Abstract
This study investigated the use of androgen receptor (AR) reporter gene assay data in a non-animal exposure-led risk assessment in which in vitro anti-androgenic activity and exposure data were put into context using a naturally occurring comparator substance with a history of dietary consumption. First, several dietary components were screened to identify which selectively interfered with AR signaling in vitro, using the AR CALUX® test. The IC50 values from these dose-response data together with measured or predicted human exposure levels were used to calculate exposure: activity ratios (EARs) for the dietary components and a number of other well-known anti-androgenic substances. Both diindolylmethane (DIM) and resveratrol are specifically acting dietary anti-androgens. The EARs for several anti-androgens were therefore expressed relative to the EAR of DIM, and how this ‘dietary comparator ratio’ (DCR) approach may be used to make safety decisions was assessed using an exposure-led case study for an anti-androgenic botanical ingredient. This highlights a pragmatic approach which allows novel chemical exposures to be put into context against dietary exposures to natural anti-androgenic substances. The DCR approach may have utility for other modes of action where appropriate comparators can be identified.
Keywords: androgen receptor, risk assessment, in vitro approaches, dietary comparison
Performing safety risk assessments that are based on perturbations in cellular signaling pathways rather than adverse effects in animal studies requires the use of multiple tools and approaches (Krewski et al., 2010). Ensuring risk assessments are protective for all relevant health effects means that pathways associated with cellular stress responses as well as with specific targets such as nuclear receptors need to be considered (Middleton et al., 2017). A Molecular Initiating Event (MIE), is the initial interaction between a molecule and a biomolecule or biosystem that can be causally linked to an outcome via a pathway (Allen et al., 2014). Reporter gene assays are useful tools in discovering or confirming the MIEs that may be associated with a specific chemical exposure, and therefore have an important role in the development of human-relevant mechanistic toxicological risk assessments. One example of a receptor-mediated MIE is androgen receptor (AR) antagonism. We have previously described how a non-animal risk assessment for anti-androgenic effects could be developed and the central importance of the AR to this strategy (Dent et al., 2015).
A number of tools are already available to characterize the effects of chemical exposure on many of the MIEs relevant for perturbation of the hypothalamus-pituitary-testicular (HPT) axis, including AR (ant)agonism. However, not all the tools needed to make the link between in vitro anti-androgenic activity and an adverse health effect in humans are available. These include higher tier in vitro tools able to distinguish endocrine activity from adversity and computational models describing the human HPT axis. Such higher tier tools may not be necessary where there is a low probability of exposure and effect concentrations overlapping. This principle has already been employed to compare high-throughput exposure data and bioactivity information from the ToxCast program (Wetmore et al., 2015), and such in vitro to in vivo extrapolation approaches are considered robust enough to be used for testing prioritization (Wambaugh et al., 2018). Comparison of AC50 or IC50 values from human-relevant in vitro assays with human plasma exposures is therefore gaining popularity as a method of performing mechanistic human safety risk assessments. In addition to broad screening using multiple in vitro assays representing different modes of action, this approach has also been used specifically for endocrine activity using data for estrogen receptor agonism and androgen receptor antagonism (Dancik et al., 2015). One question facing toxicologists performing risk assessments based on new approaches is whether extrapolating from in vitro AC50 or IC50 values is protective of human health, and what ‘margin of exposure’ is sufficient between the in vitro point of departure and the predicted or measured plasma exposure level to assure human safety? One way to address these questions is to put margins of exposure derived from in vitro only risk assessments into context against margins of exposure for comparator substances with the same mode of action. One method that has been proposed for estrogen agonists is to calculate the exposure: activity ratios (EARs) for test substances, and directly compare these with the EAR of a comparator with a history of dietary exposure (Becker et al., 2014, 2015). In that approach for estrogen agonists, the phytoestrogen genistein was selected as the comparator. An EAR for genistein was calculated by dividing the human plasma concentration of genistein at steady state (determined from several studies examining plasma exposure following consumption of soy products) by a measure of in vitro activity for the estrogen signaling pathway. Based on the assumption that normal dietary exposure to phytoestrogens is low risk, the EARs for genistein were then compared with EARs calculated for other estrogen agonists to provide ‘relative estrogenic activity exposure quotients.’ Such an approach shows promise because it considers exposure alongside bioactivity data, and because it is focuses on the assessment of human safety risk rather than an attempt to replicate the results of rodent toxicology studies (Dent et al., 2018; Krewski et al., 2010). We therefore applied similar techniques to investigate the utility of this approach for anti-androgenic materials. To provide the measure of anti-androgenic activity we selected the AR CALUX® assay (Sonneveld et al., 2005) as a human relevant and highly specific reporter gene assay for AR agonists and antagonists (van der Burg et al., 2010).
The objectives of this work were to:
Test a number of dietary components in the AR CALUX® assay to identify a comparator that could be used to help put the exposure and activity of anti-androgens into context.
Estimate plasma exposures in humans to the dietary comparator to allow EARs for other anti-androgens to be put into context.
Use a case study to investigate whether this approach could be used to arrive at a safety decision for perturbations in AR signaling for an ingredient in a consumer product without the need to generate animal data.
MATERIALS AND METHODS: AR CALUX® ASSAY
Test and reference substances
Common dietary constituents tested in the AR CALUX® assay were genistein, resveratrol, diindolylmethane (DIM), quercetin, and rutin. Case study ingredients were andrographolide (AG) and bakuchiol. Reference substances were dihydrotestosterone (DHT), flutamide, and 2-hydroxyflutamide. All test or reference substances were obtained from Sigma, with the exception of DHT which was either prepared as a concentration series in DMSO by Bio Detection Systems B.V. (BDS, Amsterdam, The Netherlands) using DHT supplied by Steraloids Inc. (purity > 98%) or supplied by Sigma (purity ≥ 99%) and prepared as a concentration series in DMSO in-house. The purity of all test substances was ≥ 95%.
Cell culture
AR CALUX® cells were obtained and used under license from BDS. The cells were cultured in growth medium comprised of Dulbecco’s Modified Eagle Medium (DMEM/F12, Thermofisher 31331028) containing 7.5% heat inactivated fetal calf serum (FCS), 1% non-essential amino acid solution (NEAA, Sigma), and 10 000 U/ml penicillin/10 000 µg/ml streptomycin (Sigma). During subculture, once per week 200 µg/ml G418 (gentamycin, Sigma) solution was added to the medium. The assay was performed in Phenol Red-free DMEM/F12 medium (Thermofisher 21041025) containing 5% charcoal stripped FCS (Gibco), 1% NEAA, and 10 000 U/ml Penicillin/10 000 µg/ml streptomycin.
AR CALUX® assay method
The assay was conducted in a GLP compliant laboratory using test methods based on previously reported procedures (Sonneveld et al., 2005). On Day 1 of the assay, cells were seeded in white, clear-bottomed 96-well plates at a density of 1 × 105 cells/ml in assay medium, 100 µl of cell suspension per well. Plates were incubated for at least 16 h (37°C 5% CO2). On Day 2, wells were checked to ensure 50%–90% confluency. Medium was removed and 200 µl of the test substance or reference standard in assay medium was added to triplicate wells. All test/reference substances were tested in both the agonism and antagonism assay, with the exception of flutamide and 2-hydroxyflutamide which were only tested in the antagonism assay. In the agonism assay, each plate included a DHT concentration series (1 × 10−12 to 1 × 10−7 M) for quality control purposes as well as the concentration range of the test substance. The concentration range for each test substance was determined by performing a cytotoxicity evaluation to ensure that the highest concentration tested did not cause any changes in cell number or morphology, and descending concentrations were set at half log intervals or closer if required to investigate a steep dose-response. Cell number was assessed using the Sigma Cell Counting Kit 8, and morphology was evaluated by light microscopy. For the antagonism assay, the medium was supplemented with a non-saturating level of DHT approximating to the EC50 of this ligand (3 × 10−10 M). Each plate included a flutamide concentration series (1 × 10−9 to 1 × 10−5 M) for quality control purposes as well as the concentration range of the test substance. Plates were incubated for 24 h. On Day 3, the luciferase assay was performed, using the ONE-Glo Luciferase Assay System (Promega). Luminescence was measured in a Tecan Safire plate reader. In cases where antagonism was observed (determined as at least a 20% decrease in relative induction of the test substance at a non-cytotoxic concentration) a specificity control assay was performed to ensure the decrease in relative induction was not due to a non-specific effect on eg general cellular health. This was done by assessing whether the decrease in relative induction was reversible in a saturating concentration of the ligand (DHT). Therefore, in the specificity control assay each plate included the concentration range of the test substance in medium containing the non-saturating level of DHT, the concentration range of the test substance in a saturating level of DHT (3 × 10−8 M), which approximates to the EC50 × 100. A single concentration of flutamide (1 × 10−5 M) in medium containing each level of DHT was also included to serve as a reference. Each experiment comprised at least 3 independent replicates.
Data interpretation
Correction for background luminescence was performed by subtracting the relative luminescence of the control (DMSO only) wells for each plate. The results were expressed relative to the reference standard, which was DHT for the agonism assay (luciferase expression at highest DHT concentration =100%) and Flutamide for the antagonism assay (luciferase expression at highest Flutamide concentration = 0%). Data were analyzed using GraphPad Prism and plotted as mean values ± SEM. Dose-response modeling was performed on log transformed data using the nonlinear variable slope (four parameters) equation (four-parameter logistic curve) in GraphPad for either stimulation (agonism assay) or inhibition (antagonism assay) according to the equation: Y = Bottom of dose-response curve + (Top of dose response curve-Bottom)/{1+10^[(LogIC50-X) × HillSlope]} using a least squares (ordinary) fit with a maximum of 1000 iterations.
The antagonism assay was considered negative when there was < 20% inhibition (relative induction ≥ 80%) at all doses, which was a cut-off suggested by the assay vendor and used to interpret ERα CALUX data (OECD, 2016). Where the antagonism assay showed inhibition of at least 20% (relative induction ≤ 80%) the specificity control assay described above was performed for all test substances with the exception of hydroxyflutamide, which is a well-known specifically acting anti-androgen. For specifically acting anti-androgens there is a clear right shift in the dose-response between the non-saturating and the saturating concentration of DHT (see flutamide curves in Figure 2). Where this right shift was observed, or where the test substance no longer showed an inhibition of luciferase induction at the saturating concentration, this provided evidence that the decrease in relative induction at the non-saturating concentration was reversible and test substance was considered to be a specifically acting anti-androgen. However, if the dose-response remained the same the inhibition of luciferase was considered to be due to a non-specific effect, and the test substance was not considered to be a specifically acting anti-androgen. This was evaluated for at least 3 individual replicates.
Figure 2.
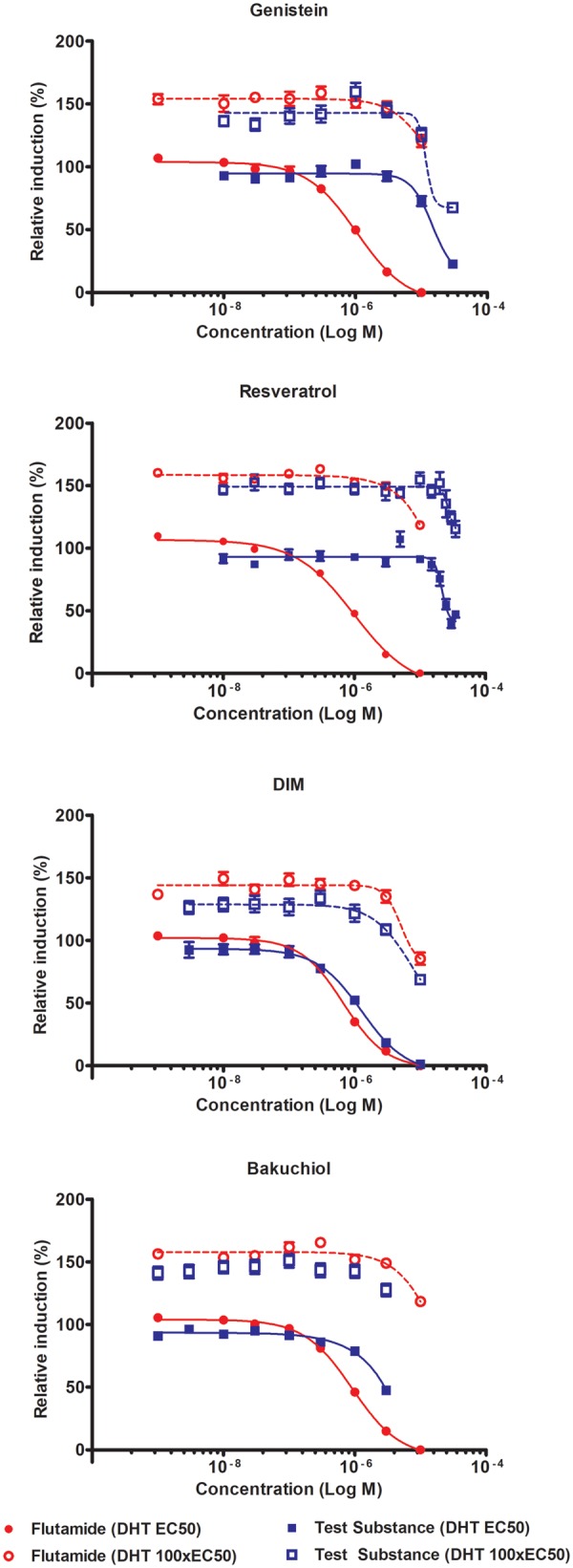
Androgen receptor antagonism and specificity control results for genistein, DIM, resveratrol, and bakuchiol. Each graph represents mean data from at least 3-independent experiments, error bars ± SEM. No model curve shown for bakuchiol at DHT 100×EC50 as chosen model did not meet goodness of fit criteria.
EXPOSURE: ACTIVITY PROFILING
Exposure: activity profiling was performed using a similar approach to that proposed for estrogenic responses (Becker et al., 2014, 2015). First, a suitable comparator was identified from the dietary components tested in the assay as a substance which showed a specific effect on the AR signaling pathway. The only dietary components that showed these characteristics were resveratrol and 3,3-diindolylmethane (DIM). DIM has previously been proposed as a promising dietary comparator for exposure to anti-androgens (Becker et al., 2014, 2015), and because the dose-response for DIM was more typical of a AR antagonist this was selected as the comparator (see Results section). An exposure: activity ratio was therefore calculated for DIM using the predicted total plasma exposure and in vitro anti-androgenic activity data. The IC50 was selected as it is considered the most appropriate metric to use in EAR calculations (Becker et al., 2015):
Because DIM exposure varies widely between individuals (Fujioka et al., 2016), to give a representation of this variability, EARs were calculated using PBBK (physiologically based biokinetic) modeling for individuals showing high, mean, and low plasma exposures. A sub-population of concern with regards to perturbations in AR signaling is pregnant women, due to the risk of serious and irreversible harm associated with blockade of AR signaling during the fetal masculinization programming window (Macleod et al., 2010). We therefore modeled plasma exposure to females of childbearing age to provide the benchmark EAR.
EARs were calculated for the remaining test substances using the same equation. Where exposure data allowed, EARs for the remaining test substances were also calculated to describe the variability in human exposures. Where the dose-response was not sufficiently well described to confidently set the IC50, the concentration at which the response of the test substance equalled 50% of the maximum response of the reference standard (flutamide) was calculated and this value (termed the PC50) was used instead (OECD, 2016). EARs were also calculated using AR CALUX® IC50 values found in the literature for the anti-androgens p,p′-DDE, vinclozolin, methoxychlor, HPTE, and BPA (Sonneveld et al., 2005; Suzuki et al., 2011; Wang et al., 2014).
Dietary comparator ratios (DCRs) were calculated based on the ratio of the EAR for the test substance to the EAR for DIM:
In considering these comparisons it should be noted that some of the EARs were calculated using serum or plasma exposure measured in males, most notably flutamide and hydroxyflutamide. The purpose of including these substances was to illustrate ‘high risk’ DCRs, encompassing exposures that are intended to completely suppress AR signaling in humans (in the case of flutamide and its active metabolite hydroxyflutamide, adult males suffering from prostate cancer). Complete suppression of AR signaling following flutamide administration to pregnant rats has been shown to cause serious and irreversible adverse effects on their male offspring (Macleod et al., 2010). It is therefore considered that the DCRs determined for flutamide and hydroxyflutamide would indicate a high probability of impacting AR signaling in all populations including pregnant women.
Variability in DCR was expressed where data allowed the range of variability in human exposure to be characterized by calculating DCRs for the highest EARTest substance/the lowest EARDIM, the mean (or where appropriate median) EARTest substance/the mean EARDIM and the lowest EARTest substance/the highest EARDIM. Where the range of variability was not available (eg, for flutamide and hydroxyflutamide) the variability in DCR was expressed by calculating this parameter for the mean EARTest substance/the lowest, mean, and highest EARDIM (see Supplementary Materials for more detail and all calculations).
EXPOSURE ASSESSMENT
It was only necessary to perform exposure assessments for those test substances showing anti-androgenic activity in the AR CALUX® assay, because EARs cannot be calculated for substances showing no activity. The human plasma or serum exposures that were used in the EAR calculations were either found in the literature or generated using a PBBK model. References used to provide the exposure data are summarized in Table 1, and full details of the exposure data or predictions used and all EAR and DCR calculations are provided in the Supplementary Material.
Table 1.
Exposure Data Used in Calculation of EARs (See Supplementary Materials for All Exposure Data and Predictions Used)
| Substance (Description) | Exposure Data Description | Reference |
|---|---|---|
| DIM (metabolite of glucobrassicin, widely consumed in cruciferous vegetables) | PBBK model predicting DIM plasma exposure (Cmax) following consumption of 50 g brussels sprouts | Reported here (see Supplementary Materials) |
| Resveratrol (present in skin of berries including grapes) | Human pharmacokinetic data describing Cmax following exposure to 25 mg resveratrol. This represents a high level of dietary intake (Presta et al., 2009) but is well below the level used as a food supplement (Raederstorff et al., 2013). | Goldberg et al. (2003) |
| Flutamide and hydroxyflutamide (prostate cancer drug and its active metabolite) | Human pharmacokinetic data describing Cmax at steady state following repeated exposure therapeutic dose of flutamide | Radwanski et al. (1989) |
| BPA (industrial chemical) | Predicted plasma concentration at steady state (Css) based on kinetic modeling at human exposures of 4 µg/kg/day (the Tolerable Daily Intake [TDI] set by European Food Safety Authority [EFSA]) | Wetmore et al. (2012) |
| Vinclozolin (plant protection product) | Predicted plasma Css based on kinetic modeling at human exposures of 25 µg/kg/day (the Reference Dose [RfD] set by the US Environmental Protection Agency [EPA]) | Wetmore et al. (2012) |
| Methoxychlor (plant protection product) | Predicted Css based on kinetic modeling at human exposures of 5 µg/kg/day (the RfD set by the U.S. EPA) | Wetmore et al. (2012) |
| HPTE (metabolite of methoxychlor) | Predicted Css based on kinetic modeling at human exposures of 5 µg/kg/day (the RfD for methoxychlor set by the U.S. EPA) | Wetmore et al. (2012) |
| p,p′-DDE (metabolite of the insect control agent DDT) | Human biomonitoring describing serum levels of populations exposed in the United States in the 1950s and 1960s and a population exposed in a DDT-sprayed area in South Africa in 2003–2005 | Longnecker et al. (2002), Bhatia et al. (2005), and Aneck-Hahn et al. (2006) |
| Bakuchiol (risk assessment case study) | PBBK model predicting bakuchiol plasma exposure (Cmax) following once-daily use of a body lotion or a shampoo containing this substance at 0.5% (hypothetical products) | Reported here (see Supplementary Materials) |
No exposure assessment was performed for AG, which was negative in the AR CALUX® assay (see AR CALUX® Assay section).
RESULTS
AR CALUX® Assay
A summary of the results for AR CALUX® assays is shown in Table 2. None of the test substances showed a positive response in the agonism assay, whereas in each experiment DHT gave a consistent positive response with very little variability between experimental replicates.
Table 2.
AR CALUX® Assay Results
| Substance | Agonism Assay |
Antagonism Assay |
Antagonism Assay IC50 |
|---|---|---|---|
| Positive or Negative (+/−) | (µM)a | ||
| Flutamide | NT | + | 0.876 |
| Hydroxyflutamide | NT | + | 0.0282 |
| Genistein | — | − | — |
| Resveratrol | — | + | 21.7 |
| Rutin hydrate | — | − | — |
| Quercetin hydrate | — | − | — |
| DIM | — | + | 1.27 |
| Bakuchiol | — | + | 2.85b |
| AG | — | − | — |
All values presented to 3 significant figures.
NT, not tested.
Best-fit IC50 from at least 3-independent experiments at a non-saturating concentration of DHT.
PC50 value presented as a reliable IC50 value could not be obtained from the dose-response.
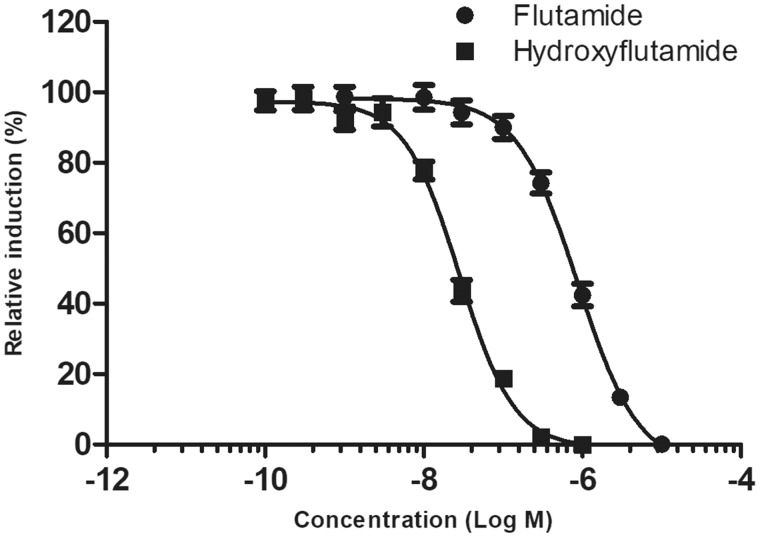
As expected, flutamide was less potent in the antagonism assay than was its active metabolite 2-hydroxyflutamide (Table 2, Figure 1).
Figure 1.
Androgen receptor antagonism results for hydroxyflutamide, mean data from 3 independent experiments, error bars ±SEM.
Quercetin, rutin, and AG gave negative results in the antagonism assay, because in all 3 replicates there was less than a 20% reduction in relative luciferase induction at any concentration. Flutamide (which was run on all plates) showed the expected antagonistic response.
Genistein met the criteria to progress to a specificity control assay (20% reduction in relative induction). A specificity control assay was therefore performed to ensure this was due to a specific effect on the AR signaling pathway, which showed the reduction in relative induction of luciferase for flutamide was reversible in the presence of a saturating concentration of DHT for all 3 replicates (Figure 2). However, for genistein the dose-response showed no right shift in the presence of a saturating concentration of DHT. This indicates that the reduced luciferase expression was due to an effect unrelated to the AR signaling pathway, and genistein was not acting as a specific AR antagonist in this assay, highlighting the value of the specificity control assay.
Resveratrol showed a clear reduction in relative induction, and the dose-response was so steep that additional experiments were performed to ensure the full dose-response could be described (Figure 2). The steep dose-response curve for resveratrol did complicate data interpretation, but overall the data indicated that the effect on relative induction was considered at least partly reversible with a slight increase in IC50 from 2.17 × 10−5 to 2.73 × 10−5 M.
DIM showed a clear reduction in relative luciferase induction, meeting the criteria for specificity control testing. Addition of the saturating concentration of DHT clearly shifted the dose-response to the right (Figure 2), increasing the IC50 from 1.27 × 10−6 to 7.50 × 10−6 M, indicating the effect on luciferase expression was reversible and that DIM was acting as a potent and specific AR antagonist. DIM was therefore selected as the dietary comparator, primarily because the dose-response was more clearly typical of an AR antagonist than was the dose-response for resveratrol. In addition, although some studies have shown a health protective effect of either resveratrol or red wine (Baur and Sinclair, 2006), the safety of liberal consumption of crucifers is less contentious than consumption of red wine. The assumption is that although DIM is a potent anti-androgen, normal dietary consumption of cruciferous vegetables is not expected to cause adverse effects in humans relating to disturbance of AR signaling.
Bakuchiol showed a clear dose-dependent reduction in relative luciferase induction (Figure 2), with the 2 highest concentrations resulting in > 20% reduction. GraphPad was unable to make a full dose-response fit to the bakuchiol data, meaning a reliable IC50 for bakuchiol could not be calculated. Additional (higher) concentrations of bakuchiol would be necessary to fully describe the dose-response, but because the highest concentration of 3 µM was close to the cytotoxic dose range a higher dose was not tested, and instead a mean PC50 value across all 6 replicates was calculated (Table 2). The relationship between the relative induction values of bakuchiol at the non-saturating concentration of the ligand (DHT) with the saturating concentration confirmed that addition of the saturating concentration reversed the effect on luciferase induction in all 6 replicates (Figure 2). Therefore, the specificity control assay did indicate that the reduction in relative luciferase induction was a specific effect on AR-mediated signaling.
In Vitro to In Vivo Extrapolation
A comparison of the in vitro points of departure (IC50 or for bakuchiol PC50) including upper and lower 95% CI where this could be calculated, and the in vivo exposure data or predictions are shown in Figure 3. For only 2 substances were the predicted or measured systemic exposures greater than the in vitro points of departure: hydroxyflutamide and p,p′-DDE exposure values from one study (Aneck-Hahn et al., 2006). For all other case substance exposures, the in vitro point of departure was greater than the predicted or measured systemic exposure.
Figure 3.
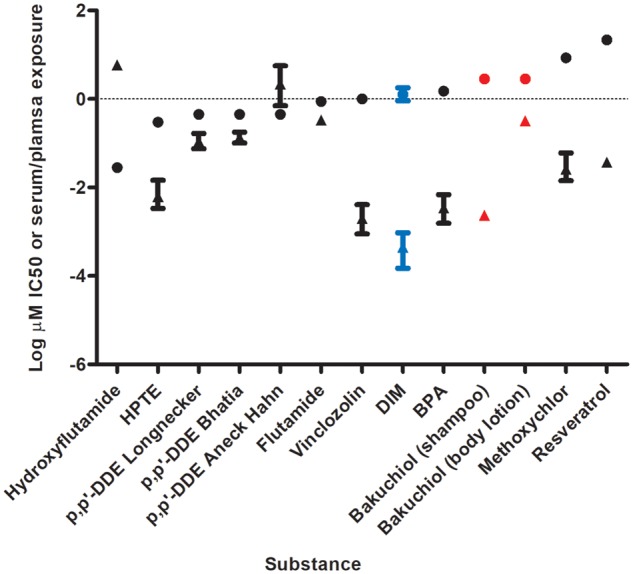
Comparison of AR CALUX® point of departure (IC50 or PC50) and measured or predicted serum or plasma exposure. Circles represent IC50 values, triangles represent serum, or plasma exposure. Dietary comparator (DIM) in blue, case study ingredient (bakuchiol) with hypothetical exposure scenarios in red. Bakuchiol uses PC50 rather than IC50. For exposure data, where values for uncertainty (eg, 95%CI) or variability (eg, percentile exposure) were published these are as described in the Supplementary Materials.
Dietary Comparator Ratios (DCRs)
Calculations showing the individual EARs and corresponding DCRs are detailed in the Supplementary Materials, and a comparison of the resulting DCRs is shown in Figure 4. Due to its low bioavailability, the mean DCR for DIM was the lowest calculated. The DCR range for resveratrol, vinclozolin, BPA, and methoxychlor overlapped with the DCR range for DIM. Aside from the shampoo case study (see Case Study Risk Assessment section), all other substance exposures provided DCRs that did not overlap with the range for DIM, with hydroxyflutamide providing the highest value, with a mean DCR of 594 000.
Figure 4.
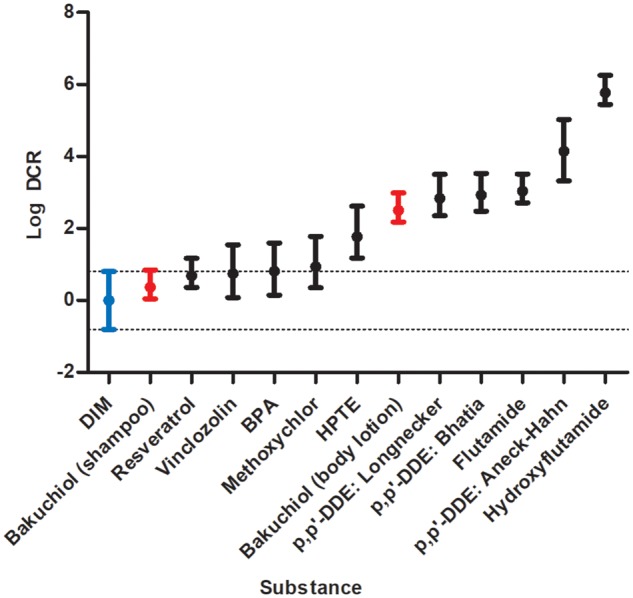
Dietary comparator ratios (DCRs). Dietary comparator (DIM) in blue, case study ingredient (bakuchiol) with hypothetical exposure scenarios in red.
Case Study Risk Assessment
Once we had determined the DCRs for the substances described above, we considered how this approach could be used to assist safety decision making using a hypothetical case study, the use of bakuchiol or AG in a body lotion or shampoo at 0.5%. In a safety risk assessment for a real consumer product other MIEs and pathways would also need to be considered, but because the purpose of this case study was to evaluate the DCR methodology we concentrated solely on AR antagonism.
AG was negative in both the agonism and antagonism assay and was therefore not taken forward as the subject of the risk assessment case study. The positive result in the AR CALUX® (antagonism) assay for bakuchiol was clear, although this substance was amongst the least potent anti-androgens tested (Table 2; Figure 3). The exposure assessment based on worst-case consumer exposure to 0.5% bakuchiol in a body lotion or shampoo predicted plasma exposure for adult females of 0.320 and 0.00234 µM, respectively. The in vitro point of departure (in this case the PC50) was below these predicted human exposure levels. To further put this margin into context, the DCRs for bakuchiol in body lotion and shampoo were calculated (Figure 4). The mean DCR for bakuchiol body lotion was 151, which is in a similar range to the p,p′-DDE exposures included in the benchmarking. The mean DCR for bakuchiol at 0.5% in shampoo was 2.34.
DISCUSSION
Using the EAR for DIM as a comparator with other substances by calculating their DCR is a pragmatic risk ranking approach, whereby DCRs below 1 provide strong assurance that adverse effects in humans relating to perturbations in AR signaling are very unlikely for that exposure scenario. It is important to note that a DCR greater than 1 does not necessarily indicate high risk. If that were the case consumption of anything more than 50 g brussels sprouts would be considered by this approach to be harmful (see Supplementary Material for DIM exposure modeling). However, the closer the DCR is to the anti-androgenic drug flutamide and its active metabolite hydroxyflutamide, the greater the risk. This is because systemic exposure to hydroxyflutamide following therapeutic use of flutamide is intended to completely supress AR signaling, and as such significant health effects relating to AR signaling would be expected in any population exposed to these levels. Use of DCRs for risk ranking requires careful consideration of the mode of action of the substance being risk assessed and how this compares with the dietary comparator.
The range of DCRs for resveratrol, BPA, vinclozolin, and methoxychlor overlapped with the range of DCRs for DIM. The DCRs for the methoxychlor metabolite HPTE were outside the range of DCRs for DIM. The exposure assessments for BPA, vinclozolin, and methoxychlor were based on the assumption that the reference dose or TDI for these substances was ingested, and HPTE was based on the assumption that the reference dose of methoxychlor was ingested and completely converted to HPTE, which is clearly worst-case. Because the reference doses or TDI for BPA, vinclozolin, and methoxychlor were set to be protective of all adverse effects on human health (including those relating to AR signaling in both adults and the developing fetus) although some were > 1, the DCR for these substances are also likely to represent a ‘region of safety.’ The exposure assessment for resveratrol was based on consumption of 25 mg/day. Although some have described this as representing a moderate intake of red wine (Walle, 2011), depending on the variety it is possible that well over 600 ml of red wine may need to be consumed to reach this level of intake (Presta et al., 2009). The DCR approach suggests that even at this level of intake the resveratrol present in the wine is unlikely to have any significant AR-mediated adverse effects.
The progression from an MIE to an adverse outcome is dependent on the magnitude and duration of the initial interaction, and transient activation of an MIE or a key event may not result in an adverse outcome. It is therefore important to understand these dose-dependent transitions (or ‘tipping points’) to ensure the risk assessment is relevant to the protection of human health (Slikker et al., 2004a,b). From our data it is not possible to accurately determine a tipping point, that, if reached would indicate a transition from adaptation to adversity. When considering whether it was feasible to set a tipping point, we investigated whether exposures to p,p′-DDE, the active metabolite of the organochlorine pesticide DDT could provide some useful insights. Exposures to DDT and p,p′-DDE have been associated with a number of adverse health effects, including adverse developmental/reproductive effects relating to the AR signaling pathway such as cryptorchidism and hypospadias, and numerous epidemiology studies have been performed examining the link between serum p,p′-DDE levels and adverse outcomes (Bonde et al., 2016). Case-control studies investigating the relationship between exposure to p,p′-DDE and birth defects which were based on data collected in the United States in the 1950s and 1960s with median maternal serum levels in the control groups of 34.3 µg/l (Longnecker et al., 2002) or 43 µg/l (Bhatia et al., 2005) have failed to show a conclusive association between exposure and hypospadias or cryptorchidism. Studies performed in areas where DDT is still used for malarial control have shown some associations between adult (male) serum levels of p,p′-DDE and sperm quality/quantity. For instance, in one cross-sectional study of 311 adult men from a DDT-sprayed area in South Africa with a median serum p,p′-DDE level of 697 µg/l, exposure was associated with impaired sperm motility, sperm cytoplasmic droplets, reduced ejaculate volume, and oligozoospermia (Aneck-Hahn et al., 2006). We calculated EARs and DCRs for these p,p′-DDE exposure scenarios using published AR CALUX® data (Suzuki et al., 2011) to see how they compared, and only the exposure data from the high exposure study in South Africa (Aneck-Hahn et al., 2006) exceeded the IC50 for p,p′-DDE. When interpreting these data, it is important to remember that the case-control studies reflected maternal serum exposure, whereas the cross-sectional study reflected adult male serum exposure. Therefore, it is not appropriate to use these data to define a transition from ‘endocrine activity’ to ‘endocrine disruption.’ They are however informative for the purposes of risk ranking. It should be noted that although most studies investigating the effects of lower exposures to DDT or p,p′-DDE (eg, recent studies in developed countries) do not show any associations between either maternal or adult male exposure and birth defects or impaired sperm, some have shown an association. For example, one well conducted study has shown an association between maternal serum exposures of around 1 ng/ml and hypospadias (Rignell-Hydbom et al., 2012). Whether the association seen in that study was due to p,p′-DDE exposure is not clear, especially given the large number of studies at similar exposure levels which have not found associations (Carmichael et al., 2010; Giordano et al., 2010). These include investigations into the potential for long-term effects of in utero exposure to p,p′-DDE. For example, in a well-designed study 176 male offspring from a Danish cohort of women, there was no relationship between maternal p,p′-DDE levels and long term consequences on male reproductive health with median maternal serum levels of 8 pmol/ml (2.54 ng/ml) (Vested et al., 2014). Given the contentious nature of potential low dose effects we have focused our evaluation on populations exposed to high levels of p,p′-DDE. This is in line with the conclusions of the US Agency for Toxic Substances and Disease Registry, which concluded that if a relationship between p,p′-DDE exposure and adverse reproductive/developmental outcomes in humans exists, it is found in populations exposed to high DDT concentrations (ATSDR, 2002, 2008).
AG was negative in the AR CALUX® assay, which was surprising given the existing in vitro and in vivo data. Although several rat male fertility studies on either Andrographis paniculata or AG have shown no adverse effects (Allan et al., 2009; Burgos et al., 1997), others have shown marked adverse effects on fertility (Akbarsha et al., 2000; Akbarsha and Murugaian, 2000; Sattayasai et al., 2010). AG is also reported to affect androgen signaling in prostate cancer cell lines (Liu et al., 2011), suggesting an ability to reduce AR expression at the transcriptional level, inhibit nuclear translocation of AR, inhibit the formation of stabilizing complexes with the co-chaperone Hsp90, slow the growth of C4-2 prostate cancer cells, and induce apoptosis. This was the reason for including AG in this evaluation. The lack of response seen in our study likely reflects differences between AR CALUX® cells and C4-2 cells, which were originally derived from LNCaP prostate cancer cells (Wu et al., 1994). Given these conflicting data, logical next steps for the evaluation of AG include assessing the reproducibility of the findings in C4-2 cells and assessing whether metabolism of AG could account for differences between these cell types.
Because different assays may provide different AC50 or IC50 values for the same test substance, it is important to ensure that all data used for a specific mode of action are comparable, ie, produced in the same assay system, and that dose-response information from that system are reproducible. The available pre-validation data on the AR CALUX® assay shows that the average IC50s were within a factor of 3 between 2 laboratories (van der Burg et al., 2010), and in our study where the same substance (flutamide) was used our IC50 value was similar to the published range. In the pre-validation study the average IC50 values for flutamide following 6 or 7 experiments were 0.399 and 0.516 µM for laboratory 1 and laboratory 2 respectively, and our IC50 value was 0.876 µM.
Although the in vitro point of departure for bakuchiol (in this case the PC50) was below the predicted human exposure levels following use in both shampoo and body lotion, it was close for body lotion (approximately 10-fold below). The DCR for bakuchiol at 0.5% in body lotion was in a similar range to the DCRs calculated for the p,p′-DDE exposures included in the benchmarking, and the DCR for bakuchiol at 0.5% in shampoo overlapped with range of DCRs for DIM. With the current predictions, exposure to bakuchiol at 0.5% in a body lotion suggests the possibility that AR signaling may be perturbed in consumers, indicating the need for a more detailed evaluation in higher-tier models. Alternatively, exposure to bakuchiol at 0.5% in a shampoo appears low risk for this mode of action.
Sources of Uncertainty
As with any risk assessment, there are several uncertainties with this approach that need to be understood to enable informed safety decision making. These include the reliance on predicted plasma exposures for the dietary comparator DIM. However, as described in the Supplementary Materials, sufficient data were available to build a model which correlated well with measured human plasma levels following administration of a known quantity of absorption-enhanced DIM and confidence that these predictions are suitable for the purpose of this investigation is high.
The skin penetration parameters used in the bakuchiol PBBK model were all predicted, and no human kinetic data were available to assess the performance of the model, meaning confidence in these predictions is much lower than confidence in the DIM model (see Supplementary Materials). Further data generation, especially in vitro skin penetration, plasma protein binding, and hepatocyte clearance would refine the exposure model, and obtaining human kinetic data to evaluate the performance of the model would greatly increase confidence in the model predictions.
In this study, plasma Cmax was used as the measure of exposure. Some substances, like resveratrol and DIM are very rapidly cleared, whereas others, like p,p′-DDE are very persistent. This is significant because clearance of anti-androgens is an important determinant in their efficacy (Gao et al., 2005). DCR values must therefore be considered alongside the overall pharmacokinetic profile of the substance being evaluated. In other words, substances in the ‘region of safety’ which are much more persistent than DIM and the other benchmark substances may require further evaluation.
In Figure 3, we were able to characterize the level of uncertainty or variability for some but not all bioactivity or exposure data. As described in the Supplementary Materials, a measure of uncertainty or variability was available in the exposure data for all test substances apart from flutamide, hydroxyflutamide, and resveratrol. A measure of uncertainty or variability was presented in the bioactivity data we generated, because in Figure 3 the best-fit and lower and upper 95% confidence intervals (CI) of the IC50 were presented. However, because there was very little variability in the data the error bars are generally not visible on the logarithmic scale. The lack of 95% CI for the published AR CALUX® data is therefore not considered to be a significant contributor to uncertainty within the risk assessment.
A number of anti-androgenic substances, including flutamide, methoxychlor, and vinclozolin, have metabolites which are more potent than the parent. This exposes a potential weakness in the way we performed the AR CALUX® assay, ie, without metabolic activation. This refinement has been described (Mollergues et al., 2017) and would be a useful additional test to include to provide further information, firstly on whether a metabolite of AG could cause transcriptional effects in the AR pathway, and also whether a metabolite of bakuchiol could be more active than its parent.
The in vitro to in vivo comparisons we have performed were based on total concentration rather than free concentration in test media and plasma, which in general are considered a more appropriate dose metric for in vitro to in vivo extrapolation (Groothuis et al., 2015). Therefore, any comparison for a substance that shows different kinetics in vitro and in vivo (eg, those that are extensively bound to plastic or serum) will be flawed. A lot of the substances we tested would be expected to be extensively bound both in vitro and in vivo. We considered the physico-chemical properties of our test and reference substances, and in particular our key comparator, DIM. Based on this evaluation we determined that in vitro and in vivo exposures to free DIM (and the other test substances) are likely to be within one order of magnitude of their nominal concentration, although analytical determination of free DIM in the assay medium and plasma protein binding would provide further confirmation of this.
CONCLUSION
Historically, reporter gene assays for endocrine modes of action have been used to prioritize chemicals for follow-up in subsequent in vivo studies, to assess whether the endocrine activity seen in vitro translates to an in vivo adverse effect and to set a point of departure (eg, a no-observed-adverse-effect level) for risk assessment. One of the objectives of this study was to investigate use of exposure data at an earlier step in this paradigm to prevent the need to generate animal data on chemicals with low activity relative to their associated human exposures. We found that the use of DCRs is a pragmatic approach which allows novel chemical exposures (as described by the bakuchiol case study) to be put into context against normal dietary exposure to anti-androgens such as DIM, and against other anti-androgenic chemicals. The DCR approach may have utility for other modes of action where appropriate comparators can be identified.
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank Andrew White, David Sanders, Beate Nicol, and Carl Westmoreland (Unilever) and Lydia Jonker, Harrie Besselink, and Peter Behnisch (BioDetection Systems) for their technical advice and helpful insights. The authors declare there are no conflicts of interest.
FUNDING
This work was funded by Unilever as part of Unilever’s ongoing effort to develop new ways of assuring consumer safety.
REFERENCES
- Akbarsha M. A., Latha P. N., Murugaian P. (2000). Retention of cytoplasmic droplet by rat cauda epididymal spermatozoa after treatment with cytotoxic and xenobiotic agents. J. Reprod. Fertil. 120, 385–390. [DOI] [PubMed] [Google Scholar]
- Akbarsha M. A., Murugaian P. (2000). Aspects of the male reproductive toxicity/male antifertility property of andrographolide in albino rats: Effect on the testis and the cauda epididymidal spermatozoa. Phytother. Res. 14, 432–435. [DOI] [PubMed] [Google Scholar]
- Allan J. J., Pore M. P., Deepak M., Murali B., Mayachari A. S., Agarwal A. (2009). Reproductive and fertility effects of an extract of Andrographis paniculata in male Wistar rats. Int. J. Toxicol. 28, 308–317. [DOI] [PubMed] [Google Scholar]
- Allen T. E. H., Goodman J., Gutsell S., Russell P. J. (2014). Defining molecular initiating events in the adverse outcome pathway framework for risk assessment. Chem. Res. Toxicol. 27, 2100–2112. [DOI] [PubMed] [Google Scholar]
- Aneck-Hahn N. H., Schulenburg G. W., Bornman M. S., Farias P., De Jager C. (2006). Impaired semen quality associated with environmental DDT exposure in young men living in a malaria area in the Limpopo Province, South Africa. J. Androl. 28, 423–434. [DOI] [PubMed] [Google Scholar]
- ATSDR. (2002). Toxicological Profile for DDT, DDE, DDD U.S. Department of Health and Human Services, Agency for Toxic Substances and Disease Registry, Atlanta, Georgia. Available at: https://www.atsdr.cdc.gov/ToxProfiles/tp35.pdf, last accessed October 15, 2018
- ATSDR. (2008). Addendum to the Toxicological Profile for DDT, DDE, DDD U.S. Department of Health and Human Services, Agency for Toxic Substances and Disease Registry, Atlanta, Georgia. Available at: https://www.atsdr.cdc.gov/toxprofiles/ddt_addendum.pdf, last accessed October 15, 2018.
- Baur J. A., Sinclair D. A. (2006). Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 5, 493–506. [DOI] [PubMed] [Google Scholar]
- Becker R. A., Friedman K. P., Simon T. W., Marty M. S., Patlewicz G., Rowlands J. C. (2015). An exposure, activity profiling method for interpreting high-throughput screening data for estrogenic activity—Proof of concept. Regul. Toxicol. Pharmacol. 71, 398–408. [DOI] [PubMed] [Google Scholar]
- Becker R. A., Hays S. M., Kirman C. R., Aylward L. L., Wise K. (2014). Interpreting estrogen screening assays in the context of potency and human exposure relative to natural exposures to phytoestrogens. Birth Defects Res. B Dev. Reprod. Toxicol. 101, 114–124. [DOI] [PubMed] [Google Scholar]
- Bhatia R., Shiau R., Petreas M., Weintraub J. M., Farhang L., Eskenazi B. (2005). Organochlorine pesticides and male genital anomalies in the child health and development studies. Environ. Health Perspect. 113, 220–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonde J. P., Flachs E. M., Rimborg S., Glazer C. H., Giwercman A., Ramlau-Hansen C. H., Hougaard K. S., Høyer B. B., Hærvig K. K., Petersen S. B., et al. (2016). The epidemiologic evidence linking prenatal and postnatal exposure to endocrine disrupting chemicals with male reproductive disorders: A systematic review and meta-analysis. Hum. Reprod. Updat. 23, 104–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgos R. A., Caballero E. E., Sánchez N. S., Schroeder R. A., Wikman G. K., Hancke J. L. (1997). Testicular toxicity assesment of Andrographis paniculata dried extract in rats. J. Ethnopharmacol. 58, 219–224. [DOI] [PubMed] [Google Scholar]
- Carmichael S. L., Herring A. H., Sjödin A., Jones R., Needham L., Ma C., Ding K., Shaw G. M. (2010). Hypospadias and halogenated organic pollutant levels in maternal mid-pregnancy serum samples. Chemosphere 80, 641–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dancik Y., Troutman J. A., Jaworska J. (2015). Estimation of in vivo dose of dermally applied chemicals leading to estrogen/androgen receptor-mediated toxicity from in vitro data—Illustration with four reproductive toxicants. Reprod. Toxicol. 55, 50–63. [DOI] [PubMed] [Google Scholar]
- Dent M. P., Amaral R. T., Da Silva P. A., Ansell J., Boisleve F., Hatao M., Hirose A., Kasai Y., Kern P., Kreiling R., et al. (2018). Principles underpinning the use of new methodologies in the risk assessment of cosmetic ingredients. Comput. Toxicol. 7, 20–26. [Google Scholar]
- Dent M. P., Carmichael P. L., Jones K. C., Martin F. L. (2015). Towards a non-animal risk assessment for anti-androgenic effects in humans. Environ. Int. 83, 94–106. [DOI] [PubMed] [Google Scholar]
- Fujioka N., Ransom B. W., Carmella S. G., Upadhyaya P., Lindgren B. R., Roper-Batker A., Hatsukami D. K., Fritz V. A., Rohwer C., Hecht S. S. (2016). Harnessing the power of cruciferous vegetables: Developing a biomarker for brassica vegetable consumption using urinary 3,3′-diindolylmethane. Cancer Prev. Res. 9, 788–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W., Bohl C. E., Dalton J. T. (2005). Chemistry and structural biology of androgen receptor. Chem. Rev. 105, 3352–3370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano F., Abballe A., De Felip E., di Domenico A., Ferro F., Grammatico P., Ingelido A. M., Marra V., Marrocco G., Vallasciani S., et al. (2010). Maternal exposures to endocrine disrupting chemicals and hypospadias in offspring. Birth Defects Res. A Clin. Mol. Teratol. 88, 241–50 [DOI] [PubMed] [Google Scholar]
- Goldberg D. M., Yan J., Soleas G. J. (2003). Absorption of three wine-related polyphenols in three different matrices by healthy subjects. Clin. Biochem. 36, 79–87. [DOI] [PubMed] [Google Scholar]
- Groothuis F. A., Heringa M. B., Nicol B., Hermens J. L. M., Blaauboer B. J., Kramer N. I. (2015). Dose metric considerations in in vitro assays to improve quantitative in vitro-in vivo dose extrapolations. Toxicology 332, 30–40. [DOI] [PubMed] [Google Scholar]
- Krewski D., Acosta D., Andersen M., Anderson H., Bailar J. C., Boekelheide K., Brent R., Charnley G., Cheung V. G., Green S., et al. (2010). Toxicity testing in the 21st century: A vision and a strategy. J. Toxicol. Environ. Health B Crit. Rev. 13, 51–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Nadiminty N., Tummala R., Chun J. Y., Lou W., Zhu Y., Sun M., Evans C. P., Zhou Q., Gao A. C. (2011). Andrographolide targets androgen receptor pathway in castration-resistant prostate cancer. Genes Cancer 2, 151–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longnecker M. P., Klebanoff M. A., Brock J. W., Zhou H., Gray K. A., Needham L. L., Wilcox A. J. (2002). Maternal serum level of 1,1-dichloro-2,2-bis(p-chlorophenyl)ethylene and risk of cryptorchidism, hypospadias, and polythelia among male offspring. Am. J. Epidemiol. 155, 313–322. [DOI] [PubMed] [Google Scholar]
- Macleod D. J., Sharpe R. M., Welsh M., Fisken M., Scott H. M., Hutchison G. R., Drake A. J., van den Driesche S. (2010). Androgen action in the masculinization programming window and development of male reproductive organs. Int. J. Androl. 33, 279–287. [DOI] [PubMed] [Google Scholar]
- Middleton A., Cooper S., Cull T., Stark R., Adeleye Y., Boekelheide K., Clewell R., Jennings P., Guo J., Liu C., et al. (2017). Case studies in cellular stress: Defining adversity/adaptation tipping points. Appl. Vitr. Toxicol. 3, 199–210. [Google Scholar]
- Mollergues J., van Vugt-Lussenburg B., Kirchnawy C., Bandi R. A., van der Lee R. B., Marin-Kuan M., Schilter B., Fussell K. C. (2017). Incorporation of a metabolizing system in biodetection assays for endocrine active substances. ALTEX 34389–398. [DOI] [PubMed] [Google Scholar]
- OECD. (2016). Test No. 455: Performance-Based Test Guideline for Stably Transfected Transactivation In Vitro Assays to Detect Estrogen Receptor Agonists and Antagonists. OECD, Paris (OECD Guidelines for the Testing of Chemicals, Section 4).
- Presta M. A., Bruyneel B., Zanella R., Kool J., Krabbe J. G., Lingeman H. (2009). Determination of flavonoids and resveratrol in wine by turbulent-flow chromatography-LC-MS. Chromatographia 69, 167–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radwanski E., Perentesis G., Symchowicz S., Zampaglione N. (1989). Single and multiple dose pharmacokinetic evaluation of flutamide in normal geriatric volunteers. J. Clin. Pharmacol. 29, 554–558. [DOI] [PubMed] [Google Scholar]
- Raederstorff D., Kunz I., Schwager J. (2013). Resveratrol, from experimental data to nutritional evidence: The emergence of a new food ingredient. Ann. N.Y. Acad. Sci. 1290, 136–141. [DOI] [PubMed] [Google Scholar]
- Rignell-Hydbom A., Lindh C. H., Dillner J., Jönsson B. A. G., Rylander L. (2012). A nested case-control study of intrauterine exposure to persistent organochlorine pollutants and the risk of hypospadias. PLoS One 7, e44767.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattayasai J., Srisuwan S., Arkaravichien T., Aromdee C. (2010). Effects of andrographolide on sexual functions, vascular reactivity and serum testosterone level in rodents. Food Chem. Toxicol. 48, 1934–1938. [DOI] [PubMed] [Google Scholar]
- Slikker W., Andersen M. E., Bogdanffy M. S., Bus J. S., Cohen S. D., Conolly R. B., David R. M., Doerrer N. G., Dorman D. C., Gaylor D. W. (2004a). Dose-dependent transitions in mechanisms of toxicity. Toxicol. Appl. Pharmacol. 201, 203–225. [DOI] [PubMed] [Google Scholar]
- Slikker W., Andersen M. E., Bogdanffy M. S., Bus J. S., Cohen S. D., Conolly R. B., David R. M., Doerrer N. G., Dorman D. C., Gaylor D. W. (2004b). Dose-dependent transitions in mechanisms of toxicity: Case studies. Toxicol. Appl. Pharmacol. 201, 226–294. [DOI] [PubMed] [Google Scholar]
- Sonneveld E., Jansen H. J., Riteco J. A. C., Brouwer A., van der Burg B. (2005). Development of androgen- and estrogen-responsive bioassays, members of a panel of human cell line-based highly selective steroid-responsive bioassays. Toxicol. Sci. 83, 136–148. [DOI] [PubMed] [Google Scholar]
- Suzuki G., Tue N. M., Van Der Linden S., Brouwer A., van der Burg B., van Velzen M., Lamoree M., Someya M., Takahashi S., Isobe T., et al. (2011). Identification of major dioxin-like compounds and androgen receptor antagonist in acid-treated tissue extracts of high trophic-level animals. Environ. Sci. Technol. 45, 10203–10211. [DOI] [PubMed] [Google Scholar]
- van der Burg B., Winter R., Man H., Vangenechten C., Berckmans P., Weimer M., Witters H., van der Linden S. (2010). Optimization and prevalidation of the in vitro AR CALUX® method to test androgenic and antiandrogenic activity of compounds. Reprod. Toxicol. 30, 18–24. [DOI] [PubMed] [Google Scholar]
- Vested A., Ramlau-Hansen C. H., Olsen S. F., Bonde J. P., Støvring H., Kristensen S. L., Halldorsson T. I., Rantakokko P., Kiviranta H., Ernst E. H., et al. (2014). In utero exposure to persistent organochlorine pollutants and reproductive health in the human male. Reproduction 148, 635–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walle T. (2011). Bioavailability of resveratrol. Ann. N.Y. Acad. Sci. 1215, 9–15. [DOI] [PubMed] [Google Scholar]
- Wambaugh J. F., Hughes M. F., Ring C. L., MacMillan D. K., Ford J., Fennell T. R., Black S. R., Snyder R. W., Sipes N. S., Wetmore B. A., et al. (2018). Evaluating in vitro-in vivo extrapolation of toxicokinetics. Toxicol. Sci. 163, 152–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Rijk J. C. W., Besselink H. T., Houtman R., Peijnenburg A. A. C. M., Brouwer A., Rietjens I. M. C. M., Bovee T. F. H. (2014). Extending an in vitro panel for estrogenicity testing: The added value of bioassays for measuring antiandrogenic activities and effects on steroidogenesis. Toxicol. Sci. 141, 78–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetmore B. A., Wambaugh J. F., Allen B., Ferguson S. S., Sochaski M. A., Setzer R. W., Houck K. A., Strope C. L., Cantwell K., Judson R. S., et al. (2015). Incorporating high-throughput exposure predictions with dosimetry-adjusted in vitro bioactivity to inform chemical toxicity testing. Toxicol. Sci. 148, 121–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetmore B. A., Wambaugh J. F., Ferguson S. S., Sochaski M. A., Rotroff D. M., Freeman K., Clewell H. J., Dix D. J., Andersen M. E., Houck K. A., et al. (2012). Integration of dosimetry, exposure, and high-throughput screening data in chemical toxicity assessment. Toxicol. Sci. 125, 157–174. [DOI] [PubMed] [Google Scholar]
- Wu H.-C., Hsieh J.-T., Gleave M. E., Brown N. M., Pathak S., Chung L. W. K. (1994). Derivation of androgen-independent human LNCaP prostatic cancer cell sublines: Role of bone stromal cells. Int. J. Cancer 57, 406–412. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.