Abstract
This study was conducted to investigate the effects of methionine restriction (MR) on growth performance, insulin sensitivity, and hepatic and muscle glucose metabolism in intrauterine growth retardation (IUGR) pigs at 49 and 105 d of age. At weaning (day 21), 30 female normal birth weight (NBW) piglets were fed control diets with adequate methionine (NBW-CON), whereas 60 female IUGR piglets were fed either the control diets (IUGR-CON) or MR diets which were 30% reduced in methionine (IUGR-MR) (n = 6 replicates (pens) with five piglets per replicate). At 49 and 105 d of age, one pig with a BW near to the mean of each replication was selected for biochemical analysis. Compared with NBW-CON pigs, IUGR-CON pigs exhibited lower relative daily gain (RDG) and homeostasis model assessment of insulin resistance (HOMA-IR) index at day 49 (P < 0.05), but higher RDG and HOMA-IR index at day 105 (P < 0.05). Hepatic phosphoenolpyruvate carboxykinase and glucose-6-phosphatase (G6Pase) activities were higher in IUGR-CON than NBW-CON pigs at both days 49 and 105 (P < 0.05), while hepatic glycogen synthase and glycogen phosphorylase activities were lower in IUGR-CON pigs at both two ages (P < 0.05). In addition, compared with NBW-CON pigs, IUGR-CON pigs (105-d old) had lower protein kinase B phosphorylation (PKB/Akt) in liver (P < 0.05), but not in muscle (P > 0.05). Compared with IUGR-CON pigs, IUGR-MR pigs had lower RDG at day 49, less blood glucose at day 105, and lower HOMA-IR index at both days 49 and 105 (P < 0.05). Additionally, compared with IUGR-CON pigs, MR decreased IUGR-MR pigs’ hepatic G6Pase activities and increased their hepatic glycogen contents at day 105 (P < 0.05), as well as increased their hepatic and muscle PKB/Akt phosphorylation (P < 0.05). In conclusion, the ability of dietary MR to restrict IUGR pigs’ growth and to reduce blood glucose appeared, respectively, in earlier and later period, but MR improved IUGR pigs’ insulin sensitivity at both days 49 and 105.
Keywords: glucose metabolism, insulin sensitivity, intrauterine growth retardation, methionine restriction, postnatal growth, young pig
INTRODUCTION
Intrauterine growth retardation (IUGR) is usually defined as a failure of the fetus to achieve the intrinsic growth potential (Rosenberg, 2008), affecting approximately 7% to 15% of all newborns in human (Saleem et al., 2011) and 15% to 20% in pig production (Quiniou et al., 2002; Su et al., 2007). Individuals born with IUGR are at high risks of chronic metabolic diseases because of changes in the structures and functions of important metabolic organs during fetal period (Barker, 2012). A great number of human epidemiological investigations and animals researches have demonstrated that IUGR individuals are prone to the development of type 2 diabetes mellitus (T2DM), which was associated with IUGR-induced insulin resistance, increased hepatic glucose production, and reduced hepatic and/or muscle glycogen synthesis (Simmons et al., 2001; Selak et al., 2003; Martin-Gronert and Ozanne, 2007; Whincup et al., 2008; Li et al., 2010). Additionally, it has been found that the catch-up growth occurring in postnatal life was in a close relation with the progress of insulin resistance and glucose metabolic disorder in IUGR individuals (Simmons et al., 2001; Morrison et al., 2010). On the contrary, inhibition of postnatal catch-up could help to attenuate IUGR-induced adverse effects on insulin sensitivity and metabolic activities (Singhal et al., 2003; Lim et al., 2011).
Dietary methionine restriction (MR) is a nutritional intervention technique, which decreases methionine content in diets. Besides slowing aging and improving metabolic health in normal birth weight (NBW) animals (Ables et al., 2012; Lees et al., 2014; Stone et al., 2014), our previous study has also showed that MR decreased BWs, improved insulin sensitivity, and reversed hyperglycemia in 180-d-old IUGR pigs, which suggested that dietary MR may be beneficial to lower IUGR-induced high risk of T2DM (Ying et al., 2017). Early postnatal stage is a key window where the IUGR-induced programming can be reversed (Vickers et al., 2005; Hochberg et al., 2011), so it is worth further research about the effects of MR on young IUGR pigs, especially at different ages. Therefore, this study was performed to investigate the effects of MR on growth, blood glucose concentration, insulin sensitivity, and hepatic and muscle glucose metabolism in IUGR pigs at 49 and 105 d of age, which can also investigate potential different effects at these two ages. Because the high similarity of anatomy between pigs and human and the suitability of using pigs as an animal model for human T2DM (Bellinger et al., 2006), this study may provide some information for relevant research in human.
METHODS AND MATERIALS
Animals and Treatments
During the preparation, healthy pregnant sows with similar expected date of confinement (<3 d) and parity (second and third) were preselected and fed a commercial diet according to professional production standard. At farrowing, approximately 90 sows that had similar litter sizes (11 to 13 piglets) and IUGR offspring were further selected. The birth weight and sex of each piglet (Landrace × Yorkshire) were recorded carefully. In each litter, one female NBW piglet (~1.52 kg) and two same-sex naturally occurring IUGR littermates (~0.87 kg) were preselected according to their birth weights (D’inca et al., 2010); specifically, a newborn piglet with a birth weight near the mean (within 0.5 SD) was defined as normal NBW piglet, whereas a 2 SD lower birth weight was identified as IUGR piglet. At weaning (21 d of age), 30 female NBW piglets were randomly selected and fed control diets, whereas their IUGR littermates were randomly assigned to the control diets or MR diets. Thereafter, 30 female NBW piglets and 60 same-sex IUGR piglets were allocated to three groups: NBW-CON group (~6.55 kg), IUGR-CON group (~4.85 kg), and IUGR-MR (~4.84 kg); each group consisted of six replicates (pens) with five piglets per replicate. The control diets were formulated with adequate methionine according to the NRC (NRC, 2012), and the MR diets were 30% reduced in methionine. The two kinds of diets were isonitrogenous by adjusting with l-alanine. The composition and nutrient levels of the diets are given in Supplementary Table 1. Feed and water were provided ad libitum. The BW and feed consumption of pigs were recorded approximately every 2 wk on the basis of pen (including those at 49 and 105 d of age). Then the relative daily gain (RDG; BW gain (g) · day (d)−1· starting BW (kg)−1), relative daily feed intake (RDFI; feed consumption (g) · day (d)−1· mean BW (kg)−1), and G:F (BW gain (g) · feed consumption (g)−1) were calculated. All experiments were approved by the Institutional Animal Care and Use Committee of Nanjing Agricultural University (NJAU-CAST-2015-098).
Sample Collection
At 49 and 105 d of age, after overnight fasting, one pig with a BW near to the mean of each replication was selected. Then approximately 10 mL heparinized blood samples were taken by jugular venepuncture, and the pigs were sacrificed by intramuscular injection of sodium pentobarbital (50 mg/kg BW). Liver tissues (left lobe) and skeletal muscle samples (left semitendinosus) were immediately collected and stored in liquid nitrogen for future biochemical assay. Plasma was obtained by centrifuging at 2,000 × g for 10 min at 4 °C and stored at −80 °C until biochemical assay.
Determinations of Plasma Hormones and Glucose Concentrations
Plasma glucose concentration was determined by using a commercial kit (361500; Rongsheng Biotechnology Company, Shanghai, China). Plasma insulin and glucagon concentrations were measured by using Insulin RIA Kit and Glucagon RIA Kit (Beijing North Institute of Biological Technology, Beijing, China). Plasma IGF-1 was determined by using an enzyme-linked immunosorbent kit (CSBE06829p; Cusabio Biotech Company, Wuhan, China). Insulin sensitivity was evaluated by using the homeostasis model assessment of insulin resistance (HOMA-IR = [fasting glucose (mmol/L) × fasting insulin (μU/mL)]/22.5) (Matthews et al., 1985).
Assessment of Hepatic and Muscle Glucose Metabolism
The activities of phosphoenolpyruvate carboxykinase (PEPCK), glycogen synthase (GYS), and glycogen phosphorylase (GYP) in liver, and muscle GYS activity were measured by using commercial kits based on colorimetric method. Kits for PEPCK (PEPCK-1-Y), GYS (GCS-1-Y), and GYP (GPA-1-Y) were purchased from Suzhou Comin Biotechnology Company, Suzhou, China).
Hepatic and muscle glycogen contents were measured by using commercial kits according to the anthracenone method (A043; Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Hepatic glucose-6-phosphatase (G6Pase) activity was assayed according to a previous method (Jia et al., 2012) with slight modifications. Liver tissues were homogenized (1:9, w/v) in 0.25 mol/L sucrose solution, and supernatant were obtained by centrifuged at 20,000 × g for 10 min at 4 °C. For determination of enzyme activity, the supernatant was added into an assay mixture (26.5 mmol/L glucose-6-phosphate and 1.8 mmol/L EDTA) and incubated at 37 °C for 10 min, and then the reaction was quenched by a final concentration of 4% perchloric acid. After that, the inorganic phosphate content in the supernatant was measured using a Pi detection kit (C006-3; Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The G6Pase activity was calculated as release rate of inorganic phosphate (nmol·min−1·mg prot−1).
Additionally, total protein concentrations used in the calculation of enzymes’ activities were determined by commercial kits based on BCA method (A045-3; Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Assay of Hepatic and Muscle mRNA Expressions
Total RNA was isolated from approximately 50 mg snap-frozen samples (9108; Takara Biotechnology, Japan) and reverse-transcribed into complementary DNA (RR037A; Takara Biotechnology, Dalian, China) by using commercial kits. Real-time PCR was carried out on a QuantStudio 5 real-time PCR system (Applied Biosystems, Foster City, USA). The SYBR Green PCR assay system was 20 µL in total, consisted of 10 µL SYBR Premix Ex Taq, 0.4 µL of the forward and reverse primers, 0.4 µL of ROX reference dye, 6.8 µL of double-distilled H2O, and 2 µL of cDNA template. The reaction conditions were as follows: 30 s at 95 °C, 40 cycles of 5 s at 95 °C, and 30 s at 60 °C. The relative mRNA expression was calculated using the 2−ΔΔCt method after normalization to GAPDH (Pfaffl, 2001). The values of NBW-CON group were used as a calibrator. The primer sequences are shown in Supplementary Table 2.
Assay of Hepatic and Muscle Protein Kinase B (PKB/Akt) Phosphorylation Levels
The procedure of western blot was conducted according to our previous study (Ying et al., 2017). Proteins were extracted from approximately 50 mg tissues by grinding in RIPA lysis containing protease and phosphatase inhibitors. Equal amounts of protein (70 μg/lane) were separated by SDS–PAGE and then transferred to activated polyvinylidene difluoride membranes. The membranes were blocked using 5% bovine serum albumin dissolved in TBST (TBST; 0.1 % Tween-20, 100 mM Tris–HCl, and 150 mM NaCl, pH 8.0). Primary antibodies for p-PKB/Akt (ser473) (#9271; Cell Signaling Technology, Boston) and total PKB/Akt (#9272; Cell Signaling Technology), and goat-anti-rabbit secondary antibody were purchased from Cell Signaling Technology. The usage of antibodies was according to productions’ instruction. The blots were developed using a chemiluminescence kit (Millipore Corporation, Billerica), and the visualized bands were obtained by a Luminescent Image Analyzer LAS-4000 system (Fujifilm Company, Tokyo, Japan). After that, the intensity of the bands was quantified by Gel-Pro Analyzer 4.0 software (Media Cybernetics, Rockville).
Statistical Analysis
Data were analyzed by SPSS 20.0 statistical software (SPSS, Chicago) and presented as means ± SEs. After analysis of homogeneity test, statistical differences between groups were determined via one-way ANOVA analysis and Tukey’s post hoc test for multiple comparisons. Differences were considered statistically significant at P < 0.05.
RESULTS
Growth Performance
From 21 to 49 d of age, IUGR decreased (P < 0.05) the RDG and G:F of IUGR-CON group when compared with those of NBW-CON group (Table 1), but did not cause significant changes in RDFI (P > 0.05). Compared with IUGR-CON group, dietary MR decreased IUGR-MR group’ RDG and G:F (P < 0.05), but also did not affect RDFI (P > 0.05).
Table 1.
Effects of dietary methionine restriction on the growth performance of intrauterine growth retardation pigs at 49 and 105 d of age
| Item1 | NBW-CON | IUGR-CON | IUGR-MR |
|---|---|---|---|
| Days 21 to 492 | |||
| RDG, g·d−1·kg−1 | 58.17 ± 1.38a | 51.31 ± 1.30b | 42.65 ± 1.43c |
| RDFI, g·d−1·kg−1 | 47.83 ± 1.18b | 50.25 ± 1.21ab | 53.07 ± 1.61a |
| G:F | 0.67 ± 0.02a | 0.60 ± 0.02b | 0.50 ± 0.01c |
| Days 50 to 1053 | |||
| RDG, g·d−1·kg−1 | 32.99 ± 1.53b | 44.48 ± 1.59a | 45.64 ± 1.68a |
| RDFI, g·d−1·kg−1 | 38.04 ± 0.48b | 44.65 ± 1.22a | 45.59 ± 0.67a |
| G:F | 0.45 ± 0.01 | 0.44 ± 0.01 | 0.44 ± 0.01 |
a–cWithin a row, means with different superscript letters differ, P < 0.05.
1NBW-CON = normal birth weight pigs fed control diets; IUGR-CON = intrauterine growth restriction pigs fed control diets; IUGR-MR = intrauterine growth restriction pigs fed methionine restriction diets; RDG = relative daily gain (BW gain (g)· day (d)−1 · starting BW (kg)−1); RDFI = relative daily feed intake (feed consumption (g) · day (d)−1 · mean BW (kg)−1); G:F = feed efficiency (BW gain (g) · feed consumption (g)−1).
2Results are presented as mean ± SE (n = 6 replicates (pens) with five piglets per replicate).
3Results are presented as mean ± SE (n = 6 replicates (pens) with four piglets per replicate).
From 50 to 105 d of age, IUGR increased (P < 0.05) RDG and RDFI in IUGR-CON group when compared with NBW-CON group, but had no significant effects on G:F (P > 0.05). Compared with IUGR-CON group, MR did not significantly affect these three parameters in IUGR-MR group (P > 0.05).
Plasma Glucose and Hormone Concentrations
At 49 d of age, the levels of plasma insulin, glucagon, IGF-1, and HOMA-IR index were lower in IUGR-CON group than NBW-CON group (P < 0.05), whereas there were no significant differences in plasma glucose concentrations between the two groups (P > 0.05) (Table 2). Compared with IUGR-CON group, IUGR-MR group had a decreased plasma IGF-1 concentration and lower HOMA-IR index (P < 0.05), but similar plasma glucose, insulin, and glucagon concentrations (P > 0.05).
Table 2.
Effects of dietary methionine restriction on the plasma glucose and hormone levels of intrauterine growth retardation pigs at 49 and 105 d of age1
| Item2 | NBW-CON | IUGR-CON | IUGR-MR |
|---|---|---|---|
| Day 49 | |||
| Glucose, mmol/L | 7.68 ± 0.70 | 6.72 ± 0.64 | 6.07 ± 0.48 |
| Insulin, pmol/L | 169.47 ± 13.09a | 119.19 ± 9.49b | 83.63 ± 13.22b |
| Glucagon, pg/mL | 236.27 ± 12.97a | 178.96 ± 16.02b | 149.88 ± 13.87b |
| IGF-1, ng/mL | 68.72 ± 2.92a | 53.62 ± 2.90b | 39.34 ± 3.39c |
| HOMA-IR | 8.06 ± 0.36a | 5.06 ± 0.48b | 3.21 ± 0.60c |
| Day 105 | |||
| Glucose, mmol/L | 5.09 ± 0.52ab | 6.47 ± 0.45a | 4.43 ± 0.32b |
| Insulin, pmol/L | 116.69 ± 9.56ab | 152.65 ± 13.52a | 107.20 ± 10.52b |
| Glucagon, pg/mL | 135.61 ± 16.05 | 150.11 ± 18.88 | 124.83 ± 12.85 |
| IGF-1, ng/mL | 30.98 ± 3.14b | 44.96 ± 3.08a | 32.49 ± 4.52b |
| HOMA-IR | 3.71 ± 0.36b | 6.27 ± 0.68a | 3.10 ± 0.52b |
a–cWithin a row, means with different superscript letters differ, P < 0.05.
1Results are presented as mean ± SE (n = 6).
2NBW-CON = normal birth weight pigs fed control diets; IUGR-CON = intrauterine growth restriction pigs fed control diets; IUGR-MR = intrauterine growth restriction pigs fed methionine restriction diets; IGF-1 = insulin-like growth factor 1; HOMA-IR = homeostasis model assessment of insulin resistance.
At 105 d of age, IUGR dramatically elevated IUGR-CON group’s plasma IGF-1 concentration and the HOMA-IR index when compared with NBW-CON group (P < 0.05), but did not cause significant changes in plasma glucose, insulin, and glucagon concentrations (P > 0.05). Compared with IUGR-CON group, MR decreased IUGR-MR group’s plasma glucose, insulin, IGF-1, and HOMA-IR levels (P < 0.05), but did not influence plasma glucagon concentrations (P > 0.05).
Hepatic Gluconeogenic Enzyme Activities
At both 49 and 105 d of age, the activities of hepatic PEPCK and G6Pase were greater in IUGR-CON group than NBW-CON group (P < 0.05) (Table 3). MR alleviated IUGR-induced increases in the G6Paes activity at 105 d of age (P < 0.05), but did not affect PEPCK activity at 105 d of age and G6Pase activity at 49 and 105 d of age (P > 0.05).
Table 3.
Effects of dietary methionine restriction on the hepatic gluconeogenic enzyme activities of intrauterine growth retardation pigs at 49 and 105 d of age1
| Item2 | NBW-CON | IUGR-CON | IUGR-MR |
|---|---|---|---|
| Day 49 | |||
| PEPCK, nmol·min−1·mg prot−1 | 28.03 ± 1.62b | 48.02 ± 1.92a | 43.87 ± 1.34a |
| G6Pase, nmol·min−1·mg prot−1 | 154.63 ± 47.47b | 257.43 ± 33.39a | 201.58 ± 44.04ab |
| Day 105 | |||
| PEPCK, nmol·min−1·mg prot−1 | 20.16 ± 0.79b | 25.15 ± 1.22a | 22.45 ± 1.25ab |
| G6Pase, nmol·min−1·mg prot−1 | 118.95 ± 15.26b | 236.85 ± 25.77a | 158.69 ± 18.17b |
a,bWithin a row, means with different superscript letters differ, P < 0.05.
1Results are presented as mean ± SE (n = 6).
2NBW-CON = normal birth weight pigs fed control diets; IUGR-CON = intrauterine growth restriction pigs fed control diets; IUGR-MR = intrauterine growth restriction pigs fed methionine restriction diets; PEPCK = phosphoenolpyruvate carboxykinase; G6Pase = glucose-6-phosphatase.
Hepatic Glycogen Metabolism
At 49 d of age, IUGR-CON pigs exhibited decreased hepatic GYS and GYP activities (P < 0.05), but similar hepatic glycogen contents (P > 0.05) when compared with NBW-CON pigs (Table 4). Compared with IUGR-CON pigs, MR increased IUGR-MR pigs’ hepatic GYS activities (P < 0.05), but did not affected their hepatic glycogen contents and GYP activities (P > 0.05).
Table 4.
Effects of dietary methionine restriction on the hepatic glycogen metabolism of intrauterine growth retardation pigs at 49 and 105 d of age1
| Item2 | NBW-CON | IUGR-CON | IUGR-MR |
|---|---|---|---|
| Day 49 | |||
| Glycogen, mg/g tissue | 15.84 ± 3.53 | 14.49 ± 3.57 | 16.93 ± 3.39 |
| GYS, nmol·min−1·mg prot−1 | 23.31 ± 3.94a | 17.43 ± 3.99b | 25.93 ± 2.82a |
| GYP, nmol·min−1·mg prot−1 | 9.27 ± 0.83a | 6.39 ± 0.58b | 6.23 ± 0.79b |
| Day 105 | |||
| Glycogen, mg/g tissue | 8.23 ± 0.82ab | 7.12 ± 0.75b | 10.53 ± 0.87a |
| GYS, nmol·min−1·mg prot−1 | 16.61 ± 1.18b | 14.36 ± 0.97b | 22.63 ± 1.46a |
| GYP, nmol·min−1·mg prot−1 | 16.73 ± 0.90a | 11.75 ± 0.76b | 14.14 ± 0.47b |
a,bWithin a row, means with different superscript letters differ, P < 0.05.
1Results are presented as mean ± SE (n = 6).
2NBW-CON = normal birth weight pigs fed control diets; IUGR-CON = intrauterine growth restriction pigs fed control diets; IUGR-MR = intrauterine growth restriction pigs fed methionine restriction diets; GYS = glycogen synthase; GYP = glycogen phosphorylase.
At 105 d of age, IUGR-CON pigs had decreased hepatic GYP activities (P < 0.05), but similar hepatic glycogen contents and GYS activities (P > 0.05) when compared with NBW-CON pigs. Compared with IUGR-CON pigs, MR elevated IUGR-MR pigs’ hepatic glycogen contents and GYS activities (P < 0.05) without affecting hepatic GYP activities (P > 0.05).
Muscle Glycogen Synthesis
At 49 d of age, IUGR-CON pigs had lower muscle glycogen contents and GYS activities (P < 0.05) when compared with NBW-CON pigs (Table 5). MR enhanced the muscle GYS activities (P < 0.05) but did not affect the muscle glycogen contents (P > 0.05) of IUGR-MR pigs when compared with those of IUGR-CON pigs.
Table 5.
Effects of dietary methionine restriction on the muscle glycogen synthesis of intrauterine growth retardation pigs at 49 and 105 d of age1
| Item2 | NBW-CON | IUGR-CON | IUGR-MR |
|---|---|---|---|
| Day 49 | |||
| Glycogen, mg/g tissue | 4.50 ± 0.35a | 3.04 ± 0.33b | 3.54 ± 0.44ab |
| GYS, nmol·min−1·mg prot−1 | 92.48 ± 7.66a | 46.45 ± 5.66c | 71.31 ± 4.51b |
| Day 105 | |||
| Glycogen, mg/g tissue | 1.75 ± 0.18 | 1.94 ± 0.21 | 1.62 ± 0.20 |
| GYS, nmol·min−1·mg prot−1 | 67.56 ± 3.58 | 70.56 ± 4.42 | 55.76 ± 5.43 |
a–cWithin a row, means with different superscript letters differ, P < 0.05.
1Results are presented as mean ± SE (n = 6).
2NBW-CON = normal birth weight pigs fed control diets; IUGR-CON = intrauterine growth restriction pigs fed control diets; IUGR-MR = intrauterine growth restriction pigs fed methionine restriction diets; GYS = glycogen synthase.
At 105 d of age, both muscle glycogen contents and muscle GYS activities were similar between the three groups (P > 0.05).
Hepatic and Muscle mRNA Expressions
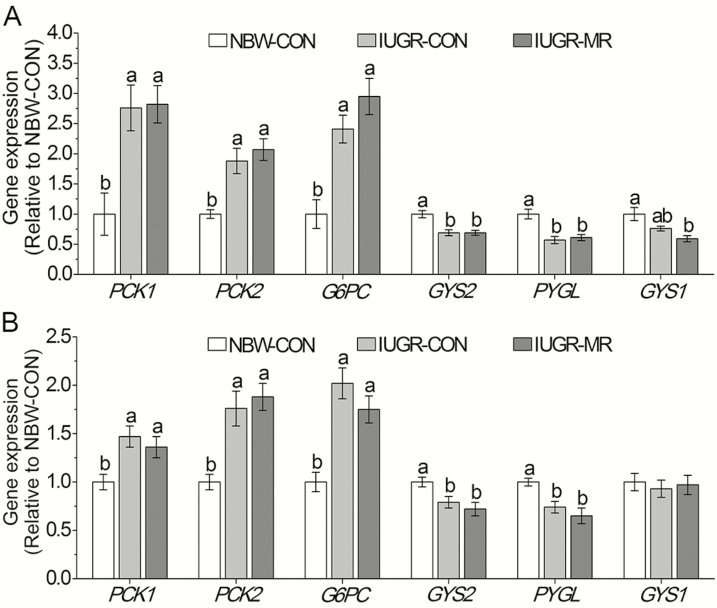
At both 49 and 105 d of age, IUGR-CON pigs had greater mRNA expressions (P < 0.05) of hepatic phosphoenolpyruvate carboxykinase 1 (PCK1), phosphoenolpyruvate carboxykinase 2 (PCK2), and glucose-6-phosphatase catalytic subunit (G6PC), but lower mRNA expressions (P < 0.05) of hepatic GYS 2 and GYP, as well as a similar muscle GYS1 mRNA expression (P > 0.05) when compared with NBW-CON pigs (Figure 1). MR had no effects on these mRNA expressions when IUGR-CON pigs and IUGR-MR pigs were compared (P > 0.05).
Figure 1.
Effects of dietary methionine restriction on the hepatic and muscle mRNA expressions of intrauterine growth retardation pigs at 49 (A) and 105 (B) d of age. The column and its bar represented mean and SE (n = 6), respectively. Means without a common letter differ, P < 0.05. NBW-CON = normal birth weight pigs fed control diets; IUGR-CON = intrauterine growth restriction pigs fed control diets; IUGR-MR = intrauterine growth restriction pigs fed methionine restriction diets; PCK1 = phosphoenolpyruvate carboxykinase 1; PCK2 = phosphoenolpyruvate carboxykinase 2; G6PC = glucose-6-phosphatase catalytic subunit; GYS2 = glycogen synthase 2 (liver); PYGL = liver glycogen phosphorylase; GYS2 = glycogen synthase 1 (muscle).
Hepatic and Muscle PKB/Akt Phosphorylation Levels
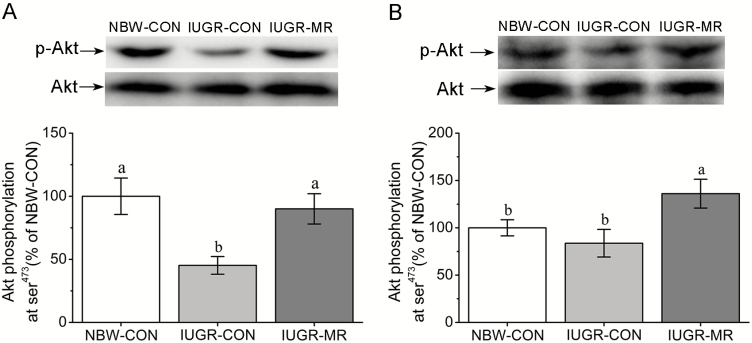
As shown in Figure 2, IUGR-CON pigs had lower PKB/Akt (ser473) phosphorylation levels in liver (P < 0.05), but not in muscle (P > 0.05) when compared with NBW-CON pigs. MR elevated hepatic and muscle PKB/Akt (ser473) phosphorylation levels when IUGR-CON pigs and IUGR-MR pigs were compared (P < 0.05).
Figure 2.
Effects of dietary methionine restriction on the phosphorylation levels of hepatic (A) and muscle (B) protein kinase B (PKB/Akt) of intrauterine growth retardation pigs at 105 d of age. The column and its bar represented mean and SE (n = 6), respectively. Means without a common letter differ, P < 0.05. NBW-CON = normal birth weight pigs fed control diets; IUGR-CON = intrauterine growth restriction pigs fed control diets; IUGR-MR = intrauterine growth restriction pigs fed methionine restriction diets.
DISCUSSION
In the present study, IUGR decreased IUGR-CON pigs’ growth rates during the period from 21 to 49 d of age, but caused growth rates’ increases from 50 to 105 d of age, reflected by the data of RDG. G:F is indicator reflecting the capacity of utilizing nutrients for body growth. Su et al.’s study showed that IUGR decreased 49-d-old pigs’ G:F by impairing their intestinal structure and function, which consequently reduced these pigs’ growth rates (Su et al., 2017). Therefore, in our study, the decreased G:F of IUGR-CON pigs from days 21 to 49 may be responsible for the reduced growth rates during this period and the recovered G:F together with the increased feed intake (reflected by increased RDFI) of IUGR-CON pigs from days 50 to 105 were important reasons for the increased growth rates of these elder IUGR-CON pigs. In parallel with the contradictory changes in IUGR-CON pigs’ growth rates at different ages, IUGR-CON pigs’ also exhibited a decreased plasma IGF-1 level at 49 d of age, but an increased one at 105 d of age. IGF-1 is an important hormone, which is closely related with pre- and postnatal growth (Cianfarani et al., 2006). Previous studies showed that IUGR lowered fetal arterial IGF-1 levels (Thorn et al., 2009) and that postnatal catch-up growth was associated with increased blood IGF-1 (Özkan et al., 1999; Cianfarani et al., 2002). Therefore, it was likely that the decreased IGF-1 of 49-d-old IUGR-CON pigs derived from fetal defects and that the increased IGF-1 of 105-d-old IUGR-CON pigs was associated with accelerated growth of these pigs. However, further researches are needed to investigate the mechanism for the relation between increased IGF-1 and accelerated growth in IUGR individuals. Similarly, the inconsistent changes in the plasma insulin and glucagon concentrations between 49- and 105-d-old IUGR-CON pigs may be also related with their changed growth rates.
Blood glucose homeostasis is a result of an intricate balance, which is mainly controlled by exogenous glucose uptake, endogenous glucose production, and glucose removal from the blood. Hepatic glucose production (gluconeogenesis and glycogen hydrolysis) and hepatic and muscle glycogen storage are the important pathways for endogenous glucose production and blood glucose removal, respectively (Sharabi et al., 2015). Our previous study found that the combination of increased hepatic gluconeogenesis (reflected by increased gluconeogenic enzymes’ activities) and decreased muscle glycogen contents resulted in hyperglycemia in 180-d-old IUGR pigs (Ying et al., 2017). However, in the present study, the same combination found in 49-d-old IUGR-CON pigs did not affect blood glucose concentrations. These contradictory findings made us further notice the decreased G:F of the 49-d-old IUGR-CON pigs, which may suggest reduced absorption of exogenous nutrients (i.e., glucose). Therefore, the reason why the 49-d-old IUGR-CON pigs could maintain normal blood glucose concentrations despite of increased hepatic gluconeogenesis and decreased muscle glycogen may be related to the reduced absorption of exogenous glucose, and such reduced absorption of exogenous glucose could also partly explain why muscle glycogen was only reduced in the 49-d-old IUGR-CON pigs, but not in 105-d-old IUGR-CON pigs. In addition, blood glucose concentrations of IUGR-CON pigs were also unchanged at 105 d of age. Such age-related changes in blood glucose concentrations were also found in Simmons et al. (2001) study and Poore and Fowden (2002) study where IUGR animals only developed hyperglycemia at old ages but not at young ages.
Despite the effects of IUGR on IUGR-CON pigs’ the growth rates and blood glucose concentrations were different between days 49 and 105, IUGR consistently increased these pigs’ hepatic PEPCK and G6Pase activities at the two ages. Such increased hepatic PEPCK and G6Pase activities were also found in newborn IUGR pigs (Jia et al., 2012) and adult IUGR pigs (Ying et al., 2017) and rats (Nyirenda et al., 1998). Therefore, all these results suggested that IUGR-induced effects on hepatic gluconeogenic enzymes’ activities were consistent irrespective of different ages. Real-time PCR data showed the mRNA expressions of PEPCK-encoding gene (i.e., PCK1 and PCK2) and G6Pase-encoding gene (i.e., G6PC) were increased in the IUGR-CON pigs of both ages, which may be, respectively, responsible for the increased PEPCK and G6Pase activities of these pigs. Insulin-induced down-regulation of gene expression is a major pathway to reduce hepatic gluconeogenic enzymes’ amount and activities, in which PKB/Akt phosphorylation is a key step (Barthel and Schmoll, 2003). Although this study only measured the PKB/Akt phosphorylation at day 105, the found decreases in the hepatic PKB/Akt phosphorylation of IUGR-CON pigs could partly explain the up-regulated mRNA expressions of the hepatic PCK1, PCK2, and G6PC in these pigs, which were also previously proved in 180-d-old IUGR pigs (Ying et al., 2017). Interestingly, although IUGR decreased IUGR-CON pigs’ hepatic PKB/Akt phosphorylation, it did not change these pigs’ muscle PKB/Akt phosphorylation levels, which may be because that liver was more susceptible to insulin resistance than skeletal muscle (Kraegen et al., 1991).
Besides the age-related changes in the growth rates and blood glucose concentrations of IUGR-CON pigs, IUGR even caused completely opposite effects on insulin sensitivity between 49- and 105-d-old IUGR-CON pigs, reflected by data of HOMA-IR index (Matthews et al., 1985); namely, the insulin sensitivity of IUGR-CON pigs was improved at 49 d of age but decreased at 105 d of age. During intrauterine period, IUGR fetal had less access to nutrients and therefore they developed selective adaptations, including enhanced insulin sensitivity to achieve a greater nutrient storage (Thorn et al., 2009). Therefore, we presumed that this selective adaptation could persist until early postnatal stage and resulted in the improved insulin sensitivity of IUGR-CON pigs at 49 d of age; however, with age, catch-up growth and excessive feed intake, IUGR-CON pigs eventually turned from insulin hypersensitivity to insulin resistance in late life.
Similar with the effects of MR on NBW animals (Ables et al., 2012; Stone et al., 2014), MR decreased the RDG and G:F of IUGR-CON pigs during the period from 21 to 49 d of age, which may be associated with increased energy expenditure caused by MR (Malloy et al., 2006). However, such an ability of MR to inhibit IUGR pigs’ growth disappeared during the period from 50 to 105 d and the period from 106 to 180 d (RDG of IUGR-CON pigs: 17.27 ± 0.79 vs. RDG of IUGR-MR pigs: 17.91 ± 0.57; P > 0.05; unpublished) of age. Because the period from 50 to 105 d and the period from 106 to 180 d (RDG of NBW-CON pigs: 13.72 ± 0.38 vs. RDG of IUGR-CON pigs: 17.27 ± 0.79; P < 0.05; unpublished) of age were the times when IUGR-CON pigs developed postnatal catch-up growth, it was likely that the inhibition of MR in the postnatal growth of IUGR pigs was eliminated by the effect of catch-up growth.
In the present study, MR decreased IUGR-MR pigs’ blood glucose concentrations at 105 d of age, but did not affect this parameter at day 49. The decreased blood glucose of 105-d-old IUGR-MR pigs may be associated with the increased hepatic glycogen contents of these pigs, which may be caused by the enhanced hepatic GYS activity, because GYS is a rate-limiting enzyme of glycogen synthesis. Insulin signal is an important regulator of GYS activity, in which PKB/Akt phosphorylation is a pivotal trigger (Barthel and Schmoll, 2003). Therefore, the increased PKB/Akt phosphorylation of the 105-d-old IUGR-MR pigs may be the main reason for the increased hepatic GYS activities of these pigs when the hepatic GYS-encoding gene (i.e., GYS 2) was not affected by MR. In addition to the increased hepatic glycogen contents of the 105-d-old IUGR-MR pigs, the decreased hepatic G6Pase activity may also play a role in decreasing the blood glucose of these 105-d-old IUGR-MR pigs by reducing hepatic gluconeogenesis, because G6Pase is the rate-limiting enzyme catalyzing the final step of gluconeogenesis (Barthel and Schmoll, 2003). However, although 49-d-old IUGR-MR pigs also had increased hepatic GYS activities as the 105-d-old IUGR-MR pigs, such increased GYS activities did not result in increased hepatic glycogen contents in the 49-d-old IUGR-MR pigs. This unchanged hepatic glycogen content of the 49-d-old IUGR-MR pigs may be associated with the unchanged hepatic G6Pase activities of these pigs, because as the last enzyme of hepatic gluconeogenesis (Barthel and Schmoll, 2003), a decrease in G6Pase activity may stimulate hepatic glycogen synthesis by increasing hepatic glucose-6-phosphate which is the substrate of glycogen synthesis (Ferrer et al., 2003). Meanwhile, increased consumption of glucose-6-phosphorate via glycogen synthesis could in return reduce G6Pase activity by decreasing G6Pase’s access to this substrate, which could be the reason for the different changes in the G6Pase activities between the 49- and 105-d-old IUGR-MR pigs especially when MR did not affect the mRNA expression of G6Pase encoding gene. In addition to decreasing blood glucose concentrations of 105-d-old IUGR-MR pigs, MR also decreased blood glucose, increased hepatic glycogen, and inhibited hepatic G6Pase activities in 180-d-old IUGR pigs (Ying et al., 2017). All these results together may imply time-dependent effects of MR on IUGR pigs’ glucose metabolism and that the occurrence of these effects may somehow have a relationship with catch-up growth of IUGR pigs.
Although MR caused different effects on IUGR pigs’ growth and glucose metabolism at different ages, MR consistently improved IUGR pigs’ capacity to control blood glucose concentration, reflected by the decreased HOMA-IR index of IUGR-MR pigs (Matthews et al., 1985). Additionally, MR produced no effect on the IUGR pigs’ mRNA expressions of different enzymes involved in gluconeogenesis and glycogen metabolism no matter the ages of these, which suggested that the effects of MR glucose metabolic enzymes did not involve in gene regulation.
In conclusion, dietary MR caused different effects on the growth rates and the glucose homeostasis between 49- and 105-d-old IUGR pigs; namely, MR did not affect IUGR pigs’ blood glucose concentrations at 49 d of age, but it lowered this parameter in 105-d-old IUGR pigs, which was associated with increased hepatic glycogen synthesis and decreased hepatic gluconeogenesis. Additionally, MR improved IUGR pigs’ insulin sensitivity at both days 49 and 105. It was also worthwhile to mention IUGR-induced different changes that IUGR decreased growth rates and improved insulin sensitivity in 49-d-old IUGR-CON pigs, but resulted in opposite results in 105-d-old IUGR-CON pigs.
Conflict of interest statement. None declared.
Supplementary Material
ACKNOWLEDGMENT
The authors thank their laboratory colleagues for their assistance.
Footnotes
This work was supported by the National Natural Science Foundation of China (grant number 31572418) and National Key Research and Development Program of China (018YFD0501100).
LITERATURE CITED
- Ables G. P., Perrone C. E., Orentreich D., and Orentreich N.. 2012. Methionine-restricted C57BL/6J mice are resistant to diet-induced obesity and insulin resistance but have low bone density. PLoS One 7:e51357. doi: 10.1371/journal.pone.0051357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D. J. P. 2012. Developmental origins of chronic disease. Public Health 126:185–189. doi: 10.1016/j.puhe.2011.11.014 [DOI] [PubMed] [Google Scholar]
- Barthel A., and Schmoll D.. 2003. Novel concepts in insulin regulation of hepatic gluconeogenesis. Am. J. Physiol. Endocrinol. Metab. 285:E685–E692. doi: 10.1152/ajpendo.00253.2003 [DOI] [PubMed] [Google Scholar]
- Bellinger D. A., Merricks E. P., and Nichols T. C.. 2006. Swine models of type 2 diabetes mellitus: insulin resistance, glucose tolerance, and cardiovascular complications. ILAR J. 47:243–258. doi: 10.1093/ilar.47.3.243 [DOI] [PubMed] [Google Scholar]
- Cianfarani S., Geremia C., Scott C. D., and Germani D.. 2002. Growth, IGF system, and cortisol in children with intrauterine growth retardation: is catch-up growth affected by reprogramming of the hypothalamic-pituitary-adrenal axis?Pediatr. Res. 51:94–99. doi: 10.1203/00006450-200201000-00017 [DOI] [PubMed] [Google Scholar]
- Cianfarani S., Ladaki C., and Geremia C.. 2006. Hormonal regulation of postnatal growth in children born small for gestational age. Horm. Res. 65 (Suppl. 3):70–74. doi: 10.1159/000091509 [DOI] [PubMed] [Google Scholar]
- D’Inca R., Kloareg M., Gras-Le Guen C., and Le Huërou-Luron I.. 2010. Intrauterine growth restriction modifies the developmental pattern of intestinal structure, transcriptomic profile, and bacterial colonization in neonatal pigs. J. Nutr. 140:925–931. doi: 10.3945/jn.109.116822 [DOI] [PubMed] [Google Scholar]
- Ferrer J. C., Favre C., Gomis R. R., Fernández-Novell J. M., García-Rocha M., de la Iglesia N., Cid E., and Guinovart J. J.. 2003. Control of glycogen deposition. FEBS Lett. 546:127–132. doi: 10.1016/S0014-5793(03)00565-9 [DOI] [PubMed] [Google Scholar]
- Hochberg Z., Feil R., Constancia M., Fraga M., Junien C., Carel J. C., Boileau P., Le Bouc Y., Deal C. L., Lillycrop K.,. et al. 2011. Child health, developmental plasticity, and epigenetic programming. Endocr. Rev. 32:159–224. doi: 10.1210/er.2009-0039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y., Cong R., Li R., Yang X., Sun Q., Parvizi N., and Zhao R.. 2012. Maternal low-protein diet induces gender-dependent changes in epigenetic regulation of the glucose-6-phosphatase gene in newborn piglet liver. J. Nutr. 142:1659–1665. doi: 10.3945/jn.112.160341 [DOI] [PubMed] [Google Scholar]
- Kraegen E. W., Clark P. W., Jenkins A. B., Daley E. A., Chisholm D. J., and Storlien L. H.. 1991. Development of muscle insulin resistance after liver insulin resistance in high-fat-fed rats. Diabetes. 40:1397–1403. doi: 10.2337/diab.40.11.1397 [DOI] [PubMed] [Google Scholar]
- Lees E. K., Król E., Grant L., Shearer K., Wyse C., Moncur E., Bykowska A. S., Mody N., Gettys T. W., and Delibegovic M.. 2014. Methionine restriction restores a younger metabolic phenotype in adult mice with alterations in fibroblast growth factor 21. Aging Cell 13:817–827. doi: 10.1111/acel.12238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., He Y., Qi L., Jaddoe V. W., Feskens E. J., Yang X., Ma G., and Hu F. B.. 2010. Exposure to the Chinese famine in early life and the risk of hyperglycemia and type 2 diabetes in adulthood. Diabetes 59:2400–2406. doi: 10.2337/db10-0385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim K., Armitage J. A., Stefanidis A., Oldfield B. J., and Black M. J.. 2011. IUGR in the absence of postnatal “catch-up” growth leads to improved whole body insulin sensitivity in rat offspring. Pediatr. Res. 70:339–344. doi: 10.1203/PDR.0b013e31822a65a3 [DOI] [PubMed] [Google Scholar]
- Malloy V. L., Krajcik R. A., Bailey S. J., Hristopoulos G., Plummer J. D., and Orentreich N.. 2006. Methionine restriction decreases visceral fat mass and preserves insulin action in aging male Fischer 344 rats independent of energy restriction. Aging Cell 5:305–314. doi: 10.1111/j.1474-9726.2006.00220.x [DOI] [PubMed] [Google Scholar]
- Martin-Gronert M. S., and Ozanne S. E.. 2007. Experimental IUGR and later diabetes. J. Intern. Med. 261:437–452. doi: 10.1111/j.1365-2796.2007.01800.x [DOI] [PubMed] [Google Scholar]
- Matthews D. R., Hosker J. P., Rudenski A. S., Naylor B. A., Treacher D. F., and Turner R. C.. 1985. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 28:412–419. doi: 10.1007/BF00280883 [DOI] [PubMed] [Google Scholar]
- Morrison J. L., Duffield J. A., Muhlhausler B. S., Gentili S., and McMillen I. C.. 2010. Fetal growth restriction, catch-up growth and the early origins of insulin resistance and visceral obesity. Pediatr. Nephrol. 25:669–677. doi: 10.1007/s00467-009-1407-3 [DOI] [PubMed] [Google Scholar]
- NRC 2012. Nutrient requirement of swine. 11th rev. ed. Washington, DC:National Academic Science. [Google Scholar]
- Nyirenda M. J., Lindsay R. S., Kenyon C. J., Burchell A., and Seckl J. R.. 1998. Glucocorticoid exposure in late gestation permanently programs rat hepatic phosphoenolpyruvate carboxykinase and glucocorticoid receptor expression and causes glucose intolerance in adult offspring. J. Clin. Invest. 101:2174–2181. doi: 10.1172/JCI1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özkan H., Aydın A., Demir N., Erci T., and Büyükgebiz A.. 1999. Associations of IGF-I, IGFBP-1 and IGFBP-3 on intrauterine growth and early catch-up growth. Neonatology. 76:274–282. doi: 10.1159/000014169 [DOI] [PubMed] [Google Scholar]
- Pfaffl M. W. 2001. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 29:e45. doi: 10.1093/nar/29.9.e45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poore K. R., and Fowden A. L.. 2002. The effect of birth weight on glucose tolerance in pigs at 3 and 12 months of age. Diabetologia 45:1247–1254. doi: 10.1007/s00125-002-0849-y [DOI] [PubMed] [Google Scholar]
- Quiniou N., Dagorn J., and Gaudre D.. 2002. Variation of piglets’ birth weight and consequences on subsequent performance. Livest. Prod. Sci. 78:63–70. doi: 10.1016/S0301-6226(02)00181-1 [DOI] [Google Scholar]
- Rosenberg A. 2008. The IUGR newborn. Semin. Perinatol. 32:219–224. doi: 10.1053/j.semperi.2007.11.003 [DOI] [PubMed] [Google Scholar]
- Saleem T., Sajjad N., Fatima S., Habib N., Ali S. R., and Qadir M.. 2011. Intrauterine growth retardation–small events, big consequences. Ital. J. Pediatr. 37:41. doi: 10.1186/1824-7288-37-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selak M. A., Storey B. T., Peterside I., and Simmons R. A.. 2003. Impaired oxidative phosphorylation in skeletal muscle of intrauterine growth-retarded rats. Am. J. Physiol. Endocrinol. Metab. 285:E130–E137. doi: 10.1152/ajpendo.00322.2002 [DOI] [PubMed] [Google Scholar]
- Sharabi K., Tavares C. D., Rines A. K., and Puigserver P.. 2015. Molecular pathophysiology of hepatic glucose production. Mol. Aspects Med. 46:21–33. doi: 10.1016/j.mam.2015.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons R. A., Templeton L. J., and Gertz S. J.. 2001. Intrauterine growth retardation leads to the development of type 2 diabetes in the rat. Diabetes 50:2279–2286. doi: 10.2337/diabetes.50.10.2279 [DOI] [PubMed] [Google Scholar]
- Singhal A., Fewtrell M., Cole T. J., and Lucas A.. 2003. Low nutrient intake and early growth for later insulin resistance in adolescents born preterm. Lancet 361:1089–1097. doi: 10.1016/S0140-6736(03)12895-4 [DOI] [PubMed] [Google Scholar]
- Stone K. P., Wanders D., Orgeron M., Cortez C. C., and Gettys T. W.. 2014. Mechanisms of increased in vivo insulin sensitivity by dietary methionine restriction in mice. Diabetes 63:3721–3733. doi: 10.2337/db14-0464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su G., Lund M. S., and Sorensen D.. 2007. Selection for litter size at day five to improve litter size at weaning and piglet survival rate. J. Anim. Sci. 85:1385–1392. doi: 10.2527/jas.2006-631 [DOI] [PubMed] [Google Scholar]
- Su W., Zhang H., Ying Z., Li Y., Zhou L., Wang F., Zhang L., and Wang T.. 2017. Effects of dietary L-methionine supplementation on intestinal integrity and oxidative status in intrauterine growth-retarded weanling piglets. Eur. J. Nutr. 57:2735–2745. doi: 10.1007/s00394-017-1539-3 [DOI] [PubMed] [Google Scholar]
- Thorn S. R., Regnault T. R., Brown L. D., Rozance P. J., Keng J., Roper M., Wilkening R. B., Hay W. W. Jr, and Friedman J. E.. 2009. Intrauterine growth restriction increases fetal hepatic gluconeogenic capacity and reduces messenger ribonucleic acid translation initiation and nutrient sensing in fetal liver and skeletal muscle. Endocrinology 150:3021–3030. doi: 10.1210/en.2008-1789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers M. H., Gluckman P. D., Coveny A. H., Hofman P. L., Cutfield W. S., Gertler A., Breier B. H., and Harris M.. 2005. Neonatal leptin treatment reverses developmental programming. Endocrinology 146:4211–4216. doi: 10.1210/en.2005-0581 [DOI] [PubMed] [Google Scholar]
- Whincup P. H., Kaye S. J., Owen C. G., Huxley R., Cook D. G., Anazawa S., Barrett-Connor E., Bhargava S. K., Birgisdottir B. E., Carlsson S.,. et al. 2008. Birth weight and risk of type 2 diabetes: a systematic review. Jama 300:2886–2897. doi: 10.1001/jama.2008.886 [DOI] [PubMed] [Google Scholar]
- Ying Z., Zhang H., Su W., Zhou L., Wang F., Li Y., Zhang L., and Wang T.. 2017. Dietary methionine restriction alleviates hyperglycemia in pigs with intrauterine growth restriction by enhancing hepatic protein kinase B signaling and glycogen synthesis. J. Nutr. 147:1892–1899. doi: 10.3945/jn.117.253427 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.