Abstract
Benzene is a ubiquitous pollutant associated with hematotoxicity but its metabolic effects are unknown. We sought to determine if and how exposure to volatile benzene impacted glucose handling. We exposed wild type C57BL/6 mice to volatile benzene (50 ppm × 6 h/day) or HEPA-filtered air for 2 or 6 weeks and measured indices of oxidative stress, inflammation, and insulin signaling. Compared with air controls, we found that mice inhaling benzene demonstrated increased plasma glucose (p = .05), insulin (p = .03), and HOMA-IR (p = .05), establishing a state of insulin and glucose intolerance. Moreover, insulin-stimulated Akt phosphorylation was diminished in the liver (p = .001) and skeletal muscle (p = .001) of benzene-exposed mice, accompanied by increases in oxidative stress and Nf-κb phosphorylation (p = .025). Benzene-exposed mice also demonstrated elevated levels of Mip1-α transcripts and Socs1 (p = .001), but lower levels of Irs-2 tyrosine phosphorylation (p = .0001). Treatment with the superoxide dismutase mimetic, TEMPOL, reversed benzene-induced effects on oxidative stress, Nf-κb phosphorylation, Socs1 expression, Irs-2 tyrosine phosphorylation, and systemic glucose intolerance. These findings suggest that exposure to benzene induces insulin resistance and that this may be a sensitive indicator of inhaled benzene toxicity. Persistent ambient benzene exposure may be a heretofore unrecognized contributor to the global human epidemics of diabetes and cardiovascular disease.
Keywords: benzene, insulin resistance, diabetes, cardiovascular disease, risk factor
The incidence of type 2 diabetes (T2D) is growing worldwide and is expected to continue to increase in the future (Tuomi et al., 2014; Zimmet et al., 2014). Although some of the rise of T2D prevalence is attributable to excessive caloric intake, minimal physical activity, and an increased life span, the development of T2D is multifactorial and may also be influenced by environmental factors (Bhatnagar, 2009; Willett, 2002). Indeed, it has been estimated that between 60% and 70% of T2D could be attributed to environmental, or non-genetic factors (Talmud et al., 2010). Whereas such factors, other than socioeconomic status and lifestyle choices, that contribute to the incidence, progression and severity of T2D are not clearly identified, a growing body of work has linked insulin resistance with exposure to air pollution. Acute exposure to particulate matter air pollution (PM) is associated with a transient increase in insulin resistance (Sun et al., 2009) and chronic exposures to high levels of PM have been linked with higher prevalence of diabetes and insulin resistance in humans (Brook et al., 2016; Kelishadi et al., 2009). Similarly, insulin resistance, the prevalence of T2D and mortality are also associated with long-term exposure to traffic pollution (Andersen et al., 2012; Kramer et al., 2010). The plausibility of the association between PM and T2D is supported by animal studies that show exposure of mice to concentrated ambient particles results in vascular and systemic insulin resistance (Brook et al., 2013; Haberzettl et al., 2016a). Collectively, these findings inform a new paradigm wherein inhalation of air pollutants can induce and exacerbate insulin resistance and metabolic disease.
Air pollution is a complex mixture of particles (ultrafines, PM2.5, PM10) gases, metals, and volatile organic compounds (VOCs, eg, benzene, acrolein, butadiene, and xylene). Whereas some components, such as PM2.5 and ozone, are well monitored and well-studied, less is known about the levels and toxicity of VOCs, that individually or collectively may have significant health impacts. Although the effects of ambient levels of VOCs are infrequently studied, occupational VOC exposures have received considerable attention. Benzene can be particularly abundant in occupational settings (> 30 ppm) (Lin et al., 2007; Vermeulen et al., 2005) and levels can reach several hundred ppm in certain industrial scenarios (Wong and Fu, 2005). Benzene is also present at high levels in vehicle emissions and cigarette smoke (> 50 ppm). The health effects of high levels of benzene have been studied extensively and its hematotoxic and leukemic effects are well known (ATSDR, 2005a; Galbraith et al., 2010; IARC, 1987, 1982b). Whereas little is known about the cardiovascular and metabolic effects of benzene, acute benzene poisoning in humans and inhalation by rats have been associated with ventricular fibrillation (Avis and Hutton, 1993) and arrhythmia (Magos et al., 1990). In addition a statistical association has been found between levels of urinary benzene metabolites in elderly adults and insulin resistance (Choi et al., 2014). Nevertheless, studies directly addressing the metabolic effects of benzene exposure are lacking.
Although benzene itself is relatively inert, its metabolism by the hepatic enzyme CYP2E1 generates superoxide anions (O2−), hydroxyl radicals (OH) and hydrogen peroxide (H2O2) (Caro and Cederbaum, 2004; Shen et al., 1996) and it is believed that this oxidative stress contributes to the inflammatory and cytotoxic effects of benzene on multiple tissues (Badham et al., 2010; Verma and Rana, 2008). The role of CYP2E1-generated reactive oxygen species (ROS) in pathophysiological outcomes is supported by studies demonstrating that CYP2E1 over-expression causes steatosis whereas its knockout protects from weight gain and insulin resistance in mice fed a high-fat diet (Schattenberg et al., 2005; Zong et al., 2012).
Because benzene metabolism produces ROS, and oxidative stress and inflammation are causative in the development and progression of insulin resistance and T2D (Bashan et al., 2009; Ceriello and Motz, 2004; Evans et al., 2005), we sought to determine whether exposure to benzene could lead to metabolic disturbances resulting in glucose intolerance and insulin resistance. We found that exposure of mice to volatile benzene-induced insulin resistance in the liver and skeletal muscle that was associated with Nf-κb activation, Mip-1α expression, and Socs1-mediated inhibition of Akt-phosphorylation. These results provide the first mechanistic evidence that exposure to benzene induces insulin resistance in mice and suggest that exposure to benzene may be a previously unrecognized risk factor for the development of insulin resistance and subsequent metabolic disease in humans.
MATERIALS AND METHODS
Benzene exposure
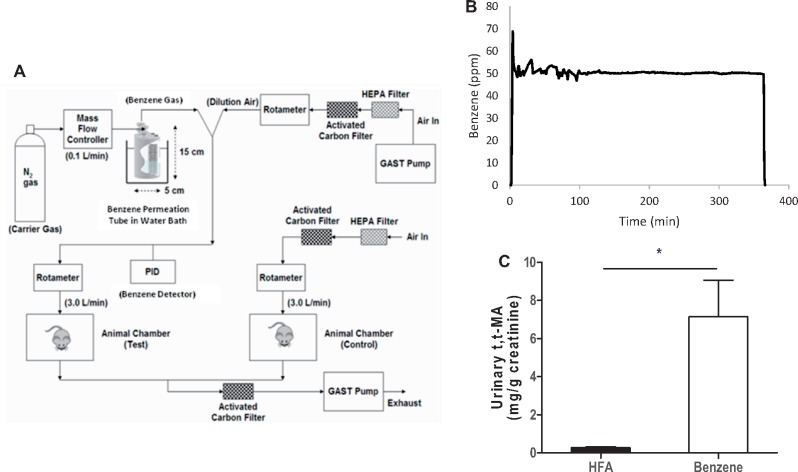
Ten-week old male C57BL/6 mice were obtained from Jackson Laboratories and were provided normal chow and water ad libitum throughout exposures. In some experiments, mice were placed on drinking water containing 1 mM TEMPOL (4-Hydroxy TEMPO: Sigma-Aldrich) starting 3 days prior to initiation of exposure and continuing throughout the exposure duration. Drinking water was changed daily. Benzene atmospheres were generated from liquid benzene (Sigma-Aldrich) in a KIN-TEK Analytical, Inc permeation tube (Figure 1A). A carrier gas (N2) was delivered to the permeation tube at 100 ml/min and diluted with HEPA- and charcoal-filtered room air (HFA; 3 L/min) and diluted gas directed to an exposure unit. Flow was distributed through a fine mesh screen of a custom cyclone-type top (Teague Enterprises) that distributed air within 10% of the mean concentration at six locations in the cage. Throughout an exposure, benzene concentrations were continuously monitored (Figure 1B) using an in-line photoionization detector (ppb RAE: Rae Industries) upstream of the exposure unit. Mice were exposed to 50 ppm benzene for 6 h/day for 2 or 6 weeks or to 10 ppm benzene for 6 h/day for 2 weeks. Mice exposed to HFA only were used as a control. Exposures in individual animals were assessed by measuring the urinary levels of the benzene metabolite, muconic acid, t,t-MA (Figure 1C). All procedures were approved by the University of Louisville Institutional Animal Care and Use Committee.
Figure 1.
Benzene inhalation exposures. A, Illustrated is a schematic of the benzene and air exposure system. Benzene was released from a certified permeation tube (Kin-Tek) in a constant temperature water bath into low flow N2 carrier gas (100 ml/min) and diluted to desired level with HEPA-filtered room air (HFA). Benzene concentration (ppm) was monitored continuously with an inline photoionization detector (PID) upstream of the exposure chamber. For control air exposure, HFA was delivered (matched to flow rate as benzene) to a separate, air-only exposure chamber. Exhaust air from benzene and HFA exposure chambers was passed through additional carbon and HEPA filters placed inside a certified fume hood before external exhaust. B, Representative PID data trace of benzene level recorded inline (1 min averages) prior, during and after a 6 h exposure. C, To assess internal exposure, urine collected from mice exposed to either benzene or HFA for 14 days was analyzed for the benzene-specific metabolite, t,t-MA. Shown are the mean t,t-MA levels normalized to creatinine. N = 10 total mice; *p < .05.
Complete blood cell counts and plasma biochemistry
At end of the exposure protocol, mice were euthanized with sodium pentobarbital (150 mg/kg body weight; i.p. 100 μl of solution in PBS). Peripheral blood was collected in 0.2 M EDTA-coated syringes by cardiac puncture and an aliquot of 25 µl was used for complete blood count on a Hemavet 950FS (Coulter). Plasma levels of total protein, albumin, alanine aminotransferase (ALT), and aspartate aminotransferase (AST) were measured on a COBAS MIRA-plus analyzer (Roche, New Jersey).
Analysis of urinary metabolites
Urine was collected in chilled tubes from mice housed in metabolic cages for 18 h. The urine was then centrifuged at 400 × g for 5 min and the supernatant stored at −20°C. Aliquots of these samples (20 µl) were mixed with an equal volume of 0.5 M sodium phosphate, pH 8.0 containing 10 µM t,t-MA as an internal standard and incubated with 130 µl 0.1 M PFBBr for 60 min at 50°C, and then extracted with 300 µl hexane. The extract (100 µl) was removed and analyzed on an Agilent 6890 N GC-MS with chemical ionization ion source. Six-point calibration curves were used to calculate the concentrations of t,t-MA (2.5 µM to 100 µM). Levels of urinary t,t-MA were normalized to levels of creatinine, which were measured on a COBAS MIRA-plus analyzer with Infinity Creatinine Reagent (Thermo Fisher Scientific, Massachusetts) (Srivastava et al., 2011).
In vivo assessment of glucose handling
Plasma glucose was measured following a 6 h fast using a standard glucose meter (Accu-check, Aviva) and glucose test strips (Accu-check, Aviva Plus). Fasting plasma insulin was measured by ELISA (Mercodia). HOMA-IR was calculated using the equation: HOMA-IR = (FPG [mg/dl]*FPI [mIU/l])/405, whereas HOMA-β was determined using the equation: HOMA-β = (20*FPI [mIU/l])/(FPG [mmol/l]-3.5) (Matthews et al., 1985). For glucose tolerance tests, (GTT) mice were fasted for 6 h and d-glucose (1 mg/g body weight, i.p.) was given as previously described in McGuinness et al. (2009). Insulin tolerance tests (ITT) were performed on unfasted animals by injection of Humulin R (Eli Lilly; 1.0 U/kg body weight). Areas under the curve (AUC; GTT) and area above the curve (AAC; ITT) were calculated using the trapezoid rule with subtraction of baseline glucose area (Conklin et al., 2017). Organ-specific insulin signaling was examined by injecting animals with saline or insulin (1.5 U kg/g body weight, i.p.) 15 min prior to euthanasia, followed by organ extraction and preservation in liquid nitrogen. Levels of phospho-Akt were measured in homogenates by Western blotting. To analyze levels of pIrs2, 200 µg of tissue homogenate was used in a PathScan ELISA kit (Cell Signaling). Data (absorbance values at 450 nm) were normalized to filtered air controls.
Western blotting and qPCR
Liver and skeletal muscle homogenates were prepared in chilled RIPA buffer using a Dounce homogenizer and then centrifuged at 4°C for 10 min at 17 000 × g. The supernatants were collected and protein concentrations were measured using a Bradford assay. For analysis of Nfκb p65 and phospho-Nfκb p65, nuclear extracts were prepared using the Episeeker Nuclear Extraction kit (Abcam). Western blotting with specific antibodies (Supplementary Table 1) was performed to analyze the levels of Akt, phospho-Akt Ser473, Nfκb p65, phospho-Nfκb p65 Ser536, Socs1, and Socs3. Images were acquired using a Typhoon 7000 FLA imaging system (GE Healthcare) and band intensities were quantified using the ImageJ software (NIH.gov). For quantitative rtPCR, RNA was extracted from frozen tissues using the miRNEasy isolation kit (Qiagen). Levels of Mip-1α, IL-1β, IL-6, and Tnfα were then measured using specific primers (Integrated DNA Technologies; Supplementary Table 2) and the Universal SYBR Green PCR Master Mix (Stratagene) on an Applied Biosystems 7900HT Fast Real Time instrument. GAPDH was used as an internal control.
Immunohistochemistry
Formalin-fixed, paraffin-embedded liver sections (4 µm) were de-paraffinized and rehydrated by sequential immersion in a graded series of alcohol and water. The sections were heated in a microwave oven for 10 min in 10 mM sodium citrate buffer for epitope retrieval. After washing with PBS, pH 7.4, sections were blocked with 10% fetal calf serum for 30 min and then incubated with an anti-CD68 antibody (Abcam) at a 1:20 dilution for 18 h at 4°C, followed by incubation with TRITC-conjugated goat anti-rabbit IgG for 60 min at room temperature. Stained sections were visualized on a Nikon Eclipse Ti fluorescent microscope using a 20× objective and images were acquired.
Measurements of oxidative stress
Glutathione (GSH) levels were measured in frozen liver and skeletal muscle samples using the BIOXYTECH GSH-412TM Colorimetric Determination Glutathione Kit (Oxis Research). To measure intracellular GSH in leukocytes, cell preparations were incubated with monochlorobimane (40 μM) for 20 min at room temperature and immunofluorescence was analyzed on an LSRII flow cytometer (Becton Dickinson). To measure the lipid peroxidation product malondialdehyde (MDA), a Lipid Peroxidation (MDA) Assay Kit (Sigma) was used.
Statistics
Data are presented as mean ± standard error of the mean. All data were analyzed using GraphPad Prism version 5.0 software (GraphPad Software, La Jolla, California). Data from inhalation exposure experiments are derived from multiple exposures. The data were analyzed and compared by two-tailed Student’s t tests, except where comparisons were made across more than two groupings using one-way ANOVA as appropriate. A p value of < .05 was considered statistically significant.
RESULTS
Systemic Effects of Benzene Exposure
Benzene is a ubiquitous pollutant found at high levels in some workplaces (> 30 ppm) (Lin et al., 2007; Vermeulen et al., 2005) in tobacco products (20–100 ppm per puff) (Appel et al., 1990) and in some vehicle exhausts (> 50 ppm). To mimic these conditions in examining the metabolic effects of benzene inhalation, mice were exposed to 50 ppm volatile benzene or HFA for 6 h/day and hematological and biochemical outcomes were analyzed. Initial exposures were for 2 weeks, in keeping with some other studies which demonstrated hematological disruption at this exposure duration (Aoyama, 1986; Cronkite et al., 1989). Additional exposures were performed for 6 weeks. After 2 weeks of exposure, benzene only affected neutrophil and red blood cell counts (Supplementary Table 3). However, mice that were exposed to benzene for 6 weeks had significantly lower levels of circulating white blood cells, neutrophils, lymphocytes, monocytes, and platelets than mice breathing filtered air (Supplementary Table 3). Two weeks of exposure to benzene also increased the liver:body weight ratio (Supplementary Table 3), but had no effect on overall body weight or growth. Benzene-exposed mice also demonstrated significantly elevated plasma ALT and AST levels compared with mice breathing filtered air (Supplementary Table 3). In comparison with control mice, mice exposed to benzene for 6 weeks had higher levels of plasma albumin (Supplementary Table 3). These observations suggest that exposure to 50 ppm benzene not only affects hematology but also induces mild liver injury in mice.
Benzene Exposure and Glucose Handling
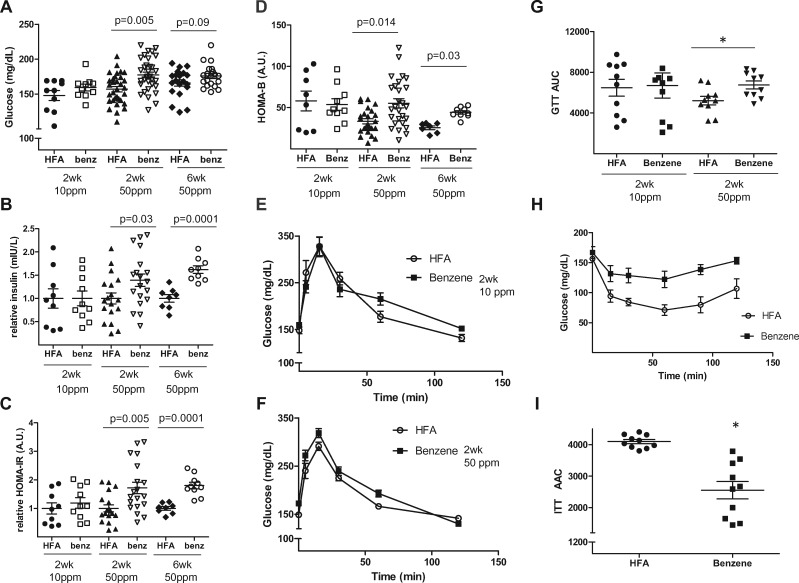
Given the marked systemic effects of benzene, we next examined its effects on glucose handling. We found that mice exposed to 50 ppm benzene for 2 weeks had a 1.13-fold higher level of fasting plasma glucose (Figure 2A) and a 1.39-fold higher level of fasting plasma insulin (Figure 2B) than mice breathing filtered air, suggesting that exposure to benzene might induce insulin resistance. To quantify this effect, we calculated HOMA-IR scores, that were significantly higher in benzene-exposed mice than control mice (Figure 2C). As with HOMA-IR, the values of HOMA-β were also significantly higher in benzene-exposed mice (Figure 2D). Mice exposed to filtered air or a lower dose of benzene (10 ppm) for 2 weeks demonstrated no differences in fasting plasma glucose (Figure 2A), fasting plasma insulin (Figure 2B), HOMA-IR scores (Figure 2C) and HOMA-β scores (Figure 2D). Mice exposed to 50 ppm benzene for a longer duration (6 weeks) had stronger, statistically significant differences versus their air counterparts in both insulin levels (p = .0001 vs p = .03; Figure 2B) and HOMA-IR scores (p = .001 vs 0.005; Figure 2C). Similarly, GTT in mice exposed to 50 ppm benzene (Figure 2F), but not 10 ppm benzene (Figure 2E) had a modest, but significant increase in the GTT AUC (Figure 2G) relative to control mice. Congruently, the ITT (Figure 2H) and AAC calculations (Figure 2I) showed an appreciable decrement in insulin responsiveness in mice inhaling 50 ppm benzene, indicating that more insulin was required to sequester glucose than in control mice. Given that 10 ppm benzene had no effect on glucose handling and that a 2 weeks of exposure at 50 ppm was effective, all further experiments were done using this level and exposure duration.
Figure 2.
Benzene exposure induces glucose intolerance. Mice were exposed to HEPA-filtered air (HFA) or 10 ppm (n = 10) or 50 ppm benzene for 2 weeks (n = 34) or 6 weeks (n = 20) as indicated and several indices of glucose handling were measured. Illustrated are levels of fasting plasma glucose (A), and levels of fasting plasma insulin (B) and calculated HOMA-IR scores (C) normalized to HFA controls. Also illustrated are calculated HOMA-β values (D). Mice exposed for 2 weeks to 10 ppm benzene (E) or 50 ppm benzene (F) were subjected to glucose tolerance tests and areas under the curve calculated (G; n = 10). Mice exposed for 2 weeks to 50 ppm benzene were also subjected to insulin tolerance tests (H; n = 10) and areas above the curve calculated (I). *p < .05.
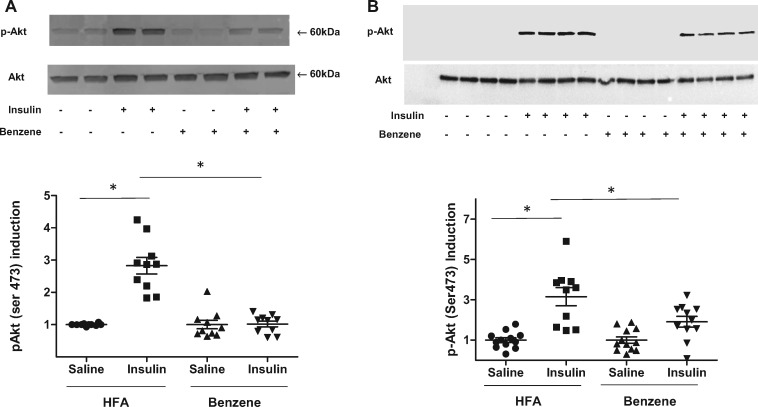
Because benzene exposure perturbs glucose homeostasis and induces a state of systemic glucose intolerance, we examined whether exposure to benzene interfered with insulin signaling. For this, we injected exposed animals with insulin 15 min before euthanasia and then analyzed Akt phosphorylation in insulin-sensitive organs. We found a marked increase in Akt phosphorylation in the liver of air-exposed mice (2.82-fold induction), but an attenuated increase in mice exposed to benzene (1.02-fold increase) (Figure 3A). Similar changes were observed in skeletal muscle, which also showed significant deficits in insulin-induced Akt phosphorylation in benzene-exposed mice (Figure 3B). Collectively, these observations suggest that exposure to benzene induces organ level insulin resistance that likely contributed to systemic glucose and insulin intolerance.
Figure 3.
Benzene exposure attenuates insulin-stimulated Akt phosphorylation. Mice exposed to HFA or 50 ppm benzene for 2 weeks were injected with saline or insulin 15 min before euthanasia. Levels of phosphorylated-Akt were measured in homogenates of liver (A; n = 10) and skeletal muscle (B; n = 9–16) by Western blotting. Levels of total Akt were also examined by Western blotting. Illustrated are representative blots (upper) and the calculated induction of insulin-stimulated Akt phosphorylation (lower); *p < .05.
Benzene Exposure Induces Oxidative Stress and Inflammation
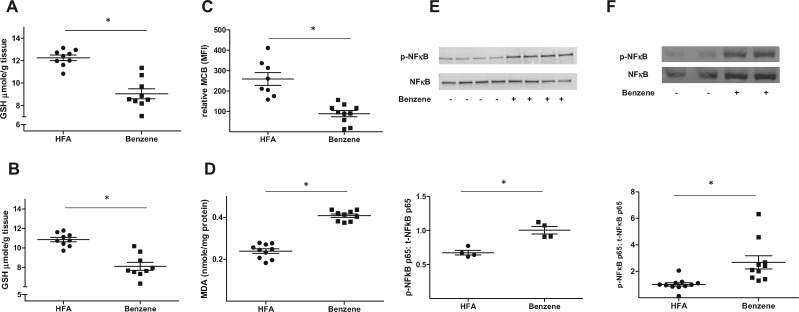
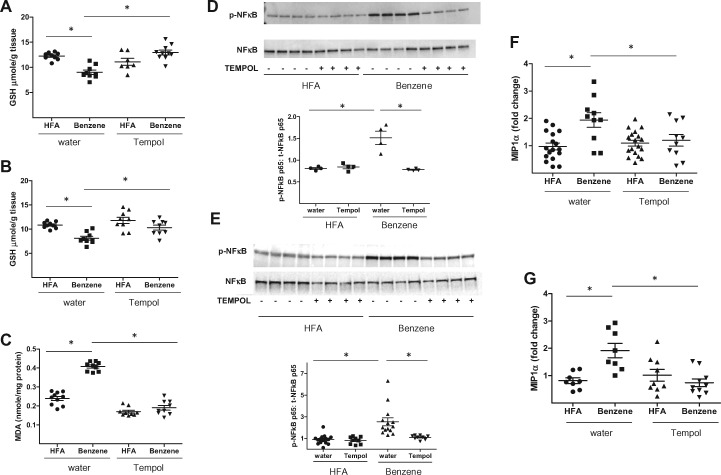
To gain insight into the mechanisms by which benzene exposure caused liver and skeletal muscle insulin resistance, we first examined whether such exposures induced oxidative stress. As ROS neutralize reduced glutathione (GSH), we directly measured levels of GSH. These measurements revealed that the levels of GSH in liver homogenates decreased from 12.2 ± 0.7 µmoles/g tissue in air-exposed mice to 9.0 ± 1.3 µmoles/g tissue in benzene-exposed mice (Figure 4A). A similar decrease in GSH levels was observed in skeletal muscle (Figure 4B). To examine the effects of benzene on GSH levels in blood leukocytes, we loaded these cells with monochlorobimane (MCB), a fluorescent GSH-binding dye, and measured changes in fluorescence by flow cytometry (Haberzettl et al., 2018). As shown in Figure 4C, median MCB fluorescence (ie, GSH levels) was significantly higher in leukocytes from air-exposed mice than from benzene-exposed mice (259 ± 31 vs 89 ± 15 A.U.). Taken together, these results show that in several organs and blood, benzene exposure depleted GSH, indicating systemic oxidative stress. The notion that benzene induces oxidative stress was further corroborated by the observation that hepatic levels of malondialdehyde (MDA), a lipid peroxidation product, were higher in the benzene-exposed animals relative to controls (0.41 ± 0.01 vs 0.24 ± 0.01 nmol/mg protein) (Figure 4D). As oxidative stress can trigger inflammation, we next examined markers of inflammation by assessing changes in the phosphorylation of the p65 subunit of Nfκb. We found in both the liver (Figure 4E) and skeletal muscle (Figure 4F) that phosphorylation of p65 was increased following exposure to benzene. To further support the idea that benzene exposure caused liver inflammation, we examined the presence of monocytes/macrophages in that tissue by immunohistochemistry. Whereas liver sections obtained from mice breathing HFA showed little positive staining with an anti-CD68 antibody (Supplementary Figure 1A) those sections from mice inhaling benzene showed stronger staining with this antibody (Supplementary Figure 1B).
Figure 4.
Benzene exposure induces tissue-specific oxidative stress and inflammation. Mice exposed to HFA or 50 ppm benzene for 2 weeks were euthanized and levels of GSH were measured in homogenates of liver (A; n = 9) and skeletal muscle (B; n = 9). In the same mice, MCB fluorescence was measured in circulating lymphocyte preparations (C; n = 8–10) and MDA was measured in liver homogenates (D; n = 10). Nfκb p65 phosphorylation and total Nfκb p65 levels in nuclear extracts of liver (E; n = 4) and skeletal muscle (F; n = 10) were measured by Western blotting. Representative blots (upper panels) and normalized data (lower panels) are illustrated; *p < .05.
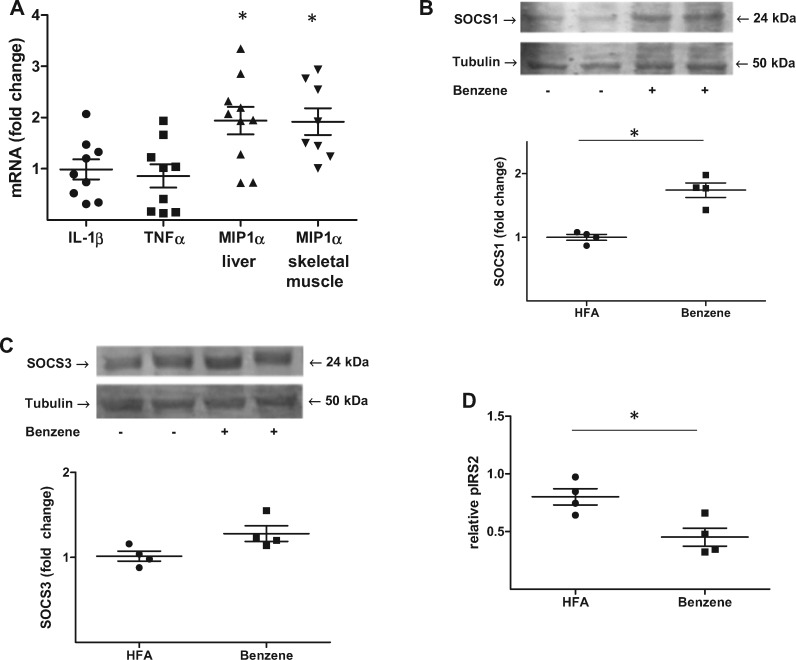
As phosphorylation of p65 results in increased expression of several cytokines, we measured transcript levels of a panel of cytokines by quantitative rtPCR. Whereas hepatic levels of IL-1β and Tnfα were unaffected after 2 weeks of exposure, there was a significant (1.94-fold) increase in levels of Mip-1α in benzene-exposed mice relative to air-exposed mice (Figure 5A). Benzene-exposure also increased Mip-1α in skeletal muscle (Figure 5A). Because Mip-1α increases transcription of Socs1 and Socs3, which are known to attenuate insulin signaling through inhibition of Irs phosphorylation (Qin et al., 2008), we next measured hepatic levels of these proteins. These measurements showed that there was a significant increase in Socs1 (1.74-fold) in benzene-exposed mice (Figure 5B) and a trending increase in Socs3 (1.28-fold, p = .058) (Figure 5C). Finally, because Socs1 can inhibit Irs-2 phosphorylation, we examined the phosphorylation of this protein. We found a significant decrease in insulin-stimulated tyrosine phosphorylation of Irs-2 in the liver of benzene-exposed mice (Figure 5D). Collectively, these observations show that benzene-induced oxidative stress and inflammation may limit insulin responsiveness (Irs-2 phosphorylation) through up-regulation of Socs1.
Figure 5.
Benzene exposure suppresses Irs-2 phosphorylation. Mice exposed to HFA or 50 ppm benzene for 2 weeks were euthanized and quantitative rtPCR performed on RNA preparations of liver and skeletal muscle. Illustrated are the fold changes in select transcripts in benzene-exposed mice relative to HFA-exposed mice (A; n = 8–10); *p < .05. Protein levels of Socs1 (B; n = 4) and Socs3 (C; n = 4) were determined in liver homogenates by Western blot analysis. Tubulin levels were used as a loading control. Illustrated are representative blots (upper panels) and normalized data (lower panels). Levels of insulin-stimulated phospho-Irs2 were also determined in liver homogenates using a specific ELISA (D; n = 4); *p < .05.
TEMPOL Attenuated the Effects of Benzene
To determine if a reduction of oxidative stress would mitigate the effects of benzene, we placed mice on drinking water containing the anti-oxidant TEMPOL (4-hydroxy TEMPO). To examine the efficacy of this treatment, we first measured organ GSH levels. We found that TEMPOL attenuated GSH depletion in the liver of benzene-exposed mice (Figure 6A). GSH-depletion in skeletal muscle was also attenuated by TEMPOL (Figure 6B). To measure oxidative stress more directly, we quantified MDA levels. Benzene-exposed mice receiving TEMPOL-containing drinking water had significantly lower concentrations of hepatic MDA than benzene-exposed animals drinking normal water (0.19 vs 0.41 nmole/mg protein) (Figure 6C). Treatment with TEMPOL also mitigated inflammation as evidenced by decreased levels of phosphorylated Nfκb p65 in both the liver (Figure 6D) and skeletal muscle (Figure 6E). Likewise, levels of Mip-1α transcripts in liver (Figure 6F) and skeletal muscle (Figure 6G) were decreased in the benzene-exposed animals drinking TEMPOL-containing water. Taken together, these observations suggest that inflammation in benzene-exposed mice was likely attributable to oxidative stress.
Figure 6.
Treatment with TEMPOL attenuates benzene-induced oxidative stress and inflammation. Mice drinking normal water or water supplemented with 1 mM TEMPOL were exposed to HFA or 50 ppm benzene for 2 weeks. After euthanasia levels of GSH were determined in homogenates of liver (A; n = 7–10) and skeletal muscle (B; n = 9). MDA levels were also measured in liver homogenates (C; n = 9–10). The levels of phospho-Nfκb and total Nfκb were measured in nuclear extracts of liver (D; n = 4) and skeletal muscle (E; n = 9–14) by Western blotting. Illustrated are representative blots (upper panels) and normalized data (lower panels). Using RNA preparations from the same tissues, quantitative rtPCR was used to quantify the levels of Mip-1α transcripts in liver (F) and skeletal muscle (G). Illustrated are the fold changes using GAPDH as a housekeeping control gene. *p < .05.
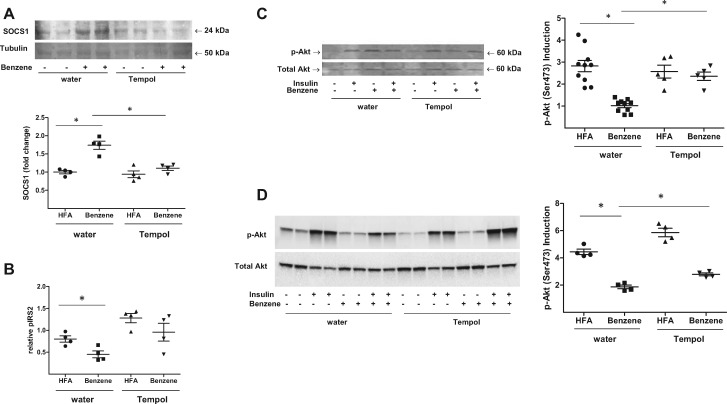
Because TEMPOL limited benzene-induced inflammation, we next determined if this treatment would also limit the effects of benzene on insulin signaling. First, we observed that benzene-exposed mice receiving TEMPOL had decreased expression of Socs1 in the liver compared with mice drinking normal water (Figure 7A). Second, corresponding to decreased Socs1 expression, we found that benzene-exposed, TEMPOL-treated animals exhibited increased insulin-stimulated Irs-2 tyrosine phosphorylation relative to benzene-exposed mice drinking normal water (Figure 7B). Third, we observed that benzene-exposed, TEMPOL-treated animals maintained their responsiveness to insulin and had higher levels of Akt phosphorylation in both the liver (Figure 7C) and skeletal muscle (Figure 7D) than benzene-treated mice drinking normal water.
Figure 7.
TEMPOL treatment restores insulin signaling in benzene-exposed mice. Mice drinking normal water or water supplemented with 1 mM TEMPOL were exposed to HFA or 50 ppm benzene for 2 weeks. After euthanasia, the abundance of Socs1 protein was measured in liver homogenates by Western blotting (A; n = 4). Tubulin levels were used as a loading control. Illustrated are representative blots (upper panels) and normalized data (lower panels). Levels of insulin-stimulated phospho-Irs-2 from liver homogenates were also determined using a specific ELISA (B; n = 4). Levels of phospho-Akt and total Akt were determined by Western blotting in homogenates of liver (C; n = 5–10), and skeletal muscle (D; n = 4) from mice variably treated with insulin as indicated. Illustrated are representative blots (left panels) and the calculated induction of insulin-stimulated Akt phosphorylation (right panels); *p < .05.
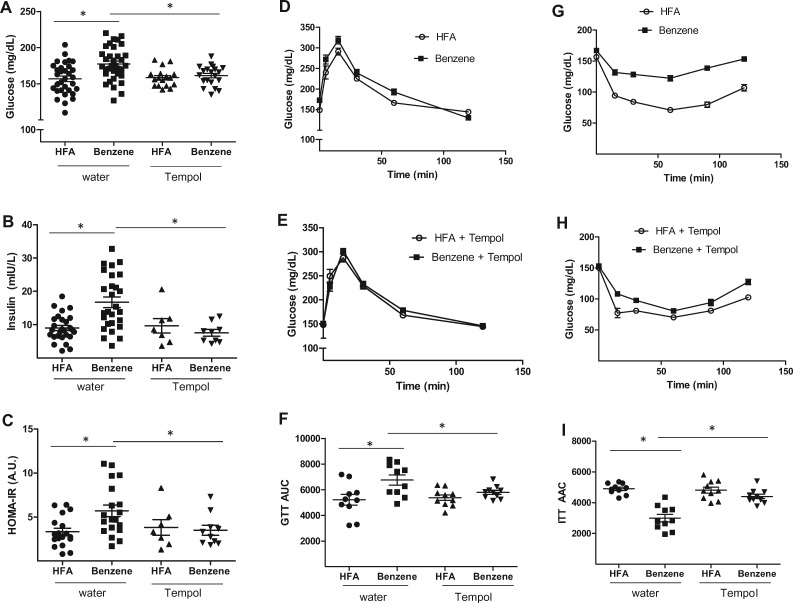
Finally, we determined whether treatment with TEMPOL would prevent the effects of benzene on glucose handling. We found that benzene-exposed mice receiving TEMPOL had significantly lower levels of fasting plasma glucose (Figure 8A) and fasting plasma insulin (Figure 8B) than did their benzene-exposed counterparts drinking normal water. TEMPOL treatment also lowered HOMA-IR scores in benzene-treated mice (Figure 8C), and these mice displayed glucose tolerant responses to either a glucose or an insulin bolus in GTT (Figs. 8D–F) and ITT (Figs. 8G–I) assays, respectively. These observations suggest that benzene-induced insulin resistance is mediated by oxidative stress.
Figure 8.
TEMPOL treatment restores glucose tolerance in benzene-exposed mice. Mice drinking normal water or water supplemented with 1 mM TEMPOL were exposed to HFA or 50 ppm benzene for 2 weeks. At that time, fasting plasma glucose (A; n = 17–34) and fasting plasma insulin (B; n = 7–27), were measured and HOMA-IR scores calculated (C). Glucose handling in these mice was also assessed in glucose tolerance tests (D, E; n = 10) and insulin tolerance tests (G, H; n = 10). Illustrated are representative curves (D, E, G, H) and calculated areas under the curve (F; AUC) or above the curve (I; AAC); *p < .05.
DISCUSSION
The major finding of this study is that inhalation of volatile benzene induces insulin resistance in mice, which could be attributed to the development of systemic oxidative stress. Consistent with this idea, treatment with the antioxidant TEMPOL prevented oxidative stress, inflammation, and insulin resistance in benzene-exposed mice. Furthermore, our results suggest that benzene-induced oxidative stress led to insulin resistance by establishing a state of chronic inflammation, perhaps due to Nfκb activation, increased transcription of Mip-1α and Socs1 expression, thereby inhibiting insulin-stimulated Irs-2 phosphorylation. Collectively, these observations identify inhaled benzene as a new environmental cause of insulin resistance and reveal a clear mechanism linking insulin signaling to oxidative stress and inflammation in benzene-exposed mice.
Multiple factors including physical inactivity, poor diet, and a family history of diabetes contribute to the development of systemic insulin resistance and T2D in humans (Rajagopalan and Brook, 2012). In addition, there is growing evidence supporting connections between environmental factors and insulin resistance. For example, exposure to chemicals (eg, p,p′-dichlorodiphenyldichloroethylene) (Jaacks and Staimez, 2015; Rosenbaum et al., 2017), inhalation of PM2.5 (Brook et al., 2016; Haberzettl et al., 2016a) or ozone (Bass et al., 2013; Vella et al., 2015), and frequent exposure to additional traffic-related air pollutants (NOx and CO) (Kramer et al., 2010; Thiering et al., 2013) have all been reported to promote peripheral insulin resistance. Likewise, cigarette smoking has also been associated with diabetes and higher insulin use (Kramer et al., 2010; Madsbad et al., 1980; Thiering et al., 2013). Supporting this notion, this study identifies another common pollutant, benzene, which also promotes an insulin-resistant phenotype.
Benzene is a common constituent of polluted air and is present in high concentrations in automobile exhaust, gasoline, paints, adhesives, and solvents (Mohamed et al., 2002; Weisel, 2010). It is also present in cigarette smoke (ATSDR, 2005b; Korte et al., 2000), hookah (Kassem et al., 2014), and e-cigarettes (Lee et al., 2017). Occupational exposure to high levels of benzene has been linked to the development of acute myeloid and acute nonlymphocytic leukemias, whereas lower exposure (< 1 ppm) has been associated with hematoxic effects and lymphohematopoietic cancers (IARC, 1982a). Whereas the cardiovascular and metabolic effects of benzene have been less well studied, our studies showing that exposure to benzene decreases insulin sensitivity suggest the metabolic effects of benzene in humans may be just as pervasive as its well-documented hematoxic and leukemic effects.
Our mechanistic studies show that inhalation of benzene induces oxidative stress and inflammation, establishing a state of systemic insulin resistance and glucose intolerance. The connection between inflammation and insulin resistance has been well studied and may proceed through multiple mechanisms (Hotamisligl, 2006; Ronn et al., 2007). One causative mechanism suggested by our studies is the up-regulation of Socs proteins which attenuate downstream signaling from Irs proteins either by increasing their ubiquitination and degradation, or by competitively binding to sites of tyrosine phosphorylation, thus blocking interaction with downstream effectors (Ueki et al., 2004). We found that benzene induced a significant up-regulation of Socs1, and a near significant up-regulation of Socs3. Hence both Socs1, which limits signaling through Irs-2, and Socs3, which limits signaling through Irs-1, may contribute to the development of insulin resistance in benzene-exposed mice. The more prominent role of Socs1 in our studies is consistent with the benzene-induced up-regulation of Mip-1α, an inflammatory cytokine that preferentially regulates Socs1 (Qin et al., 2008; Rui et al., 2002; Ueki et al., 2004).
The oxidative stress induced by benzene inhalation likely arises from ROS generated at multiple points in its metabolism (Supplementary Figure 2) by the hepatic enzyme CYP2E1 (Gut et al., 1996; Powley and Carlson, 2000). The catalytic activity of this enzyme produces several ROS, primarily superoxide, and also lower amounts of hydrogen peroxide and hydroxyl radicals (Kuthan and Ullrich, 1982; Loida and Sligar, 1993; Snyder et al., 1993). Additional ROS is produced during the conversion of hydroquinone to 1,4-benzoquinone (BQ) via myeloperoxidase. Consistent with the notion that superoxide generated by CYP2E1 mediates the pathological outcomes of benzene, we found that treatment with the superoxide dismutase mimetic, TEMPOL, mitigated benzene-induced inflammation, and conferred protection from insulin resistance. A significant role for CYP2E1-generated ROS in inducing insulin resistance is supported by studies showing that CYP2E1−/− mice are protected from weight gain and insulin resistance after being placed on a high-fat diet (Zong et al., 2012) and that these mice are resistant to benzene toxicity (Carlson, 2004). Moreover, individuals with deficiencies for anti-oxidant enzymes are more susceptible to benzene-induced toxicity (Wan et al., 2006; Ye et al., 2015), suggesting that ROS production and subsequent generation of oxidative stress are critical mediators of benzene toxicity.
Further studies are needed to more completely define the mechanisms of benzene-induced insulin resistance. For example, it is uncertain whether the inhibitory effects of benzene on insulin signaling persist or abate in the absence of exposure. Some prior studies on benzene hematotoxicity suggest these effects have some degree of reversibility, depending upon exposure dose and duration (Cronkite et al., 1989). However, there is ample evidence suggesting that benzene can induce epigenetic alterations which may contribute to its long-term carcinogenic effects (Baccarelli and Bollati, 2009; Fenga et al., 2016). Thus, it remains a possibility that similar epigenetic effects may promote persistent metabolic defects. Another limitation of this study is that it does not address a real life exposure situation, wherein gases (eg, benzene) are often mixed with particles and metals. Exposure to PM2.5 itself contributes to insulin resistance through a pulmonary, oxidative stress-dependent mechanism (Haberzettl et al., 2016b). Thus, it is not clear if benzene could exacerbate the effects of PM2.5 or vice versa. This is particularly important because the pulmonary locus of a pure benzene exposure (upper airway) is different from the exposure locus of a benzene-particle mixture (respiratory zone). Finally, whereas our data are consistent with the idea that oxidative stress is largely causative in benzene-induced insulin resistance, we cannot discount the contribution of other mechanisms. For instance, certain benzene metabolites can form protein and DNA adducts (Rappaport et al., 2005; Xie et al., 2005) which may also contribute to aberrations in glucose handling. The presence and identity of any such adducts resulting from our exposure protocols are currently unknown.
In summary, our data show that exposure to volatile benzene can induce metabolic disorders. These findings are consistent with our previous work showing that benzene exposure in humans was associated with increased cardiovascular disease risk scores and deficits of vascular reparative cells (Abplanalp et al., 2017). Moreover, because benzene is present in both combustible and non-combustible tobacco products, it may be an important contributor to the metabolic toxicity of these products. This study raises the possibility that occupational exposure to benzene may be associated with heightened risk for the development of metabolic disease in exposed workers. Therefore, increased workplace surveillance is required to minimize such exposures. Finally, given that benzene is ubiquitous in urban environments and is generated in high concentrations in automobile exhaust, it is tempting to speculate the current high prevalence of insulin resistance and diabetes in urban areas may be in part linked to benzene exposure and that minimizing benzene emissions may be a readily implementable strategy to assist in decreasing the global burden of insulin resistance and diabetes.
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank Xiaoping Li, Patrick Whang, Michelle Snyder, Whitney Theis, and Millie Winner for technical assistance. The authors have nothing to disclose.
FUNDING
This work was supported by grants from the National Institutes of Health [RO1 ES019217 to TEO, RO1 HL122676 to DJC, P54 HL120163, P30 RR029846, and R01 ES029846 to AB and P42 ES023716 and R01 HL-95593 to SS].
REFERENCES
- Abplanalp W., DeJarnett N., Riggs D. W., Conklin D. J., McCracken J. P., Srivastava S., Xie Z., Rai S., Bhatnagar A., O'Toole T. E. (2017). Benzene exposure is associated with cardiovascular disease risk. PLoS One 12, e0183602.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen Z. J., Raaschou-Nielsen O., Ketzel M., Jensen S. S., Hvidberg M., Loft S., Tjonneland A., Overvad K., Sorensen M. (2012). Diabetes incidence and long-term exposure to air pollution: A cohort study. Diabetes Care 35, 92–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoyama K. (1986). Effects of benzene inhalation on lymphocyte subpopulations and immune response in mice. Toxicol. Appl. Pharmacol. 85, 92–101. [DOI] [PubMed] [Google Scholar]
- Appel B. R., Guirguis G., Kim I. S., Garbin O., Fracchia M., Flessel C. P., Kizer K. W., Book S. A., Warriner T. E. (1990). Benzene, benzo(a)pyrene, and lead in smoke from tobacco products other than cigarettes. Am. J. Public Health 80, 560–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ATSDR. (2005a). Toxicological profile for benzene. Chemical Agents and Related Occupations. p 7.
- ATSDR. (2005b). Toxicological profile for benzene. Chemical Agents and Related Occupations. p 273.
- Avis S. P., Hutton C. J. (1993). Acute benzene poisoning: A report of three fatalities. J. Forensic Sci. 38, 599–602. [PubMed] [Google Scholar]
- Baccarelli A., Bollati V. (2009). Epigenetics and environmental chemicals. Curr. Opin. Pediatr. 21, 243–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badham H. J., Renaud S. J., Wan J., Winn L. M. (2010). Benzene-initiated oxidative stress: Effects on embryonic signaling pathways. Chem. Biol. Interact. 184, 218–221. [DOI] [PubMed] [Google Scholar]
- Bashan N., Kovsan J., Kachko I., Ovadia H., Rudich A. (2009). Positive and negative regulation of insulin signaling by reactive oxygen and nitrogen species. Physiol. Rev. 89, 27–71. [DOI] [PubMed] [Google Scholar]
- Bass V., Gordon C. J., Jarema K. A., MacPhail R. C., Cascio W. E., Phillips P. M., Ledbetter A. D., Schladweiler M. C., Andrews D., Miller D. (2013). Ozone induces glucose intolerance and systemic metabolic effects in young and aged Brown Norway rats. Toxicol. Appl. Pharmacol. 273, 551–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar A. (2009). Could dirty air cause diabetes? Circulation 119, 492–494. [DOI] [PubMed] [Google Scholar]
- Brook R. D., Sun Z., Brook J. R., Zhao X., Ruan Y., Yan J., Mukherjee B., Rao X., Duan F., Sun L. (2016). Extreme air pollution conditions adversely affect blood pressure and insulin resistance: The air pollution and cardiometabolic disease study. Hypertension 67, 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook R. D., Xu X., Bard R. L., Dvonch J. T., Morishita M., Kaciroti N., Sun Q., Harkema J., Rajagopalan S. (2013). Reduced metabolic insulin sensitivity following sub-acute exposures to low levels of ambient fine particulate matter air pollution. Sci. Total Environ. 448, 66–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson G. (2004). Influence of selected inhibitors on the metabolism of the styrene metabolite 4-vinylphenol in wild-type and CYP2E1 knockout mice. J. Toxicol. Environ. Health A 67, 905–909. [DOI] [PubMed] [Google Scholar]
- Caro A. A., Cederbaum A. I. (2004). Oxidative stress, toxicology, and pharmacology of CYP2E1. Annu. Rev. Pharmacol. Toxicol. 44, 27–42. [DOI] [PubMed] [Google Scholar]
- Ceriello A., Motz E. (2004). Is oxidative stress the pathogenic mechanism underlying insulin resistance, diabetes, and cardiovascular disease? The common soil hypothesis revisited. Arterioscler. Thromb. Vasc. Biol. 24, 816–823. [DOI] [PubMed] [Google Scholar]
- Choi Y. H., Kim J. H., Lee B. E., Hong Y. C. (2014). Urinary benzene metabolite and insulin resistance in elderly adults. Sci. Total Environ. 482–483, 260–268. [DOI] [PubMed] [Google Scholar]
- Conklin D. J., Malovichko M. V., Zeller I., Das T. P., Krivokhizhina T. V., Lynch B. H., Lorkiewicz P., Agarwal A., Wickramasinghe N., Haberzettl P. (2017). Biomarkers of chronic acrolein inhalation exposure in mice: Implications for tobacco product-induced toxicity. Toxicol. Sci. 158, 263–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronkite E. P., Drew R. T., Inoue T., Hirabayashi Y., Bullis J. E. (1989). Hematotoxicity and carcinogenicity of inhaled benzene. Environ. Health Perspect. 82, 97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J. L., Maddux B. A., Goldfine I. D. (2005). The molecular basis for oxidative stress-induced insulin resistance. Antioxid. Redox Signal. 7, 1040–1052. [DOI] [PubMed] [Google Scholar]
- Fenga C., Gangemi S., Costa C. (2016). Benzene exposure is associated with epigenetic changes (Review). Mol. Med. Rep. 13, 3401–3405. [DOI] [PubMed] [Google Scholar]
- Galbraith D., Gross S. A., Paustenbach D. (2010). Benzene and human health: A historical review and appraisal of associations with various diseases. Crit. Rev. Toxicol. 40(Suppl. 2), 1–46. [DOI] [PubMed] [Google Scholar]
- Gut I., Nedelcheva V., Soucek P., Stopka P., Vodicka P., Gelboin H. V., Ingelman-Sundberg M. (1996). The role of CYP2E1 and 2B1 in metabolic activation of benzene derivatives. Arch. Toxicol. 71, 45–56. [DOI] [PubMed] [Google Scholar]
- Haberzettl P., Conklin D. J., Abplanalp W. T., Bhatnagar A., O’Toole T. E. (2018). Inhalation of fine particulate matter impairs endothelial progenitor cell function via pulmonary oxidative stress. Arterioscler. Thromb. Vasc. Biol. 38, 131–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberzettl P., McCracken J. P., Bhatnagar A., Conklin D. J. (2016a). Insulin sensitizers prevent fine particulate matter-induced vascular insulin resistance and changes in endothelial progenitor cell homeostasis. Am. J. Physiol. Heart Circ. Physiol. 310, H1423–H1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haberzettl P., O’Toole T. E., Bhatnagar A., Conklin D. J. (2016b). Exposure to fine particulate air pollution causes vascular insulin resistance by inducing pulmonary oxidative stress. Environ. Health Perspect. 124, 1830–1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligl G. (2006). Inflammation and metabolic disorders. Nature 444, 860–867. [DOI] [PubMed] [Google Scholar]
- IARC. (1982a). Benzene. IARC Monogr. Eval. Carcinog. Risks Hum. 29, 93–128. [Google Scholar]
- IARC. (1982b). Some industrial chemicals and dyestuffs. IARC Mongr. Eval. Carcinog. Risk Chem. Hum. 7, 1–398. [PubMed] [Google Scholar]
- IARC. (1987). Overall evaluations of carcinogenicity: An updating of IARC mongraphs volumes 1 to 42. IARC Monogr. Eval. Carcinog. Risks Hum. 1, 440. [PubMed] [Google Scholar]
- Jaacks L. M., Staimez L. R. (2015). Association of persistent organic pollutants and non-persistent pesticides with diabetes and diabetes-related health outcomes in Asia: A systematic review. Environ. Int. 76, 57–70. [DOI] [PubMed] [Google Scholar]
- Kassem N. O. F., Kassem N. O., Jackson S. R., Liles S., Daffa R. M., Zarth A. T., Younis M. A., Carmella S. G., Hofstetter C. R., Chatfield D. A., et al. (2014). Benzene uptake in Hookah smokers and non-smokers attending Hookah social events: Regulatory implications. Cancer Epidemiol. Biomarkers Prev. 23, 2793–2809. [DOI] [PubMed] [Google Scholar]
- Kelishadi R., Mirghaffari N., Poursafa P., Gidding S. S. (2009). Lifestyle and environmental factors associated with inflammation, oxidative stress and insulin resistance in children. Atherosclerosis 203, 311–319. [DOI] [PubMed] [Google Scholar]
- Korte J. E., Hertz-Picciotto I., Schulz M. R., Ball L. M., Duell E. J. (2000). The contribution of benzene to smoking-induced leukemia. Environ. Health Perspect. 108, 333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer U., Herder C., Sugiri D., Strassburger K., Schikowski T., Ranft U., Rathmann W. (2010). Traffic-related air pollution and incident type 2 diabetes: Results from the SALIA cohort study. Environ. Health Perspect. 118, 1273–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuthan H., Ullrich V. (1982). Oxidase and oxygenase function of the microsomal cytochrome P450 monooxygenase system. Eur. J. Biochem. 126, 583–588. [DOI] [PubMed] [Google Scholar]
- Lee M. S., LeBouf R. F., Son Y. S., Koutrakis P., Christiani D. C. (2017). Nicotine, aerosol particles, carbonyls and volatile organic compounds in tobacco- and menthol-flavored e-cigarettes. Environ. Health 16, 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y.-S., Vermeulen R., Tsai C. H., Waidyanatha S., Lan Q., Rothman N., Smith M. T., Zhang L., Shen M., Li G., et al. (2007). Albumin adducts of electrophilic benzene metabolites in benzene-exposed and control workers. Environ. Health Perspect. 115, 28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loida P. J., Sligar S. G. (1993). Molecular recognition in cytochrome P-450: Mechanism for the control of uncoupling reactions. Biochemistry 32, 11530–11538. [DOI] [PubMed] [Google Scholar]
- Madsbad S., McNair P., Christensen M. S., Christiansen C., Faber O. K., Binder C., Transbol I. (1980). Influence of smoking on insulin requirement and metbolic status in diabetes mellitus. Diabetes Care 3, 41–43. [DOI] [PubMed] [Google Scholar]
- Magos G. A., Lorenzana-Jimenez M., Vidrio H. (1990). Toluene and benzene inhalation influences on ventricular arrhythmias in the rat. Neurotoxicol. Teratol. 12, 119–124. [DOI] [PubMed] [Google Scholar]
- Matthews D. R., Hosker J. P., Rudenski A. S., Naylor B. A., Treacher D. F., Turner R. C. (1985). Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28, 412–419. [DOI] [PubMed] [Google Scholar]
- McGuinness O. P., Ayala J. E., Laughlin M. R., Wasserman D. H. (2009). NIH experiment in centralized mouse phenotyping: The Vanderbilt experience and recommendations for evaluating glucose homeostasis in the mouse. Am. J. Physiol. Endocrinol. Metab. 297, E849–E855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed M. F., Kang D., Aneja V. P. (2002). Volatile organic compounds in some urban locations in United States. Chemosphere 47, 863–882. [DOI] [PubMed] [Google Scholar]
- Powley M. W., Carlson G. P. (2000). Cytochromes P450 involved with benzene metabolism in hepatic and pulmonary microsomes. J. Biochem. Mol. Toxicol. 14, 303–309. [DOI] [PubMed] [Google Scholar]
- Qin H., Niyongere S. A., Lee S. J., Baker B. J., Benveniste E. N. (2008). Expression and functional significance of Socs-1 and Socs-3 in astrocytes. J. Immunol. 181, 3167–3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan S., Brook R. D. (2012). Air pollution and type 2 diabetes: Mechanistic insights. Diabetes 61, 3037–3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappaport S. M., Waidyanatha S., Yeowell-O’Connell K., Rothman N., Smith M. T., Zhang L., Qu Q., Shore R., Li G., Yin S. (2005). Protein adducts as biomarkers of human benzene metabolism. Chem. Biol. Interact. 153–154, 103–109. [DOI] [PubMed] [Google Scholar]
- Ronn S. G., Billestrup N., Mandrup-Poulsen T. (2007). Diabetes and suppressors of cytokine signaling proteins. Diabetes 56, 541–548. [DOI] [PubMed] [Google Scholar]
- Rosenbaum P. F., Weinstock R. S., Silverstone A. E., Sjodin A., Pavuk M. (2017). Metabolic syndrome is associated with exposure to organochlorine pesticides in Anniston, AL, United States. Environ. Int. 108, 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rui L., Yuan M., Frantz D., Shoelson S., White M. F. (2002). Socs-1 and Socs-3 block insulin signaling by ubiquitin-mediated degradation of IRS1 and IRS2. J. Biol. Chem. 277, 42394–42398. [DOI] [PubMed] [Google Scholar]
- Schattenberg J. M., Wang Y., Singh R., Rigoli R. M., Czaja M. J. (2005). Hepatocyte CYP2E1 overexpression and steatohepatitis lead to impaired hepatic insulin signaling. J. Biol. Chem. 280, 9887–9894. [DOI] [PubMed] [Google Scholar]
- Shen Y., Shen H. M., Shi C. Y., Ong C. N. (1996). Benzene metabolites enhance reactive oxygen species generation in HL60 human leukemia cells. Hum. Exp. Toxicol. 15, 422–427. [DOI] [PubMed] [Google Scholar]
- Snyder R., Witz G., Goldstein B. D. (1993). The toxicology of benzene. Environ. Health Perspect. 100, 293–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava S., Sithu S. D., Vladykovskaya E., Haberzettl P., Hoetker D. J., Siddiqui M. A., Conklin D. J., D'Souza S. E., Bhatnagar A. (2011). Oral exposure to acrolein exacerbates atherosclerosis in apoE-null mice. Atherosclerosis 215, 301–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Q., Yue P., Deiuliis J. A., Lumeng C. N., Kampfrath T., Mikolaj M. B., Cai Y., Ostrowski M. C., Lu B., Parthasarathy S., et al. (2009). Ambient air pollution exaggerates adipose inflammation and insulin resistance in a mouse model of diet-induced obesity. Circulation 119, 538–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmud P. J., Hingorani A. D., Cooper J. A., Marmot M. G., Brunner E. J., Kumari M., Kivimaki M., Humphries S. E. (2010). Utility of genetic and non-genetic risk factors in prediction of type 2 diabetes: Whitehall II prospective cohort study. BMJ 340, b4838.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiering E., Cyrys J., Kratzsch J., Meisinger C., Hoffmann B., Berdel D., von Berg A., Koletzko S., Bauer C. P., Heinrich J. (2013). Long-term exposure to traffic-related air pollution and insulin resistance in children: Results from the GINIplus and LISAplus birth cohorts. Diabetologia 56, 1696–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomi T., Santoro N., Caprio S., Cai M., Weng J., Groop L. (2014). The many faces of diabetes: A disease with increasing heterogeneity. Lancet 383, 1084–1094. [DOI] [PubMed] [Google Scholar]
- Ueki K., Kondo T., Kahn C. R. (2004). Suppressor of cytokine signaling 1 (Socs-1) and Socs-3 cause insulin resistance through inhibition of tyrosine phosphorylation of insulin receptor substrate proteins by discrete mechanisms. Mol. Cell. Biol. 24, 5434–5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vella R. E., Pillon N. J., Zarrouki B., Croze M. L., Koppe L., Guichardant M., Pesenti S., Chauvin M. A., Rieusset J., Geloen A., et al. (2015). Ozone exposure triggers insulin resistance through muscle c-Jun N-terminal kinase activation. Diabetes 64, 1011–1024. [DOI] [PubMed] [Google Scholar]
- Verma Y., Rana S. V. (2008). Modulation of CYP4502E1 and oxidative stress by testosterone in liver and kidney of benzene treated rats. Indian J. Exp. Biol. 46, 568–572. [PubMed] [Google Scholar]
- Vermeulen R., Lan Q., Zhang L., Gunn L., McCarthy D., Woodbury R. L., McGuire M., Podust V. N., Li G., Chatterjee N., et al. (2005). Decreased levels of CXC-chemokines in serum of benzene-exposed workers identified by array-based proteomics. Proc. Natl. Acad. Sci. U.S.A. 102, 17041–17046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan J. X., Zhang Z. B., Guan J. R., Cao D. Z., Ye R., Jin X. P., Xia Z. L. (2006). Genetic polymorphism of toxicant-metabolizing enzymes and prognosis of Chinese workers with chronic benzene poisoning. Ann. N.Y. Acad. Sci. 1076, 129–136. [DOI] [PubMed] [Google Scholar]
- Weisel C. P. (2010). Benzene exposure: An overview of monitoring methods and their findings. Chem. Biol. Interact. 184, 58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett W. C. (2002). Balancing life-style and genomics research for disease prevention. Science 296, 695–698. [DOI] [PubMed] [Google Scholar]
- Wong O., Fu H. (2005). Exposure to benzene and non-Hodgkin lymphoma, an epidemiologic overview and an ongoing case-control study in Shanghai. Chem. Biol. Interact. 153–154, 33–41. [DOI] [PubMed] [Google Scholar]
- Xie Z., Zhang Y., Guliaev A. B., Shen H., Hang B., Singer B., Wang Z. (2005). The p-benzoquinone DNA adducts derived from benzene are highly mutagenic. DNA Repair (Amst) 4, 1399–1409. [DOI] [PubMed] [Google Scholar]
- Ye L. L., Zhang G. H., Huang J. W., Li Y., Zheng G. Q., Zhang D. T., Zhou L. F., Tao X. D., Zhang J., Ye Y. J., et al. (2015). Are polymorphisms in metabolism protective or a risk for reduced white blood cell counts in a Chinese population with low occupational benzene exposures? Int. J. Occup. Environ. Health 21, 232–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmet P. Z., Magliano D. J., Herman W. H., Shaw J. E. (2014). Diabetes: A 21st century challenge. Lancet Diabetes Endocrinol. 2, 56–64. [DOI] [PubMed] [Google Scholar]
- Zong H., Armoni M., Harel C., Karnieli E., Pessin J. E. (2012). Cytochrome P-450 CYP2E1 knockout mice are protected against high-fat diet-induced obesity and insulin resistance. Am. J. Physiol. Endocrinol. Metab. 302, E532–E539. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.