Abstract
The postpartum period is associated with structural and functional plasticity in brain regions involved in parenting. While one study identified an increase in grey matter volume during the first four months among new mothers, little is known regarding the relationship between cortical thickness across postpartum months and perceived adjustment to parenthood. In this study of 39 socioeconomically diverse first-time new mothers, we examined the relations among postpartum months, cortical thickness, and parental self-efficacy. We identified a positive association between postpartum months and cortical thickness in the prefrontal cortex including the superior frontal gyrus extending into the medial frontal and orbitofrontal gyri, in the lateral occipital gyrus extending into the inferior parietal and fusiform gyri, as well as in the caudal middle frontal and precentral gyri. The relationship between cortical thickness and parental self-efficacy was specific to the prefrontal regions. These findings contribute to our understanding of the maternal brain in the first six months postpartum and provide evidence of a relationship between brain structure and perceived adjustment to parenthood.
Keywords: maternal brain, cortical thickness, parental self-efficacy, postpartum period
1. Introduction
Brain structure may play an important role in parenting for new mothers. Non-human studies provide evidence for morphological changes during the early postpartum period as critical for the onset and maintenance of maternal behaviors (Hillerer et al., 2014; Leuner et al., 2010). Similarly, in human mothers, structural increase (assessed by grey matter volume) in brain regions involved in parenting has been observed during the first four months postpartum (Kim et al., 2010). However, the study includes new mothers with limited socioeconomic diversity [i.e. mostly high socioeconomic status (SES) and Caucasian]. The current study aims to expand evidence of associations between parenthood and brain morphology, assessed by cortical thickness, in a socioeconomically diverse sample of new mothers. Moreover, the current study aims to extend evidence of brain structure-behavior associations by examining whether cortical thickness is associated with new mothers’ self-efficacy in parenting.
According to both animal and human studies, the parental brain may undergo changes to support high levels of parental motivation during the early postpartum period (Lonstein et al., 2015). Parenting requires constant care and attention to infants over an extended period of time. The physical, psychological, and economical demands can overwhelm new parents during the postpartum period (Nystrom and Ohrling, 2004). Infants are entirely dependent on their parents for survival, thus it is evolutionarily critical for new parents to have strong motivation and ability to provide caregiving despite the demands and challenges. Furthermore, infants’ needs evolve as they grow older. They spend less time crying and fussing, and more time playing and socially interacting, and by 6 months become more mobile (Bornstein, 2002). Flexibility of the maternal brain during the postpartum period may support development of different interaction styles in response to the rapidly changing needs of infants. The increased amount of complex social interactions involving multi-sensory cues (e.g. visual, auditory, tactile) between mothers and infants during the later postpartum months may also enhance the structure and function of the maternal brain over time.
Animal models have generated strong evidence for an association between changes in hormones such as oxytocin and steroid hormones, as well as interactions with pups, and neuronal morphological changes in brain regions involved in sensory information processing and maternal behaviors, including the olfactory bulb, hypothalamus, parietal cortex, somatosensory cortex, and prefrontal cortex (Fleming and Korsmit, 1996; Kinsley et al., 2008; Leuner and Sabihi, 2016; Xerri et al., 1994). Female rodents that previously avoided pups exhibit strong maternal motivation and behaviors toward their own pups during the postpartum period. These changes in behavior provide support for brain plasticity. Continuous interactions with pups maintain and enhance further brain plasticity in the maternal brain (Lonstein et al., 2015; Rosenblatt, 1994). Structural changes during the postpartum period occur through several different mechanisms including cell proliferation, neurogenesis, increased synaptic densities and dendritic growth. For example, rodent mothers exhibit dendritic growth of pyramidal neurons in the medial prefrontal cortex (Leuner and Gould, 2010) and neurogenesis in the olfactory bulb (Kopel et al., 2012). In the hippocampus of lactating rats, cell proliferation is suppressed but spine density increases during the postpartum period (Pawluski et al., 2016).
Consistent with animal findings, human mothers display increased grey matter from the first to fourth month postpartum, in brain regions related to maternal motivation (e.g. the midbrain, amygdala, striatum), sensory information processing (e.g. the superior temporal cortex, precentral and postcentral cortex), executive control and emotion regulation (e.g. the prefrontal cortex), and social cognition (e.g. the insula, parietal cortex) (Kim et al., 2010). Human mothers exhibit enhanced functional responses in these brain regions to their own infant cues, such as cry sounds and faces (Barrett et al., 2012; Kim et al., 2016; Nitschke et al., 2004), and such enhanced neural responses correlate with mothers’ sensitive caregiving behaviors during interactions with their own infant (Atzil et al., 2011; Hipwell et al., 2015; Kim et al., 2011b; Musser et al., 2012).
While many studies have examined neural activation in the human maternal brain, to the best of the authors’ knowledge, understanding of the associations between brain structure and human mothering is limited. Current evidence for structural changes in the maternal brain is also inconsistent. Kim et al. (2010) observed increases in grey matter volume when comparing the first to the fourth month postpartum. On the other hand, Hoekzema et al. (2017) reported decreases in brain structure (grey matter volume, cortical thickness, surface area) during pregnancy, then minimal changes from 2 months postpartum to 2-year follow-ups. Another paper (Oatridge et al., 2002) reported an overall brain size decrease during pregnancy, but full recovery by six months postpartum. Thus, the current study aims to address the inconsistent evidence related to human maternal brain structure during the first six months postpartum. While Kim et al. (2010) examined the grey matter volume during the first four months postpartum, the current study will focus on cortical thickness. Some argue that the surface-based analysis of cortical thickness can assess more regionally specific changes that are associated with different factors (e.g. age, disorder) compared to the volumetric analysis (Fischl and Dale, 2000). Indeed, previous studies examined cortical thickness in relation to development and environmental influences in human children (McLaughlin et al., 2014; O’Donnell et al., 2005) as well as adults (Lövdén et al., 2013; Thomas and Baker, 2013). In the current study, we examined whether the timing of the postpartum months may be associated with regional variations in cortical thickness of the human maternal brain.
Furthermore, we examined an association between cortical thickness and mothers’ adjustment to parenthood. In the current study, we focused on parental self-efficacy. Parental self-efficacy, a widely examined psychometric measure of parenthood adjustment, is a mother’s belief in her capacity to effectively manage parenting-related tasks as well as an overall perceived competence in being a parent (Teti and Gelfand, 1991). Parental self-efficacy is important because it plays a role in promoting positive mother-infant relationships (Leerkes and Crockenberg, 2002; Teti and Gelfand, 1991), and in turn positive infant developmental outcomes (Troutman et al., 2012). Although little is understood about brain region(s) responsible for parental self-efficacy, previoius work observed associations between self-efficacy in other domains such as emotion regulation and prefrontal cortex function (Goldin et al., 2009). Additionally, researchers have consistently observed new mothers’ increased prefrontal cortex activation in response to baby cues (Barrett and Fleming, 2011; Rutherford et al., 2015; Strathearn et al., 2008; Swain et al., 2017), and these responses are further associated with more sensitive parenting (Kim et al., 2011a; Musser et al., 2012; Swain et al., 2008). Moreover, new mothers with postpartum depression exhibit altered functional connectivity with the prefrontal cortex (Ho and Swain, 2017; Moses-Kolko et al., 2010), which may indicate difficulties in emotion regulation, and further influence competence as a parent. Given the role of the prefrontal cortex in high-order cognitive functions including emotion regulation, we suggest that brain structure in the prefrontal cortex is most likely to be directly associated with parental self-efficacy.
The current cross-sectional study investigates whether a positive association between later postpartum months and cortical thickness in human mothers across the first six months postpartum exists. We recruited mothers with socioeconomic diversity (i.e. around half of the participants were living in low income, and around half of the participants were an ethnic minority). While the previous study (Kim et al., 2010) included both primiparous and multiparous mothers, the current study focused only on primiparous (i.e. first-time) mothers based on evidence that parity is associated with differences in brain structure (Akbari et al., 2013). Cortical thickness does not include measurements of subcortical structure, therefore we focused our hypothesis on cortical structure. While the evidence is mixed, animal research strongly suggests postpartum structural growth in brain areas including the prefrontal cortex and brain regions involved in sensory information processing. Thus, we hypothesized that later postpartum months would be associated with greater cortical thickness in several regions including the prefrontal cortex, pre- and post-central gyri, superior temporal cortex, and inferior parietal cortex. We further hypothesize a positive association between mothers’ parental self-efficacy and cortical thickness, particularly in the prefrontal cortex.
2. Material and Methods
2.1. Participants
To ensure the socioeconomic diversity of the sample, participants were recruited through brochures and flyers made available in WIC (Women, Infant, and Children) centers, Colorado-state Prenatal Plus programs, and midwifery clinics in the Denver metro area. Selection criteria included: (1) age 18–40; (2) primiparous; (3) English-speaking; (4) IQ (assessed by the Wechsler Abbreviated Scale of Intelligence; WASI) > 70; (5) income-to-needs ratio (comparing family income during the past 12 months to the annually adjusted federal poverty line) below 6.5; (6) no pregnancy-related or infant medical illnesses involving more than a one-night stay in the neonatal intensive-care unit (NICU); (7) no current or historical psychiatric/neurological illness other than depression or anxiety diagnoses (to keep a controlled but ecologically valid community sampling approach); and (8) no contraindications to MRI scanning.
After removing one participant with evidence of movement (i.e. ringing artifacts) in the structural brain image, we included thirty-nine first-time mother participants in the analysis. The sample was socioeconomically diverse – 48.7% was low income (income-to-needs ratio < 2) and 51.2% had ethnic minority backgrounds.
2.2. Procedures
New mothers who expressed interest in the study were screened using the eligibility criteria. If the mother was eligible, a home visit was scheduled. During the home visit, the participant completed a demographic and family income interview administered by a trained researcher, as well as the WASI and self-reported questionnaires. Within a few weeks following the home visit, participants completed a neuroimaging session at the University of Colorado Boulder neuroimaging center. The mean interval between the home visit and neuroimaging session was approximately 3 weeks. Participants received monetary compensation after each visit. The procedures were approved by the Institutional Review Board of the University of Denver.
2.3. Measures
2.3.1. Maternal Self-Efficacy Scales (MSES).
Parenting-related self-efficacy in mothers was assessed using the MSES (Teti and Gelfand, 1991). The scale consists of 10 items on a scale of 1 (not good at all) to 4 (very good). Nine of the ten items assess mothers’ feelings of efficacy on specific activities of infant caregiving including soothing, feeding, bathing, understanding what the baby wants and enjoys, and maintaining interactions with the baby. The tenth item assesses mothers’ global feelings of efficacy in mothering. Scores from the ten items were summed to indicate parenting-related self-efficacy (Teti and Gelfand, 1991). The scale has good reliability and validity (Teti and Gelfand, 1991) and has been used in many studies with new mothers during the first postpartum year (Jones and Prinz, 2005; Leerkes and Crockenberg, 2002; Reece and Harkless, 1998; Schneewind and Pfeiffer, 1995; Teti and Gelfand, 1991; Troutman et al., 2012). In the current study, Cronbach’s alpha was 0.68 for the MSES.
2.3.2. Beck Depression Inventory (BDI).
Depressive symptoms were assessed using the BDI (Beck et al., 1988). The BDI consists of 21 items, with each item answered on a scale of 0 (symptom is absent) to 3 (symptom is severe). The total scores of all items indicates depressed mood. The questionnaire has also been widely used across diverse demographic backgrounds including the perinatal population (Gotlib et al., 1989; O’Hara et al., 1984; O’hara and Swain, 1996). For the sample, Cronbach’s alpha on the BDI was 0.82.
2.4. Neuroimaging
2.4.1. MRI Acquisition
High resolution T1-weighted magnetization prepared rapid gradient-echo (MPRAGE) images were acquired using a 3T Siemens Magnetom Tim Trio scanner. A 32-channel phased-array coil collected sagittal planes with the following parameters: 192 sagittal slices, TR = 2530 ms, TE = 1.64 ms, flip angle = 7°, FOV = 256 mm2 and voxel size 1 × 1 × 1 mm. Visual inspection for each image was conducted to check for excessive motion artifacts. One participant was excluded based upon this inspection; thus a total of 39 participants were included in the following image processing and analysis.
2.4.2. Image Processing
Cortical thickness analysis was conducted using the Freesurfer Image Analysis Suite 5.3 (http://surfer.nmr.mgh.harvard.edu/). T1-weighted images were entered into the Freesurfer recon-all pipeline which conducts: motion correction, intensity bias field removal, skull-stripping to remove any non-brain structures, and transformation into Talairach space. Representations of gray matter/white matter boundaries and cortical surface were reconstructed. The difference between the outer gray matter boundary (the pial surface) to the white/gray boundary is calculated at each point across the cortex (Dale et al., 1999; Dale and Sereno, 1993). This process involves triangular tessellation of the reconstructed surface into approximately 160,000 vertices per hemisphere. Local cortical thickness is computed as the difference between spatially equivalent vertices in the pial and gray/white matter surfaces. Cortical thickness measures are computed in native space and transformed into a spherical representation and registered into a common spherical atlas. As a result, cortical thickness maps are constructed from an average surface (‘fsaverage’) which is based on the MNI305 atlas. Following the standard procedure, maps were smoothed with 10 mm (FWHM) Gaussian kernel to reduce measurement noise and small scale registration errors for the vertex based analysis (Fischl and Dale, 2000). For each of the surface reconstructions, visual quality control of the reconstruction of the white matter and pial surfaces slice-by-slice for axial, sagittal, and coronal views was performed, and no image was edited manually to maintain objectivity.
2.5. Statistical Analysis
A whole-brain surface analysis was conducted using Freesurfer’s GLM analysis tools (mri_glmfit). At the group level, a general linear model was fit at each of the surface vertices. The model included the postpartum month at the time of the scan as the variable of interest and participants’ age and race (a dichotomous variable – white/non-white) as nuisance covariates (to rule out variability in cortical thickness based on age and race). Cluster-wise corrections for multiple comparisons were conducted at the whole brain level using a null-z permutation-based strategy with 10,000 iterations at a vertex-wise threshold p < .05 (1.3) and a cluster-wise probability (CWP) p < .05. This was conducted using Freesurfer’s tool mri_glmfit-sim in which a Monte Carlo simulation measures the maximum cluster size under the null-hypothesis.
Using SPSS (SPSS, Inc., Chicago, Ill), the posthoc analysis was conducted to examine the associations between postpartum months and cortical thickness in the suprathreshold regions after controlling for additional covariates – income-to-needs ratio and BDI scores – to rule out their potential role in predicting cortical thickness. In addition, we conducted a whole-brain analysis including income-to-needs ratio and BDI scores as additional covariates to confirm the associations between postpartum months and cortical thickness after controlling for the covariates.
Lastly, we used zero-order correlation, then separate multiple regressions to test the associations among cortical thickness and parental self-efficacy, controlling for postpartum months, income-to-needs ratio, and BDI scores.
3. Results
3.1. Demographic Variables
Table 1 includes descriptive statistics for the participants. Two of the participants were taking antidepressants. Postpartum months were not associated with demographic or psychometric variables described in Table 1, ps > 0.10 (Supplementary Table 1). Parental self-efficacy scores were also not associated with demographic or psychometric variables described in Table 1, ps > 0.10.
Table 1.
Demographic information for the sample.
| N (%) | Mean ± SD | Range | |
|---|---|---|---|
| Postpartum months at time of scan | 3.96 ± 1.58 | 0.89 - 6.96 | |
| Maternal age (years) | 24.41 ± 5.21 | 18 - 36 | |
| Maternal race/ethnicity | |||
| Caucasian | 19 (48.7) | ||
| Hispanic | 12 (30.7) | ||
| African-American | 2 (5.1) | ||
| Other | 6 (15.3) | ||
| Infant sex (female) | 23 (58.9) | ||
| Income-to-needs ratio | 2.46 ± 1.48 | 0.44 - 6.34 | |
| Depressive mood (BDI) | 6.97 ± 5.63 | 0 - 26 | |
| Parental self-efficacy (MSES) | 34.87 ± 3.09 | 27 - 40 | |
| Mood disorder history (Yes) | 4 (10.2) | ||
| Current intake of antidepressant or psychoactive medications | 2 (0.5) | ||
| Breastfeeding (and/or daily pump; but not exclusive formula feeding) | 38 (97.4) | ||
| Right handedness* | 33 (84.6) | ||
| Time away from own infant per week (hours) | 11.63 ± 13.27 | 0-40 | |
| Maternal working status (working full- or part-time) | 11 (28.2) | ||
| Interval between home and MRI visit (months) | 0.85 ± 0.68 | 0.07 - 3.42 |
one participant’s handedness data was missing.
3.2. Associations between Postpartum Months and Cortical Thickness
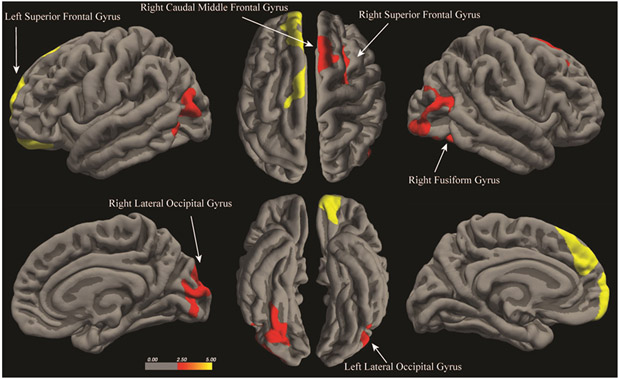
Consistent with the hypothesis, positive associations between postpartum months and cortical thickness were found in both hemispheres, controlling for mother’s own age and race. In the left hemisphere, later postpartum months were associated with greater cortical thickness in two clusters – (1) the superior frontal gyrus including the medial frontal and orbitofrontal gyri, (2) the lateral occipital gyrus including the middle and inferior temporal gyri, and inferior parietal gyrus (Figure 1 and Table 2; Supplementary Figure 1). In the right hemisphere, later postpartum months were associated with greater cortical thickness in four clusters – (1) the superior frontal gyrus, (2) caudal middle frontal gyrus including the precentral gyrus, (3) the fusiform gyrus, (4) the lateral occipital gyrus including the middle temporal and inferior parietal gyri (Figure 1 and Table 3; Supplementary Figure 2).
Figure 1.
Results of the whole-brain surface analysis identifying brain regions in which cortical thickness is positively associated with postpartum months (Cluster-wise probability corrected, p < 0.05).
Table 2.
Regions showing positive associations between postpartum months and cortical thickness in the left hemisphere. (BA = Brodmann Area, CWP = cluster-wise probability)
| Peak Vertex | Size (mm2) |
MNI X |
MNI Y | MNI Z |
CWP | Peak BA |
Additional Regions included in the Cluster |
|---|---|---|---|---|---|---|---|
| Superior Frontal Gyrus | 1894.27 | −12.2 | 64.2 | 10.2 | 0.0001 | 10 | Lateral and Medial Orbitofrontal Gyri |
| Lateral Occipital Gyrus | 1059.58 | −43.4 | −77.6 | 9.7 | 0.0058 | 19 | Middle/Inferior Temporal, Inferior Parietal Gyri |
Table 3.
Regions showing positive associations between postpartum months and cortical thickness in the right hemisphere. (BA = Brodmann Area, CWP = cluster-wise probability)
| Peak Vertex | Size (mm2) |
MNI X |
MNI Y |
MNI Z |
CWP | Peak BA |
Additional Regions included in the Cluster |
|---|---|---|---|---|---|---|---|
| Fusiform Gyrus | 916.79 | 31.3 | −74.1 | −11.4 | 0.0188 | 19 | Lateral Occipital Gyrus |
| Superior Frontal Gyrus | 1063.79 | 7 | 5.7 | 61.9 | 0.0067 | 6 | -- |
| Caudal Middle Frontal Gyrus | 824.59 | 25.6 | 7.6 | 46.2 | 0.0357 | 8 | Precentral Gyrus |
| Lateral Occipital Gyrus | 1063.68 | 33.6 | −100.4 | −7.5 | 0.0067 | 18 | Lateral Occipital, Inferior Parietal, Middle Temporal Gyri |
Posthoc analysis revealed that the positive associations in all clusters above were significant after including additional covariates – income-to-needs ratio and depressive symptoms, Bs ≥ 0.57, ps < 0.001. The whole-brain model including the additional covariates – income-to-needs ratio and depressive symptoms – confirmed that all the clusters in Tables 2 and 3 are significant after controlling for the additional covariates. In this model, an effect of income-to-needs ratio was identified in the left superior parietal gyrus, suggesting a positive association between cortical thickness in this region and income-to-needs ratio. No significant main effect of the depressive symptoms was identified.
There were no negative associations between postpartum months and cortical thickness. Thus, no region was identified to show a decrease in cortical thickness across postpartum months.
3.3. Associations between Cortical Thickness and Parental Self-Efficacy
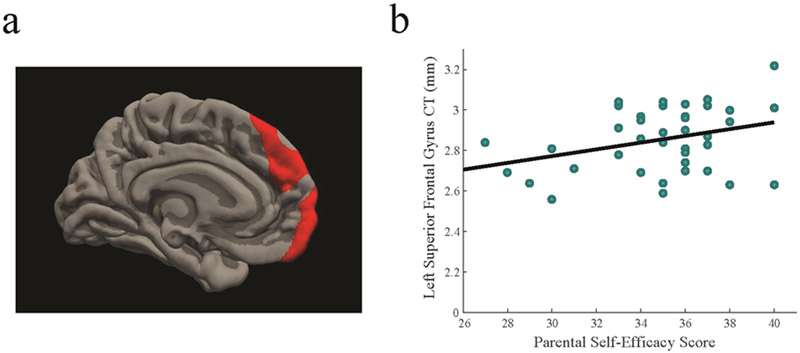
From the analysis of the associations with postpartum months, we identified three clusters in the prefrontal cortex – left superior/medial frontal gyrus (Table 2), right superior frontal gyrus, and right caudal middle frontal gyrus (Table 3). All three clusters were positively correlated with higher parental self-efficacy, rs(37) > 0.32, ps < 0.05 (Figure 2; Supplementary Table 1). After controlling for the postpartum months, income-to-needs ratio, and BDI scores, higher parental self-efficacy was associated with greater cortical thickness in the three clusters – left superior/medial frontal gyrus (B= 0.41, p < 0.05), right superior frontal gyrus (B =0.53, p < 0.01) and right caudal middle frontal gyrus (B= 0.50, p < 0.05). We explored the associations with parental self-efficacy in the other suprathreshold regions, but no significant association was identified (Supplementary Table 1). Thus, in this study, the associations with parental self-efficacy were specific to greater cortical thickness in the prefrontal regions.
Figure 2.
(a) Significant cluster identified in the left superior frontal gyrus extending into the medial frontal and orbitofrontal gyri; (b) a scatter plot of the relationship between the left superior frontal gyrus and parental self-efficacy (CT = cortical thickness);
4. Discussion
The current study examined the associations between motherhood and brain structure during the first six months postpartum among first-time mothers with socioeconomically diverse backgrounds. We found that greater cortical thickness in several brain regions, including the medial and lateral prefrontal, fusiform, and occipital gyri, was associated with later postpartum months. The positive associations between postpartum months and cortical thickness were significant after controlling for mothers’ age, race, socioeconomic status, and depressive symptoms. Furthermore, greater cortical thickness of the prefrontal cortex was associated with higher levels of parental self-efficacy. These findings suggest that brain morphology during the early postpartum period may support greater perceived self-efficacy in parenting among human first-time mothers with socioeconomically diverse backgrounds.
Animal and human mother research highlight the important role of the prefrontal cortex in parenting. The medial prefrontal cortex has direct anatomical connections to key subcortical regions involved in maternal motivation – hypothalamus, amygdala, ventral tegmental area, and striatum (Hoover and Vertes, 2007; Lonstein et al., 2015). In rodents, lesions and disruption in the medial prefrontal cortex lead to deficits in sensitive maternal behaviors such as pup retrievals and licking behaviors (Afonso et al., 2007; Febo et al., 2010). Researchers also observed post-partum neuronal growth specifically in the medial prefrontal cortex. Mother rats had more dendritic spines in the medial prefrontal cortex compared to virgin female rats, and neuronal growth in mother rats was linked to enhanced cognitive flexibility (Leuner and Gould, 2010). In humans, high-order cognitive functions involve both the medial and lateral prefrontal cortex including integration of reward and sensory information, executive function, and emotion regulation (Alvarez and Emory, 2006; O’Doherty, 2004; Wager et al., 2008), and in turn, may contribute to sensitive parenting. Human mothers showed increased grey matter volume in both medial and lateral prefrontal regions during the first four months postpartum (Kim et al., 2010), and increased responses in these brain regions to their own baby’s cues compared to other baby’s cues correlated with sensitive maternal behaviors (Atzil et al., 2011; Kim et al., 2011b; Musser et al., 2012). Thus, the current study extends previous evidence of the medial and lateral prefrontal cortex role in parenting by demonstrating positive associations between cortical thickness and later postpartum months.
We also observed greater cortical thickness in later postpartum months in regions that are involved in visual information processing and motor responses. The lateral occipital cortex, inferior parietal cortex, and fusiform gyrus assist in perceiving and processing visual information, particularly social stimuli such as faces (Gauthier et al., 1999; Haxby et al., 1996). The caudal middle frontal gyrus and precentral gyrus include premotor and primary motor cortices which contribute to planning and executing motor responses to stimuli (Chouinard and Paus, 2006; Rizzolatti et al., 1996). During the early postpartum period, interactions with infants provide a rich array of olfactory, auditory, somatosensory, and visual information to mothers. New mothers typically exhibit increased sensitivity toward their infant’s somatosensory cues including odors, faces, and cry sounds (Stallings et al., 2001; Thompson-Booth et al., 2014; Wiesenfeld and Malatesta, 1982). In rodents, neural reconstruction of the olfactory bulb, parietal lobe, and somatosensory cortex were associated with the expression of maternal behaviors during the postpartum period (Kinsley et al., 2008). Our findings of greater cortical thickness in these regions involved in social and somatosensory information processing during the early postpartum period are consistent with previous findings in human mothers, including increased grey matter volume (Kim et al., 2010) and enhanced brain activation in response to own infant cues (Nishitani et al., 2014).
We found that parental self-efficacy was specifically associated with cortical thickness in prefrontal areas. Self-efficacy involves high-level cognitive processes, including metacognition (Bandura, 1993), which involves prefrontal cortex functions (Baird et al., 2013). The other regions involved in visual information processing and motor responses are involved in providing inputs to the prefrontal cortex and performing specific tasks of caregiving. As the prefrontal cortex is involved in integrating sensorimotor information from other regions and regulating thoughts and behaviors, cortical thickness in this region was most likely to be directly associated with parental self-efficacy. In fMRI studies, researchers often observe increased activation in the prefrontal cortex in response to infant cues, however reduced activation among new mothers with greater difficulties adjusting to parenthood contrasts this common observation (Ho and Swain, 2017; Laurent and Ablow, 2011; Swain et al., 2017). Thus, the current finding of a positive association between parental self-efficacy and cortical thickness in the prefrontal cortex provides important insight into understanding the role of prefrontal cortex morphology in the adjustment to parenthood.
We should note there is evidence that brain volumes decrease during pregnancy (Hoekzema et al., 2016; Oatridge et al., 2002) and remain low at a two-year post-pregnancy follow up (Hoekzema et al., 2017). This appears to contradict our evidence of increases in maternal brain volume (Kim et al., 2010) and an association between later postpartum months and regional cortical thickness in the postpartum period, however, it is possible that maternal brain structure exhibits flexibility and undergoes alternated increases and decreases following childbirth. There is evidence that brain volumes decrease during pregnancy but rebound shortly after childbirth. Hoekzema et al. (2017) associate structural decrease with enhanced pruning driven by elevated levels of estrogen during pregnancy, but estrogen drops to pre-pregnancy levels soon after delivery. At the same time, levels of other hormones that are important for maternal motivation, including oxytocin, increase during the early postpartum period (Rosenblatt, 2002). Increased levels of oxytocin together with consistent interactions with offspring play a critical role in functional and anatomical changes in the maternal brain during the early postpartum period (Lonstein et al., 2015). Animal models provide strong evidence of structural enhancement in the prefrontal cortex. In lactating dams, the medial PFC undergoes morphological growth including increased dendritic length, branching and increased numbers of spines on dendrites, which is further associated with enhanced behavioral flexibility (Hillerer et al., 2014; Leuner and Gould, 2010). Moreover, rodent studies suggest that even during pregnancy, increased neurogenesis occurs in regions important for parenting, such as the olfactory bulb (Furuta and Bridges, 2005; Shingo et al., 2003). In one study with human mothers, decreased brain size during pregnancy recovered to baseline by six months postpartum (Oatridge et al., 2002). Thus, while we cannot rule out a possibility that some of the increase in cortical thickness may reflect recovery from pregnancy, we speculate that the positive associations between parental self-efficacy and cortical thickness in specific regions in the current study may also reflect morphological remodeling to support parenting competence during the first six months postpartum. A follow-up study examining brain structure every few months over several years will be critical for developing a clear understanding of brain plasticity in the human maternal brain.
Brain regions showing grey matter volume increase in a previous study (Kim et al., 2010) and cortical thickness increase in this study are largely overlapping, particularly in the medial and lateral prefrontal cortex, the precentral gyrus, and inferior parietal gyrus. However, a greater number of brain regions showed grey matter volume increase in the previous study (Kim et al., 2010). For example, while the anterior cingulate cortex plays a role in emotion regulation and social cognition (Etkin et al., 2011) and was positively associated with postpartum months in the 2010 study on grey matter volume, we did not find this association with cortical thickness. Other regions that showed increased volume, but not thickness, across postpartum months include the anterior cingulate gyrus, insula, precuneus, and the superior temporal gyrus. On the other hand, the current study revealed an association between later postpartum months and greater cortical thickness in the occipital gyrus, which was not identified in the previous study. Differences between the two studies may involve several factors. First, grey matter volume increase may reflect morphological change mechanisms other than cortical thickening, such as an increase in surface area (Winkler et al., 2010). It is possible that the regions identified in Kim et al. (2010), but not the current study, undergo surface area increase while cortical thickness remains constant. Second, the current study has a wider postpartum window (0–6 months) compared to Kim et al. (2010) (0–4 months). Thus, differences may be related to the timing of structural changes in specific regions. Third, participants in the current study have far more diverse socioeconomic and racial/ethnic backgrounds compared to the participants in Kim et al. (2010). Therefore, the different findings may reflect differences in the culture, environment, and demographic backgrounds of the participants. Lastly, while Kim et al. (2010) reported grey matter volume increase in subcortical regions over the first few months postpartum, the current study did not allow for examination of subcortical areas. Future studies of a larger sample should investigate anatomical changes in both cortical and subcortical regions to replicate previous findings, and to examine associations with parenting efficacy.
While we identified significant associations among cortical thickness in the prefrontal regions and parental efficacy, we did not investigate a potential mediating role of cortical thickness in the links between postpartum months and parental self-efficacy. The current study has a cross-sectional design, so it is not advisable to conduct the mediation analysis (Maxwell and Cole, 2007; Maxwell et al., 2011). Therefore, the research design limits the interpretation of temporal relationships among postpartum months, cortical thickness, and parental self-efficacy. The cross-sectional analysis provides only correlations with postpartum months, and does not assess an increase from a baseline measurement of cortical thickness or self-efficacy. It is not possible to directly infer changes over time, indicative of brain plasticity, from the results. We also cannot rule out the possibility that women with higher self-efficacy before childbirth have greater cortical thickness in the prefrontal cortex. Thus, longitudinal designs, optimally including pre-pregnancy and pregnancy scans as well as multiple postnatal scans at different postpartum months, are critically needed to assess changes in cortical thickness, and thus the brain plasticity that is specifically associated with changes between pregnancy and the postpartum experience. Furthermore, future studies should consider connectivity measures among brain structures. Structural connectivity assessed by diffusion tensor imaging (DTI) and functional connectivity assessed by brain network analysis will provide important insight into how the connections between subcortical and cortical regions change over time during the early postpartum period to support parenting.
The results of the current study should be considered in light of other limitations. First, although the statistical threshold levels used in the current study (both vertex-wise and cluster-wise thresholds less than 0.05) have also been used in other studies (e.g.(Ahveninen et al., 2013; King et al., 2015; Robinson et al., 2015; Schneider et al., 2014; Sneve et al., 2015; Takaya et al., 2016; Tchistiakova et al., 2014; Zorlu et al., 2017), we recognize those thresholds are relatively optimistic. Therefore, findings from the current study should be interpreted with caution until future studies use larger samples and more conservative thresholds (e.g. vertex-wise threshold p < 0.005 or .001). Second, it is important to consider other factors that change over the first six months postpartum. For example, as infants get older, mothers are likely to have better quality sleep and less fatigue, which may be associated with changes in the cortical structure. Thus, examining the impact of sleep and fatigue on maternal brain structure in future studies is imperative. Finally, future studies may consider other populations to expand our understanding. These may include new fathers and parents who are at risk for low self-efficacy, such as those with mood disorders.
5. Conclusions
The current study suggests that the first six months postpartum may constitute a sensitive period for associations between brain structure and motherhood. Consistent with the previous findings on grey matter volumes, we observed positive associations between cortical thickness and postpartum months among new mothers. Moreover, the current study extended evidence of human maternal brain morphology in a socioeconomically diverse sample. The associations between prefrontal cortical thickness and parental self-efficacy may have particularly important implications for interventions. Interventions could increase parental self-efficacy which may in turn positively impact mother-infant relationships and parenting behaviors (Jones and Prinz, 2005). High-order cognitive functions that involve prefrontal activation such as cognitive flexibility and self-regulation may be particularly susceptible to intervention efforts for increasing parental self-efficacy based on the significant associations with brain morphology in the prefrontal cortex.
Supplementary Material
Acknowledgments
This work was supported by the National Institute of Child Health and Human Development [R01HD090068; R21HD078797]; the Professional Research Opportunity for Faculty (PROF) and Faculty Research Fund (FRF), University of Denver; and the Victoria S. Levin Award For Early Career Success in Young Children’s Mental Health Research, Society for Research in Child Development (SRCD). The authors declare that they have no conflicts of interest in the research. The authors thank families who participate in the study and individuals who support recruitment. The authors also wish to acknowledge Amy Anderson, Lindsay Blanton, Christian Capistrano, Christina Congleton, Tanisha Crosby-Attipoe, Andrew Erhart, Victoria Everts, Rachel Gray, Claire Jeske, Laura Jeske, Daniel Mason, and Nanxi Xu for research assistance.
References
- Afonso VM, Sison M, Lovic V, Fleming AS, 2007. Medial prefrontal cortex lesions in the female rat affect sexual and maternal behavior and their sequential organization. Behavioral Neuroscience 121, 515–526. [DOI] [PubMed] [Google Scholar]
- Ahveninen J, Huang S, Belliveau JW, Chang W-T, Hämäläinen M, 2013. Dynamic oscillatory processes governing cued orienting and allocation of auditory attention. Journal of Cognitive Neuroscience 25, 1926–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbari EM, Shams S, Belay HT, Kaiguo M, Razak Z, Kent CF, Westwood T, Sokolowski MB, Fleming AS, 2013. The effects of parity and maternal behavior on gene expression in the medial preoptic area and the medial amygdala in postpartum and virgin female rats: A microarray study. Behavioral Neuroscience 127, 913–922. [DOI] [PubMed] [Google Scholar]
- Alvarez JA, Emory E, 2006. Executive function and the frontal lobes: a meta-analytic review. Neuropsychology review 16, 17–42. [DOI] [PubMed] [Google Scholar]
- Atzil S, Hendler T, Feldman R, 2011. Specifying the neurobiological basis of human attachment: brain, hormones, and behavior in synchronous and intrusive mothers. Neuropsychopharmacology 36, 2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird B, Smallwood J, Gorgolewski KJ, Margulies DS, 2013. Medial and lateral networks in anterior prefrontal cortex support metacognitive ability for memory and perception. The Journal of Neuroscience 33, 16657–16665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandura A, 1993. Perceived self-efficacy in cognitive development and functioning. Educational psychologist 28, 117–148. [Google Scholar]
- Barrett J, Fleming AS, 2011. Annual Research Review: All mothers are not created equal: neural and psychobiological perspectives on mothering and the importance of individual differences. Journal of Child Psychology and Psychiatry and Allied Disciplines 52, 368–397. [DOI] [PubMed] [Google Scholar]
- Barrett J, Wonch KE, Gonzalez A, Ali N, Steiner M, Hall GB, Fleming AS, 2012. Maternal affect and quality of parenting experiences are related to amygdala response to infant faces. Soc Neurosci 7, 252–268. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Garbin MG, 1988. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clinical Psychology Review 8(1), 77–100. [Google Scholar]
- Bornstein MH, 2002. Parenting Infants In: Bornstein MH (Ed.), Handbook of Parenting Erlbaum, Mahwah, N.J., 1, pp. 3–43. [Google Scholar]
- Chouinard PA, Paus T, 2006. The primary motor and premotor areas of the human cerebral cortex. The neuroscientist 12, 143–152. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI, 1999. Cortical surface-based analysis: I. Segmentation and surface reconstruction. Neuroimage 9, 179–194. [DOI] [PubMed] [Google Scholar]
- Dale AM, Sereno MI, 1993. Improved localizadon of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: a linear approach. Journal of Cognitive Neuroscience 5, 162–176. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R, 2011. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends Cogn Sci 15, 85–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febo M, Felix-Ortiz AC, Johnson TR, 2010. Inactivation or inhibition of neuronal activity in the medial prefrontal cortex largely reduces pup retrieval and grouping in maternal rats. Brain Research 1325, 77–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Dale AM, 2000. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences 97, 11050–11055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming AS, Korsmit M, 1996. Plasticity in the maternal circuit: effects of maternal experience on Fos-Lir in hypothalamic, limbic, and cortical structures in the postpartum rat. Behavioral Neuroscience 110, 567. [DOI] [PubMed] [Google Scholar]
- Furuta M, Bridges RS, 2005. Gestation-induced cell proliferation in the rat brain. Developmental Brain Research 156, 61–66. [DOI] [PubMed] [Google Scholar]
- Gauthier I, Tarr MJ, Anderson AW, Skudlarski P, Gore JC, 1999. Activation of the middle fusiform’face area’increases with expertise in recognizing novel objects. Nature Neuroscience 2, 568–573. [DOI] [PubMed] [Google Scholar]
- Goldin PR, Manber-Ball T, Werner K, Heimberg R, Gross JJ, 2009. Neural Mechanisms of Cognitive Reappraisal of Negative Self-Beliefs in Social Anxiety Disorder. Biological Psychiatry 66, 1091–1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlib IH, Whiffen VE, Mount JH, Milne K, Cordy NI, 1989. Prevalence rates and demographic characteristics associated with depression in pregnancy and the postpartum. Journal of Consulting and Clinical Psychology 57, 269. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Ungerleider LG, Horwitz B, Maisog JM, Rapoport SI, Grady CL, 1996. Face encoding and recognition in the human brain. Proceedings of the National Academy of Sciences 93, 922–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillerer KM, Jacobs VR, Fischer T, Aigner L, 2014. The maternal brain: an organ with peripartal plasticity. Neural Plast 2014, 574159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipwell AE, Guo C, Phillips ML, Swain JE, Moses-Kolko EL, 2015. Right Frontoinsular Cortex and Subcortical Activity to Infant Cry Is Associated with Maternal Mental State Talk. Journal of Neuroscience 35, 12725–12732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho SS, Swain JE, 2017. Depression alters maternal extended amygdala response and functional connectivity during distress signals in attachment relationship. Behavioural Brain Research 325, 290–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekzema E, Barba-Müller E, Pozzobon C, Picado M, Lucco F, García-García D, Soliva JC, Tobeña A, Desco M, Crone EA, 2017. Pregnancy leads to long-lasting changes in human brain structure. Nature Neuroscience 20, 287–296. [DOI] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP, 2007. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Structure and Function 212, 149–179. [DOI] [PubMed] [Google Scholar]
- Jones TL, Prinz RJ, 2005. Potential roles of parental self-efficacy in parent and child adjustment: A review. Clinical Psychology Review 25, 341–363. [DOI] [PubMed] [Google Scholar]
- Kim P, Capistrano C, Congleton C, 2016. Socioeconomic disadvantages and neural sensitivity to infant cry: role of maternal distress. Soc Cogn Affect Neurosci 11, 1597–15607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Feldman R, Mayes LC, Eicher V, Thompson N, Leckman JF, Swain JE, 2011a. Breastfeeding, brain activation to own infant cry, and maternal sensitivity. Journal of child psychology and psychiatry 52, 907–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Feldman R, Mayes LC, Eicher V, Thompson N, Leckman JF, Swain JE, 2011b. Breastfeeding, brain activation to own infant cry, and maternal sensitivity. Journal of Child Psychology and Psychiatry and Allied Disciplines 52, 907–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Leckman JF, Mayes LC, Feldman R, Wang X, Swain JE, 2010. The plasticity of human maternal brain: Longitudinal changes in brain anatomy during the early postpartum period. Behavioral Neuroscience 124, 695–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JA, Geisler D, Ritschel F, Boehm I, Seidel M, Roschinski B, Soltwedel L, Zwipp J, Pfuhl G, Marxen M, 2015. Global cortical thinning in acute anorexia nervosa normalizes following long-term weight restoration. Biological Psychiatry 77, 624–632. [DOI] [PubMed] [Google Scholar]
- Kinsley CH, Bardi M, Karelina K, Rima B, Christon L, Friedenberg J, Griffin G, 2008. Motherhood induces and maintains behavioral and neural plasticity across the lifespan in the rat. Archives of Sexual Behavior 37, 43–56. [DOI] [PubMed] [Google Scholar]
- Kopel H, Schechtman E, Groysman M, Mizrahi A, 2012. Enhanced synaptic integration of adult-born neurons in the olfactory bulb of lactating mothers. The Journal of Neuroscience 32, 7519–7527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent HK, Ablow JC, 2011. A cry in the dark: depressed mothers show reduced neural activation to their own infant’s cry. Soc Cogn Affect Neurosci 7, 125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leerkes EM, Crockenberg SC, 2002. The Development of Maternal Self-Efficacy and Its Impact on Maternal Behavior. Infancy 3, 227–247. [DOI] [PubMed] [Google Scholar]
- Leuner B, Glasper ER, Gould E, 2010. Parenting and plasticity. Trends in Neurosciences 33, 465–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Gould E, 2010. Dendritic growth in medial prefrontal cortex and cognitive flexibility are enhanced during the postpartum period. The Journal of Neuroscience 30, 13499–13503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Sabihi S, 2016. The birth of new neurons in the maternal brain: hormonal regulation and functional implications. Frontiers in Neuroendocrinology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonstein JS, Lévy F, Fleming AS, 2015. Common and divergent psychobiological mechanisms underlying maternal behaviors in non-human and human mammals. Hormones and Behavior 73, 156–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lövdén M, Wenger E, Mårtensson J, Lindenberger U, Bäckman L, 2013. Structural brain plasticity in adult learning and development. Neuroscience and Biobehavioral Reviews 37, 2296–2310. [DOI] [PubMed] [Google Scholar]
- Maxwell SE, Cole DA, 2007. Bias in cross-sectional analyses of longitudinal mediation. Psychological methods 12, 23–44. [DOI] [PubMed] [Google Scholar]
- Maxwell SE, Cole DA, Mitchell MA, 2011. Bias in cross-sectional analyses of longitudinal mediation: Partial and complete mediation under an autoregressive model. Multivariate Behavioral Research 46, 816–841. [DOI] [PubMed] [Google Scholar]
- McLaughlin KA, Sheridan MA, Winter W, Fox NA, Zeanah CH, Nelson CA, 2014. Widespread reductions in cortical thickness following severe early-life deprivation: a neurodevelopmental pathway to attention-deficit/hyperactivity disorder. Biological Psychiatry 76, 629–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses-Kolko EL, Perlman SB, Wisner KL, James J, Saul AT, Phillips ML, 2010. Abnormally reduced dorsomedial prefrontal cortical activity and effective connectivity with amygdala in response to negative emotional faces in postpartum depression. American Journal of Psychiatry 167, 1373–1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musser ED, Kaiser-Laurent H, Ablow JC, 2012. The neural correlates of maternal sensitivity: An fMRI study. Developmental Cognitive Neuroscience 2, 428–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishitani S, Kuwamoto S, Takahira A, Miyamura T, Shinohara K, 2014. Maternal Prefrontal Cortex Activation by Newborn Infant Odors. Chemical Senses 39, 195–202. [DOI] [PubMed] [Google Scholar]
- Nitschke JB, Nelson EE, Rusch BD, Fox AS, Oakes TR, Davidson RJ, 2004. Orbitofrontal cortex tracks positive mood in mothers viewing pictures of their newborn infants. Neuroimage 21, 583–592. [DOI] [PubMed] [Google Scholar]
- Nystrom K, Ohrling K, 2004. Parenthood experiences during the child’s first year: literature review. Journal of Advanced Nursing 46, 319–330. [DOI] [PubMed] [Google Scholar]
- O’Donnell S, Noseworthy MD, Levine B, Dennis M, 2005. Cortical thickness of the frontopolar area in typically developing children and adolescents. Neuroimage 24, 948–954. [DOI] [PubMed] [Google Scholar]
- O’Hara MW, Neunaber DJ, Zekoski EM, 1984. Prospective study of postpartum depression: Prevalence, course, and predictive factors. Journal of Abnormal Psychology 93, 158–171. [DOI] [PubMed] [Google Scholar]
- O’hara MW, Swain AM, 1996. Rates and risk of postpartum depression-a meta-analysis. International review of psychiatry 8, 37–54. [Google Scholar]
- O’Doherty JP, 2004. Reward representations and reward-related learning in the human brain: insights from neuroimaging. Current opinion in neurobiology 14, 769–776. [DOI] [PubMed] [Google Scholar]
- Oatridge A, Holdcroft A, Saeed N, Hajnal JV, Puri BK, Fusi L, Bydder GM, 2002. Change in brain size during and after pregnancy: study in healthy women and women with preeclampsia. American Journal of Neuroradiology 23, 19–26. [PMC free article] [PubMed] [Google Scholar]
- Pawluski JL, Lambert KG, Kinsley CH, 2016. Neuroplasticity in the maternal hippocampus: Relation to cognition and effects of repeated stress. Hormones and Behavior 77, 86–97. [DOI] [PubMed] [Google Scholar]
- Reece SM, Harkless G, 1998. Self-Efficacy, Stress, and Parental Adaptation: Applications to the Care of Childbearing Families. Journal of Family Nursing 4, 198–215. [Google Scholar]
- Rizzolatti G, Fadiga L, Gallese V, Fogassi L, 1996. Premotor cortex and the recognition of motor actions. Cognitive brain research 3, 131–141. [DOI] [PubMed] [Google Scholar]
- Robinson ME, Lindemer ER, Fonda JR, Milberg WP, McGlinchey RE, Salat DH, 2015. Close‐range blast exposure is associated with altered functional connectivity in Veterans independent of concussion symptoms at time of exposure. Human Brain Mapping 36, 911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenblatt J, 1994. Psychobiology of maternal behavior: contribution to the clinical understanding of maternal behavior among humans. Acta Paediatrica 83, 3–8. [DOI] [PubMed] [Google Scholar]
- Rosenblatt JS, 2002. Hormonal bases of parenting in mammals Handbook of parenting 2, 31–60. [Google Scholar]
- Rutherford HJV, Wallace NS, Laurent HK, Mayes LC, 2015. Emotion regulation in parenthood. Developmental Review 36, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneewind KA, Pfeiffer P, 1995. Impact of family processes on control beliefs. Self-efficacy in changing societies, 114–148. [Google Scholar]
- Schneider CE, White T, Hass J, Geisler D, Wallace SR, Roessner V, Holt DJ, Calhoun VD, Gollub RL, Ehrlich S, 2014. Smoking status as a potential confounder in the study of brain structure in schizophrenia. Journal of Psychiatric Research 50, 84–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shingo T, Gregg C, Enwere E, Fujikawa H, Hassam R, Geary C, Cross JC, Weiss S, 2003. Pregnancy-stimulated neurogenesis in the adult female forebrain mediated by prolactin. Science 299, 117–120. [DOI] [PubMed] [Google Scholar]
- Sneve MH, Grydeland H, Nyberg L, Bowles B, Amlien IK, Langnes E, Walhovd KB, Fjell AM, 2015. Mechanisms underlying encoding of short-lived versus durable episodic memories. The Journal of Neuroscience 35, 5202–5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallings J, Fleming AS, Corter C, Worthman C, Steiner M, 2001. The effects of infant cries and odors on sympathy, cortisol, and autonomic responses in new mothers and nonpostpartum women. Parenting 1, 71–100. [Google Scholar]
- Strathearn L, Li J, Fonagy P, Montague PR, 2008. What’s in a smile? Maternal brain responses to infant facial cues. Pediatrics 122, 40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE, Ho SS, Rosenblum KL, Morelen D, Dayton CJ, Muzik M, 2017. Parent-child intervention decreases stress and increases maternal brain activity and connectivity during own baby-cry: An exploratory study. Development and Psychopathology 29, 535–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE, Tasgin E, Mayes LC, Feldman R, Constable RT, Leckman JF, 2008. Maternal brain response to own baby-cry is affected by cesarean section delivery. Journal of Child Psychology and Psychiatry 49, 1042–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaya S, Liu H, Greve DN, Tanaka N, Leveroni C, Cole AJ, Stufflebeam SM, 2016. Altered anterior posterior connectivity through the arcuate fasciculus in temporal lobe epilepsy. Human Brain Mapping 37, 4425–4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchistiakova E, Anderson ND, Greenwood CE, MacIntosh BJ, 2014. Combined effects of type 2 diabetes and hypertension associated with cortical thinning and impaired cerebrovascular reactivity relative to hypertension alone in older adults. NeuroImage: Clinical 5, 36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teti DM, Gelfand DM, 1991. Behavioral competence among mothers of infants in the first year: the mediational role of maternal self‐efficacy. Child Development 62, 918–929. [DOI] [PubMed] [Google Scholar]
- Thomas C, Baker CI, 2013. Teaching an adult brain new tricks: a critical review of evidence for training-dependent structural plasticity in humans. Neuroimage 73, 225–236. [DOI] [PubMed] [Google Scholar]
- Thompson-Booth C, Viding E, Mayes LC, Rutherford HJ, Hodsoll S, McCrory E, 2014. I Can’t Take My Eyes Off of You: Attentional Allocation to Infant, Child, Adolescent and Adult Faces in Mothers and Non-Mothers. PLoS One 9, e109362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troutman B, Moran TE, Arndt S, Johnson RF, Chmielewski M, 2012. Development of parenting self-efficacy in mothers and infatns with high negative emotionality. Infant Mental Health Journal 33,45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wager TD, Davidson ML, Hughes BL, Lindquist MA, Ochsner KN, 2008. Prefrontal-subcortical pathways mediating successful emotion regulation. Neuron 59, 1037–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiesenfeld AR, Malatesta CZ, 1982. Infant distress: Variables affecting responses of caregivers and others. Parenting: Its causes and consequences, 123–139. [Google Scholar]
- Winkler AM, Kochunov P, Blangero J, Almasy L, Zilles K, Fox PT, Duggirala R, Glahn DC, 2010. Cortical thickness or grey matter volume? The importance of selecting the phenotype for imaging genetics studies. Neuroimage 53, 1135–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xerri C, Stern JM, Merzenich MM, 1994. Alterations of the cortical representation of the rat ventrum induced by nursing behavior. Journal of Neuroscience 14, 1710–1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorlu N, Cropley VL, Zorlu PK, Delibas DH, Adibelli ZH, Baskin EP, Esen ÖS, Bora E, Pantelis C, 2017. Effects of cigarette smoking on cortical thickness in major depressive disorder. Journal of Psychiatric Research 84, 1–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.