Abstract
Various functions of dietary sphingolipids have been reported; however, little is known about marine sphingolipids. Ceramide 2-aminoethylphosphonate (CAEP), an abundant sphingolipid in marine mollusks, frequently has a unique triene type of sphingoid base [2-amino-9-methyl-4,8,10-octadecatriene-1,3-diol (d19:3)]. We previously reported that dietary CAEP prepared from the skin of squid was digested in the intestinal mucosa of mice via ceramides to yield free sphingoid bases. How dietary CAEP is then used in the body remains unclear. Here, we investigated the absorption of dietary CAEP using a lipid absorption assay on the lymph collected from rats with thoracic duct cannulation. Our results reveal that sphingoid bases derived from CAEP, including d16:1, d18:1, and d19:3, were detected in the lymph after administration of CAEP. Lymphatic recovery of d19:3 was lower than that of other sphingoid bases. A large fraction of the absorbed sphingoid bases was present as complex sphingolipids, whereas a smaller portion was present in the free form. Fatty acids in ceramide moieties found in the lymph were partially different from dietary CAEP, which indicates that sphingoid bases derived from CAEP could be, at least in part, resynthesized into complex sphingolipids. Future studies should elucidate the metabolism of sphingoid bases derived from CAEP.
Keywords: dietary sphingolipids, lymph cannulation, intestinal digestion, sphingolipid metabolism
Sphingolipids, one of the major families of lipids, are composed of a ceramide formed by a sphingoid base bound to a fatty acid on a 2-amide group. The polar head portion of sphingolipids and the molecular structures of ceramides are diverse and vary between species (1). Because sphingolipids are elements of cellular membranes, they are ubiquitous in various organisms, including those consumed as food products (2, 3).
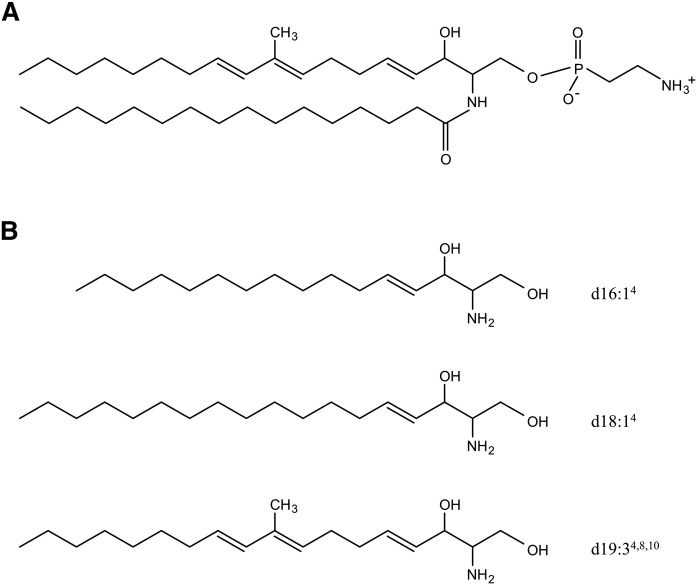
Ceramide 2-aminoethylphosphonate (CAEP) is a general sphingolipid of marine origin (Fig. 1A) (4). CAEP is widely encountered in marine invertebrates (5), including mollusks such as squid (6) and shellfish (7), which are consumed globally. CAEPs are rarely found in mammals. Unlike the C-O-P linkage encountered in the polar head of major sphingophospholipids, such as sphingomyelin, a phosphorus atom of 2-aminoethylphosphonate, a polar head of CAEP, is directly bound to a carbon atom (C-P bond) (8). Sphingolipids having C-P bonds, including CAEP, are sphingophosphonolipids.
Fig. 1.
Typical chemical structure of CAEP (d19:3/16:0) (A) and structures of sphingoid bases derived from CAEP (B). Shorthand name designations are as described by Karlsson (1).
The structures of sphingoid bases in CAEP frequently contain trienes and odd-numbered carbon chains that are typical of marine invertebrates and different from those in mammals. The major sphingoid bases in mammals are saturated or monounsaturated, even-numbered carbon chains. In addition to sphingosine (d18:1), which is a principal sphingoid base, sphinganine (d18:0) and phytosphingosine (t18:0) are commonly encountered in mammals (9). Hexadeca-4-sphingenine (d16:1) is often found in milk sphingomyelin (10, 11). In contrast, CAEP consists of not only d18:1 and d16:1, but also a unique triene-type sphingoid base, 2-amino-9-methyl-4,8,10-octadecatriene-1,3-diol (d19:3) (12–14) (Fig. 1B).
Because ceramides and their constituents are known to play important roles as intracellular mediators (15), these metabolites from dietary marine sphingolipids could reasonably affect biological functions. Recently, dietary sphingolipids were reported to modulate epidermal ceramides, resulting in improved skin barrier function (16). Free sphingoid bases originating from dietary sphingolipids also upregulate mRNA expression of ceramide synthases in normal human foreskin keratinocytes (17). Dietary sphingolipids exhibit antitumor potential in the gut (18, 19). This antitumor activity may be due to digestion products derived from dietary sphingolipids; sphingoid bases show significant cytotoxicity against colon cancer cell lines via induction of apoptosis (20, 21). Moreover, d19:3 from marine products reportedly induces apoptosis in human hepatoma HepG2 cells (22). Dietary marine sphingolipids having distinctive structures are also expected to have biological activity.
To elucidate the mechanism underlying dietary effects of sphingolipids, further knowledge of their digestion and absorption is important. Dietary glucosylceramide and sphingomyelin can be hydrolyzed to their components (sphingoid bases, fatty acids, and the polar head group) by intestinal enzymes. These hydrolysis products can be absorbed from the digestive tract (23–25). The majority of sphingoid bases absorbed by the gastrointestinal tract are metabolized to fatty acids and incorporated into glycerolipids, while smaller fractions remain in free form or are resynthesized to complex sphingolipids (26, 27). We previously reported that dietary CAEP was also digested to free sphingoid bases via ceramides by the small intestinal mucosa of mice. Moreover, CAEP containing d19:3 was more easily digested by the intestinal mucosa than CAEP containing other sphingoid bases (28). However, how dietary CAEP is absorbed and utilized in the body remains unclear. Several reports indicate that dietary sphingolipids, such as glucosylceramide and sphingomyelin, can be absorbed from the intestine into the lymph after degradation to free sphingoid bases and fatty acids (11, 25, 26). In the present study, we evaluated the absorption of dietary CAEP by using a rat thoracic duct cannulation method that is useful for evaluating absorption of lipids and their metabolites in vivo (29).
MATERIALS AND METHODS
Chemicals
Triolein and sodium taurocholate were purchased from Nacalai Tesque Inc., Ltd. (Kyoto, Japan). Essential fatty acid-free BSA was obtained from Sigma Chemical Co. (St. Louis, MO). O-phthalaldehyde (OPA) was purchased from Wako Pure Chemical, Ltd. (Osaka, Japan). Hydrogen chloride-methanol reagent (5–10%) for esterification was obtained from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). Other chemicals and solvents were of reagent grade, except for the HPLC-grade solvents used for HPLC analysis.
Preparation of CAEP
CAEP was purified from crude lipids extracted from the skin from jumbo flying squid, Dosidicus gigas, which were kindly donated by Dr. Saito (Ishikawa Prefectural University, Japan), as described previously (28). Purity of CAEP was 98%, as determined by HPLC equipped with an evaporative light-scattering detector (28). The composition of sphingoid bases in the CAEP was measured by HPLC analysis with fluorescence detection (Shimadzu Co., Kyoto, Japan) after acid hydrolysis (28, 30). Constituent sphingoid bases were 41.4% d19:3, 28.6% d16:1, and 12.9% d18:1, with smaller fractions of others. Methyl-esterified fatty acids prepared from CAEP were analyzed by gas-liquid chromatography (GC-14B, Shimadzu) using a CP-SIL 88 capillary column (60 m × 0.25 mm, 0.2 μm, Agilent Technologies, Santa Clara, CA) and a flame ionization detector (Shimadzu) according to the method of Yunoki et al. with slight modifications (31). The injector and detector temperatures were both 240°C. The oven temperature was initially 170°C and was increased by 4°C/min, then held at 225°C for 20 min. Fatty acid methyl esters (FAMEs) were identified by comparing their retention times with a FAME standard (Funakoshi Co., Tokyo, Japan).
Thoracic duct cannulation in rats
This study was conducted in conformity with the policies and procedures detailed in the Animal Experiment Guidelines of Tohoku University (approval no. 2015AgA-001). This animal experiment is necessary to elucidate intestinal absorption of dietary CAEP, including digestion processes. No serious adverse events were observed in this study. Surgeries and maintenance of rats and all other procedures were performed as described previously (25). Male Sprague-Dawley rats (10 weeks old) were obtained from Japan SLC (Hamamatsu, Japan) and were housed in stainless-steel wire-mesh cages in a room kept at 23 ± 1°C with a 12 h light-dark cycle. After acclimatization for 1 week with both MF Standard Rodent Chow (Oriental Yeast, Tokyo, Japan) and distilled water ad libitum, rats were anesthetized with isoflurane, a cannula (SV35, Natume Co., Tokyo, Japan) was inserted into their left thoracic lymph duct to collect lymphatic fluid, and a catheter (SP-55, Natume) was inserted into their stomach. After surgery, each rat was placed in a restraining cage in a warm recovery room. A physiological solution containing 139 mmol/l glucose and 85 mmol/l NaCl was infused continuously overnight at a rate of 3 ml/h through the stomach catheter, and the same solution was also provided as drinking water. The next morning, rats were divided into two groups (CAEP group, n = 4; control group, n = 3). After collection of the lymph for 2 h as a blank control, rats were infused with 3 ml of an emulsion as a single bolus through the stomach catheter. Test emulsion containing 5 mg of CAEP, 200 mg of triolein, 200 mg of sodium taurocholate, and 50 mg of BSA was prepared by ultrasonication (11, 25). BSA was included in the emulsion to more completely disperse the water-insoluble ingredients (32). Control experiments were performed using the same emulsion without CAEP (control group). After infusion of emulsions, infusion of the glucose:NaCl solution was continued. The lymph was collected in an EDTA-containing tube for analysis at the following intervals after infusion: −2 to 0, 0–1, 1–2, 2–3, 3–4, 4–5, and 5–6 h. The lymph was stored at −30°C until analyzed.
Lipid extraction
Lipids were extracted from each lymph sample as previously reported (25). The alkali-stable fraction, prepared by saponification of lipids to remove glycerolipids, was analyzed to quantify free sphingoid bases (the free sphingoid base fraction) and to identify ceramide molecular species. A portion of the alkali-stable fraction of each lymph extract was hydrolyzed with aqueous methanolic 1 M HCl at 70°C for 18 h (30). Efficiencies of acid hydrolysis of ceramide (d18:1/24:0) and sphingomyelin (d18:1/16:0) were 95.7 ± 4.2% and 82.2 ± 0.2%, respectively (n = 3). Free sphingoid bases liberated by acid hydrolysis of complex sphingolipids were analyzed using HPLC to quantify total sphingolipids (the total sphingoid base fraction) in lymph.
HPLC analysis with fluorescence detection
OPA derivatives of sphingoid bases in the free sphingoid base fraction were analyzed using an HPLC system equipped with a fluorescence detector (Shimadzu) as previously described (28). Sphingoid bases were identified by mass spectroscopy using an LCMS-2010 EV (Shimadzu) equipped with an ESI interface (Shimadzu). The MS was operated under the following conditions: probe voltage, 1.50 kV; CDL temperature, 250°C; block heater temperature, 200°C; nebulizer gas flow, 1.5 L/min; and MS range, m/z 420–520.
In the analysis of the total sphingoid base fraction, HPLC conditions were slightly modified to separate peaks of sphingoid bases isomerized by acid hydrolysis. The TSKgel ODS-100Z column (2.0 mm × 250 mm; inner diameter, 5 μm; Tosoh, Tokyo, Japan) was eluted using a binary gradient consisting of acetonitrile as mobile phase A and water as mobile phase B. The gradient profile used was: 0–60 min, 70–20% B; 60–65 min, 20–0% B; and 65–90 min, 0% B. Increases in the levels of the sphingoid bases from the blank control after CAEP administration were corrected by the levels in the lymph after administration of control emulsion at each time point. Each sphingoid base was quantified with a calibration curve developed with an analytical standard (d-erythro-sphingosine, Avanti Polar Lipids, Inc., Alabaster, AL) (24, 25, 28).
LC/MS analyses
To identify complex sphingolipids in lymph, the alkaline-stable fraction of the lymph extracts was analyzed to obtain structural information using an HPLC system coupled to an ion-trap TOF mass spectrometer (LC/MS-IT-TOF; Shimadzu) equipped with an atmospheric pressure chemical ionization (APCI) or ESI interface (Shimadzu). HPLC separation was performed as previously reported (28). Ceramides were analyzed by LCMS-IT-TOF according to the method of Sugawara et al. (33) with minor modifications. The MS was operated under the following conditions: probe voltage, 4.50 kV; CDL temperature, 200°C; block heater temperature, 200°C; nebulizer gas flow, 2.0 l/min; MS and MS2 ion accumulation time, 10 and 100 ms respectively; MS range, m/z 400–700; MS2 range, m/z 200–350; and CID parameters, 60% energy and 100% collision gas (argon).
For structural analysis of sphingomyelin, ceramide, and hexosylceramide, [M + H – H2O – phosphocholine]+, [ M + H – H2O]+, and [ M + H – H2O – hexose]+, respectively, in positive-scan mode was used to obtain product ions by MS/MS analysis. Typical signals of d16:1 (m/z 236.3), d18:1 (m/z 264.3), and d19:3 (m/z 274.3), characteristic for sphingoid bases present in CAEP, were observed as product ions using auto MS/MS detection mode. Pairs of structurally specific product ions of sphingoid bases and their precursor ions were used to identify ceramide molecules.
Statistical analysis
Data are reported as means ± standard errors. Statistical analysis was performed by two-way ANOVA with repeated measures using StatView software (SAS Institute, Cary, NC). Data were considered as statistically significant at P < 0.05. Post hoc power analysis, with α = 0.05, used G*Power 3.1.9.2 (34, 35), a software program that estimates statistical power.
RESULTS
Recovery of sphingoid bases from the dietary CAEP in lymph
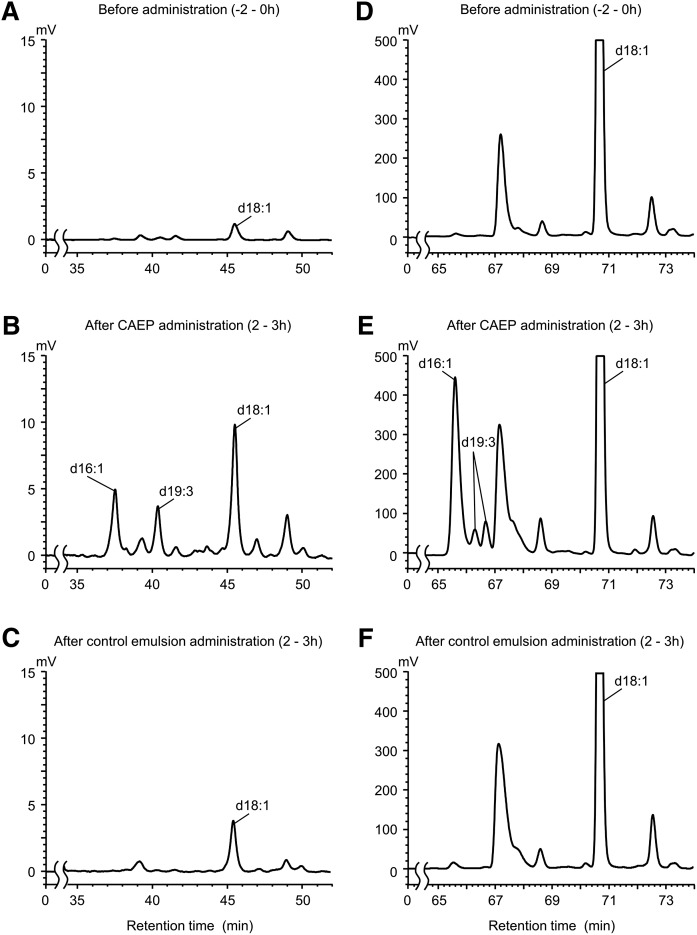
Lymph output was not altered by treatment (data not shown). Sphingoid bases, including d16:1, d19:3, and d18:1, the dominant constituents of CAEP, were definitively detected in the free sphingoid base fraction of the lymph extracts from treated animals (Fig. 2A–C). Endogenous d18:1 was detected in the lymph prior to the administration of the emulsions (Fig. 2A), and its level increased following the administration of the control emulsion (Fig. 2C). d19:3 and d16:1 were detected only in the lymph after administration of CAEP (Fig. 2B). Peaks ascribed to d19:3, d16:1, and d18:1 were prominently increased in the total sphingoid base fraction (Fig. 2D–F). Compared with chromatograms for the free sphingoid base fraction, d19:3 was detected as multiple peaks after acid hydrolysis. Molecular ion peaks were found at m/z 486.3, which indicates a d19:3 OPA derivative [M + H]+. Some sphingoid bases with branched chains or dienes are isomerized by acid hydrolysis (9).
Fig. 2.
HPLC chromatograms of sphingoid bases in rat lymph. Alkali-stable fraction (free sphingoid base fraction) extracted from the rat lymph before (A) and after (B) administration of CAEP or control emulsion (C). Acid hydrolysis fraction (total sphingoid base fraction) extracted from the rat lymph before (D) and after (E) administration of CAEP or control emulsion (F). The peak of each sphingoid base was detected by fluorescence after OPA derivatization (334 nm/440 nm).
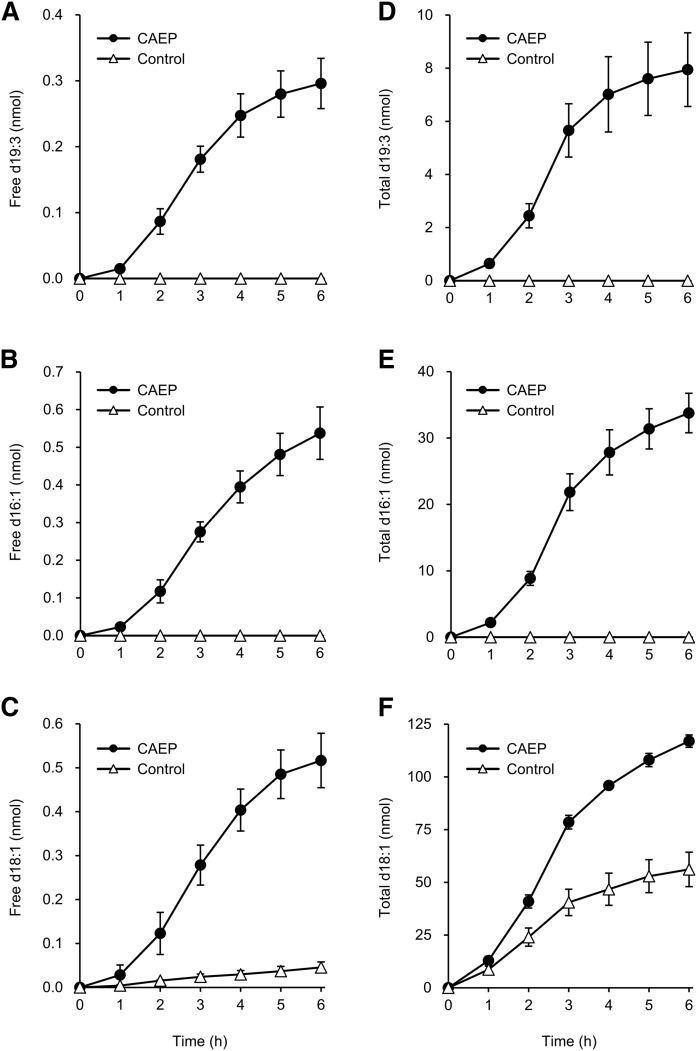
Cumulative amounts of d19:3, d16:1, and d18:1 detected in each fraction are shown in Fig. 3. (n = 4). Cumulative recoveries of sphingoid bases from dietary CAEP in the 6 h after administration were calculated as 0.009 ± 0.001% for d19:3 and 0.023 ± 0.004% for d16:1 in the free sphingoid base fraction. For the total sphingoid base fraction, recoveries were 0.25 ± 0.05% for d19:3 and 1.46 ± 0.15% for d16:1. Levels of d18:1 were also increased in both free and total sphingoid base fractions following administration of control emulsion and appeared to be of endogenous origin (Fig. 3C, F). However, the levels in the control group were significantly lower than those in the CAEP group. Putative recoveries of d18:1 in the free and total sphingoid base fractions during 6 h after administration were 0.052 ± 0.007% and 6.10 ± 0.34%, respectively, corrected by subtraction of the level in the lymph after administration of control emulsion.
Fig. 3.
Cumulative amounts of each sphingoid base in the lymph of rats infused with CAEP or control emulsion. Free sphingoid base fraction: d19:3 (A), d16:1 (B), and d18:1 (C). Total sphingoid base fraction: d19:3 (D), d16:1 (E), and d18:1 (F). Data are reported as means ± SEs (CAEP group, n = 4; control group, n = 3).
Identification of molecular species of sphingolipids in rat lymph after the administration of CAEP
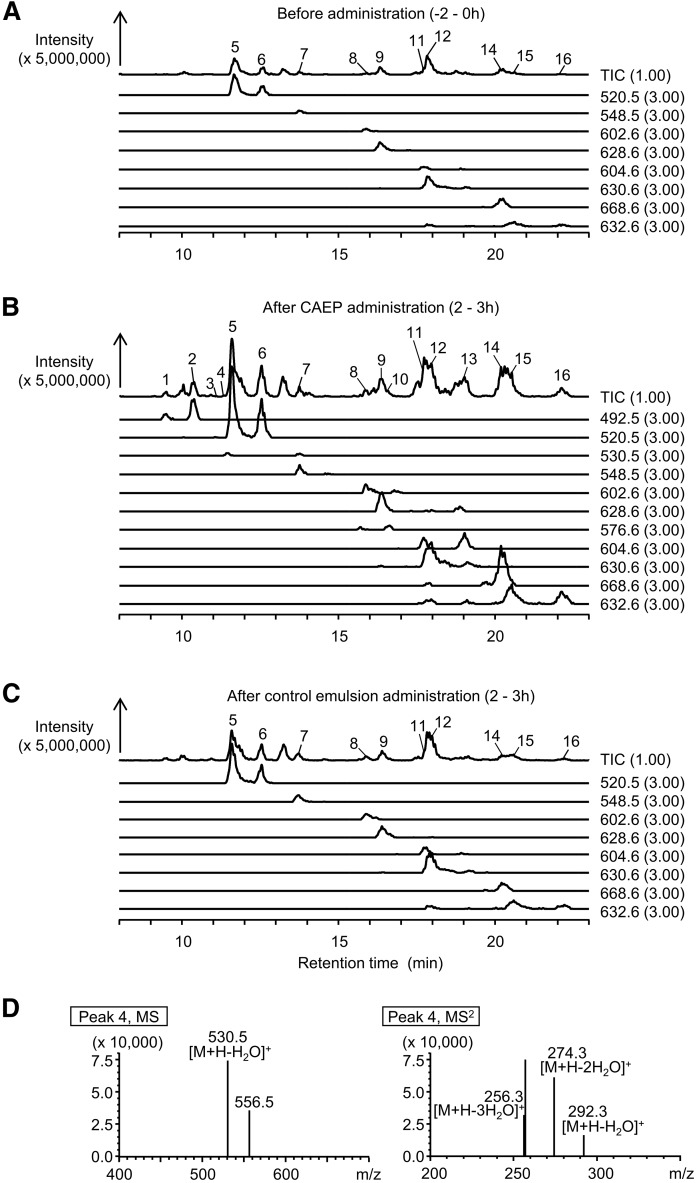
Total and selected ion chromatograms of sphingomyelins, ceramides, and hexosylceramides in the lymph by LC/MS-IT-TOF are shown in Fig. 4. Precursor/product ion pairs found for sphingomyelin, ceramide, and hexosylceramide in the lymph extracts are shown in Table 1. [M + H – H2O – phosphocholine]+ and [M + H – H2O – hexose]+ as precursor ions for sphingomyelin and hexosylceramide, respectively, were detected by APCI analysis, because the polar head moiety was easily released. To discriminate these ions from [M + H – H2O]+ as free ceramides, we confirmed ions of sphingomyelin and hexosylceramide [M + H]+ using an ESI probe. [M + H]+ of sphingomyelin (m/z 675.5, peak 1; m/z 703.6, peak 5; m/z 731.6, peak 7; m/z 785.7, peak 8; m/z 811.7, peak 9; m/z 787.7, peak 11; m/z 813.7, peak 12; m/z 815.7, peak 15) and hexosylceramide (m/z 700.6, peak 3) were detected with this analysis. Complex sphingolipids composed of d16:1, d18:1, and d19:3, the major components of CAEP, were identified in the lymph after CAEP administration. The fatty acid moiety of these molecules was mainly palmitic acid, which is the most abundant fatty acid of CAEP. The fatty acid composition of CAEP was as follows: 69.7% 16:0, 7.6% 22:1, 7.2% 18:0, 3.5% 16:1, 2.1% 20:0, 2.1% 20:1, 1.3% 24:1, and other fatty acids in smaller fractions. Sphingomyelin (d16:1/16:0, peak 1), ceramide (d16:1/16:0, peak 2; d19:3/16:0, peak 4; d16:1/22:0, peak 10; d16:1/24:0, peak 13), and hexosylceramide (d18:1/16:0, peak 3) shown in Fig. 4 were detected only after CAEP administration. Ceramide molecules, d16:1/16:0 (peak 2), d19:3/16:0 (peak 4), and d18:1/16:0 (peak 6), were similar to the ceramide backbone of CAEP. However, d16:1/22:0 (peak 10) and d16:1/24:0 (peak 13) had a different fatty acid moiety from the CAEP constituent. d19:3 in sphingomyelin and hexosylceramide was not detected with methods used in the LC/MS-IT-TOF analysis. Sphingomyelin molecules (d18:1/16:0, peak 5; d18:1/22:0, peak 11; d18:1/24:1, peak 12; d18:1/24:0, peak 15) and ceramide molecules (d18:1/16:0, peak 6; t18:0/16:0, peak 14) were detected in the lymph without administration of CAEP, indicating that these molecules were endogenous (Fig. 4A, C). However, after CAEP administration, these analytes were present in greater amounts than observed in controls (Fig. 4B). The d18:1 sphingolipids, which increased after administration of CAEP, are the same sphingolipids found in endogenous lipids. These results indicate that absorbed sphingoid bases could be, at least in part, resynthesized into complex sphingolipids.
Fig. 4.
LC/MS-IT-TOF analysis of the alkali-stable fraction extracted from the rat lymph before (A) and after (B) administration of CAEP or control emulsion (C). Total ion and selected ion chromatograms are illustrated. Each peak (1–16) was identified as sphingomyelin, ceramide, or hexosylceramide by using precursor/product ion pairs. The putative structures of sphingomyelins are shown as follows: peak 1, d16:1/16:0; peak 5, d18:1/16:0; peak 7, d18:1/18:0; peak 8, d18:1/22:1; peak 9, d18:1/24:2; peak 11, d18:1/22:0; peak 12, d18:1/24:1; peak 15, d18:1/24:0. The putative structures of ceramides shown as follows: peak 2, d16:1/16:0; peak 4, d19:3/16:0; peak 6, d18:1/16:0; peak 10, d16:1/22:0; peak 13, d16:1/24:0; peak 14, t18:0/24:0; and peak 16, d18:1/24:0. The putative structure of peak 3 is hexosylceramide (d18:1/24:0). D: MS and MS/MS spectra of the components of peak 4.
TABLE 1.
Identification of sphingolipid molecular species from the lymph after CAEP administration by APCI-MS analysis
| Molecule | Precursor Ion m/z | Product Ion m/z | Peak Area | Peak |
| Sphingomyelin | ||||
| d16:1/16:0 | 492.5 | 236.3 | 1,025,212 | 1 |
| d18:1/16:0 | 520.5 | 264.3 | 13,912,825 | 5 |
| d18:1/18:0 | 548.5 | 264.3 | 1,392,451 | 7 |
| d18:1/22:1 | 602.6 | 264.3 | 1,718,386 | 8 |
| d18:1/24:2 | 628.6 | 264.3 | 4,365,934 | 9 |
| d18:1/22:0 | 604.6 | 264.3 | 1,764,411 | 11 |
| d18:1/24:1 | 630.6 | 264.3 | 7,659,060 | 12 |
| d18:1/24:0 | 632.6 | 264.3 | 5,813,556 | 15 |
| Ceramide | ||||
| d16:1/16:0 | 492.5 | 236.3 | 3,768,386 | 2 |
| d19:3/16:0 | 530.5 | 274.3 | 267,245 | 4 |
| d18:1/16:0 | 520.5 | 264.3 | 7,509,368 | 6 |
| d16:1/22:0 | 576.6 | 236.3 | 692,972 | 10 |
| d16:1/24:0 | 604.6 | 236.3 | 3,412,062 | 13 |
| t18:0/24:0 | 668.6 | 264.3, 282.3, 300.3 | 11,017,591 | 14 |
| d18:1/24:0 | 632.6 | 264.3 | 3,316,077 | 16 |
| Hexosylceramide | ||||
| d18:1/16:0 | 520.5 | 264.3 | 32,697 | 3 |
DISCUSSION
Taking into consideration the daily intake of dietary sphingolipids by humans (2, 3), the dosage used in this study was not excessive for evaluating intestinal absorption of CAEP. Total recovery of CAEP was higher than recovery of other dietary glycosphingolipids, such as plant glucosylceramide and sea cucumber cerebroside, reported previously (25, 36), but was similar to recovery of milk sphingomyelin (11). Dietary phosphosphingolipids may be more easily absorbed than dietary glycosphingolipids because of differences in digestibility. We previously found that hydrolysis of CAEP by the intestinal mucosa was similar to hydrolysis of sphingomyelin at pH 9.0 and occurred more rapidly at pH 7.2 (28). Conversely, low lymphatic recovery of glycosphingolipids may be attributed to the relatively low capacity for hydrolysis of sphingolipids in the intestine (24).
In this study, we show that unique sphingoid bases derived from CAEP, including d19:3 and d16:1, can be absorbed from the intestine; these sphingoid bases were found only in the lymph after administration of CAEP. Studies have shown that d16:1, which is a major sphingoid base in bovine milk, is a suitable marker for evaluating lymphatic absorption of dietary milk sphingomyelin (11, 37). Because increased levels of d18:1 in the lymph after administration of control emulsion appeared to be increased in endogenous lipids, its putative recovery after administration of CAEP was calculated by subtracting the value of d18:1 in the lymph of control rats (11, 25, 36). Recovery of d16:1 was higher than recovery of d19:3, although intestinal digestion of CAEP containing d19:3 was greater than digestion of other species (28). The increase in the amount of d18:1 in the lymph after CAEP administration may also be due to de novo synthesis, but the increment of d18:1 was higher than recovery of d19:3. Our previous studies indicated that P-glycoprotein (P-gp), which is an ATP-dependent drug efflux pump (38), contributes to selective absorption of d18:1, the major sphingoid base in mammals (24, 25, 39). P-gp is widely distributed in mammalian tissues and is present on the apical surfaces of the intestine (40, 41). Efflux by P-gp in the intestine might contribute to relatively low recovery of d19:3 from dietary CAEP in lymph. This lower absorption rate of d19:3 is consistent with our previous reports on intestinal absorption of dietary plant glucosylceramide containing sphingadienine (d18:2) and sea cucumber cerebroside containing d17:1 and d19:2 (25, 36, 39). Recovery of d16:1 from dietary milk sphingomyelin was shown to be 0.6–4.2% (11) and was relatively higher than recovery of sphingoid bases other than d18:1. Our present results also indicated that the recovery of d16:1 in the lymph is higher than that of d19:3. d16:1, unlike d19:3, may be minimally affected P-gp in the intestine because of its structural similarity to d18:1.
Sphingoid bases such as d18:1 can be converted to sphingosine-1-phosphate (S1P) by phosphorylation at the C-1 position (42). S1P is then cleaved into phosphoethanolamine and the corresponding fatty aldehydes by sphingosine phosphate lyase (43). Fatty aldehydes are oxidized to fatty acids and then incorporated, primarily, into glycerolipids (26, 44). Recently, phytosphingosine was reported to also be metabolized to odd-numbered carbon chain fatty acids and incorporated into glycerolipids (45). It remains unclear, however, if odd-numbered carbon chain sphingoid bases evaluated in this study are utilized and resynthesized to sphingolipids. Similar to other sphingoid bases, it is speculated that a part of d19:3 in dietary CAEP might be metabolized to fatty acids after absorption. Detailed metabolism of unique sphingoid bases such as d19:3 deserves further study. In a previous report, d18:2, a typical sphingoid base in plants, was detected transiently in plasma of adult mice after gavage administration (46). Additional studies focusing on d19:3 and its metabolites in plasma are also needed for a detailed understanding of mechanisms of intestinal absorption of CAEP.
Previous studies indicated that C16-ceramides containing sphingoid bases originating from dietary sphingolipids, such as d18:2 and d17:1, were found in the lymph after ingestion (25, 36, 39). In this study, the C16-ceramides d16:1 and d19:3 were detected and d18:1 was detected in increased amounts in the lymph after ingestion of CAEP. Because palmitic acid is not only a constituent of CAEP but is also abundant in vivo, palmitate-containing molecules might result from resynthesis from a fraction of free sphingoid bases formed from dietary CAEP in the digestive tract during absorption. Ceramides having d16:1 and a different fatty acid from the original CAEP constituent were also found. Sphingomyelin containing d16:1 was also detected. Thus, sphingoid base molecules with unique structures derived from dietary sphingolipids can be absorbed and incorporated into ceramide. Moreover, our results indicate that a part of the ceramide molecule can be hexosylated or modified with phosphocholine. These complex sphingolipids might be transported by lipoproteins, because sphingolipids are a component of lipoprotein and ceramides are the predominant sphingolipids found in the VLDL in the rat lymph and blood (3, 47, 48).
In conclusion, we demonstrate that dietary CAEP is, at least in part, absorbed into the lymph, and some fraction may be salvaged to form other sphingolipids. These findings provide novel insights into the utilization of CAEP, a marine sphingolipid used as a food ingredient. Further studies are needed to elucidate the metabolism of unique sphingoid bases derived from CAEP.
Acknowledgments
The authors thank Dr. Saito and Dr. Itonori for providing valuable materials.
Footnotes
Abbreviations:
- APCI
- atmospheric pressure chemical ionization
- CAEP
- ceramide 2-aminoethylphosphonate
- d16:1
- hexadeca-4-sphingenine
- d18:1
- sphingosine
- d19:3
- 2-amino-9-methyl-4,8,10-octadecatriene-1,3-diol
- IT-TOF
- ion-trap TOF mass spectrometer
- OPA
- o-phthalaldehyde
- P-gp
- P-glycoprotein
- t18:0
- phytosphingosine
This work was supported by the Japan Society for the Promotion of Science KAKENHI Grants JP15J01143 and JP16H04923. The authors declare that no conflicts of interest exist.
REFERENCES
- 1.Karlsson K. A. 1970. Sphingolipid long chain bases. Lipids. 5: 878–891. [DOI] [PubMed] [Google Scholar]
- 2.Sugawara T., and Miyazawa T.. 1999. Separation and determination of glycolipids from edible plant sources by high-performance liquid chromatography and evaporative light-scattering detection. Lipids. 34: 1231–1237. [DOI] [PubMed] [Google Scholar]
- 3.Vesper H., Schmelz E. M., Nikolova-Karakashian M. N., Dillehay D. L., Lynch D. V., and Merrill A. H. Jr.. 1999. Sphingolipids in food and the emerging importance of sphingolipids to nutrition. J. Nutr. 129: 1239–1250. [DOI] [PubMed] [Google Scholar]
- 4.Simon G., and Rouser G.. 1967. Phospholipids of the sea anemone: quantitative distribution; absence of carbon-phosphorus linkages in glycerol phospholipids; structural elucidation of ceramide aminoethylphosphonate. Lipids. 2: 55–59. [DOI] [PubMed] [Google Scholar]
- 5.Mukhamedova Kh. S., and Glushenkova A. I.. 2000. Natural phosphonolipids. Chem. Nat. Compd. 36: 329–341. [Google Scholar]
- 6.Saito H., and Ishikawa S.. 2012. Characteristic of lipids and fatty acid compositions of the neon flying squid, Ommastrephes bartramii. J. Oleo Sci. 61: 547–564. [DOI] [PubMed] [Google Scholar]
- 7.Hori T., Arakawa I., and Sugita M.. 1967. Distribution of ceramide 2-aminoethylphosphonate and ceramide aminoethylphosphate (sphingoethanolamine) in some aquatic animals. J. Biochem. 62: 67–70. [DOI] [PubMed] [Google Scholar]
- 8.Moschidis M. C. 1984. Phosphonolipids. Prog. Lipid Res. 23: 223–246. [DOI] [PubMed] [Google Scholar]
- 9.Karlsson K. A. 1970. On the chemistry and occurrence of sphingolipid long-chain bases. Chem. Phys. Lipids. 5: 6–43. [DOI] [PubMed] [Google Scholar]
- 10.Byrdwell W. C., and Perry R. H.. 2007. Liquid chromatography with dual parallel mass spectrometry and 31P nuclear magnetic resonance spectroscopy for analysis of sphingomyelin and dihydrosphingomyelin. II. Bovine milk sphingolipids. J. Chromatogr. A. 1146: 164–185. [DOI] [PubMed] [Google Scholar]
- 11.Morifuji M., Higashi S., Oba C., Ichikawa S., Kawahata K., Yamaji T., Itoh H., Manabe Y., and Sugawara T.. 2015. Milk phospholipids enhance lymphatic absorption of dietary sphingomyelin in lymph-cannulated rats. Lipids. 50: 987–996. [DOI] [PubMed] [Google Scholar]
- 12.Jin W., Rinehart K. L., and Jares-Erijman E. A.. 1994. Ophidiacerebrosides: cytotoxic glycosphingolipids containing a novel sphingosine from a sea star. J. Org. Chem. 59: 144–147. [Google Scholar]
- 13.Ohashi Y., Tanaka T., Akashi S., Morimoto S., Kishimoto Y., and Nagai Y.. 2000. Squid nerve sphingomyelin containing an unusual sphingoid base. J. Lipid Res. 41: 1118–1124. [PubMed] [Google Scholar]
- 14.Facchini L., Losito I., Cataldi T. R., and Palmisano F.. 2016. Ceramide lipids in alive and thermally stressed mussels: an investigation by hydrophilic interaction liquid chromatography-electrospray ionization Fourier transform mass spectrometry. J. Mass Spectrom. 51: 768–781. [DOI] [PubMed] [Google Scholar]
- 15.Hannun Y. A., and Obeid L. M.. 2008. Principles of bioactive lipid signalling: lessons from sphingolipids. Nat. Rev. Mol. Cell Biol. 9: 139–150. [DOI] [PubMed] [Google Scholar]
- 16.Morifuji M., Oba C., Ichikawa S., Ito K., Kawahata K., Asami Y., Ikegami S., Itoh H., and Sugawara T.. 2015. A novel mechanism for improvement of dry skin by dietary milk phospholipids: effect on epidermal covalently bound ceramides and skin inflammation in hairless mice. J. Dermatol. Sci. 78: 224–231. [DOI] [PubMed] [Google Scholar]
- 17.Duan J., Sugawara T., Hirose M., Aida K., Sakai S., Fujii A., and Hirata T.. 2012. Dietary sphingolipids improve skin barrier functions via the upregulation of ceramide synthases in the epidermis. Exp. Dermatol. 21: 448–452. [DOI] [PubMed] [Google Scholar]
- 18.Duan R. D., and Nilsson Å.. 2009. Metabolism of sphingolipids in the gut and its relation to inflammation and cancer development. Prog. Lipid Res. 48: 62–72. [DOI] [PubMed] [Google Scholar]
- 19.Schmelz E. M. 2004. Sphingolipids in the chemoprevention of colon cancer. Front. Biosci. 9: 2632–2639. [DOI] [PubMed] [Google Scholar]
- 20.Aida K., Kinoshita M., Sugawara T., Ono J., Miyazawa T., and Ohnishi M.. 2004. Apoptosis inducement by plant and fungus sphingoid bases in human colon cancer cells. J. Oleo Sci. 53: 503–510. [Google Scholar]
- 21.Sugawara T., Zaima N., Yamamoto A., Sakai S., Noguchi R., and Hirata T.. 2006. Isolation of sphingoid bases of sea cucumber cerebrosides and their cytotoxicity against human colon cancer cells. Biosci. Biotechnol. Biochem. 70: 2906–2912. [DOI] [PubMed] [Google Scholar]
- 22.Hossain Z., Sugawara T., and Hirata T.. 2013. Sphingoid bases from sea cucumber induce apoptosis in human hepatoma HepG2 cells through p-AKT and DR5. Oncol. Rep. 29: 1201–1207. [DOI] [PubMed] [Google Scholar]
- 23.Schmelz E. M., Crall K. J., Larocque R., Dillehay D. L., and Merrill A. H. Jr.. 1994. Uptake and metabolism of sphingolipids in isolated intestinal loops of mice. J. Nutr. 124: 702–712. [DOI] [PubMed] [Google Scholar]
- 24.Sugawara T., Kinoshita M., Ohnishi M., Nagata J., and Saito M.. 2003. Digestion of maize sphingolipids in rats and uptake of sphingadienine by Caco-2 cells. J. Nutr. 133: 2777–2782. [DOI] [PubMed] [Google Scholar]
- 25.Sugawara T., Tsuduki T., Yano S., Hirose M., Duan J., Aida K., Ikeda I., and Hirata T.. 2010. Intestinal absorption of dietary maize glucosylceramide in lymphatic duct cannulated rats. J. Lipid Res. 51: 1761–1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nilsson Å. 1968. Metabolism of sphingomyelin in the intestinal tract of the rat. Biochim. Biophys. Acta. 164: 575–584. [DOI] [PubMed] [Google Scholar]
- 27.Wakashima T., Abe K., and Kihara A.. 2014. Dual functions of the trans-2-enoyl-CoA reductase TER in the sphingosine 1-phosphate metabolic pathway and in fatty acid elongation. J. Biol. Chem. 289: 24736–24748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomonaga N., Manabe Y., and Sugawara T.. 2017. Digestion of ceramide 2-aminoethylphosphonate, a sphingolipid from the jumbo flying squid Dosidicus gigas, in mice. Lipids. 52: 353–362. [DOI] [PubMed] [Google Scholar]
- 29.Bollman J. L., Cain J. C., and Grindlay J. H.. 1948. Techniques for the collection of lymph from the liver, small intestine, or thoracic duct of the rat. J. Lab. Clin. Med. 33: 1349–1352. [PubMed] [Google Scholar]
- 30.Gaver R. C., and Sweeley C. C.. 1965. Methods for methanolysis of sphingolipids and direct determination of long-chain bases by gas chromatography. J. Am. Oil Chem. Soc. 42: 294–298. [DOI] [PubMed] [Google Scholar]
- 31.Yunoki K., Kukino O., Nadachi Y., Fujino T., and Ohnishi M.. 2008. Separation and determination of functional complex lipids from chicken skin. J. Am. Oil Chem. Soc. 85: 427–433. [Google Scholar]
- 32.Vahouny G. V., Fawal I., and Treadwell C. R.. 1957. Factors facilitating cholesterol absorption from the intestine via lymphatic pathways. Am. J. Physiol. 188: 342–346. [DOI] [PubMed] [Google Scholar]
- 33.Sugawara T., Aida K., Duan J., and Hirata T.. 2010. Analysis of glucosylceramides from various sources by liquid chromatography-ion trap mass spectrometry. J. Oleo Sci. 59: 387–394. [DOI] [PubMed] [Google Scholar]
- 34.Erdfelder E., Faul F., and Buchner A.. 1996. GPOWER: A general power analysis program. Behav. Res. Methods Instrum. Comput. 28: 1–11. [Google Scholar]
- 35.Faul F., Erdfelder E., Buchner A., and Lang A. G.. 2009. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav. Res. Methods. 41: 1149–1160. [DOI] [PubMed] [Google Scholar]
- 36.Duan J., Ishida M., Aida K., Tsuduki T., Zhang J., Manabe Y., Hirata T., and Sugawara T.. 2016. Dietary cerebroside from sea cucumber (Stichopus japonicus): absorption and effects on skin barrier and cecal short-chain fatty acids. J. Agric. Food Chem. 64: 7014–7021. [DOI] [PubMed] [Google Scholar]
- 37.Morifuji M., Kitade M., Oba C., Fukasawa T., Kawahata K., Yamaji T., Manabe Y., and Sugawara T.. 2017. Milk fermented by lactic acid bacteria enhances the absorption of dietary sphingomyelin in rats. Lipids. 52: 423–431. [DOI] [PubMed] [Google Scholar]
- 38.Juranka P. F., Zastawny R. L., and Ling V.. 1989. P-glycoprotein: multidrug-resistance and a superfamily of membrane-associated transport proteins. FASEB J. 3: 2583–2592. [DOI] [PubMed] [Google Scholar]
- 39.Fujii A., Manabe Y., Aida K., Tsuduki T., Hirata T., and Sugawara T.. 2017. Selective absorption of dietary sphingoid bases from the intestine via efflux by P-glycoprotein in rats. J. Nutr. Sci. Vitaminol. (Tokyo). 63: 44–50. [DOI] [PubMed] [Google Scholar]
- 40.Thiebaut F., Tsuruo T., Hamada H., Gottesman M. M., Pastan I., and Willingham M. C.. 1987. Cellular localization of the multidrug-resistance gene product P-glycoprotein in normal human tissues. Proc. Natl. Acad. Sci. USA. 84: 7735–7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Croop J. M., Raymond M., Haber D., Devault A., Arceci R. J., Gros P., and Housman D. E.. 1989. The three mouse multidrug resistance (mdr) genes are expressed in a tissue-specific manner in normal mouse tissues. Mol. Cell. Biol. 9: 1346–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stoffel W., and Assmann G.. 1970. Metabolism of sphingosine bases, XV1. Enzymatic degradation of 4t-sphingenine 1-phosphafe (sphingosine 1-phosphate) to 2t-hexadecen-l-al and ethanolamine phosphate. Hoppe Seylers Z. Physiol. Chem. 351: 1041–1049. [DOI] [PubMed] [Google Scholar]
- 43.Stoffel W., Bauer E., and Stahl J.. 1974. The metabolism of sphingosine bases in Tetrahymena pyriformis: sphingosine kinase sphingosine-1-phosphate lyase. Hoppe Seylers Z. Physiol. Chem. 355: 61–74. [DOI] [PubMed] [Google Scholar]
- 44.Kihara A. 2014. Sphingosine 1-phosphate is a key metabolite linking sphingolipids to glycerophospholipids. Biochim. Biophys. Acta. 1841: 766–772. [DOI] [PubMed] [Google Scholar]
- 45.Kondo N., Ohno Y., Yamagata M., Obara T., Seki N., Kitamura T., Naganuma T., and Kihara A.. 2014. Identification of the phytosphingosine metabolic pathway leading to odd-numbered fatty acids. Nat. Commun. 5: 5338. [DOI] [PubMed] [Google Scholar]
- 46.Suh J. H., Makarova A. M., Gomez J. M., Paul L. A., and Saba J. D.. 2017. An LC/MS/MS method for quantitation of chemopreventive sphingadienes in food products and biological samples. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 1061–1062: 292–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chapman M. J. 1986. Comparative analysis of mammalian plasma lipoproteins. Methods Enzymol. 128: 70–143. [DOI] [PubMed] [Google Scholar]
- 48.Merrill A. H. Jr., Lingrell S., Wang E., Nikolova-Karakashian M., Vales T. R., and Vance D. E.. 1995. Sphingolipid biosynthesis de novo by rat hepatocytes in culture. Ceramide and sphingomyelin are associated with, but not required for, very low density lipoprotein secretion. J. Biol. Chem. 270: 13834–13841. [DOI] [PubMed] [Google Scholar]