLC instrument.
In the clinical proof-of-concept study, concentrations of 6MP metabolites were similar to or slightly lower than those observed in previous studies in pediatric ALL patients.12,36 Although a limited number of patient samples were included in the cross-validation with an established assay in another laboratory, our method was comparable with the said other LC-MS/MS method. This result verifies the use of generally accepted cutoff values for determining treatment efficacy and hepatotoxicity.15,17 In our results, the RBC concentrations of 6MMP were stronger correlated with an increase in liver enzyme activities than 6TG, possibly because the methylation pathway was more activated than the salvage pathway. In previous research, an increase in TPMT activity during chemotherapy and a decrease after chemotherapy was observed.5 Induction of the methylation pathway could be influenced by 6MP but not the salvage pathway. This may indicate that 6MMP might be a better marker for appropriate drug dosing, which would make it an important candidate for therapeutic drug monitoring in 6MP-treated patients. When measured using a microelectronic device that checked opening of the pill bottle, the observed mean compliance rate was approximately 90%, but the values were lower in certain groups, including elderly and single mother caregivers.37,38 Lower treatment compliance rates were closely associated with higher relapse rates.8,37 Therefore, 6MP metabolite measurements may also help to identify noncompliance and encourage proper medication intake in pediatric ALL patients after remission. To the best of our knowledge, this is the first report about the application 6MP metabolite quantification in Korean pediatric patients with ALL.
Interestingly, the concentration of 6TG was higher in the older than the younger patients in this study. This phenomenon seems to be a distinct characteristic of 6TG concentration, as 6MP dose and 6MMP concentration were not increased. Previous studies did not report an association between survival and age,13,39 but a recent pharmacokinetics study also discovered an age-related increase in 6TG, but not in 6MMP concentrations, which is consistent with our findings.36 The cause of this relation is not well understood. TPMT activity did not change significantly with age, except in RBC for newborns and in cord blood, which had 1.6-fold higher TPMT activity than RBCs from adults and children.40,41 This age-related increase in 6TG might be due to age-related changes in 6MP metabolism. Therefore, it can be speculated that younger patients may need larger doses of 6MP to achieve the same concentrations of 6TG as older patients. Additional research is required to establish the scientific basis of this phenomenon and to reveal its clinical significance.
We could not find any statistically significant correlation between concentrations of 6MP metabolites and WBC or ANC. Previous studies reported an inverse correlation between 6TG and WBC and/or ANC in pediatric patients with ALL.34,42,43 However, most of these studies were conducted about 30 years ago and, in some cases, repeatedly measured 6MP metabolites in a single patient, and then the mean value was used for establishing clinical correlations. More recently, a multicenter study suggested that variation in 6TG was associated with treatment compliance, continuity of prescription, and 6MP dose intensity (consequently risk of relapse).8 Although several studies have questioned the clinical usefulness of the monitoring of 6MP metabolites,11,44 it is still widely used for monitoring compliance. In our study, 6MP metabolites and complete blood count were measured once or twice in most patients, so that it was difficult to estimate the average value or the degree of variation.
The prominent factor affecting myelosuppression in this study was the duration of maintenance therapy. In the multivariate analysis, other drug-related factors did not significantly affect WBC or ANC, whereas duration of the maintenance therapy turned out to be a significant factor. One explanation for the duration-related decreased myelosuppression may have been that the compliance decreased over time, which was well described in a study using the electromonitoring system of pill bottle.37 Another possibility was that myelosuppression could not be achieved efficiently because of the resistance to antimetabolite drugs. TPMT activity increased in patients receiving chemotherapy and decreased after discontinuation of therapy.5 Perhaps, metabolism of 6MP, including TPMT activity, increased further with the longer period of drug use, suggesting that the degree of myelosuppression gradually decreased even with the same drug doses.
Liver enzymes were associated with the 6MP metabolites in the correlation analysis, but the association was not significant in the multivariate analysis with adjustment for age, 6MP, MTX dose, and duration of treatment. Similar to previous reports,45 6MMP concentrations of more than 5700 pmol/8 × 108 RBC were associated with ALT elevation, but ALT elevation was also significantly affected by the 6MP dose, MTX dose, and treatment duration. Multivariate analysis showed that MTX dose and duration of treatment were the most predictive factors for ALT and AST elevation. This decrease in hepatotoxicity with treatment duration might be explained with similar concepts of duration-related decreased myelosuppression, such as noncompliance or resistance to the drugs. Nonetheless, the time included in the study after maintenance therapy varied from patient to patient, and the interval between measurement times was not constant between patients, which limited the interpretation of the results.
CONCLUSION
In conclusion, we developed and validated an assay for the quantification of 6MP metabolites in RBC using LC-MS/MS, and this method was the first that was applied to Korean pediatric patients with ALL. Said assay showed reliable performance in the range of concentrations detected in patient RBCs. The concentrations of 6TG increased with age, whereas the doses of 6MP and 6MMP were not associated with age. Elevated activities of ALT and AST were associated with higher 6MP metabolite concentrations, but this association was not evident in the multivariate analysis. ANC and WBC were higher, whereas ALT and AST were lower, respectively, with longer duration of maintenance treatment, which may be due to noncompliance or resistance to drugs over time.
Footnotes
The authors declare no conflict of interest.
H. J. Kang and S. H. Song designed research. S. Y. Moon, J.-H. Lim, E.-H. Kim, and Y. Nam performed experiments for validation and application. K. S. Yu, K. H. Lee, and J. H. Song assisted with manuscript preparation. K. T. Hong, J. Y. Choi, C. R. Hong, H. Kim, H. J. Kang, and H. Y. Shin managed and collected patients for application. S. Y. Lee helped the comparison study between institutions.
REFERENCES
- 1.Schmiegelow K, Nielsen SN. Mercaptopurine/methotrexate maintenance therapy of childhood acute lymphoblastic leukemia : clinical facts and fiction. J Pediatr Hematol Oncol. 2014;36:503–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Karran P, Attard N. Thiopurines in current medical practice: molecular mechanisms and contributions to therapy-related cancer. Nat Rev Cancer. 2008;8:24–36. [DOI] [PubMed] [Google Scholar]
- 3.Relling MV, Gardner EE, Sandborn WJ, et al. Clinical pharmacogenetics implementation consortium guidelines for thiopurine methyltransferase genotype and thiopurine dosing: 2013 update. Clin Pharmacol Ther. 2013;93:324–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Evans WE, Horner M, Chu YQ, et al. Altered mercaptopurine metabolism, toxic effects, and dosage requirement in a thiopurine methyltransferase-deficient child with acute lymphocytic leukemia. J Pediatr. 1991;119:985–989. [DOI] [PubMed] [Google Scholar]
- 5.Lennard L, Lilleyman JS, Van LJWR. Genetic variation in response to 6-mercaptopurine for childhood acute lymphoblastic leukaemia. Lancet. 1990;336:225–229. [DOI] [PubMed] [Google Scholar]
- 6.Schutz E, Von Ahsen N, Oellerich M. Genotyping of eight thiopurine methyltransferase mutations: three-color multiplexing, “two-color/shared” anchor, and fluorescence-quenching hybridization probe assays based on thermodynamic nearest-neighbor probe design. Clin Chem. 2000;46:1728–1737. [PubMed] [Google Scholar]
- 7.Bassan R, Hoelzer D. Modern therapy of acute lymphoblastic leukemia. J Clin Oncol. 2011;29:532–543. [DOI] [PubMed] [Google Scholar]
- 8.Bhatia S, Landier W, Hageman L, et al. Systemic exposure to thiopurines and risk of relapse in children with acute lymphoblastic leukemia: a children’s oncology group study. JAMA Oncol. 2015;1:287–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmiegelow K, Al-Modhwahi I, Andersen MK, et al. Methotrexate/6-mercaptopurine maintenance therapy influences the risk of a second malignant neoplasm after childhood acute lymphoblastic leukemia: results from the NOPHO ALL-92 study. Blood. 2009;113:6077–6084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schmiegelow K, Schroder H, Schmiegelow M. Methotrexate and 6-mercaptopurine maintenance therapy for childhood acute lymphoblastic leukemia: dose adjustments by white cell counts or by pharmacokinetic parameters? Cancer Chemother Pharmacol. 1994;34:209–215. [DOI] [PubMed] [Google Scholar]
- 11.Balis FM, Holcenberg JS, Poplack DG, et al. Pharmacokinetics and pharmacodynamics of oral methotrexate and mercaptopurine in children with lower risk acute lymphoblastic leukemia: a joint children’s cancer group and pediatric oncology branch study. Blood. 1998;92:3569–3577. [PubMed] [Google Scholar]
- 12.Lilleyman JS, Lennard L. Mercaptopurine metabolism and risk of relapse in childhood lymphoblastic leukaemia. Lancet. 1994;343:1188–1190. [DOI] [PubMed] [Google Scholar]
- 13.Schmiegelow K, Schroder H, Gustafsson G, et al. Risk of relapse in childhood acute lymphoblastic leukemia is related to RBC methotrexate and mercaptopurine metabolites during maintenance chemotherapy. J Clin Oncol. 1995;13:345–351. [DOI] [PubMed] [Google Scholar]
- 14.Lancaster D, Lennard L, Lilleyman J. Profile of non-compliance in lymphoblastic leukaemia. Arch Dis Child. 1997;76:365–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dervieux T, Meyer G, Barham R, et al. Liquid chromatography-tandem mass spectrometry analysis of erythrocyte thiopurine nucleotides and effect of thiopurine methyltransferase gene variants on these metabolites in patients receiving azathioprine/6-mercaptopurine therapy. Clin Chem. 2005;51:2074–2084. [DOI] [PubMed] [Google Scholar]
- 16.Hedeland RL, Hvidt K, Nersting J, et al. DNA incorporation of 6-thioguanine nucleotides during maintenance therapy of childhood acute lymphoblastic leukaemia and non-Hodgkin lymphoma. Cancer Chemother Pharmacol. 2010;66:485–491. [DOI] [PubMed] [Google Scholar]
- 17.Nygaard U, Toft N, Schmiegelow K. Methylated metabolites of 6-mercaptopurine are associated with hepatotoxicity. Clin Pharmacol Ther. 2004;75:274–281. [DOI] [PubMed] [Google Scholar]
- 18.Lennard L, Maddocks JL. Assay of 6-thioguanine nucleotide, a major metabolite of azathioprine, 6-mercaptopurine and 6-thioguanine, in human red blood cells. J Pharm Pharmacol. 1983;35:15–18. [DOI] [PubMed] [Google Scholar]
- 19.Shipkova M, Armstrong VW, Wieland E, et al. Differences in nucleotide hydrolysis contribute to the differences between erythrocyte 6-thioguanine nucleotide concentrations determined by two widely used methods. Clin Chem. 2003;49:260–268. [DOI] [PubMed] [Google Scholar]
- 20.de Graaf P, Vos RM, de Boer NHK, et al. Limited stability of thiopurine metabolites in blood samples: relevant in research and clinical practise. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:1437–1442. [DOI] [PubMed] [Google Scholar]
- 21.Dervieux T, Boulieu R. Simultaneous determination of 6-thioguanine and methyl 6-mercaptopurine nucleotides of azathioprine in red blood cells by HPLC. Clin Chem. 1998;44:551–555. [PubMed] [Google Scholar]
- 22.Yoo IY, Lee K, Ji OJ, et al. Evaluation of stability of thiopurine metabolites using a validated LC-MS/MS method. Ann Lab Med. 2018;38:255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matuszewski BK, Constanzer ML, Chavez-Eng CM. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC−MS/MS. Anal Chem. 2003;75:3019–3030. [DOI] [PubMed] [Google Scholar]
- 24.Lee MN, Kang B, Choi SY, et al. Relationship between azathioprine dosage, 6-thioguanine nucleotide levels, and therapeutic response in pediatric patients with IBD treated with azathioprine. Inflamm Bowel Dis. 2015;21:1054–1062. [DOI] [PubMed] [Google Scholar]
- 25.CLSI. User Verification of Performance for Precision and Trueness; Approved Guideline-Second Edition. EP15-A2. Wayne, PA: Clinical and Laboratory Standards Institute; 2005. [Google Scholar]
- 26.CLSI. Evaluation of Detection Capability for Clinical Laboratory Measurement Procedures; Approved Guideline-Second Edition. EP17-A2. Wayne, PA: Clinical and Laboratory Standards Institute; 2012. [Google Scholar]
- 27.Relling MV, Hancock ML, Boyett JM, et al. Prognostic importance of 6-mercaptopurine dose intensity in acute lymphoblastic leukemia. Blood. 1999;93:2817–2823. [PubMed] [Google Scholar]
- 28.Lennard L, Singleton HJ. High-performance liquid chromatographic assay of the methyl and nucleotide metabolites of 6-mercaptopurine: quantitation of red blood cell 6-thioguanine nucleotide, 6-thioinosinic acid and 6-methylmercaptopurine metabolites in a single sample. J Chromatogr B Biomed Sci Appl. 1992;583:83–90. [DOI] [PubMed] [Google Scholar]
- 29.Cuffari C, Theoret Y, Latour S, et al. 6-Mercaptopurine metabolism in Crohn’s disease: correlation with efficacy and toxicity. Gut. 1996;39:401–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kirchherr H, Shipkova M, von Ahsen N. Improved method for therapeutic drug monitoring of 6-thioguanine nucleotides and 6-methylmercaptopurine in whole-blood by LC/MSMS using isotope-labeled internal standards. Ther Drug Monit. 2013;35:313–321. [DOI] [PubMed] [Google Scholar]
- 31.Hofmann U, Heinkele G, Angelberger S, et al. Simultaneous quantification of eleven thiopurine nucleotides by liquid chromatography-tandem mass spectrometry. Anal Chem. 2012;84:1294–1301. [DOI] [PubMed] [Google Scholar]
- 32.Raja MJ, Kavitha JR, Kumar KP, et al. Simultaneous determination of azathioprine and its metabolite 6-mercaptopurine in human plasma using solid phase extraction-evaporation and liquid chromatography-positive electrospray tandem mass spectrometry. Int Curr Pharm J. 2012;1:342–352. [Google Scholar]
- 33.Al-Ghobashy MA, Hassan SA, Abdelaziz DH, et al. Development and validation of LC–MS/MS assay for the simultaneous determination of methotrexate, 6-mercaptopurine and its active metabolite 6-thioguanine in plasma of children with acute lymphoblastic leukemia: correlation with genetic polymorphism. J Chromatogr B Anal Technol Biomed Life Sci. 2016;1038:88–94. [DOI] [PubMed] [Google Scholar]
- 34.Chrzanowska M, Kolecki P, Duczmal-Cichocka B, et al. Metabolites of mercaptopurine in red blood cells: a relationship between 6-thioguanine nucleotides and 6-methylmercaptopurine metabolite concentrations in children with lymphoblastic leukemia. Eur J Pharm Sci. 1999;8:329–334. [DOI] [PubMed] [Google Scholar]
- 35.Dervieux T, Boulieu R. Identification of 6-methylmercaptopurine derivative formed during acid hydrolysis of thiopurine nucleotides in erythrocytes, using liquid chromatography-mass spectrometry, infrared spectroscopy, and nuclear magnetic resonance assay. Clin Chem. 1998;44:2511–2515. [PubMed] [Google Scholar]
- 36.Adam de Beaumais T, Fakhoury M, Medard Y, et al. Determinants of mercaptopurine toxicity in paediatric acute lymphoblastic leukemia maintenance therapy. Br J Clin Pharmacol. 2011;71:575–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bhatia S, Landier W, Shangguan M, et al. Nonadherence to oral mercaptopurine and risk of relapse in Hispanic and non-Hispanic white children with acute lymphoblastic leukemia: a report from the Children’s Oncology Group. J Clin Oncol. 2012;30:2094–2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lennard L, Welch J, Lilleyman JS. Intracellular metabolites of mercaptopurine in children with lymphoblastic leukaemia: a possible indicator of non-compliance? Br J Cancer. 1995;72:1004–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lennard L, Lilleyman JS. Variable mercaptopurine metabolism and treatment outcome in childhood lymphoblastic leukemia. J Clin Oncol. 1989;7:1816–1823. [DOI] [PubMed] [Google Scholar]
- 40.McLeod HL, Relling MV, Liu Q, et al. Polymorphic thiopurine methyltransferase in erythrocytes is indicative of activity in leukemic blasts from children with acute lymphoblastic leukemia. Blood. 1995;85:1897–1902. [PubMed] [Google Scholar]
- 41.McLeod HL, Krynetski EY, Wilimas JA, et al. Higher activity of polymorphic thiopurine S-methyltransferase in erythrocytes from neonates compared to adults. Pharmacogenetics. 1995;5:281–286. [DOI] [PubMed] [Google Scholar]
- 42.Lennard L, Keen D, Lilleyman JS. Oral 6-mercaptopurine in childhood leukemia: parent drug pharmacokinetics and active metabolite concentrations. Clin Pharmacol Ther. 1986;40:287–292. [DOI] [PubMed] [Google Scholar]
- 43.Schmiegelow K, Bruunshuus I. 6-Thioguanine nucleotide accumulation in red blood cells during maintenance chemotherapy for childhood acute lymphoblastic leukemia, and its relation to leukopenia. Cancer Chemother Pharmacol. 1990;26:288–292. [DOI] [PubMed] [Google Scholar]
- 44.Reinshagen M, Schütz E, Armstrong VW, et al. 6-Thioguanine nucleotide-adapted azathioprine therapy does not lead to higher remission rates than standard therapy in chronic active Crohn disease: results from a randomized, controlled, open trial. Clin Chem. 2007;53:1306–1314. [DOI] [PubMed] [Google Scholar]
- 45.Dubinsky MC, Lamothe S, Yang HY, et al. Pharmacogenomics and metabolite measurement for 6-mercaptopurine therapy in inflammatory bowel disease. Gastroenterology. 2000;118:705–713. [DOI] [PubMed] [Google Scholar]