Supplemental Digital Content is available in the text.
Keywords: canagliflozin; cardiovascular diseases; diabetes mellitus, type 2; ischemic attack, transient; stroke
Abstract
Background and Purpose—
This study reports the detailed effects of canagliflozin on stroke, stroke subtypes, and vascular outcomes in participants with and without cerebrovascular disease (stroke or transient ischemic attack) at baseline from the CANVAS (Canagliflozin Cardiovascular Assessment Study) Program.
Methods—
The CANVAS Program, comprising 2 similarly designed and conducted clinical trials, randomly assigned 10 142 participants with type 2 diabetes mellitus and high cardiovascular risk to canagliflozin or placebo. Its primary outcome was a composite of major adverse cardiovascular events. The main outcome of interest for this report was fatal or nonfatal stroke. Additional exploratory outcomes were stroke subtypes and other vascular outcomes defined according to standard criteria.
Results—
There were 1 958 (19%) participants with prior stroke or transient ischemic attack at baseline. These individuals were older, more frequently women, and had higher rates of heart failure, atrial fibrillation, and microvascular disease (all P<0.001) compared with those without such a history. There were 309 participants with stroke events during follow-up (123 had prior stroke or transient ischemic attack at baseline and 186 did not), at a rate of 7.93/1000 patient-years among those assigned canagliflozin and 9.62/1000 patient-years among placebo (hazard ratio, 0.87; 95% CI, 0.69–1.09). Analysis of stroke subtypes found no effect on ischemic stroke (n=253, hazard ratio, 0.95; 95% CI, 0.74–1.22), a significant reduction for hemorrhagic stroke (n=30, hazard ratio, 0.43; 95% CI, 0.20–0.89) and no effect on undetermined stroke (n=29, hazard ratio, 1.04; 95% CI, 0.48–2.22). Effects on other cardiovascular outcomes were comparable among participants with and without stroke or transient ischemic attack at baseline.
Conclusions—
There were too few events in the CANVAS Program to separately define the effects of canagliflozin on stroke, but benefit is more likely than harm. The observed possible protective effect for hemorrhagic stroke was based on small numbers but warrants further investigation.
Clinical Trial Registration—
URL: https://www.clinicaltrials.gov. Unique identifiers: NCT01032629 and NCT01989754.
The prevalence of type 2 diabetes mellitus has doubled over the past 3 decades and is likely to affect a half a billion people in the next 3 decades.1 Given that new medications for diabetes mellitus could potentially be given to some tens of millions of people, it is vital that these medications are safe, and in particular, not associated with an increased vascular risk.2,3 The sodium glucose co-transporter 2 (SGLT2) inhibitors have recently emerged as important new treatments for diabetes mellitus. The mechanism of action, by reducing the reuptake of glucose in the kidney, lowers blood glucose, with other favorable effects on biomarkers, particularly weight loss.4 Evidence of the effect of SGLT2 inhibitors on vascular events has come from 2 trial programs, the EMPA-REG OUTCOME trial (Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients) testing empagliflozin5 and the CANVAS (Canagliflozin Cardiovascular Assessment Study) Program trials testing canagliflozin.6 In the EMPA-REG OUTCOME study, there was a nonsignificant increase in the risk of stroke (hazard ratio [HR], 1.18; 95% CI, 0.89–1.56), and in the CANVAS Program, there was a nonsignificant decrease in the risk of stroke (HR, 0.87; 95% CI, 0.69–1.09). Extensive subsidiary analyses of EMPA-REG OUTCOME have not identified any adverse effect of empagliflozin that might have caused an increase in stroke risk.7 Given that type 2 diabetes mellitus is associated with an approximate doubling in the risk of stroke compared with people without diabetes mellitus,8 it is important to understand more about the effects of these drugs on stroke and whether those with established cerebrovascular disease have any additional risks or benefits compared with those without. The aim of this study was to explore the detailed effects of canagliflozin on stroke, stroke subtypes, and other vascular outcomes among CANVAS Program participants and to analyze whether these effects differed for those with and without a history of cerebrovascular disease (stroke or transient ischemic attack [TIA]) at baseline.
Methods
Program Design
The study design, characteristics of participants, and the main results of the CANVAS Program have previously been published.6,9–11 In brief, the CANVAS Program, comprising 2 similarly designed and conducted trials—CANVAS and CANVAS-R (CANVAS-Renal)—was designed to assess the cardiovascular and renal safety and efficacy of canagliflozin, and how any potential benefits might balance against risks. There were 667 centers in 30 countries in the 2 trials that were scheduled for joint close-out and analysis when at least 688 cardiovascular events and a minimum of 78 weeks follow-up had been accrued for the last randomized participant, which occurred in February 2017.
Data from the CANVAS Program will be made available in the public domain via the Yale University Open Data Access Project (YODA; http://yoda.yale.edu/) once the product and relevant indication studied have been approved by regulators in the United States and European Union and the study has been completed for 18 months. The trial protocols and statistical analysis plans were published along with the primary CANVAS Program manuscript.6
Participants
Participants in the CANVAS Program were those with type 2 diabetes mellitus (glycated hemoglobin [HbA1c] ≥7.0% and ≤10.5%), aged ≥30 years with a history of symptomatic atherosclerotic cardiovascular disease, or ≥50 years with ≥2 risk factors for cardiovascular disease (duration of diabetes mellitus ≥10 years, systolic blood pressure [BP] >140 mmHg while on one or more antihypertensive agents, current smoker, microalbuminuria or macroalbuminuria, or high-density lipoprotein-cholesterol [HDL-C] <1 mmol/L). Patients treated with insulin and those with mild to moderate renal failure (estimated glomerular filtration rate [eGFR] ≥30 mL/min per 1.73 m2) were included. For the analyses presented in this report, a baseline diagnosis of cerebrovascular disease was based on a self-report of prior stroke or TIA.
Randomization, Treatment, and Follow-Up
After a 2-week, single-blind, placebo run-in period, participants were randomized centrally through an interactive web response system using a computer-generated randomization schedule prepared by the study sponsor using randomly permuted blocks. Participants in CANVAS were assigned in a 1:1:1 ratio to canagliflozin 300 mg, canagliflozin 100 mg, or matching placebo, and participants in CANVAS-R were randomly assigned in a 1:1 ratio to canagliflozin or matching placebo, administered at an initial dose of 100 mg daily with optional uptitration to 300 mg from week 13. Participants and all study staff were masked to individual treatment allocations until the completion of the study. Use of other background therapy for glycemic management, prevention of stroke and other cardiovascular outcomes, and other diseases was according to best practice instituted in line with local guidelines.
Participants were followed after randomization in a face-to-face follow-up that was scheduled for 3 visits in the first year and at 6-month intervals thereafter, with alternating telephone follow-up between face-to-face assessments. Every follow-up included inquiry about primary and secondary outcome events and serious adverse events. Serum creatinine measurement with eGFR was performed at least every 26 weeks in both trials. Participants who prematurely discontinued study treatment continued scheduled follow-up wherever possible, with extensive efforts made to obtain full outcome data for all during the final follow-up window that spanned from November 2016 to February 2017.
Outcomes
The main outcome of interest for this report is fatal or nonfatal stroke. These were originally part of the primary composite outcome of major adverse cardiovascular events (nonfatal stroke, nonfatal myocardial infarction, or cardiovascular death) used for the CANVAS Program.6 Additional exploratory outcomes for this report were fatal stroke; nonfatal stroke; ischemic stroke, hemorrhagic stroke, and stroke of undetermined type; TIA; stroke or TIA; major adverse cardiovascular events (nonfatal stroke, nonfatal myocardial infarction, or cardiovascular death); fatal or nonfatal myocardial infarction; hospitalized heart failure; cardiovascular death; all-cause mortality; progression of albuminuria (defined as >30% increase in albuminuria and a change from either normoalbuminuria to microalbuminuria or macroalbuminuria or from microalbuminuria to macroalbuminuria); and serious decline in kidney function (defined as a 40% reduction in eGFR sustained for at least 2 consecutive measures, end-stage kidney disease, or death from renal causes). Possible intermediate markers of stroke risk were also analyzed, which included systolic BP, diastolic BP, body weight, HbA1c, cholesterol, triglycerides, hematocrit, urinary albumin-to-creatinine ratio, eGFR, and adverse events of atrial fibrillation reported during follow-up.
Endpoint Adjudication Committees (online-only Data Supplement) adjudicated all cardiovascular outcomes, renal outcomes, and deaths, with stroke events adjudicated by experienced stroke physicians (online-only Data Supplement). Stroke was defined using the 2013 American Heart Association/American Stroke Association criteria.12 Ischemic and hemorrhagic stroke were determined by the neuroimaging findings, while undetermined stroke represented a clinical stroke without acute imaging to confirm the cause. TIAs were defined as a transient impairment of neurological function lasting <24 hours and without evidence of stroke on any acute neuroimaging. The Endpoint Adjudication Committee reviewed all suspected strokes and transient neurological events (including TIAs) as originally reported by the site investigators to determine whether the event met the criteria for a stroke. A reported TIA event could be adjudicated as a stroke event and it was removed from the analysis of TIA events if this was the case. Therefore, TIA events included in the analyses within this report were those not adjudicated to be stroke events.
Statistical Analysis
Categorical variables were summarized as the number of patients (with corresponding percentages), and continuous variables were summarized as the mean and SD or the median and interquartile range. Differences in baseline characteristics between participants with a history of cerebrovascular disease compared with participants with no history of cerebrovascular disease were evaluated using generalized Cochran-Mantel-Haenszel test, ANOVA, or the Wilcoxon rank-sum test. Efficacy analyses were based upon the full, integrated dataset and the intent-to-treat approach, with the comparison being between all participants assigned to canagliflozin (regardless of dose) and all participants assigned to placebo. Analyses were based on the occurrence of the first event under investigation. The trial was powered to detect an effect on the primary composite outcome and not for the analyses of stroke. Annualized incidence rates per 1000 patient-years of follow-up were calculated for all outcomes in addition to HRs and 95% CIs determined from Cox regression models, with treatment as the exploratory variable, and factors of trial and history of cardiovascular disease included in the model. We tested the homogeneity of treatment effects across the 2 contributing trials using P values for interactions, and the same approach was used for testing comparability of effects across subgroups defined by baseline participant characteristics. Sensitivity analyses of fatal or nonfatal stroke were performed according to whether the events occurred on-treatment or within 7, 30, or 90 days of treatment discontinuation. Effects of canagliflozin on continuous intermediate markers of stroke risk were analyzed using an ANCOVA model with treatment as an independent effect and adjusting for trial and baseline value. Change in the continuous intermediate marker from baseline to the last measurement throughout the trials and the difference of canagliflozin compared with placebo in the least squares means were estimated from the model. For atrial fibrillation, the HR with 95% CI was estimated from the same Cox regression model that was used to determine effects on stroke. Analyses were performed using SAS Enterprise Guide version 7.1.
Standard Protocol Approvals, Registrations, and Patient Consents
The protocols for the 2 trials were approved by the ethics committees at each site. All participants provided written informed consent.
Results
There were 10 142 patients in the CANVAS Program (Figure I in the online-only Data Supplement), and the mean follow-up time was 188.2 weeks. Mean age was 63.3 years, 35.8% were women, mean duration of diabetes mellitus was 13.5 years, and 65.6% had a history of cardiovascular disease. A total of 1 958 (19.3%) participants reported a history of cerebrovascular disease (stroke or TIA) at baseline. These participants were significantly different from other trial participants in most aspects of demography, disease history, and medication for the management of stroke risks, though the absolute magnitude of the differences was mostly small (Table I in the online-only Data Supplement). Atrial fibrillation was reported at baseline in 8.6% of those with cerebrovascular disease compared with 5.4% among those without.
Effects of Canagliflozin on Stroke, TIA, and Stroke Subtypes
There were 309 trial participants with a fatal or nonfatal stroke recorded during follow-up (123 had prior stroke or TIA at baseline and 186 did not), at a rate of 7.93/1000 patient-years among those assigned canagliflozin and 9.62/1000 patient-years among those assigned placebo (HR, 0.87; 95% CI, 0.69–1.09; Figures 1 and 2). Hemorrhagic stroke was uncommon (30 events), with an observed reduction in risk for those allocated to canagliflozin compared with placebo (HR, 0.43; 95% CI, 0.20–0.89; Figures 1 and 3). The rate of ischemic stroke was also lower among those treated with canagliflozin compared with placebo (n=253; HR, 0.95; 95% CI, 0.74–1.22), but this did not reach statistical significance. The rate of undetermined stroke events (n=29) was similar in both groups (HR, 1.04; 95% CI, 0.48–2.22). Point estimates of effect were consistent and below unity for fatal stroke (n=39; HR, 0.84; 95% CI, 0.44–1.59), nonfatal stroke (n=274; HR, 0.90; 95% CI, 0.71–1.15), TIA (n=88; HR, 0.86; 95% CI, 0.56–1.32), and the composite of stroke or TIA (n=377; HR, 0.89; 95% CI, 0.73–1.10), but none of these individual results were statistically significant. The estimate of effect of canagliflozin on stroke risk did not vary with the time since the last dose of randomized treatment (Figure II in the online-only Data Supplement). The use of antithrombotic agents at baseline had no effect on the risk of hemorrhagic stroke (HR, 0.63; 95% CI, 0.22–1.76).
Figure 1.
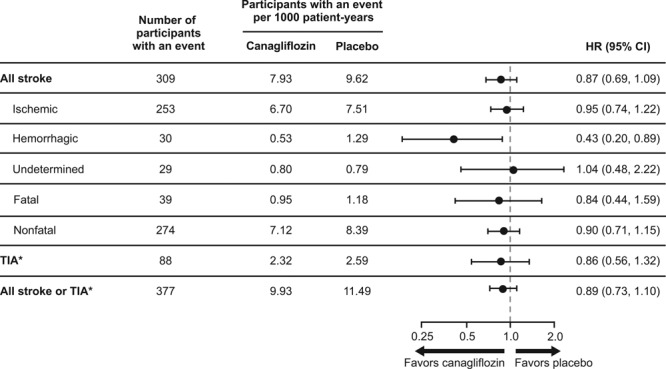
Effects of canagliflozin on stroke and TIA. *TIAs were not adjudicated but based upon adverse event reports made by site investigators. Incomplete ascertainment of TIAs is possible because adverse event reporting in the Canagliflozin Cardiovascular Assessment Study (CANVAS) Program was streamlined from January 2014 to capture only serious adverse events and adverse events leading to discontinuation, and TIA events considered by the site investigator as nonserious would not be captured after this time. HR indicates hazard ratio; and TIA, transient ischemic attack.
Figure 2.
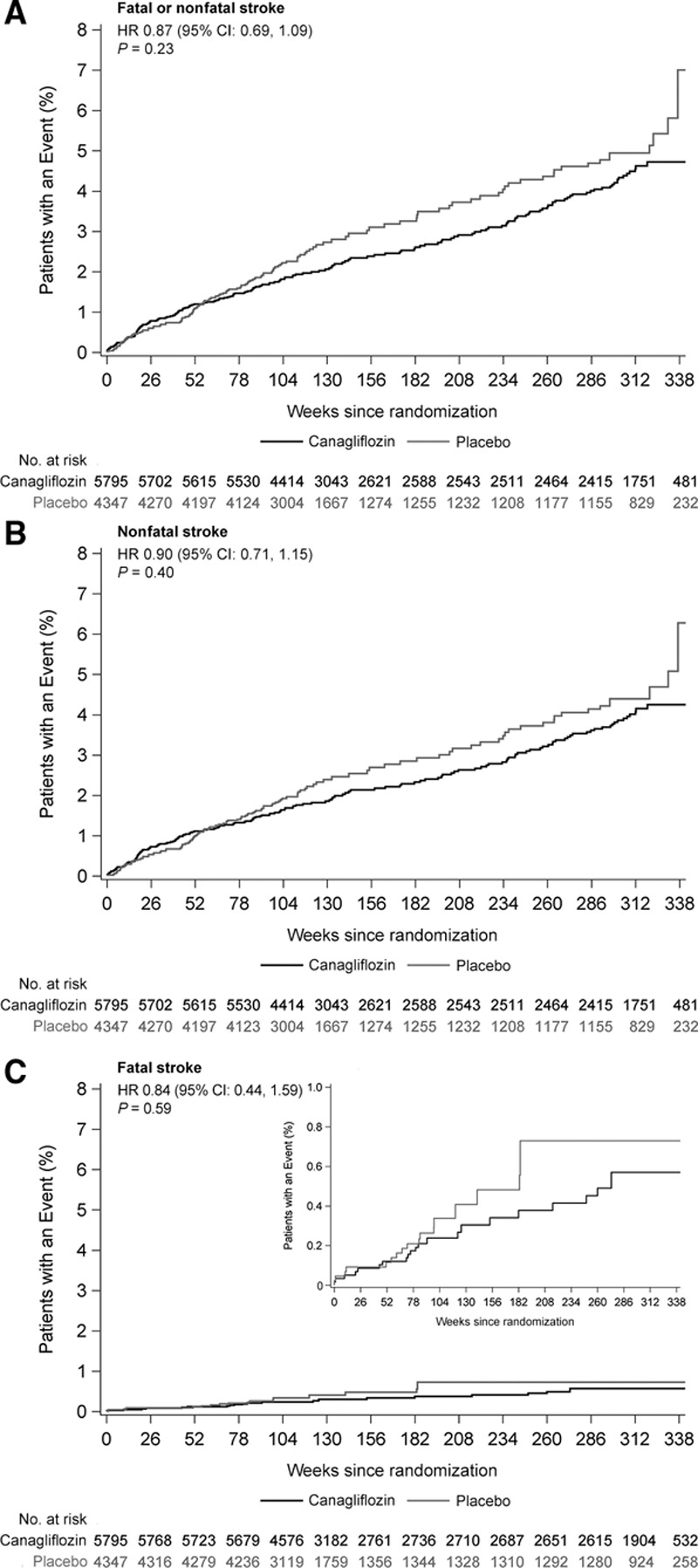
Effects of canagliflozin on fatal and nonfatal stroke. A, Fatal or nonfatal stroke. B, Nonfatal stroke. Reprinted from Neal et al6 with permission. Copyright ©2017, Massachusetts Medical Society. C, Fatal stroke. HR indicates hazard ratio.
Figure 3.
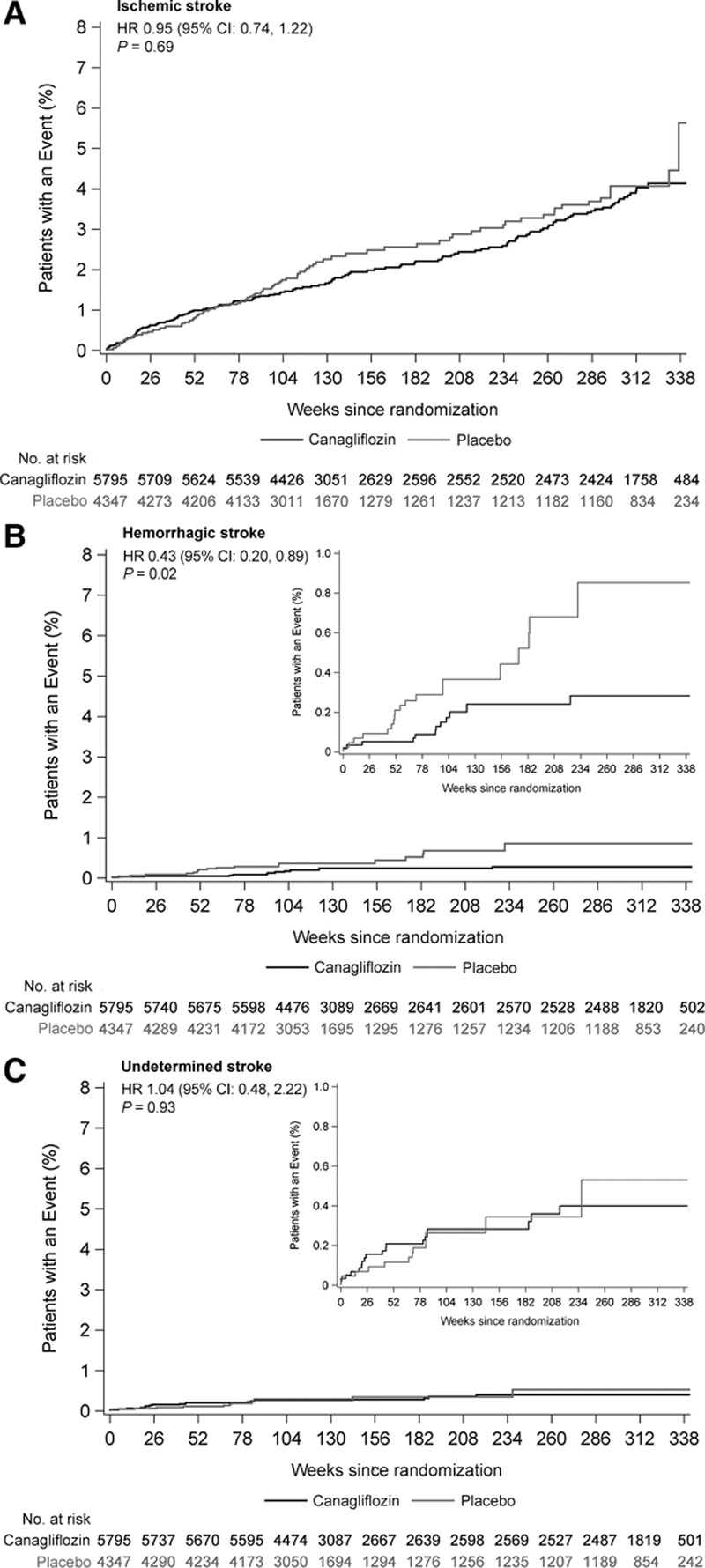
Effects of canagliflozin on stroke subtypes. A, Ischemic stroke. B, Hemorrhagic stroke. C, Undetermined stroke. HR indicates hazard ratio.
Effects on Possible Intermediate Markers of Stroke Risk
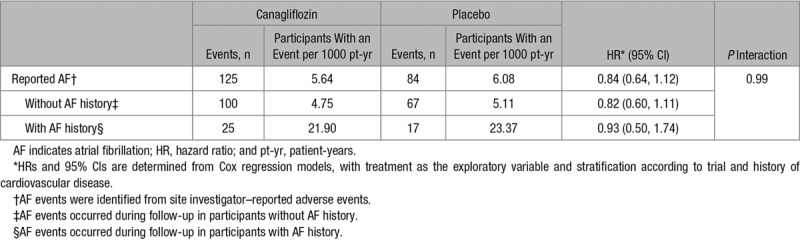
There were favorable effects of canagliflozin compared with placebo on systolic BP, diastolic BP, body weight, HbA1c, HDL-C, urinary albumin-to-creatinine ratio, and eGFR. Small increases were observed for hematocrit, low-density lipoprotein-cholesterol (LDL-C), total cholesterol, and triglycerides with null effects on the ratio of HDL-C to LDL-C (Table 1). There was no detectable effect of canagliflozin compared with placebo on atrial fibrillation (HR, 0.84; 95% CI, 0.64–1.12), which was also true for the subsets of participants with and without atrial fibrillation history at baseline (P interaction=0.99; Table 2).
Table 1.
Effects of Canagliflozin on Possible Intermediate Markers of Stroke Risk
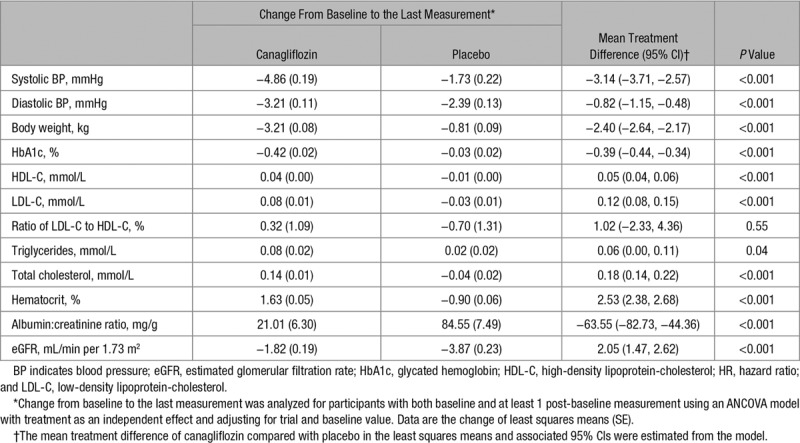
Table 2.
Effects of Canagliflozin on Atrial Fibrillation
Effects of Canagliflozin on Stroke in Patient Subgroups
Effects of treatment on stroke were similar in CANVAS and CANVAS-R (HR, 0.93; 95% CI, 0.69–1.27 versus HR, 0.80; 95% CI, 0.56–1.13; P interaction=0.51) and for most other participant subgroups (Figure 4). The exceptions were subsets defined by age (P interaction=0.006), eGFR (P interaction=0.005), and use of antithrombotic therapy (P interaction=0.04), which indicated greater protection in older patients, those with lower eGFR, and those reporting antithrombotic use.
Figure 4.
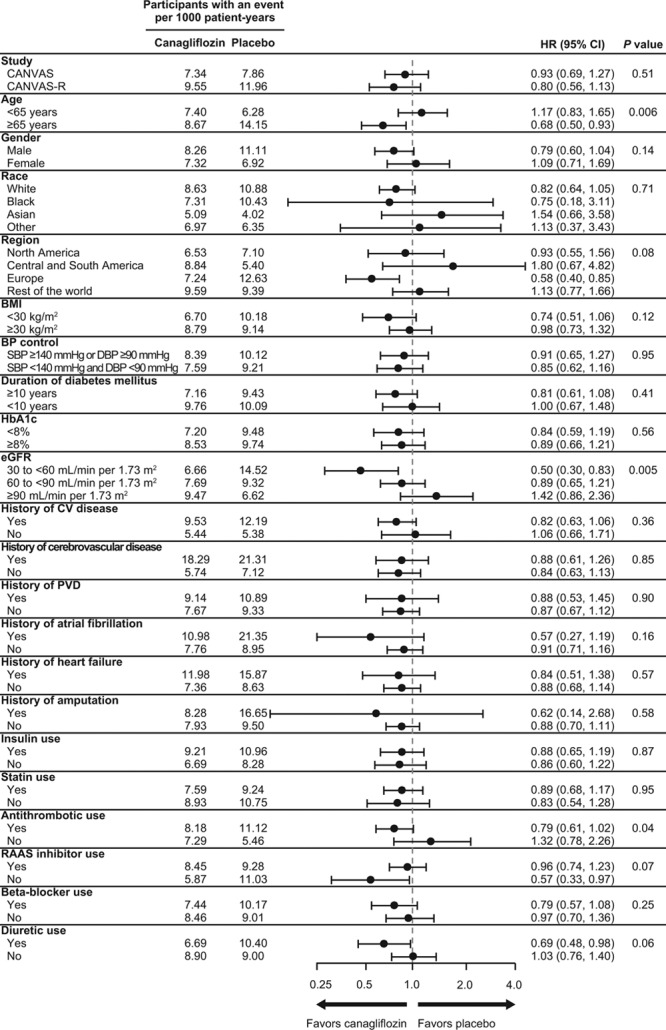
Effects of canagliflozin on stroke in patient subgroups. BMI indicates body mass index; BP, blood pressure; CANVAS, Canagliflozin Cardiovascular Assessment Study; CANVAS-R, CANVAS-Renal; CV, cardiovascular; DBP, diastolic blood pressure; eGFR, estimated glomerular filtration rate; HbA1c, glycated hemoglobin; PVD, peripheral vascular disease; RAAS, renin-angiotensin-aldosterone system; and SBP, systolic BP.
Effects of Canagliflozin on Cardiovascular, Kidney, and Death Outcomes in Patients With and Without Cerebrovascular Disease at Baseline
Patients with stroke or TIA at baseline were at higher absolute risk of subsequent stroke and all other vascular outcomes, with 123 stroke events occurring in those 1 958 patients with prior stroke or TIA versus 186 in the 8 184 patients without. The proportional effects of canagliflozin compared with placebo were comparable in patients with and without cerebrovascular disease at baseline for cardiovascular, kidney, and death outcomes (all P interaction >0.19; Figure 5).
Figure 5.
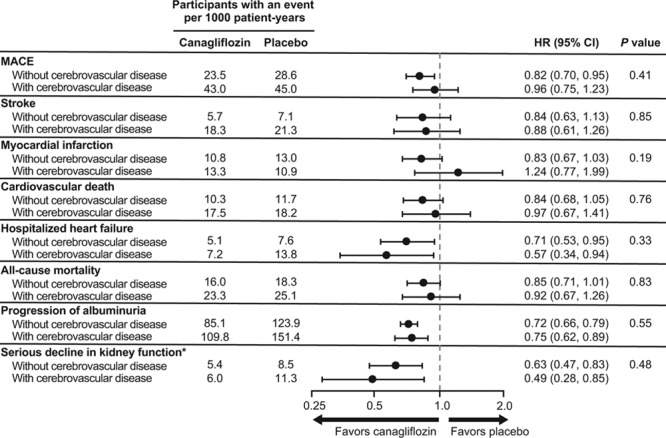
Effects of canagliflozin on cardiovascular and renal outcomes in patient with and without cerebrovascular (stroke or TIA) disease at baseline. eGFR indicates estimated glomerular filtration rate; HR, hazard ratio; MACE, major adverse cardiovascular events (nonfatal stroke, nonfatal myocardial infarction, or cardiovascular death); and TIA, transient ischemic attack. *Composite of 40% reduction in eGFR sustained for at least 2 consecutive measures, end-stage kidney disease, or death from renal causes.
Discussion
In the CANVAS Program, the composite of nonfatal stroke, nonfatal myocardial infarction, and cardiovascular death was significantly reduced, with a favorable (but not statistically significant) reduction in each of the 3 components.6 Similarly, in these analyses, our primary outcome of stroke (fatal or nonfatal), although with a favorable point estimate of effect, was not statistically significant. The CANVAS Program was not powered to examine the individual contributions of stroke, myocardial infarction, and cardiovascular death to the primary outcome, but the observed effect on stroke events is consistent with that initially anticipated on the basis of the known BP-lowering effect of the compound.13 Analysis according to pathological stroke subtype identified separately statistically significant protection against hemorrhagic stroke, albeit with small numbers, though there was no clear effect on ischemic stroke or undetermined stroke. The hemorrhagic stroke result, if confirmed, could be consistent with a BP-lowering mechanism of stroke prevention with canagliflozin, at least in part, as hemorrhagic stroke is more strongly determined by higher BP than ischemic stroke.13
Stroke, in particular, but also most other cardiovascular outcomes and death, occurred more frequently in patients with a baseline history of stroke or TIA compared with those without, though both sets of participants experienced comparable proportional reductions in the risks of these outcomes with use of canagliflozin.14
The effects of canagliflozin were broadly similar across a wide range of other participants, such as those using established treatments for the prevention of stroke, such as BP-lowering therapy, and patients of different ethnic backgrounds. The borderline significant interaction of canagliflozin treatment and stroke prevention with baseline use of an antithrombotic is likely to reflect a chance finding consequent upon the many comparisons made rather than a real effect. There was no corresponding evidence of an interaction by use of acetylsalicylic acid or anticoagulant therapy for stroke in EMPA-REG OUTCOME, though those participants all had a baseline history of cardiovascular disease and baseline use of these agents was greater.7 By contrast, the significance level of the interactions of canagliflozin and stroke with age and eGFR make chance a less likely explanation, though the strong correlation between age and lower eGFR mean that these observations may not be independent of one another. A biological explanation for a greater effect of canagliflozin on stroke reduction in older compared with younger individuals or among individuals with impaired compared with preserved renal function is unknown, though comparable trends were noted in EMPA-REG OUTCOME.7 The CREDENCE trial (Canagliflozin and Renal Endpoints in Diabetes With Established Nephropathy Clinical Evaluation), which was done in patients with impaired renal function, will provide significant additional insight into these effects and may provide an indication of mechanism.15
Beneficial effects of canagliflozin on stroke might be anticipated based upon the BP lowering achieved with SGLT2 inhibition, since BP reduction is well known to significantly reduce both first and recurrent stroke, with greater reduction for hemorrhagic stroke.16–20 Theoretical risks of hypoperfusion attributable to hypovolemia or hypotension21 have not been observed in prior large trials of stroke prevention, and there was no evidence for such effects in the CANVAS Program. Hemorrhagic stroke is especially BP dependent,13 so while the positive effect on this outcome observed in the CANVAS Program was based on relatively few events, a positive finding for hemorrhagic stroke is consistent with stroke epidemiology and clinical trials, albeit larger in magnitude than might have been expected for the observed BP reduction. Potential mechanisms for BP lowering with SGLT2 inhibition include natriuresis, osmotic diuresis (leading to volume depletion), and reduction in body weight.22 The changes in lipid parameters with canagliflozin would tend to favor hemorrhagic stroke prevention, though effects on cholesterol were small. Additional anti-atherosclerotic effects of SGLT2 inhibition mediated through effects on glucose and obesity may also contribute to protection against stroke in the longer term.23 There was no evidence of an adverse effect mediated through hemoconcentration, with favorable directions of effect for strokes of ischemic as well as hemorrhagic origin. Likewise, there was no evidence of any adverse effect of withdrawal of randomized treatment on stroke risk,7 with constant HRs observed for strokes occurring on-treatment and at various intervals after treatment discontinuation.
The findings reported here are strengthened by the rigorous design and conduct of the trial, the prespecification of stroke as an outcome of interest, and the careful adjudication of all potential stroke events according to recognized subtypes by an expert committee. This included screening all events reported as TIA for possible stroke events by the Endpoint Adjudication Committee, which resulted in additional stroke events being identified. Our study has limitations, the chief one being that the study was not powered to detect significant differences in total stroke events. The possible difference in effects by subtype of stroke needs to be interpreted with caution as a consequence but warrants further investigation. A further weakness is that TIAs were not themselves adjudicated, but all TIA events were screened by the stroke adjudicators to ensure stroke had not been misreported as TIA. Incomplete ascertainment of TIAs is possible because adverse event reporting in the CANVAS Program was streamlined from January 2014 to capture only serious adverse events and adverse events leading to discontinuation, and TIA events considered by the site investigator as nonserious would not be captured after this time. Missing TIA events should, however, be distributed nondifferentially between active and control groups and should not bias our results.
The unfavorable direction of effect reported for stroke in the EMPA-REG OUTCOME trial was not observed within the CANVAS Program, with a nonsignificant lower rate of all stroke events in those treated with canagliflozin and an indication of a possible beneficial effect for hemorrhagic stroke. The EMPA-REG OUTCOME trial recorded only about half as many hemorrhagic strokes as the CANVAS Program and did not report the effect of empagliflozin on that outcome, so comparability of the effects of the compounds on hemorrhagic stroke cannot be determined. Additional data from ongoing trials of SGLT2 inhibitors will provide further insight, and the CREDENCE trial, in particular, should clarify whether the effects of SGLT2 inhibition on stroke are enhanced in patients with chronic kidney disease.
Conclusions
The CANVAS Program demonstrated a reduction in the primary composite outcome of nonfatal stroke, nonfatal myocardial infarction, and cardiovascular death. There were too few events to separately define the effects of canagliflozin on stroke, but these analyses show that benefit is more likely than harm. The observed possible protective effect for hemorrhagic stroke was based on small numbers but warrants further investigation.
Acknowledgments
We thank all investigators, study teams, and patients for participating in these studies. We thank the following people for their contributions to the statistical monitoring/analyses and the protocol development, safety monitoring, and operational implementation over the duration of both studies: Lyndal Hones, Lucy Perry, Sharon Dunkley, Tao Sun, Hsiaowei Deng, Qiang Li, Severine Bompoint, Laurent Billot, Mary Lee, Joan Lind, Roger Simpson, Mary Kavalam, Terry Barrett, Ed Connell, Michele Weidner-Wells, Jacqueline Yee, Dainius Balis, Frank Vercruysse, Elisa Fabbrini, Nicole Meyers, Gary Meininger, and Norm Rosenthal. Medical writing support was provided by Kimberly Dittmar, PhD, of MedErgy. Drs Zhou and Rådholm contributed to the analysis and interpretation of the data, and the revision of the article. Drs Lindley, Shaw, and Desai contributed to the design of the study, the interpretation of the data, and the drafting and revising of the article. Drs Jenkins, Watson, and Oh contributed to the interpretation of the data and the revision of the article. Drs Perkovic, Mahaffey, de Zeeuw, Fulcher, Matthews, and Neal contributed to the design of the study, acquisition and interpretation of the data, and the revision of the article. All authors approved the final version of the article for submission.
Sources of Funding
Supported by Janssen Research & Development, LLC; ClinicalTrials.gov identifiers, NCT01032629, NCT01989754. Medical writing support was funded by Janssen Global Services, LLC. Canagliflozin has been developed by Janssen Research & Development, LLC, in collaboration with Mitsubishi Tanabe Pharma Corporation.
Disclosures
Dr Zhou reports receiving overseas visiting funding from Ren Ji Hospital, School of Medicine, Shanghai Jiao Tong University and a Scientia PhD Scholarship from the University of New South Wales, Sydney. Dr Lindley reports research support from the National Health and Medical Research Council of Australia and was a paid adjudicator for the CANVAS Program trials. Dr Rådholm reports receiving funding from a County Council of Östergötland International Fellowship. Dr Jenkins was a paid adjudicator for the CANVAS Program trials and has received payment for lectures and advisory boards for Novartis, TEVA, and Allergan. Dr Watson was a paid adjudicator for the CANVAS Program trials. Dr Perkovic reports receiving research support from the Australian National Health and Medical Research Council (Senior Research Fellowship and Program Grant); serving on steering committees for AbbVie, Boehringer Ingelheim, GlaxoSmithKline, Janssen, Novartis, and Pfizer; and serving on advisory boards and speaking at scientific meetings for AbbVie, Astellas, AstraZeneca, Bayer, Baxter, Bristol-Myers Squibb, Boehringer Ingelheim, Durect, Eli Lilly, Gilead, GlaxoSmithKline, Janssen, Merck, Novartis, Novo Nordisk, Pfizer, Pharmalink, Relypsa, Retrophin, Roche, Sanofi, Servier, and Vitae. The financial disclosures of Dr Mahaffey can be viewed at http://med.stanford.edu/profiles/kenneth-mahaffey. Dr de Zeeuw reports serving on advisory boards and as a speaker for Bayer, Boehringer Ingelheim, Fresenius, and Mitsubishi Tanabe; serving on steering committees and/or as a speaker for AbbVie and Janssen; and serving on data safety and monitoring committees for Bayer. Dr Fulcher reports receiving research support from Novo Nordisk and serving on advisory boards and as a consultant for Janssen, Novo Nordisk, Boehringer Ingelheim, and Merck Sharp & Dohme. Drs Shaw, Oh, and Desai report being full-time employees of Janssen Research & Development, LLC. Dr Matthews reports receiving research support from Janssen; serving on advisory boards and as a consultant for Novo Nordisk, Novartis, Eli Lilly, Sanofi-Aventis, Janssen, and Servier; and giving lectures for Novo Nordisk, Servier, Sanofi-Aventis, Eli Lilly, Novartis, Janssen, Mitsubishi Tanabe, and Aché Laboratories. Dr Neal reports receiving research support from the Australian National Health and Medical Research Council Principal Research Fellowship and from Janssen, Roche, Servier, and Merck Schering Plough; and serving on advisory boards and involvement in continuing medical education programs for Abbott, Janssen, Novartis, Pfizer, Roche, and Servier, with any consultancy, honoraria, or travel support paid to his institution.
Supplementary Material
Footnotes
A complete list of investigators in the CANVAS Program is provided in the online-only Data Supplement.
The online-only Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/STROKEAHA.118.023009.
References
- 1.Chen L, Magliano DJ, Zimmet PZ. The worldwide epidemiology of type 2 diabetes mellitus–present and future perspectives. Nat Rev Endocrinol. 2011;8:228–236. doi: 10.1038/nrendo.2011.183. doi: 10.1038/nrendo.2011.183. [DOI] [PubMed] [Google Scholar]
- 2.Kernan WN, Viscoli CM, Furie KL, Young LH, Inzucchi SE, Gorman M, et al. IRIS Trial Investigators. Pioglitazone after ischemic stroke or transient ischemic attack. N Engl J Med. 2016;374:1321–1331. doi: 10.1056/NEJMoa1506930. doi: 10.1056/NEJMoa1506930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marso SP, Bain SC, Consoli A, Eliaschewitz FG, Jódar E, Leiter LA, et al. SUSTAIN-6 Investigators. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834–1844. doi: 10.1056/NEJMoa1607141. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 4.Vasilakou D, Karagiannis T, Athanasiadou E, Mainou M, Liakos A, Bekiari E, et al. Sodium-glucose cotransporter 2 inhibitors for type 2 diabetes: a systematic review and meta-analysis. Ann Intern Med. 2013;159:262–274. doi: 10.7326/0003-4819-159-4-201308200-00007. doi: 10.7326/0003-4819-159-4-201308200-00007. [DOI] [PubMed] [Google Scholar]
- 5.Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. EMPA-REG OUTCOME Investigators. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 6.Neal B, Perkovic V, Mahaffey KW, de Zeeuw D, Fulcher G, Erondu N, et al. CANVAS Program Collaborative Group. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med. 2017;377:644–657. doi: 10.1056/NEJMoa1611925. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 7.Zinman B, Inzucchi SE, Lachin JM, Wanner C, Fitchett D, Kohler S, et al. EMPA-REG OUTCOME Investigators (Empagliflozin Cardiovascular Outcome Event Trial in Type 2 Diabetes Mellitus Patients) Empagliflozin and cerebrovascular events in patients with type 2 diabetes mellitus at high cardiovascular risk. Stroke. 2017;48:1218–1225. doi: 10.1161/STROKEAHA.116.015756. doi: 10.1161/STROKEAHA.116.015756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarwar N, Gao P, Seshasai SR, Gobin R, Kaptoge S, Di AE, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375:2215–2222. doi: 10.1016/S0140-6736(10)60484-9. doi: 10.1016/S0140-6736(10)60484-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Neal B, Perkovic V, de Zeeuw D, Mahaffey KW, Fulcher G, Stein P, et al. Rationale, design, and baseline characteristics of the Canagliflozin Cardiovascular Assessment Study (CANVAS)–a randomized placebo-controlled trial. Am Heart J. 2013;166:217.e11–223.e11. doi: 10.1016/j.ahj.2013.05.007. doi: 10.1016/j.ahj.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 10.Neal B, Perkovic V, Matthews DR, Mahaffey KW, Fulcher G, Meininger G, et al. CANVAS-R Trial Collaborative Group. Rationale, design and baseline characteristics of the CANagliflozin cardioVascular Assessment Study-Renal (CANVAS-R): a randomized, placebo-controlled trial. Diabetes Obes Metab. 2017;19:387–393. doi: 10.1111/dom.12829. doi: 10.1111/dom.12829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neal B, Perkovic V, Mahaffey KW, Fulcher G, Erondu N, Desai M, et al. CANVAS Program collaborative group. Optimizing the analysis strategy for the CANVAS Program: a prespecified plan for the integrated analyses of the CANVAS and CANVAS-R trials. Diabetes Obes Metab. 2017;19:926–935. doi: 10.1111/dom.12924. doi: 10.1111/dom.12924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A, et al. American Heart Association Stroke Council, Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular and Stroke Nursing; Council on Epidemiology and Prevention; Council on Peripheral Vascular Disease; Council on Nutrition, Physical Activity and Metabolism. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:2064–2089. doi: 10.1161/STR.0b013e318296aeca. doi: 10.1161/STR.0b013e318296aeca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lawes CM, Rodgers A, Bennett DA, Parag V, Suh I, Ueshima H, et al. Asia Pacific Cohort Studies Collaboration. Blood pressure and cardiovascular disease in the Asia Pacific region. J Hypertens. 2003;21:707–716. doi: 10.1097/00004872-200304000-00013. doi: 10.1097/01.hjh.0000052492.18130.07. [DOI] [PubMed] [Google Scholar]
- 14.Mahaffey KW, Neal B, Perkovic V, de Zeeuw D, Fulcher G, Erondu N, et al. CANVAS Program Collaborative Group. Canagliflozin for primary and secondary prevention of cardiovascular events: results from the CANVAS Program (Canagliflozin Cardiovascular Assessment Study). Circulation. 2018;137:323–334. doi: 10.1161/CIRCULATIONAHA.117.032038. doi: 10.1161/CIRCULATIONAHA.117.032038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jardine MJ, Mahaffey KW, Neal B, Agarwal R, Bakris GL, Brenner BM, et al. CREDENCE study Investigators. The Canagliflozin and Renal Endpoints in Diabetes With Established Nephropathy Clinical Evaluation (CREDENCE) study rationale, design, and baseline characteristics. Am J Nephrol. 2017;46:462–472. doi: 10.1159/000484633. doi: 10.1159/000484633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turnbull F Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of different blood-pressure-lowering regimens on major cardiovascular events: results of prospectively-designed overviews of randomised trials. Lancet. 2003;362:1527–1535. doi: 10.1016/s0140-6736(03)14739-3. doi: 10.1016/S0140-6736(03)14739-3. [DOI] [PubMed] [Google Scholar]
- 17.Turnbull F, Neal B, Algert C, Chalmers J, Chapman N, Cutler J, et al. Blood Pressure Lowering Treatment Trialists’ Collaboration. Effects of different blood pressure-lowering regimens on major cardiovascular events in individuals with and without diabetes mellitus: results of prospectively designed overviews of randomized trials. Arch Intern Med. 2005;165:1410–1419. doi: 10.1001/archinte.165.12.1410. doi: 10.1001/archinte.165.12.1410. [DOI] [PubMed] [Google Scholar]
- 18.PROGRESS Collaborative Group. Randomised trial of a perindopril-based blood-pressure-lowering regimen among 6105 individuals with previous stroke or transient ischaemic attack. Lancet. 2001;358:1033–1041. doi: 10.1016/S0140-6736(01)06178-5. doi: 10.1016/S0140-6736(01)06178-5. [DOI] [PubMed] [Google Scholar]
- 19.Xie X, Atkins E, Lv J, Bennett A, Neal B, Ninomiya T, et al. Effects of intensive blood pressure lowering on cardiovascular and renal outcomes: updated systematic review and meta-analysis. Lancet. 2016;387:435–443. doi: 10.1016/S0140-6736(15)00805-3. doi: 10.1016/S0140-6736(15)00805-3. [DOI] [PubMed] [Google Scholar]
- 20.Emdin CA, Rahimi K, Neal B, Callender T, Perkovic V, Patel A. Blood pressure lowering in type 2 diabetes: a systematic review and meta-analysis. JAMA. 2015;313:603–615. doi: 10.1001/jama.2014.18574. doi: 10.1001/jama.2014.18574. [DOI] [PubMed] [Google Scholar]
- 21.Malyszko J, Muntner P, Rysz J, Banach M. Blood pressure levels and stroke: J-curve phenomenon? Curr Hypertens Rep. 2013;15:575–581. doi: 10.1007/s11906-013-0402-z. doi: 10.1007/s11906-013-0402-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baker WL, Smyth LR, Riche DM, Bourret EM, Chamberlin KW, White WB. Effects of sodium-glucose co-transporter 2 inhibitors on blood pressure: a systematic review and meta-analysis. J Am Soc Hypertens. 2014;8:262.e9–275.e9. doi: 10.1016/j.jash.2014.01.007. doi: 10.1016/j.jash.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 23.Inzucchi SE, Zinman B, Wanner C, Ferrari R, Fitchett D, Hantel S, et al. SGLT-2 inhibitors and cardiovascular risk: proposed pathways and review of ongoing outcome trials. Diab Vasc Dis Res. 2015;12:90–100. doi: 10.1177/1479164114559852. doi: 10.1177/1479164114559852. [DOI] [PMC free article] [PubMed] [Google Scholar]


