Abstract
Previous studies have reported an increase in the utilization of threonine (Thr) during immune system stimulation (ISS). However, increased utilization of an AA during ISS may not reflect an increased dietary requirement, as endogenous sources may supply AA to meet the need for enhanced utilization. The current study evaluated the impact of ISS on components of dietary Thr requirements, i.e., maintenance requirement and the efficiency of Thr utilization. Thirty-nine gilts (initial BW 32 ± 2.1 kg) of commercially relevant genetics were individually housed in metabolism crates and fed one of six experimental diets in which Thr was the first limiting among other AA. Three levels of dietary Thr were tested within each ISS group: 70%, 90%, and 110% of daily Thr requirements, which were estimated based on the potential of each ISS group for protein deposition (PD). Following adaptation to the experimental diets, pigs from each dietary treatment group were injected with either increasing amounts of Escherichia coli lipopolysaccharide (ISS+; 25 and 35 µg/kg BW) or saline (ISS−). Injections were given 48-h apart and whole-body nitrogen balance was measured for 72-h following the first injection. Body temperature (BT) was monitored and blood samples were collected 24 h after initiation of ISS and evaluated for measures of blood chemistry. Blood chemistry and BT results indicated an effective ISS in pigs (P < 0.03). Threonine intake increased PD in a linear fashion in both ISS groups (P < 0.01). The marginal efficiency of standardized ileal digestible (SID) Thr utilization for PD, represented by the slope, was not affected by ISS. However, ISS substantially increased the extrapolated maintenance SID Thr requirements, represented by the intercept at zero PD (ISS− vs. ISS+, −11.2 vs. −56.3 SE 13.2; P < 0.05). Collectively, our results indicated that the physiological changes associated with ISS increased the dietary SID Thr requirements for PD due to an increase in maintenance requirements.
Keywords: immune system stimulation, growing pigs, threonine requirement
INTRODUCTION
Immune system stimulation (ISS) alters AA (AA) utilization in part by repartitioning AA toward processes involved in an immune response, thus impacting AA requirements (Reeds and Jahoor, 2001; Obled, 2003; Rakhshandeh and de Lange, 2011). The metabolism of threonine (Thr) during ISS has gained attention due to its role in the synthesis of Thr-rich immune system metabolites, such as immunoglobulins, acute phase proteins, and intestinal mucins (Faure et al., 2007; Rémond et al., 2009). Mucins are major components of the basal and specific endogenous AA losses (EAAL) in pigs, accounting for up to 60% of total intestinal nitrogen (N) losses (Moughan, 1999; NRC, 2012). Considering that Thr accounts for 16% to 20% of crude mucin, increased synthesis and secretion of mucins may impact Thr requirements in growing pigs during ISS (Lien et al., 1997). This idea was supported by a study from Stuart et al. that reported a 1.5-fold increase in plasma Thr flux, a measure of Thr utilization, in pigs challenged with porcine reproductive and respiratory syndrome virus (Stuart et al., 2015). This study also reported a significant reduction in apparent ileal digestibility of dietary N, which can be associated with increased EAAL in these pigs. Thus, increases in the utilization of Thr for synthesis of immune system metabolites contribute, in part, to reduced lean tissue accretion during ISS and may impact sustainable pork production. Despite these findings, no study, to our knowledge, has directly evaluated the impact of systemic ISS on the specific measures of Thr requirements in growing pigs, i.e., the requirements for maintenance and the efficiency of Thr utilization for protein deposition (PD). Therefore, the current study evaluated the effects of ISS on dietary Thr requirements for PD in growing pigs. We hypothesized that ISS increases Thr utilization for biological processes involved in an immune response, therefore impacting dietary Thr requirements for PD.
MATERIALS AND METHODS
All methods and procedures for this experiment were reviewed and approved by the Texas Tech University Animal Use and Care Committee (ACUC approval number 16038-05). All animal trials were conducted at the Texas Tech University Swine Research Center (New Deal, TX).
General Design of the Study
A total of 39 PIC gilts (Pig Improvement Company North America, TN; initial BW 32 ± 2.1 kg) were obtained from the TTU breeding herd and housed individually in stainless steel adjustable metabolism crates (1.7 × 0.7 m, with the tenderfoot flooring) in an environmentally controlled facility (temperature 18–22 ○C). Following a 4-d adaptation to the experimental diets and environment, ISS was induced by i.m. injection of increasing amounts of Escherichia coli lipopolysaccharide (ISS+; 25 and 35 µg/kg BW; LPS strain 055:B5; Sigma-Aldrich, St. Louis, MO; n = 24; de Lange et al., 1989). Pigs in the control group (ISS−; n = 15) received sterile saline i.m. (ISS−; n = 15). Injections were given 48-h apart and whole-body N-balance was measured for 72-h following the first injection of either LPS or saline. The initial dose of LPS was increased by 29% for the subsequent injection to overcome tolerance to LPS. We have shown that this model of ISS elicits a mild immune response with moderate clinical symptoms, such as a low degree fever and mild-to-moderate lethargy (Rakhshandeh and de Lange, 2012). Pigs in the ISS− group received i.m. injections of sterile saline to account for the stress induced by injection. Three levels of dietary standardized ileal digestible (SID) Thr were evaluated within each ISS group. Blood samples were collected 24-h after the first injection of either LPS or saline and assayed for measures of blood chemistry. Infrared (IR) thermography was performed to monitor body temperature (BT) during the course of the study.
Diets and Feeding
For each ISS group, diets were formulated based on the nutrient requirements that were predicted using the NRC Swine model and based on performance variables determined in previous studies (NRC, 2012; Rakhshandeh et al., 2012, 2014; Rakhshandeh and de Lange, 2012; de Ridder et al., 2012; Stuart et al., 2016). The performance variables included a mean initial BW of 25 kg, an ADFI of 1.3 kg/d for ISS− and 0.9 kg/d for ISS+, and PD of 100 and 60 g/d for ISS− and ISS+, respectively (NRC, 2012). Within each ISS group three levels of dietary SID Thr were evaluated: 70% (L1), 90% (L2), and 110% (L3) of established Thr requirements for maximum PD. In all of the experimental diets, ratios of essential SID AA to Thr (AA:Thr) exceeded the recommendations of NRC Swine by 20% to ensure Thr was the first limiting among other AA (Table 1; Wang and Fuller, 1989; NRC, 2012). Diets L1 and L3 for each ISS group were prepared in single batches, and L2 diets were achieved by blending equal parts of L1 and L3. A constant ratio between dietary N and Lys, as well as dietary N and Thr, was maintained across all experimental diets (Table 1). All experimental diets were isoenergetic and contained 14 MJ/kg of metabolizable energy. The experimental diets were fortified with vitamins and minerals to surpass the requirements recommended by NRC Swine (Table 1; NRC, 2012). Titanium dioxide (0.25% TiO2; Bobette Boyer Hall Technologies, St. Louis) was included in all diets as an indigestible marker to determine nutrient digestibility. Pigs were fed either 1,309 or 906 g/d (as-fed basis) divided into two equal meals, which was fed twice per day (08:00 and 17:00 hours) and allowed free access to water. The feed allowance was slightly restricted (5%) to avoid feed refusal and minimize feed waste (Tables 1 and 2).
Table 1.
Ingredient composition and nutrient contents of experimental diets
| Treatment | ||||
|---|---|---|---|---|
| ISS− | ISS+ | |||
| L11 | L32 | L11 | L32 | |
| Ingredient composition, g/kg as fed basis | ||||
| Corn | 823 | 656 | 860 | 716 |
| Soybean meal | 121 | 290 | 86.5 | 232 |
| Hydrogenated vegetable fat | 10 | 10 | 10 | 10 |
| Lysine HCl | 7.9 | 12.4 | 6.8 | 10.7 |
| dl-Methionine | 2.3 | 3.6 | 1.96 | 3.08 |
| l-Threonine | 4.0 | 6.3 | 3.53 | 5.55 |
| l-Tryptophan | 1.4 | 2.1 | 1.19 | 1.86 |
| Limestone | 9.6 | 8 | 9 | 7.5 |
| Dicalcium phosphate | 8.6 | 7.1 | 8 | 6.8 |
| Salt | 5 | 5 | 5 | 5 |
| Vitamin and mineral premix2 | 15 | 15 | 15 | 15 |
| Titanium dioxide | 2.5 | 2.5 | 2.5 | 2.5 |
| Calculated nutrient content, g/kg as fed basis | ||||
| Metabolizable energy MJ/kg | 13.8 | 13.8 | 13.8 | 13.8 |
| CP (N × 6.25) | 106.3 | 166.9 | 93.1 | 146.4 |
| SID lysine | 7.88 | 12.39 | 6.81 | 10.71 |
| SID methionine | 2.46 | 4.22 | 2.07 | 3.64 |
| SID methionine + cysteine | 4.46 | 7.00 | 3.91 | 6.15 |
| SID threonine | 3.99 | 6.27 | 3.53 | 5.55 |
| SID tryptophan | 1.36 | 2.11 | 1.18 | 1.90 |
| SID valine | 5.09 | 8.09 | 4.48 | 7.05 |
| Calcium | 6.13 | 6.15 | 5.60 | 5.59 |
| STTD P3 | 2.81 | 2.88 | 2.60 | 2.68 |
| Nitrogen: lysine | 2.73 | 2.70 | 2.80 | 2.72 |
| Nitrogen: threonine | 5.38 | 5.30 | 5.39 | 5.26 |
1Thirty-nine gilts (initial BW 32 ± 2.1 kg) were subjected to one of two health states: immune system stimulated (ISS+; n = 24), and healthy control (ISS−; n = 15). Repeated intramuscular injection of E. coli lipopolysaccharide (25 and 35 µg/kg BW) was used to induce ISS. Diets were formulated to contain 70% (level 1, L1), 90% (L2), and 110% (L3) of daily standardized ileal digestible (SID) Thr requirements for each ISS group, which were estimated based on the potential of each group for PD according to the NRC Swine model (NRC, 2012). In all experimental diets, Thr was the first limiting among other AA. Diets L1 and L3 for each ISS group were prepared in single batches and L2 diets were achieved by blending equal parts of L1 and L3.
2Provided the following amounts of vitamins and trace minerals (per kilogram of diet): vitamin A, 10,075 IU; vitamin D3, 1,100 IU; vitamin E, 83 IU; vitamin K (as menadione), 3.7 mg; d-pantothenic acid, 58.5 mg; riboflavin, 18.3 mg; choline, 2209.4 mg; folic acid, 2.2 mg; naicin, 73.1 mg; thiamin, 7.3 mg; pyridoxine, 7.3 mg; vitamin B12, 0.1 mg; D-biotin, 0.4; Cu, 12.6 mg; Fe, 100 mg; Mn, 66.8 mg; Zn, 138.4 mg; Se, 0.3 mg; I, 1.0 mg; S, 0.8 mg; Mg, 0.0622%; Na, 0.0004%; Cl, 0.0336%; Ca, 0.0634%, P, 0.003%; K, 0.0036%.
3Standardized total tract digestible phosphorous.
Table 2.
Formulated and analyzed total AA content of experimental diets (%; as-fed basis)1, 2
| ISS− | ISS+ | |||||||
|---|---|---|---|---|---|---|---|---|
| L1 formulated |
L1 analyzed |
L3 formulated |
L3 analyzed |
L1 formulated |
L1 analyzed |
L3 formulated |
L3 analyzed |
|
| Isoleucine | 0.52 | 0.54 | 0.87 | 0.85 | 0.44 | 0.45 | 0.75 | 0.75 |
| Leucine | 1.26 | 1.22 | 1.75 | 1.68 | 1.16 | 1.11 | 1.58 | 1.52 |
| Lysine | 0.89 | 0.94 | 1.40 | 1.38 | 0.77 | 0.79 | 1.21 | 1.26 |
| Methionine | 0.28 | 0.27 | 0.46 | 0.39 | 0.24 | 0.21 | 0.40 | 0.36 |
| Cysteine | 0.25 | 0.25 | 0.35 | 0.32 | 0.23 | 0.21 | 0.32 | 0.29 |
| Phenylalanine | 0.63 | 0.63 | 1.01 | 0.95 | 0.56 | 0.53 | 0.88 | 0.84 |
| Threonine | 0.50 | 0.51 | 0.78 | 0.78 | 0.44 | 0.43 | 0.69 | 0.68 |
| Tryptophan | 0.16 | 0.16 | 0.25 | 0.24 | 0.14 | 0.12 | 0.22 | 0.21 |
| Valine | 0.61 | 0.59 | 0.97 | 0.90 | 0.54 | 0.50 | 0.85 | 0.81 |
1Thirty-nine gilts (initial BW 32 ± 2.1 kg) were subjected to one of two health states: immune system stimulated (ISS+; n = 24), and healthy control (ISS−; n = 15). Repeated intramuscular injection of E. coli lipopolysaccharide (25 and 35 µg/kg BW) was used to induce ISS. Diets were formulated to contain 70% (level 1, L1), 90% (L2), and 110% (L3) of daily standardized ileal digestible (SID) Thr requirements for each ISS group, which were estimated based on the potential of each group for PD according to the NRC Swine model (NRC, 2012). In all experimental diets, Thr was the first limiting among other AA. Diets L1 and L3 for each ISS group were prepared in single batches, and L2 diets were achieved by blending equal parts of L1 and L3.
2Analysis of the AA contents for the diets was performed at the Agricultural Experiment Station Chemical Laboratories at the University of Missouri-Columbia, MO.
Observations, Sampling, and Chemical Analysis
Eye temperature was measured at times 0, 2, 4, 6, 8, 10, 24, 48, 50, and 72 h post LPS injection using a FLIR E40 digital camera (FLIR Systems, Inc., Wilsonville, OR), as described by Petry et al.(2017). The IR resolution for each picture was set at the maximum resolution of 160 × 120. The emissivity value used was 0.98, which is the recommendation for biological tissues. Multiple pictures were taken and an average of three pictures with the best quality, in terms of focus and precision, were analyzed. Nitrogen balance was conducted as described by Rakhshandeh et al. for 72 h after the first injection of LPS or saline (Rakhshandeh et al., 2014). In brief, urine was collected via collection trays underneath each crate that funneled urine into tared, lidded buckets containing sufficient amounts of 3 N HCl to maintain urine pH <3. From each 24-h urine collection, 10% of the urine volume was pooled for each pig and stored at 4 °C until analyzed for total N contents. Feed waste and vomit for each pig were collected in trays beneath each feeder, oven dried, cooled in a desiccator, and weighed to accurately determine daily feed intake. Fecal samples were collected twice daily and stored in sealed bags at −20 °C until further processing. At the end of the N-balance period, feces were thawed, pooled for each pig, thoroughly homogenized using a mixer and kept at −20 °C until subsampled and lyophilized. Blood samples, which were collected by external jugular venipuncture 24-h post initial LPS or saline injection, were immediately evaluated for blood chemistry parameters using an i-STAT Handheld Analyzer (Abaxis Inc., CA) with an i-STAT CHEM8+ cartridge.
Fecal samples were lyophilized, pulverized, and thoroughly mixed before further analysis. Titanium dioxide and DM of fecal and feed samples were determined in triplicate and according to standard Association of Official Analytical Chemists procedures (AOAC, 1997). The N content of feces, urine, and feed samples were quantified in triplicate using a LECO-Trumac N analyzer (Leco Co., NV) according to standard Association of Official Analytical Chemists procedures (AOAC, 1997). AA analysis of the diets was performed at the Agricultural Experiment Station Chemical Laboratories at the University of Missouri-Columbia, MO. Dietary AA were determined by cation-exchange chromatography coupled with post-column ninhydrin derivatization and quantitation (AOAC, 1997).
Calculation and Statistical Analysis
Apparent total tract digestibility of dietary N, which was determined using the indicator technique and TiO2 as an indigestible marker, was used to compute fecal N excretion (Rakhshandeh et al., 2013). Nitrogen retention (g/d) was calculated as balance between the net N intake (feed N minus feed waste N) and N excretion (fecal plus urinary N). Protein deposition was calculated as retained N × 6.25 (Rakhshandeh et al., 2014).The following linear regression model was used to estimate maintenance SID Thr requirements and the marginal efficiency of SID Thr utilization for PD for each ISS group:
The regression coefficient a is used to estimate extrapolated maintenance SID Thr requirements (g/d), i.e., SID Thr intake when PD equals zero, calculated as −a/b. The regression coefficient b (g PD/g SID Thr intake) represents the marginal efficiency of utilization of SID Thr intake for PD (Fuller et al., 1989; Rakhshandeh et al., 2014).
Statistical analysis was carried out using SAS software version 9.4 (SAS Institute, NC). Normality and homogeneity of variances were confirmed using the Univariate procedure (PROC UNIVARIATE). Outliers were determined as any value that differed from the treatment mean by ±2 SD. Data were analyzed in a complete randomized design with pigs as an experimental unit, diet as a fixed effect and pig within crate as a random effect using mixed procedure (PROC MIXED). Dietary Thr intake was used as a co-variate for determining the fixed effect of ISS on measures of blood chemistry and BT and when appropriate (P > 0.10), a reduced model was used. For parameters such as BT that were measured over time, repeated measurements analysis of variance was used. An appropriate covariance structure was selected for analyses by fitting the model with the structure, which provided the ‘best’ fit, based on Akaike information criterion and Schwarz Bayesian criterion. Tukey–Kramer was used for multiple comparisons test. To evaluate the linear and quadratic effects of SID Thr intake on measures of performance and dietary N utilization, a polynomial orthogonal contrast was tested for each ISS group. Regression procedure (PROC REG) was used to test the differences in regression parameters (a and b) between ISS− and ISS+ groups when PD was regressed on SID Thr intake. Values are reported as least square means with their SEs. Treatment effects were considered significant at P ≤ 0.05. A tendency towards a significant difference between treatment means was considered at P ≤ 0.10. A power test was used to determine the number of animals per treatment group, since ISS results in a higher variability in N-balance. The coefficient of variance was 5; the percent difference from the control was decided at 10; and P ≤ 0.05 with a power of 90%.
RESULTS
General Observations
All pigs readily consumed experimental diets and showed signs of good health prior to the study. The ISS+ pigs displayed clinical signs of disease such as lethargy, fever, and vomiting. However, ISS did not affect the intake of the daily feed allowance during the post-ISS period. The vomitus and feed waste collected amounted to <2% of the feed allowance. Data from one pig (ISS+, L2) were excluded from the study due to a severe immune response to LPS injection that included severe lethargy and anorexia (refusing to eat for over 24 h). Analyzed dietary nutrient contents fell within anticipated values (±15%) that were derived from NRC (2012). Calculated values of dietary contents were used for the interpretation of results
Measures of Immune Function, Hematology, and Blood Chemistry
The main effects of ISS on measures of immune function, hematology, and blood chemistry are presented in table 3. Threonine intake had no effect on measures of immune function, hematology, and blood chemistry. Immune system stimulation increased eye temperature by 0.62 ± 0.117 °C (P < 0.01). Relative to ISS− pigs, blood urea nitrogen (BUN), and creatinine levels, as well as anion gap (AnionGAP), were higher in ISS+ pigs (P < 0.03). Hemoglobin (Hb), hematocrit (HCT), and blood glucose levels were not affected by ISS (P > 0.10).
Table 3.
Main effects of immune system stimulation (ISS) on eye temperature and blood parameters in growing pigs1, 2
| Parameter | ISS− | ISS+ | SEM | P-value |
|---|---|---|---|---|
| Number of pigs | 15 | 23 | ||
| Eye temperature, °C | 38.4 | 39.0 | 0.08 | 0.01 |
| Hematology | ||||
| Hemoglobin, g/dL | 10.8 | 9.5 | 1.19 | 0.33 |
| Hematocrit, % PCV3 | 31.8 | 27.9 | 3.51 | 0.34 |
| Blood chemistry | ||||
| Blood urea nitrogen, mg/dL | 4.7 | 24.2 | 7.61 | 0.03 |
| Glucose, mg/dL | 97.4 | 100.3 | 4.79 | 0.74 |
| Creatinine | 1.1 | 2.6 | 0.67 | 0.05 |
| Acid/base | ||||
| AnionGAP4, mEg/L | 20.4 | 22.6 | 0.59 | 0.03 |
1Data are the least square means ± the largest SEM. Pigs were injected with either increasing amounts of E. coli lipopolysaccharide (ISS+; 25 and 35 µg/kg BW) or saline (ISS−), given 48 h apart. One pig was excluded from the study due to sever reaction to ISS.
2Results for eye temperature represent the best estimate of mean obtained during ISS based on the repeated-measures ANOVA. Infrared thermography was used to monitor eye temperature at 0, 2, 4, 6, 8, 10, 24, 48, 50, and 72 h post-ISS. Hematology, blood chemistry and acid/base data were obtained 24 h post-ISS.
3PCV = packed cell volume.
4AnionGAp = anion gap.
Growth Performance and N Utilization
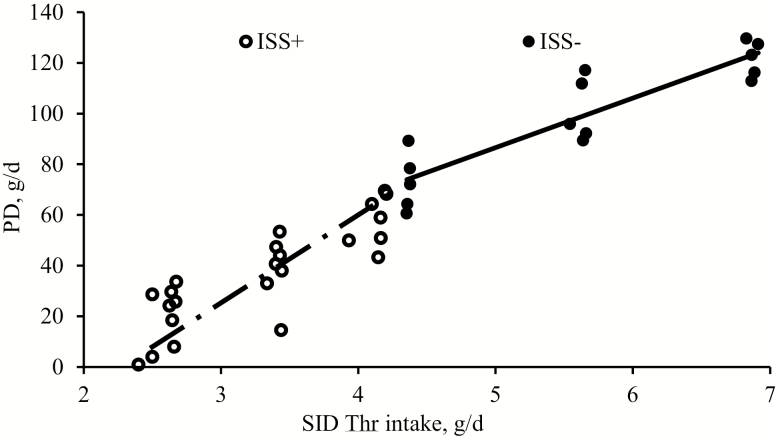
The effects of ISS on growth performance and N utilization are presented in Table 4. BW of the animals did not differ at the beginning of the study (32 ± 2.2 kg; P > 0.20). Final BW was lower in pigs that received the ISS+ diets compared to those that received ISS− diets (average of 33 vs. 36 ± 1.2 kg; P < 0.01). Daily DMI was affected by daily dietary allowance (P < 0.01). As anticipated, increasing the daily Thr allowance increased the SID Thr intake in a linear fashion in both ISS groups (P < 0.01). An increase in SID Thr intake improved the ATTD of N in ISS+ pigs linearly (P = 0.02), but it did not affect the ATTD of N in ISS− pigs (P = 0.70). In both ISS groups, dietary N intake increased linearly with SID Thr intake (P < 0.01). Higher SID Thr intake increased the urinary N excretion in ISS− pigs in a linear fashion (P < 0.04), while it had no effect on urinary N excretion in ISS+ pigs (P > 0.69). No significant difference was observed in urinary N excretion between ISS groups (P > 0.20). Total N excretion (i.e., urine and feces) linearly increased with SID Thr intake in ISS− pigs (P < 0.01), but not in ISS+ pigs. Dietary SID Thr intake had no effect on total N excretion in ISS+ pigs (P = 0.23). In both ISS groups, SID Thr intake increased the daily PD in a linear fashion (P < 0.01). Nitrogen retention-to-N intake (N retention: N intake) was significantly lower (38%) in ISS+ pigs as determined by a multiple range comparison (P = 0.03). Nitrogen retention: N intake was not different among levels of Thr intake in the ISS− group (P = 0.42); however, the ratio was significantly lower in pigs with the lowest level of Thr intake in the ISS+ group (P < 0.01). The comparison of linear regression parameters (Table 5) seen when relating PD (g/d) to SID Thr intake (g/d) indicated that the marginal efficiency of SID Thr utilization for PD, represented by the slope, was not affected by ISS (P = 0.10). However, ISS substantially increased the extrapolated maintenance SID Thr requirements, represented by the intercept at zero PD in Figure 1 (P < 0.05; Table 5).
Table 4.
Effects of immune system stimulation (ISS) and standardized ileal digestible (SID) threonine (Thr) intake on final BW and dietary nitrogen utilization in growing pigs1
| Treatment2 | Contrast3 (P <) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Health status | ISS− | ISS+ | SEM | P < | ISS− | ISS+ | ||||||
| Dietary Thr level | L1 | L2 | L3 | L1 | L2 | L3 | Lin. | Quad. | Lin. | Quad. | ||
| n | 5 | 5 | 5 | 8 | 7 | 8 | — | — | — | — | — | — |
| Final BW, kg | 35bc | 37a | 37ab | 33c | 34c | 33c | 1.18 | 0.01 | 0.64 | 0.96 | 0.87 | 0.88 |
| DMI, kg/d | 1.09a | 1.10a | 1.10a | 0.68b | 0.75b | 0.70b | 0.05 | 0.01 | 0.58 | 0.85 | 0.42 | 0.71 |
| SID Thr intake, g/d | 4.4c | 5.6b | 6.9a | 2.4e | 3.4df | 3.9cd | 0.24 | 0.01 | 0.01 | 0.80 | 0.01 | 0.43 |
| ATTD4 of N, % | 70.2ab | 76.5a | 73.8ab | 56.7c | 69.4b | 68.9b | 3.96 | 0.01 | 0.74 | 0.12 | 0.02 | 0.13 |
| N utilization, g/d | ||||||||||||
| N intake | 23.0c | 29.7b | 36.4a | 13.0e | 18.1d | 20.4d | 1.28 | 0.01 | 0.01 | 0.80 | 0.01 | 0.43 |
| Urinary N excretion | 4.5 | 6.5 | 7.4 | 5.7 | 6.4 | 5.8 | 1.02 | 0.28 | 0.04 | 0.61 | 0.70 | 0.97 |
| Total N excretion | 11.3bc | 13.5b | 16.9a | 11.1c | 11.9bc | 12.bc | 0.95 | 0.01 | 0.05 | 0.18 | 0.23 | 0.56 |
| PD5 (N × 6.25), g/d | 72.9c | 101.3b | 121.7a | 11.8f | 38.7e | 52.1d | 5.77 | 0.01 | 0.01 | 0.24 | 0.01 | 0.41 |
| N retention: N intake | 0.51a | 0.54a | 0.54a | 0.15c | 0.34b | 0.41b | 0.04 | 0.01 | 0.81 | 0.19 | 0.10 | 0.38 |
1Data are the least square means ± the largest SEM. Thirty-nine gilts (initial BW 32 ± 2.1 kg) were subjected to one of two health states: immune system stimulated (ISS+; n = 24) and healthy control (ISS−; n = 15). Repeated intramuscular injection of E. coli lipopolysaccharide (LPS; 25 and 35 µg/kg BW) were used to induce ISS. Diets were formulated to contain 70% (level 1, L1), 90% (L2), and 110% (L3) of daily standardized ileal digestible (SID) Thr requirements for each ISS group, which were estimated based on the potential of each group for PD according to the NRC Swine model (NRC, 2012). In all experimental diets, Thr was the first limiting among other AA. Diets L1 and L3 for each ISS group were prepared in single batches and L2 diets were achieved by blending equal parts of L1 and L3. Whole-body nitrogen balance (N-balance) was measured for 72 h following the first injection of either LPS or saline. One pig was excluded from the study due to sever reaction to ISS.
2Different superscripts represent statistically significant differences at P < 0.05 for each treatment.
3Linear (Lin.) and quadratic (Quad.) effects of SID Thr intake on measures of performance and dietary N utilization were tested using a polynomial orthogonal contrast.
4ATTD = apparent total tract digestibility of dietary nitrogen (N). The indicator technique was used to determine dietary N digestibility using titanium dioxide as an indigestible marker.
5PD = protein (N × 6.25) deposition.
Table 5.
Impact of immune system stimulation (ISS) on parameters representing the linear relationship between standardized ileal digestible (SID) Threonine (Thr) intake and protein deposition (PD) in growing pigs1, 2
| ISS− | ISS+ | SEM | P | |
|---|---|---|---|---|
| Intercept (PD at 0 SID Thr intake) | −11.2 | −56.3 | 13.20 | 0.05 |
| Slope (g PD/g SID Thr intake) | 20.0 | 28.0 | 3.94 | 0.10 |
| R 2 | 0.81 | 0.71 |
1Data of regression analysis using a one-slope model: y = a + b(x), where y is the PD (g/d), a (intercept) can be used for mathematical estimation of extrapolated maintenance requirement (−a/b), b (slope) represents the marginal efficiency of Thr utilization for PD and x is the daily Thr intake (g/d) when SAA intake is below the requirements for maximum PD.
2 Thirty-nine gilts (initial BW 32 ± 2.1 kg) were fed one of six experimental diets in which Thr was the first limiting among other AA. Diets were formulated to contain 70 (level 1, L1), 90 (L2), and 110 (L3) % of daily SID Thr requirements for each ISS group, which were estimated based on the potential of each group for PD according to NRC Swine model (NRC, 2012). Pigs were injected with either increasing amounts of E. coli lipopolysaccharide (ISS+; 25 and 35 µg/kg BW) or saline (ISS−), given 48 h apart, while measuring whole-body N-balance for 72 h following the first LPS or saline injection. One pig was excluded from the study due to sever reaction to ISS.
Figure 1.
Effects of immune system stimulation (ISS) and standardized ileal digestible threonine (SID Thr) intake on whole-body protein deposition (PD) in growing pigs. Thirty-nine gilts (initial BW 32 ± 2.1 kg) were fed one of six experimental diets in which Thr was the first limiting among other AA. Diets were formulated to contain 70 (level 1, L1), 90 (L2), and 110 (L3) % of daily SID Thr requirements for each ISS group, which were estimated based on the potential of each group for PD according to NRC Swine model (NRC, 2012). Pigs were injected with either increasing amounts of E. coli lipopolysaccharide (ISS+, ○; 25 and 35 µg/kg BW; n = 23) or saline (ISS−, ●; n = 15), given 48 h apart, while measuring whole-body N-balance for 72 h after the first injection of LPS or saline. One pig was excluded from the study due to sever reaction to ISS.
DISCUSSION
The main objective of the present study was to evaluate the impact of mild clinical ISS on dietary SID Thr requirements for PD in growing pigs. To our knowledge, this study is the first to evaluate the impact of systemic ISS on the maintenance requirements for and marginal efficiency of Thr utilization for PD in growing pigs. Since the efficiency of the first limiting AA for PD can be affected by the dietary AA balance, we used serial dilutions of dietary protein to generate dietary treatments that avoided confounding Thr intake levels with changes in dietary AA balance (Wang and Fuller, 1989). In this study Thr was first limiting AA in all diets, which was achieved by ensuring that the ratio of all other essential SID AA to Thr exceeded the recommendations of NRC Swine (NRC, 2012). Previous studies have confirmed that when the ratio of essential AA to a target essential AA exceeds the optimum ratio for maximum PD by 20%, the target AA becomes the first limiting among all other essential AA (Wang and Fuller, 1989; de Ridder et al., 2012; Rakhshandeh et al., 2014). Therefore, in the present study, the observed change in PD was a function of the change in available Thr and not the overall supply of essential AA. In this study, we did not pair-feed the control (ISS−) animals since reduced feed intake can influence the maintenance requirements and possibly the efficiency of the utilization of the first limiting AA for PD in non-immune challenged pigs (Fuller et al., 1989). The main component of the maintenance requirement for AA is intestinal EAAL, which is a function of DMI (NRC, 2012). Therefore, in non-immune challenged pair-fed pigs the maintenance requirements for SID Thr would likely decrease, compared to the ISS− group in the current study. Furthermore, moderate feed restriction is known to decrease the rate of inevitable catabolism of AA, which, consequently, leads to an increase in the efficiency of the first limiting AA for utilization (de Lange, 2003). Thus, the marginal efficiency of SID Thr utilization for PD would likely have increased in pair-fed animals, compared to the ISS group in our study. These two components (the maintenance requirement for Thr along with the efficiency of Thr utilization) might lead to reduced dietary SID Thr requirements in pair-fed animals (NRC, 2012). The level of daily feed allowance for the ISS+ pigs was determined using previous studies that observed a constant quantitative decrease in feed intake with the same model of ISS (Rakhshandeh et al., 2012, 2014; Rakhshandeh and de Lange, 2012; de Ridder et al., 2012; Stuart et al., 2016).
In the current study, ISS was induced by repeated i.m. injections of increasing amounts of LPS. We previously have shown that this model of ISS induces a relatively mild immune response that mimics the impact of mild clinical chronic disease on protein and AA utilization (Rakhshandeh and de Lange, 2012; de Ridder et al., 2012; Rakhshandeh et al., 2014). In the present study, LPS-induced ISS elicited a febrile response, as determined by eye temperature. This technique was used as a less invasive measure of the febrile response, which indicates the effectiveness of ISS, and correlates to core BT (Petry et al., 2017). Eye temperature remained elevated for the entire course of the study in ISS+ pigs, suggesting persistent, elevated core BT, an indicator of a systemic immune response (Petry et al., 2017). The pro-inflammatory cytokine IL-1β serves as the main endogenous pyrogen in initiating the febrile response and works synergistically with IL-6 and TNF-α (Johnson, 1997). Therefore, an increase in eye temperature suggests that ISS in the current study may have been mediated by pro-inflammatory cytokines. We also observed a significant increase in the levels of urea N and creatinine in the blood of ISS+ pigs. Measures of BUN, the primary metabolite derivative of AA catabolism, and blood creatinine, an indicator for increased skeletal muscle degradation, have been associated with a reduced efficiency of AA utilization and protein accretion during ISS (Hosten, 1990). The latter is consistent with our findings, in which we observed a significant reduction in N retention: N intake ratio, and suggest a decrease in the efficiency of dietary N utilization for whole-body PD in ISS+ pigs (Rakhshandeh et al., 2014). We also observed an increase in AnionGAP in ISS+ pigs. Importantly, ISS did not impact the plasma electrolyte balance that was calculated from levels of Na+, K+, and Cl− (data not shown). Therefore, higher AnionGAP in ISS+ pigs most likely indicates an increased level of lactic acid in the blood, which would reflect a shift from aerobic to anaerobic glycolytic metabolism. This shift usually occurs during the acute phase response of systemic inflammation (De Backer, 2003), further indicating the presence of ISS. These metabolic changes during ISS are often characterized by reduced blood glucose levels during the post-absorptive state, due to enhanced glucose uptake by immune cells as their preferred source of energy (Kominsky et al., 2010; Delmastro-Greenwood and Piganelli, 2013; Pearce and Pearce, 2013). However, in the current study, ISS had no effect on blood glucose levels, since our pigs were in the absorptive state when blood samples were collected. Long-term and severe inflammatory responses in various species are characterized by reduced Hb levels and HCT. The latter occurs as a result of pro-inflammatory cytokine-mediated interference with reticuloendothelial iron transport, decreased sensitivity of erythron to erythropoietin, and reduced erythrocyte survival during severe inflammation (Goyette et al., 2004). The lack of effect of ISS on Hb levels and HCT in our study is likely because only a mild inflammatory response was stimulated by our model of ISS. Collectively, these results indicated that repeated injection of increasing amounts of LPS induced an effective ISS in our study.
Reduced protein gain and increased N loss are characteristics of an immune response (Reeds and Jahoor, 2001; Obled, 2003). Based on the experimental design in the current study, pigs in each ISS group were fed to their estimated requirements, thus mitigating the potentially confounding effect of lower SID Thr intake on PD. However, reduced PD in the ISS+ pigs can be attributed, predominantly, to hyperactivation of the immune system, since N retention per unit of N intake was significantly lower in these animals compared to ISS− pigs. In previous studies using the same model of ISS, we have shown that while PD was reduced in ISS+ pigs, it remained unaffected over time in pair-fed ISS− pigs (de Ridder et al., 2012; Rakhshandeh et al., 2014).
The efficiency of utilization of the first limiting AA becomes reduced when the levels of this AA rise above that required for maximum PD (Williams et al., 1997; Möhn et al., 2000). Therefore, in the present study, the marginal efficiency of dietary SID Thr utilization for PD was determined using three levels of Thr intake, two of which were below those needed to maximize PD in each ISS group. The marginal efficiency of utilization of Thr for PD, represented by the slope, was numerically higher in ISS+ pigs than ISS− pigs, but it did not reach the levels of statistical significance (1 g of additional SID Thr intake supported 19.5 ± 2.6 and 28.0 ± 3.9 g/d PD in the ISS− and ISS+ pigs, respectively; P = 0.10). The efficiency of SID Thr utilization in ISS− pigs was probably affected by the energy intake being slightly limiting in this group, especially at L3 (Möhn et al., 2000). These results are in general agreements with the findings of Fuller et al. who used N-balance to estimate the marginal efficiency of Thr intake for PD (Fuller et al., 1989). According to NRC (2012), whole-body protein contains ~3.8% Thr in non-immune challenged pigs. Assuming that this percentage remained unchanged in ISS+ pigs, the mean efficiency of utilization of SID Thr intake for Thr retention in PD can be estimated at 0.90 for both ISS groups. This value is much higher than estimates suggested by NRC Swine and de Lange et al. (de Lange et al., 2001; NRC, 2012) and can be attributed predominantly to the systemic overestimation of PD values when using conventional N-balance methods (Fuller et al., 1989; de Lange et al., 2001). However, the above assumption may require further verification, as we and others have shown that ISS can alter the AA profile of whole-body PD (Breuillé et al., 2006; Rakhshandeh et al., 2009).
The daily requirement for Thr represents the sum of the requirements for maintenance functions and for PD (Fuller et al., 1989; NRC, 2012). In the current study, the relationship between PD and SID Thr intake generated a higher estimate of maintenance SID Thr requirements in the ISS+ pigs than in the ISS− pigs (1.9 ± 0.40 g/d; using a common partial efficiency of Thr utilization of 23.8 for the ISS− and ISS+; average b coefficients in Table 4). This higher estimate directly contributes to the observed impact of ISS on PD at all levels of dietary Thr intake. Maintenance requirements for absorbed Thr serve to replace gut endogenous and integument Thr losses, replace losses due to the minimum plus inevitable catabolism of Thr, and the use of Thr for synthesis of immune system metabolites (Fuller et al., 1989; Hahn and Baker, 1995; Rakhshandeh and de Lange, 2011). Among these, increased Thr utilization for mucin synthesis, a major component of gut EAAL, is likely the pathway that is the greatest quantitative contributor to increased Thr requirements during ISS (Faure et al., 2006; Rémond et al., 2009). Threonine-rich mucins are the major component of mucus produced by Brunner’s glands and goblet cells, which are found in the epithelial lining of the intestine and the respiratory tract. Greater numbers of goblet cells are found in the large intestine than in the small intestine (Kim and Ho, 2010). Therefore, when evaluating the effects of ISS on gut endogenous Thr losses, and thus Thr requirements, the contribution of the large intestine to total intestinal Thr loses must be considered. Overall, the observed increase in the Thr requirement for maintenance in the current study agrees with reports from other studies that showed enhanced Thr utilization at intestinal tissue and whole-body levels during ISS (Faure et al., 2003, 2006; Dharmani et al., 2009; Rémond et al., 2009). Furthermore, our results suggest that an increase in the dietary SID Thr requirements for PD during ISS is due to increased extrapolated Thr maintenance requirements, which is probably due to enhanced utilization of Thr for the synthesis of mucins and other immune system metabolites
CONCLUSIONS AND IMPLICATIONS
Repeated injection with increasing amounts of LPS elicited effective immune system stimulation, allowing evaluation of the impact of hyperactivation of the immune system on dietary threonine requirements in growing pigs. Immune system stimulation increased dietary standardized ileal digestible threonine requirements for protein deposition by increasing the maintenance requirements for threonine in growing pigs at a fixed level of energy intake. Thus, immune challenged pigs require higher levels of threonine in their diets to maintain protein deposition levels similar to those during non-immune challenged states. Increased maintenance requirements during ISS may be largely attributed to the enhanced utilization of absorbed threonine for synthesis of immune system metabolites, particularly mucins. These findings warrant further studies to directly evaluate the impact of disease on endogenous AA losses from both the small and large intestines in growing pigs.
Conflict of interest statement. None declared.
ACKNOWLEDGMENTS
The current study was presented in part in the proceedings of the American Society of Animal Science meeting (Midwest section 2017), Omaha, NE, USA. The authors express their deep gratitude to Professor Cornelius F.M. de Lange for his intellectual contributions to this study. The present study was funded by Texas Tech University (internal funds). This research is a publication from the Department of Animal and Food Sciences, Texas Tech University. This study was conducted at the Texas Tech University Swine Research facility.
LITERATURE CITED
- AOAC 1997. Official methods of analysis. 16th ed. Washington, DC:AOAC. [Google Scholar]
- Breuillé D., Béchereau F., Buffière C., Denis P., Pouyet C., and Obled C.. 2006. Beneficial effect of amino acid supplementation, especially cysteine, on body nitrogen economy in septic rats. Clin. Nutr. 25:634–642. doi: 10.1016/j.clnu.2005.11.009 [DOI] [PubMed] [Google Scholar]
- De Backer D. 2003. Lactic acidosis. Intensive Care Med. 29:699–702. doi: 10.1007/s00134-003-1746-7 [DOI] [PubMed] [Google Scholar]
- Delmastro-Greenwood M. M. and Piganelli J. D.. 2013. Changing the energy of an immune response. Am. J. Clin. Exp. Immunol. 2:30–54. doi: pmc/articles/PMC3714201/ [PMC free article] [PubMed] [Google Scholar]
- Dharmani P., V. Srivastava V. Kissoon-Singh, and Chadee K.. 2009. Role of intestinal mucins in innate host defense mechanisms against pathogens. J. Innate Immun. 1:123–135. doi: 10.1159/000163037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure M., F. Choné C. Mettraux J. P. Godin F. Béchereau J. Vuichoud I. Papet D. Breuillé, and Obled C.. 2007. Threonine utilization for synthesis of acute phase proteins, intestinal proteins, and mucins is increased during sepsis in rats. J. Nutr. 137:1802–1807. doi: 10.1093/jn/137.7.1802 [DOI] [PubMed] [Google Scholar]
- Faure M., C. Mettraux D. Moennoz J. P. Godin J. Vuichoud F. Rochat D. Breuillé C. Obled, and Corthésy-Theulaz I.. 2006. Specific amino acids increase mucin synthesis and microbiota in dextran sulfate sodium-treated rats. J. Nutr. 136:1558–1564. doi: 10.1093/jn/136.6.1558 [DOI] [PubMed] [Google Scholar]
- Faure M., D. Moënnoz F. Montigon C. Mettraux S. Mercier E. J. Schiffrin C. Obled D. Breuillé, and Boza J.. 2003. Mucin production and composition is altered in dextran sulfate sodium-induced colitis in rats. Dig. Dis. Sci. 48:1366–1373. doi: 10.1023/A:1024175629909. [DOI] [PubMed] [Google Scholar]
- Fuller M. F., R. McWilliam T. C. Wang, and Giles L. R.. 1989. The optimum dietary amino acid pattern for growing pigs. 2. Requirements for maintenance and for tissue protein accretion. Br. J. Nutr. 62:255–267. doi: 10.1079/BJN19890009 [DOI] [PubMed] [Google Scholar]
- Goyette R. E., N. S. Key, and Ely E. W.. 2004. Hematologic changes in sepsis and their therapeutic implications. Semin. Respir. Crit. Care Med. 25:645–659. doi: 10.1055/s-2004-860979 [DOI] [PubMed] [Google Scholar]
- Hahn J. D. and Baker D. H.. 1995. Optimum ratio to lysine of threonine, tryptophan, and sulfur amino acids for finishing swine. J. Anim. Sci. 73:482–489. doi:10.2527/1995.732482x [DOI] [PubMed] [Google Scholar]
- Hosten A. O. 1990. BUN and creatinine. In: Walker H. K., Hall W. D., and J. W. Hurst, editors. Clinical methods: the history, physical, and laboratory examinations. 3rd ed. Boston:Butterworths; p. 874–878. [PubMed] [Google Scholar]
- Johnson R. W. 1997. Inhibition of growth by pro-inflammatory cytokines: an integrated view. J. Anim. Sci. 75:1244–1255. doi:10.2527/1997.7551244x [DOI] [PubMed] [Google Scholar]
- Kim Y. S. and Ho S. B.. 2010. Intestinal goblet cells and mucins in health and disease: recent insights and progress. Curr. Gastroenterol. Rep. 12:319–330. doi: 10.1007/s11894-010-0131-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kominsky D. J., E. L. Campbell, and Colgan S. P.. 2010. Metabolic shifts in immunity and inflammation. J. Immunol. 184:4062–4068. doi: 10.4049/jimmunol.0903002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lange C.F.M. 2003. Animal effects on amino acid catabolism and protein accretion in visceral organs in pigs. Proceedings of 9; th DPP. Edmonton, Alberta, Canada: University of Alberta. p. 243–261. [Google Scholar]
- de Lange C. F., A. M. Gillis, and Simpson G. J.. 2001. Influence of threonine intake on whole-body protein deposition and threonine utilization in growing pigs fed purified diets. J. Anim. Sci. 79:3087–3095. doi:10.2527/2001.79123087x [DOI] [PubMed] [Google Scholar]
- de Lange C. F., W. C. Sauer R. Mosenthin, and Souffrant W. B.. 1989. The effect of feeding different protein-free diets on the recovery and amino acid composition of endogenous protein collected from the distal ileum and feces in pigs. J. Anim. Sci. 67:746–754. doi:10.2527/jas1989.673746x [DOI] [PubMed] [Google Scholar]
- Lien K. A., W. C. Sauer, and Fenton M.. 1997. Mucin output in ileal digesta of pigs fed a protein-free diet. Z. Ernahrungswiss. 36:182–190. doi: 10.1007/BF01611398 [DOI] [PubMed] [Google Scholar]
- Möhn S., A. M. Gillis P. J. Moughan, and de Lange C. F.. 2000. Influence of dietary lysine and energy intakes on body protein deposition and lysine utilization in the growing pig. J. Anim. Sci. 78:1510–1519. doi:10.2527/2000.7861510x [DOI] [PubMed] [Google Scholar]
- Moughan P. J. 1999. Protein metabolism in the growing pig. In: I. Kyriazakis, editor. Quantative biology of the pig. 1st ed. Wallingford, UK:CABI Publishing; p. 299–331. [Google Scholar]
- NRC 2012. Nutrient requirements of swine. 11th ed. Washington, DC:The National Academic Press. [Google Scholar]
- Obled C. 2003. Amino acid requirements in inflammatory states. Can. J. Anim. Sci. 83:365–373. doi: 10.4141/A03-021 [DOI] [Google Scholar]
- Pearce E. L. and Pearce E. J.. 2013. Metabolic pathways in immune cell activation and quiescence. Immunity. 38:633–643. doi: 10.1016/j.immuni.2013.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petry A., W. McGilvray A. R. Rakhshandeh, and Rakhshandeh A.. 2017. Technical note: assessment of an alternative technique for measuring body temperature in pigs. J. Anim. Sci. 95:3270–3274. doi: 10.2527/jas.2017.1566 [DOI] [PubMed] [Google Scholar]
- Rakhshandeh A., J. C. Dekkers B. J. Kerr T. E. Weber J. English, and Gabler N. K.. 2012. Effect of immune system stimulation and divergent selection for residual feed intake on digestive capacity of the small intestine in growing pigs. J. Anim. Sci. 90(Suppl. 4):233–235. doi: 10.2527/jas.53976 [DOI] [PubMed] [Google Scholar]
- Rakhshandeh A., J. K. Htoo N. Karrow S. P. Miller, and de Lange C. F.. 2014. Impact of immune system stimulation on the ileal nutrient digestibility and utilisation of methionine plus cysteine intake for whole-body protein deposition in growing pigs. Br. J. Nutr. 111:101–110. doi: 10.1017/S0007114513001955 [DOI] [PubMed] [Google Scholar]
- Rakhshandeh A., Htoo J., and de Lange C. F. M.. 2009. Impact of immune system stimulation and sulfur amino acid intake on amino acid composition of selected tissues in pigs. Can. J. Anim. Sci. 89:172. doi:10.4141/CJAS09300 [Google Scholar]
- Rakhshandeh A., and de Lange C. F. M.. 2011. Immune system stimulation in the pig: effect on performance and implications for amino acid nutrition. In: R. J., Van Barnevled, editor. Manipulating pig production XIII. Werribee, VIC, Austrilia:Australasian Pig Science Association Incorporation; p. 31–46. [Google Scholar]
- Rakhshandeh A. and de Lange C. F.. 2012. Evaluation of chronic immune system stimulation models in growing pigs. Animal. 6:305–310. doi: 10.1017/S1751731111001522 [DOI] [PubMed] [Google Scholar]
- Rakhshandeh A., Weber T. E., Dekkers J. C. M., Tuggle C. K., Kerr B. J., and Gabler N.. 2013. Impact of systemic immune system stimulation on intestinal integrity and function in pigs. FASEB J. 27(suppl. 1):867.2–867.2. https://www.fasebj.org/doi/abs/10.1096/fasebj [Google Scholar]
- Reeds P. J., and Jahoor F.. 2001. The amino acid requirements of disease. Clin. Nutr. 1:15–22. doi: 10.1054/clnu.2001.0402 [DOI] [Google Scholar]
- Rémond D., Buffière C., Godin J.-P., Mirand P. P., Obled C., Papet I., Dardevet D., Williamson G., Breuillé D., and Faure M.. 2009. Intestinal inflammation increases gastrointestinal threonine uptake and mucin synthesis in enterally fed minipigs. J. Nutr. 139:720–726. doi: 10.3945/jn.108.101675 [DOI] [PubMed] [Google Scholar]
- de Ridder K., C. L. Levesque J. K. Htoo, and de Lange C. F.. 2012. Immune system stimulation reduces the efficiency of tryptophan utilization for body protein deposition in growing pigs. J. Anim. Sci. 90:3485–3491. doi: 10.2527/jas.2011-4830 [DOI] [PubMed] [Google Scholar]
- Stuart W., Burkey T. E., Gabler N. K., Schwartz K., Klein D., Dawson J. A., Pendleton A. R., de Lange C. F. M., and Rakhshandeh A.. 2016. Immune system stimulation (ISS) induced by E. coli lipopolysaccharide (LPS) alters amino acid metabolism in growing pigs. J. Anim. Sci. 94:51–52 (Abstract) [Google Scholar]
- Stuart W., Burkey T. E., Gabler N. K., Schwartz K., de Lange C. F. M., Klein D., Dawson J. A., and Rakhshandeh A.. 2015. Infection with porcine reproductive and respiratory syndrome virus (PRRSV) affects body protein deposition and alters amino acid metabolism in growing pigs. J. Anim. Sci. 93:855 (Abstract) [Google Scholar]
- Wang T. C., and Fuller M. F.. 1989. The optimum dietary amino acid pattern for growing pigs 1. Experiments by amino acid deletion. Br. J. Nutr. 62:77–89. doi: 10.1079/BJN19890009 http://www.journals.cambridge.org/abstract_S0007114589000929 [DOI] [PubMed] [Google Scholar]
- Williams N. H., T. S. Stahly, and Zimmerman D. R.. 1997. Effect of chronic immune system activation on body nitrogen retention, partial efficiency of lysine utilization, and lysine needs of pigs. J. Anim. Sci. 75:2472–2480. doi: 1997.7592481x [DOI] [PubMed] [Google Scholar]