Abstract
Rationale:
Concomitant cryoglobulinemic vasculitis and cold agglutinin disease (CAD) is an extremely uncommon clinical scenario. The role of bortezomib in the treatment of cryoglobulinemic vasculitis needs further investigation.
Patient concerns:
A 72-year-old Chinese woman presented with a 25-year history of cyanosis of the extremities after cold exposure, which worsened and was accompanied with purpuric skin lesions and proteinuria in recent years. Laboratory data demonstrated hemolysis. Cold agglutinin and cryoglobulin tests were positive. There was no evidence for malignancies after blood, image, and pathologic tests.
Diagnoses:
Concomitant cryoglobulinemic vasculitis and CAD.
Interventions:
The patient was treated with bortezomib-based regimen, including bortezomib, cyclophosphamide, and dexamethasone.
Outcomes:
The patient responded well to the treatment. Both symptoms and laboratory tests significantly improved. The patient's condition was in a state of sustained remission in the 6-month follow-up.
Lessons:
This rare case promotes further understanding of these 2 diseases and suggests that bortezomib is a promising treatment in type I cryoglobulinemic vasculitis.
Keywords: bortezomib, cold agglutinin disease, cryoglobulinemic vasculitis
1. Introduction
Cryoglobulins are immunoglobulins (Igs) that reversibly precipitate when the temperature is lower than 37°C. According to the presence of monoclonal and/or polyclonal Igs, cryoglobulinemia is classified into 3 types. Type I cryoglobulins are made up of a pure monoclonal Ig, generally either IgM or IgG. Types II and III are so-called mixed cryoglobulinemias. Type II cryoglobulins consist of a mixture of monoclonal IgM and polyclonal IgG, while type III cryoglobulins are composed of polyclonal IgM and IgG.[1] Most cases of cryoglobulinemia have a known underlying disease, which can be roughly grouped into infections, autoimmune disorders, and malignancies. Treatment should be focused on the underlying disease if possible.[2]
Cold agglutinins are autoantibodies that agglutinate red blood cells at an optimum temperature of 3°C to 4°C. Cold agglutinin disease (CAD) affects about 15% of patients with autoimmune hemolytic anemia. The most common symptom was acrocyanosis triggered by cold. Nearly 90% patients with CAD are mediated by monoclonal or polyclonal IgM, while the rest are caused by IgG, IgA, or light chains. CAD can be seen in the postinfectious setting, connective tissue diseases, and lymphoproliferative disorders. Early diagnostic evaluation and treatment improve outcomes in CAD.[3]
Herein, we report a rare case with concomitant cryoglobulinemic vasculitis and CAD without known underlying disease. No similar case had been reported before. Using bortezomib-based regimen, we successfully treated this patient. We report this case for further understanding of these 2 cold-activated diseases.
2. Case report
A 72-year-old Chinese woman presented with a 25-year history of cyanosis of the extremities after cold exposure (Fig. 1A), which worsened and was accompanied with purpuric skin lesions (Fig. 1B) and proteinuria in recent years. The patient came to our hospital in 1995, 2 years after the development of the disease. Laboratory investigations revealed a hemoglobin level of 96 g/L, plasma-free hemoglobin concentrations of 31.4 mg/dL and a reticulocyte percentage of 7.2%. Cold agglutinin test was 1:64 at 4°C and the precipitate was dissolved on heating to 20°C. Throat swabs detected mycoplasma pneumoniae antibody and X-ray revealed suspectable inflammation in the inferior lobe of right lung. With no evidence of lymphoproliferative diseases, bone marrow biopsy showed that the red blood cells assembled and piled up. The remaining laboratory tests were within normal range (Table 1). Notably, asymptomatic presence of cryoglobulins could be detected in the serum. Finally, cold agglutinin syndrome, probably caused by mycoplasma infection, was diagnosed after excluding other etiologies. Meanwhile, cryoglobulinemia with no end-organ damage existed. The patient was treated with prednisone and roxithromycin. Between 1996 and 2014, the patient worked as a diplomat in San Francisco, New York, and Vancouver, respectively. By keeping herself warm, cyanosis did not relapse. Laboratory tests showed that her hemoglobin level ranged between 131 and 154 g/L during this period.
Figure 1.
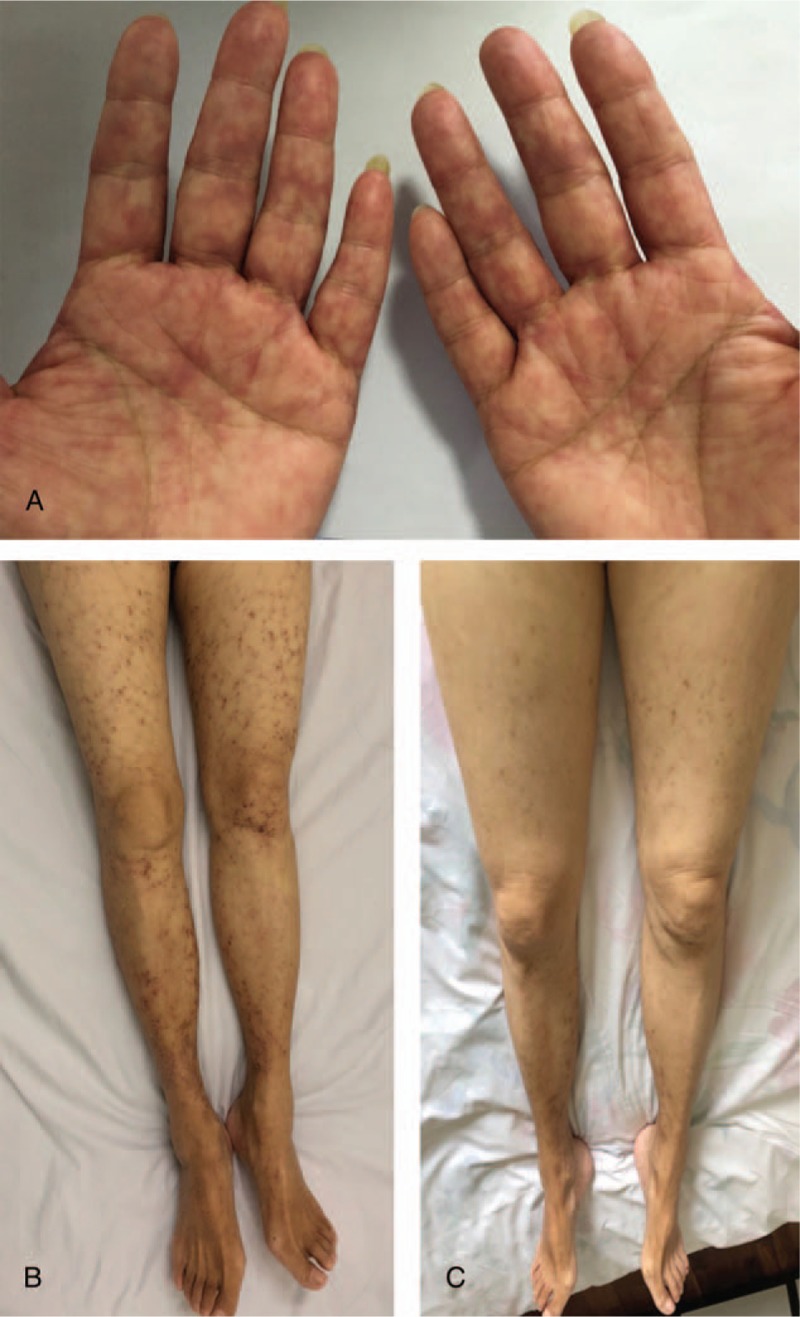
(A) Cyanosis of hands after cold exposure. (B) Purpuric skin lesions in May 2018. (C) Dramatically improved skin lesions after 2 cycles of bortezomib-based therapy.
Table 1.
Laboratory data.
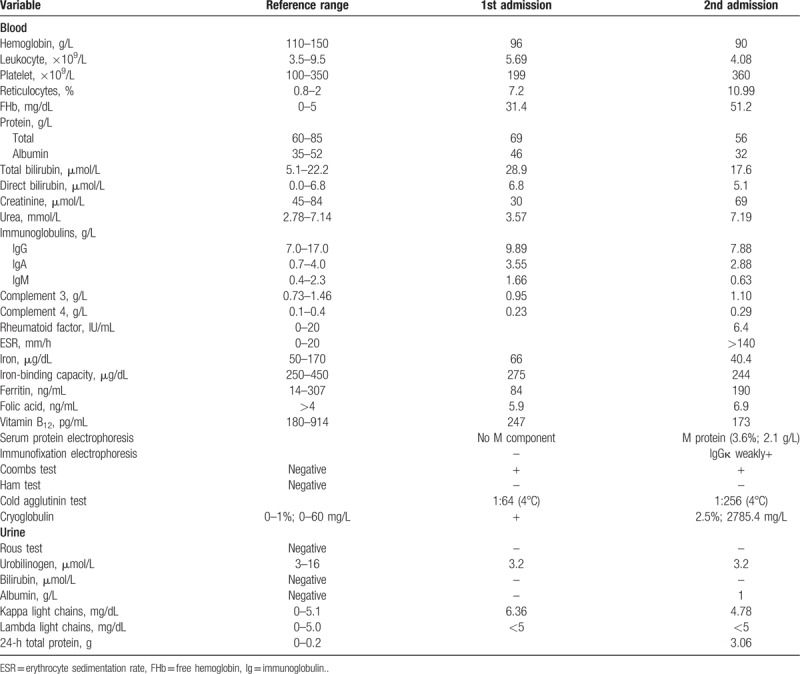
In 2014, purpuric skin lesions (mainly on the lower limbs, sometimes on the arms, chest, and neck), proteinuria, and cyanosis of increasing frequency and severity developed. The patient was admitted to our hospital again in March 2018. Similar to the 1st admission, her hemoglobin level was 90 g/L. Meanwhile, her 24-hour urine total protein was 3.06 g. More comprehensive examinations were conducted this time. Hepatitis A, B, and C virus, cytomegalovirus, Epstein–Barr virus, human parvovirus B19, and human immunodeficiency virus serologies were negative, as were antinuclear antibodies, antineutrophil cytoplasmic antibodies, antiextractable nuclear antigen antibodies, and lupus anticoagulant studies. Complement and Ig were within normal range. M protein (3.6%; 2.1 g/L) was found and immunofixation electrophoresis revealed that IgGκ was slightly positive. Cold agglutinin test was 1:256 at 4°C and the precipitate could be dissolved at 20°C. Serum type-I IgGκ cryoglobulin was detected (cryocrit: 2.5%; absolute cryoglobulin concentration: 2785.4 mg/L). The other test results are shown in Table 1. Positron emission tomography revealed no evidence for underlying malignancies. No waveform could be elicited in the left gastrocnemius nerve by electromyogram. Pulmonary function test demonstrated the reduction of diffusion function. Echocardiogram revealed left atrial enlargement, mild mitral and tricuspid insufficiency, mild pulmonary hypertension, and decreased left ventricular relaxation function.
Bone marrow biopsy, renal biopsy, and skin biopsy were done. Red blood cells could still be seen gathering and piling up together in the bone marrow biopsy (Fig. 2). Proportion of the phlogocytes slightly elevated. Immunohistochemical test revealed: CD3 (−), CD20 (scattered B cell+), CD38 (a few phlogocytes+), CD79a (−), CD138 (a few phlogocytes+), EMA (−), Ki-67 (index 15%), Mum-1 (−), PAX-5 (−). Flowcytometry of the bone marrow revealed no abnormal immunophenotypic pattern. The results of fluorescence in situ hybridization and genetic test for leukemia were negative. Additionally, she also underwent percutaneous renal biopsy, the results of which were in accordance with the manifestation of cryoglobulinemic vasculitis with kidney. Skin biopsy was also performed on the lesion of her right leg (Fig. 3). Hyaline thrombus was seen in the lumen of blood vessels.
Figure 2.
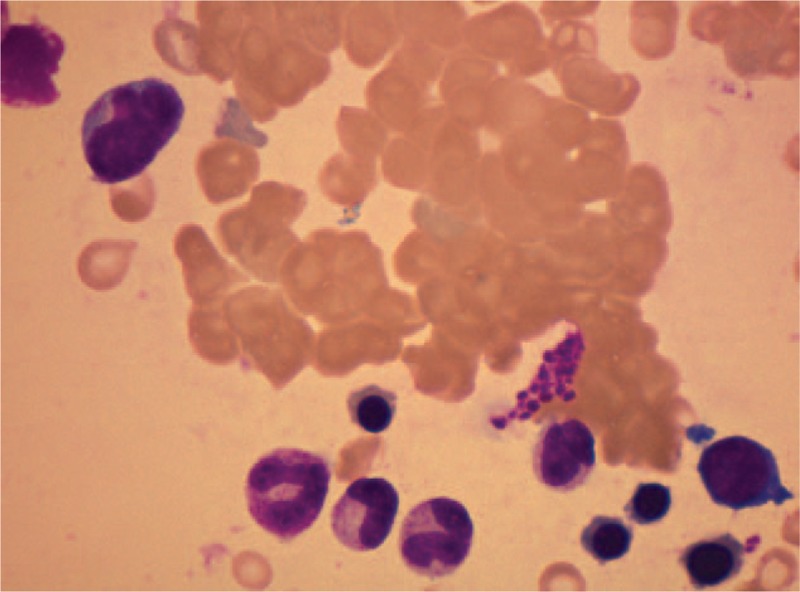
Bone marrow biopsy showed red blood cells gathering and piling up together, with no evidence of malignancies.
Figure 3.
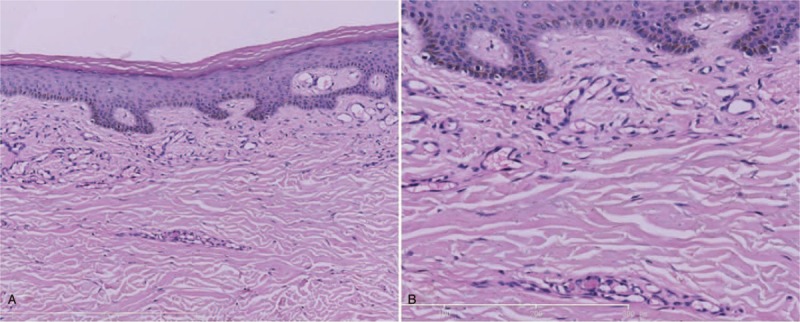
Skin biopsy showing hyaline thrombus in the lumen of blood vessels (A, ×100; B, ×400).
After finishing the examinations, concomitant cryoglobulinemic vasculitis and CAD were diagnosed. Bortezomib at a dose of 1.3 mg/m2, cyclophosphamide at a dose of 300 mg/m2, and dexamethasone at a dose of 40 mg were used on days 1, 8, 15, and 22 every 35 days. After 2 cycles of therapy, the hemoglobin, plasma-free hemoglobin, reticulocyte values together with M protein returned to the normal ranges. Meanwhile, both qualitative and quantitative tests for cryoglobulins were negative. In addition, the cutaneous rash dramatically improved (Fig. 1C) and 24-hour urine total protein decreased to 0.03 g, suggesting the remission of cryoglobulinemic vasculitis. The patient's condition was in a state of sustained remission in the 6-month follow-up. No further medication was given during this period.
3. Discussion
Cryoglobulins are Igs that precipitate in vitro at temperatures lower than 37°C. Only a small proportion of these patients have organ damage caused by cryoglobulins via 2 main pathways: vascular sludging or immune-mediated mechanisms. Currently, there are limited epidemiologic studies on the prevalence of cryoglobulinemia. The incidence is reportedly 1:100,000 persons with a female to male ratio of 3:1.[4] CAD is a rare and poorly understood disorder affecting 15% patients with autoimmune hemolytic anemia. Approximately 90% cold agglutinins are cold-reactive IgM directed against antigens on the red blood cells.[5] Thus, the coexistence of cryoglobulinemic vasculitis and CAD is an extremely uncommon scenario. To the best of our knowledge, this was the 1st case with concomitant cryoglobulinemic vasculitis and CAD. As for treatment, the patient was successfully treated with bortezomib, cyclophosphamide, and dexamethasone. We believe that this case uniquely illustrates its rare medical condition from the following aspects.
First, to the best of our knowledge, this is the 1st case with concomitant cryoglobulinemic vasculitis and CAD. On one hand, laboratory test demonstrated the presence of a measurable amount of cryoglobulin and the component of cryoglobulin was monoclonal IgGκ. Indicators of cryoglobulinemic thrombosis with skin were proven after biopsy. While no established diagnostic criteria existed, cryoglobulinemic vasculitis was diagnosed after a careful consideration of combined clinical, laboratory, and pathologic data. No evidence of clonal hematologic diseases, autoimmune diseases or infection was discovered. Complement was normal in our case, which may be explained the study of Petersen and colleagues which illustrated normal complement may be caused by specific IgG subclasses.[6] On the other hand, CAD was diagnosed considering clinical manifestations, existence of hemolysis, positive direct antiglobulin test, and cold agglutinin titer ≥64 at 4°C. Both cryoglobulinemic vasculitis and CAD are rare diseases, which makes the coexistence extremely uncommon.
Second, considering the type of cryoglobulins, results of immunofixation electrophoresis and Igs within normal range, it is likely that the CAD was also mediated by cold-reactive IgG in this case, although the patient did not have clinical manifestations significantly different from the IgM-mediated CADs. We have to admit that it is a pity we cannot testify this hypothesis due to the limitations of our laboratory. Cold agglutinins in the usual cases are IgMκ anti-I autoantibodies. There are rare examples of IgG cold agglutinins.[7] Silberstein and colleagues reported six patients with IgG-reactive chronic CAD responsive to glucocorticoids and splenectomy, which were purported useless in CAD.[8] We thought it was prednisone rather than roxithromycin that worked in the 1st treatment, too. Because the disease had been existing for 2 years before the 1st admission, the likelihood of infection was rather small. However, IgG has always been recognized as an incomplete antibody. The mechanism and treatment of IgG-mediated CAD needs further investigation.
Finally, successful treatment was done with bortezomib-based regimen. For cryoglobulinemia, high-grade evidence regarding the appropriate approach to treatment is limited at present because of the low incidence. Recommendations are mostly expert opinion.[9] In type I cryoglobulinemia, the use of rituximab is controversial due to the lack of efficacy against CD-20-negative plasma cells.[10] Bortezomib is a proteasome inhibitor that inhibits angiogenesis and production of paraproteins.[11] Bortezomib has been shown effective in cryoglobulinemic vasculitis associated with multiple myeloma.[10] Ramirez and colleagues confirmed the efficacy of bortezomib-based regimen as a treatment for refractory type I cryoglobulinemic vasculitis associated with monoclonal gammopathy of undetermined significance.[12] For CAD, patients may respond well to rituximab, especially in primary CAD.[3] However, optimal treatment of the underlying disorder is essential when feasible. Cytotoxic agents can be useful for both malignancies and CAD.[13] As for our patient, M protein was detected in the 2nd admission. Although no underlying disease was diagnosed, plasma cell dyscrasia was the most suspicious cause of concomitant type I cryoglobulinemic vasculitis and CAD after excluding infections and autoimmune disorders. Thus, the patient was treated with bortezomib, cyclophosphamide, and dexamethasone. In our case, bortezomib was proven effective regardless of the underlying disease in type I cryoglobulinemic vasculitis. Larger controlled studies are warranted for a detailed treatment strategy, although great challenges exist because of the low incidence.
4. Conclusion
In summary, it is worth noting that this is probably the 1st case with concomitant cryoglobulinemic vasculitis and CAD. Meanwhile, with a long duration of disease and follow-up time for more than 20 years, no known underlying disease could be diagnosed. Furthermore, bortezomib-based regimen was effective in our patient, indicating that bortezomib can be promising in type I cryoglobulinemic vasculitis.
Author contributions
Conceptualization: Xiao-hang Liu.
Data curation: Meng Zhang.
Investigation: Fan Jin.
Resources: Lu Zhang.
Supervision: Lu Zhang.
Writing – original draft: Xiao-hang Liu, Mei-xi Liu, Fan Jin.
Writing – review & editing: Meng Zhang, Lu Zhang.
Footnotes
Abbreviations: CAD = cold agglutinin disease, ESR = erythrocyte sedimentation rate, Ig = immunoglobulin.
Written, informed consent was obtained from the patient to use the content and imaging material for publication.
The authors have no funding and conflicts of interest to disclose.
References
- [1].Brouet JC, Clauvel JP, Danon F, et al. Biologic and clinical significance of cryoglobulins: a report of 86 cases. Am J Med 1974;57:775–88. [DOI] [PubMed] [Google Scholar]
- [2].Ramos-Casals M, Stone JH, Cid MC, et al. The cryoglobulinaemias. Lancet 2012;379:348–60. [DOI] [PubMed] [Google Scholar]
- [3].Swiecicki PL, Hegerova LT, Gertz MA. Cold agglutinin disease. Blood 2013;122:1114–21. [DOI] [PubMed] [Google Scholar]
- [4].Takada S, Shimizu T, Hadano Y, et al. Cryoglobulinemia. Mol Med Rep 2012;6:3–8. [DOI] [PubMed] [Google Scholar]
- [5].Berentsen S, Randen U, Tjønnfjord GE. Cold agglutinin-mediated autoimmune hemolytic anemia. Hematol Oncol Clin North Am 2015;29:455–71. [DOI] [PubMed] [Google Scholar]
- [6].Petersen T, Riviere S, Malbos S, et al. Subclasses of monoclonal (type I) immunoglobulin G cryoglobulins: report on two distinct cases with myeloma. Clin Lab 2018;64:615–8. [DOI] [PubMed] [Google Scholar]
- [7].Silberstein LE, Shoenfeld Y, Schwartz RS, et al. A combination of IgG and IgM autoantibodies in chronic cold agglutinin disease: immunologic studies and response to splenectomy. Vox Sang 1985;48:105–9. [DOI] [PubMed] [Google Scholar]
- [8].Silberstein LE, Berkman EM, Schreiber AD. Cold hemagglutinin disease associated with IgG cold-reactive antibody. Ann Intern Med 1987;106:238–42. [DOI] [PubMed] [Google Scholar]
- [9].Muchtar E, Magen H, Gertz MA. How I treat cryoglobulinemia. Blood 2017;129:289–98. [DOI] [PubMed] [Google Scholar]
- [10].Terrier B, Karras A, Kahn JE, et al. The spectrum of type I cryoglobulinemia vasculitis: new insights based on 64 cases. Medicine (Baltimore) 2013;92:61–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Besada E, Vik A, Koldingsnes W, et al. Successful treatment with bortezomib in type-1 cryoglobulinemic vasculitis patient after rituximab failure: a case report and literature review. Int J Hematol 2013;97:800–3. [DOI] [PubMed] [Google Scholar]
- [12].Ramirez GA, Campochiaro C, Salmaggi C, et al. Bortezomib in type I cryoglobulinemic vasculitis: are we acting too late? Intern Med 2015;54:1119–23. [DOI] [PubMed] [Google Scholar]
- [13].Berentsen S, Tjønnfjord GE. Diagnosis and treatment of cold agglutinin mediated autoimmune hemolytic anemia. Blood Rev 2012;26:107–15. [DOI] [PubMed] [Google Scholar]


