Abstract
Eight cecally cannulated Quarter Horses were used in a replicated 4 × 4 Latin square experiment conducted in four 14-d periods to determine effects of sodium caseinate (casein) on hindgut fermentation and fiber digestion. During each period, horses were assigned to one of four treatments consisting of control (water; CON), 0.125 g casein/kg BW (LOW), 0.25 g casein/kg BW (MED), or 0.5 g casein/kg BW (HI). Casein was solubilized in 800 mL water and dosed directly into the cecum at 0700 and 1900 hours using a metal dosing syringe. Smooth Bromegrass hay (CP 8.50%), water, and salt were provided ad libitum. New hay was fed at 0700 and 1900 hours, and orts were recorded at 1900 daily. During the final 3 d of each period, cecal digesta were collected every 6 h, pH was measured, and samples were frozen for subsequent analyses of VFA and NH3 concentrations. Feed intake during the final 4 d of each period was recorded. Feces were collected during the 3-d sampling period, pooled, subsampled, and frozen. Fecal samples were analyzed for pH and used to determine digestibilities of DM, OM, NDF, and ADF. Statistical analyses were performed via the GLIMMIX procedure of SAS 9.4. Linear and quadratic effects of sodium caseinate on pH, VFA concentrations, and apparent digestibility were assessed by SAS. Digestibilities of DM, OM, ADF, and NDF were unaffected by treatment (P > 0.40). Horses dosed with CON and MED treatments had greater cecal pH than those fed LOW or HI treatments (P < 0.01). Cecal NH3 concentrations increased linearly in response to the amount of casein administered (P < 0.01). Cecal NH3 decreased 6 h after dosing and addition of new hay, regardless of treatment (P < 0.01). Total cecal VFA were unaffected by treatment (P > 0.10), but VFA changed over time with the greatest concentrations observed 6 h after treatments were administered and introduction of new hay (P < 0.01). Treatment did not affect DMI (P ≥ 0.17). In this experiment, cecal infusions of sodium caseinate had minimal to no effect on fermentation parameters or fiber degradation in the horse. A type II error may have occurred due to small population size or the medium quality hay fed to these horses provided sufficient N for microbial fermentation.
Keywords: cecal fermentation, fiber digestion, horse, sodium caseinate
INTRODUCTION
In ruminants, supplemental degradable intake protein improves microbial fermentation and utilization of low-quality forages (Köster et al., 1996). Both protein and nonprotein N enhance fiber digestion and DMI in cattle consuming forages containing <7% CP (Moore and Kunkle, 1995; Köster et al., 1996). Providing supplemental N to ruminal microorganisms subjected to a protein-limited environment allows fibrolytic bacteria to generate additional peptides, AA, and ammonia, which ultimately lead to improved fermentation of feedstuffs and, consequently, animal performance.
Although feed efficiency is not of primary concern in the horse industry, proper digestion of dietary fiber is critical to gut health, energy supply, and overall well-being. In fact, consumption of poor quality forages is associated with reduced DM and NDF degradation, which may lead to impaction colic and inadequate nutrient availability.
Both the rumen and cecum are sites of vast microbial ecosystems that require N for proper growth and function. In the horse, however, the small intestine is located proximal to the primary site of fermentation. As a result, the quantity of dietary N reaching the microbial population of the hindgut is less than that presented to microorganisms in the forestomach of ruminants. Given these similarities and dissimilarities, it can be hypothesized that N may be limiting to carbohydrate digestion in the cecae of horses fed low or moderate quality forages, effectively limiting digestibility of DM, NDF, and ADF. The purpose of this study was to determine if protein supplemented as sodium caseinate directly into the cecum would improve digestion and utilization of a cool season grass hay.
MATERIALS AND METHODS
Animals, Facilities, and Diets
All procedures were approved by the Kansas State University Institutional Animal Care and Use Committee. Eight mature Quarter Horses (four geldings, four mares) with an initial mean BW of 515 ± 15.4 kg and previously fitted with cecal cannulae (Beard et al., 2011) were used in a replicated 4 × 4 (treatment × horse) Latin square design.
Horses were individually housed in 3.05 m × 3.66 m stalls bedded with pine shavings and turned out for 15 to 30 min per day into a drylot during noncollection days. Water and Smooth Bromegrass hay (Table 1) were provided ad libitum, with hay provided at 0700 and 1900 hours each day. Refusals of hay were recorded at 1900 hours. Hay samples were collected throughout the study with a hay core sampler (#07190, AgraTronix, Streetsboro, OH). Upon completion of the study, samples were mixed and a sub-sample collected for proximate analysis.
Table 1.
Analysis of Smooth Bromegrass hay provided to horses ad libitum1,2
| Item | Amount |
|---|---|
| DM, % | 90.40 |
| OM, % | 91.38 |
| CP, % | 8.50 |
| NDF, % | 58.60 |
| ADF, % | 34.90 |
| Non-fiber carbohydrates, % Lignin, % |
20.30 4.60 |
| Crude fat, % | 3.20 |
| Digestible energy, Mcal/kg | 2.59 |
| Ca, % | 0.50 |
| P, % | 0.15 |
| Mg, % | 0.11 |
| K, % | 2.29 |
| AIA, % | 2.62 |
1Proximate analysis using wet chemistry (Dairy One Forage Lab, Ithaca, NY).
2DM basis.
Treatments
Treatments consisted of a control (CON; water only), 0.125 g sodium caseinate/kg BW (LOW; Erie Foods International, Inc., Rochelle, IL), 0.25 g sodium caseinate/kg BW (MED), and 0.50 g sodium caseinate/kg BW (HI). Sodium caseinate (casein) was solubilized in 800 mL distilled water using a 4-L heavy duty blender (Waring Commercial, Torrington, CT). Solutions were prepared daily and kept at 2 °C until time of application. Treatments were brought to room temperature and then administered to horses via cecal cannulae using a 500-mL dosing syringe. Horses were dosed with designated treatments twice daily (0700 and 1900 hours) for 14 d. At the conclusion of each 14-d period, horses were switched to their next respective treatment (Table 2). With four periods, the experiment lasted a total of 56 d.
Table 2.
Quantity of sodium caseinate (g/kg BW) administered by period1
| Horse | Period 1 | Period 2 | Period 3 | Period 4 |
|---|---|---|---|---|
| 1 | 0 | 0.125 | 0.25 | 0.50 |
| 2 | 0 | 0.125 | 0.25 | 0.50 |
| 3 | 0.125 | 0.25 | 0.50 | 0 |
| 4 | 0.125 | 0.25 | 0.50 | 0 |
| 5 | 0.25 | 0.50 | 0 | 0.125 |
| 6 | 0.25 | 0.50 | 0 | 0.125 |
| 7 | 0.50 | 0 | 0.125 | 0.25 |
| 8 | 0.50 | 0 | 0.125 | 0.25 |
1Horses in the control group were administered 800 mL distilled water via cecal cannulae. Sodium caseinate was solubilized in 800 mL distilled water and administered via cecal cannulae two times daily, and each period was 14 d.
Sample Collection
Starting at 0700 on day 12 of each period, cecal digesta were collected from each horse via gravity flow every 6 h (0700, 1300, 1900 and 0100 hours) for 3 d (days 12, 13, 14, and 0100 hours on day 15). In the first treatment period, samples from the final collection time (0100 hours on day 15) were not obtained and, therefore, not included in the analysis. Cecal fluid was immediately strained through four layers of cheesecloth, placed into 500-mL containers (Specimen Storage Containers, #14955117A, Fisher Scientific, Pittsburg, PA), and frozen (−18 °C) for later analyses. Pine shavings were removed from stalls and cleaned on day 12 to allow the collection of total fecal output from stall floors from days 12 to 14. At the conclusion of the 72-h sampling period, feces were mixed by hand and a 100-g subsample from each horse was obtained. Fecal samples were frozen (−18 °C) for later analyses.
Sample Analyses
Strained cecal fluid was immediately measured for pH after collection using a portable pH meter (Thermo Scientific Orion 3 Star Portable pH Meter, Waltham, MA). From each sample, four 1-mL aliquots of cecal fluid were transferred by pipette into microcentrifuge tubes and deproteinated with 25% (w/v) meta-phosphoric acid at a 4:1 ratio (fluid: meta-phosphoric acid). Samples were frozen (−18 °C) and saved for later analyses of VFA and NH3.
Deproteinated cecal samples were thawed and centrifuged at 17,000 × g for 15 min. The aqueous supernatant was transferred to gas chromatography vials in duplicate and analyzed for VFA concentrations using an Agilent 7890 gas chromatograph (Agilent Technologies, Santa Clara, CA) equipped with a DB-WAX capillary column (30 m × 0.53 mm × 0.5 mm film thickness; Sigma-Aldrich, St. Louis, MO) and flame ionization detector. Volatile fatty acids were quantified by comparison to known standards (Supelco Volatile Fatty Acid Standard Mix; Sigma-Aldrich) containing acetate, propionate, isobutyrate, butyrate, isovalerate, valerate, isocaproate, caproate, and heptanoate. Deproteinized cecal samples were also analyzed, in duplicate, for NH3 concentrations using a Technicon AutoAnalyzer3 (Technicon Instruments Corporation, Tarrytown, NY; Technicon Industrial Method #512-77T; Broderick and Kang, 1980).
Thawed fecal samples were dried at 55 °C using a forced air oven for 24 h, air-equilibrated, and weighed. Hay and fecal samples were ground using a Wiley Mill (Model 4, Thomas Scientific, Swedesboro, NJ) until they passed through a 1-mm screen. A 1-g aliquot of each ground sample was used to determine DM, ash, and OM according to the protocols of the National Forage Testing Association (Undersander et al., 1993).
Neutral detergent fiber and nonsequential ADF concentrations in hay and fecal matter were determined using an ANKOM200/220 Fiber Analyzer (ANKOM Technology, Macedon, NY) and the protocols established by Van Soest et al. (1991). Acid insoluble ash (AIA) concentrations were determined according to the protocol established by Van Keulen and Young (1977). Acid insoluble ash was used as an indigestible marker to determine nutrient digestibility using the following equation:
Statistical Analyses
All statistical analyses were performed using the GLIMMIX procedure of SAS 9.4 (SAS Institute Inc., Cary, NC). Digestibilities and fecal pH were analyzed using fixed effects of treatment and period with a random effect of horse. Cecal VFA, NH3, and pH were analyzed using a random effect of horse and fixed effects of treatment, time, and treatment by time interaction. DMI was analyzed using the fixed effects of treatment, period, and treatment by period, with the random effect of horse. Degrees of freedom were determined using the Kenward–Rogers approximation. Significance was declared at P ≤ 0.05 and a tendency considered at 0.05 < P ≤ 0.10. Differences among least-squares means were determined using the PDiff option of SAS. Linear and quadratic effects of the sodium caseinate on pH, VFA concentrations, apparent nutrient digestibility were assessed by SAS.
RESULTS
Cecal and Fecal pH
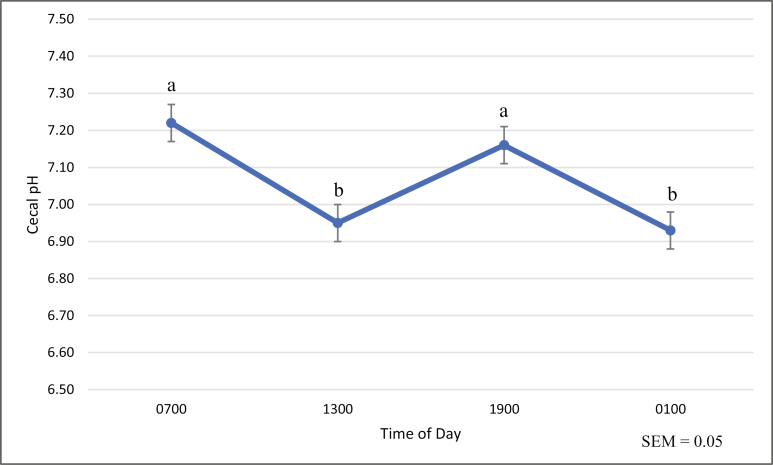
Mean cecal pH in horses dosed with LOW (7.04 ± 0.05) and HI (7.01 ± 0.05) treatments were less (P < 0.01) than that of control horses (7.11 ± 0.05; Table 3). A time effect was also observed, with cecal pH being less (P < 0.01) at 1300 (6.95 ± 0.05) and 0100 hours (6.93 ± 0.05) when compared to 0700 (7.22 ± 0.05) and 1900 hours (7.16 ± 0.05; Fig. 1). There was no treatment by time interaction detected. There were no treatment differences in fecal pH (Table 3).
Table 3.
Effect of cecally infused sodium caseinate on cecal pH, cecal VFA concentrations, cecal NH3 concentrations, and fecal pH in horses consuming a cool season grass hay1,2
| Item | CON | LOW | MED | HI | SEM | P-value3 |
|---|---|---|---|---|---|---|
| Cecum | ||||||
| pH | 7.11a | 7.04b | 7.11a | 7.01c | 0.05 | <0.01 |
| Acetate, mM | 43.20 | 44.50 | 43.20 | 44.10 | 4.40 | 0.99 |
| Propionate, mM | 11.50 | 11.89 | 11.51 | 12.51 | 1.25 | 0.91 |
| Acetate:propionate ratio | 4.35 | 4.29 | 4.31 | 4.02 | 0.21 | 0.58 |
| Butryate, mM | 4.90 | 5.50 | 4.75 | 5.60 | 0.66 | 0.66 |
| Isovalerate, mM | 0.06a | 0.04a | 0.05a | 0.12b | 0.05 | <0.01 |
| Isobutyrate, mM | 0.07a,b | 0.04a | 0.07a,b | 0.10b | 0.05 | 0.02 |
| Total VFA, mM | 59.98 | 62.83 | 60.24 | 63.53 | 6.30 | 0.96 |
| NH3, mg/100 mL | 0.56 | 0.71 | 0.99 | 1.48 | 0.17 | <0.01 |
| Fecal pH4 | 6.74 | 6.72 | 6.83 | 6.83 | 0.14 | 0.84 |
1Horses in the CON group were administered 800 mL of distilled water via cecal cannulae. Horses in the LOW, MED, and HI group were administered 0.125, 0.25, and 0.5 g/kg BW sodium caseinate, respectively, solubilized in 800 mL of distilled water, via cecal cannulae. Concentrations given represent the mean of samples taken at 0700, 1300, 1900, and 0100 hours on days 12, 13, and 14 of the dosing period.
2Values with different superscripts differ (P < 0.05).
3 P-value for overall model F-test.
4pH of pooled fecal samples representing d 12, 13, and 14 of each period.
Figure 1.
Effect of time on cecal pH†,‡
†Horses were provided ad libitum Smooth Bromegrass hay and water. New hay was offered at 0700 and 1900 hours. ‡Means across all treatment groups (CON, MED, MED, and HI) on days 12 to 14. Horses in the CON group were administered 800 mL of distilled water via cecal cannulae. Horses in the LOW, MED, and HI group were administered 0.125, 0.25, and 0.5 g/kg BW sodium caseinate, respectively, solubilized in 800 mL of distilled water, via cecal cannulae. a,bValues with different letters differ (P < 0.01).
Cecal VFA
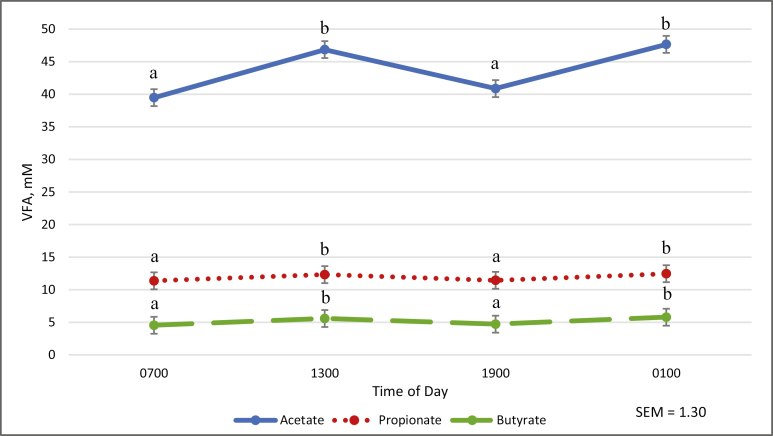
Administration of casein had no effect on cecal concentrations of acetate, propionate, or butyrate, or acetate to propionate ratio (A:P; Table 3). Similarly, total VFA concentrations were unaffected by treatment (Table 3). Isovalerate was elevated (P < 0.05; Table 3) in horses dosed with HI treatment compared to all other treatments. Isobutyrate was less (P < 0.05) with LOW treatment when compared to HI (Table 3). Acetate, propionate, and butyrate were elevated (P < 0.05) at 1300 and 0100 hours when compared to 0700 and 1900 hours (Fig. 2.)
Figure 2.
Effect of time on cecal acetate, proprionate, and butyrate concentrations†,‡†Mean cecal VFA concentration across all treatment groups. ‡There was no treatment by time interaction (P > 0.10). a,bMeans within a line with different letter differ (P < 0.01).
Feed Intake and Digestibility
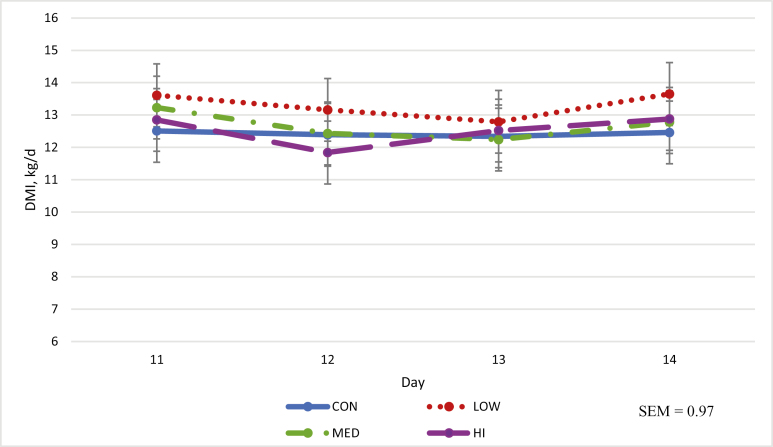
DMI was unaffected by treatment (Fig 3). Treatment had no effect on digestibilities of DM, NDF, ADF, or OM (Table 4).
Figure 3.
Effect of cecally infused sodium caseinate on voluntary DMI†,‡,a,b,c‡Horses in the CON group were administered 800 mL of distilled water via cecal cannulae. Horses in the LOW, MED, and HI group were administered 0.125, 0.25, and 0.50 g/kg BW sodium caseinate, respectively, solubilized in 800 mL of distilled water, via cecal cannulae. aNo effect of treatment (P > 0.05). bNo effect of day (P > 0.05). cNo day by treatment interaction (P > 0.05).
Table 4.
Apparent digestibilities of Smooth Bromegrass hay, %
| Treatment1 | DM | OM | NDF | ADF |
|---|---|---|---|---|
| Control | 46.30 | 46.26 | 44.78 | 45.47 |
| LOW | 43.85 | 43.85 | 42.16 | 43.88 |
| MED | 48.28 | 48.37 | 47.19 | 47.80 |
| HI | 45.51 | 45.37 | 43.62 | 44.45 |
| SEM | 2.36 | 2.45 | 2.94 | 3.04 |
| P-value2 | 0.41 | 0.44 | 0.44 | 0.62 |
1Horses in the control group were administered 800 mL of distilled water via cecal cannulae. Horses in the LOW, MED, and HI groups were administered 0.125, 0.25, and 0.5 g/kg BW sodium casein, respectively, solubilized in 800 mL distilled water, via cecal cannulae.
2 P-value for overall model F-test.
Cecal Ammonia Concentrations
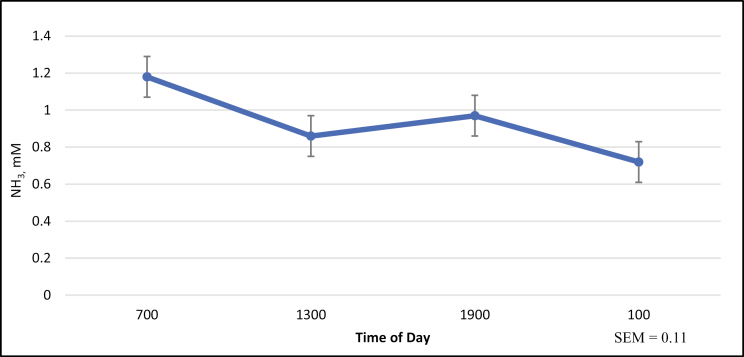
Cecal NH3 concentrations increased (P < 0.01) linearly as concentrate of casein increased (Table 3). Additionally, there was an effect of time on NH3 concentrations with concentrations being greatest at 0700 hours and lowest at 0100 hours (P < 0.01; Fig 4).
Figure 4.
Effect of time on cecal NH3 concentration†,‡†Mean cecal NH3 concentration across all treatment (CON, LOW, MED, and HI) groups. Horses in the CON group were administered 800 mL of distilled water via cecal cannulae. Horses in the LOW, MED, and HI group were administered 0.125, 0.25, and 0.50 g/kg BW sodium caseinate, respectively, solubilized in 800 mL of distilled water, via cecal cannulae. ‡No treatment by time interaction (P > 0.10). a,b,cValues with different superscripts differ (P < 0.05).
DISCUSSION
Within 6 h of consumption, feedstuffs reach the cecum in horses (De Fombelle, 2003) where they undergo microbial fermentation, resulting in VFA production and a subsequent decrease in cecal pH. This was confirmed in the current study when acetate, propionate, and butyrate were elevated, and pH was reduced at both sampling periods (1300 and 0100 hours) that occurred 6 h following feeding (0700 and 1900 hours). These observations were thus expected and attributed to meal consumption.
Mean cecal pH ranged from 7.01 to 7.11 and was similar to values reported in other studies (Goodson et al., 1988; Medina et al., 2002). Therefore, despite differences noted between treatment groups in mean cecal pH, the physiological significance is probably minimal. Although not significant, horses in the LOW and HI treatment groups had numerically greater (≈3 mM) cecal concentrations of total VFA when compared to CON and MED, which may explain differences observed in cecal pH.
While there were no differences between treatments in digestibilities of DM, NDF, ADF, or OM, a pattern similar to that of changes in cecal VFA concentrations emerged. The same numerical differences were observed where apparent digestibilities in the CON and MED horses were numerically greater (≈2% to 3%) when compared to LOW and HI horses. This contradicts previous data collected in our laboratory (unpublished data) in which we observed increases (P < 0.05) in disappearances of DM, NDF, and ADF when casein was provided to cultures containing equine cecal microorganisms provided with native prairie hay. It is important to note, however, that the in vitro experiment utilized native prairie hay containing 4.8% CP, which was less than the 8.5% CP of Smooth Bromegrass hay used in the current in vivo experiment. Reitnour and Salsbury (1972) reported that horses cecally infused with varying protein sources (fishmeal, soybean meal, and linseed meal) had similar DM digestibilities when compared to horses consuming a basal diet containing 6.1% CP. The authors hypothesized that there were no treatment differences due to increased passage rate caused by cecal distension, as 600 mL of water were required to administer the slurry (Reitnour and Salsbury, 1972). In the present study, 800 mL of water were used to administer treatments. While this may have impacted passage rate, we believe that this is unlikely given the large volume (16 to 68 L) of the equine cecum (Ross and Hanson, 1992). It is more probable that the CP content of the Smooth Bromegrass hay (8.5%) provided to horses in the current experiment satisfied the needs of fibrolytic microbes for ammonia, thus supplemental protein was not necessary nor beneficial, having no effect on fiber digestion nor DMI. This explanation is supported by findings in cattle, whereby supplemental protein increases DM disappearance and DMI of low-quality feedstuffs, but the same effect is not observed when CP of the hay fed exceeds 7% (McCollum and Horn, 1990; Minson 1990).
When comparing mean fecal and cecal pH, fecal values were less. Because greater concentrations of lactate-producing bacteria are found in feces, this finding was not unexpected. In fact, others have reported decreased fecal pH compared to cecal pH in horses (Douthit et al., 2014). These findings provide further evidence that fecal pH is not reflective of cecal parameters (Drougol et al., 2012).
As casein is readily soluble, it is logical to assume that as casein levels increased, more NH3 would be produced from fermentation processes. This was confirmed in the current experiment, as there was a linear increase in NH3 in response to increasing casein. Intraruminal infusions of casein have resulted in similar increases in ruminal NH3–N concentrations (Slyter et al., 1979). Nelson and Tyznik (1971) reported that cecal NH3 was greatest (10.8 mg/100 mL) 1 h postprandially and declined to 5.0 mg/100 mL at 6 h in horses fed casein. This aligns with our results, insofar as NH3 concentrations were decreased 6 h after feeding and dosing.
CONCLUSION
In summary, varying levels of casein administered intracecally to horses consuming a medium-quality bromegrass hay had minimal effects on fermentation parameters or fiber degradation. It is likely that the forage consumed by the horses provided sufficient N to cecal microbes. It is unclear as to whether the same results would occur using a poorer quality forage <8.5% CP. Give the numerical patterns which occurred across several response variables, it is equally as likely that a Type II error occurred due to a low population size. Indeed, managing a herd of cecally cannulated horses presents challenges not normally encountered with their uncannulated counterparts. As a result, increasing the population size was not an option.
LITERATURE CITED
- Beard W. L., Slough T. L., and Gunkel C. D.. 2011. Technical note: a 2-stage cecal cannulation technique in standing horses. J. Anim. Sci. 89:2425–2429. doi: 10.2527/jas.2010-3718 [DOI] [PubMed] [Google Scholar]
- Broderick G., and Kang J.. 1980. Automated simultaneous determination of ammonia and total amino acids in ruminal fluid and in vitro media. J. Dairy Sci. 63:64–75. [DOI] [PubMed] [Google Scholar]
- De Fombelle A., Varloud M., Goachet A. G., Jacotot E., Philippeau C., Drogoul C., and Julliand V.. 2003. Characterization of the microbial and biochemical profile of the different segments of the digestive tract in horses given two distinct diets. J. Anim. Sci. 77:293–304. [Google Scholar]
- Goodson J., Tyznik W. J., Cline J. H., and Dehority B. A.. 1988. Effects of an abrupt diet change from hay to concentrate on microbial numbers and physical environment in the cecum of the pony. Appl. Environ. Microbiol. 54:1946–1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köster H. H., Cochran R. C., Titgemeyer E. C., Vanzant E. S., Abdelgadir I., and St-Jean G.. 1996. Effect of increasing degradable intake protein on intake and digestion of low-quality, tallgrass-prairie forage by beef cows. J. Anim. Sci. 74:2473–2481. [DOI] [PubMed] [Google Scholar]
- McCollum F. T. III, and Horn G. W.. 1990. Protein supplementation of grazing livestock: a review. Prof. Anim. Sci. 6:1−16. doi:10.15232/S1080-7446(15)32251-8 [Google Scholar]
- Medina B., Girard I. D., Jacotot E., and Julliand V.. 2002. Effect of a preparation of Saccharomyces cerevisiae on microbial profiles and fermentation patterns in the large intestine of horses fed a high fiber or a high starch diet. J. Anim. Sci. 80:2600–2609. doi:10.1093/ansci/80.10.2600 [DOI] [PubMed] [Google Scholar]
- Minson D. J. 1990. Forage in ruminant nutrition. 1st ed. San Diego, CA:Academic Press. [Google Scholar]
- Moore J. E., Kunkle W. E.. 1995. Improving forage supplementation programs for beef cattle. 6th. Florida Rumin. Nutr. Symp. 65–75. [Google Scholar]
- Nelson D. D., and Tyznik W. J.. 1971. Protein and nonprotein nitrogen utilization in the horse. J. Anim. Sci. 32:68–73. doi: 10.2527/jas1971.32168x [DOI] [PubMed] [Google Scholar]
- Reitnour C. M., and Salsbury R. L.. 1972. Digestion and utilization of cecally infused protein by the equine. J. Anim. Sci. 35:1190–1193. [DOI] [PubMed] [Google Scholar]
- Ross W. R., and Hanson R. R.. 1992. Large intestine. In: J. A., Auer, editor, Equine surgery., Philadelphia, PY:W.B. Sauders; p. 379–406. [Google Scholar]
- Slyter L., Satter L., and Dinius D.. 1979. Effect of ruminal ammonia concentration on nitrogen utilization by steers. J. Anim. Sci. 48: 906–912. doi:10.2527/jas1979.484906x [Google Scholar]
- Undersander D., Mertens D. R., and Thiex N.. 1993. Forage analyses procedures. Omaha, NE:National Forage Testing Association. [Google Scholar]
- Van Keulen J., and Young B.. 1977. Evaluation of acid-insoluble ash as a natural marker in ruminant digestibility studies. J. Anim. Sci. 44: 282–287. doi:10.2527/jas1977.442282x [Google Scholar]
- Van Soest P. V., Robertson J., and Lewis B.. 1991. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 74: 3583–3597. doi:10.3168/jds.S0022-0302(91)78551-2 [DOI] [PubMed] [Google Scholar]