Abstract
Phosphoinositide-specific phospholipases C (PI-PLCs) are involved in signaling pathways related to critical cellular functions, such as cell cycle regulation, cell differentiation, and gene expression. Nuclear PI-PLCs have been studied as key enzymes, molecular targets, and clinical prognostic/diagnostic factors in many physiopathologic processes. Here, we summarize the main studies about nuclear PI-PLCs, specifically, the imbalance of isozymes such as PI-PLCβ1 and PI-PLCζ, in cerebral, hematologic, neuromuscular, and fertility disorders. PI-PLCβ1 and PI-PLCɣ1 affect epilepsy, depression, and bipolar disorder. In the brain, PI-PLCβ1 is involved in endocannabinoid neuronal excitability and is a potentially novel signature gene for subtypes of high-grade glioma. An altered quality or quantity of PI-PLCζ contributes to sperm defects that result in infertility, and PI-PLCβ1 aberrant inositide signaling contributes to both hematologic and degenerative muscle diseases. Understanding the mechanisms behind PI-PLC involvement in human pathologies may help identify new strategies for personalized therapies of these conditions.
Keywords: nucleus, myelodysplastic syndromes, fertility, phospholipase C, brain, myotonic dystrophy
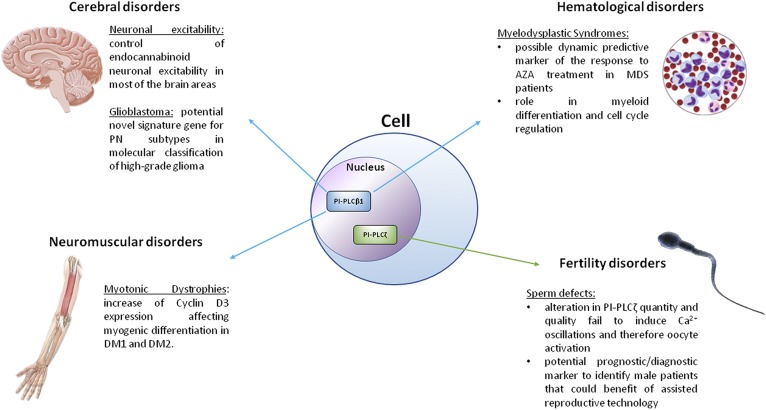
Nuclear phosphoinositide-specific phospholipases C (PI-PLCs) are a group of enzymes that hydrolyze phosphatidylinositol 4,5-biphosphate [PI(4,5)P2] to inositol 1,4,5-trisphosphate (IP3) and diacylglycerol (DAG) that, in turn, are key second messengers involved in the activation of several signaling pathways related to many critical cellular functions (1, 2). Indeed, nuclear PI-PLCs play pivotal roles in cell cycle regulation (3, 4), cell proliferation, cell differentiation, membrane trafficking, and gene expression (5, 6). Recent evidence on the role of nuclear PI-PLC signaling in cell cycle regulation and cell proliferation paved the way to new fields of research related to different strategic physiopathological mechanisms in many cellular systems and diseases. This is the case of PI-PLCβ1, which plays a role in the physiopathology of brain disorders (along with PI-PLCγ1), hematological malignancies, and neuromuscular diseases, but also of PI-PLC-ζ, which has been associated with fertility disorders. All in all, the imbalance of nuclear PI-PLC isoenzymes, such as PI-PLCβ1 and PI-PLC-ζ, can lead to pathology (7).
CEREBRAL DISORDERS
PI-PLCs are very expressed in different brain areas and have been related to many brain disorders (8). PI-PLCβ1 localizes at high concentration in the hippocampus, amygdala, lateral septum, olfactory bulb, and cerebral cortex (9). Indeed, PI metabolism has been related to neurotrasmission, cortical development, and synaptic plasticity (10, 11). PI-PLC has been linked to epilepsy and to schizophrenia in PI-PLCβ1 knocked-out mice (12). Losses of PI-PLCβ1 were also detected in patients with epileptic encephalopathies, schizophrenia, and bipolar disorders (13–15). Specific neurotransmitters (NTs) and hormones trigger PI-PLC pathways, resulting in different activities in distinct brain regions (12). Another PI-PLC, PI-PLCγ1, has been correlated to neuronal cell migration and synaptic plasticity, and its activity has been suggested to be of some relevance in epilepsy, Huntington disease, depression, bipolar disorders, and Alzheimer disease (12).
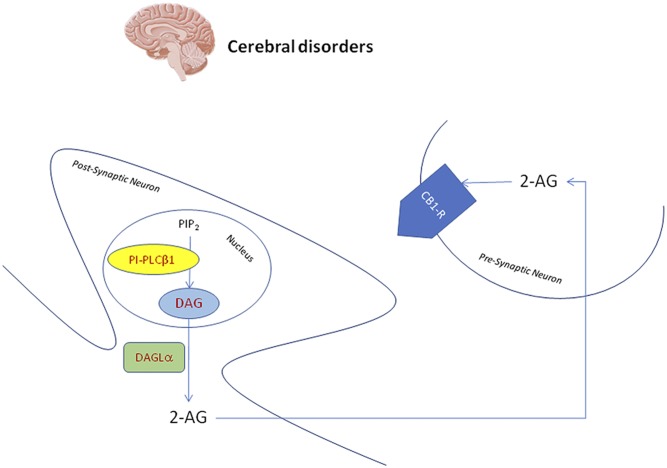
Specifically at nuclear level, PI-PLCβ1 seems to be pivotal in the control of endocannabinoid neuronal excitability in most of the brain areas (14). As Fig. 1 shows, it is essential in the synthesis of DAG, for which hydrolysis catalyzed by DAG-lipase α (DAGLα) leads to 2-arachidonyl-glycerol (2-AG), the most represented CB1 cannabinoid receptor endogenous agonist. Released from the postsynaptic neuron, 2-AG can activate CB1 cannabinoid receptors on the presynaptic sites repressing other NT release (16). Moreover, PI-PLCβ1 can respond to two separate signals: depolarization and receptor activation, maintaining the brain inhibitory circuits through 2-AG (17). Interestingly, this pathway related to PI-PLCβ1/DAGLα/2-AG seems to be specifically localized at neuronal nuclear level. In the adult rat brain, double immunofluorescence staining and confocal laser scanning showed an overlapping pattern of both PI-PLCβ1 and DAGLα with nuclear speckles marker SC-35 and NeuN/Fox3. In addition, PI-PLCβ1 was also highly expressed in neuronal nuclear regions full of PI(4,5)P2. The colocalization with nuclear pore complex and lamin B1 was instead not found (16). There is also evidence of the relation between 2-AG and nuclear PPARγ as a receptor or a prostaglandin precursor (17). Furthermore, considering the pivotal role of nuclear PI-PLCβ1 in cell cycle regulation and the controversial and the complicated role of the cell cycle in several CNS insults and pathologies, it would be very fascinating to study nuclear PI-PLCβ1 functions during diverse brain injuries (17).
Fig. 1.
Nuclear PI-PLC β1 is essential in the synthesis of nuclear DAG. Possibly, this nuclear DAG is hydrolyzed by DAGLα to produce 2-AG. Released from the postsynaptic neuron, 2-AG can in turn activate cannabinoid receptor type 1 (CB1-R) on the presynaptic sites, repressing other NT release.
Also, in glioma, the two isoforms of PI-PLCβ1 localize differently, in the cytosol and in the nucleus of C6 glioma cells, respectively (18). PI-PLCβ1 was also shown to be transited into the nucleus among C6 glial cell and Neuro2A cell (mouse neuroblastoma cell line) under stimuli (19). Glioblastoma multiforme (GBM), also known as grade IV glioma, is the most common and most aggressive form of astrocytic cancer among primary brain tumors. It has a documented molecular heterogeneity and is rapidly fatal (20). The incidence of GBM is about 6 cases per 100,000 people/year. This type of malignancy often has a rapid progression (about 2–3 months) (21). The therapy is still based on a neurosurgical, chemotherapeutical, radiotherapeutical approach without any successful targeted therapeutic strategy to date (22, 23). Many pieces of evidence suggest a role of inositide metabolism in GBM tumorigenesis due to the interactions of several molecules such as diacylglicerol kinases, PI-PLCs, and phosphoinositide 5-phosphatase (24–26). Indeed, at both the cytoplasmic and nuclear level, the lipid signaling molecules control several pivotal mechanisms of cell proliferation, cell migration, cell cycle, and apoptosis (27). New data analyses of The Cancer Genome Atlas (TCGA) and four independent Gene Expression Omnibus datasets revealed a correlation between differential expression of PI-PLCβ1 and glioma pathological grades. PI-PLCβ1 is a potential novel signature gene for proneural (PN) subtypes in molecular classification of high-grade glioma, because its gene expression correlates with known PN subtype signature genes (25). Kaplan-Meier survival curves based on differential PI-PLCβ1 gene expression from the Repository for Molecular Brain Neoplasia Data (REMBRANDT) and TCGA cohorts also demonstrate that a high level of PI-PLCβ1 expression is associated with patient’s long-term survival. More specifically, PI-PLCβ1 gene expression level correlates the best with PN glioma signature gene receptor tyrosine-protein kinase erbB-4 (ERBB4) (25). ERBB4 protein is a tyrosine-protein kinase and a member of the epidermal growth factor receptor subfamily, which contributes to glioma pathogenesis. However, PI-PLCβ1 microarray probes only target and bind to common cDNA region of both “a” and “b” isoforms. Considering that these two isoforms are different in their C terminals, microarray data based on current PI-PLCβ1 probes could not differentiate the two isoforms. If the transcription of one PI-PLCβ1 isoform is the predominant isoform, the minor component of overall PI-PLCβ1 signal may change the conclusion reached in this study: the higher the glioma grade, the lower the PI-PLCβ1 expression. It will be possible for one isoform to gain its signal strength along with pathological grades. Further investigations are, therefore, required to better understand these studies: the neuronal nuclear localization and the mechanisms of PI-PLCβ1 and its related molecules in different pathophysiological brain functions could open new research perspectives.
HEMATOLOGICAL DISORDERS
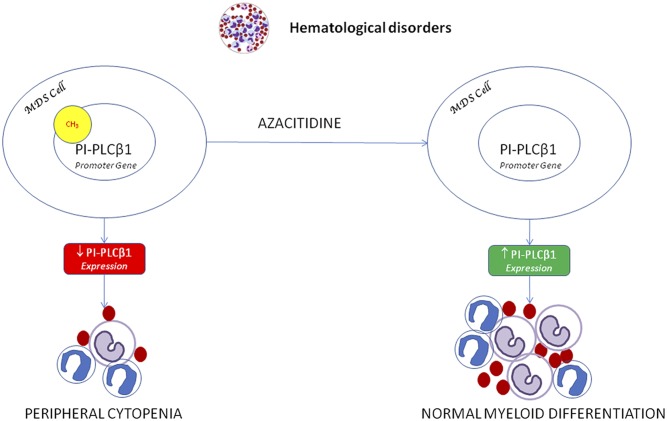
Among the hematological disorders, both MDSs and acute myeloid leukemia (AML) have been related to nuclear inositide signaling (28–30). Specifically, MDSs have been associated with nuclear PI-PLC pathways (31–33). MDSs are a group of pathologies characterized by hematopoietic stem cell alteration with heterogeneous characteristics and different clinical effects. Thirty percent of MDS patients can evolve into AML. The International Prognostic Scoring System and the World Health Organization Classification-Based Scoring System, mainly based on the blast number and karyotype, divide MDS patients into higher and lower risk of AML progression. The evidence of a correlation between the presence of a PI-PLCβ1 monoallelic gene deletion and the progression of MDS to AML opened new perspectives of research (34). The gene encoding for PI-PLCβ1 is on chromosome 20p12. Higher-risk MDS patients should undergo allogeneic hematopoietic stem cell transplantation, but for those who are not candidates, hypomethylating agents (HMAs), such azacitidine (AZA), are now the first therapeutic choice (35). HMAs can also be used in lower-risk MDS patients to reduce anemia or other cytopenias. AZA is indeed both a hypomethylating and a direct cytotoxic agent for abnormal hematopoietic cells. MDS patients with a higher risk of AML evolution show a reduction in the expression of the nuclear PI-PLCβ1 variant. PI-PLCβ1b can physiologically regulate the cell cycle G1 phase progression; therefore, the drastic reduction of nuclear PI-PLCβ1 expression could alter the normal cell cycle in MDS patients. Nuclear PI-PLCβ1 in MDS is also epigenetically relevant (36, 37). Several studies have shown that PI-PLCβ1 is a molecular target for AZA (38, 39). Higher- and lower-risk patients that respond to the treatment have shown an early increase of nuclear PI-PLCβ1 expression, a reduction of PI-PLCβ1 promoter methylation, an induction of normal myeloid differentiation, and a better prognosis (40). Moreover, the increase of PI-PLCβ1 expression and the reduction in PI-PLCβ1 promoter methylation are not only related to a favorable clinical response, but also to a durable response to the treatment and a myeloid induction (Fig. 2). This effect is particularly interesting, because, as AZA treatment needs several cycles to observe a clinical response, the molecular response could specifically and rapidly predict the future patients’ outcome during hypomethylating therapies. In this way, it would be possible to personalize MDS patients’ therapies in order to differentiate the ones who would benefit from the treatment from the ones who would be refractory to the treatment and thus could avoid it (41). Furthermore, several studies showed that in higher-risk MDS patients, there is an inverse correlation between PI-PLCβ1 and protein kinase B, also known as Akt (42). The constitutive activation of Akt, through phosphorylation, determines a decrease in MDS cell apoptosis that can be reverted after PI-PLCβ1 increase due to the hypomethylating therapies. Indeed PI-PLCβ1 increase could reduce the level of p-Akt, inducing a higher level of apoptosis (43). This mechanism is not fully understood, but a possible explanation could be that, as PI(4,5)P2 is a common substrate of both PI-PLCβ1 and PI3Ks, the alterations in PI-PLCβ1 levels could cause PI(4,5)P2 hydrolysis, reducing its availability for PI3K. This could eventually inhibit Akt activation (44).
Fig. 2.
Nuclear PI-PLC β1 expression, increased by AZA sensitivity on PI-PLCβ1 promoter methylation (CH3), induces myeloid differentiation in MDS cells.
More recently, a study on the effect of lenalidomide on 16 patients with lower-risk MDS and deletion of the long arm of chromosome 5 [del(5q)], as well as del(5q) and nondel(5q) hematopoietic cell lines, focused on erythropoiesis, cell cycle, and PI-PLCβ1/protein kinase C-α (PKC-α) signaling (45). Indeed, nuclear PI-PLCβ1 is a negative regulator of erythroid differentiation (46), and it specifically targets PKC-α, which, in turn, has been associated with proliferation and differentiation of human erythroleukemia cells (47). In this new study, in MDS, lenalidomide induced PI-PLCβ1a to localize primarily in the cytoplasm, where it is not directly associated with inhibition of erythroid differentiation. The data on the cell lines showed that lenalidomide specifically induced the expression of PI-PLCβ1 in the cytoplasm of Namalwa CSN.70 [i.e., del(5q) cells]. In these cells, a nuclear translocation of PKC-α, associated with erythropoiesis, was also detected. These results better explain the role of PI-PLCβ1/PKC-α signaling in erythropoiesis and lead to a better comprehension of the lenalidomide effect on del(5q) MDS, opening the way to innovative, targeted therapies (45). These mechanisms underline the potential of nuclear PI-PLCβ1 to become a prognosis stratification marker and a treatment-predictive outcome marker in patients with MDS.
NEUROMUSCULAR DISORDERS
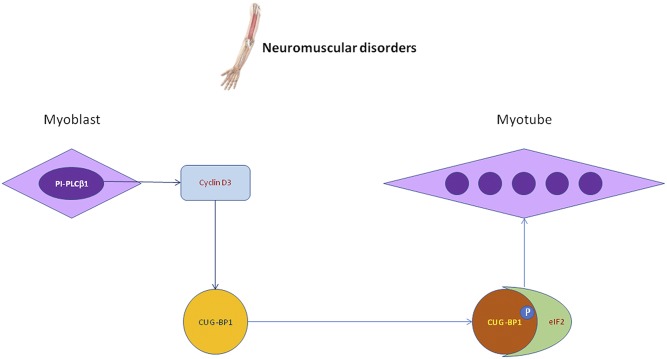
Among the neuromuscular disorders, myotonic dystrophies (DMs) are autosomal dominant neuromuscular degenerative disorders characterized by a variable clinical picture and a slow progressive course. The clinical picture is characterized by myotonia, loss of muscle mass, cataracts, defects in the cardiac conduction system, endocrine abnormalities, and cognitive deficits in congenital cases. These diseases are classified into DM type I (DM1) and DM type II (DM2). Both DM are determined by DNA tandem repeats that result in aberrant RNA accumulation in the nucleus that causes alteration in RNA-binding protein localization. In DM1, the mutated gene is called DM protein kinase and encodes a myosin kinase expressed in skeletal muscles. In DM2, there is a defect in zinc finger protein 9 (48). CUG triplet repeat RNA-binding protein 1 (CUGBP1) plays a central role in alternative splicing of specific target genes and can interact with eukaryotic initiation factor 2 (eIF2) and cyclin D3 inducing normal myogenic differentiation. Indeed, cyclin D3 mediates the phosphorylation of CUGBP1 (49), thus increasing the interactions of CUGBP1 with eIF2 during normal myogenesis (Fig. 3). This process can be altered in DM, where CUGBP1-eIF2 interactions are reduced in DM-differentiating cells, with an impaired muscle differentiation (50). Nevertheless, in DM1, ectopic expression of cyclin D3 helps the increase of the CUGBP1-eIF2 complex, improving the myogenic differentiation marker expression (49). Moreover, during the myogenesis in DM cells, there is a decrease of PI-PLCβ1 expression that could be related to the reduction in cyclin D3 transcription and induction. Interestingly, the normalization of PI-PLCβ1 expression in DM1 and DM2 myoblasts can cause a cyclin D3 expression increase, determining a partially restored phenotype of the myotubes (50). Both PI-PLCβ1 and cyclin D3 could be investigated for future possible molecular therapies in order to induce a correct skeletal muscle differentiation in DM. Indeed, the role of PI-PLCβ1 and cyclin D3 seem to be pivotal in physiological myogenic differentiation (51, 52).
Fig. 3.
Nuclear PI-PLC β1 increased catalytic activity is necessary to induce cyclin D3, which, in turn, mediates the phosphorylation of CUGBP1 and increases the formation of CUGBP1-eIF2 complex, thus leading to myogenic differentiation and myotube formation.
FERTILITY DISORDERS
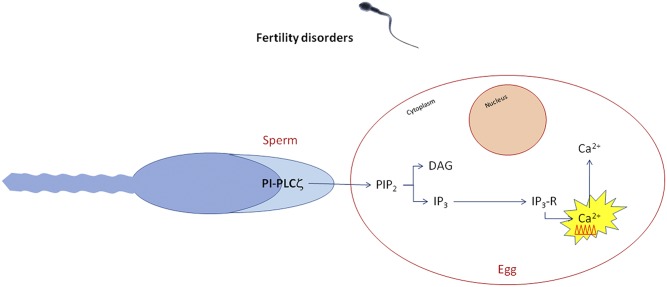
The principal cause of infertility after intracytoplasmatic sperm injection is considered to be the alteration of oocyte activation mechanisms, of which sperm defects seem to be the main cause (53). Sperm defects have been associated to another PI-PLC isozyme related to nuclear activities: PI-PLCζ, a specific sperm protein involved in nuclear infertility mechanisms (54). PI-PLCζ has been specifically connected with molecular oocyte activation, but this PI-PLC nuclear translocation mechanism still remains unknown (Fig. 4). PI-PLCζ is essential in inducing Ca2+ release via the IP3 pathway (55). Ca2+ oscillations within the oocyte have been related to many processes responsible for its activation, such as the cortical granule exocytosis, the release of meiotic arrest, the gene expression regulation, the recruitment of maternal mRNA, the pronuclear formation, and the initiating of embryogenesis (53). The specific mechanism of these reactions is still unclear, but the central role of Ca2+ is fundamental for the beginning of embryogenesis (53). cRNA PI-PLCζ and recombinant protein microinjections in mice and cattle oocyte determine Ca2+ oscillations and oocyte activation (55). Moreover, infertile human sperm with an alteration in PI-PLCζ quantity and quality fail to induce Ca2+ oscillations and therefore oocyte activation (53). Therefore, for these reasons, it is possible that PI-PLCζ could be pivotal in fertility processes and could be considered a prognostic/diagnostic molecular marker in order to identify male patients that could benefit of assisted reproductive technology. Moreover, even if Ca2+ ionophores are the main agent for artificial oocyte activation, PI-PLCζ could be a safer potential therapeutic agent (53). These studies could open new possibilities of research in the field of fertility disorders in relation to the inositide signaling world.
Fig. 4.
Calcium oscillates and ionophores regulate fertility treatments: IP3 production and subsequent Ca2+ oscillations are triggered by a sperm-derived soluble protein, which is released to the oocyte cytoplasm immediately after sperm-egg fusion.
CONCLUSIONS
All in all, the role of PI-PLCs in nuclear signaling appears to be relevant in that the activity or expression of imbalanced nuclear PI-PLCs is associated with control and development of diseases. Figure 5 summarizes the involvement of PI-PLCβ1 and PI-PLCζ in the pathologies of mammals and mainly of humans.
Fig. 5.
Schematic diagram of the role of nuclear PI-PLCβ1 and PI-PLCζ in pathologies.
Footnotes
Abbreviations:
- 2sAG
- 2-arachidonyl-glycerol
- AML
- acute myeloid leukemia
- AZA
- azacitidine
- CUGBP1
- CUG triplet repeat RNA-binding protein 1
- DAG
- diacylglycerol
- DAGLα
- diacylglycerol-lipase α
- del(5q)
- deletion of the long arm of chromosome 5
- GBM
- glioblastoma multiforme
- DM
- myotonic dystrophy
- eIF2
- eukaryotic initiation factor 2
- IP3
- inositol-1,4,5-triphosphate
- MDS
- myelodysplastic syndrome
- NT
- neurotransmitter
- PI3K
- phosphoinositide 3-kinase
- PI(4
- 5)P2, phosphatidylinositol 4,5-bisphosphate
- PI-PLC
- phosphoinositide-specific phospholipase C
- PKC-α
- protein kinase C-α
- PN
- proneural
This work was supported by the Intesa San Paolo Foundation and Ministero dell’Istruzione, dell’Università e della Ricerca PRIN 2015 grants.
The online version of this article (available at http://www.jlr.org) contains a supplement.
REFERENCES
- 1.Cocco L., Follo M. Y., Manzoli L., and Suh P. G.. 2015. Phosphoinositide-specific phospholipase C in health and disease. J. Lipid Res. 56: 1853–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cocco L., Martelli A. M., and Gilmour R. S.. 1994. Inositol lipid cycle in the nucleus. Cell. Signal. 6: 481–485. [DOI] [PubMed] [Google Scholar]
- 3.Poli A., Fiume R., Baldanzi G., Capello D., Ratti S., Gesi M., Manzoli L., Graziani A., Suh P. G., Cocco L., et al. 2017. Nuclear localization of diacylglycerol kinase alpha in K562 cells is involved in cell cycle progression. J. Cell. Physiol. 232: 2550–2557. [DOI] [PubMed] [Google Scholar]
- 4.Vitale M., Matteucci A., Manzoli L., Rodella L., Mariani A. R., Zauli G., Falconi M., Billi A. M., Martelli A. M., Gilmour R. S., et al. 2001. Interleukin 2 activates nuclear phospholipase Cbeta by mitogen-activated protein kinase-dependent phosphorylation in human natural killer cells. FASEB J. 15: 1789–1791. [DOI] [PubMed] [Google Scholar]
- 5.Ratti S., Ramazzotti G., Faenza I., Fiume R., Mongiorgi S., Billi A. M., McCubrey J. A., Suh P. G., Manzoli L., Cocco L., et al. 2018. Nuclear inositide signaling and cell cycle. Adv. Biol. Regul. 67: 1–6. [DOI] [PubMed] [Google Scholar]
- 6.Ramazzotti G., Faenza I., Fiume R., Matteucci A., Piazzi M., Follo M. Y., and Cocco L.. 2011. The physiology and pathology of inositide signaling in the nucleus. J. Cell. Physiol. 226: 14–20. [DOI] [PubMed] [Google Scholar]
- 7.Yang Y. R., Follo M. Y., Cocco L., and Suh P. G.. 2013. The physiological roles of primary phospholipase C. Adv. Biol. Regul. 53: 232–241. [DOI] [PubMed] [Google Scholar]
- 8.Yang Y. R., Kang D. S., Lee C., Seok H., Follo M. Y., Cocco L., and Suh P. G.. 2016. Primary phospholipase C and brain disorders. Adv. Biol. Regul. 61: 80–85. [DOI] [PubMed] [Google Scholar]
- 9.Fukaya M., Uchigashima M., Nomura S., Hasegawa Y., Kikuchi H., and Watanabe M.. 2008. Predominant expression of phospholipase Cbeta1 in telencephalic principal neurons and cerebellar interneurons, and its close association with related signaling molecules in somatodendritic neuronal elements. Eur. J. Neurosci. 28: 1744–1759. [DOI] [PubMed] [Google Scholar]
- 10.Raben D. M., and Barber C. N.. 2017. Phosphatidic acid and neurotransmission. Adv. Biol. Regul. 63: 15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Spires T. L., Molnar Z., Kind P. C., Cordery P. M., Upton A. L., Blakemore C., and Hannan A. J.. 2005. Activity-dependent regulation of synapse and dendritic spine morphology in developing barrel cortex requires phospholipase C-beta1 signalling. Cereb. Cortex. 15: 385–393. [DOI] [PubMed] [Google Scholar]
- 12.Yang Y. R., Jung J. H., Kim S. J., Hamada K., Suzuki A., Kim H. J., Lee J. H., Kwon O. B., Lee Y. K., Kim J., et al. 2017. Forebrain-specific ablation of phospholipase Cgamma1 causes manic-like behavior. Mol. Psychiatry. 22: 1473–1482. [DOI] [PubMed] [Google Scholar]
- 13.Kurian M. A., Meyer E., Vassallo G., Morgan N. V., Prakash N., Pasha S., Hai N. A., Shuib S., Rahman F., Wassmer E., et al. 2010. Phospholipase C beta 1 deficiency is associated with early-onset epileptic encephalopathy. Brain. 133: 2964–2970. [DOI] [PubMed] [Google Scholar]
- 14.Lo Vasco V. R., Cardinale G., and Polonia P.. 2012. Deletion of PLCB1 gene in schizophrenia-affected patients. J. Cell. Mol. Med. 16: 844–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lo Vasco V. R., Longo L., and Polonia P.. 2013. Phosphoinositide-specific phospholipase C beta1 gene deletion in bipolar disorder affected patient. J. Cell Commun. Signal. 7: 25–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montaña M., García del Caño G., López de Jesús M., González-Burguera I., Echeazarra L., Barrondo S., and Sallés J.. 2012. Cellular neurochemical characterization and subcellular localization of phospholipase C beta1 in rat brain. Neuroscience. 222: 239–268. [DOI] [PubMed] [Google Scholar]
- 17.García del Caño G., Montaña M., Aretxabala X., González-Burguera I., López de Jesus M., Barrondo S., and Sallés J.. 2014. Nuclear phospholipase C-beta1 and diacylglycerol LIPASE-alpha in brain cortical neurons. Adv. Biol. Regul. 54: 12–23. [DOI] [PubMed] [Google Scholar]
- 18.Bahk Y. Y., Song H., Baek S. H., Park B. Y., Kim H., Ryu S. H., and Suh P. G.. 1998. Localization of two forms of phospholipase C-beta1, a and b, in C6Bu-1 cells. Biochim. Biophys. Acta. 1389: 76–80. [DOI] [PubMed] [Google Scholar]
- 19.Aisiku O. R., Runnels L. W., and Scarlata S.. 2010. Identification of a novel binding partner of phospholipase cbeta1: translin-associated factor X. PLoS One. 5: e15001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wick W., Osswald M., Wick A., and Winkler F.. 2018. Treatment of glioblastoma in adults. Ther. Adv. Neurol. Disord. 11: 1756286418790452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wick W., and Platten M.. 2018. Understanding and treating glioblastoma. Neurol. Clin. 36: 485–499. [DOI] [PubMed] [Google Scholar]
- 22.Jhanwar-Uniyal M., Gillick J. L., Neil J., Tobias M., Thwing Z. E., and Murali R.. 2015. Distinct signaling mechanisms of mTORC1 and mTORC2 in glioblastoma multiforme: a tale of two complexes. Adv. Biol. Regul. 57: 64–74. [DOI] [PubMed] [Google Scholar]
- 23.Olmez I., Love S., Xiao A., Manigat L., Randolph P., McKenna B. D., Neal B. P., Boroda S., Li M., Brenneman B., et al. 2018. Targeting the mesenchymal subtype in glioblastoma and other cancers via inhibition of diacylglycerol kinase alpha. Neuro-oncol. 20: 192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dominguez C. L., Floyd D. H., Xiao A., Mullins G. R., Kefas B. A., Xin W., Yacur M. N., Abounader R., Lee J. K., Wilson G. M., et al. 2013. Diacylglycerol kinase alpha is a critical signaling node and novel therapeutic target in glioblastoma and other cancers. Cancer Discov. 3: 782–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lu G., Chang J. T., Liu Z., Chen Y., Li M., and Zhu J. J.. 2016. Phospholipase C beta 1: a candidate signature gene for proneural subtype high-grade glioma. Mol. Neurobiol. 53: 6511–6525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramos A. R., Elong Edimo W., and Erneux C.. 2018. Phosphoinositide 5-phosphatase activities control cell motility in glioblastoma: two phosphoinositides PI(4,5)P2 and PI(3,4)P2 are involved. Adv. Biol. Regul. 67: 40–48. [DOI] [PubMed] [Google Scholar]
- 27.Ratti S., Mongiorgi S., Ramazzotti G., Follo M. Y., Mariani G. A., Suh P. G., McCubrey J. A., Cocco L., and Manzoli L.. 2017. Nuclear inositide signaling via phospholipase C. J. Cell. Biochem. 118: 1969–1978. [DOI] [PubMed] [Google Scholar]
- 28.Poli A., Billi A. M., Mongiorgi S., Ratti S., McCubrey J. A., Suh P. G., Cocco L., and Ramazzotti G.. 2016. Nuclear phosphatidylinositol signaling: focus on phosphatidylinositol phosphate kinases and phospholipases C. J. Cell. Physiol. 231: 1645–1655. [DOI] [PubMed] [Google Scholar]
- 29.Mongiorgi S., Finelli C., Yang Y. R., Clissa C., McCubrey J. A., Billi A. M., Manzoli L., Suh P. G., Cocco L., and Follo M. Y.. 2016. Inositide-dependent signaling pathways as new therapeutic targets in myelodysplastic syndromes. Expert Opin. Ther. Targets. 20: 677–687. [DOI] [PubMed] [Google Scholar]
- 30.Mongiorgi S., Follo M. Y., Yang Y. R., Ratti S., Manzoli L., McCubrey J. A., Billi A. M., Suh P. G., and Cocco L.. 2016. Selective activation of nuclear PI-PLCbeta1 during normal and therapy-related differentiation. Curr. Pharm. Des. 22: 2345–2348. [DOI] [PubMed] [Google Scholar]
- 31.Manzoli L., Mongiorgi S., Clissa C., Finelli C., Billi A. M., Poli A., Quaranta M., Cocco L., and Follo M. Y.. 2014. Strategic role of nuclear inositide signalling in myelodysplastic syndromes therapy. Mini Rev. Med. Chem. 14: 873–883. [PubMed] [Google Scholar]
- 32.Cocco L., Faenza I., Follo M. Y., Ramazzotti G., Gaboardi G. C., Billi A. M., Martelli A. M., and Manzoli L.. 2008. Inositide signaling: nuclear targets and involvement in myelodysplastic syndromes. Adv. Enzyme Regul. 48: 2–9. [DOI] [PubMed] [Google Scholar]
- 33.Cocco L., Follo M. Y., Faenza I., Bavelloni A., Billi A. M., Martelli A. M., and Manzoli L.. 2007. Nuclear inositide signaling: an appraisal of phospholipase C beta 1 behavior in myelodysplastic and leukemia cells. Adv. Enzyme Regul. 47: 2–9. [DOI] [PubMed] [Google Scholar]
- 34.Follo M. Y., Finelli C., Clissa C., Mongiorgi S., Bosi C., Martinelli G., Baccarani M., Manzoli L., Martelli A. M., and Cocco L.. 2009. Phosphoinositide-phospholipase C beta1 mono-allelic deletion is associated with myelodysplastic syndromes evolution into acute myeloid leukemia. J. Clin. Oncol. 27: 782–790. [DOI] [PubMed] [Google Scholar]
- 35.Finelli C., Follo M. Y., Stanzani M., Parisi S., Clissa C., Mongiorgi S., Barraco M., and Cocco L.. 2016. Clinical impact of hypomethylating agents in the treatment of myelodysplastic syndromes. Curr. Pharm. Des. 22: 2349–2357. [DOI] [PubMed] [Google Scholar]
- 36.Jhanwar S. C. 2015. Genetic and epigenetic pathways in myelodysplastic syndromes: a brief overview. Adv. Biol. Regul. 58: 28–37. [DOI] [PubMed] [Google Scholar]
- 37.Follo M. Y., Mongiorgi S., Finelli C., Clissa C., Ramazzotti G., Fiume R., Faenza I., Manzoli L., Martelli A. M., and Cocco L.. 2010. Nuclear inositide signaling in myelodysplastic syndromes. J. Cell. Biochem. 109: 1065–1071. [DOI] [PubMed] [Google Scholar]
- 38.Follo M. Y., Finelli C., Mongiorgi S., Clissa C., Bosi C., Testoni N., Chiarini F., Ramazzotti G., Baccarani M., Martelli A. M., et al. 2009. Reduction of phosphoinositide-phospholipase C beta1 methylation predicts the responsiveness to azacitidine in high-risk MDS. Proc. Natl. Acad. Sci. USA. 106: 16811–16816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Follo M. Y., Russo D., Finelli C., Mongiorgi S., Clissa C., Fili C., Colombi C., Gobbi M., Manzoli L., Piazzi M., et al. 2012. Epigenetic regulation of nuclear PI-PLCbeta1 signaling pathway in low-risk MDS patients during azacitidine treatment. Leukemia. 26: 943–950. [DOI] [PubMed] [Google Scholar]
- 40.Cocco L., Finelli C., Mongiorgi S., Clissa C., Russo D., Bosi C., Quaranta M., Malagola M., Parisi S., Stanzani M., et al. 2015. An increased expression of PI-PLCbeta1 is associated with myeloid differentiation and a longer response to azacitidine in myelodysplastic syndromes. J. Leukoc. Biol. 98: 769–780. [DOI] [PubMed] [Google Scholar]
- 41.Cocco L., Manzoli L., Faenza I., Ramazzotti G., Yang Y. R., McCubrey J. A., Suh P. G., and Follo M. Y.. 2016. Modulation of nuclear PI-PLCbeta1 during cell differentiation. Adv. Biol. Regul. 60: 1–5. [DOI] [PubMed] [Google Scholar]
- 42.Ruzzene M., Bertacchini J., Toker A., and Marmiroli S.. 2017. Cross-talk between the CK2 and AKT signaling pathways in cancer. Adv. Biol. Regul. 64: 1–8. [DOI] [PubMed] [Google Scholar]
- 43.Follo M. Y., Finelli C., Mongiorgi S., Clissa C., Chiarini F., Ramazzotti G., Paolini S., Martinelli G., Martelli A. M., and Cocco L.. 2011. Synergistic induction of PI-PLCbeta1 signaling by azacitidine and valproic acid in high-risk myelodysplastic syndromes. Leukemia. 25: 271–280. [DOI] [PubMed] [Google Scholar]
- 44.Follo M. Y., Manzoli L., Poli A., McCubrey J. A., and Cocco L.. 2015. PLC and PI3K/Akt/mTOR signalling in disease and cancer. Adv. Biol. Regul. 57: 10–16. [DOI] [PubMed] [Google Scholar]
- 45.Poli A., Ratti S., Finelli C., Mongiorgi S., Clissa C., Lonetti A., Cappellini A., Catozzi A., Barraco M., Suh P. G., et al. 2018. Nuclear translocation of PKC-alpha is associated with cell cycle arrest and erythroid differentiation in myelodysplastic syndromes (MDSs). FASEB J. 32: 681–692. [DOI] [PubMed] [Google Scholar]
- 46.Manzoli L., Billi A. M., Gilmour R. S., Martelli A. M., Matteucci A., Rubbini S., Weber G., and Cocco L.. 1995. Phosphoinositide signaling in nuclei of Friend cells: tiazofurin down-regulates phospholipase C beta 1. Cancer Res. 55: 2978–2980. [PubMed] [Google Scholar]
- 47.Bavelloni A., Poli A., Fiume R., Blalock W., Matteucci A., Ramazzotti G., McCubrey J. A., Cocco L., and Faenza I.. 2014. PLC-beta 1 regulates the expression of miR-210 during mithramycin-mediated erythroid differentiation in K562 cells. Oncotarget. 5: 4222–4231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cho D. H., and Tapscott S. J.. 2007. Myotonic dystrophy: emerging mechanisms for DM1 and DM2. Biochim. Biophys. Acta. 1772: 195–204. [DOI] [PubMed] [Google Scholar]
- 49.Salisbury E., Sakai K., Schoser B., Huichalaf C., Schneider-Gold C., Nguyen H., Wang G. L., Albrecht J. H., and Timchenko L. T.. 2008. Ectopic expression of cyclin D3 corrects differentiation of DM1 myoblasts through activation of RNA CUG-binding protein, CUGBP1. Exp. Cell Res. 314: 2266–2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Faenza I., Blalock W., Bavelloni A., Schoser B., Fiume R., Pacella S., Piazzi M., D’Angelo A., and Cocco L.. 2012. A role for PLCbeta1 in myotonic dystrophies type 1 and 2. FASEB J. 26: 3042–3048. [DOI] [PubMed] [Google Scholar]
- 51.Ramazzotti G., Faenza I., Fiume R., Billi A. M., Manzoli L., Mongiorgi S., Ratti S., McCubrey J. A., Suh P. G., Cocco L., et al. 2017. PLC-beta1 and cell differentiation: an insight into myogenesis and osteogenesis. Adv. Biol. Regul. 63: 1–5. [DOI] [PubMed] [Google Scholar]
- 52.Ramazzotti G., Faenza I., Gaboardi G. C., Piazzi M., Bavelloni A., Fiume R., Manzoli L., Martelli A. M., and Cocco L.. 2008. Catalytic activity of nuclear PLC-beta(1) is required for its signalling function during C2C12 differentiation. Cell. Signal. 20: 2013–2021. [DOI] [PubMed] [Google Scholar]
- 53.Amdani S. N., Yeste M., Jones C., and Coward K.. 2016. Phospholipase C zeta (PLCzeta) and male infertility: clinical update and topical developments. Adv. Biol. Regul. 61: 58–67. [DOI] [PubMed] [Google Scholar]
- 54.Kashir J., Nomikos M., and Lai F. A.. 2018. Phospholipase C zeta and calcium oscillations at fertilisation: the evidence, applications, and further questions. Adv. Biol. Regul. 67: 148–162. [DOI] [PubMed] [Google Scholar]
- 55.Saunders C. M., Larman M. G., Parrington J., Cox L. J., Royse J., Blayney L. M., Swann K., and Lai F. A.. 2002. PLC zeta: a sperm-specific trigger of Ca(2+) oscillations in eggs and embryo development. Development. 129: 3533–3544. [DOI] [PubMed] [Google Scholar]