Abstract
Rationale:
The potential efficacy of apatinib in patients with advanced triple-negative breast cancer (TNBC) has been observed in a previous phase II clinical study. However, there is no study to evaluate its efficacy and safety in TNBC patients with brain metastasis (BM). Here we report one case that apatinib exhibited excellent antitumor effects in a breast cancer patient with brain metastasis, with no serious treatment-associated with adverse event.
Patient concerns:
In this case report, one Chinese woman who was diagnosed with stage IV TNBC with multiple bone, lung, and brain metastases was unable to tolerate chemotherapy and refused whole-brain radiation therapy (WBRT) due to her poor physical condition. She had previously undergone radical mastectomy and intravenous chemotherapy.
Diagnoses:
Triple-negative breast cancer.
Interventions:
The patient underwent left radical mastectomy with ipsilateral axillary lymph node dissection, and the following adjuvant chemotherapy, but developed multiple bone, lung, and brain metastases. Due to her poor physical condition, chemotherapy was not eligible for her. And she refused WBRT and chose to take low-dose apatinib (250 mg, oral, daily) monotherapy.
Outcomes:
After 2 months of treatment, the symptom of headache and vomiting relieved and all the brain metastases (BMs) lesions disappeared.
Lessons:
Low-dose apatinib monotherapy may be an alternative treatment for patients with poor physical condition. Preclinical and clinical studies should be conducted to further evaluate the mechanism and efficacy of apatinib in the treatment of BM from TNBC, as well as to explore the optimal dose of the drug.
Keywords: apatinib, brain metastasis, triple-negative breast cancer
1. Introduction
Triple-negative breast cancer (TNBC) is highly malignant and has a high tendency to metastasize to the brain. Data from the Dana-Farber Cancer Institute showed that nearly half of all metastatic TNBC patients experienced metastasis to the brain before death.[1] One recent Chinese study reported that the incidence of brain metastasis (BM) in metastatic TNBC patients was 29% (127/433).[2] TNBC-BM patients are intractable and usually have poor prognosis with a short median survival time of about half a year, even if they are treated with current standard treatment regimens.[1,2] And also many TNBC-BM patients are not tolerant to the toxicities resulting from traditional chemotherapy. The development of effective treatment regimens for TNBC-BM patients is urgent unmet medical needs.
Apatinib, an orally administered small-molecule targeted drug, has potential antiangiogenic and antineoplastic effects by blocking the intracellular ATP-binding site of VEGFR-2. The efficacy of this drug has been evaluated by phase II and III clinical trials,[3,4] and apatinib has been approved as third-line treatment for advanced gastric cancer patients in October 2014 in China. In recent years, a series studies have shown that apatinib shows encouraging antitumor activities in several solid tumors, including non-small cell lung cancer and breast cancer.[5–9] However, the efficacy of apatinib monotherapy in TNBC-BM patients has not been reported yet. Herein, we reported one TNBC-BM patient who responded to low-dose (250 mg, QD, oral) apatinib.
2. Case report
In June 2014, a 51-year-old Chinese woman underwent left radical mastectomy with ipsilateral axillary lymph node dissection in our hospital. The pathological diagnosis and stage was T1N1M0 stage IIA breast cancer. The genetic subtype was triple-negative. Two weeks after surgery, as adjuvant therapy, the patient received chemotherapy of paclitaxel combined with epirubicin four times. In December 2015, multiple metastases in the bone were detected by both whole-body bone scanning and computed tomography (CT), no local tumor recurrence or metastatic lesions in other organs was found. Then the patient received one cycle of gemcitabine and carboplatin chemotherapy. The patient reported that her pain was significantly relieved, but the treatment was stopped because of severe adverse events (experienced one grade four bone marrow suppression and one severe hepatic injury). The patients complained aggravated pain, in March 2016, she received tegafur and lumbar radiotherapy treatments (VMAT, 30 Gy/10F/3 Gy). The medical timeline is outlined in Figure 1.
Figure 1.
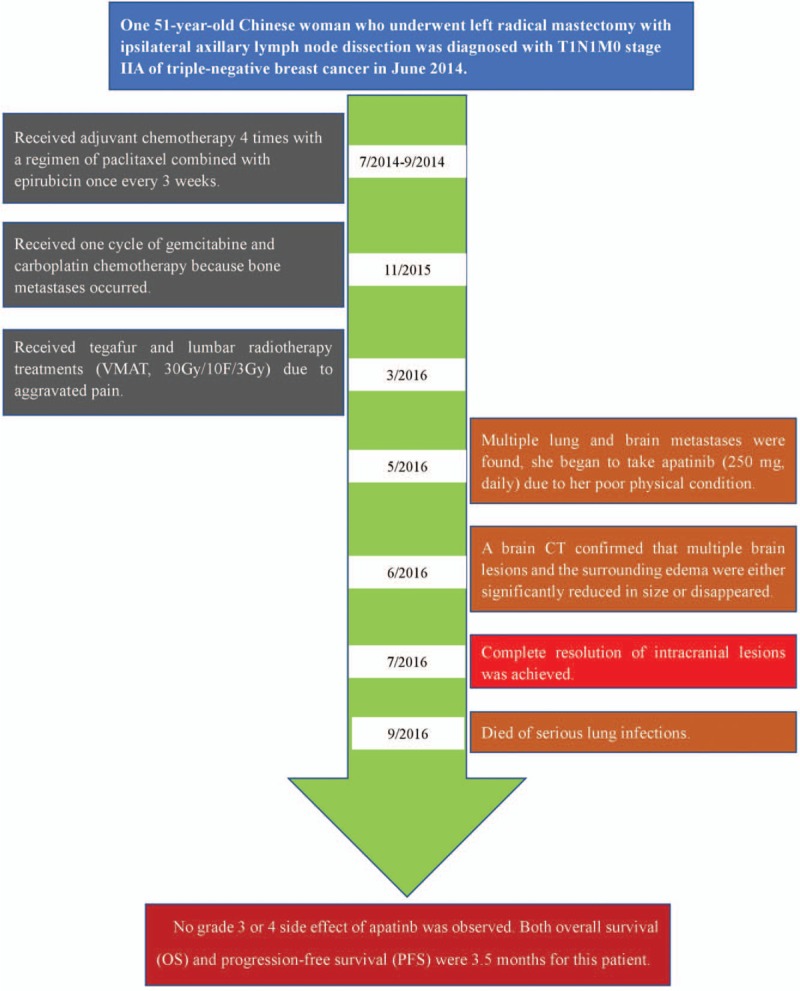
Timeline of interventions and outcomes.
In May 2016, the patient complained headache and frequent vomiting. BMs and surrounding edema were found by brain CT examination (Fig. 2A). At the same time, multiple lung metastases were also found by chest CT. Considering her poor physical condition (PS 4), we thought chemotherapy was not eligible for her, and recommended to receive whole-brain radiation therapy (WBRT) or targeted therapy. She refused WBRT and chose to take apatinib (250 mg, daily) since May 10, 2016. After 1 month of apatinib monotherapy, she reported that the severity of headache and vomiting relieved, and her performance status was also improved (PS 3). The brain CT examination showed that multiple brain lesions and the surrounding edema were either significantly reduced in size or disappeared (Fig. 2B). After 1 month of continued treatment, all the intracranial lesions disappeared (Fig. 2C). During the whole treatment period, no grade 3 or 4 adverse event was observed. Unfortunately, the patient died because of serious lung infections. Both overall survival (OS) and progression-free survival (PFS) were 3.5 months for this patient.
Figure 2.
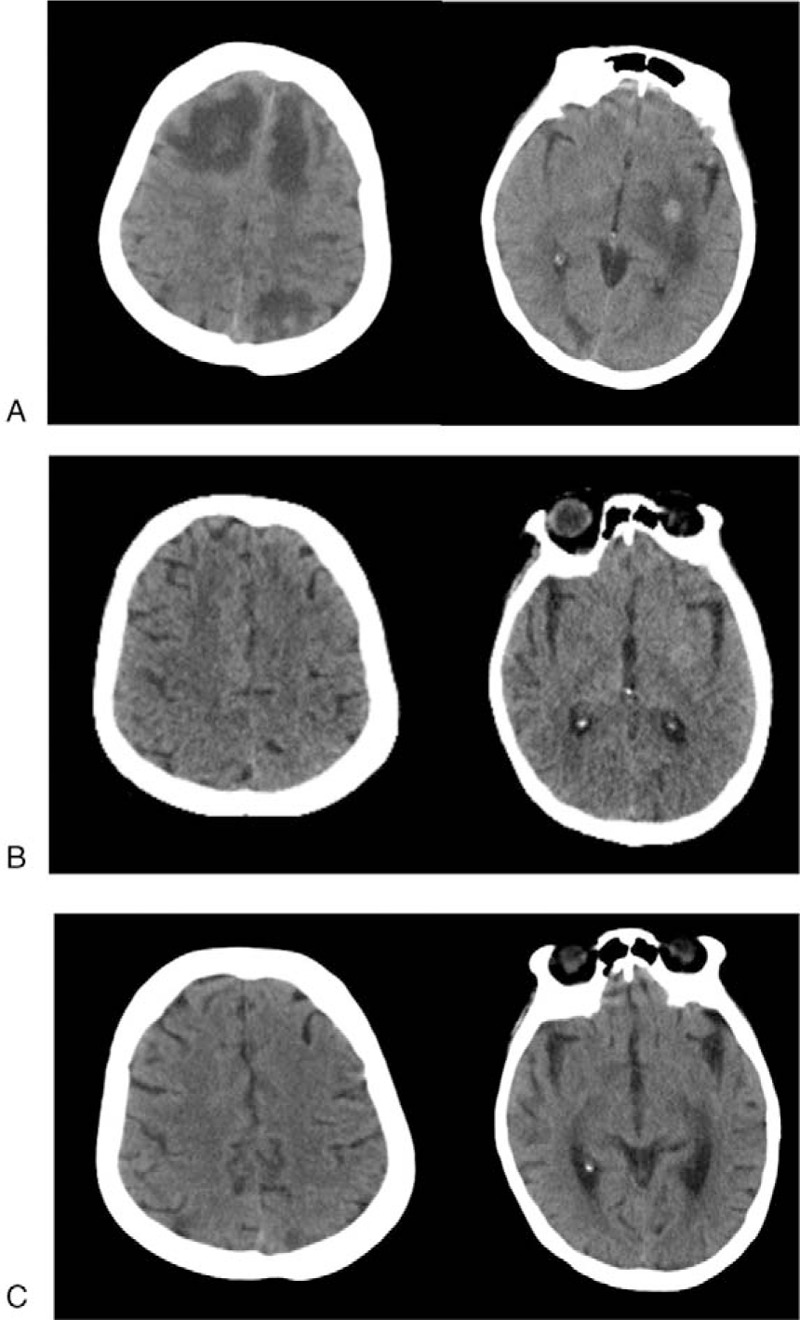
CT of response to apatinib monotherapy: (A) before single-agent apatinib, (B) after 1 month of single-agent apatinib, and (C) after 2 months of single-agent apatinib.
3. Discussion
The multicenter phase II study that was conducted to explore therapeutic doses of apatinib indicated that a dose of 500 mg/day, rather than 750 mg/day, should be recommended for heavily pretreated metastatic TNBC patients because of toxicity.[7] However, treatment with the lower dosage of apatinib (500 mg, daily) did not reduce the toxicities, the rates of drug interruption and dose reduction were 25.4% and 32.2%, respectively.[7] It is still pertinent to find the best dose of apatinib in the treatment of TNBC, especially for patients with poor physical condition. To the best of our knowledge, this is the first report to describe the successful use of low-dose apatinib (250 mg, daily) monotherapy to treat TNBC-BM patient with poor physical condition. In this report, 250 mg apatinib treatment exhibited obvious efficacy for the BM lesions.
Most conventional chemotherapeutic drugs and humanized monoclonal antibodies are not effective at treating patients with BM due to the presence of the blood-brain barrier (BBB).[10–12] For example, trastuzumab is one type of macromolecular agent that has difficulty crossing the BBB in vitro models of brain metastases from breast cancer, with low concentration in the cerebrospinal fluid and rare antitumor activity for intracranial lesions in patients with human epidermal growth factor receptor (HER2)-positive breast cancer.[13,14] As is known, antiangiogenesis has become one of the important modules of current cancer therapy. Antiangiogenic drugs targeting vascular endotheliocytes may avoid the obstacle of the BBB. Bevacizumab, an antiangiogenic monoclonal antibody with a large molecular weight, has shown efficacy when combined with chemotherapy in the treatment of patients with NSCLC and asymptomatic untreated brain metastases, with an overall response rate (ORR) of 61.2% in intracranial lesions, a median PFS of 6.7 months [95% confidence interval (CI), 5.7–7.1] and a median overall survival (OS) rate of 16 months.[15] Similarly, as an inhibitor of the VEGF/VEGFR pathway in vascular endothelial cells, apatinib achieved good results in the treatment of metastatic TNBC patients with BM that have been exhibited in another document[16] and our present report.
Compared with traditional chemotherapy drugs, tyrosine kinase inhibitors (TKIs) have a low molecular weight and could permeate the BBB.[17] The epidermal growth factor receptor TKIs (eg, gefitinib, erlotinib, afatinib, and Osimertinib) have shown activity in the treatment of brain metastases in patients with NSCLC, achieved response rates of 60%–80% and a complete response rate as high as 40%.[18,19] Besides the activity in antiangiogenesis, as one of the small molecule TKIs, apatinib was proven to be able to induce tumor cells apoptosis in vitro tests.[20–23] When used for the treatment of intracerebral tumors, apatinb may pass through the BBB easily and then directly induce neoplastic cell apoptosis.
Evidence revealed that metastatic cancer cells could produce VEGF, which binding to VEGFR2, and induce the disruption of the BBB, facilitate a series of physiological changes including edema. Blocking of this signaling pathway could decrease clinical severity and tissue damage.[12,24–26] One case reported that apatinib can obviously reduce refractory radiation-induced brain edema.[26] In this present report, apatinib also showed activity for elimination of multiple BMs, as well as the surrounding edema after short-term treatment. We speculate that apatinib might have the ability to repair the BBB by blocking the intracellular ATP-binding site of VEGFR2.
In conclusion, low-dose apatinib monotherapy exerted excellent efficacy in the treatment of symptomatic BMs in one TNBC patient, the possible mechanism might be through antiangiogenesis, pro-apoptosis of tumor cells, and BBB repair. This suggests that low-dose apatinib monotherapy may be an efficient alternative for patients with poor physical condition. Preclinical and clinical studies should be developed to further explore the mechanism, efficacy, and appropriate dose of apatinib in treating brain-metastasized breast cancer.
Acknowledgments
The authors thank the family members of this patient for their agreement to publication of this report.
Author contributions
Conceptualization: Yu-Ming Jia.
Data curation: Shan-Bing Wang, Kai-Jian Lei.
Investigation: Ting Li, Mao-Qiong Jiang.
Methodology: Shan-Bing Wang.
Project administration: Kai-Jian Lei, Yu-Ming Jia.
Writing – original draft: Ting Li, Shan-Bing Wang.
Writing – review & editing: Ting Li, Shan-Bing Wang.
Footnotes
Abbreviations: BBB = blood-brain barrier, BM = brain metastasis, BMs = brain metastases, ORR = overall response rate, OS = overall survival, PFS = progression-free survival, PS = performance status score, TKIs = tyrosine kinase inhibitors, TNBC = triple-negative breast cancer, TNBC-BM = triple-negative breast cancer with brain metastasis.
Shan-Bing Wang contributed equally to this work, and is the co-first author of this article.
The authors have no conflicts of interest to disclose.
References
- [1].Lin NU, Claus E, Sohl J, et al. Sites of distant recurrence and clinical outcomes in patients with metastatic triple-negative breast cancer: high incidence of central nervous system metastases. Cancer 2008;113:2638–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Jin J, Gao Y, Zhang J, et al. Incidence, pattern and prognosis of brain metastases in patients with metastatic triple negative breast cancer. BMC Cancer 2018;18:446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Li J, Qin S, Xu J, et al. Randomized, double-blind, placebo-controlled phase III trial of Apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J Clin Oncol 2016;34:1448–54. [DOI] [PubMed] [Google Scholar]
- [4].Li J, Qin S, Xu J, et al. Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from a randomized, placebo-controlled, parallel-arm, phase II trial. J Clin Oncol 2013;31:3219–25. [DOI] [PubMed] [Google Scholar]
- [5].Li X, Le L, Han L, et al. Short-term efficacy and safety of apatinib in advanced squamous cell carcinoma of the lung. Indian J Cancer 2017;54:547–9. [DOI] [PubMed] [Google Scholar]
- [6].Wu D, Liang L, Nie L, et al. Efficacy, safety and predictive indicators of apatinib after multilines treatment in advanced nonsquamous nonsmall cell lung cancer: apatinib treatment in nonsquamous NSCLC. Asia Pac J Clin Oncol 2018;14:446–52. [DOI] [PubMed] [Google Scholar]
- [7].Hu X, Zhang J, Xu B, et al. Multicenter phase II study of apatinib, a novel VEGFR inhibitor in heavily pretreated patients with metastatic triple-negative breast cancer. Int J Cancer 2014;135:1961–9. [DOI] [PubMed] [Google Scholar]
- [8].Hu X, Cao J, Hu W, et al. Multicenter phase II study of apatinib in non-triple-negative metastatic breast cancer. BMC Cancer 2014;14:820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wang L, Liang L, Yang T, et al. A pilot clinical study of apatinib plus irinotecan in patients with recurrent high-grade glioma: clinical trial/experimental study. Medicine (Baltimore) 2017;96:e9053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Cordon-Cardo C, O’Brien JP, Boccia J, et al. Expression of the multidrug resistance gene product (P-glycoprotein) in human normal and tumor tissues. J Histochem Cytochem 1990;38:1277–87. [DOI] [PubMed] [Google Scholar]
- [11].Gerstner ER, Fine RL. Increased permeability of the blood-brain barrier to chemotherapy in metastatic brain tumors: establishing a treatment paradigm. J Clin Oncol 2007;25:2306–12. [DOI] [PubMed] [Google Scholar]
- [12].Fidler IJ. The biology of brain metastasis: challenges for therapy. Cancer J 2015;21:284–93. [DOI] [PubMed] [Google Scholar]
- [13].Terrell-Hall TB, Nounou MI, El-Amrawy F, et al. Trastuzumab distribution in an in-vivo and in-vitro model of brain metastases of breast cancer. Oncotarget 2017;8:83734–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mutlu H, Buyukcelik A. The combination of weekly trastuzumab plus vinorelbine may be preferable regimen in HER-2 positive breast cancer patients with brain metastasis. J Oncol Pharm Pract 2015;21:310–2. [DOI] [PubMed] [Google Scholar]
- [15].Besse B, Le Moulec S, Mazieres J, et al. Bevacizumab in patients with nonsquamous non-small cell lung cancer and asymptomatic, Untreated Brain Metastases (BRAIN): a nonrandomized, phase II study. Clin Cancer Res 2015;21:1896–903. [DOI] [PubMed] [Google Scholar]
- [16].Hu T, Liu C, Li Q, et al. Apatinib + CPT-11 + S-1 for treatment of refractory brain metastases in patient with triple-negative breast cancer case report and literature review. Medicine (Baltimore) 2018;97:e349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Stewart DJ, Erasmus JJ. Erlotinib accumulation in brain metastases from non-small cell lung cancer: visualization by positron emission tomography in a patient harboring a mutation in the epidermal growth factor receptor. J Thorac Oncol 2011;6:1149–51. [DOI] [PubMed] [Google Scholar]
- [18].Dempke WC, Edvardsen K, Lu S, et al. Brain metastases in NSCLC - are TKIs changing the treatment strategy? Anticancer Res 2015;35:5797–806. [PubMed] [Google Scholar]
- [19].Ballard P, Yates JW, Yang Z, et al. Preclinical comparison of Osimertinib with other EGFR-TKIs in EGFR-mutant NSCLC brain metastases models, and early evidence of clinical brain metastases activity. Clin Cancer Res 2016;22:5130–40. [DOI] [PubMed] [Google Scholar]
- [20].Zhang H, Cao Y, Chen Y, et al. Apatinib promotes apoptosis of the SMMC-7721 hepatocellular carcinoma cell line via the PI3K/Akt pathway. Oncol Lett 2018;15:5739–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Cheng X, Feng H, Wu H, et al. Targeting autophagy enhances apatinib-induced apoptosis via endoplasmic reticulum stress for human colorectal cancer. Cancer Lett 2018;431:105–14. [DOI] [PubMed] [Google Scholar]
- [22].Qiu H, Li J, Liu Q, et al. Apatinib, a novel tyrosine kinase inhibitor, suppresses tumor growth in cervical cancer and synergizes with Paclitaxel. Cell Cycle 2018;17:1235–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Liu K, Ren T, Huang Y, et al. Apatinib promotes autophagy and apoptosis through VEGFR2/STAT3/BCL-2 signaling in osteosarcoma. Cell Death Dis 2017;8:e3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Yano S, Shinohara H, Herbst RS, et al. Expression of vascular endothelial growth factor is necessary but not sufficient for production and growth of brain metastasis. Cancer Res 2000;60:4959–67. [PubMed] [Google Scholar]
- [25].Argaw AT, Asp L, Zhang J, et al. Astrocyte-derived VEGF-A drives blood-brain barrier disruption in CNS inflammatory disease. J Clin Invest 2012;122:2454–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Hu WG, Weng YM, Dong Y, et al. Apatinib in refractory radiation-induced brain edema: a case report. Medicine (Baltimore) 2017;96:e7358. [DOI] [PMC free article] [PubMed] [Google Scholar]


