Abstract
This study was aimed to estimate the survival rate characteristics of 2887 hepatocellular carcinoma (HCC) patients in the single-center and identify useful prognostic factors to help in the clinical management of patients with HCC.
Two thousand eight hundred eighty-seven patients diagnosed with HCC at the Fourth Hospital of Hebei Medical University, China, between January 2002 and December 2015 were involved.
The causes and baseline characteristics were summarized. Median survival time was 9.0 (20.0) months. Overall, HCC patients showed 1-, 2-, 3-, and 5-year survival rates equal to 49.3%, 35.3%, 26.6%, and19.5% respectively. The median survival time of HCC patients first hospitalized in 2009 to 2015 was higher than those in 2002 to 2008. The results showed that the Barcelona clinic liver cancer (BCLC) stage and Tumor size were independent prognostic factors to HCC patients.
The survival rate of HCC patients has increased in recent years, but the overall survival rate and the prognosis were poor.
Keywords: hepatocellular carcinoma, prognostic factors, survival analysis, survival rate characteristics
1. Introduction
Hepatocellular carcinoma (HCC) shows a grave morbidity and mortality and is considered to be a serious threat to human health and life. HCC represents the second most common cause of death from cancer all over the world.[1,2] Many effective treatment modalities for HCC has been used in clinical treatment such as hepatectomy, transhepatic arterial chemotherapy and embolization, radio frequency ablation, and the survival rates of HCC patients is increased.[3,4] It has been raised that HCC has many prognostic factors such as sex, Child-Pugh class, the clinical presentation of ascites, cough, fatigue, and the presence of metastases. However, there still lack of big data and more accurate clinical researches.[5,6]
We investigated the clinical data of patients treated in our institution for HCC between 2002 and 2015 and compared OS between 2002 and 2008 or between 2009 and 2015. By performing survival analysis and identifying the possible prognostic factors, we aimed to identify useful prognostic factors to help in the clinical management of patients with HCC.
2. Materials and methods
This retrospective study involved 2887 patients diagnosed with HCC at the Fourth Hospital of Hebei Medical University, China, between January 2002 and December 2015. Within this final cohort of patients, HCC was diagnosed in accordance with the guidelines for HCC in 2001.[7] This study was reviewed by China Clinical Trial Registry, and the approval number was ChiCTR-PRC-16007997.
All included cases were first summarized and then analyzed. Our primary endpoint was overall survival (OS). Our second aim was to investigate OS between 2002 and 2008 or between 2009 and 2015, depending on when the patients were first admitted into hospital. Then, we retrospectively analyzed 5 clinical-pathological characteristics which may affect the prognostic of patients with HCC, including Child-Pugh class, Barcelona clinic liver cancer (BCLC) stage, tumor size, portal vein tumor thrombus (PVTT), and therapy. The therapies were classified as follows: surgical treatment, trans-catheter arterial chemo-embolization (TACE) only, radiofrequency ablation (RFA)/microwave coagulation therapy (MCT) only, surgical treatment +TACE, or conservative treatment only. Finally, we used the meaningful variables from univariate analysis in multivariate Cox model analysis to confirm the significant and independent impact of HCC survival time.
In the study, we took the factors which may be related to the prognosis of HCC or controversial in other studies into analysis, and defined the number of tumor nodules, and the presence of PVTT, according to abdominal ultrasound, abdominal computed tomography or magnetic resonance imaging findings, or surgical observations. The patients were followed up until December 2015 or death. We defined survival time as the time elapsed between the first month in hospital to the date of death or last follow up.
All statistical analyses were performed with SPSS software version 20, Chicago, IL. The Kaplan–Meier method was used to estimate survival, while the log-rank test was used to compare survival rates between the 2 groups. The Cox proportional hazards model was used for the multivariate analysis of significant survival variables from Kaplan–Meier method. Statistical significance was determined at P <.05.
3. Results
3.1. Causes and general characteristics
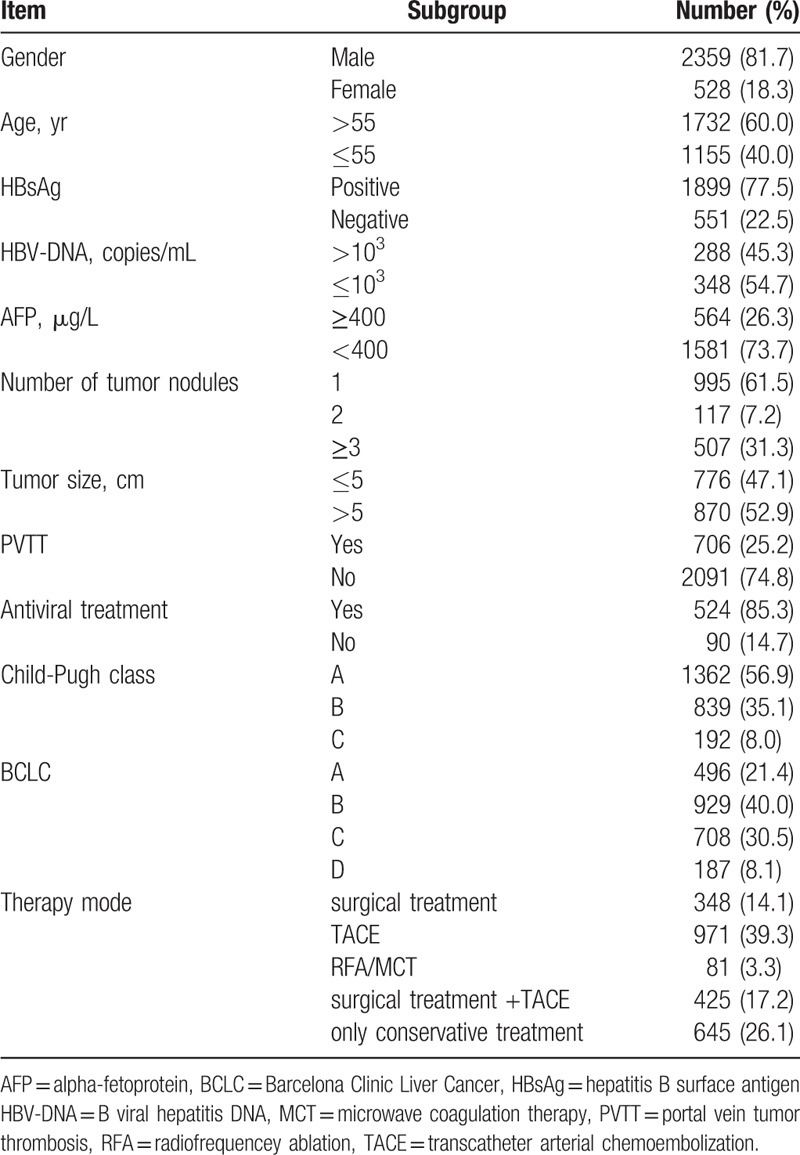
The causes and baseline characteristics are summarized in Table 1. Male gender (81.7%) was predominant, and the mean age was 57.7 ± 10.4 years; 1732 HCC patients (60.0%) were over 55 years of age. From 2450 patients, 1899 (77.5%) were positive for hepatitis B surface antigen (HBsAg). B viral hepatitis DNA (HBV-DNA) (≤103 copies/mL) was encountered in 348/636 patients (54.7%), and alpha-fetoprotein (AFP) (≤400 μg/L) was encountered in 1581/2145 patients (73.7%). Data showed that 995 HCC patients (61.5%) had single tumor nodules, 117 patients (7.2%) had 2 tumor nodules, and 507 patients (31.3%) had 3 or more. Tumor size was greater than 5 cm in 870 HCC patients (52.9%) and 706/2797 HCC patients (25.2%) had positive PVTT. Out of 614 HCC patients, 524 (85.3%) received antiviral treatment. In total, 1362 HCC patients (56.9%) were Child–Pugh class A, 839 (35.1%) were Child–Pugh class B,192 (8.0%) were Child–Pugh class C, 496 (21.4%) were BCLC A, 929 (40.0%) were BCLC B, 708 (30.5%) were BCLC C, and 187 (8.1%) were BCLC D. In terms of therapy, 348 HCC patients (14.1%) underwent surgical treatment only, 970 patients (39.3%) underwent TACE, 81 patients (3.3%) underwent RFA or MCT, 425 patients (17.2%) underwent surgical treatment and TACE, while 645 patients (26.1%) received only conservative treatment.
Table 1.
The cause and general characteristics.
3.2. Prognosis
3.2.1. OS
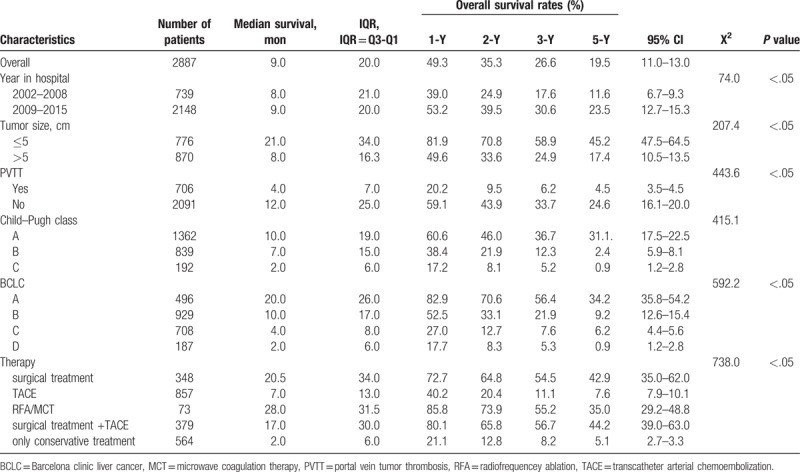
Median survival time was 9.0 (20.0) months. Overall, HCC patients showed 1-, 2-, 3-, and 5-year survival rates equal to 49.3%, 35.3%, 26.6%, and 19.5%, respectively (Table 2).
Table 2.
Overall survival and the impact on the prognosis of hospitalized year.
3.2.2. The impact of hospitalization year upon patient prognosis
Patients were divided into 2 groups according to the year they first attended our hospital, for 2002 to 2008 and 2009 to 2015, respectively. The median survival time for these 2 periods were 8.0 (21.0) months and 9.0 (20.0) months, respectively. For these time periods, the 1-year OS rates were 37.6% and 49.9%, the 2-year OS rates were 22.1% and 36.0%, the 3-year OS rates were 14.5% and 27.4%, and the 5-year OS rates were 8.8% and 12.0%, respectively, A log-rank test showed statistically significant differences between groups (X2 = 52.3, P <.05). Please refer to Table 2.
3.2.3. The impact of tumor size upon patient prognosis
The median survival time of patients with a tumor size ≤5 cm or >5 cm were 21.0 (34.0) months and 8.0 (16.3) months, respectively. HCC patients with a tumor size ≤5 cm had higher survival rates. A log-rank test showed statistically significant differences between groups (X2 = 207.4, P <.05). Refer to Table 2 and Figure 1.
Figure 1.
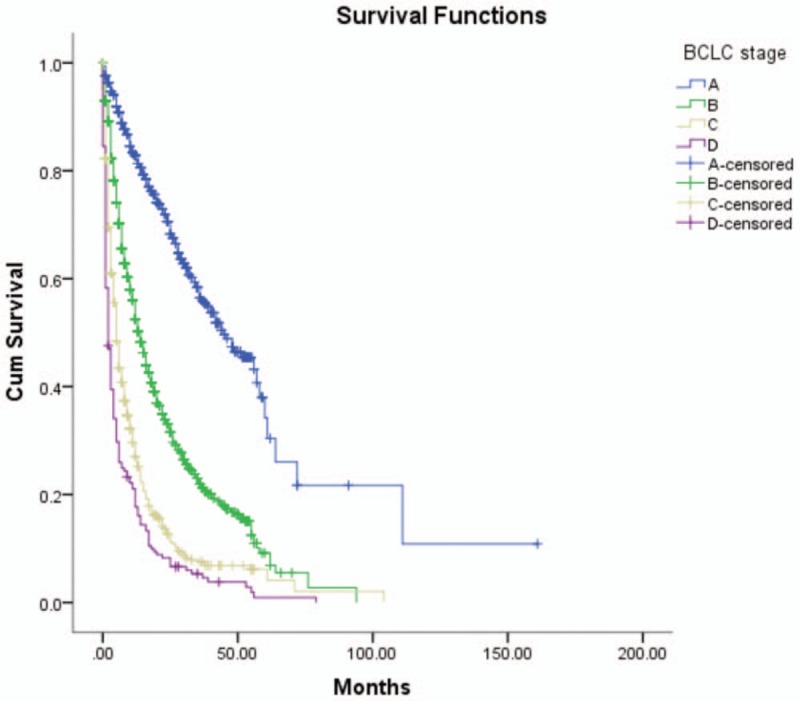
Survival rates comparison of tumor size.
3.2.4. The impact of portal vein tumor thrombosis (PVTT) upon patient prognosis
The median survival time of patients with PVTT and not respectively were: 4.0 (7.0) months and 12.0 (25.0) months. The HCC patients with PVTT had a shorter survival rates. Log-rank test showed differences between groups was statistically significant (X2 = 443.6, P <.05). Refer to Table 2.
3.2.5. The impact of Child–Pugh class upon patient prognosis
The median survival time of HCC patients with Child–Pugh class A, B, and C were 10.0 (19.0) months, 7.0 (15.0) months and 2.0 (6.0) months, respectively. The 1-year OS rates were 60.6%, 38.4%, and 17.2%, the 2-year OS rates were 46.0%, 21.9%, and 8.1%, the 3-year OS rates were 36.7%, 12.3%, and 5.2%, while the 5-year OS rates were 31.1%, 2.4%, and 0.9%, respectively. A log-rank test showed statistically significant differences between groups (X2 = 415.1, P <.05). Please refer to Table 2.
3.2.6. The impact of BCLC stage upon patient prognosis
The assessment of BCLC impact was possible in 2320/2887 patients (80.4%); there was insufficient clinical data available for the remaining 567 patients. The median survival time of HCC patients with BCLC stage A, B, C, and D were 20.0 (26.0) months, 10.0 (17.0) months, 4.0 (8.0) months, and 2.0 (6.0) months, respectively. The 1-year OS rates were 82.9%, 52.5%, 27.0%, and 17.7%, 2-year OS rates were 70.6%, 33.1%, 12.7%, and 8.3%, 3-year OS rates were 56.4%, 21.9%, 7.6%, and 5.3%, while the 5-year OS rates were 34.2%, 9.2%, 6.2%, and 0.9%, respectively. The log-rank test showed statistically significant differences between groups (X2 = 592.2, P <.05). Please refer to Table 2 and Figure 2.
Figure 2.
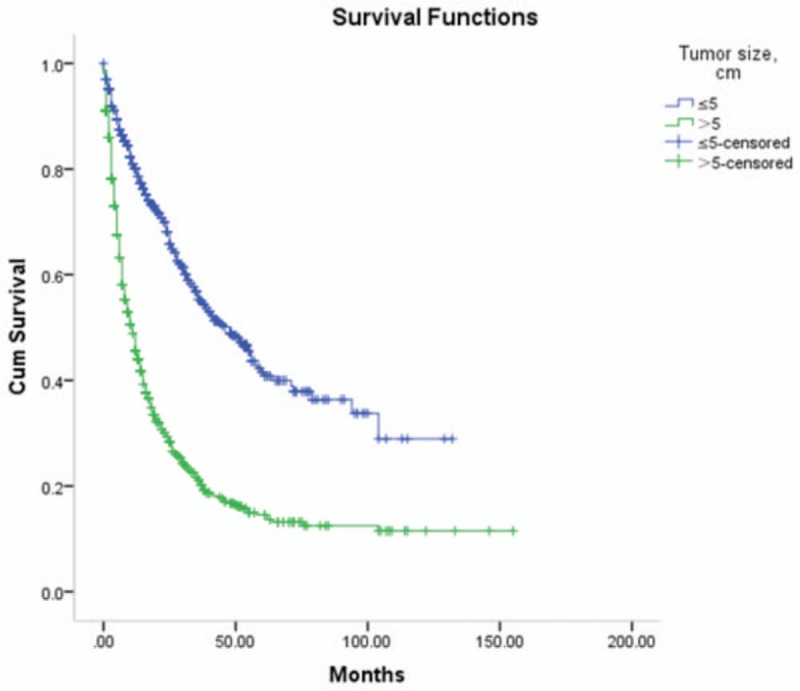
Survival rates comparison of BCLC stage. BCLC = Barcelona Clinic Liver Cancer.
3.2.7. The impact of different modes of therapy upon patient prognosis
The 5-year survival rates of HCC patients receiving surgical treatment only, TACE, RFA/MCT, surgical treatment combined with TACE, or conservative treatment only, were significantly different (P <.05). Patients undergoing surgical resection, with or without TACE, had higher survival rates than the other groups (Table 2).
3.2.8. Prognostic factors
Kaplan–Meier analysis showed that the following factors significantly affected the prognosis of HCC patients (P <.05): hospitalization year, HBsAg, AFP level, number of tumor nodules, tumor size, PVTT, antiviral treatment, Child–Pugh class, BCLC stage, and mode of therapy. In contrast, gender, age, and HBV-DNA did not affect the prognosis of patients with HCC (P >.05).
Next, we used the meaningful variables (HBsAg, AFP level, number of tumor nodules, tumor size, PVTT, antiviral treatment, Child–Pugh class, BCLC stage, and mode of therapy) in multivariate Cox model analysis. This confirmed the identification of prognostic factors for HCC, which were significant and independent. Results showed that BCLC stage (95% CI: 1.6–2.9, P <.05) and tumor size (95% CI: 2.1–5.6, P <.05) were independent prognostic factors for HCC (Table 3).
Table 3.
Multivariate analysis of prognostic variables affecting the survival of 2887 HCC patients.

4. Discussion
The high incidence and mortality rate of HCC has attracted much attention, but there has been very limited research undertaken with regard to prognostic factors for this disease, particularly in large sample sizes.[8,9] This present study involved the retrospective analysis of 2887 HCC patients from the Fourth Hospital of Hebei Medical University between 2002 and 2015. Our hospital is a provincial hospital with a sufficient number of HCC patients to represent the therapeutic levels in the region, thus overcoming the shortcomings of previous publications involving a limited number of cases and short follow-up times. Consequently, we hoped to be able to provide useful reference data for the prevention and treatment of HCC.
In our study, the median survival time of HCC patients first hospitalized in 2009 to 2015 was higher than those in 2002 to 2008. This suggests that the quality of healthcare has improved over recent years. For example, videography and early cancer screening have made it possible to diagnose cancer much earlier, and have led to improved cure rates. In addition, Jung et al reported that there has been an improvement in terms of patients being more attentive to further treatment.[10] However, further research is necessary to exclude potential confounding factors.
In our analysis of Child–Pugh class, Kaplan–Meier survival analysis showed that the survival rate of patients with Child–Pugh A was significantly higher than the other 2 classes. This means, that patients with a gradual deterioration of Child–Pugh class, shower shorter survival times, and had a worse prognosis.
Our data also showed that BCLC stage was a comprehensive evaluation criteria for tumor score, liver function and the general condition of HCC patients. This criterion only not only evaluates tumors, but can also guide treatment decisions for HCC patients. Our data showed that prognosis became significantly worse as BCLC stage increased; this finding was in accord with that of Shang.[11]
Some studies have also shown that the clinical characteristics of a tumor might be an important determinant of recurrence and metastasis of HCC, and that recurrence was directly related to survival time.[12,24] For example, tumor number and size both increase the difficulty of surgical resection, reduce success rates, make treatment selection more limited, and lead to an increased chance of recurrence and metastasis. One previous study of liver cancer patients with recurrence after resection observed that tumor size was an independent factor affecting the prognosis of HCC patients.[13] This previous report was consistent with our own current data; we also showed that tumor size is an independent prognostic factor for HCC.
PVTT forms when HCC invades the portal vein; the incidence of PVTT in HCC patients is 40.0%,[14] and reflects the progression of HCC. During the slow process of PVTT formation, there is a reduction in portal blood flow. Since this does not completely block blood flow in the portal vein, the body activates compensatory mechanisms which result in the formation of collateral circulation around the PVTT to maintain liver blood supply. PVTT is not only an early manifestation of liver metastases, but can also promote tumor metastasis. Meanwhile, it can also cause or aggravate portal hypertension when a portal tumor thrombus occurs in the portal vein, which then causes a series of complications such as gastrointestinal bleeding, portal hypertension stomach, and other such consequences. The results of this present study concluded that the survival rate of HCC patients with PVTT was lower than the group without PVTT, an observation which is consistent with the views of Huang.[15]
The treatment of liver cancer includes surgery, interventional therapy, radiation therapy, chemotherapy, biological therapy, immunotherapy, and so on.[16–18]. Surgical resection remains the most effective treatment method for HCC, and because of the biological characteristics of HCC, radiation therapy, and chemotherapy are not sensitive; furthermore, liver cirrhosis and liver dysfunction limit the dosage and combination of chemotherapies.[10] Interventional therapy in the clinical treatment of HCC has been widely used, and remains a common treatment for advanced and non-resectable HCC. Interventional therapy includes TACE, hepatic arterial embolization and hepatic arterial infusion; the 5-year survival rate after treatment with hepatic arterial embolization or TACE is known to range between 5% and 15%.[19] Kagawa considered that TACE, combined with RFA therapy, significantly increased the survival rate of patients.[20] In the present study, the patients who underwent surgery with or without TACE and RFA/MCT had a higher survival rate than the other patient groups. Furthermore, the survival rate of surgical resection combined with TACE was higher than TACE alone. The survival rate of patients treated conservatively was lower than for other treatments.
Individual circumstances should also be considered when selecting the most appropriate treatments for HCC patients. Wang reported that HBV-DNA was an important factor in the survival rate of liver cancer.[21] Chuma also concluded that patients with low levels of HBV-DNA (below 104 copies/mL) had a significantly higher survival rate than those with higher levels.[22] In this study, HBV-DNA level was not shown to be a significant prognostic factor for HCC patients, although this may have been due to the relatively small number of cases or other factors. Antiviral therapy can improve liver function, and secure more opportunities for the further treatment of HCC. Furthermore, because the virus might be activated in the treatment of HCC, consensus now agrees that antiviral therapy should be part of the treatment plan.[23] The antiretroviral therapy adopted in our study program includes lamivudine, telbivudine, entecavir, adefovir, lamivudine or telbivudine combined with adefovir dipivoxil. We concluded that antiviral therapy was a significant prognostic factor for HCC, and was thus consistent with the findings of Guo.[24]
Sang et al. analyzed 257 HCC patients from January 2000 to December 2003 and found that the severity of symptoms, Child–Pugh classification, AFP level, tumor size, PVTT, and TNM stage were independent prognostic factors for HCC.[23] Wang et al further reported that Child–Pugh class, vascular invasion, and treatment methods were closely related to the prognosis of HCC patients in their analysis of 306 HCC cases between January 2003 and January 2005.[25] In our current study, we showed that the following were significant prognostic factors for HCC: hospitalization year, HBsAg, AFP level, Child–Pugh class, number of tumor nodules, tumor size, PVTT, BCLC stage, antiviral treatment, and mode of therapy.
There are some limitations, however, which need to be considered when interpreting the results of our present study. To a certain extent, retrospective studies are not as superior as a prospective study, though X2 tests show no selection and follow-up bias. It was concluded that male gender (81.7%) was predominant, and gender did not affect the prognosis of patients with HCC (P > .05), which is consistent with the result of Sang Seok Lee.[26,27] Prognostic factors for HCC are more complex than we first thought, and it is possible that these factors interact with one another. Consequently, future studies should involve a more detailed analysis of prognostic factors.
5. Conclusion
In recent years, the survival rate of HCC patients has increased, although OS rate remains low, and prognosis is poor. Hospitalization year, HBsAg, AFP level, Child–Pugh class, number of tumor nodules, tumor size, PVTT, BCLC stage, antiviral treatment, and mode of therapy represent significant prognostic factors for HCC. BCLC stage and tumor size represent independent prognostic factors of HCC.
Author contributions
Conceptualization: Chunyan Wang.
Data curation: Chunyan Wang, Shengmian Li.
Formal analysis: Chunyan Wang.
Investigation: Chunyan Wang.
Methodology: Chunyan Wang.
Resources: Chunyan Wang, Shengmian Li.
Software: Chunyan Wang.
Supervision: Shengmian Li.
Validation: Chunyan Wang.
Visualization: Chunyan Wang.
Writing – original draft: Chunyan Wang.
Writing – review & editing: Chunyan Wang, Shengmian Li.
Shengmian Li orcid: 0000-0003-4455-2942.
Footnotes
Abbreviations: AFP = alpha-fetoprotein, BCLC = Barcelona clinic liver cancer, HBsAg = Hepatitis B surface antigen, HBV-DNA = B viral hepatitis DNA, HCC = hepatocellular carcinoma, MCT = microwave coagulation therapy, OS = overall survival, PVTT = portal vein tumor thrombosis, RFA = radiofrequencey ablation, TACE = transcatheter arterial chemoembolization.
The authors report no conflicts of interest.
References
- [1].Chacko Shinu, Samanta Subir. Hepatocellular carcinoma: a life-threatening disease. Biomed Pharmacother 2016;84:1679–88. [DOI] [PubMed] [Google Scholar]
- [2].He YT, Liang D, Li DJ, et al. Estimated cancer incidence and mortality in Hebei province, 2012. Chin J Cancer Res 2016;28:286–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Toyoda H, Kumada T, Kiriyama S, et al. Changes in the characteristics and survival rate of hepatocellular carcinoma from 1976 to 2000: analysis of 1365 patients in a single institution in Japan. Cancer 2004;100:2415–21. [DOI] [PubMed] [Google Scholar]
- [4].Ryder SD. British Society of Gastroenterology. Guidelines for the diagnosis and treatment of hepatocellular carcinoma (HCC) in adults. Gut 2003;52:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Wu CY, Chen YJ, Ho HJ, et al. Association between nucleoside analogues and risk of hepatitis B virus-related hepatocellular carcinoma recurrence following liver reserction. JAMA 2012;308:1906–14. [DOI] [PubMed] [Google Scholar]
- [6].Zeeneldin A, Salem SE, Darwish A, et al. Untreated hepatocellular carcinoma in Egypt: outcome and prognostic factors. J Hepatocell Carcinoma 2015;V2N:3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Yang BH, Xia JL. Diagnosis and treatment of primary liver cancer. Chin J Hepatol 2001;9:324. [Google Scholar]
- [8].Han DH, Choi GH, Kim DH, et al. Single center experience (ten years) with surgical resection for treating hepatocellular carcinoma: strategies for improving the long-term survival after resection. Korean J Hepatobiliary Pancreat Surg 2008;12:245–53. [Google Scholar]
- [9].Lee JK, Chung YH, Song BC, et al. Recurrences of hepatocellular carcinoma following initial remission by transcatheter arterial chemoembolization. J Gastroenterol Hepatol 2002;17:52–8. [DOI] [PubMed] [Google Scholar]
- [10].Yan D, Li H. Hepatic artery interventional therapy in combination with other means in treatment with hepatocellular carcinoma of portal vein tumor thrombus. J Pract Oncol 2011;32:335–6. [Google Scholar]
- [11].Shang CY, Su HY, Lu J, et al. Prognostic factors affecting TACE treatment in patients with primary hepatic carcinoma. Mod Oncol 2012;19:2466–9. [Google Scholar]
- [12].Zeeneldin AA, Salem SE, Tabashy RH, et al. Transarterial chemoembolization for the treatment of hepatocellular carcinoma: A single center experience including 221 patients. J Egypt Nat Cancer Inst 2013;25:143–50. [DOI] [PubMed] [Google Scholar]
- [13].Sun HC, Tang ZY, Ma ZC. Clinico-pathological factors influencing the disease-free survival of patients with hepatocellular carcinoma after radical operation. Chin J Hepatobiliary Surg 2000;6:7. [Google Scholar]
- [14].Pirisi M, Avellini C, Fabris C, et al. Portal vein thrombosis in hepatocellular carcinoma: age and sex distribution in an autopsy study. J CancerResClinOncol 1998;124:397–400. [DOI] [PubMed] [Google Scholar]
- [15].Huang JQ, Peng NH, Zou QQ, et al. Cox model analysis of prognostic factors after radical hepatectomy for primary hepatocellular carcinoma. Cancer Res Prevent Treat 2009;36:137–9. [Google Scholar]
- [16].Dai Liu, Kevin F, Staveley-O’Carroll, et al. Immune-based therapy clinical trials in hepatocellular carcinoma. J Clin Cell Immunol 2015;6: [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Pinter M, Peck-Radosavljevic M. Review article: systemic treatment of hepatocellular carcinoma. Aliment Pharmacol Ther 2018;48:598–609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Zhou J, Sun HC, Wang Z, et al. Guidelines for diagnosis and treatment of primary liver cancer in china (2017 edition). Liver Cancer 2018;7:235–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Wu MC, Wu D. Progress and Prospects of primary liver cancer treatment. Cancer Progress 2005;5:410–2+422. (in Chinese). [Google Scholar]
- [20].Kagawa T, Koizumi J, Kojima S, et al. Transcatheter arterial chemoembolization plus radiofrequency ablation therapy for early stage hepatocellular carcinoma:comparison with surgical resection. Cancer 2010;116:3638–44. [DOI] [PubMed] [Google Scholar]
- [21].Wang WH, Cao JB. Antiviral therapy for hepatitis B virus-associated hepatocellular carcinoma. Shijie Huaren Xiaohua Zazhi 2013;21:415–20. [Google Scholar]
- [22].Chuma M, Hige S, Kamiyama T. The influence of hepatitis B DNA level and antiviral therapy on recurrence after initial curative treatment in patients with hepatocellular carcinoma. J Gastroenterol 2009;44:991–9. [DOI] [PubMed] [Google Scholar]
- [23].Dai CL, Zhao Y. Comprehensive treatment for primary liver cancer. Chin J Bases Clin General Surg 2014;21:133–7. [Google Scholar]
- [24].Guo HH, Chao Y, Li Y. Antiviral therapy-related clinical efficacy for primary liver cancer patients with hepatitis B. Chin J Gerontol 2011;31:3016–8. [Google Scholar]
- [25].Wang ST, Zhou DH, Lin LZ. Multiple factors analysis of advanced primary liver cancer. Hebei Med J 2011;33:2261–2. [Google Scholar]
- [26].Lee SS, Shin HS, Kim HJ, et al. Analysis of prognostic factors and 5-year survival rate in patients with hepatocellular carcinoma: a single-center experience. Korean J Hepatol 2012;18:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Rawla P, Sunkara T, Muralidharan P, et al. Update in global trends and aetiology of hepatocellular carcinoma. Contemp Oncol (Pozn) 2018;22:141–50. [DOI] [PMC free article] [PubMed] [Google Scholar]


