Abstract
ABSTRACT: To provide insight into maternal recognition of pregnancy control in equids, the mitogenic and developmental effects of endometrium-expressed growth factors (epidermal growth factor (EGF), fibroblast growth factor 2 (FGF2), hepatocyte growth factor (HGF), and insulin-like growth factor-1 (IGF-1)) were examined in equine iTr cells, an equine trophectoderm cell line. Initial western blots revealed that HGF and IGF-1 stimulate phosphorylation of AKT serine/threonine kinase 1 (AKT1) and EGF, FGF2, or HGF resulted in phosphorylation of both extracellular signal-regulated kinase 1 (ERK1) and ERK2. Mitotic activity was stimulated (P < 0.05) by EGF, FGF2, and HGF. Chemical disruption of mitogen-activated protein kinase kinases 1 and 2 (MEK1/2) phosphorylation suppresses (P < 0.05) proliferation in control and growth factor treated cells demonstrating a dependence on ERK1/2 for mitotic activity. Treatment of iTr cells with EGF or HGF in the presence of an AKT1 inhibitor prohibits (P < 0.05) growth factor stimulated proliferation. The effect of EGF, FGF2, HGF, and IGF-1 on steroid biosynthetic enzyme gene expression, including prostaglandin-endoperoxide synthase 2 (PTGS2), was determined by real-time PCR. Neither EGF, FGF2, nor IGF-1 affected PTGS2 expression while HGF caused a two-fold increase (P < 0.05) in expression. Co-supplementation with HGF and an AKT1 inhibitor did not block PTGS2 expression, whereas providing an MEK1/2 inhibitor prevented (P < 0.05) the HGF-mediated increase in PTGS2. These results provide novel evidence of a role for HGF in equine trophectoderm proliferation and prostaglandin biosynthesis.
Keywords: hepatocyte growth factor, proliferation, prostaglandin, trophectoderm
INTRODUCTION
Early embryogenesis in the horse is unique amongst domestic animals. Following fertilization of the ova, the embryo travels through the oviduct and enters the uterus at 6 d postovulation, whereas nonfertilized ova are not expelled into the uterus (Betteridge et al., 1982). Soon thereafter the blastocyst is surrounded by a thickened glycocalyx capsule that protects the embryo as it moves throughout the uterus because of intrauterine contractions that are mediated by prostaglandins E and F, a requirement for establishment of pregnancy (McDowell et al., 1988; Stout and Allen, 2001). By day 10 of development, an embryonic disc is present, and gastrulation is complete by days 14 to 16, when fixation within an uterine horn occurs (Gaivão et al., 2014; Klein, 2015).
Both capsule formation and uterine mobility are hallmarks of equid pregnancy establishment, but the underlying mechanism(s) of equine maternal recognition of pregnancy (MRP) remain largely unresolved. What is known is that manipulation of prostaglandin F2α (PGF2α) secretion is the apparent key to MRP in mares, and that the MRP signal(s) should exist by day 14 postovulation to prevent endometrial PGF2α from lysing the corpus luteum (Boerboom et al., 2004). Uterine fluids at days 14 to 16 postovulation contain less PGF2α than cycling mares (Berglund et al., 1982). In vitro exposure of endometrial explants to embryo secretions from this time period decreased prostaglandin synthetase 2 (PTGS2) (Ealy et al., 2010). At day 18 postovulation, uterine fluids contain PGF2α concentrations that are similar to cyclic mares undergoing luteolysis, suggesting that the equine embryo does not prevent PGF2α secretion during pregnancy, but delays it (Stout and Allen, 2002). However, little is known about the actual MRP signal or group of signals. Dialysis of conditioned media from equine embryos at day 14 blastocysts and co-cultured with equine endometrial explants determined that a protein of 1 to 6 kDa will inhibit PGF2α synthesis (Sharp et al., 1989). Others report that the anti-luteolytic activity released by embryos was refractile to proteinase treatment but was removed by charcoal treatment, indicating that the proposed MRP was a lipophilic, nonpolar compound (Ababneh et al., 2000). One cannot discount, however, the possibility that two or more MRP signals exist in the mare. The trophectoderm (i.e., outermost layer of embryonic cells) synthesizes and releases both cytokines and hormones during the MRP timeframe, and apparently several of these secreted factors are capable of suppressing prostaglandin F2α (PGF2α) secretion. The question that remains unanswered is which signal or set of signals are essential for the establishment of pregnancy.
Cytokines and growth factors within the uterine microenvironment support the growth and development of the conceptus prior to implantation. In other species, many of these same signaling molecules also facilitate MRP either directly by affecting the expression of the MRP signal or indirectly by promoting embryogenesis, and specifically early placental development (Spencer et al., 2004). For example, culture of bovine blastocysts with fibroblast growth factor 2 (FGF2) causes an increase in the production and release of interferon tau (IFNT), the MRP factor for ruminants (Michael et al., 2006; Cooke et al., 2009). Other endometrial factors, such as epidermal growth factor (EGF) and insulin-like growth factor 1 (IGF-1) stimulate bovine trophoblast cell proliferation and increase bovine blastocyst size (Dilly et al., 2010; Xie et al., 2017; Mesalam et al., 2018). Also, treatment of early stage embryos with IGF-1 initiates intracellular signals that protect the developing blastocyst from an environmental heat insult (Bonilla et al., 2011). The mare endometrium produces FGF2 (de Ruijter-Villani et al., 2013), VEGF (Silva et al., 2011), EGF (Allen et al., 2017), IGF-1 and IGF-2 (Sessions-Bresnahan et al., 2018) as well as the cytokines LIF (de Ruijter-Villani et al., 2015), IL6, TNFα, and IL1β (Sessions-Bresnahan et al., 2018). Their participation and requirement in early embryogenesis in the horse remain undefined.
We propose that describing how various endometrial-derived growth factors influence trophoblast cell proliferation and prostaglandin gene expression may provide new clues into the embryo-derived mechanisms that control MRP in horses. Our hypothesis was tested using iTr cells, an equine trophoblast cell line created through a partial cellular reprogramming event, that are similar in morphology to trophoblast cells and share a common genetic signature with native trophectoderm (TE) (Reinholt et al., 2017).
MATERIALS AND METHODS
Animals and Biopsy
All animal protocols were reviewed and approved by the Virginia Polytechnic Institute and State University Institutional Animal Care and Use Committee. Mares (n = 3) were evaluated daily by transrectal ultrasonography to map follicular development and ovulation. Mares were sedated (Xylazine, MWI Animal Health, Boise, ID) prior to biopsy collection. Endometrial biopsies were recovered when the follicle diameter reached 40 mm or greater, as measured by ultrasonography, and at day 8 postovulation by insertion of a sterile alligator-type biopsy device through the cervix into the uterus and physical resection of tissue (~75 mg). Tissue was briefly rinsed with ice-cold sterile phosphate-buffered saline (PBS) and snap frozen in liquid nitrogen for RNA isolation. Liver RNA was isolated from archived samples obtained from healthy horses euthanized at the Virginia-Maryland College of Veterinary Medicine.
Cell Culture
Induced trophoblasts (iTr) were created by reprogramming umbilical cord matrix mesenchymal stem cells with human Oct4, Sox2, KLF4 and c-Myc (Reinholt et al., 2017). Transcriptome analysis indicates that the cells contain the core TE lineage network, steroidogenesis genes, and capsule forming genes. Based on morphology and gene expression profiles, the cells appear functionally equivalent to native TE. Induced Tr cells are cultured on tissueware coated with extracellular matrix (Matrigel, Corning, Corning, NY) in high glucose Dulbecco’s modified Eagle medium supplemented with 15% fetal bovine serum, 10 mM nonessential AA, 2 mm glutamine, 55 µM β-mercaptoethanol, 1% penicillin-streptomycin and 0.5% gentamicin. All media and supplements were purchased from Invitrogen (Thermofisher, Carlsbad, CA). For the measurement of proliferation, cells were serum starved overnight followed by treatment with 10 ng/mL bovine FGF2, human EGF, human hepatocyte growth factor (HGF), or human IGF-1 for 24 h. Growth factors were purchased from R&D Systems (Minneapolis, MN). During the final 2 h of culture, 10 µM 5-ethynyl-2ʹ-deoxyuridine (EdU) was included for the detection of DNA synthesis. Chemical inhibition of AKT serine/threonine kinase 1 (AKT1) or mitogen-activated protein kinase kinase 1 and 2 (MEK1/2) was accomplished by supplementation of the culture media with 1 nM MK2206 and 1 nM PD0325901, respectively. Cells were fixed with 4% paraformaldehyde in PBS and EdU was detected by click chemistry (Click-iT EdU AlexaFluor 488, Invitrogen). Nuclei were detected following incubation with 10 µg/mL Hoechst 33245 (Invitrogen). Total nuclei and EdU-positive nuclei were visualized with epifluorescence (Eclipse TS100, Nikon, Melville, NY) and 10 representative images were captured at 200-fold magnification with a CoolSNAP HQ2 camera (Photometrics, Tucson, AZ) with shutter speed controlled by NIS Elements software (Nikon). Total cells and EdU(+) nuclei were enumerated within the images and a mitotic index was calculated as EdU(+)/Hoechst(+) × 100. No differences in proliferation were observed between 10 and 100 ng/mL for any growth factor (data not shown) thus, all experiments used the lesser concentration.
RNA Isolation and Polymerase Chain Reaction
Total RNA was extracted and purified from iTr cells and endometrial tissue using TRIzol (Ambion, Austin, TX). Contaminating DNA was removed with DNase I (Ambion). A High-Capacity cDNA Reverse Transcription Kit (ThermoFisher) was used to convert RNA into cDNA, according to the manufacturer’s instructions. Real-time PCR reactions were performed with Power SYBR Green PCR Master Mix (ThermoFisher) and gene-specific primers (Table 1) using an Eppendorf Realplex thermocycler (Eppendorf, Hamburg, Germany). The fold change for all the samples was calculated using the 2−△△Ct method. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as an internal control; GAPDH Ct values were unaffected by treatment. Verification that a single amplicon was produced was completed by electrophoresis through 2% agarose gels impregnated with SYBR Safe DNA gel stain (Thermofisher) in 40 mM Tris–acetate buffer (pH 8.3) containing 1 mM ethylenediaminetetraacetic acid. Amplicons were visualized at 280 nm on a BioDoc-It (Analytik Jena, Upland, CA).
Table 1.
Gene-specific primer sequences for PCR
| Gene | Sequence, 5ʹ to 3ʹ | Size, bp |
|---|---|---|
| CYP11A | F-AACGTTACCGAGATGCTGGC | 159 |
| R-CACCATCGTGCTCGTGTCTC | ||
| GAPDH | F-CCACCCCTAACGTGTCAGTC | 150 |
| R-AATCGCAGGAGACAACCTGG | ||
| PTGES2 | F-TGATCTGGCTGTGTATGGCG | 164 |
| R-ATCTTCCGTTGCCTCTCGTC | ||
| PTGES3 | F-CACGTTCATTCTCCGTCCTCG | 196 |
| R-GCAGTCGACTCTTCTCCGTTG | ||
| PTGS2 | F-GCTTGTTCCAGACGAGCAGG | 188 |
| R-GGGATGCCAGTGATAGAGCG | ||
| EGF | F-TTCTGGACTGATATGGGGATT | 126 |
| R-GGCATCGCACCAATACAACTT | ||
| FGF2 | F-TGCTATGAAGGAAGATGGAAG | 132 |
| R-TTATACTGCCCCGTTCGTTTC | ||
| HGF | F-CGCTACGAAGTCTGTGACATTCC | 70 |
| R-TTCCCCATTGCAGGTCATG | ||
| IGF-1 | F-GAAGCAATGGGAAAAATCAGC | 89 |
| R-AGGTAGAAGAGATGTGAGGAG |
Western Blot
Cells were washed with ice-cold PBS and lysed with radioimmunoprecipitation assay (Thermofisher) buffer containing protease and phosphatase inhibitors (Halt; Thermofisher). Insoluble material was removed by centrifugation at 10,000 × g for 5 min at 4 °C. Protein concentration was measured colorimetrically (BCA Assay, Thermofisher). Proteins were diluted in loading buffer containing SDS (Thermofisher) and heated at 95 °C for 5 min. Equivalent amounts of protein were electrophoretically separated through denaturing 10% polyacrylamide gels (NuPAGE, Thermofisher). Proteins were transferred to nitrocellulose (iBlot2 Dry Transfer, Thermofisher). Membranes were incubated with 10 mM Tris, pH 8.0, 150 mM NaCl (TBS) containing 2% bovine serum albumin (BSA) and 0.1% Tween-20 (TBST) for 30 min at room temperature to block nonspecific antigen sites. Subsequently, membranes were incubated at 4 ℃ overnight with anti-AKT1 (pan 11E7; Cell Signaling Technologies (CST), Danvers, MD), anti-phosphoAKT1S473 (D9E; CST), anti-extracellular signal-regulated kinases 1 and 2 (ERK1/2) (137F5; CST), anti-phosphoERK1/2 (197G2; CST), or anti-α/β-tubulin (2148; CST) diluted 1:2,000 in TBS containing 2% BSA. Blots were washed extensively with TBST followed by incubation with donkey anti-rabbit IgG-peroxidase (Invitrogen) diluted 1:2,000 in TBS containing 2% BSA for 45 min at room temperature. After washing with PBS, immunocomplexes were visualized by chemiluminescence (SuperSignal Chemiluminescence, Thermofisher) using a BioDoc-It (Analytik Jena, Upland, CA). The experiments were repeated in triplicate and a representative blot is shown.
Statistics
Data were analyzed by one- or two-way ANOVA using Prism 7 (Graphpad Software, La Jolla, CA). Technical replicates (triplicate wells) were averaged for each biological replicate (n = 3) prior to calculation of means and SEM. Post-test comparisons were performed using Tukey’s adjustment to analyze pre-planned comparisons between groups. A minimum of three replicates of each experiment was performed. Significance was set at P < 0.05.
RESULTS
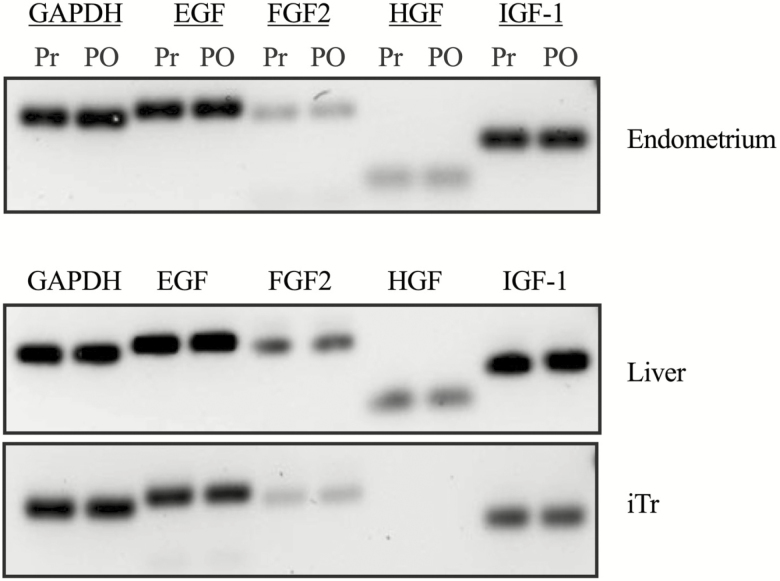
Expression of the EGF, FGF2, HGF, and IGF-1 was examined by RT-PCR using total RNA isolated from the endometrium, archived adult liver tissue and iTr cells. Expression of EGF, FGF2, HGF and IGF-1 was detected in endometrium and liver (positive control) RNA isolates (Figure 1). Expression of EGF, FGF2 and IGF-1 was detected in iTr cells, whereas HGF was not detected.
Figure 1.
Expression of potential embryokines in equine endometrium, liver, and iTr cells. Total RNA was isolated from adult mare liver and pre- (Pr) and postovulation (PO) endometrium, and equine iTr cells, reverse transcribed and amplified with gene-specific primers for EGF, FGF2, HGF, IGF-1, and glyceraldehyde 3-phosphate dehydrogenase (GAPDH). Representative gel from a single mare endometrium sample is shown. Liver amplicons from two horses and iTr amplicons from two passages are shown in the lower panels. Amplicons were separated through Syber-safe agarose gels and imaged.
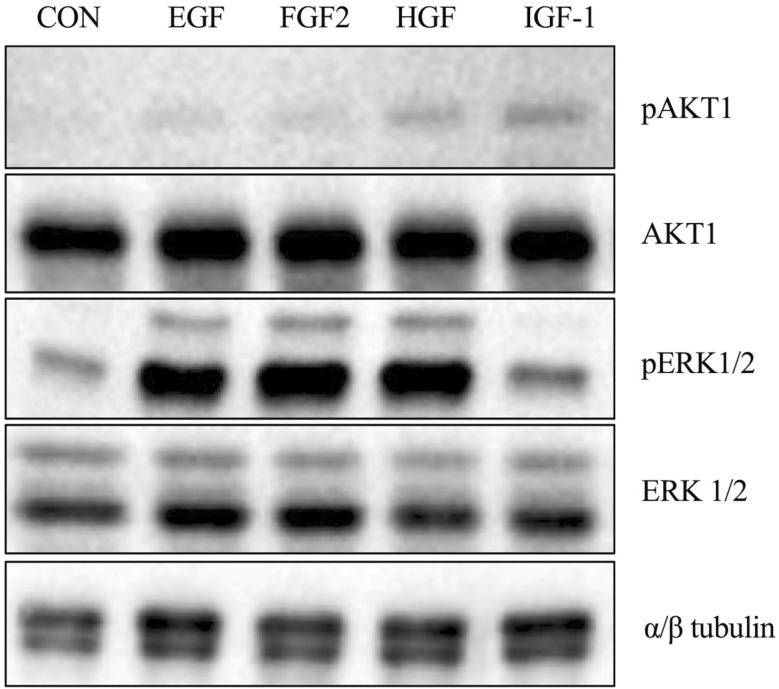
As a first step to ensure that the ligands can affect iTr biology, each growth factor was examined for its ability to phosphorylate AKT1 and ERK1/2, two prominent intracellular signaling effectors of RTKs. Serum-starved equine iTr cells were treated with recombinant EGF, FGF2, HGF, or IGF-1 and analyzed with total and phospho-specific antibodies to AKT1 and ERK1/2. Weak phosphorylation of AKT1 was observed in response to EGF, FGF2, HGF, or IGF-1 exposure (Figure 2). In contrast, substantial amounts of phosphorylated ERK1/2 were detected following treatment with EGF, FGF2, and HGF. Direct phosphorylation of ERK1/2 in response to IGF-I was no different than control. No differences in the relative amounts of total AKT, ERK1/2, or α/β tubulin proteins were noted.
Figure 2.
Phosphorylation of AKT1 and ERK1/2 in response to selected growth factors. Equine iTr cells were serum starved and treated with 10 ng/mL of EGF, FGF2, HGF, or IGF-1 for 20 min prior to lysis. Proteins were separated through SDS-PAGE, transferred to nitrocellulose and analyzed by western blot for total and phosphorylated version of AKT and ERK1/2. Tubulin expression was monitored as a loading control.
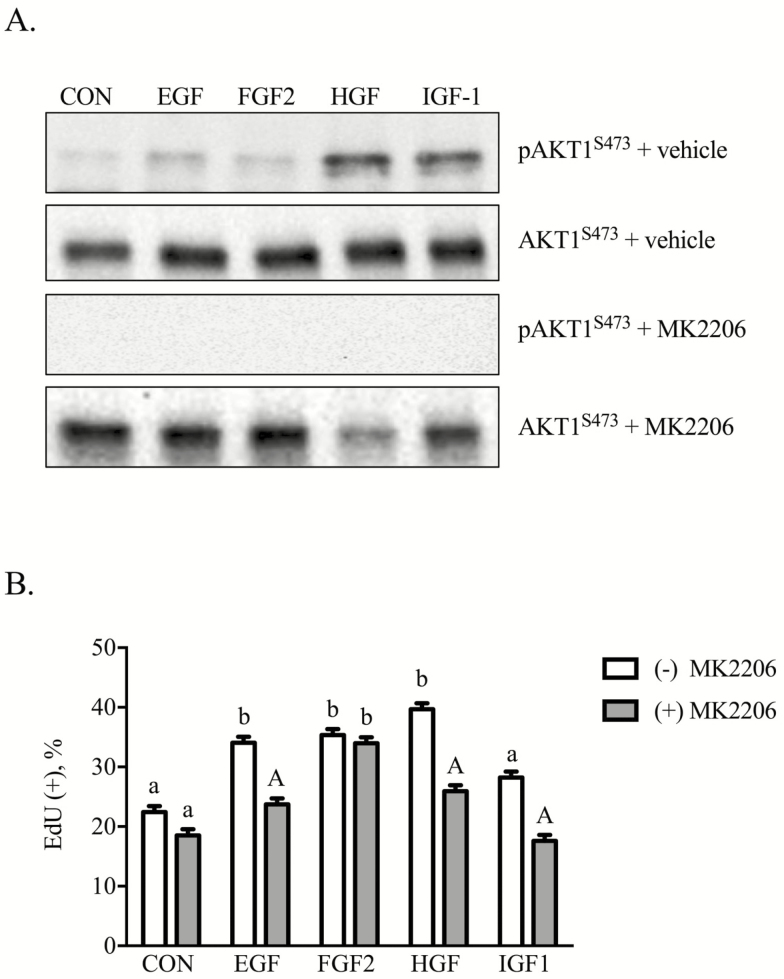
Initial experiments examined the role of AKT1 as a mediator of iTr proliferation. In brief, equine iTr cells were treated with each growth factor in the presence or absence of the AKT inhibitor, MK2206. Weak phosphorylation of AKT1S473 occurred in response to EGF, FGF2, HGF, and IGF-1 (Figure 3A). Phosphorylation was inhibited completely by inclusion of 1 nM MK2206 in the culture media. The next experiment examined whether these growth factors influence iTr proliferation. Results revealed that EGF, FGF2, HGF, and IGF-1 increased (P < 0.05) the percentage of cells incorporating EdU (Figure 3B). Supplementation of the culture media with the individual growth factors and 10 nM MK2206 suppressed (P < 0.05) EGF, HGF, and IGF-1-directed proliferation. The AKT inhibitor affected neither control nor FGF2-mediated cell division.
Figure 3.
Equine iTr cells proliferate in response to EGF, HGF, and IGF-1 in an AKT1-dependent manner. Equine iTr cells were treated with the respective growth factors in the presence or absence of 1 nM MK2206, an AKT inhibitor. Lysates were analyzed by western blot for total and phosphorylated AKT1 (A). Parallel plates of cells were treated in a similar manner with 5-ethynyl-2ʹ-deoxyuridine (EdU) included 2 h prior to fixation. Total and EdU(+) cells were enumerated (B). Means and SEMs of three replicate experiments shown. Different letters represent significance at P < 0.05. Capital letters indicate significance within treatment at P < 0.05.
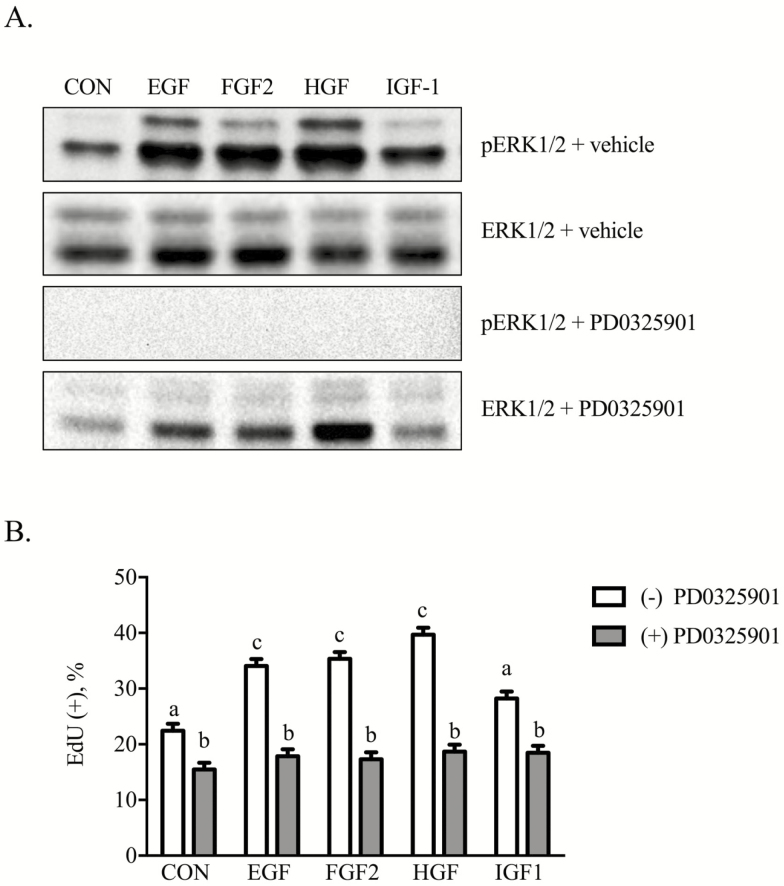
The importance of ERK1/2 signals as mediators of mitogenic effects was evaluated. Phosphorylation of ERK1/2 occurred in response to EGF, FGF2, HGF, and IGF-1, although to varying intensity levels (Figure 4A). Supplementation with 1 nM PD0325901 suppressed (P < 0.05) both basal and growth factor-initiated phosphorylation of ERK1/2 as well as proliferation (Figure 4B).
Figure 4.
Equine iTr cells require MEK/ERK signals for proliferation. Equine iTr cells were treated with the respective growth factors in the presence or absence of 1 nM PD0325901, an MEK inhibitor. Lysates were analyzed by western blot for total and phosphorylated ERK1/2 (A). Parallel plates of cells were treated in a similar manner with EdU included 2 h prior to fixation. Total and EdU(+) cells were enumerated (B). Means and SEMs of three replicate experiments shown. Different letters represent significance at P < 0.05.
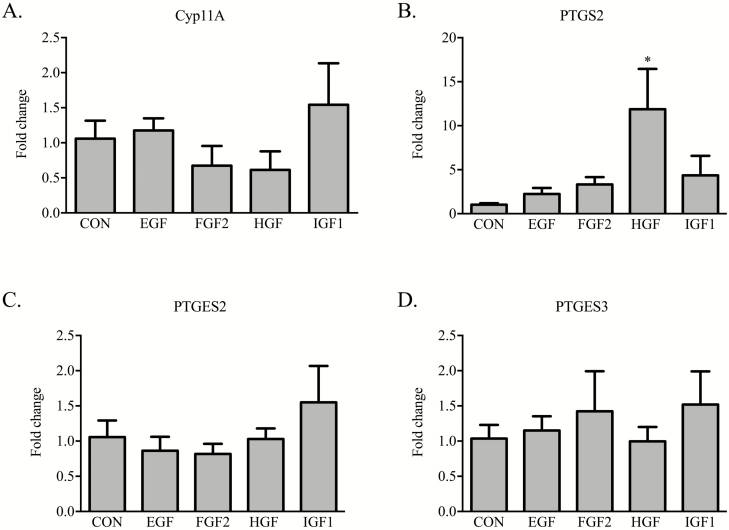
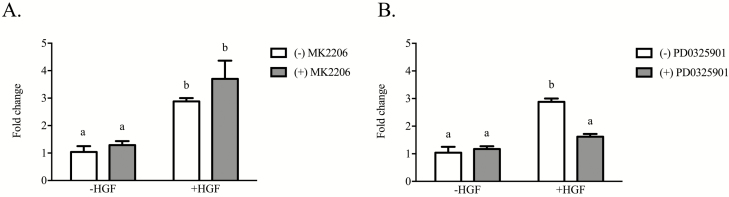
A final set of studies examined whether the growth factors under investigation affect gene expression of enzymes controlling prostaglandin E2 and F2α production. Treatment of iTr with either EGF, FGF2, HGF, or IGF-1 failed to alter basal transcript amounts for cytochrome P450 family 11 subfamily A member 1 (CYP11A), prostaglandin E synthase 2 (PTGES2) and PTGES3 (Figures 5A, C, D, respectively). Treatment of iTr cells with HGF caused a 10-fold increase (P < 0.05) in PTGS2 mRNA abundance. Neither IGF-1, FGF2, nor EGF elicited an effect on PTGS2 (P = 0.25, 0.30, 0.76, respectively; Figure 5B). Media supplementation with 1 nM MK2206 (AKT inhibitor) did not affect either basal transcription of PTGS2 or the HGF-directed response (Figure 6A). By contrast, iTr cells incubated with HGF and PD0325901 (MEK1/2 inhibitor) resulted in a reduction (P < 0.05) in PTGS2 mRNA content to levels comparable to controls (Figure 6B).
Figure 5.
Growth factor treatment of equine iTr cells differentially affects steroidogenic gene expression. Equine iTr cells were cultured for 36 h with 10 ng/mL of EGF, FGF2, HGF, or IGF-1. Total RNA was isolated, reverse transcribed, and analyzed by real-time PCR for Cyp11A, PTGS2, PTGES2, and PTGES3 mRNA abundance. Fold change calculated relative to CON cells treated with vehicle only. Mean and SEM of three replicate experiments shown. *Significance at P < 0.05.
Figure 6.
HGF stimulates PTGS2 expression through an MEK-dependent signaling pathway. Equine iTr cells were cultured for 36 h with 10 ng/mL of HGF in the presence or absence of 1 nM MK2206 (A) or 1 nM PD0325901 (B). Total RNA was isolated, reverse transcribed, and analyzed by real-time PCR for PTGES2 mRNA abundance. Fold change calculated relative to CON cells treated with vehicle only. Mean and SEM of three replicate experiments shown. *Significance at P < 0.05.
DISCUSSION
The incidence of pregnancy loss in the mare is 7.9%, and the majority of these losses occur within the first 42 d postovulation (Rose et al., 2018). The underlying causes of these losses remain largely unknown due to our limited understanding of embryology and MRP in the horse. Communication between the uterine endometrium and the TE of the developing blastocyst is a critical determinant in establishing pregnancy. Transcriptome analysis of the early TE reveals the presence of conventional RTK signaling systems as well as chemokine and interferon cell signaling genes (Iqbal et al., 2014; Reinholt et al., 2017).
This work determined that iTr cells can respond to FGF2. Fibroblast growth factor receptors 1, 2, and 3 are expressed in TE with a temporal increase over the initial 21 d of the pregnancy that coincide with an increase in FGF2 expression within the endometrium (de Ruijter-Villani et al., 2013). Treatment of iTr cells with FGF2 caused an increase in cell proliferation, similar to that observed in bovine trophoblast cells and blastocysts (Xie et al., 2017). The ligand-initiated weak phosphorylation of AKT1, and AKT1 was not involved in either mitotic responses or steroidogenesis enzyme expression in equine iTr cells. Thus, the intracellular signaling pathway used by FGF2 for equine trophoblast proliferation remains unknown. It is possible that FGF2 utilizes MEK1/2-dependent pathways, but we were unable to tease apart mitosis in the absence of ERK1/2 activity, which is an absolute requirement for iTr cell division.
Insulin-like growth factor-1 contributes to multiple events in early embryogenesis and trophoblast biology specifically. Treatment of primary TE cells isolated from day 12 porcine embryos with an equivalent amount of IGF (20 ng/mL) does not affect proliferation but does promote migration (Jeong et al., 2014). Proliferation was increased by 60% over controls following treatment of porcine trophoblasts with 100 ng/mL, indicating that a greater concentration is needed to elicit an effect. In a similar manner, 50 ng/mL of IGF-1 is required to stimulate proliferation of bovine CT1 trophoblast cells (Xie et al., 2017). Initial experiments treating iTr cells with either 10 or 100 ng/mL of IGF-1 did not show a difference in mitotic index thus, we chose to use the lesser concentration (data not shown). The absence of a mitogenic effect and a direct effect on steroidogenic enzyme gene expression is not due to the inability to initiate intracellular signaling events as both AKT1 and ERK1/2 are phosphorylated in response to IGF-1. The phosphatidylinositide 3 kinase (PI3K)/AKT/mTOR pathway underlies both autophagy and cell survival (Heras-Sandoval et al., 2014). Activation of either or both of these cellular processes likely contributes to early events within the equine blastocyst to promote viability. Future efforts examining the importance of IGF-1 as a mediator of early embryo survival in the mare are necessary.
Ligand-initiated receptor recruitment of PI3Ks to the plasma membrane followed by AKT phosphorylation and formation of the PI3K/AKT/ mTOR signalosome underlies cell proliferation, metabolism, and growth (Laplante and Sabatini, 2012). Ablation of individual components (PI3K p110α, PI3K p110β, and mTORC) and downstream effectors (Sin1) of the signaling module is embryonic lethal in mice (Yu and Cui, 2016). Activation of the module by EGF and HGF triggers equine iTr proliferation and offers a potential pathway for early embryo development in the horse. In support of this premise, EGF improves bovine embryo quality in a serum-free media in vitro (Mesalam et al., 2018). Culture of bovine blastocysts with EGF affected neither total blastomere number nor CDX2(+) trophoblast numbers but worked synergistically with FGF2 and IGF-I to increase both parameters (Xie et al., 2017). Proliferation of trophoblast cells derived from bovine placentomes is increased by EGF treatment through a pathway that involves both MEK1/2 and PI3K activity (Dilly et al., 2010). The discrepant results likely reflect differences in developmental age of the cell types.
The most striking result of the experiments was the observation that HGF initiated ERK1/2 signals and this resulted in a 10-fold increase in PTGS2 expression. The enzymatic actions of PTGS2 convert arachidonic acid to PGH2, a precursor to PGE2 and PGF2α. Equine embryos secrete and release PGE2 and PGF2α into the uterine milieu, and this contributes to smooth muscle contraction within the uterus and subsequent embryo mobility (Watson and Sertich, 1989; Stout and Allen, 2002). Inhibition of PTGS2 does not affect uterine tone but substantially reduces embryo motility within the uterus in mares (Stout and Allen, 2001). Our results fill a void through identification of HGF, a growth factor secreted by the endometrium, as a modulator of trophoblast PTGS2 expression and subsequent PG production during early pregnancy. These actions are dependent upon the MEK/ERK signaling module. By contrast, HGF signals through the PI3K/AKT module are critical for trophoblast proliferation. The capacity to use divergent signaling networks to control distinct cellular functions makes HGF an intriguing possible modulator of MRP. The absence of a detectable difference in HGF pre- and postovulation suggests that the source of the growth factor may be the inner cell mass (ICM). Secretion of HGF as a paracrine factor from the ICM may initiate a cascade of events causing increased PTGS2 expression and downstream synthesis and release of PGE2, a myometrium contraction hormone, from the TE. Alternatively, while the total amount of HGF produced by the endometrium may remain unchanged by pregnancy status, the amounts of bioactive HGF may be substantially different. The growth factor is synthesized and secreted as pro-HGF requiring proteolytic cleavage by HGF-A to create the functional peptide (Fajardo-Puerta et al., 2016). Production of active HGF may promote PG production by the TE supporting embryo mobility and resulting in a successful pregnancy. Although the source of HGF remains unknown, our results suggest that the embryokine facilitates trophectoderm expansion during the preimplantation stage of gestation while ensuring continued mobility prior to fixation.
Maternal recognition of pregnancy in the mare remains a mystery but likely involves endometrial-derived growth factor communication with the outer trophectoderm layer of the embryo. Results presented herein provide evidence that EGF, FGF2, HGF, and IGF-1 may support trophoblast proliferation during early equine embryogenesis. More importantly, HGF-mediated signals uniquely affect both trophoblast proliferation and steroidogenesis gene transcription supporting a role for this endometrial-derived growth factor as a novel facilitator of MRP in the horse.
Conflict of interest statement. None declared.
Footnotes
1This research was supported by the Paul Mellon Professorship fund to SEJ.
LITERATURE CITED
- Ababneh M. M., Troedsson M. H., Michelson J. R., and Seguin B. E.. 2000. Partial characterization of an equine conceptus prostaglandin inhibitory factor. J. Reprod. Fertil. Suppl.:607–613. [PubMed] [Google Scholar]
- Allen W. R., Gower S., and Wilsher S.. 2017. Localisation of epidermal growth factor (EGF), its specific receptor (EGF-R) and aromatase at the materno-fetal interface during placentation in the pregnant mare. Placenta 50:53–59. doi: 10.1016/j.placenta.2016.12.024 [DOI] [PubMed] [Google Scholar]
- Berglund L. A., Sharp D. C., Vernon M. W., and Thatcher W. W.. 1982. Effect of pregnancy and collection technique on prostaglandin F in the uterine lumen of pony mares. J. Reprod. Fertil. Suppl. 32:335–341. [PubMed] [Google Scholar]
- Betteridge K. J., Eaglesome M. D., Mitchell D., Flood P. F., and Beriault R.. 1982. Development of horse embryos up to twenty two days after ovulation: observations on fresh specimens. J. Anat. 135:191–209. [PMC free article] [PubMed] [Google Scholar]
- Boerboom D., Brown K. A., Vaillancourt D., Poitras P., Goff A. K., Watanabe K., Doré M., and Sirois J.. 2004. Expression of key prostaglandin synthases in equine endometrium during late diestrus and early pregnancy. Biol. Reprod. 70:391–399. doi: 10.1095/biolreprod.103.020800 [DOI] [PubMed] [Google Scholar]
- Bonilla A. Q., Ozawa M., and Hansen P. J.. 2011. Timing and dependence upon mitogen-activated protein kinase signaling for pro-developmental actions of insulin-like growth factor 1 on the preimplantation bovine embryo. Growth Horm. IGF Res. 21:107–111. doi: 10.1016/j.ghir.2011.03.003 [DOI] [PubMed] [Google Scholar]
- Cooke F. N., Pennington K. A., Yang Q., and Ealy A. D.. 2009. Several fibroblast growth factors are expressed during pre-attachment bovine conceptus development and regulate interferon-tau expression from trophectoderm. Reproduction 137:259–269. doi: 10.1530/REP-08-0396 [DOI] [PubMed] [Google Scholar]
- Dilly M., Hambruch N., Haeger J. D., and Pfarrer C.. 2010. Epidermal growth factor (EGF) induces motility and upregulates MMP-9 and TIMP-1 in bovine trophoblast cells. Mol. Reprod. Dev. 77:622–629. doi: 10.1002/mrd.21197 [DOI] [PubMed] [Google Scholar]
- Ealy A. D., Eroh M. L., and Sharp D. C. III. 2010. Prostaglandin H synthase type 2 is differentially expressed in endometrium based on pregnancy status in pony mares and responds to oxytocin and conceptus secretions in explant culture. Anim. Reprod. Sci. 117:99–105. doi: 10.1016/j.anireprosci.2009.03.014 [DOI] [PubMed] [Google Scholar]
- Fajardo-Puerta A. B., Mato Prado M., Frampton A. E., and Jiao L. R.. 2016. Gene of the month: HGF. J. Clin. Pathol. 69:575–579. doi: 10.1136/jclinpath-2015-203575 [DOI] [PubMed] [Google Scholar]
- Gaivão M. M. F., Rambags B. P. B., and Stout T. A. E.. 2014. Gastrulation and the establishment of the three germ layers in the early horse conceptus. Theriogenology. 82:354–365. doi: 10.1016/j.theriogenology.2014.04.018 [DOI] [PubMed] [Google Scholar]
- Heras-Sandoval D., Pérez-Rojas J. M., Hernández-Damián J., and Pedraza-Chaverri J.. 2014. The role of PI3K/AKT/mtor pathway in the modulation of autophagy and the clearance of protein aggregates in neurodegeneration. Cell. Signal. 26:2694–2701. doi: 10.1016/j.cellsig.2014.08.019 [DOI] [PubMed] [Google Scholar]
- Iqbal K., Chitwood J. L., Meyers-Brown G. A., Roser J. F., and Ross P. J.. 2014. RNA-seq transcriptome profiling of equine inner cell mass and trophectoderm. Biol. Reprod. 90:61. doi: 10.1095/biolreprod.113.113928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong W., Song G., Bazer F. W., and Kim J.. 2014. Insulin-like growth factor I induces proliferation and migration of porcine trophectoderm cells through multiple cell signaling pathways, including protooncogenic protein kinase 1 and mitogen-activated protein kinase. Mol. Cell. Endocrinol. 384:175–184. doi: 10.1016/j.mce.2014.01.023 [DOI] [PubMed] [Google Scholar]
- Klein C. 2015. Novel equine conceptus–endometrial interactions on Day 16 of pregnancy based on RNA sequencing. Reprod. Fertil. Dev. 28:1712–1720. doi: 10.1071/RD14489 [DOI] [PubMed] [Google Scholar]
- Laplante M., and Sabatini D. M.. 2012. Mtor signaling in growth control and disease. Cell 149:274–293. doi: 10.1016/j.cell.2012.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDowell K. J., Sharp D. C., Grubaugh W., Thatcher W. W., and Wilcox C. J.. 1988. Restricted conceptus mobility results in failure of pregnancy maintenance in mares. Biol. Reprod. 39:340–348. doi: 10.1095/biolreprod39.2.340 [DOI] [PubMed] [Google Scholar]
- Mesalam A., Lee K.-L., Khan I., Chowdhury M. M. R., Zhang S., Song S.-H., Joo M.-D., Lee J.-H., Jin J.-I., and Kong I.-K.. 2018. A combination of bovine serum albumin with insulin-transferrin-sodium selenite and/or epidermal growth factor as alternatives to fetal bovine serum in culture medium improves bovine embryo quality and trophoblast invasion by induction of matrix metalloproteinases. Reprod. Fertil. Dev. in press. doi: 10.1071/RD18162 [DOI] [PubMed] [Google Scholar]
- Michael D. D., Alvarez I. M., Ocón O. M., Powell A. M., Talbot N. C., Johnson S. E., and Ealy A. D.. 2006. Fibroblast growth factor-2 is expressed by the bovine uterus and stimulates interferon-tau production in bovine trophectoderm. Endocrinology 147:3571–3579. doi: 10.1210/en.2006-0234 [DOI] [PubMed] [Google Scholar]
- Reinholt B. M., Bradley J. S., Jacobs R. D., Ealy A. D., and Johnson S. E.. 2017. Tissue organization alters gene expression in equine induced trophectoderm cells. Gen. Comp. Endocrinol. 247:174–182. doi: 10.1016/j.ygcen.2017.01.030 [DOI] [PubMed] [Google Scholar]
- Rose B. V., Firth M., Morris B., Roach J. M., Wathes D. C., Verheyen K. L. P., and de Mestre A. M.. 2018. Descriptive study of current therapeutic practices, clinical reproductive findings and incidence of pregnancy loss in intensively managed thoroughbred mares. Anim. Reprod. Sci. 188:74–84. doi: 10.1016/j.anireprosci.2017.11.011 [DOI] [PubMed] [Google Scholar]
- de Ruijter-Villani M., van Boxtel P. R., and Stout T. A.. 2013. Fibroblast growth factor-2 expression in the preimplantation equine conceptus and endometrium of pregnant and cyclic mares. Theriogenology 80:979–989. doi: 10.1016/j.theriogenology.2013.07.024 [DOI] [PubMed] [Google Scholar]
- de Ruijter-Villani M., Deelen C., and Stout T. A. E.. 2015. Expression of leukaemia inhibitory factor at the conceptus? maternal interface during preimplantation development and in the endometrium during the oestrous cycle in the mare. Reprod. Fertil. Dev. 28:1642. doi: 10.1071/RD14334 [DOI] [PubMed] [Google Scholar]
- Sessions-Bresnahan D. R., Heuberger A. L., and Carnevale E. M.. 2018. Obesity in mares promotes uterine inflammation and alters embryo lipid fingerprints and homeostasis. Biol. Reprod. 152:4158. doi: 10.1093/biolre/ioy107 [DOI] [PubMed] [Google Scholar]
- Sharp D. C., McDowell K. J., Weithenauer J., and Thatcher W. W.. 1989. The continuum of events leading to maternal recognition of pregnancy in mares. J. Reprod. Fertil. Suppl. 37:101–107. [PubMed] [Google Scholar]
- Silva L. A., Klein C., Ealy A. D., and Sharp D. C.. 2011. Conceptus-mediated endometrial vascular changes during early pregnancy in mares: an anatomic, histomorphometric, and vascular endothelial growth factor receptor system immunolocalization and gene expression study. Reproduction 142:593–603. doi: 10.1530/REP-11-0149 [DOI] [PubMed] [Google Scholar]
- Spencer T. E., Burghardt R. C., Johnson G. A., and Bazer F. W.. 2004. Conceptus signals for establishment and maintenance of pregnancy. Anim. Reprod. Sci. 82–83:537–550. doi: 10.1016/j.anireprosci.2004.04.014 [DOI] [PubMed] [Google Scholar]
- Stout T. A., and Allen W. R.. 2001. Role of prostaglandins in intrauterine migration of the equine conceptus. Reproduction 121:771–775. [PubMed] [Google Scholar]
- Stout T. A., and Allen W. R.. 2002. Prostaglandin E(2) and F(2 alpha) production by equine conceptuses and concentrations in conceptus fluids and uterine flushings recovered from early pregnant and dioestrous mares. Reproduction 123:261–268. [PubMed] [Google Scholar]
- Watson E. D., and Sertich P. L.. 1989. Prostaglandin production by horse embryos and the effect of co-culture of embryos with endometrium from pregnant mares. J. Reprod. Fertil. 87:331–336. doi: 10.1530/jrf.0.0870331 [DOI] [PubMed] [Google Scholar]
- Xie M., McCoski S. R., Johnson S. E., Rhoads M. L., and Ealy A. D.. 2017. Combinatorial effects of epidermal growth factor, fibroblast growth factor 2 and insulin-like growth factor 1 on trophoblast cell proliferation and embryogenesis in cattle. Reprod. Fertil. Dev. 29:419–430. doi: 10.1071/RD15226 [DOI] [PubMed] [Google Scholar]
- Yu J. S. L., and Cui W.. 2016. Proliferation, survival and metabolism: the role of PI3K/AKT/mTOR signalling in pluripotency and cell fate determination. Development. 143:3050–3060. doi: 10.1242/dev.137075 [DOI] [PubMed] [Google Scholar]