Supplemental Digital Content is available in the text
Keywords: autonomic nervous system, heart rate control, parasympathetic nervous system, standing position, sympathetic nervous system, thyroid diseases
Abstract
Subclinical hypothyroidism (SCHypo) is associated with autonomic disturbances that can interfere in physiological responses. This study was designed to evaluate linear and nonlinear variables of heart rate variability (HRV) following postural change, comparing subjects with SCHypo to euthyroid subjects.
HRV analyses were performed in 5-minute time series collected in the supine and standing positions from a subsample of 855 participants of the ELSA-Brasil study. The cardiac autonomic nervous function was evaluated by linear time and frequency domain analyses (SDNN, RMSSD, LFms2, HFms2, and LF/HF ratio) as well as by nonlinear symbolic dynamics (0, 1, and 2 V).
After exclusions, 509 (92.0%) euthyroid and 44 (8.0%) SCHypo participants were eligible for analyses. At the baseline supine rest measurement, the 0 V symbolic pattern was higher (27.7 vs 25.4, P = .02) and 2 V was lower (18.0 vs 22.9, P = .02) than in the euthyroid group. Comparing the variation between positions, the 0 V pattern showed a lower delta in SCHypo than in Euthyroid subjects (8.0 vs 10.8%, P = .04).
SCHypo presented lower sympathetic and parasympathetic tonus at rest and a blunted sympathetic response to active postural change, marked by reduced variation in the 0 V of symbolic analysis (SA). Additionally, it is suggested that SA of HR dynamics is an alternative and, possibly, a more sensitive method for cardiac autonomic assessment following orthostatism in this population.
1. Introduction
The thyroid gland and the Autonomic Nervous System (ANS) are closely linked by their controlling center, the hypothalamus, and also by their effects on the cardiovascular system.[1,2] In addition, there are ANS sympathetic fibers from the sympathetic superior ganglion among the fibers innervating the thyroid gland follicular cells.[3]
The ANS is the main controller of the heart. Its sympathetic branch sends stimuli to increase the heart rate (HR), while the parasympathetic pathways decrease the HR. A synchronic and complex mechanism of interactions between the 2 ANS branches produces fluctuations in heartbeat intervals. Generally, the greater the interbeat variations, the better the neurovegetative control and the better the cardiovascular system functionality, representing a good capacity to adapt and respond to internal and external stimuli.[4–6]
As the cardiovascular system is a major target for thyroid hormone action,[6] all aspects of cardiovascular homeostasis are affected by thyroid hormones, including myocardial contractility, cardiac rhythm, peripheral vascular resistance, and blood flow.[7] Autonomic disturbances are common in patients with thyroid diseases, even in subclinical conditions,[8,9] and it has been suggested that clinical impairments in subclinical hypothyroidism (SCHypo) may precede cardiac dysfunctions.[10–12] However, some regulatory function disturbances of the ANS in subclinical thyroid dysfunctions may be slightly perceptible, requiring some stimulus to appear. The orthostatic maneuver is a useful method for evaluating physiological adaptive mechanisms in response to changes in body position. In healthy subjects, there is a shift in autonomic balance from parasympathetic predominance, at rest, to sympathetic control while standing.[12–14] The hypothesis is that the cardiac autonomic response to postural change will be attenuated or absent in subjects with SCHypo. To the best of our knowledge, no other study has analyzed cardiac autonomic control following orthostatism in SCHypo patients.
Heart rate variability (HRV) has been increasingly used as a tool to assess the capacity of the ANS to influence the cardiovascular system, particularly the heartbeats.[15–17] Several computer-assisted procedures have been developed for this purpose, considering linear and nonlinear methods of analyses. The SA, proposed by Porta et al,[18,19] is a nonlinear method that seems to be very appropriate for assessing autonomic modulation. It has the advantage of being suitable for short HR time series, and for providing a robust sympathetic representative index.
This study evaluates the autonomic responses to postural change using linear and nonlinear analysis of heart rate variability (HRV), comparing subjects with SCHypo to euthyroid subjects, using baseline data from the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil).
2. Materials and methods
2.1. Study design
This study is a cross-sectional analysis of the ELSA-Brasil baseline data, collected from August 2008 to December 2010. All subjects were fasting for at least 12 hour and refrained from smoking for approximately 2 hour before ECG recording, which was the time between arriving at the investigation center and proceeding cardiac exams. All recordings were performed between 8.00 am and 1.00 pm Afterward, other physical exams were performed, including blood pressure (BP) and anthropometric measures.
For the HRV analyses, an electrocardiogram (ECG) was always obtained in the morning (between 8:00 am and noon) to avoid circadian differences. Short-term recordings were performed for 10 minutes in the supine position and 10 minutes in the standing position. For the orthostatic maneuver, patients were verbally instructed to stand up, and then to stand still, without moving or talking.
2.2. Participants
The ELSA-Brasil protocol, published in full elsewhere,[20–22] included 15,105 civil servants, aged 35 to 74 years, from 6 institutions of 6 different Brazilian cities. This research is composed of a subsample of 855 participants, from whom HRV data were collected in the supine and standing positions. Participants with not validated HRV, no information about thyroid function, underuse of drugs with thyroid effects, beta-blockers, or any medication that could interfere with thyroid function, such as amiodarone, carbamazepine, carbidopa, phenytoin, furosemide, haloperidol, heparin, interferon, levodopa, lithium, metoclopramide, primidone, rifampicin, and valproic acid, were excluded.[23] Thyroid information of participants with central hypothyroidism or with history of radiotherapy was not included in the databank, therefore, not included in the analysis. In this subsample, no subject reported history of surgical procedures interfering with ANS.
The protocol was approved at all 6 centers by the Institutional Review Boards for research involving human participants, according to the Declaration of Helsinki. Written informed consent was obtained from all the participants.
2.3. Definition of thyroid function
Thyroid stimulating hormone (TSH) and free thyroxine (FT4) were measured by a third generation immunoenzymatic assay (Siemens, Deerfield, IL) in serum obtained from venous blood samples after overnight fasting and centrifugation. To avoid selection bias, TSH was measured in all the participants, and not only in those with previously known TSH dysfunctions. FT4 was only evaluated in participants who presented altered TSH levels. In this study, the SCHypo group was classified based on the use of any medication for the treatment of thyroid disorders, TSH levels above the reference range of 0.4 to 4.0 mIU/l, and FT4 concentration between 0.8 and 1.9 ng/dl (analytical sensitivity of 0.3 ng/dl).
Overt hypothyroidism group was classified based on the use of medication for the treatment of thyroid disorders or TSH levels above the reference range and FT4 concentration lower than 0.8 ng/dl. Subclinical hyperthyroidism subjects were those with TSH under the reference values and FT4 between the normal range and overt hyperthyroidism participants were classified by TSH lower and FT4 higher than the reference range.
This criteria is similar to the criteria used in the National Health and Nutrition Examination Survey (NHANES III)[24] and is recommended by Surks et al[25]
As the purpose of this study is to assess HRV in SCHypo, subjects with overt hypothyroidism, subclinical hyperthyroidism or overt hyperthyroidism were not considered in the analyses.
2.4. Heart rate variability
The protocol used to record and analyze HRV in the ELSA-Brasil study has been published elsewhere.[20] The ECG was obtained in a temperature-controlled room (21–24°C) and was sampled at 250 Hz with a digital electrocardiograph (Micromed, Brazil), consistent with international standards for the measurement of HRV.[5] The WinCardio software (version 4.4a) was used to generate the R-R interval series from a selected lead (usually Lead II) associated with a higher R-wave amplitude. The artifact detection and spectral analytic techniques were the same as those used by Dantas et al,[26] in which the R-R series were automatically preprocessed to remove ectopic beats and artifacts, and linear interpolation was used to replace any removed beats, in accordance with the following criteria: each R–R interval was compared to a reference called R–Raverage. The R–Raverage was calculated as the average of the last 10 normal R–R intervals. R–R intervals smaller than 80% of the R–Raverage or greater than 120% of the R–Raverage were considered as ectopic beats, removed from the R–R series, and replaced by linear interpolation. If R–R series changed by more than 20%, they were excluded from analyses. Participants with nonrecorded or nonvalidated HRV were excluded from analysis.
Short-term HRV analyses were carried out on validated and artifact-free R–R series using MATLAB customized software.[17] For this purpose, 5-minute segments were extracted from each 10-minute R–R series. Based on the criteria of greater stability, a standardized 5-minute segment of data was analyzed from 2 minutes and 30 seconds to 7 minutes and 30 seconds. Each segment was preprocessed aiming to remove trends, subtracting the values of a linear regression function from the R–R series. All the analyses were performed in the supine and standing positions, for both linear (time and frequency domains) and nonlinear methods (symbolic dynamics). The time domain analysis included mean HR (in beats per minute, bpm), standard deviation of NN interval (SDNN, ms), percentage of successive NN differences >50 ms (pNN50, ms) and the root mean square of successive differences between adjacent normal R-R intervals (RMSSD, ms). Frequency domain analysis was carried out using autoregressive modeling, estimated by the Yule–Walker method, using the recursive algorithm of Levinson–Durbin. Then, the high-frequency (HF) (0.15–0.40 Hz), low-frequency (LF) (0.04–0.1 Hz), and LF/HF ratio were calculated.
The nonlinear analysis based on symbolic dynamics was carried out in the same R–R interval sequences as those used for the time and power spectral analysis, as fully described by Porta et al[13] Briefly, the RR series was transformed into a sequence of symbols from 0 to 5, by dividing the range of the values of each sequence into 6 equal segments. Then, all combinations of three symbols were translated into only one of the following 3 possible patterns:
Sequences with no variation 0 V: when all 3 symbols were equal (e.g., 000, 111, 222,...), corresponding to sympathetic cardiac modulation;[13]
Patterns with 1 variation or 1 V: when 2 consecutive symbols were identical and the remnant was different (e.g., 445, 133);
Sequences with 2 variations between successive symbols or 2 V (e.g., 123, 321, 213, or 143) corresponding to parasympathetic cardiac modulation.[27]
At the end, the percentages of 0, 1, and 2 V patterns were computed for the whole sequence. The concept behind this pattern construction is based on different latencies and time courses of the ANS branches, that is slow modulations of sympathetic (less variation of R–R intervals in a period of time) and fast modulations of parasympathetic (more variation of R–R intervals in a period of time).[13,17,19]
2.5. Other measurements
Each participant underwent an interview with strict quality control, conducted by trained personnel, at the Research Center for clinical exams, in accordance with standard protocols.[28] Questionnaires addressed age, sex, and self-reported smoking status. All prescription and over-the-counter pill containers were reviewed for medications taken in the 15 days prior. Height and weight were measured with the participant wearing light clothes, and body-mass index was calculated as weight divided by height in square meters. BP measurements were taken using a validated Omron HEM 705CPINT oscillometric device. Three measurements were taken at 1-minute intervals. The mean of the last 2 BP measurements was considered as the clinical BP.
In this cross-sectional analysis, hypertension was defined as the use of medication to treat hypertension, or systolic BP ≥140 mm Hg, or diastolic BP ≥90 mm Hg.[29] Diabetes was defined as a previous medical history of diabetes, use of medication to treat diabetes, fasting plasma glucose ≥126 mg/dl, as measured by the hexokinase method (ADVIA 1200, Siemens); 2 hours plasma glucose ≥200 mg/dl in the glucose tolerance test; or HbA1C ≥6.5% using high-performance liquid chromatography (HPLC) (Bio-Rad Laboratories, Hercules, CA).
2.6. Statistical analysis
Using Minitab 18 software (Minitab, Paris, France), we calculated a power greater than 80% with an alpha of 0.05 for a sample size of 44 subjects, with a mean difference of 16 ms and standard deviation of ±15 ms in SDNN.
Before statistical analyses, the normality was checked by the Shapiro-Wilk test and the homogeneity of variances by the Levene test. Outliers were assessed by the boxplot method, using “fences” of 3 interquartile ranges above and below the third and the first quartiles as limits for non-outlier observations.[30] Continuous variables were expressed as mean and standard deviations or median and interquartile ranges (IQR). The groups were compared using the non-paired Student t test or Mann–Whitney test, as appropriate, after assessing normality assumptions. Categorical variables were expressed as proportions and compared using the chi-square test.
To compare the groups in relation to the response to the postural change, the delta between positions (HRV variable in the standing position - HRV variable in the supine position) was calculated for each index and analyzed using the Mann–Whitney test, as the data presented non-parametric distribution.
The correlation between the LF/HF ratio and each of the symbolic dynamic patterns (0, 1, and 2 V) was tested using Spearman rank correlation analysis. The association of HRV variables with TSH, FT4, and anti-TPO levels was analyzed by Spearman rank correlation analysis and multivariate linear regression.
All analyses were performed using SPSS 22.0 (IBM, Chicago, IL). All tests were 2-tailed, with a P value of <.05 being considered as significant.
3. Results
3.1. Participant characteristics
After exclusions (missing information about thyroid function: N = 5; overt hypothyroidism N = 86; subclinical hyperthyroidism N = 9; and overt hyperthyroidism N = 4; not validated HRV: N = 101; using medication that alters thyroid function: N = 32; using beta-blocker medication N = 65), from the subsample of 855 participants from whom HRV data were collected in the supine and standing positions, 553 were eligible for analysis. Of this subsample, 509 (92.0%) subjects were euthyroid and 44 (8.0%) SCHypo. The flowchart in Figure 1 shows all the exclusions.
Figure 1.
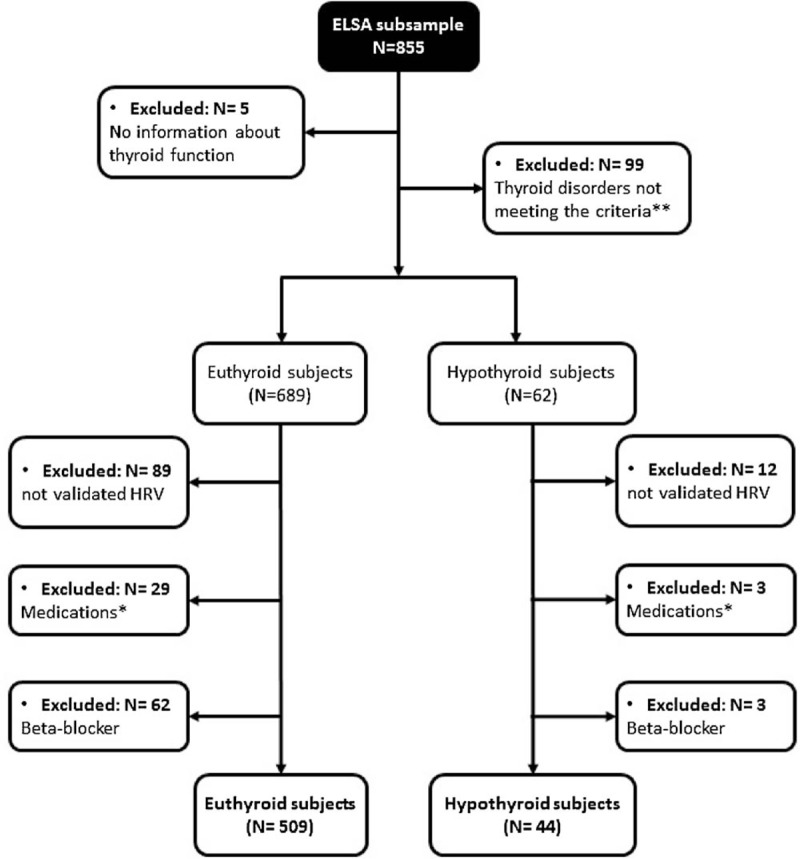
Study Flowchart. ∗Medications (N = 32): drugs with thyroid effects or that could interfere with thyroid function. ∗∗Subclinical Hyperthyroidism (N = 9), Overt Hyperthyroidism (N = 4), Overt Hypothyroidism (N = 86).
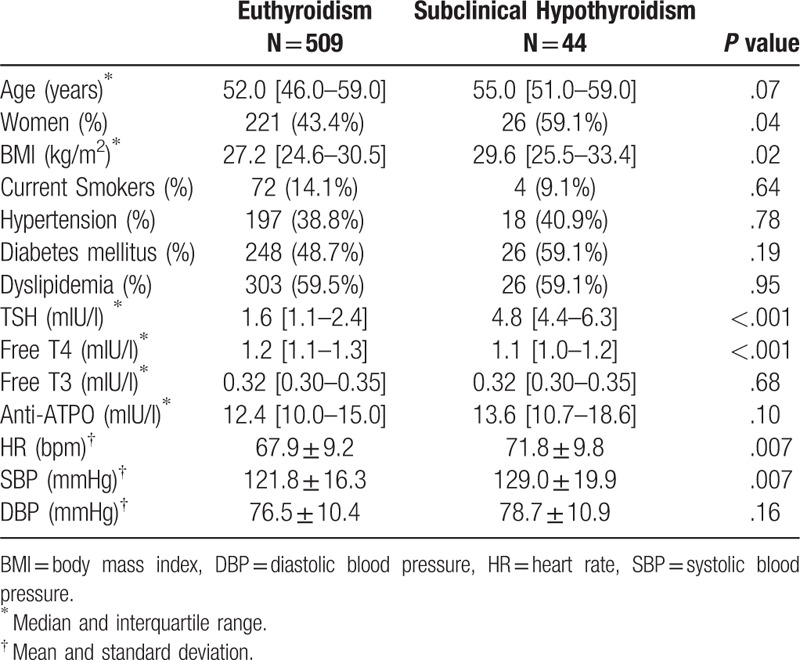
Table 1 shows that the SCHypo group presented higher frequency of women, higher BMI, HR, and systolic BP in comparison to Euthyroid subjects. Both groups were similar in terms of age and clinical characteristics. As expected, statistically significant higher levels of TSH were found in the SCHypo group (P < .001).
Table 1.
Anthropometric, clinical, and cardiovascular characteristics of the sample, comparing subclinical hypothyroidism and euthyroidism.
3.2. Effects of SCHypo on HR variability in the supine position and during active postural change (Table 2)
Table 2.
Median and interquartile range of HRV variables, in the supine and standing, and deltas between positions, according to the presence of subclinical hypothyroidism.
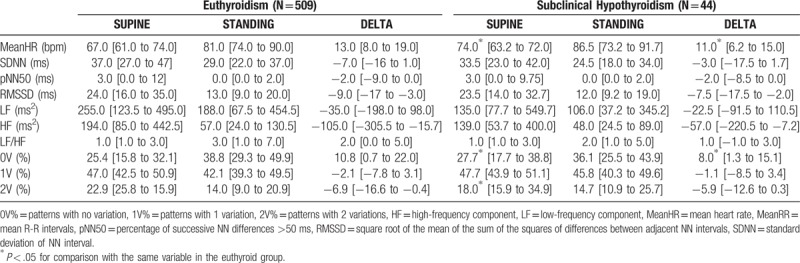
In the supine rest position, SCHypo presented significantly higher 0 V (27.7% vs. 25.4%; P = .02) and lower 2 V (18.0% vs 22.9%; P = .02) patterns than the euthyroidism group. No differences were detected in linear HRV variables (Table 2).
The response to orthostatism was analyzed using delta values between the supine and standing positions, comparing the SCHypo group with the euthyroid group (Table 2). The data show that all indices presented higher deltas in the latter, but only the 0 V pattern indicated a significant difference (14.0% in Euthyroidism vs 6.8% in SCHypo; P = .04).
We performed comparisons between euthyroidism and overt hypothyroidism groups and the results showed that overt hypothyroid subjects presented lower LF in supine (150.0 vs 255, P = .03), lower SDNN (26.0 vs 29.0, P = .04) and higher 1 V (47.1 vs 42.1, P = .01) after standing, and smaller 1 V delta (−1.0 vs −2.1, P = .04) in comparison to euthyroid participants (see Table 1, Supplemental Content, which shows the HRV variables of overt treated and non-treated hypothyroidism subjects).
Considering the SCHypo group, the Spearman rank correlation and the multivariate linear regression analyses detected no correlation between HRV variables and TSH, FT4 and anti-TPO (see Tables 2 and 3, Supplemental Content, which shows the correlation coefficients and multivariate regression for SCHypo subjects). For overt hypothyroidism subjects, only a weak correlation was found between LF/HF ratio and FT4 and the multivariate linear regression showed a relationship between ln (RMSSD) and FT4, which disappear after adjustment for multiple comparisons (see Table 4 and 5, Supplemental Content, which shows the correlation coefficients and multivariate regression for overt hypothyroidism subjects).
In the supine position, Spearman correlation analysis showed that LF/HF ratio presented significant positive correlation with 0 V (r = 0.53, P < .001) and negative correlation with 1 V (r = −0.16, P = < .001) and 2 V (r = −0.50, P < .001). In relation to delta values, this correlation slightly decreased to r = 0.33 (P = <.001), r = −0.13 (P = .001), and r = −0.33 (P < .001), respectively.
4. Discussion
Our results showed a lower variation in cardiac autonomic responses in patients with SCHypo, compared to the normal group. The main finding of this study is that these subjects present autonomic impairments, either in the supine rest position or after postural change.
The SCHypo group showed significantly higher sympathetic and lower parasympathetic modulation at baseline conditions, represented by the 0 V and 2 V patterns, respectively. This condition was also detected by Galetta et al,[11] but using linear indices. In this study, no linear-based HRV variable was able to detect this scenario, suggesting that the nonlinear SA might represent a valid alternative and may be more sensitive for assessing sympathetic and parasympathetic modulations in SCHypo subjects.[13,17]
Although the time and frequency domains are the most explored and studied methods in clinical routines, as well as in scientific research, they have major limitations. Since they provide information about 2 consecutive heartbeat fluctuations around the mean,[31] the beat-to-beat variations are too generalized. As well, analysis of spectral components is described as being susceptible to influence from several biological variables, such as baroreflex[32] and breathing.[33]
In linear phenomena, a change in 1 condition is expected to have a direct and proportional effect on the connected system. However, in physiological systems, this is unlikely to occur and the changes are not proportional, causing major effects or only minor modifications.[34] Organ systems are nonlinear and, in relation to the heart, every beat is at least slightly different from the others.[35] Since linear methods describe only part of HRV, it has been proposed that nonlinear calculations also explain some features of HRV.[17]
Therefore, nonlinear methods of HRV analysis were proposed as a complementary tool to evaluate cardiac autonomic modulation. According to Schumacker (2004),[36] these methods may offer advantages over linear techniques in identifying and quantifying the modulation of 2 interactions among neurocardiac control mechanisms. SA of HR dynamics has the potential to detect these non-proportional fluctuations in autonomic control, that is, nonreciprocal changes in sympathovagal modulations or changes with different magnitudes. This may explain why a significant delta difference is found only in the symbolic dynamic analysis, since changing from the horizontal to the vertical body position does not necessarily promote a shift from parasympathetic predominance to sympathetic control in the same proportion.
Following the orthostatic maneuver, SCHypo participants presented significantly lower variation in the HR and 0 V pattern of SA, which suggests blunted sympathetic modulation. When standing, the HR and cardiac output must increase properly, decreasing the HR variability. This maintains the BP, despite the subtle blood volume transfer to the lower part of the body, seeking to maintain cerebral blood perfusion.[12,37] Thus, HRV behavior in response to postural change has been used as a sensitive measure of autonomic control.[14] Our result shows that the sympathetic activation, necessary to compensate the effects of postural change, does not occur as it does in subjects without thyroid dysfunctions. On the other hand, the parasympathetic modulation while standing does not appear to be affected by isolated high TSH levels, despite the reduced tonus at baseline rest conditions.
According to Carnethon et al,[14] altered HRV responses to postural change can be detected before manifestations of autonomic damage, such as postural hypotension, and may occur early in the course of cardiovascular diseases. Additionally, in the prospective study of Parle et al,[38] out of 76 patients with SCHypo, 30 (40%) individuals developed overt hypothyroidism and began thyroxine replacement treatment during follow-up. In view of this, the results of our HRV analysis in individuals with subclinical hypothyroid reinforces that this is an intermediate state between euthyroidism and overt hypothyroidism, not only in terms of thyroid hormones but also related to the existence of further cardiovascular impairments.[12,39] These findings may be extrapolated to the clinical practice, calling physicians’ attention to early signs of other system dysfunctions as a consequence of thyroid diseases, as well as their non-classical risk factors.
The autonomic impairments in hypothyroidism-related states are mainly related to an increased sympathetic function. The mechanism may be associated with elevated levels of thyrotropin releasing hormone, which directly influences the sympathetic outflow within the central nervous system.[40] However, despite high norepinephrine release from sympathetic nerves, there is a reduced receptor responsiveness to catecholamines at cardiac and peripheral levels.[41,42] Additionally, in hypothyroid states, there is an increased vasomotor tone, since thyroid hormones have profound effects on systemic vascular resistance, which may influence HRV through baroreceptor modulation.[41]
Increased sympathetic activity and altered sensitivity to catecholamine at the receptor level promotes an altered energy metabolism. Thus, lipid profile abnormalities occurred commonly in hypothyroidism [43,44] may contribute to even higher sympathovagal imbalance. This probably is the link between coronary artery disease and hypothyroidism, which adds further dimensions to the cardiovascular significance of the thyroid disturbances.[43,45,46] Moreover, an impaired parasympathetic drive with an augmented sympathetic tone, as verified in SCHypo subjects, is a favorable scenario for hypertension prevalence or development.[47] This leads to a direct effect on peripheral arterial resistance and possibly increases renin level and activity to increase BP.[48] All the exposed reinforces that even subclinical dysfunctions must have physician's attention regarding cardiovascular impairments.
Correlation analysis between LF/HF ratio and symbolic dynamic variables showed a positive correlation with 0 V and negative correlation with 2 V in the supine position and in relation to delta values. The correlation coefficients were basically equal for both indices, which suggests that this ratio did not reflect the shift to sympathetic dominance and being consistent with Billman et al,[49] who concluded that LF/HF data cannot accurately quantify cardiac sympathovagal balance either in health or disease.
We must explain that overt hypothyroid subjects were not included in the main analysis of this study because almost the entire sample was under treatment. The ELSA-Brasil cohort's participants have wide access to health systems, thus 59 out of 64 subjects with overt hypothyroid (92%) have been treated by levothyroxine. Only 5 (8%) were newly diagnosed untreated patients and were oriented to search for an endocrinologist. Therefore, as it has been reported that the replacement treatment with levothyroxine may cause an improvement in autonomic abnormalities following euthyroidism restoration,[42,50–52] it is not possible to conclude whether the results represent the medication effect or the hypothyroid state dysfunction itself.
The main factor that differentiates this study from others in the literature is the size of the total sample. Participants enrolled in the ELSA-Brasil study were apparently healthy subjects, making this sample more reflective of the country's population, as it is not restricted to a clinical sample from a general practice or specialized service.
As limitations of this study, we include the absence of postural orthostatic tachycardia syndrome data and BP measurements following orthostatism, which could provide information about circulatory disorders and postural hypotension, providing a more precise source of data about other clinical outcomes. Additionally, other maneuvers were not performed in this data collection, such as the Valsalva maneuver, controlled respiration rate, hand grip test, cold pressor test, and others that could have improved the autonomic function assessment in this population.
In conclusion, this study contributes additional evidence of cardiovascular variability during an autonomic maneuver in SCHypo disease. Subjects with SCHypo presented higher sympathetic and lower vagal tonus at supine rest, with blunted sympathetic autonomic responses to active postural change. Additionally, the SA of HR dynamics is found to be a possible alternative and, perhaps, a more sensitive method for cardiac autonomic assessment at rest and during physiological stress in this population.
Acknowledgments
We are very grateful to all ELSA-Brasil participants and research staff.
Author contributions
Conceptualization: Rosangela Akemi Hoshi, Eduardo M Dantas, Isabela Bensenor.
Data curation: Rosangela Akemi Hoshi, Rodrigo V Andreao, Itamar S Santos, Eduardo M Dantas, Jose G Mill, Paulo A Lotufo.
Formal analysis: Rosangela Akemi Hoshi, Itamar S Santos, Jose G Mill, Isabela Bensenor.
Funding acquisition: Jose G Mill, Paulo A Lotufo, Isabela Bensenor.
Investigation: Rosangela Akemi Hoshi, Rodrigo V Andreao, Eduardo M Dantas, Paulo A Lotufo, Isabela Bensenor.
Methodology: Rosangela Akemi Hoshi, Rodrigo V Andreao, Itamar S Santos, Eduardo M Dantas, Jose G Mill, Paulo A Lotufo, Isabela Bensenor.
Project administration: Rosangela Akemi Hoshi.
Supervision: Rosangela Akemi Hoshi, Itamar S Santos, Eduardo M Dantas, Isabela Bensenor.
Writing – original draft: Rosangela Akemi Hoshi, Isabela Bensenor.
Writing – review & editing: Rosangela Akemi Hoshi, Rodrigo V Andreao, Eduardo M Dantas, Jose G Mill, Paulo A Lotufo, Isabela Bensenor.
Rosangela Akemi Hoshi orcid: 0000-0002-4034-0451.
Rosangela Akemi Hoshi orcid: 0000-0002-4034-0451.
Supplementary Material
Footnotes
Abbreviations: 0V = patterns with no variation, 1V = patterns with one variation, 2V = patterns with two variations, ANS = Autonomic Nervous System, BP = blood pressure, ECG = Electrocardiogram, FT3 = free triiodothyronine, FT4 = free thyroxine, HF = high frequency, HR = heart rate, HRV = Heart rate variability, LF = low frequency, pNN50 = percentage of successive NN differences > 50 ms, RMSSD = root mean square of successive differences between adjacent normal R-R intervals, RRi = R-R intervals, SA = symbolic analysis, SCHypo = subclinical hypothyroidism, SDNN = standard deviation of consecutive RRi, TSH = thyroid stimulating hormone.
This study was funded by the Brazilian Ministry of Health (Science and Technology Department) and the Brazilian Ministry of Science and Technology (FINEP and CNPq) (grant numbers 01 06 0010.00 RS, 01 06 0212.00 BA, 01 06 0300.00 ES, 01 06 0278.00, MG, 01 06 0115.00 SP, and 01 06 0071.00 RJ). This sub-study was also funded by the Sao Paulo Research Foundation, Proc Fapesp no. 2015/17213–2.
Supplemental Digital Content is available for this article.
The authors declare that they have no conflict of interest.
References
- [1].Bhat AN, Kalsotra L, Yograj S. Autonomic reactivity with altered thyroid status. JK Sci 2007;9:70–4. [Google Scholar]
- [2].Reeves JW, Fisher AJ, Newman MG, et al. Sympathetic and hypothalamic-pituitary-adrenal asymmetry in generalized anxiety disorder. Psychophysiology 2016;53:951–7. doi:10.1111/psyp.12634. [DOI] [PubMed] [Google Scholar]
- [3].Sundler F, Grunditz T, Håkanson R, et al. Innervation of the thyroid. A study of the rat using retrograde tracing and immunocytochemistry. Acta Histochem 1989;37:191–8. [PubMed] [Google Scholar]
- [4].Billman GE. Heart rate variability - a historical perspective. Front Physiol 2011;2:1–3. doi:10.3389/fphys.2011.00086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Task Force of ESC and NASPE. Heart rate varibility: standards of measurement, physiological interpretationand clinical use. Circulation 1996;93:1043–65. doi:10.1161/01.CIR.93.5.1043. [PubMed] [Google Scholar]
- [6].Maor E, Kivity S, Kopel E, et al. Differences in heart rate profile during exercise among subjects with subclinical thyroid disease. Thyroid 2013;23:1226–32. doi:10.1089/thy.2013.0043. [DOI] [PubMed] [Google Scholar]
- [7].Petretta M, Bonaduce D, Spinelli L, et al. Cardiovascular haemodynamics and cardiac autonomic control in patients with subclinical and overt hyperthyroidism. Eur J Endocrinol 2001;145:691–6. doi:10.1530/eje.0.1450691. [DOI] [PubMed] [Google Scholar]
- [8].Pearce EN. Update in Lipid Alterations in Subclinical Hypothyroidism. J Clin Endocrinol Metab 2012;97:326–33. doi:10.1210/jc.2011-2532. [DOI] [PubMed] [Google Scholar]
- [9].Miranda ÉJFP, Hoshi RA, Bittencourt MS, et al. Relationship between heart rate variability and subclinical thyroid disorders of the Brazilian Longitudinal Study of Adult Health (ELSA-Brasil). Brazilian J Med Biol Res 2018;51:1–8. doi:10.1590/1414-431x20187704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Galetta F, Franzoni F, Fallahi P, et al. Changes in autonomic regulation and ventricular repolarization induced by subclinical hyperthyroidism. Biomed Pharmacother 2010;64:546–9. doi:10.1016/j.biopha.2009.10.001. [DOI] [PubMed] [Google Scholar]
- [11].Galetta F, Franzoni F, Fallahi P, et al. Heart rate variability and QT dispersion in patients with subclinical hypothyroidism. Biomed Pharmacother 2006;60:425–30. doi:10.1016/j.biopha.2006.07.009. [DOI] [PubMed] [Google Scholar]
- [12].Goichot B, Brandenberger G, Vinzio S, et al. Sympathovagal response to orthostatism in overt and in subclinical hyperthyroidism. J Endocrinol Invest 2004;27:348–52. [DOI] [PubMed] [Google Scholar]
- [13].Porta A, Tobaldini E, Guzzetti S, et al. Assessment of cardiac autonomic modulation during graded head-up tilt by symbolic analysis of heart rate variability. Am J Physiol Heart Circ Physiol 2007;293:H702-8.doi:10.1152/ajpheart.00006.2007. [DOI] [PubMed] [Google Scholar]
- [14].Carnethon MR, Liao D, Evans GW, et al. Correlates of the shift in heart rate variability with an active postural change in a healthy population sample: the atherosclerosis risk in communities study. Am Heart J 2002;143:808–13. doi:10.1067/mhj.2002.121928. [DOI] [PubMed] [Google Scholar]
- [15].Bravi A, Longtin A, Seely AJ. Review and classification of variability analysis techniques with clinical applications. Biomed Eng Online 2011;10:90doi:10.1186/1475-925X-10-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Francesco B, Maria Grazia B, Emanuele G, et al. Linear and nonlinear heart rate variability indexes in clinical practice. Comput Math Methods Med 2012;2012:219080doi:10.1155/2012/219080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Dantas EM, Andreao RV, da Silva VJ, et al. Comparison between symbolic and spectral analyses of short-term heart rate variability in a subsample of the ELSA-Brasil study. Physiol Meas 2015;36:2119–34. doi:10.1088/0967-3334/36/10/2119. [DOI] [PubMed] [Google Scholar]
- [18].Porta A, Guzzetti S, Montano N, et al. Entropy, entropy rate, and pattern classification as tools to typify complexity in short heart period variability series. IEEE Trans Biomed Eng 2001;48:1282–91. [DOI] [PubMed] [Google Scholar]
- [19].Guzzetti S, Borroni E, Garbelli PE, et al. Symbolic dynamics of heart rate variability: a probe to investigate cardiac autonomic modulation. Circulation 2005;112:465–70. doi:10.1161/CIRCULATIONAHA.104.518449. [DOI] [PubMed] [Google Scholar]
- [20].Mill JG, Pinto K, Griep RH, et al. Medical assessments and measurements in ELSA-Brasil. Rev Saude Publica 2013;47:54–62. doi:10.1590/S0034-8910.2013047003851. [DOI] [PubMed] [Google Scholar]
- [21].Aquino EML, Barreto SM, Bensenor IM, et al. Brazilian longitudinal study of adult health (ELSA-Brasil): objectives and design. Am J Epidemiol 2012;175:315–24. doi:10.1093/aje/kwr294. [DOI] [PubMed] [Google Scholar]
- [22].Schmidt MI, Duncan BB, Mill JG, et al. Cohort profile: longitudinal study of adult health (ELSA-Brasil). Int J Epidemiol 2015;44:68–75. doi:10.1093/ije/dyu027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Singer P. Braverman LE, Cooper D. Primary Hipothyroidism due to other causes. Werner & Ingbar's The Thyroid: A Fundamental and Clinical Text. Philadelphia: Lippincott–Williams & Wilkins; 2005. 553–5. [Google Scholar]
- [24].Hollowell JG, Staehling NW, Flanders WD, et al. Serum TSH, T 4, and thyroid antibodies in the United States population (1988 to 1994): national health and nutrition examination survey (NHANES III). J Clin Endocrinol Metab 2002;87:489–99. doi:10.1210/jcem.87.2.8182. [DOI] [PubMed] [Google Scholar]
- [25].Surks MI, Ortiz E, Daniels GH, et al. Subclinical thyroid disease: scientific review and guidelines for diagnosis and management. JAMA 2004;291:228–38. doi:10.1001/jama.291.2.228. [DOI] [PubMed] [Google Scholar]
- [26].Dantas E, Sant’Anna ML, Andreão RV, et al. Spectral analysis of heart rate variability with the autoregressive method: what model order to choose? Comput Biol Med 2012;42:164–70. doi:10.1016/j.compbiomed.2011.11.004. [DOI] [PubMed] [Google Scholar]
- [27].Porta A, D’Addio G, Pinna G, et al. Symbolic analysis of 24 h holter heart period variability series: comparison between normal and heart failure patients. Comput Cardiol 2005;32:575–8. [Google Scholar]
- [28].Bensenor IM, Griep RH, Pinto KA, et al. Rotinas de organizacao de exames e entrevistas no centro de investigacao ELSA-Brasil. Rev Saude Publica 2013;47suppl 2:37–47. doi:10.1590/S0034-8910.2013047003780. [DOI] [PubMed] [Google Scholar]
- [29].Chor D, Luiz Pinho Ribeiro A, Sá Carvalho M, et al. Prevalence, awareness, treatment and influence of socioeconomic variables on control of high blood pressure: results of the ELSA-Brasil study. PLoS One 2015;10:e0127382doi:10.1371/journal.pone.0127382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Brunoni AR, Kemp AH, Dantas EM, et al. Heart rate variability is a trait marker of major depressive disorder: evidence from the sertraline vs. electric current therapy to treat depression clinical study. Int J Neuropsychopharmacol 2013;16:1937–49. doi:10.1017/S1461145713000497. [DOI] [PubMed] [Google Scholar]
- [31].Stein PK, Domitrovich PP, Huikuri HV, et al. Traditional and nonlinear heart rate variability are each independently associated with mortality after myocardial infarction. J Cardiovasc Electrophysiol 2005;16:13–20. doi:10.1046/j.1540-8167.2005.04358.x. [DOI] [PubMed] [Google Scholar]
- [32].Goldstein DS, Bentho O, Park M-Y, et al. Low-frequency power of heart rate variability is not a measure of cardiac sympathetic tone but may be a measure of modulation of cardiac autonomic outflows by baroreflexes. Exp Physiol 2011;96:1255–61. doi:10.1113/expphysiol.2010.056259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Malliani A, Pagani M, Lombardi F, et al. Cardiovascular neural regulation explored in the frequency domain. Circulation 1991;84:482–92. [DOI] [PubMed] [Google Scholar]
- [34].Ernst G. Heart-rate variability—more than heart beats? Front Public Health 2017;5:1–2. doi:10.3389/fpubh.2017.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Piskorski J, Guzik P. Filtering Poincaré plots. Comput Methods Sci Technol 2005;11:39–48. doi:10.12921/cmst.2005.11.01.39-48. [Google Scholar]
- [36].Schumacher A. Linear and nonlinear approaches to the analysis of R-R interval variability. Biol Res Nurs 2004;5:211–21. doi:10.1177/1099800403260619. [DOI] [PubMed] [Google Scholar]
- [37].Akatsu J, Kumashiro M, Miyake S, et al. Differences in heart rate variability between young and elderly normal men during graded head up tilt. Ind Health 1999;37:68–75. doi:10.2486/indhealth.37.68. [DOI] [PubMed] [Google Scholar]
- [38].Parle JV, Maisonneuve P, Sheppard MC, et al. Franklyn J a. Prediction of all-cause and cardiovascular mortality in elderly people from one low serum thyrotropin result: a 10-year cohort study. Lancet 2001;358:861–5. doi:10.1016/S0140-6736(01)06067-6. [DOI] [PubMed] [Google Scholar]
- [39].Luboshitzky R, Aviv A, Herer P, et al. Risk factors for cardiovascular disease in women with subclinical hypothyroidism. Thyroid 2002;12:421–5. doi:10.1089/105072502760043512. [DOI] [PubMed] [Google Scholar]
- [40].Polikar R, Burger A, Scherrer U, et al. The Thyroid and the Heart. Circulation 1993;87:1435–41. doi:10.1161/01.CIR.87.5.1435. [DOI] [PubMed] [Google Scholar]
- [41].Cacciatori V, Gemma ML, Bellavere F, et al. Power spectral analysis of heart rate in hypothyroidism. Eur J Endocrinol 2000;143:327–33. doi: 10.1530/eje.0.1430327. [DOI] [PubMed] [Google Scholar]
- [42].Cacciatori V, Bellavere F, Pezzarossa A, et al. Power Spectral Hyperthyroidism. J Clin Endocrinol Metab 1996;81:2828–35. [DOI] [PubMed] [Google Scholar]
- [43].O’Brien T, Katz K, Hodge D, et al. The effect of the treatment of hypothyroidism and hyperthyroidism on plasma lipids and apolipoproteins AI, AII and E. Clin Endocrinol (Oxf) 1997;46:17–20. doi:10.1046/j.1365-2265.1997.d01-1753.x. [DOI] [PubMed] [Google Scholar]
- [44].Heemstra KA, Burggraaf J, Van Der Klaauw AA, et al. Short-term overt hypothyroidism induces sympathovagal imbalance in thyroidectomized differentiated thyroid carcinoma patients. Clin Endocrinol (Oxf) 2010;72:417–21. doi:10.1111/j.1365-2265.2009.03655.x. [DOI] [PubMed] [Google Scholar]
- [45].Mustafa G, Kursat FM, Ahmet T, et al. The relationship between erythrocyte membrane fatty children. Rev Port Cardiol 2017;36:499–508. doi:10.1016/j.repc.2016.10.013. [DOI] [PubMed] [Google Scholar]
- [46].Kahaly GJ. Cardiovascular and atherogenic aspects of subclinical hypothyroidism. Thyroid 2000;10:665–79. doi:10.1089/10507250050137743. [DOI] [PubMed] [Google Scholar]
- [47].Wu JS, Lu FH, Yang YC, et al. Epidemiological study on the effect of pre-hypertension and family history of hypertension on cardiac autonomic function. J Am Coll Cardiol 2008;51:1896–901. doi:10.1016/j.jacc.2007.12.053. [DOI] [PubMed] [Google Scholar]
- [48].Liao D, Cai J, Barnes RW, et al. Association of cardiac autonomic function and the development of hypertension - the ARIC study. Am J Hypertens 1996;7061:1147–56. [DOI] [PubMed] [Google Scholar]
- [49].Billman GE. The LF/HF ratio does not accurately measure cardiac sympatho-vagal balance. Front Physiol 2013;20:26doi:10.3389/fphys.2013.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Galetta F, Franzoni F, Fallahi P, et al. Changes in heart rate variability and QT dispersion in patients with overt hypothyroidism. Eur J Endocrinol 2008;158:85–90. doi:10.1530/EJE-07-0357. [DOI] [PubMed] [Google Scholar]
- [51].Inukai T, Kobayashi I, Kobayashi T, et al. Parasympathetic nervous system activity in hypothyroidism determined by R-R interval variations on electrocardiogram. J Intern Med 1990;228:431–4. doi:10.1111/j.1365-2796.1990.tb00259.x. [DOI] [PubMed] [Google Scholar]
- [52].Bhat AN, Yograj S, Bahl R. Alteration in autonomic reactivity from hypothyroid to euthyroid status. Indian J Clin Anat Physiol 2016;3:377doi:10.5958/2394-2126.2016.00086.4. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


