Abstract
Angus × Hereford calves (n = 159; 87 heifers and 72 steers) were ranked by sex, BW, and age, and assigned to one of three vaccination schemes against bovine respiratory disease (BRD): (i) vaccination at weaning (day 0) and revaccination at feedyard arrival (day 30; CON, n = 53), (ii) vaccination 15 d before weaning (day −15) and revaccination 15 d before feedyard arrival (day 15; EARLY, n = 53), and (iii) vaccination 15 d after weaning (day 15) and revaccination 15 d after feedyard arrival (day 45; DELAYED, n = 53). Calves were maintained on pasture from days −15 to 29, transported (day 30) for 480 km to a commercial growing feedyard, and moved (day 180) to an adjacent finishing yard where they remained until slaughter (day 306). Calf BW was recorded on two consecutive days (days −15, −14, 0, 1, 29, 30, 75, 76, 179, and 180), which were averaged for BW gain calculation. Calves were assessed for BRD signs daily from days 0 to 306. Blood samples were collected on days −15, 0, 15, 30, 45, 60, and 75. No treatment effects were detected (P ≥ 0.49) for BW responses and carcass characteristics (P ≥ 0.32). Serum titers against bovine viral diarrhea type 1 were greater (P ≤ 0.05) in EARLY vs. CON and DELAYED from days 15 to 45, and greater (P < 0.01) in CON vs. DELAYED on days 30 and 45. Serum titers against bovine herpesvirus-1 were greater (P < 0.01) in EARLY vs. CON and DELAYED on days 0 and 30, and greater (P < 0.01) in EARLY and CON vs. DELAYED on days 15 and 45. Serum titers against bovine respiratory syncytial virus were greater (P = 0.05) in EARLY vs. CON on day 0, greater (P = 0.04) in CON vs. DELAYED on day 15, and greater (P ≤ 0.03) in EARLY and CON vs. DELAYED from days 30 to 60. Serum titers against parainfluenza3 virus were greater (P ≤ 0.04) in EARLY vs. DELAYED on days 30 and 45, and greater (P < 0.01) in CON vs. DELAYED on day 30. Incidence of BRD was less (P = 0.04) in EARLY vs. CON and DELAYED, and similar (P = 0.99) between CON and DELAYED. Therefore, altering the time of vaccination and revaccination against BRD to provide both doses prior to feedlot entry altered serum antibody responses to BRD pathogens, and alleviated the incidence of this disease in feedlot cattle.
Keywords: feedlot cattle, health, performance, respiratory diseases, vaccination
INTRODUCTION
The bovine respiratory disease (BRD) complex is the most important cause of morbidity and mortality in U.S. feedlots (NASS, 2006), and management to mitigate incidence of BRD is warranted for optimal welfare and productivity of feedlot cattle (Duff and Galyean, 2007). Preconditioning programs are examples of such management (Martin et al., 1999), in which calves generally receive vaccination against BRD pathogens at weaning and revaccination 30 d later upon feedlot arrival (England et al., 2009). However, vaccine efficacy is reduced when administered to highly stressed animals (Blecha et al., 1984), whereas weaning and feedlot entry are major stressors to cattle (Cooke, 2017). Vaccination against BRD pathogens has also been shown to impair cattle performance (Arthington et al., 2013; Rodrigues et al., 2015). For these reasons, altering the time of vaccination against BRD has been investigated as an approach to enhance vaccine efficacy, immunity to BRD, and performance in feedlot cattle.
Richeson et al. (2008) reported that delaying BRD vaccination by 14 d after feedlot arrival increased seroconversion to bovine herpesvirus-1 (BHV-1) during feedlot receiving. However, the majority of BRD cases occur within the first 14 d upon feedlot entry (Kirkpatrick et al., 2008), and delaying vaccination may not provide full immunological protection to newly received cattle. In turn, Lippolis et al. (2016) reported that advancing the time of BRD vaccination, by providing the first dose prior to weaning and revaccination during a 30-d preconditioning program, enhanced cattle performance and antibody response to bovine viral diarrhea viruses (BVDV) and Mannheimia haemolytica (MH) during feedlot receiving. Nevertheless, these authors failed to report substantial treatment impacts on BRD incidence, given that morbidity rates were not as prevalent when compared to commercial feedlots (Snowder et al., 2006; Marques et al., 2016).
Lippolis et al. (2016) concluded that additional research was warranted to validate their results in high-stress feedlot environments, including evaluation of a greater number of animals, antibody response to vaccination against other BRD viruses, and cattle performance until slaughter. Based on this rationale, we hypothesized that advancing the time of vaccination against BRD pathogens, by providing both doses prior to feedlot entry, mitigates BRD incidence and promotes performance of cattle in a commercial feedlot system. Therefore, this experiment compared the impacts of advancing, delaying, or vaccinating against BRD at the time of weaning and feedlot entry on serum antibody titers against BRD pathogens, health responses, and performance of cattle managed in commercial feedyards until slaughter.
MATERIALS AND METHODS
This experiment was divided into a preweaning (days −15 to −1), preconditioning (days 0 to 29), feedlot growing (days 30 to 179), and finishing (days 180 to 306) phases. All animals were cared for in accordance with acceptable practices and experimental protocols reviewed and approved by the Oregon State University, Institutional Animal Care and Use Committee (#4913).
Animals and Treatments
One hundred and fifty-nine Angus × Hereford calves (steers, n = 72; heifers, n = 87) were utilized in this experiment. On day −15, calves were ranked by sex, BW, and age (initial BW = 192 ± 2 kg, initial age = 173 ± 1 d), and assigned to one of three treatments: (i) vaccination at weaning (day 0) and revaccination at feedyard entry (day 30; CON, n = 53), (ii) vaccination 15 d before weaning (day −15) and revaccination 15 d before feedyard entry (day 15; EARLY, n = 53), and (iii) vaccination 15 d after weaning (day 15) and revaccination 15 d after feedyard entry (day 45; DELAYED, n = 53). Treatment groups contained 29 heifers and 24 steers each, and were balanced for initial calf BW and age. Vaccines administered to calves during the experiment were against Clostridium and MH (2 mL s.c. injection of One Shot Ultra 7; Zoetis, Florham Park, NJ), BHV-1, BVDV types 1 and 2, parainfluenza3 virus (PI3), and bovine respiratory syncytial virus (BRSV; 2 mL s.c. injection of Bovi-Shield Gold 5; Zoetis). Calves not receiving vaccination were administered 4 mL (s.c.) of sterile saline. Moreover, all calves received these vaccines at ~45 d of age (branding) according to vaccination guidelines for cow–calf operations (England, 2009; USDA, 2010). Dams were vaccinated against Clostridium and MH (2 mL s.c. injection of Ultra 7; Zoetis) at calf branding, and against BHV-1, BVDV types 1 and 2, PI3, BRSV, Leptospira, and Campylobacter fetus (5 mL s.c. injection of Bovi-Shield Gold FP 5 VL5 HB; Zoetis) at weaning.
From days −15 to −1, calves remained with their respective dams in a single semi-arid rangeland pasture (Ganskopp and Bohnert, 2009). Dams were multiparous, and their age at the beginning of the experiment was 5.9 ± 0.2 yr for CON, 6.1 ± 0.2 yr for DELAYED, and 5.6 ± 0.2 yr for EARLY. Calves were weaned and administered an anthelmintic (s.c. injection at 1 mL/50 kg of BW of Dectomax; Zoetis) on day 0. From days 0 to 29 (preconditioning phase), calves were managed as a single group in a meadow-foxtail pasture (Alopecurus pratensis L.) with ad libitum access to a mixture of alfalfa (Medicago sativai L.) and meadow-foxtail hay (50:50 mixture; as-fed basis). Calves also had ad libitum access to water and a commercial mineral mix (Cattleman’s Choice, Performix Nutrition Systems, Nampa, ID) containing 14% Ca, 10% P, 16% NaCl, 1.5% Mg, 3,200 mg/kg of Cu, 65 mg/kg of I, 900 mg/kg of Mn, 140 mg/kg of Se, 6,000 mg/kg of Zn, 136,000 IU/kg of vitamin A, 13,000 IU/kg of vitamin D3, and 50 IU/kg of vitamin E. On day 30, calves were loaded into one of two double-deck commercial livestock trailer (Legend 50’ cattle liner; Barrett LLC., Purcell, OK) and transported for 480 km to a commercial growing lot (Top Cut; Echo, OR). Distribution of treatments was balanced between trailers, and calves from both trailers were transported at the same time and through the same route. At the growing lot, calves were managed as a single group and offered growing diets for ad libitum consumption (feedlot growing phase; Table 1). On day 180, calves were moved to a nearby finishing lot (Beef Northwest; Boardman, OR; 53-km distance) according to their transportation guidelines, where they continued to be managed as a single group and offered finishing diets for ad libitum consumption (feedlot finishing phase; Table 1). After arriving at the finishing lot, calves received Bovi-Shield Gold 5 (Zoetis), Vision 7 (Merck Animal Health, Kenilworth, NJ), Valbazen (Zoetis), and Bimectin pour-on (Bimeda Animal Health Inc., Oakbrook Terrace, IL). Steers were implanted with Revalor IS (Merck Animal Health) and heifers were implanted with Revalor IH (Merck Animal Health) upon arrival. Cattle were slaughtered on day 306 (Tyson Fresh Meats Inc., Pasco, WA).
Table 1.
Ingredient composition (as-fed basis) of growing and finishing diets offered to cattle
| Growing lot1 | Finishing lot2 | |||||
|---|---|---|---|---|---|---|
| Ingredients, % as-fed | A | B | A | B | C | D |
| Alfalfa hay | 0.0 | 0.0 | 23.3 | 16.7 | 8.4 | 5.0 |
| Barley | 18.0 | 17.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Corn cobbs | 0.0 | 5.3 | 0.0 | 0.0 | 0.0 | 0.0 |
| Corn silage | 10.0 | 15.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Corn stover | 0.0 | 10.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Culled French fries | 0.0 | 0.0 | 0.0 | 5.0 | 6.7 | 15.0 |
| High-moisture corn | 0.0 | 0.0 | 0.0 | 0.0 | 7.7 | 12.0 |
| Mineral and vitamin mix3,4 | 3.0 | 3.4 | 11.3 | 7.2 | 6.5 | 1.5 |
| Mixed pea/wheat/barley hay | 34.0 | 5.3 | 0.0 | 0.0 | 0.0 | 0.0 |
| Potato slurry | 13.0 | 23.0 | 0.0 | 10.0 | 12.1 | 25.0 |
| Rolled corn | 0.0 | 0.0 | 40.4 | 40.0 | 40.0 | 21.1 |
| Ryegrass silage | 22.0 | 15.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Vegetable oil | 0.0 | 0.0 | 0.0 | 0.5 | 0.9 | 0.4 |
| Wet distillers grain | 0.0 | 6.0 | 25.0 | 20.6 | 17.7 | 20.0 |
1A = offered for 10 d upon receiving; B = offered for 140 d after diet A and until transfer to the finishing lot.
2A = offered for 10 d upon receiving; B = offered for 10 d after diet A; C = offered for 10 d after diet B; D = offered until slaughter.
3Growing diets included Rumax (Performix Nutrition Systems, Nampa, ID).
4Finishing diets included a customized blend of minerals, vitamins, and feed additives (Westway Feed Products, Tomball, TX and Land O’Lakes, Inc., Saint Paul, MN).
Sampling
Samples of hay offered during the preconditioning phase were collected on day 0 and analyzed for nutrient content by a commercial laboratory (Dairy One Forage Laboratory, Ithaca, NY). Samples were analyzed by wet chemistry procedures as described by Lippolis et al. (2016). Calculations for net energy for maintenance and gain used the equations proposed by the NRC (1996). Nutritional profile of alfalfa and meadow-foxtail hay were, respectively (DM basis) 58% and 56% total digestible nutrients, 50% and 65% neutral detergent fiber, 33% and 39% acid detergent fiber, 1.16 and 1.08 Mcal/kg of for net energy for maintenance, 0.60 and 0.55 Mcal/kg of for net energy for gain, and 15.0% and 6.9% CP.
Calf full BW was recorded on two consecutive days (days −15 and −14, 0 and 1, 29 and 30, 75 and 76, and 179 and 180), and values from both days were averaged for ADG calculation. During the preconditioning phase, cattle were observed daily for BRD signs according to the DART system (Zoetis) as detailed by Souza et al. (2018). During the feedlot growing and finishing phases, cattle were also observed daily for BRD signs based on the DART system (Zoetis) and received medication according to the management criteria of the commercial feedyard. All BRD evaluators were blinded to treatment assignment to cattle, and incidence of BRD during the experiment was compiled in 7-d intervals. Blood samples were collected from all calves on days −15, 0, 15, 30, 45, 60, and 75 via jugular venipuncture into blood collection tubes (Vacutainer, 10 mL; Becton Dickinson, Franklin Lakes, NJ) containing no additives for serum collection. Blood samples were collected prior to treatment administration (days −15 to 45), and prior to the first feeding of the day (days 30 to 75). HCW was collected upon slaughter on day 306, and final BW was estimated based on HCW adjusted to 63% dressing (Loza et al., 2010). After a 24-h chill, trained personnel assessed carcass backfat thickness at the 12th-rib and LM area, whereas a USDA grader recorded all other carcass measures.
Blood Analyses
Blood samples were placed immediately on ice, centrifuged (2,500 × g for 30 min; 4 °C) for serum harvest, and stored at −80°C on the same day of collection. Serum samples collected from 18 calves per treatment (steers, n = 9; heifers, n = 9) not observed with BRD signs during the experiment (days 0 to 306) were selected for analysis of antibody titers against BRD pathogens, to ensure that this response was associated with vaccine efficacy rather than pathogenic infection (Callan, 2001; Souza et al., 2018). Samples were analyzed for antibody titers against BRSV, BHV-1, BVD-1, and PI3 using virus neutralization tests (Texas A&M Veterinary Medical Diagnostic Laboratory, Amarillo, TX). It is not certain if selected calves were indeed healthy or just asymptomatic to BRD (Richeson et al., 2008), although none of them exhibited BRD signs and clinical symptoms throughout the experimental period as mentioned previously. Antibody titers against these pathogens were evaluated based on day of the experiment (days −15 to 75) or based on equivalent days relative to the vaccination and revaccination (Lippolis et al., 2016).
Statistical Analysis
Calf was considered the experimental unit for all analyses. Quantitative data were analyzed using the MIXED procedure of SAS (SAS Inst. Inc., Cary, NC), whereas binary data were analyzed using the GLIMMIX procedure of SAS (SAS Inst. Inc.) with a binomial distribution. All data were analyzed using Satterthwaite approximation to determine the denominator degrees of freedom for the tests of fixed effects, with calf(treatment × sex) as random effect. Model statement for BW, ADG, and total morbidity and mortality rates contained the effects of treatment, sex, and the resultant interaction. Model statement for serum variables and cumulative BRD incidence contained the effects of treatment, sex, day, and all resultant interactions. The specified term for the repeated statements was day, calf(treatment × sex) was the subject, whereas the covariance structure used was first-order autoregressive based on the Akaike information criterion. Results are reported as least-square means, and were separated using the PDIFF option. Significance was set at P ≤ 0.05 and tendencies were determined if P > 0.05 and ≤0.10. Results are reported according to main effects if no interactions were significant, or according to the highest-order interaction detected.
RESULTS
Performance and Carcass Traits
No treatment effects were detected (P ≥ 0.48) for BW and ADG during the experimental period (Table 2). Such outcomes include similar (P ≥ 0.54) ADG from days 30 to 75 (1.04, 1.00, and 1.07 kg/d for CON, DELAYED, and EARLY, respectively; SEM = 0.04) and BW on day 75 (257, 254, and 254 kg of BW for CON, DELAYED, and EARLY, respectively; SEM = 4) among treatments, which corresponds to the receiving period in the growing lot. Accordingly, no treatment differences were detected (P ≥ 0.29) on carcass characteristics upon slaughter (Table 3).
Table 2.
Performance parameters of cattle vaccinated against respiratory pathogens at: (i) weaning (day 0) and at feedlot entry (day 30; CON, n = 53), (ii) 15 d before weaning (day −15) and 15 d before feedlot entry (day 15; EARLY, n = 53), and (iii) 15 d after weaning (day 15) and 15 d after feedlot entry (day 45; DELAYED, n = 53)1,2
| Item | EARLY | CON | DELAYED | SEM | P-value |
|---|---|---|---|---|---|
| Body weight, kg | |||||
| Pre-weaning (day −15) | 191 | 193 | 192 | 3 | 0.93 |
| Weaning (day 0) | 195 | 197 | 197 | 3 | 0.91 |
| Final preconditioning (day 30) | 207 | 210 | 208 | 3 | 0.85 |
| Final growing phase (day 180) | 388 | 390 | 388 | 5 | 0.96 |
| Final finishing phase (day 306) | 621 | 631 | 628 | 9 | 0.75 |
| Average daily gain, kg/d | |||||
| Pre-weaning (days −15 to −1) | 0.274 | 0.297 | 0.344 | 0.041 | 0.48 |
| Preconditioning (days 0 to 30) | 0.396 | 0.441 | 0.403 | 0.033 | 0.58 |
| Growing phase (days 31 to 180) | 1.22 | 1.20 | 1.20 | 0.02 | 0.89 |
| Finishing phase (days 181 to 306) 3 | 1.85 | 1.90 | 1.88 | 0.04 | 0.71 |
1Vaccines administered were against Clostridium and Mannheimia haemolytica (2 mL subcutaneous injection of One Shot Ultra 7; Zoetis, Florham Park, NJ), bovine herpesvirus-1, bovine viral diarrhea viruses types 1 and 2, parainfluenza3 virus, and bovine respiratory syncytial virus (2 mL subcutaneous injection of Bovi-Shield Gold 5; Zoetis). Calves also received these vaccines at 45 d of age. Calves not receiving vaccination were administered subcutaneously 4 mL of sterile saline.
2Calves were maintained in a single pasture from days −15 to 30, transported (day 30) for 480 km to a commercial growing feedyard, and moved (day 180) to an adjacent finishing yard where they remained until slaughter (day 306).
3 Calculated based on hot carcass weight (assuming 63% dressing; Loza et al., 2010).
Table 3.
Carcass characteristics of cattle vaccinated against respiratory pathogens at: (i) weaning (day 0) and at feedlot entry (day 30; CON, n = 53), (ii) 15 d before weaning (day −15) and 15 d before feedlot entry (day 15; EARLY, n = 53), and (iii) 15 d after weaning (day 15) and 15 d after feedlot entry (day 45; DELAYED, n = 53)1,2
| Item3 | EARLY | CON | DELAYED | SEM | P-value |
|---|---|---|---|---|---|
| Hot carcass weight, kg | 394 | 402 | 398 | 4 | 0.47 |
| Backfat, cm | 1.88 | 1.82 | 1.86 | 0.06 | 0.80 |
| LMarea, cm2 | 92.6 | 91.8 | 92.3 | 0.8 | 0.80 |
| KPH, % | 2.07 | 2.03 | 2.02 | 0.02 | 0.29 |
| Marbling | 487 | 484 | 479 | 10 | 0.85 |
| Yield grade | 3.66 | 3.74 | 3.72 | 0.10 | 0.81 |
| Retail product, % | 48.6 | 48.5 | 48.5 | 0.2 | 0.97 |
| Choice, % | 96.0 | 97.7 | 91.6 | 3.0 | 0.35 |
1Vaccines administered were against Clostridium and Mannheimia haemolytica (2 mL subcutaneous injection of One Shot Ultra 7; Zoetis, Florham Park, NJ), bovine herpesvirus-1, bovine viral diarrhea viruses types 1 and 2, parainfluenza3 virus, and bovine respiratory syncytial virus (2 mL subcutaneous injection of Bovi-Shield Gold 5; Zoetis). Calves also received these vaccines at 45 d of age. Calves not receiving vaccination were administered 4 mL of sterile saline subcutaneously.
2Calves were maintained in a single pasture from days −15 to 30, transported (day 30) for 480 km to a commercial growing feedyard, and moved (day 180) to an adjacent finishing yard where they remained until slaughter (day 306).
3Backfat thickness measured at the 12th rib; marbling score: 400 = Small00, 500 = Modest00; 600 = Medium00; yield grade calculated as reported by Lawrence et al. (2010); USDA retail yield equation = 51.34 − (5.78 × backfat) − (0.0093 × hot carcass weight) − (0.462 × KPH) + (0.74 × LM area area).
Serum Titers Against BRD Viruses
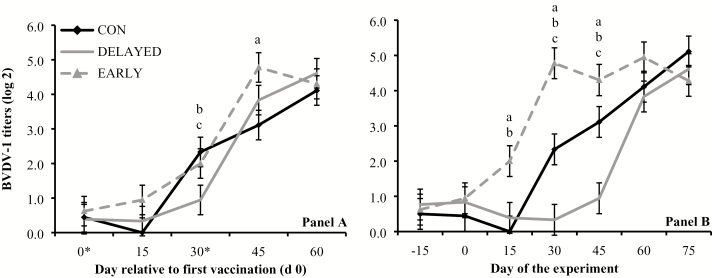
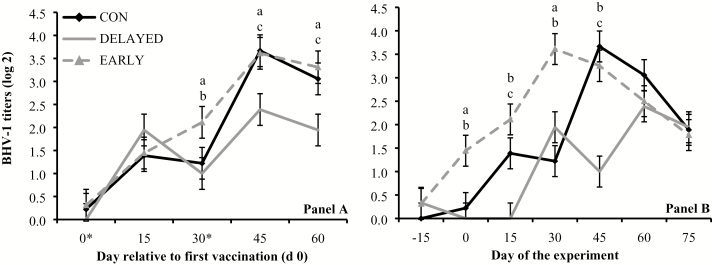
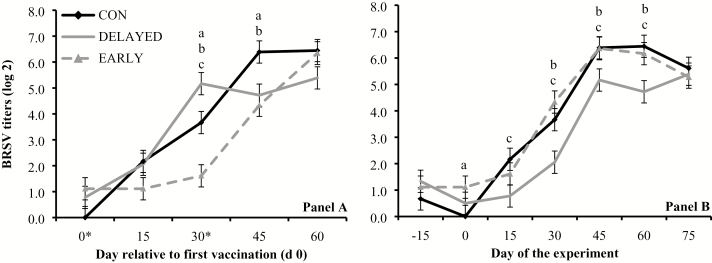
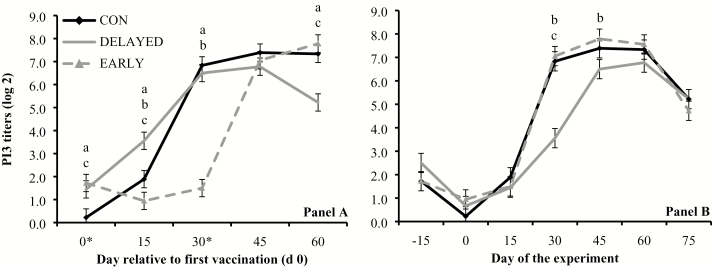
Treatment × day interactions were detected (P ≤ 0.01) for serum titers against BVD-1, BHV-1, BRSV, and PI3, when results were analyzed based on days relative to initial vaccination. Serum titers against BVD-1 were greater (P ≤ 0.05) in EARLY and CON vs. DELAYED on day 30, and greater (P < 0.01) in EARLY vs. CON on day 45 (Figure 1A). Serum titers against BHV-1 were greater (P ≤ 0.05) in EARLY vs. CON and DELAYED on day 30, and greater (P ≤ 0.02) in EARLY and CON vs. DELAYED on days 45 and 60 (Figure 2A). Serum titers against BRSV were greater (P < 0.01) in EARLY vs. CON and DELAYED on days 30 and 45, and greater (P < 0.01) in CON vs. DELAYED on day 30 (Figure 3A). Serum titers against PI3 were greater (P = 0.03) in EARLY and DELAYED vs. CON on day 0, greater (P ≤ 0.05) in DELAYED vs. CON and EARLY as well as CON vs. EARLY on day 15, greater (P < 0.01) in CON and DELAYED vs. EARLY on day 30, and greater (P < 0.01) in EARLY and CON vs. DELAYED on day 60 (Figure 4A).
Figure 1.
Serum titers against bovine viral diarrhea virus-1 (BVDV-1; titer log 2) in cattle vaccinated against respiratory pathogens at: (i) weaning (day 0) and at feedlot entry (day 30; CON, n = 53), (ii) 15 d before weaning (day −15) and 15 d before feedlot entry (day 15; EARLY, n = 53), and (iii) 15 d after weaning (day 15) and 15 d after feedlot entry (day 45; DELAYED, n = 53). Vaccines administered were against Clostridium and Mannheimia haemolytica (2 mL subcutaneous injection of One Shot Ultra 7; Zoetis, Florham Park, NJ), bovine herpesvirus-1, BVDV-1 and type 2, parainfluenza3 virus, and bovine respiratory syncytial virus (2 mL subcutaneous injection of Bovi-Shield Gold 5; Zoetis). Calves not receiving vaccination were administered 4 mL of sterile saline. Panel A reports values relative to the day of the first vaccination (day 0) within each treatment, whereas asterisks in the X-axis indicate vaccination days. Panel B reports values during the experiment (days −15 to 75). Treatment × day interactions were detected (P < 0.01). Within days, letters indicate (P ≤ 0.05): a = EARLY vs. CON, b = EARLY vs. DELAYED, c = CON vs. DELAYED.
Figure 2.
Serum titers against bovine herpesvirus-1 (BHV-1; titer log 2) in cattle vaccinated against respiratory pathogens at: (i) weaning (day 0) and at feedlot entry (day 30; CON, n = 53), (ii) 15 d before weaning (day −15) and 15 d before feedlot entry (day 15; EARLY, n = 53), and (iii) 15 d after weaning (day 15) and 15 d after feedlot entry (day 45; DELAYED, n = 53). Vaccines administered were against Clostridium and Mannheimia haemolytica (2 mL subcutaneous injection of One Shot Ultra 7; Zoetis, Florham Park, NJ), BHV-1, bovine viral diarrhea viruses types 1 and 2, parainfluenza3 virus, and bovine respiratory syncytial virus (2 mL subcutaneous injection of Bovi-Shield Gold 5; Zoetis). Calves not receiving vaccination were administered 4 mL of sterile saline. Panel A reports values relative to the day of the first vaccination (day 0) within each treatment, whereas asterisks in the X-axis indicate vaccination days. Panel B reports values during the experiment (days −15 to 75). Treatment × day interactions were detected (P ≤ 0.01). Within days, letters indicate (P ≤ 0.05): a = EARLY vs. CON, b = EARLY vs. DELAYED, c = CON vs. DELAYED.
Figure 3.
Serum titers against bovine respiratory syncytial virus (BRSV; titer log 2) in cattle vaccinated against respiratory pathogens at: (i) weaning (day 0) and at feedlot entry (day 30; CON, n = 53), (ii) 15 d before weaning (day −15) and 15 d before feedlot entry (day 15; EARLY, n = 53), and (iii) 15 d after weaning (day 15) and 15 d after feedlot entry (day 45; DELAYED, n = 53). Vaccines administered were against Clostridium and Mannheimia haemolytica (2 mL subcutaneous injection of One Shot Ultra 7; Zoetis, Florham Park, NJ), bovine herpesvirus-1, bovine viral diarrhea viruses types 1 and 2, parainfluenza3 virus, and BRSV (2 mL subcutaneous injection of Bovi-Shield Gold 5; Zoetis). Calves not receiving vaccination were administered 4 mL of sterile saline. Panel A reports values relative to the day of the first vaccination (day 0) within each treatment, whereas asterisks in the X-axis indicate vaccination days. Panel B reports values during the experiment (days −15 to 75). Treatment × day interactions were detected (P ≤ 0.01). Within days, letters indicate (P ≤ 0.05): a = EARLY vs. CON, b = EARLY vs. DELAYED, c = CON vs. DELAYED.
Figure 4.
Serum titers against parainfluzenza3 virus (PI3; titer log 2) in cattle vaccinated against respiratory pathogens at: (i) weaning (day 0) and at feedlot entry (day 30; CON, n = 53), (ii) 15 d before weaning (day −15) and 15 d before feedlot entry (day 15; EARLY, n = 53), and (iii) 15 d after weaning (day 15) and 15 d after feedlot entry (day 45; DELAYED, n = 53). Vaccines administered were against Clostridium and Mannheimia haemolytica (2 mL subcutaneous injection of One Shot Ultra 7; Zoetis, Florham Park, NJ), bovine herpesvirus-1, bovine viral diarrhea viruses types 1 and 2, PI3, and bovine respiratory syncytial virus (2 mL subcutaneous injection of Bovi-Shield Gold 5; Zoetis). Calves not receiving vaccination were administered 4 mL of sterile saline. Panel A reports values relative to the day of the first vaccination (day 0) within each treatment, whereas asterisks in the X-axis indicate vaccination days. Panel B reports values during the experiment (days −15 to 75). Treatment × day interactions were detected (P ≤ 0.01). Within days, letters indicate (P ≤ 0.05): a = EARLY vs. CON, b = EARLY vs. DELAYED, c = CON vs. DELAYED.
Treatment × day interactions were also detected (P ≤ 0.01) for serum titers against BVD-1, BHV-1, BRSV, and PI3 when results were analyzed based day of the experiment. Serum titers against BVD-1 were greater (P ≤ 0.05) in EARLY vs. CON and DELAYED from days 15 to 45, and greater (P < 0.01) in CON vs. DELAYED on days 30 and 45 (Figure 1B). Serum titers against BHV-1 were greater (P < 0.01) in EARLY vs. CON and DELAYED on days 0 and 30, and greater (P < 0.01) in EARLY and CON vs. DELAYED on days 15 and 45 (Figure 2B). Serum titers against BRSV were greater (P = 0.05) in EARLY vs. CON on day 0, greater (P = 0.04) in CON vs. DELAYED on day 15, and greater (P ≤ 0.03) in EARLY and CON vs. DELAYED from days 30 to 60 (Figure 3B). Serum titers against PI3 were greater (P ≤ 0.04) in EARLY vs. DELAYED on days 30 and 45, and greater (P < 0.01) in CON vs. DELAYED on day 30 (Figure 4B).
Health Responses
A treatment × day interaction was detected (P = 0.05) for incidence of BRD, which was greater (P ≤ 0.05) in CON vs. EARLY from days 84 to 306, and greater (P ≤ 0.05) in DELAYED vs. EARLY from days 231 to 306 (Figure 5). Overall, BRD incidence during the experimental period was less (P = 0.04) in EARLY vs. CON and DELAYED (16.9 vs. 32.1% and 32.1% of cattle diagnosed with BRD, respectively; SEM = 6.1), and similar (P = 0.99) between CON and DELAYED. No treatment differences, however, were detected (P ≥ 0.90) for incidence of cattle diagnosed with BRD that required ≥2 antimicrobial treatments (29.4%, 23.5%, and 22.2% in CON, DELAYED, and EARLY, respectively; SEM = 12.3), or number of antimicrobial treatments required upon BRD diagnosis (1.29, 1.35, and 1.33 in CON, DELAYED, and EARLY, respectively; SEM = 0.18). No other causes of morbidity were observed, and no treatment differences were detected (P = 0.88) for mortality (5.5%, 3.8%, and 3.8% in CON, DELAYED, and EARLY, respectively; SEM = 2.9).
Figure 5.
Cumulative incidence of bovine respiratory disease (BRD) signs in cattle vaccinated against respiratory pathogens at: (i) weaning (day 0) and at feedlot entry (day 30; CON, n = 53), (ii) 15 d before weaning (day −15) and 15 d before feedlot entry (day 15; EARLY, n = 53), and (iii) 15 d after weaning (day 15) and 15 d after feedlot entry (day 45; DELAYED, n = 53). Vaccines administered were against Clostridium and Mannheimia haemolytica (2 mL subcutaneous injection of One Shot Ultra 7; Zoetis, Florham Park, NJ), bovine herpesvirus-1, bovine viral diarrhea viruses types 1 and 2, parainfluenza3 virus, and bovine respiratory syncytial virus (2 mL subcutaneous injection of Bovi-Shield Gold 5; Zoetis). Cattle were maintained in a single pasture from days −15 to 30, transported (day 30) for 480 km to a commercial growing feedyard, and moved (day 180) to an adjacent finishing yard where they remained until slaughter (day 306). Cattle were observed daily for BRD signs according to the DART system (Zoetis) from days 0 to 306. A treatment × day interaction was detected (P = 0.05). Within days, letters indicate (P ≤ 0.05): a = EARLY vs. CON, and b = EARLY vs. DELAYED.
DISCUSSION
Vaccination against BRD pathogens has been shown to impair, at least transiently, performance traits in beef cattle. Arthington et al. (2013) reported reduced ADG and feed efficiency, during a 16-d evaluation period, in heifers vaccinated against MH compared with unvaccinated heifers. Rodrigues et al. (2015) administered similar vaccines as herein to beef steers, and noted that feed intake was reduced for 72 h after BRD vaccination when compared with unvaccinated cohorts. These outcomes were attributed to metabolic, inflammatory, and acute-phase responses triggered by the viral fraction and adjuvant contained in BRD vaccines, which are required for proper acquirement of protective immunity (Johnson, 1997; Tizard, 2004; Cooke, 2017). Accordingly, Lippolis et al. (2016) reported that altering the time of vaccination against BRD pathogens, in a manner that both injections are administered prior to feedlot entry, improved cattle ADG during a 45-d feedlot receiving. Richeson et al. (2008) also reported increased receiving ADG during in cattle vaccinated against BRD pathogens 14 d after feedlot entry compared with cohorts vaccinated at arrival. Similar outcomes, however, were not observed herein as ADG and BW did not differ between EARLY, CON, and DELAYED cattle, including during the 45-d receiving period in the growing lot. Consequently, no treatment differences were noted for carcass characteristic upon slaughter, such as HCW, LM area, and marbling. The aforementioned studies, however, investigated the impacts of vaccination strategies during limited periods in the feedyard, while reporting transient impacts in cattle ADG without effective changes in BW (Richeson et al. 2008; Arthington et al., 2013; Lippolis et al., 2016). Richeson et al. (2009) also reported similar ADG and BW during a 56-d receiving period in cattle administered clostridial and BRD vaccines at feedlot arrival or 14 d later. Collectively, one can conclude that altering the time of vaccination against BRD pathogens, despite transitory changes in ADG, does not impact BW and overall performance of feedlot cattle throughout the feeding period.
Cattle in this experiment received vaccination against Clostridium and BRD pathogens at the time of branding (~45 d of age), following recommendations for cow–calf health management (England, 2009; USDA, 2010). The vaccines utilized herein contained inactivated whole cultures of bacteria and a water-soluble adjuvant (One Shot Ultra 7; Zoetis), and modified-live virus strains of BHV-1, BVDV, PI3, and BRSV (Bovi-Shield Gold 5, Zoetis). It is still debatable if providing such vaccines to nursing calves at branding yields and effective immune response (Fulton et al., 2004; Cortese, 2009). Nevertheless, calves from all treatments received the exact same vaccination scheme at branding, and were equally managed from birth until the beginning of the experiment. Therefore, the immune-related differences noted among EARLY, CON, and DELAYED calves should not be associated with previous cow–calf management and vaccination history.
Serum antibody titers provide an indication of immune protection, disease prevention and vaccine efficacy in cattle (Howard et al., 1989; Bolin and Ridpath, 1990; Callan, 2001). Based on serum titers relative to day of the first vaccination, advancing or delaying BRD vaccination to circumvent the stress of weaning and feedlot entry substantially impacted vaccine response. Overall, EARLY improved antibody responses to BVDV-1 and BHV-1 within days 30 to 45 after initial vaccination, whereas the opposite was observed in DELAYED cattle. In turn, EARLY impaired antibody responses to PI3 and BRSV within 45 d after initial vaccination, whereas DELAYED improved these responses on days 15 and 30, respectively. Research investigating antibody titers against BRD viruses in feedlot cattle according to timing of vaccination are still limited and with variable results. Using a similar experimental design as herein, Lippolis et al. (2016) did not report differences among vaccination strategies in antibodies response to BVDV types I and II. Delaying vaccination against BRD by 14 d after feedlot entry did not impact antibody response to BVDV (Richeson et al., 2009), but increased seroconversion to BHV-1 during feedlot receiving (Richeson et al., 2008). To our knowledge, no research studies compared serum antibody titers against PI3 and BRSV according to timing of vaccination in feedlot cattle.
The divergence in serum antibody responses between EARLY and DELAYED noted herein, and when compared to previous research, cannot be fully clarified by this experimental design. Vaccine response is also influenced by other factors such as antibodies acquired from milk in nursing calves, or natural disease exposure and subsequent host immune response (Richeson et al., 2009; Downey et al., 2013). Although serum samples were obtained from cattle not diagnosed with BRD, and antibody response to BVDV and BHV-1 was improved in EARLY calves by weaning, the aforementioned factors may also have influenced treatment effects on serum titers. Antibody response to BHV-1, BVDV, PI3, and BRSV may be inhibited by maternal antibodies from milk, such as EARLY on day −15 of the experiment, in calves receiving a modified-live vaccine with no specific adjuvant (Ellis et al., 1996). Corroborating our results, Kirkpatrick et al. (2001) reported that passive immunity substantially interfered with antibody response to BRSV and PI3, but at a lesser extent in response to BVDV and BHV-1, in colostrum-fed dairy calves vaccinated against these pathogens with a modified-live vaccine. Nevertheless, the exact reasons by which advancing or delaying vaccination against BRD modulate antibody response and vaccine efficacy in feedlot cattle still warrant further investigation (Richeson et al., 2008; Richeson et al., 2009; Lippolis et al., 2016).
Despite the variation in antibody responses among treatments, EARLY resulted in greater serum titers and thus immune protection (Callan, 2001) against BRD viruses at feedlot entry (day 30 of the experiment). In turn, DELAYED cattle were those with less immunity to BRD viruses during the initial 15 d in the growing lot, which increased once DELAYED were revaccinated. These outcomes should be mainly attributed to number and interval between vaccinations and feedlot entry, as in Lippolis et al. (2016), rather than vaccine efficacy. Calves assigned to EARLY received the second vaccination 15 d before feedlot entry, which gave them more time to acquire immune protection against BRD viruses compared to other treatments. Accordingly, incidence of BRD was less in EARLY compared with all other treatments, corroborating with our main hypothesis and treatment effects on serum titers at feedlot arrival. It should be noted, however, that serum antibody titers were evaluated within 45 d after feedlot arrival (day 60 of the experiment), whereas treatment differences in BRD became statistically evident around day 84 of the experiment. Moreover, BRD incidence among EARLY and DELAYED only became different in the finishing lot, nearly 200 d after feedlot arrival. Hence, the benefits of EARLY in mitigating BRD cannot be fully attributed to treatment effects on antibody responses to vaccination, nor to serum antibody titers upon feedlot entry. Nevertheless, these outcomes support Lippolis et al. (2016), who noted a similar outcome during a 45-d feedlot receiving period, but without statistical differences due to limited experimental power and overall BRD incidence.
The ability of cattle diagnosed with BRD to recover from the disease was not impacted by vaccination strategy, despite treatment differences noted for serum antibody titers and BRD incidence. Incidence of cattle that required ≥2 antimicrobial treatments, number of antimicrobial treatments required upon BRD diagnosis, and mortality rates were similar among vaccination strategies. Indeed, BRD vaccines are mostly expected to prevent and control the disease, but not hasten recovery in sick animals (Edwards, 2010). More importantly, BRD incidence has major consequences to performance and carcass traits in feedlot cattle, including growth and marbling score (Schneider et al., 2009). These relationships were not observed in this experiment, given that ADG, BW, and carcass characteristics were similar between treatments. Nonetheless, the BRD complex has major consequences to production efficiency, animal welfare, and economic viability in feedlot systems (Snowder et al., 2006). Strategies to mitigate this disease, including novel vaccination strategies as investigated herein, are warranted to optimize welfare and productivity of feedlot cattle in the United States and across the globe (Callan and Garry, 2002).
In conclusion, EARLY increased serum titers against BRD viruses at feedlot entry, and alleviated the incidence of BRD during the entire feeding period compared with CON and DELAYED. Differences in serum titers among treatments at feedlot entry should not be associated with increased vaccine response in EARLY calves, but with greater interval between vaccinations and feedlot entry. This experiment, however, was not designed to elucidate all immunological benefits of EARLY, whereas feedlot performance and carcass characteristics upon slaughter were not altered by treatments. Although the vaccines utilized herein are commonly administered to feeder cattle in commercial U.S. operations (USDA, 2011), altering the time of vaccination needs to be investigated using different BRD vaccines, including inactivated and adjuvant-containing vaccines against BHV-1, BVDV, PI3, and BRSV. Nevertheless, advancing the time of vaccination against BRD pathogens to provide both doses prior to feedlot entry appears to be a valid strategy to enhance immunocompetence and alleviate BRD in feedlot cattle.
Conflict of interest statement. None declared.
Footnotes
Financial support for this research was provided by USDA-NIFA Oregon (ORE00163). Appreciation is extended to Zoetis (Florham Park, NJ) for product donation. Alice P. Brandão is supported by CAPES, Brazil (#88881.128327/2016-01).
LITERATURE CITED
- Arthington J. D., R. F. Cooke T. D. Maddock D. B. Araujo P. Moriel N. Dilorenzo, and Lamb G. C.. 2013. Effects of vaccination on the acute-phase protein response and measures of performance in growing beef calves. J. Anim. Sci. 91:1831–1837. doi: 10.2527/jas.2012-5724 [DOI] [PubMed] [Google Scholar]
- Blecha F., S. L. Boyles, and Riley J. G.. 1984. Shipping suppresses lymphocyte blastogenic responses in Angus and Brahman x Angus feeder calves. J. Anim. Sci. 59:576–583. [DOI] [PubMed] [Google Scholar]
- Bolin S. R. and Ridpath J. F.. 1990. Range of viral neutralizing activity and molecular specificity of antibodies induced in cattle by inactivated bovine viral diarrhea virus vaccines. Am. J. Vet. Res. 51:703–707. [PubMed] [Google Scholar]
- Callan R. J. 2001. Fundamental considerations in developing vaccination protocols. Bovine Pract. 34:14–22. [Google Scholar]
- Callan R. J. and Garry F. B.. 2002. Biosecurity and bovine respiratory disease. Vet. Clin. North Am. Food Anim. Pract. 18:57–77. doi: 10.1016/S0749-0720(02)00004-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R. F. 2017. Nutritional and management considerations for beef cattle experiencing stress-induced inflammation. Prof. Anim. Sci. 33:1–11. doi: 10.15232/pas.2016-01573 [DOI] [Google Scholar]
- Cortese V. S. 2009. Neonatal immunology. Vet. Clin. North Am. Food Anim. Pract. 25:221–227. doi: 10.1016/j.cvfa.2008.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downey E. D., R. G. Tait M. S. Jr Mayes C. A. Park J. F. Ridpath D. J. Garrick, and Reecy J. M.. 2013. An evaluation of circulating bovine viral diarrhea virus type 2 maternal antibody level and response to vaccination in angus calves. J. Anim. Sci. 91:4440–4450. doi: 10.2527/jas.2012-5890 [DOI] [PubMed] [Google Scholar]
- Duff G. C. and Galyean M. L.. 2007. Board-invited review: recent advances in management of highly stressed, newly received feedlot cattle. J. Anim. Sci. 85:823–840. doi: 10.2527/jas.2006-501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards T. A. 2010. Control methods for bovine respiratory disease for feedlot cattle. Vet. Clin. North Am. Food Anim. Pract. 26:273–284. doi: 10.1016/j.cvfa.2010.03.005 [DOI] [PubMed] [Google Scholar]
- Ellis, J. A., L. E. Hassard, V. S. Cortese, and P. S. Morley. 1996. Effects of perinatal vaccination on humoral and cellular immune responses in cows and young calves. J. Vet. Med. Assoc. 208:393–400. [PubMed] [Google Scholar]
- England J. 2009. Vaccination and immunization: vaccination programs for cattle operations. 605 in Cattle Producer’s Handbook. 3rd ed. University of Idaho, Moscow: Agricultural Public Distribution. [Google Scholar]
- Fulton R. W., R. E. Briggs M. E. Payton A. W. Confer J. T. Saliki J. F. Ridpath L. J. Burge, and Duff G. C.. 2004. Maternally derived humoral immunity to bovine viral diarrhea virus (BVDV) 1a, BVDV1B, BVDV2, bovine herpesvirus-1, parainfluenza-3 virus bovine respiratory syncytial virus, Mannheimia haemolytica and Pasteurella multocida in beef calves, antibody decline by half-life studies and effect on response to vaccination. Vaccine 22:643–649. doi: 10.1016/j.vaccine.2003.08.033 [DOI] [PubMed] [Google Scholar]
- Ganskopp D. C., and Bohnert D. W.. 2009. Landscape nutritional patterns and cattle distribution in rangeland pastures. Appl. Anim. Behav. Sci. 116:110–119. doi: 10.1016/j.applanim.2008.10.006 [DOI] [Google Scholar]
- Howard C. J., M. C. Clarke, and Brownlie J.. 1989. Protection against respiratory infection with bovine virus diarrhoea virus by passively acquired antibody. Vet. Microbiol. 19:195–203. [DOI] [PubMed] [Google Scholar]
- Johnson R. W. 1997. Inhibition of growth by pro-inflammatory cytokines: an integrated view. J. Anim. Sci. 75:1244–1255. doi: 10.2527/1997.7551244x [DOI] [PubMed] [Google Scholar]
- Kirkpatrick J., Fulton R. W., Burge L. J., Dubois W. R., and Payton M.. 2001. Passively transferred immunity in newborn calves, rate of antibody decay, and effect on subsequent vaccination with modified live virus vaccine. Bov. Pract. 35:47–55. [Google Scholar]
- Kirkpatrick J. G., Step D. L., Payton M. E., Richards J. B., McTague L. F., Saliki J. T., Confer A. W., Cook B. J., Ingram S. H., and Wright J. C.. 2008. Effect of age at the time of vaccination on antibody titers and feedlot performance in beef calves. J. Am. Vet. Med. Assoc. 233:136–142. doi: 10.2460/javma.233.1.136 [DOI] [PubMed] [Google Scholar]
- Lippolis K. D., R. F. Cooke K. M. Schubach A. P. Brandão L. G. da Silva R. S. Marques, and Bohnert D. W.. 2016. Altering the time of vaccination against respiratory pathogens to enhance antibody response and performance of feeder cattle. J. Anim. Sci. 94:3987–3995. doi: 10.2527/jas.2016-0673 [DOI] [PubMed] [Google Scholar]
- Loza P. L., C. D. Buckner K. J. Vander Pol G. E. Erickson T. J. Klopfenstein, and Stock R. A.. 2010. Effect of feeding combinations of wet distillers grains and wet corn gluten feed to feedlot cattle. J. Anim. Sci. 88:1061–1072. doi: 10.2527/jas.2009-2190 [DOI] [PubMed] [Google Scholar]
- Marques R. S., Cooke R. F., Rodrigues M. C., Cappellozza B. I., Larson C. K., Moriel P., and Bohnert D. W.. 2016. Effects of organic or inorganic cobalt, copper, manganese, and zinc supplementation to late-gestating beef cows on productive and physiological responses of the offspring. J. Anim. Sci. 94:1215–1226. doi: 10.2527/jas.2015-0036 [DOI] [PubMed] [Google Scholar]
- Martin S. W., E. Nagy D. Armstrong, and Rosendal S.. 1999. The associations of viral and mycoplasmal antibody titers with respiratory disease and weight gain in feedlot calves. Can. Vet. J. 40:560–7, 570. [PMC free article] [PubMed] [Google Scholar]
- NASS 2006. National Agricultural Statistics Service, Agricultural Statistics Board. Washington, DC:USDA. [Google Scholar]
- NRC 1996. Nutrient Requirements of Beef Cattle. 7th ed. Washington, DC:National Academic Press. [Google Scholar]
- Richeson J. T., Beck P. A., Gadberry M. S., Gunter S. A., Hess T. W., Hubbell D. S., and Jones C.. 2008. Effects of on-arrival versus delayed modified-live virus vaccination on health, performance, and serum infectious bovine rhinotracheitis titers of newly-received beef calves. J. Anim. Sci. 86:999–1005. doi: 10.2527/jas.2007-0593 [DOI] [PubMed] [Google Scholar]
- Richeson J. T., Kegley E. B., Gadberry M. S., Beck P. A., Powell J. G., and Jones C. A.. 2009. Effects of on-arrival versus delayed clostridial or modified live respiratory vaccinations on health, performance, bovine viral diarrhea virus type I titers, and stress and immune measures of newly received beef calves. J. Anim. Sci. 87:2409–2418. doi: 10.2527/jas.2008-1484 [DOI] [PubMed] [Google Scholar]
- Rodrigues M. C., Cooke R. F., Marques R. S., Cappellozza B. I., Arispe S. A., Keisler D. H., and Bohnert D. W.. 2015. Effects of vaccination against respiratory pathogens on feed intake, metabolic and inflammatory responses in beef heifers. J. Anim. Sci. 93:4443–4452. doi: 10.2527/jas.2015-9277 [DOI] [PubMed] [Google Scholar]
- Schneider M. J., R. G. Tait W. D. Jr Busby, and Reecy J. M.. 2009. An evaluation of bovine respiratory disease complex in feedlot cattle: impact on performance and carcass traits using treatment records and lung lesion scores. J. Anim. Sci. 87:1821–1827. doi: 10.2527/jas.2008-1283 [DOI] [PubMed] [Google Scholar]
- Snowder G. D., L. D. Van Vleck L. V. Cundiff, and Bennett G. L.. 2006. Bovine respiratory disease in feedlot cattle: environmental, genetic, and economic factors. J. Anim. Sci. 84:1999–2008. doi: 10.2527/jas.2006-046 [DOI] [PubMed] [Google Scholar]
- Sousa O. A., Cooke R. F., Brandão A. P., Schubach K. M., Schumaher T. F., Bohnert D. W., and Marques R. S.. 2018. Productive and physiological responses of feeder cattle supplemented with Yucca schidigera extract during feedlot receiving. J. Anim. Sci. 10.1093/jas/sky412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tizard I. R. 2004. Vaccines and their production. In: Merchant, T., editor. Veterinary immunology. 7th ed. Philadelphia, PA:Elsevier; p. 247–259. [Google Scholar]
- USDA 2010. Beef 2007–08, Part IV: reference of beef cow-calf management practices in the United States, 2007–08. USDA APHIS:VS, CEAH. Fort Collins, CO #523.0210. https://www.aphis.usda.gov/animal_health/nahms/beefcowcalf/downloads/beef0708/Beef0708_dr_PartIV.pdf (accessed 5 October 2018). [Google Scholar]
- USDA. 2011. Feedlot 2011, Part IV: Health and health management on US feedlots with a capacity of 1000 of more head. NAHMS Feedlot Studies: 1–100. [Google Scholar]