Abstract
An experiment was conducted to test the hypothesis that pigs fed diets supplemented with exogenous phytase reduce mucin synthesis in the small intestine, increase protein hydrolysis in the stomach, increase breakdown of phytate along the gastrointestinal tract, and increase mineral and AA digestibility. A diet based on corn, soybean meal, and canola meal was formulated to meet requirements for growing pigs except for Ca and P, which were lower than requirements. Three additional diets were formulated by adding 750, 1,500, or 3,000 units of phytase (FTU) per kilogram to the basal diet. Eight growing barrows (38.45 ± 3.06 kg) were prepared with a T-cannula in the duodenum and another T-cannula in the distal ileum. Pigs were housed individually and allotted to a replicated 4 × 4 Latin square design with four pigs and four periods in each square. Each period lasted 14 d with the initial 7 d being the adaptation period to the diets. Pigs were fed twice daily in combined amounts equal to 3.2 times the estimated requirement for maintenance energy. Results indicated that the apparent ileal digestibility (AID) and the apparent total tract digestibility (ATTD) of Ca and P increased (linear and quadratic, P ≤ 0.05) as phytase inclusion increased. However, values for AID of Ca and P were not different from values for ATTD of Ca and P, indicating that there is no net absorption of Ca and P in the hindgut. The apparent duodenal digestibility (ADD) of Ca and P was ~30% and 10% to 20%, respectively, indicating some digestion in the stomach of both Ca and P. A quadratic increase (P < 0.05) of the AID of GE was observed with the breakpoint around 1,500 FTU, but there was a negative linear (P ≤ 0.001) effect of dietary phytase on the ATTD of GE. Phytase did not affect mucin synthesis in the small intestine, protein hydrolysis in the stomach, or ileal digestibility of dispensable and indispensable AA. However, degradation of higher phytate esters (IP6 and IP5) into lower phytate esters (IP4 and IP3) and inositol increased as dietary phytase increased, indicating that it is possible to completely degrade dietary phytate if microbial phytase is included by at least 3,000 FTU in the diet. In conclusion, supplementing diets with phytase resulted in increased degradation of phytate and phytate esters and improved digestibility of Ca and P, but phytase did not change intestinal mucin synthesis, gastric protein hydrolysis, or the AID of AA.
Keywords: amino acids, mucin, phytase, phytate, pigs, protein size
INTRODUCTION
Plant phytate is an anti-nutritional factor that is believed to have negative effects on animal performance in addition to binding of Ca and P (Bedford and Walk, 2016). Binding of protein to phytate may result in reduced AA digestibility and it is, therefore, possible that inclusion of exogenous phytase in diets increases AA digestibility, which has been demonstrated in broiler chickens (Cowieson et al., 2006; Yu et al., 2012). However, in pigs, effects of microbial phytase on digestibility of AA have not been consistent (Zeng et al., 2016; She et al., 2018). Phytate may increase mucin synthesis, and thereby increase endogenous losses of AA, which reduces calculated values for digestibility of AA. Phytate may also reduce pepsin activity with a subsequent reduction in protein digestibility although this has not been experimentally demonstrated (Cowieson et al., 2006; Woyengo et al., 2010). The anti-nutritional effect of phytate may be fully or partly ameliorated by the use of exogenous phytase, which results in release of Ca and P in the intestinal tract and a subsequent increase in digestibility of Ca and P (Almeida and Stein, 2010; Gonzalez-Vega et al., 2015). However, exogenous phytase may also reduce other anti-nutritional effects of phytate and reduce the concentration of lower phytate esters, but data to demonstrate these in pigs are limited (Woyengo et al., 2010). Recently, high doses of phytase (>1,500 FTU) has been hypothesized to result in release of nutrients other than Ca and P (Wilcock and Walk, 2016), but there is limited data from pigs demonstrating this. Therefore, the objective of this experiment was to test the hypothesis that addition of increasing concentrations of an exogenous phytase to diets fed to pigs may reduce mucin synthesis in the small intestine, increase protein hydrolysis in the stomach, increase hydrolysis of phytate along the gastrointestinal tract, and increase mineral and AA digestibility.
MATERIALS AND METHODS
The protocol for the experiment was submitted to the Institutional Animal Care and Use Committee at the University of Illinois and approved prior to initiation of the experiment. Pigs that were the offspring of L 359 boars mated to Camborough females (PIC; Hendersonville, TN) were used.
Animals, Housing, Diets, and Experimental Design
A control diet based on corn, soybean meal, and canola meal was formulated to meet nutrient requirements for 25 to 50 kg growing pigs (NRC, 2012) with the exception that Ca and P were included in quantities below the requirements. Three additional diets that were similar to the basal diet with the exception that they contained 750, 1,500, or 3,000 phytase units (FTU; Quantum Blue 5G; AB Vista, Marlborough, UK) were also formulated. Titanium dioxide was included at 0.5% in all diets as an indigestible marker (Table 1).
Table 1.
Ingredient composition of experimental diets, as-fed basis
| Ingredient, % | FTU1 per kilogram | |||
|---|---|---|---|---|
| 0 | 750 | 1,500 | 3,000 | |
| Ground corn | 58.00 | 58.00 | 58.00 | 58.00 |
| Soybean meal, (48% CP) | 18.00 | 18.00 | 18.00 | 18.00 |
| Canola meal | 18.00 | 18.00 | 18.00 | 18.00 |
| Corn starch | 0.10 | 0.085 | 0.07 | 0.04 |
| Soybean oil | 4.00 | 4.00 | 4.00 | 4.00 |
| Limestone | 0.70 | 0.70 | 0.70 | 0.70 |
| Salt | 0.40 | 0.40 | 0.40 | 0.40 |
| Titanium dioxide | 0.50 | 0.50 | 0.50 | 0.50 |
| Vitamin mineral premix | 0.30 | 0.30 | 0.30 | 0.30 |
| Phytase premix2 | — | 0.015 | 0.03 | 0.06 |
1FTU = phytase units.
2Phytase premix contained 5,000 phytase units per gram (Quantum Blue 5,000; AB Vista, Marlborough, UK).
Eight growing barrows (38.45 ± 3.06 kg) were surgically prepared with a T-cannula in the duodenum and another T-cannula in the distal ileum (Stein et al., 1998). Pigs were housed individually in pens (1.2 × 1.5 m) that had smooth sides, a fully slatted tri-bar floor, a feeder, and a nipple drinker. Pigs were fed a conventional grower diet while recuperating after surgery. On day 10 postsurgery, feeding of experimental diets was initiated.
Pigs were allotted to a replicated 4 × 4 Latin square design with four pigs and four periods in each square (Kim and Stein, 2009). Within each square, one pig received each diet during each period, and no pig received the same diet more than once. Feed was provided in amounts equal to 3.2 times the estimated requirement for maintenance energy (i.e., 197 kcal ME/kg0.6; NRC, 2012). Daily feed allotments were divided into two equal meals and were fed at 0700 and 1700 hours, respectively. Water was available at all times. The BW of individual pigs was recorded at the beginning of the experiment and at the end of each period.
Each period lasted 14 d with the initial 7 d being the adaptation period to the diets. Fecal samples were collected via anal stimulation and grab sampling on days 8, 9, and 10. Each day, the pH of fecal samples was determined using a pH meter (Orion Star A111; Fisher Scientific, Waltham, MA) with the probe placed directly in the feces. A 12-mL representative fecal sample was collected each day and snap frozen in liquid N, and the remaining fecal samples were composited within the 3 collection days. Ileal digesta were collected for 8 h on days 11 and 12 starting 2 h after the morning meal had been provided. The pH of samples collected 4 and 7 h after feeding the morning meal was determined. A 12-mL sample was collected 6 h after feeding and snap frozen in liquid N. Duodenal digesta were collected on days 13 and 14. Separate samples were collected in plastic bags 20, 40, 60, 120, and 240 min after feeding the morning meal. The pH in all duodenal digesta samples was determined. A 12-mL representative sample from each collection was snap frozen in liquid N, and a pooled sample from the five collections was stored at −20 ℃. There were, therefore, five separate samples of snap frozen duodenal digesta collected from each pig, in addition to the pooled sample from the 2 collection days. All snap-frozen samples were stored at −80 ℃. On days 13 and 14, two blood samples were also collected from all pigs 30 min before, 30 min after, and 240 min after feeding the morning meal. One of the blood samples was collected in a vacutainer containing heparin, and the other was collected in a vacutainer containing ethylenediaminetetraacetic acid. Blood collected in heparin containing vacutainers was stored at −80 ℃. Blood collected in ethylenediaminetetraacetic acid containing vacutainers was immediately centrifuged using a Thermo Scientific Sorvall ST 8 centrifuge (Thermo Scientific, Waltham, MA) at 4,000 rotations per min for 13 min. After centrifugation, the plasma was extracted and stored at −80 ℃.
Analyses
All digesta and fecal samples were thawed, lyophilized, and then ground. However, before grinding, the duodenal samples from the 2 d of collection were mixed within pig and diet for each time point. Diets were analyzed for concentration of GE using an isoperibol bomb calorimeter (Model 6400; Parr Instruments, Moline, IL), DM(Method 930.15; AOAC International, 2007), and CP using the combustion procedure (Method 990.03; AOAC International, 2007) on an FP628 protein analyzer (LECO Corporation, St. Joseph, MI). Ash (Method 942.05; AOAC International, 2007) was also determined in the diets, and acid hydrolyzed ether extract was determined using 3 N HCl on the ANKOMHCl Hydrolysis System (ANKOM Feed Technology, Macedon, NY) followed by crude fat extraction with petroleum ether on an ANKOMXT15 Extractor (Method AOCS Am 5-04; ANKOM Feed Technology, Macedon, NY). Starch in diets was analyzed using the glucoamylase procedure (Method 979.10; AOAC International, 2007), and total dietary fiber was derived from the sum of analyzed values for soluble dietary fiber and insoluble dietary fiber according to Method 991.43 (AOAC International, 2007) using the Ankom Dietary Fiber Analyzer (Ankom Technology, Macedon, NY). Calcium and P were also analyzed using inductively coupled plasma spectroscopy (Method 985.01 A, B, and C; AOAC, 2007) after wet ash sample preparation [Method 975.03 B (b); AOAC International, 2007], and AA were analyzed on a Hitachi Amino Acid Analyzer (Model L8800; Hitachi High Technologies America Inc., Pleasanton, CA) using ninhydrin for postcolumn derivatization and norleucine as the internal standard. Samples were hydrolyzed with 6 N HCl for 24 h at 110 °C [Method 982.30 E (a); AOAC International, 2007] before analysis. Methionine and cysteine were analyzed as met sulfone and cysteic acid after cold performic acid oxidation overnight before hydrolysis [Method 982.30 E (b); AOAC International, 2007]. Tryptophan was determined after NaOH hydrolysis for 22 h at 110 °C [Method 982.30 E(c); AOAC International, 2007]. Phytase activity was analyzed by the ELISA method using Quantiplate Kits for Quantum Blue and phytate-P content was predicted by the Near Infra-Red Reflectance Spectroscopy method. Both analyses were completed by AB Vista in Cordova, Tennessee. Diets were also analyzed for Ti (Myers et al., 2004).
Pooled duodenal digesta, ileal digesta, and fecal samples were analyzed for Ti, DM, Ca, and P as described above, and ileal digesta were also analyzed for AA and CP. Duodenal digesta samples were also analyzed for protein size using a four-size cut-off system to quantify proteins that were less than 5 kDa, proteins that were between 5 and 20 kDa, proteins that were between 20 and 50 kDa, and proteins that were >50 kDa. This was accomplished using an extraction buffer containing Tris–HCl buffer, lithium dodecyl sulfate, and glycerol. Peptides and proteins from each sample were extracted exhaustively by dissolving the sample in buffer for 24 h, heating at 95 ℃, and centrifugation to remove insoluble protein. Samples were then analyzed using a polyacrylamide gel running MES buffer (Bio-Rad, Hercules, CA), which resolves peptides from 5 to 250 kDa. Gels were fixed and stained using SYPRO Ruby stain (Bio-Rad). The gels were scanned using a laser scanning densitometer (GE Typhoon 9400 Variable Mode Imager; Amersham Biosciences Corp., Piscataway, NJ) to document gel pictures as well as analysis for peptide/protein quantification in each sample (Rehbein and Schwalbe, 2015).
Ileal digesta were analyzed by Creative Proteomics, Shirley, NY, for concentrations of sialic acid as a marker for mucin synthesis. This was accomplished by adding 0.1 g digesta to 1 mL reagent A, which hydrolyzed the samples at 80 ℃ for 2 h, and the sample was then cooled to room temperature and dried. The sample was further dissolved with addition of 1 mL water and a Norleau dilution buffer was added for the final dilution. A 180-µL sample was transferred to a tube and 20 µL reagent B was added to derivatize for 150 min at 50 ℃ and the sample was left in the dark during derivatization. The solution was cooled and sialic acid was analyzed using HPLC.
Diets and snap frozen fecal, ileal, and duodenal samples were analyzed for phytate ester concentrations by high-pressure ion chromatography (LC-4000; JASCO, Easton, MD) using postcolumn derivitization and UV detection at 290 nanometers. Inositol concentration was determined using HPLC with pulsed amperometry on the gold electrode of an ED50 electrochemical detector (DX500 HPLC system; Dionex Corporation, Sunnyvale, CA). Analyses for phytate esters and inositol were completed at the University of East Anglia, School of Biological Sciences, UK (Laird et al., 2016).
Samples of pig blood plasma were thawed and 0.5 mL was transferred to a 2-mL centrifuge tube and 1 mL perchloric acid (1 N) was added. Samples were then stored at 4 ℃ for 30 min, centrifuged using a refrigerated centrifuge (Eppendorf Centrifuge 5427 R; Eppendorf AG, Hamburg, Germany) at 4 ℃ and 17,500 × g for 10 min. The supernatant was extracted using a 5-mL capacity syringe (Thermo Scientific National Target All-Plastic Disposable Syringes; Thermo Scientific, Waltham, MA) with a needle attached, and the needle was then replaced with a syringe filter [Kinesis Polytetrafluoroethylene (PTFE) Syringe Filters; Kinesis Inc., Vernon Hills, IL]. The content was dissipated into another tube, and this sample was analyzed for inositol at the University of East Anglia, School of Biological Sciences (UK) as explained for diets and snap frozen fecal, ileal, and duodenal samples. Blood collected in the heparin containing vacutainers was analyzed for Ca and P as explained for diets.
Calculations and Statistical Analysis
The apparent ileal digestibility (AID) of energy, AA, Ca, and P and the apparent total tract digestibility (ATTD) of energy, Ca, and P were calculated (Stein et al., 2007: NRC, 2012). The apparent duodenal digestibility (ADD) of Ca and P was also calculated by mixing digesta collected from the five time points into one sample. Concentrations of phytate and phytase in diets were expressed as percent and FTU per kg, respectively. Concentrations of phytate esters and phytase in all samples from the duodenum, the ileum, and the feces were expressed as nanomole per gram dry weight and FTU per kilogram, respectively, and sialic acid concentrations were calculated in ileal digesta samples as milligram per kilogram.
Data were analyzed using the MIXED procedure of SAS (SAS Inst. Inc., Cary, NC) with pig as the experimental unit. The univariate procedure in SAS was used to confirm normality of data and also to identify possible outliers. Least square means were calculated for each independent variable using the LSMeans statement in SAS. Contrast statements were used to determine linear and quadratic effects of phytase on values for ADD, AID, and ATTD of energy and nutrients, on the concentration of sialic acid in ileal digesta, and on protein size in duodenal digesta. For protein size, the results were converted into weight percentage (wt.%) from pixel concentration. Appropriate coefficients for unequally spaced inclusion rates of phytase were obtained using PROC IML. Level of significance and tendencies were set at P ≤ 0.05 and P < 0.10, respectively, for all statistical tests.
Effects of collection time, diet, and the interaction between collection time and diet were also determined for duodenal digesta, and blood inositol and digesta phytate esters. If the interaction between main effects was not significant, individual main effects were reported. If the interaction between main effects was significant, the parameter estimates for the second-order response surface model to increasing phytase inclusion and collection time was determined using NLREG version 6.5 (Sherrod, 2008) as described by Khuri and Cornell (1996). Parameter estimates of the model that were not significant (P > 0.10) and were not included in a significant interaction were removed from the model and the estimates were recalculated. The surface response full model was:
where Y is the dependent variable, a is the intercept, b, c, d, e, and f are the coefficients, FTU is the dietary phytase inclusion rate, and T is the collection time for duodenal digesta.
RESULTS
Nutrient Composition of Diets
The analyzed concentrations of Ca in diets containing 0, 750, 1,500, or 3,000 FTU of phytase were 0.54%, 0.53%, 0.53%, and 0.54%, respectively, and P concentrations were 0.48%, 0.46%, 0.49%, and 0.49%, respectively. These values were in agreement with formulated values. Likewise, analyzed concentrations of CP and AA were in agreement with formulated values (Table 2). The analyzed phytase in the diets was slightly greater than formulated, but the differences among diets were as intended. Concentrations of phytate, phytate esters, and inositol in all diets were as expected.
Table 2.
Analyzed nutritional composition of experimental diets, as-fed basis
| FTU1 per kilogram | ||||
|---|---|---|---|---|
| Item | 0 | 750 | 1,500 | 3,000 |
| DM, % | 86.17 | 86.47 | 86.64 | 86.69 |
| GE, kcal/kg | 4,103 | 4,106 | 4,094 | 4,093 |
| CP, % | 15.62 | 15.48 | 15.35 | 15.94 |
| Indispensable, AA | ||||
| Arg, % | 1.24 | 1.22 | 1.22 | 1.18 |
| His, % | 0.54 | 0.52 | 0.53 | 0.51 |
| Ile, % | 0.90 | 0.87 | 0.88 | 0.84 |
| Leu, % | 1.65 | 1.60 | 1.62 | 1.57 |
| Lys, % | 1.12 | 1.10 | 1.11 | 1.05 |
| Met, % | 0.32 | 0.34 | 0.32 | 0.31 |
| Phe, % | 0.97 | 0.91 | 0.92 | 0.88 |
| Thr, % | 0.79 | 0.77 | 0.78 | 0.75 |
| Trp, % | 0.24 | 0.23 | 0.22 | 0.24 |
| Val, % | 1.02 | 1.00 | 1.01 | 0.97 |
| Dispensable, AA | ||||
| Ala, % | 0.99 | 0.96 | 0.97 | 0.95 |
| Asp, % | 1.80 | 1.76 | 1.77 | 1.69 |
| Cys, % | 0.39 | 0.40 | 0.39 | 0.37 |
| Glu, % | 3.47 | 3.39 | 3.45 | 3.31 |
| Gly, % | 0.91 | 0.89 | 0.91 | 0.87 |
| Pro, % | 1.19 | 1.15 | 1.19 | 1.15 |
| Ser, % | 0.79 | 0.77 | 0.78 | 0.75 |
| Tyr, % | 0.59 | 0.56 | 0.56 | 0.55 |
| Acid hydrolyzed ether extract, % | 6.98 | 6.24 | 6.07 | 6.17 |
| Starch, % | 35.22 | 35.46 | 40.68 | 38.84 |
| Soluble dietary fiber, % | 0.70 | 1.00 | 0.70 | 0.90 |
| Insoluble dietary fiber, % | 13.10 | 13.50 | 14.00 | 13.10 |
| Total dietary fiber, % | 13.80 | 14.50 | 14.70 | 14.00 |
| Ash, % | 4.81 | 4.91 | 4.70 | 4.82 |
| Ca, % | 0.54 | 0.53 | 0.53 | 0.54 |
| P, % | 0.48 | 0.46 | 0.49 | 0.49 |
| Phytase, FTU/kg | <50 | 938 | 1,630 | 4,490 |
| Phytate-P content, % | 0.29 | 0.30 | 0.29 | 0.30 |
| Phytate esters, nmol/g (DM basis) | ||||
| IP6 | 18,477 | 19,794 | 17,534 | 19,379 |
| IP5 | 2,851 | 2,762 | 2,588 | 2,969 |
| IP4 | 342 | 298 | 260 | 511 |
| IP3 | 205 | 495 | 319 | 412 |
| Inositol, nmol/g (DM basis) | 1,580 | 1,272 | 1,228 | 1,264 |
1FTU = phytase units.
Digestibility of Calcium, Phosphorus, CP, AA, and Energy
There was no effect of phytase inclusion on the ADD of Ca but the AID and ATTD of Ca increased (linear and quadratic, P ≤ 0.001) as the inclusion of phytase increased (Table 3). For the ADD of P, there was an increase and then a decrease (quadratic, P < 0.001) as the inclusion of phytase increased and the AID and ATTD increased (linear and quadratic, P ≤ 0.001) as the inclusion of phytase increased. There were, however, no differences in the AID of AA among diets (data not shown). There was a quadratic increase and then decrease (P < 0.05) in the AID of GE with the breakpoint at around 1,500 FTU of phytase (Table 4). However, a negative linear (P ≤ 0.001) effect of phytase on the ATTD of GE was observed.
Table 3.
Apparent duodenal digestibility (ADD), apparent ileal digestibility (AID), and apparent total tract digestibility (ATTD) of Ca and P in experimental diets containing 0, 750, 1,500, or 3,000 FTU/kg
| FTU1 per kilogram | P-value | ||||||
|---|---|---|---|---|---|---|---|
| Item | 0 | 750 | 1,500 | 3,000 | SEM | Linear | Quadratic |
| Ca | |||||||
| ADD, % | 30.1 | 32.4 | 35.9 | 28.1 | 4.61 | 0.61 | 0.10 |
| AID, % | 55.1 | 71.4 | 75.4 | 79.0 | 2.28 | <0.001 | <0.001 |
| ATTD, % | 52.4 | 69.1 | 75.3 | 75.5 | 2.12 | <0.001 | <0.001 |
| P | |||||||
| ADD, % | 11.3 | 14.3 | 23.4 | 9.17 | 5.42 | 0.66 | 0.005 |
| AID, % | 26.6 | 49.7 | 59.4 | 69.0 | 1.91 | <0.001 | <0.001 |
| ATTD, % | 27.8 | 54.1 | 66.9 | 70.3 | 1.59 | <0.001 | <0.001 |
1FTU = phytase units.
Table 4.
Apparent ileal digestibility (AID) and apparent total tract digestibility (ATTD) of GE in experimental diets containing 0, 750, 1,500, or 3,000 FTU/kg
| FTU1 per kilogram | P-value | ||||||
|---|---|---|---|---|---|---|---|
| Item | 0 | 750 | 1,500 | 3,000 | SEM | Linear | Quadratic |
| AID, % | 73.4 | 73.7 | 75.4 | 72.7 | 0.8 | 0.47 | 0.009 |
| ATTD, % | 83.6 | 82.6 | 82.7 | 81.6 | 0.3 | <0.001 | 0.61 |
1FTU = phytase units.
Protein Size, Sialic Acid, and Plasma Ca and P
There were no differences in protein size in the duodenal digesta among diets and no evidence of phytase increasing gastric protein digestion was observed (Table 5). Likewise, no differences in the concentration of sialic acid were observed among diets. There was a decrease (linear, P ≤ 0.001) in plasma Ca and P from 30 min before feeding the morning meal to 240 min after the morning meal (Table 6). There was, however, no effect of phytase on the concentration of plasma Ca among diets, but plasma P increased (quadratic, P < 0.05) as dietary phytase inclusion increased (Table 7).
Table 5.
Protein size concentration (wt. %) in duodenal digesta and sialic acid concentration (DM basis) in ileal digesta of pigs fed diets containing 0, 750, 1,500, or 3,000 FTU/kg
| FTU1 per kilogram | P-value | ||||||
|---|---|---|---|---|---|---|---|
| Item | 0 | 750 | 1,500 | 3,000 | SEM1 | Linear | Quadratic |
| Protein size, kDa | |||||||
| >50, wt.% | 43.9 | 46.4 | 43.2 | 44.6 | 3.3 × 106 | 1.00 | 0.70 |
| 50–20, wt.% | 26.6 | 20.3 | 20.3 | 21.7 | 1.8 × 106 | 0.48 | 0.14 |
| 20–5, wt.% | 22.9 | 28.4 | 30.6 | 27.7 | 2.0 × 106 | 0.38 | 0.30 |
| <5, wt.% | 6.6 | 4.9 | 6.0 | 6.0 | 3.7 × 105 | 0.93 | 0.16 |
| Sialic acid concentration, mg/kg (DM) | 81.16 | 69.65 | 73.27 | 71.91 | 10.34 | 0.34 | 0.31 |
1FTU = phytase units.
Table 6.
Concentrations of plasma Ca and P 30 min before feeding the morning meal, 30 min after the morning meal, and 240 min after the morning meal1
| Item | 30 min before morning meal | 30 min after morning meal | 240 min after morning meal | SEM | Linear |
|---|---|---|---|---|---|
| Ca, mg/dL | 6.86 | 6.85 | 6.46 | 0.09 | <0.001 |
| P, mg/dL | 48.25 | 50.40 | 46.63 | 0.48 | <0.001 |
1No interaction between diet and time for blood plasma Ca and P was observed.
Table 7.
Blood Ca and P concentrations in plasma from pigs fed diets containing 0, 750, 1,500, or 3,000 FTU/kg1
| FTU2 per kilogram | P-value | ||||||
|---|---|---|---|---|---|---|---|
| Item | 0 | 750 | 1,500 | 3,000 | SEM | Linear | Quadratic |
| Ca, mg/dL | 6.77 | 6.62 | 6.89 | 6.71 | 0.14 | 0.99 | 0.88 |
| P, mg/dL | 46.94 | 48.35 | 48.96 | 49.20 | 48.34 | 0.004 | 0.04 |
1No interaction between diet and time for blood plasma Ca and P was observed.
2FTU = phytase units.
Inositol Phosphate Concentration of Diets, Plasma, and Phytate Esters
There was an increase (linear and quadratic, P ≤ 0.05) in the concentration of duodenal inositol as phytase inclusion increased (Table 8). However, inositol concentration in duodenal digesta could not be predicted using phytase inclusion and collection time in the surface response model. For ileal digesta, there was a decrease (linear and quadratic, P ≤ 0.001) in IP6 and IP5 concentrations as phytase inclusion increased and an increase and then a decrease in IP4 (quadratic, P < 0.001) and IP3 (linear and quadratic, P ≤ 0.01). There was also an increase (linear, P ≤ 0.05) in inositol in ileal digesta as phytase inclusion increased. In the feces, there were no differences among treatments for any phytate esters except for a tendency (linear and quadratic, P = 0.07) for a decreased concentration of IP6 and a decrease (linear and quadratic, P ≤ 0.001) in the concentration of IP5 as phytase inclusion increased.
Table 8.
Phytate esters and inositol (nmol/g, DM basis) in duodenal digesta, ileal digesta, and fecal samples from pigs fed diets containing 0, 750, 1,500, or 3,000 FTU/kg1
| FTU/kg2 | P-value | ||||||
|---|---|---|---|---|---|---|---|
| Item | 0 | 750 | 1,500 | 3,000 | SEM | Linear | Quadratic |
| Duodenal digesta | |||||||
| Inositol | 2,944 | 2,987 | 2,710 | 3,439 | 438 | 0.01 | 0.009 |
| Ileal digesta | |||||||
| IP6 | 66,849 | 11,736 | 7,862 | 4,860 | 2,198 | <0.001 | <0.001 |
| IP5 | 12,244 | 5,752 | 3,400 | 776 | 742 | <0.001 | <0.001 |
| IP4 | 1,483 | 23,761 | 19,763 | 7,018 | 1,515 | 0.93 | <0.001 |
| IP3 | 258 | 5,043 | 6,306 | 3,511 | 534 | 0.004 | <0.001 |
| Inositol | 5,890 | 12,748 | 11,536 | 26,321 | 2,629 | <0.001 | 0.28 |
| Fecal samples | |||||||
| IP6 | 587 | 381 | 376 | 380 | 71 | 0.07 | 0.07 |
| IP5 | 136 | 47 | 40 | 34 | 10 | <0.001 | <0.001 |
| IP4 | 33 | 28 | 34 | 26 | 6 | 0.53 | 0.72 |
| IP3 | 152 | 116 | 142 | 140 | 24 | 0.96 | 0.50 |
| Inositol | 27 | 20 | 16 | 15 | 7 | 0.17 | 0.41 |
1No interaction between diet and time for duodenal digesta inositol concentration was observed.
2FTU = phytase units.
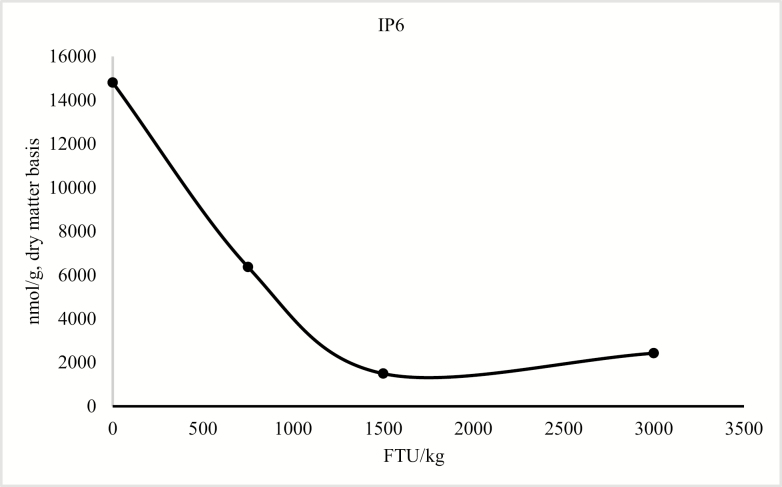
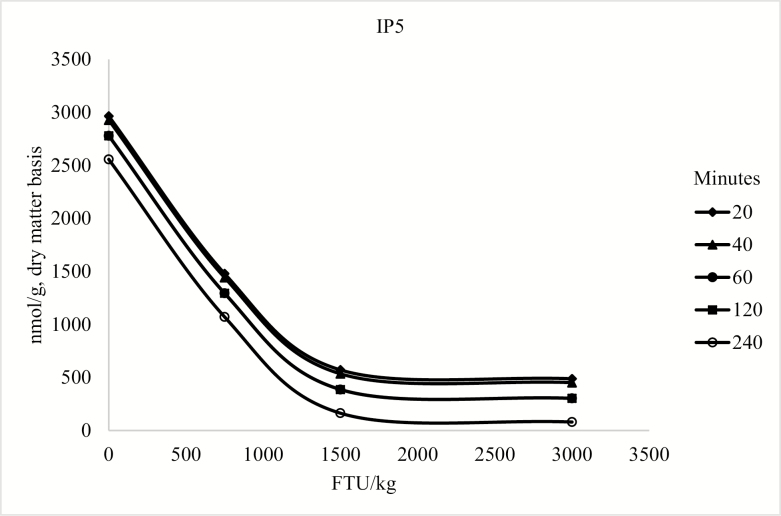
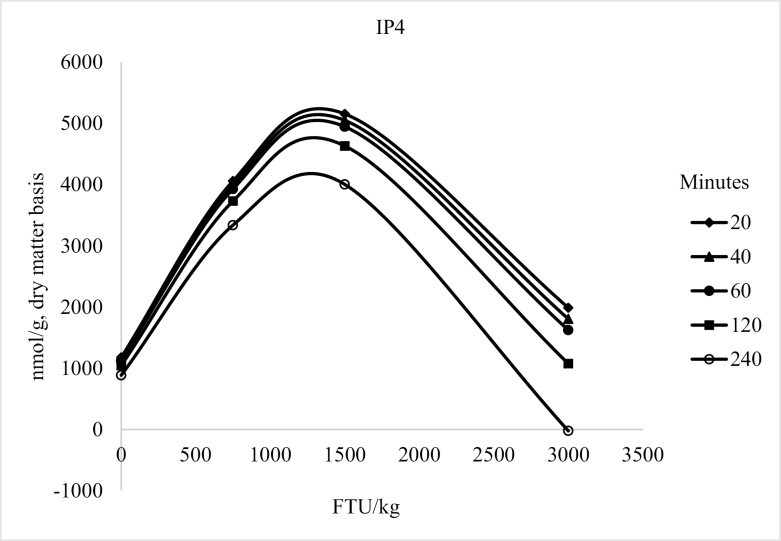
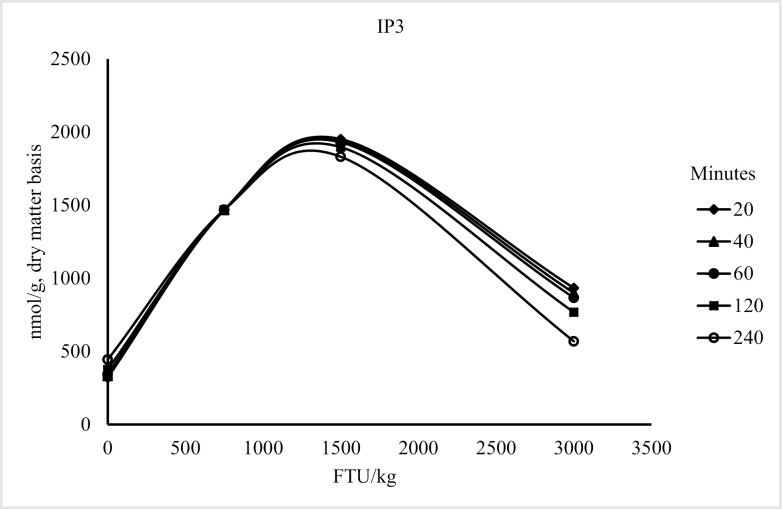
The model for the concentration of duodenal IP6 only contained the linear and quadratic terms for phytase (P < 0.05; Figure 1). For the concentration of IP5, the model included the linear and quadratic terms of phytase (P < 0.05) and the linear effect of collection time (P < 0.05; Figure 2). For duodenal IP3 and IP4 concentration, the model was reduced because the quadratic term of collection time was not significant; however, there was an interaction between phytase and collection time (P < 0.05; Figure 3 and Figure 4), but there was no effect of collection time on the duodenal digesta concentration of inositol (Table 9). For blood plasma inositol, there was a linear increase (P = 0.05) from 30 min before feeding the morning meal to 240 min after the morning meal (Table 10). A linear increase (P ≤ 0.001) and a quadratic increase (P ≤ 0.05) in plasma inositol concentrations were also observed as inclusion of phytase in the diets increased (Table 11).
Figure 1.
Predicted values, based on the quadratic effect of phytase (P < 0.05), for IP6 in pigs fed diet containing 0, 750, 1,500, or 3,000 FTU/kg of phytase. The interaction between diet and time in the model is not significant. The concentration of IP6 could be described by the following model: IP6 = 14,808.237 − 13.625 × FTU + 0.003 × FTU2 (P < 0.05).
Figure 2.
Predicted values, based on the quadratic effect of phytase (P < 0.05) and collection time (P < 0.05), for IP5 in pigs fed diet containing 0, 750, 1,500, or 3,000 FTU/kg of phytase, 20, 40, 60, 120, and 240 min after feeding the morning meal. The concentration of IP5 could be described by the following model: IP5 = 3,001.510 − 2.365 × FTU + 0.001 × FTU2 − 1.855 × T (P < 0.05).
Figure 3.
Predicted values, based on the interaction between phytase and collection time (P < 0.10), for IP4 in pigs fed diet containing 0, 750, 1,500, or 3,000 FTU/kg of phytase, 20, 40, 60, 120, and 240 min after feeding the morning meal. The concentration of IP4 could be described by the following model: IP4 = 1,204.995 + 5.085 × FTU − 0.002 × FTU2 − 1.329 × T − 0.003 × FTU × T (P < 0.05).
Figure 4.
Predicted values, based on the interaction between phytase and collection time (P < 0.10), for IP3 in pigs fed diet containing 0, 750, 1,500, or 3,000 FTU/kg of phytase, 20, 40, 60, 120, and 240 min after feeding the morning meal. The concentration of IP3 could be described by the following model: IP3 = 309.550 + 1.985 × FTU − 0.001 × FTU2 + 0.559 × T − 0.001 × FTU × T (P < 0.05).
Table 9.
Concentrations (nmol/g, DM basis) of inositol in duodenal digesta collected 20, 40, 60, 120, or 240 min after feeding pigs diets containing 0, 750, 1,500, or 3,000 FTU/kg1
| Time after feeding morning meal | P-value | |||||||
|---|---|---|---|---|---|---|---|---|
| Item | 20 | 40 | 60 | 120 | 240 | SEM | Linear | Quadratic |
| Inositol | 3,222 | 2,814 | 2,979 | 3,093 | 3,211 | 200 | 0.22 | 0.38 |
1No interaction between diet and time for duodenal digesta inositol concentration was observed.
Table 10.
Concentrations of plasma inositol 30 min before feeding the morning meal, 30 min after the morning meal, and 240 min after the morning meal1
| Item | 30 min before morning meal | 30 min after morning meal | 240 min after morning meal | SEM | Linear |
|---|---|---|---|---|---|
| Inositol, μM | 67.10 | 56.09 | 82.74 | 4.83 | 0.05 |
1No interaction between diet and time for concentration of plasma inositol was observed.
Table 11.
Inositol concentrations in plasma from pigs fed diets containing 0, 750, 1,500, or 3,000 FTU/kg1
| FTU2 per kilogram | P-value | ||||||
|---|---|---|---|---|---|---|---|
| Item | 0 | 750 | 1,500 | 3,000 | SEM | Linear | Quadratic |
| Inositol, μM | 47.99 | 65.84 | 75.96 | 83.59 | 6.41 | <0.001 | 0.04 |
1No interaction between diet and time for concentration of plasma inositol was observed.
2FTU = phytase units.
DISCUSSION
Diets
Diets were formulated with corn, soybean meal, and canola meal to provide substrate for phytase. Canola meal contains 0.65% phytate-P and corn and soybean meal contain 0.21% and 0.38%, respectively (NRC, 2012). The fact that analyzed phytate-P in the diets was close to formulated values indicate that the corn, soybean meal, and canola meal used contained phytate-P close to formulated values that were based on NRC (2012).
Calcium and Phosphorus
The ADD of Ca at all levels of phytase inclusion was ~30%, which indicates some absorption of Ca in the stomach. This value is lower than reported by Gonzalez-Vega et al. (2014) who observed an ADD of 43% for Ca from calcium carbonate. It is possible that the synthetic diets that were used by Gonzalez-Vega et al. (2014) resulted in a different degradation pattern than the more practical diets used in this experiment, which may explain this difference.
The lack of a difference between AID and ATTD of Ca, regardless of phytase level, indicates that there is no net absorption of Ca in the hindgut of pigs. This observation is in agreement with previous data (Bohlke et al., 2005; Gonzalez-Vega et al., 2014). As with Ca, the 10% to 20% ADD for P indicates that there is some absorption of P in the stomach. In addition, there was no differences between the AID and the ATTD of P, which confirmed results from previous studies (Fan et al., 2001; Shen et al., 2002; Ajakaiye et al., 2003; Bohlke et al., 2005; Dilger and Adeola, 2006), indicating that there is no net absorption of P from the hindgut of pigs. Thus, for both Ca and P, these data indicate that the majority of absorption takes place in the small intestine and some absorption also takes place in the stomach.
A quadratic effect of phytase on ATTD of Ca and P has been observed in some previous experiments (Almeida and Stein, 2012; Almeida et al., 2013; She et al., 2018). However, a linear increase in the ATTD of P has also been reported without an apparent breakpoint even if 20,000 FTU of phytase was added to the diet (Zeng et al., 2015). A linear effect of up to 2,000 FTU of phytase on the ATTD of P, but not on Ca, was also reported (Velayudhan et al., 2015). It is possible that the conflicting responses to increased dietary phytase among experiments may be the result of differences among experiments in the substrate for phytase, differences in the potency of the phytase used, or differences in the P sufficiency of the test diet. However, the linear effects of phytase on ATTD of Ca and P indicate that the phytase used in this experiment has the capacity to continue to hydrolyze ester bonds between the inositol ring in phytate and P when added to diets based on corn, soybean meal, and canola meal.
Amino Acids and Energy
The observation that there was no effect of phytase on the AID of AA is in agreement with She et al. (2018), who reported that inclusion of up to 4,000 FTU of an E. coli phytase to a corn–soybean meal-based diet had no impact on the AID of AA. Likewise, addition of up to 2,000 FTU of an E. coli phytase had no effect on the AID of the majority of AA (Liao et al., 2005; Velayudhan et al., 2015). These observations indicate that for diets based on corn and soybean meal, or corn, soybean meal, and canola meal, there appears to be no effect of phytase on the AID of AA by cannulated growing pigs.
The quadratic decrease in the AID of GE that was observed as phytase was added to the diets is in contrast with previous data, which indicated no effect of phytase on ileal digestibility of energy (Liao et al., 2005; Cervantes et al., 2011; Zeng et al., 2015). However, in other studies, an increase in the AID of GE with supplementation of phytase was observed (Velayudhan, 2015; Kiarie et al., 2016). The negative effect of phytase on the ATTD of GE that was observed in this experiment is in agreement with a recent meta-analysis for effects of microbial phytase on ATTD of GE (Torres-Pitarch et al., 2017). However, results of some studies also indicated that there was no effect of phytase on the ATTD of GE (Zeng et al, 2016; She et al., 2018), or that the ATTD of GE increased with supplementation of phytase to diets (Kiarie et al., 2016; Dersjant-Li et al., 2017). Thus, results of this and previous experiments indicate that the effect of microbial phytase on the AID and ATTD of GE is not consistent. Whether or not these differences are caused by differences among the phytases used, ingredient composition of diets, or other factors is not known, but research to determine effects of phytase on all energy-contributing nutrients is needed.
Protein Size
The lack of differences among diets in the duodenal protein size indicates that phytase does not hydrolyze protein in the stomach of growing pigs. We are not aware of previous data for the effect of phytase on the size of protein in the duodenum of pigs. However, our hypothesis that phytase, by reducing the concentration of phytate, might increase protein hydrolysis and thereby reduce protein size was rejected. This result was supported by the fact that the AID of AA was not affected by phytase, which further indicates that under the conditions of this experiment, there was no effect of phytase on protein hydrolysis in the stomach or AA absorption in the small intestine. However, we did not measure endogenous enzyme activity, but negative effects of phytate on AA digestibility, and subsequent benefits of phytase supplementation, may be associated with phytate–pepsin or phytate–trypsin interactions (Liu et al., 2009; Yu et al., 2012). The binding of phytate to free AA, however, appears less clear and requires further evaluation in vivo.
Sialic Acid
Sialic acid is a major component of mucin (Wang and Brand-Miller, 2003) and in the intestinal tract, sialic acid is mostly limited to the secretory mucins (Jass and Walsh, 2001). The lack of differences among treatments in sialic acid concentrations in the ileal digesta indicates that phytase has no effect on mucin synthesis in the intestinal tract of growing pigs. Thus, our hypothesis that phytase may reduce mucin synthesis in the small intestine of pigs was rejected. This is contrary to data indicating a decrease in mucin secretion with phytase supplementation (Kies, 2005). It is not clear what the reason for this difference between experiments may be. It is believed that mucin synthesis increases when anti-nutritional factors are present in the small intestine (Cowieson et al., 2006). However, it appears that phytate may not impact the synthesis of mucin, because despite the fact that most phytate was degraded by phytase in this experiment, no change in the synthesis of mucin as indicated by the concentration of sialic acid was observed.
Blood Calcium and Phosphorus
The reduction in blood plasma Ca and P from before feeding to 240 min after feeding indicates that bone tissue synthesis may have increased after pigs consumed their meal and then was reduced before the next meal. To our knowledge, there are no other data for the effect of phytase on the concentration of Ca and P in blood plasma over time relative to feeding. Nevertheless, the current data indicate that bone synthesis may be stimulated after a meal by the availability of energy, Ca, P, and possibly AA, and as a consequence of increased bone tissue synthesis, plasma concentrations of Ca and P are reduced 240 min after the meal.
The observation that plasma P increased as phytase was added to diets is a consequence of the increased digestibility of P as phytase was added. However, the fact that no increase in plasma Ca was observed with increasing dietary phytase, despite increased digestibility of Ca, indicates that Ca is cleared from blood at a faster rate than P, which likely is a result of the strict hormonal control of plasma Ca concentrations (Felsenfeld and Levine, 2015).
Inositol Phosphate
The decreased concentration of duodenal IP6 and IP5 and the increase and then decrease in the concentration of IP4 and IP3 as dietary phytase increased, regardless of collection time, indicates that there is degradation of phytate esters in the stomach of pigs. This observation is in agreement with results of several studies demonstrating that the main site for phytate hydrolysis in the gastrointestinal tract is the stomach (Schlemmer et al., 2001; Haraldsson et al., 2005; Blaabjerg and Poulsen, 2010). The increased duodenal inositol concentration that was observed as inclusion of phytase increased, indicates complete hydrolysis of some phytate esters in the stomach. The reduced concentration of IP6 and IP5 in the ileal digesta, the increased concentration of IP4 and IP3, and the increased concentration of ileal inositol that were observed as dietary phytase increased, indicates a progressive degradation of high phytate esters into low phytate esters and inositol as inclusion of phytase increased. In contrast with previous data, which indicated no IP6 degradation in the small intestine (Haraldsson et al., 2005), there was a reduction in IP6 in the small intestine in response to increased dietary phytase inclusion. The observation that the concentration of inositol and phytate esters was greater in ileal digesta than in the diet for pigs fed the control diet is a result of nutrient absorption in the small intestine, and therefore, a concentration of dietary inositol and phytate esters in the digesta. The lack of an effect of dietary phytase on the concentration of phytate esters and inositol in the fecal samples, and the observed degradation of phytate in the hindgut is in agreement with data indicating considerable phytate degradation in the hindgut of growing pigs due to phytase synthesized by intestinal microbes (Rutherfurd et al., 2014). However, P released after the ileum cannot be absorbed, and thus, does not contribute to the nutritional status of the pig (Dersjant-Li et al., 2015). To our knowledge, data comparing the concentration of phytate esters and inositol in intestinal contents among pigs fed diets with different dosages of supplemented phytase have not been reported. However, the current data clearly indicate increased hydrolysis of IP6 and IP5 as dietary phytase concentration increased, which resulted in increased concentrations of inositol in small intestinal digesta and in blood plasma indicating that some of the phytate was completely de-phosphorylated in the small intestine.
CONCLUSIONS
Mucin synthesis in the small intestine, protein hydrolysis in the stomach, and AA absorption were not affected by supplementation of phytase to diets fed to cannulated, growing pigs. However, phytase supplementation increased the degradation of high phytate esters into lower phytate esters and inositol in the stomach and small intestine, and results indicated that complete degradation of phytate in the stomach and small intestine is possible if the dosage of microbial phytase is increased up to at least 3,000 FTU, and this was observed as early as 20 min postfeeding. Phytase supplementation also increased the digestibility of Ca and P in the intestinal tract of pigs as phytase inclusion increased, but there was no evidence of Ca or P absorption in the hindgut of pigs. Likewise, the concentration of P in blood plasma increased with increasing dietary phytase, but the concentration of Ca in blood plasma was not affected by microbial phytase indicating strict hormonal control of plasma Ca concentrations.
Footnotes
Financial support for this research from AB Vista, Marlborough, the UK, is greatly appreciated.
LITERATURE CITED
- Ajakaiye A., Fan M. Z., Archbold T., Hacker R. R., Forsberg C. W., and Phillips J. P.. 2003. Determination of true digestive utilization of phosphorus and the endogenous phosphorus outputs associated with soybean meal for growing pigs. J. Anim. Sci. 81:2766–2775. doi: 10.2527/2003.81112766x [DOI] [PubMed] [Google Scholar]
- Almeida F. N., and Stein H. H.. 2010. Performance and phosphorus balance of pigs fed diets formulated on the basis of values for standardized total tract digestibility of phosphorus. J. Anim. Sci. 88:2968–2977. doi: 10.2527/jas.2009-2285 [DOI] [PubMed] [Google Scholar]
- Almeida F. N., and Stein H. H.. 2012. Effects of graded levels of microbial phytase on the standardized total tract digestibility of phosphorus in corn and corn coproducts fed to pigs. J. Anim. Sci. 90:1262–1269. doi: 10.2527/jas.2011-4144 [DOI] [PubMed] [Google Scholar]
- Almeida F. N., Sulabo R. C., and Stein H. H.. 2013. Effects of a novel bacterial phytase expressed in Aspergillus oryzae on digestibility of calcium and phosphorus in diets fed to weanling or growing pigs. J. Anim. Sci. Biotechnol. 4:8. doi: 10.1186/2049-1891-4-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- AOAC International 2007. Official methods of analysis of AOAC International. 18th ed. rev. 2. Hortwitz W. and G. W. Latimer Jr, editors. Gaithersburg, MD:AOAC International. [Google Scholar]
- Bedford M. R., and Walk C. L.. 2016. Reduction of phytate to tetrakisphosphate (IP4) to triphosphate (IP3), or perhaps even lower, does not remove its antinutritional properties. In: Walk C. L., Kuhn I., Stein H. H., Kidd M. T., and M. Rodehutscord, editors, Phytate destruction. Consequences for precision animal nutrition. Wageningen, The Netherlands: Wageningen Academic Publishers. p. 45–52. doi: 10.3920/978-90-8686-836-0_3 [DOI] [Google Scholar]
- Blaabjerg K., and Poulsen H. D.. 2010. Microbial phytase and liquid feeding increase phytate degradation in the gastrointestinal tract of growing pigs. Livest. Sci. 134:88–90. doi: 10.1016/j.livsci.2010.06.106 [DOI] [Google Scholar]
- Bohlke R. A., Thaler R. C., and Stein H. H.. 2005. Calcium, phosphorus, and amino acid digestibility in low-phytate corn, normal corn, and soybean meal by growing pigs. J. Anim. Sci. 83:2396–2403. doi: 10.2527/2005.83102396x [DOI] [PubMed] [Google Scholar]
- Cervantes M., Gόmez R., Fierro S., Barrera M. A., Morales A., Araiza B. A., Zijlstra R. T., Sanchez J. E., and Sauer W. C.. 2011. Ileal digestibility of amino acids, phosphorus, phytate and energy in pigs fed sorghum-based diets supplemented with phytase and Pancreatin®. J. Anim. Physiol. Anim. Nutr. 95:179–186. doi: 10.1111/j.1439-0396.2010.01038.x [DOI] [PubMed] [Google Scholar]
- Cowieson A., Acamovic J. T., and Bedford M. R.. 2006. Phytic acid and phytase: implications for protein utilization by poultry. Poult. Sci. 85:878–885. doi: 10.1093/ps/85.5.878 [DOI] [PubMed] [Google Scholar]
- Dersjant-Li Y., Awati A., Schulze H., and Partridge G.. 2015. Phytase in non-ruminant animal nutrition: a critical review on phytase activities in the gastrointestinal tract and influencing factors. J. Sci. Food Agric. 95:878–896. doi: 10.1002/jsfa.6998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dersjant-Li Y., Wealleans A. L., Barnard L. P., and Lane S.. 2017. Effect of increasing Buttiauxella phytase dose on nutrient digestibility and performance in weaned piglets fed corn or wheat-based diets. Anim. Feed Sci. Technol. 234:101–109. doi: 10.1016/j.anifeedsci.2017.09.008 [DOI] [Google Scholar]
- Dilger R. N., and Adeola O.. 2006. Estimation of true phosphorus digestibility and endogenous phosphorus loss in growing pigs fed conventional and low-phytate soybean meals. J. Anim. Sci. 84:627–634. doi: 10.2527/2006.843627x [DOI] [PubMed] [Google Scholar]
- Fan M. Z., Archbold T., Sauer W. C., Lackeyram D., Rideout T., Gao Y., de Lange C. F., and Hacker R. R.. 2001. Novel methodology allows simultaneous measurement of true phosphorus digestibility and the gastrointestinal endogenous phosphorus outputs in studies with pigs. J. Nutr. 131:2388–2396. doi: 10.1093/jn/131.9.2388 [DOI] [PubMed] [Google Scholar]
- Felsenfeld A. J., and Levine B. S.. 2015. Calcitonin, the forgotten hormone: does it deserve to be forgotten?Clin. Kidney J. 8:180–187. doi: 10.1093/ckj/sfv011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Vega J. C., Walk C. L., Liu Y., and Stein H. H.. 2014. The site of net absorption of ca from the intestinal tract of growing pigs and effect of phytic acid, ca level and ca source on ca digestibility. Arch. Anim. Nutr. 68:126–142. doi: 10.1080/1745039X.2014.892249 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Vega J. C., Walk C. L., and Stein H. H.. 2015. Effects of microbial phytase on apparent and standardized total tract digestibility of calcium in calcium supplements fed to growing pigs. J. Anim. Sci. 93:2255–2264. doi: 10.2527/jas.2014-8215 [DOI] [PubMed] [Google Scholar]
- Haraldsson A. K., Vilg J. V., Andlid T. A., Alminger M., and Sandberg A. S.. 2005. Degradation of phytate by high-phytase Saccharomyces cerevisiae strains during simulated gastrointestinal digestion. J. Agric. Food Chem. 53:5438–5444. doi: 10.1021/jf0478399 [DOI] [PubMed] [Google Scholar]
- Jass J. R., and Walsh M. D.. 2001. Altered mucin expression in the gastrointestinal tract: a review. J. Cell. Mol. Med. 5:327–351. doi: 10.1111/j.1582-4934.2001.tb00169.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khuri A. I., and Cornell J. A.. 1996. Response surfaces: designs and analyses. 2nd ed. Gainesville, FL:Marcel Dekker, Inc. [Google Scholar]
- Kiarie E., Walsh M. C., He L., Velayudhan D. E., Yin Y. L., and Nyachoti C. M.. 2016. Phytase improved digestible protein, phosphorus, and energy contents in camelina expellers fed to growing pigs. J. Anim. Sci. 94:215–218. doi: 10.2527/jas.2015-9735 [DOI] [Google Scholar]
- Kies A. K. 2005. Phytase studies in pigs and poultry. Effect on protein digestion and energy utilization [PhD dissertaion]., The Netherlands:Wageningen University. [Google Scholar]
- Kim B. G., and Stein H. H.. 2009. A spreadsheet program for making a balanced Latin square design. Rev. Colomb. Cienc. Pecu. 22:591–596. [Google Scholar]
- Laird S., Kühn I., Wilcock P., and Miller H. M.. 2016. The effects of phytase on grower pig growth performance and ileal inositol phosphate degradation. J. Anim. Sci. 94:142–145. doi: 10.2527/jas2015-9762 [DOI] [Google Scholar]
- Liao S. F., Kies A. K., Sauer W. C., Zhang Y. C., Cervantes M., and He J. M.. 2005. Effect of phytase supplementation to a low- and a high-phytate diet for growing pigs on the digestibilities of crude protein, amino acids, and energy. J. Anim. Sci. 83:2130–2136. doi: 10.2527/2005.8392130x [DOI] [PubMed] [Google Scholar]
- Liu N., Ru Y. J., Li F. D., Wang J. P., and Lei X. Q.. 2009. Effect of dietary phytate and phytase on proteolytic digestion and growth regulation of broilers. Arch. Anim. Nutr. 63:292–303. doi: 10.1080/17450390903020422 [DOI] [PubMed] [Google Scholar]
- Myers W. D., Ludden P. A., Nayigihugu V., and Hess B. W.. 2004. Technical note: a procedure for the preparation and quantitative analysis of samples for titanium dioxide. J. Anim. Sci. 82:179–183. doi: 10.2527/2004.821179x [DOI] [PubMed] [Google Scholar]
- NRC 2012. Nutrient Requirements of Swine. 11th rev. ed. Washington, DC:National Academies Press. [Google Scholar]
- Rehbein P., and Schwalbe H.. 2015. Integrated protocol for reliable and fast quantification and documentation of electrophoresis gels, protein expression and purification. Protein Expr. Purif. 110:1–6. doi: 10.1016/j.pep.2014.12.006 [DOI] [PubMed] [Google Scholar]
- Rutherfurd S. M., Chung T. K., and Moughan P. J.. 2014. Effect of microbial phytase on phytate P degradation and apparent digestibility of total P and ca throughout the gastrointestinal tract of the growing pig. J. Anim. Sci. 92:189–197. doi: 10.2527/jas.2013-6923 [DOI] [PubMed] [Google Scholar]
- Schlemmer U., Jany K. D., Berk A., Schulz E., and Rechkemmer G.. 2001. Degradation of phytate in the gut of pigs–pathway of gastro-intestinal inositol phosphate hydrolysis and enzymes involved. Arch. Tierernahr. 55:255–280. doi: 10.1080/17450390109386197 [DOI] [PubMed] [Google Scholar]
- She Y., Sparks J. C., and Stein H. H.. 2018. Effects of increasing concentrations of an Escherichia coli phytase on the apparent ileal digestibility of amino acids and the apparent total tract digestibility of energy and nutrients in corn-soybean meal diets fed to growing pigs. J. Anim. Sci. 96:2804–2816. doi: 10.1093/jas/sky152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y., Fan M. Z., Ajakaiye A., and Archbold T.. 2002. Use of the regression analysis technique to determine the true phosphorus digestibility and the endogenous phosphorus output associated with corn in growing pigs. J. Nutr. 132:1199–1206. doi: 10.1093/jn/132.6.1199 [DOI] [PubMed] [Google Scholar]
- Sherrod P. H. 2008. Nonlinear regression analysis program (NLREG) version 6.5 (advanced). Nashville, TN:Philip H. Sherrod. [Google Scholar]
- Stein H. H., Sève B., Fuller M. F., Moughan P. J., and De Lange C. F. M.. 2007. Invited review: amino acid bioavailability and digestibility in pig feed ingredients: terminology and application. J. Anim. Sci. 85:172–180. doi: 10.2527/jas.2005-742 [DOI] [PubMed] [Google Scholar]
- Stein H. H., Shipley C. F., and Easter R. A.. 1998. Technical note: a technique for inserting a T-cannula into the distal ileum of pregnant sows. J. Anim. Sci. 76:1433–1436. doi: 10.2527/1998.7651433x [DOI] [PubMed] [Google Scholar]
- Torres-Pitarch A., Hermans D., Manzanilla E. G., Bindelle J., Everaert N., Beckers Y., Torrallardona D., Bruggeman G., Gardiner G. E., and Lawlor P. G.. 2017. Effect of feed enzymes on digestibility and growth in weaned pigs: a systematic review and meta-analysis. Anim. Feed Sci. Technol. 233:145–159. doi: 10.1016/j.anifeedsci.2017.04.024 [DOI] [Google Scholar]
- Velayudhan D. E., Heo J. M., Dersjant-Li Y., Owusu-Asiedu A., and Nyachoti C. M.. 2015. Efficacy of novel 6-phytase from Buttiauxella sp. on ileal and total tract nutrient digestibility in growing pigs fed a corn-soy based diet. Anim. Feed Sci. Technol. 210:217–224. doi: 10.1016/j.anifeedsci.2015.10.005 [DOI] [Google Scholar]
- Wang B., and Brand-Miller J.. 2003. The role and potential of sialic acid in human nutrition. Eur. J. Clin. Nutr. 57:1351–1369. doi: 10.1038/sj.ejcn.1601704 [DOI] [PubMed] [Google Scholar]
- Wilcock P., and Walk C. L.. 2016. Low phytate nutrition – what is the pig and poultry industry doing to counter dietary phytate as an antinutrient and how is it being applied? In: Walk C. L., Kuhn I., Stein H. H., Kidd M. T., and M. Rodehutscord, editors, Phytate destruction. Consequences for precision animal nutrition. Wageningen, The Netherlands: Wageningen Academic Publishers. p. 87–106. doi: 10.3920/978-90-8686-836-0_3 [DOI] [Google Scholar]
- Woyengo T. A., Adeola O., Udenigwe C. C., and Nyachoti C. M.. 2010. Gastro-intestinal digesta pH, pepsin activity and soluble mineral concentration responses to supplemental phytic acid and phytase in piglets. Livest. Sci. 134:91–93. doi: 10.1016/j.livsci.2010.06.107 [DOI] [Google Scholar]
- Yu S., Cowieson A., Gilbert C., Plumstead P., and Dalsgaard S.. 2012. Interactions of phytate and myo-inositol phosphate esters (IP1-5) including IP5 isomers with dietary protein and iron and inhibition of pepsin. J. Anim. Sci. 90:1824–1832. doi: 10.2527/jas.2011-3866 [DOI] [PubMed] [Google Scholar]
- Zeng Z., Li Q., Tian Q., Zhao P., Xu X., Yu S., and Piao X.. 2015. Super high dosing with a novel buttiauxella phytase continuously improves growth performance, nutrient digestibility, and mineral status of weaned pigs. Biol. Trace Elem. Res. 168:103–109. doi: 10.1007/s12011-015-0319-2 [DOI] [PubMed] [Google Scholar]
- Zeng Z. K., Li A. Y., Zhao P. F., Xu X., Tian Q. Y., Wang H. L., Pan L., Yu S., and Piao X. S.. 2016. A new Buttiauxella phytase continuously hydrolyzes phytate and improves amino acid digestibility and mineral balance in growing pigs fed phosphorus-deficient diet. J. Anim. Sci. 94:629–638. doi: 10.2527/jas.2015-9143 [DOI] [PubMed] [Google Scholar]