Abstract
We developed a robust analytical method for quantification of malondialdehyde (MDA) in exhaled breath condensate (EBC) via derivatization with 2,4-dinitrophenylhydrazine (DNPH). The target MDA-DNPH hydrazone was separated by ultra-performance liquid chromatography using two reversed-phase analytical columns (C18 and phenyl-hexyl) inter-connected via a two-position, six-port switching valve to a single-quadrupole mass spectrometer. The target derivative was analyzed under positive electrospray ionization using single ion monitoring mode (m/z = 235 for the target derivative, and m/z =237 for its labeled isotopic analog). This pseudo two-dimensional chromatographic separation provided optimum separation conditions for the target derivative resulting in the limit of detection of 0.58 nM in EBC sample (or 36.2 pmol on-column amount), which is comparable to those reported previously using different techniques, including tandem mass spectrometry. Based on the calibration solutions, the method had a linear quantification range of 1.0–200 nM (r2 =0.998). The method showed good relative recoveries (92.2–102.0%) and acceptable precisions (3.6–12.2% for inter-day precision, and 4.3–12.4% for intra-day precision for two quality control levels, prepared from 5nM and 25nM solutions). The derivative was found to be stable at room temperature for 48 h or during analysis. The method was used to analyze 205 exhaled breath condensate samples collected from individuals from a healthy population of student athletes. MDA was detected in approximately 95% of these samples, with concentrations ranging from 1.16 to 149.63nM. The median concentration was 6.82 nM, (IQR 4.08–9.88). These data demonstrate that our method can be successfully used to measure MDA in population studies.
1. Introduction
Malondialdehyde (MDA) is perhaps the most extensively studied end-product of polyunsaturated-lipid peroxidation under radical-induced oxidative stress conditions [1–8]. MDA has been linked to oxidative stress-related health problems including chronic obstructive pulmonary disease (COPD), asthma, and cardiovascular diseases [6,9,10]. For example, increased levels of MDA in exhaled breath condensate (EBC), condensed exhalate from airway lining fluid, were observed in patients with asthma, COPD, idiopathic pulmonary fibrosis, and cystic fibrosis compared to healthy non-smokers [6,10,11]. Concentrations of MDA in EBC and urine were shown to be correlated with air pollution levels [5,12], while increased levels of MDA in EBC were associated with exposures to aerosols of nano-iron oxide [13] and nanotitanium dioxide [14] among workers. MDA concentrations were also associated with changes in lung function and inflammatory markers of individuals exposed to traffic related pollution [15]. In addition, treatment of asthma patients with anti-inflammation therapy revealed a decrease in MDA levels in EBC to those that are similar to non-asthmatic control subjects, suggesting a direct link between MDA levels and oxidative stress [16].
The majority of previous MDA measurement methods required binding of MDA with a derivatizing agent to facilitate liquid chromatographic separation and/or to allow detection using ultraviolet/visible absorption or fluorescence techniques because of the physicochemical properties of MDA (e.g., high reactivity, absence of fluorescence, and low molecular weight) [9,17]. Thiobarbituric acid has been extensively used in the analysis of MDA as a principal derivatizing agent [9]. However, the conditions of this derivatization are relatively harsh, resulting in an induction of lipid peroxidation that artificially releases more MDA into the sample’s solution. Such processes contribute to overestimation of MDA levels, and therefore complicate the interpretation and cross-validation of the results [9,17]. To prevent this issue, other derivatizing agents requiring milder conditions, have been introduced. These include 2,4-dinitrophenylhydrazine (DNPH) [3,9], diaminonaphthalene, hydralazine [18], and dansylhydrazide [19]. Still, derivatizing MDA with these agents has often resulted in poor selectivity and sensitivity, particularly with spectrophotometric and/or spectrofluorometric instruments. The limits of detection (LODs) of these methods were reported to be as high as 2.1 μM [17].
To achieve the desired sensitivity and LODs for the analysis of trace amounts of endogenous MDA, use of highly sensitive and selective instruments such as tandem mass spectrometers is preferred [20]. With tandem mass spectrometers, quantification of MDA could be conducted without derivatization [20], although derivatization is still commonly used. Chen et al. quantified the urinary MDA-DNPH derivative using a liquid chromatography-tandem mass spectrometer, but noted the lack of reproducibility of their method due to the solid phase extraction approach [21]. Also, methods based on the derivatization of MDA with pentafluorobenzyl bromide were proposed for quantification using gas chromatography coupled with single and tandem mass spectrometers with reported LODs as low as 2 amol or (2 × 10−18) mol [22]. Only a few methods have been reported that analyze MDA in EBC.
Although tandem mass spectrometers are suitable for the analysis of MDA in various biological matrices (e.g., urine, serum, and EBC), lack of instrument availability, financial resources, and/or operating expertise may limit their use, especially in low- and middle-income countries. This makes the biological analysis of MDA at low levels difficult. Therefore, we aimed to develop an alternative method using a single quadrupole mass spectrometer to analyze MDA in EBC samples. Although this instrument alone may not offer parallel selectivity to tandem mass spectrometers, it surpasses the capabilities of ultraviolet/visible absorption or fluorescence instruments using an instrument much less expensive than a tandem mass spectrometer. To improve the sensitivity and selectivity of the single-quadrupole mass spectrometer, we derivatized MDA with DNPH and separated this derivative using pseudo two-dimensional liquid chromatography, combining phenylhexyl and C18 analytical columns via an existing two-position/six-port (2P/6P) valve on the mass spectrometer. To control for potential matrix effects [23], isotope dilution quantification was used. The method was validated using pooled EBC samples and tested for its suitability by analyzing 205 individual human EBC samples.
2. Materials and methods
2.1. Chemicals and reagents
We obtained acetonitrile (HPLC grade), formic acid (88%, laboratory grade), and ethanol (200 proof) from Fisher Scientific (Hampton, NH, USA). Hydrochloric acid (30%, for ultratrace analysis), malondialdehyde tetrabutylammonium salt (96%, neat), 2,4-dinitrophenylhydrazine (97%, reagent grade), and 3,5-di-tert-4-butylhydroxytoluene (BHT) analytical standard were obtained from Sigma-Aldrich (Saint Louis, MO, USA). The internal standard, 1,1,3,3-tetraethoxypropane-1,3,-D2 (D2-TEP), was purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Water was purified using an EMD Millipore Milli-Q Ultrapure water purification system (Billerica, MA, USA).
2.2. Preparation of standard, quality control, and labeled internal standard solutions
High concentration standard stock and quality control solutions were prepared by weighing a known amount of neat standard then diluting with acetonitrile (Stock I solution). This stock solution was used to prepare an 800 nM stock solution in water weekly (Stock II solution). The stock I and II solutions were stored at −20 °C. Eightpoint calibration solutions were prepared daily from corresponding the stock II solution in Milli-Q® water. The concentrations of the calibration solutions were as follows (1.0, 2.5, 5.0, 10, 25, 50, 100, and 200 nM).
Quality control solutions (prepared at concentrations of 5.0 and 25 nM) were prepared separately in Milli-Q® water from the corresponding stock II solution. Labeled internal standard (IS) stock solution was prepared by hydrolysis of the D2-TEP standard according to a previously published protocol [24]. This labeled IS stock solution was diluted with Milli-Q® water to yield a concentration of 250 nM.
2.3. Preparation of blank, calibration, quality control, and unknown samples
EBC samples were preserved with the anti-oxidizing agent, butylated hydroxytoluene (BHT), prepared via dilution of 25 mg of BHT in 10 mL ethanol. Sample vials were pre-loaded with 5 μL of the BHT solution, and then a 250 μL aliquot of EBC was added immediately following collection. Samples were then stored at −80 °C prior to analysis. Each unknown sample was prepared according to the following procedure. First, 20 μL of labeled IS solution was added to a 250 μL glass vial insert which was placed inside a 2.5 mL amber auto-sampler vial. Second, 100 μL of unknown EBC sample was added to the same vial, followed by 20 μL of 10 mM DNPH in acetonitrile and 20 μL of 1.5 M hydrochloric acid solution, prepared in Milli-Q® water from 30% hydrochloric acid solution. Third, the vial was capped, vortex mixed, and incubated at 37 °C for 70 min.
For the calibration curve and quality control samples, 100 μL of the respective solutions was used in lieu of unknown sample. A blank sample was also prepared in a similar manner as the unknown samples, except that 100 μL of Milli-Q® water was used instead. All other chemicals remained the same. In each analytical batch, an eight-point calibration curve, two quality control samples, a blank, and 40 unknown samples were prepared together.
After derivatization, the final volume of the calibration and quality control samples was 160 μL, representing a dilution factor of 1.6 of the samples. This results in final concentrations of MDA in calibration samples of 0.625, 1.56, 3.12, 6.25, 15.6, 31.2, 62.5, and 125 nM. Similarly, the final concentrations of MDA in the quality control samples were 3.12 and 15.6 nM. It should be noted here that a 100 μL aliquot of the EBC samples and the calibration solutions undergo the same derivatization procedure resulting in the same dilution factor of 1.6. Thus, the original concentrations of the calibration solutions can be used and the final reporting units of MDA concentrations were nM or nmol/L of EBC sample.
2.4. Chromatographic separation and mass spectrometric conditions
The sample analysis was carried out using an Agilent Infinity 1290 ultra-performance liquid chromatograph (UPLC) with a G4226A auto-sampler connected to an Agilent G6150B single quadrupole mass spectrometer (Santa Clara, CA, USA). The separation was performed on the following to Agilent columns: Eclipse Plus C18 RRHD (1.8 μm 2.1 × 50 mm) and Eclipse Plus Phenyl-Hexyl (1.8 μm 2.1 × 50 mm). The C18 column temperature was set to 40 °C, while the phenyl-hexyl column temperature was set to 25 °C.
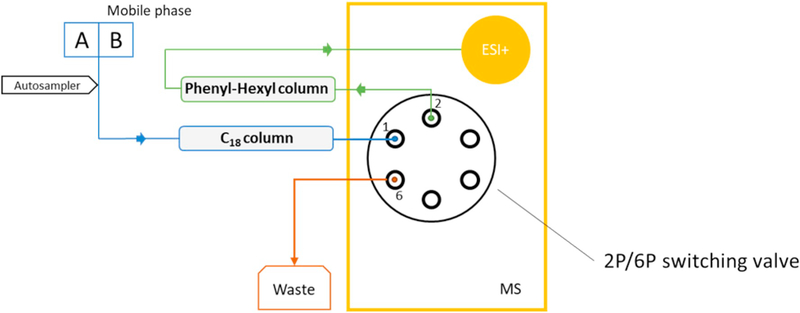
An injector program was used to facilitate injection of the samples. It was essential that the needle was washed with acetonitrile prior to sample uptake. For analysis of the EBC samples, 20 μL of each sample was injected five times into the needle loop and mixed. With this procedure, a total of 100 μL of sample volume was injected into the columns. The binary mobile phase consisted of A: Milli-Q® water with 0.05% v/v formic acid and B: acetonitrile without additives. Separation was achieved via a gradient elution program (Table 1). The total program runtime was 14.5 min. Fig. 1 illustrates the configuration of the chromatographic setup and the 2P/6P switching valve.
Table 1.
Graduated elution liquid chromatography program for analysis of malondialdehyde-2,4-dinitrophenylhydrazine in exhaled breath condensate samples.
| Time, min | Acetonitrile composition, % | Flow rate, mL/min | Valve position | Time, min | Acetonitrile composition (%) | Flow rate, mL/min | Valve position |
|---|---|---|---|---|---|---|---|
| 0.0 | 3.0 | 0.5 | To waste | 10.7 | 100 | 0.3 | To MS |
| 4.5 | 27.0 | 0.5 | To waste | 11.0 | 100 | 0.3 | To MS |
| 5.0 | 29.6 | 0.4 | To waste | 11.7 | 100 | 0.5 | To MS |
| 6.2 | 36.0 | 0.37 | To MS | 12.9 | 100 | 0.5 | To MS |
| 7.2 | 37.6 | 0.35 | To MS | 13.0 | 51 | 0.5 | To MS |
| 7.5 | 38.0 | 0.3 | To MS | 13.1 | 3.0 | 0.4 | To MS |
| 10.5 | 42.7 | 0.3 | To MS | 14.0 | 3.0 | 0.4 | To waste |
Note: During chromatographic separation, a binary mobile phase composition consisting of Milli-Q® water with 0.05% formic acid (v/v) and acetonitrile without additives was used.
Fig. 1.
Column switching configuration using an existing 2-position/6-port (2P/6P) switching valve located on a mass spectrometer.
Note: A and B represent mobile phase compositions, A: Milli-Q® water with 0.05% v/v formic acid, B: 100% acetonitrile without additives. Initial separation of malondialdehyde (MDA) from interference compounds is performed on a C18 column, where the 2P/6P valve is switched to the waste position (1 to 6). At the approximate time of elution of MDA from the C18 column, the 2P/6P valve is switched to the MS position (1 to 2) to facilitate separation of MDA on the phenyl-hexyl column and further analysis on the mass spectrometer.
The target derivative and its labeled IS derivative were ionized using positive electrospray ionization and analyzed using a mass spectrometer in single ion monitoring (SIM) mode. Capillary and nozzle voltages were set to 4000 V and 800 V, respectively. Nitrogen (99%), generated in-house using a generator from Peak Scientific Instruments Ltd. (Inchinnan, Scotland, UK), was used as a drying and sheath gas. The flow rates were set to 8.0 L/min and 12.0 L/min, respectively. Drying gas and sheath gas temperatures were set to 350 °C and 360 °C, respectively. The nebulizer pressure was set to 10 psi. The fragmentor voltage was set to 120 V, while the dwell time was set to 444 ms. The MDA-DNPH derivative was monitored at m/z of 235, while the D2-MDA-DNPH derivative was monitored at m/z of 237. The peak of the target derivative was selected based on its retention time (RT), relative retention time to labeled IS peak, peak shape, and signal-to-nose ratio.
2.5. Determination of limit of blank (LOB), LOD, and selection of the lowest calibration point
The LOB and LOD were determined based on a method described by Armbruster and Pry [25]. Briefly, the LOB was estimated through the measurement of five replicates of blank samples on five different days (total of 25 samples). The LOB was calculated using the Eq. (1) below.
| (1) |
To calculate the LOD, a set of non-replicate measurements of the lowest calibration level (prepared from the 1.0 nM solution), over 17 days, was used. The standard deviation was calculated from this set of data. The LOD was then calculated according to the Eq. (2) below.
| (2) |
2.6. Method validation
The method precision was determined according to the protocol described by Chesher [26] using two quality control levels (QCL and QCH). Replicate samples (5 samples/day at each concentration level) were prepared and analyzed daily over a 5-day period to determine inter-day and intra-day precision. The precisions are expressed as relative standard deviation (% RSD) values.
The method relative recoveries (RR) were determined, according to FDA guidelines, using a set of replicate quality control materials (QCL and QCH) prepared from the standard solutions prepared at 5.0 and 25 nM (N = 25). The RR were calculated by dividing the average, quantified values of the quality control materials by the respective, expected concentration values for both levels. The method RR were expressed as percentage values [27].
A study of autosampler storage stability was performed via duplicate injections of QCL, QCH, and pooled EBC samples, every 12 h for 48 h after derivatization. Samples were stored in the autosampler at room temperature (approximately 22 °C) between analytical runs.
Matrix effects were evaluated by comparing mean responses (peak areas) of labeled IS in matrix-free samples (calibration curve and QC) and in EBC samples, across different analytical batches. A Wilcoxon rank sum test was used for statistical comparison. A p-value of 0.05 was used as the cutoff for significance. To control for and manage matrix effects, isotope dilution quantification was used [23].
The method was used to analyze 205 exhaled breath condensate samples to assess the suitability of the method. EBC samples were collected from 138 unique adolescent subjects who were participants in after-school sports programs and agreed to participate in the Study of Air Pollution and Physical Activity (SAPPA). SAPPA was approved by the Institutional Review Boards of Emory and Georgia State Universities (Approval number #00055533). Informed consent was given by the adult participant or parents or guardians of the minor participants; minor participants gave assent to be in the study. The mean (SD) age of subjects was 16.6 (1.3) with a range of 14–19 years. Most participants were male (97 male and 41 female) and African American (134 black, 4 Hispanic, and no other races or ethnicities). EBC samples were collected on-location using an R-tube® (Respiratory Research, Charlottesville, VA). Condensation was achieved using aluminum sleeves chilled with dry ice (−68 °C). Participants exhaled through the R-tube® until approximately 1 mL of condensate was produced, typically 6–10 min. Samples were immediately aliquoted and frozen on dry ice. Many of the participants had samples collected more than once.
3. Results
3.1. Chromatographic separation conditions
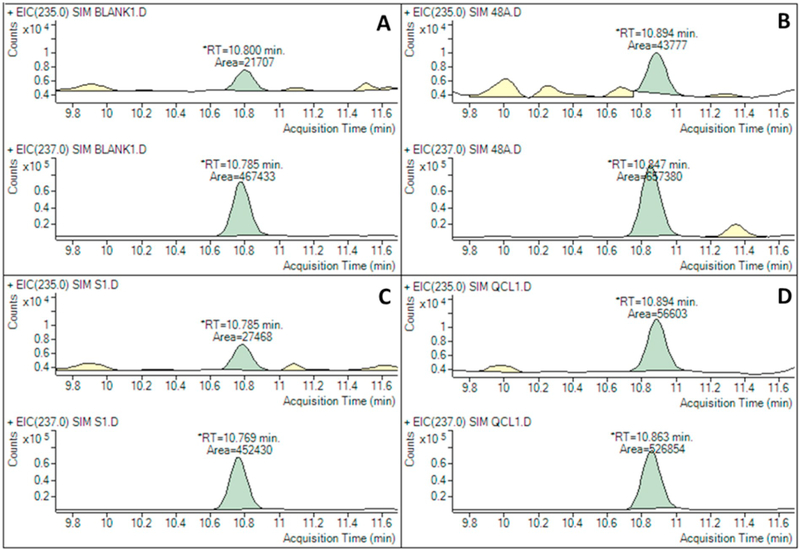
Optimal chromatographic separation conditions were achieved using a pseudo two-dimensional chromatographic configuration, placing the Agilent Eclipse Plus C18 RRHD column before the Agilent Eclipse Plus Phenyl-Hexyl analytical column. Fig. 2 shows examples of chromatograms of the target and labeled IS peaks that were extracted from EBC, lowest calibration level (matrix-free), low-level quality control (matrix-free), and blank (matrix-free) samples. All samples were analyzed using the final configuration.
Fig. 2.
Chromatograms of malondialdehyde-2,4-dinitrophenylhydrazine [MDA-DNPH (m/z = 235)] and deuterated internal standard D2-MDA-DNPH (m/z =237) derivatives
Note: A= blank, B= EBC sample (final concentration =1.63 nM), C= lowest calibration level (prepared from the 1.0 nM standard solution), D = low-level quality control (prepared from the 5nM standard solution).
A different, pseudo two-dimensional chromatographic configuration, where a Phenyl-Hexyl column was placed before a C18 column was tested. This configuration failed to achieve optimal separation, even under matrix-free conditions. In addition, with this configuration, peaks of the derivatives were broad, resulting in an increased LOD and thus reducing the applicability of this approach to the analysis of typical human EBC samples (data not shown). The current order of analytical columns, however, provides sharper peaks of derivatives and lower LODs.
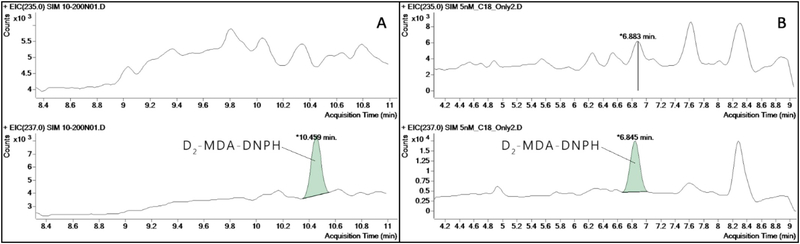
In addition, using either a C18 column or a Phenyl-Hexyl column alone provided poor separation of the target derivative against its background noise. As such, it contributed to the increased LOD that was too high to detect the biological concentrations of MDA found in EBC samples. Fig. 3 shows the chromatograms of the MDA derivatives separated using each of the two analytical columns employed.
Fig. 3.
Chromatograms of MDA derivatives found in 10 nM (A) and 5nM (B) matrix-free standards under single column separation.
Note: A= when a phenyl-hexyl column was used, B =when a C18 column was used.
3.2. Limit of blank and limit of detection
The LOB was 0.18 nM. The LOD was 0.58 nM per EBC sample (36.2 pmol on-column amount). The lowest calibration level was prepared from the 1.0 nM standard solution.
3.3. Method validation
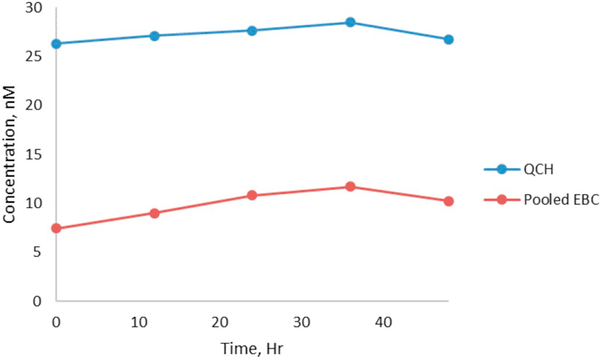
The method precisions and accuracies are summarized in Table 2. The accuracies ranged from 92% to 102%. The inter-day precisions ranged from 4.3% to 12% while the intra-day precisions were 3.6%–12%. The target and labeled IS derivatives were stable at room temperature up to 48 h after derivatization. Fig. 4 shows the plots of relative response ratios (the peak area of the target derivative divided by the peak area of the labeled IS derivative) obtained from replicates of QCL and pooled EBC samples analyzed between 0 and 48 hour post derivatization.
Table 2.
Relative recoveries and precisions of the method calculated from replicate samples prepared from two different concentrations of quality control solutions.
| Concentration levels* | Relative recovery, % | Precision, % | |
|---|---|---|---|
| Within-day | Between-day | ||
| 5.0 nM | 92 | 12 | 12 |
| 25 nM | 102 | 3.6 | 4.3 |
These concentration levels referred to the concentrations of the quality control solutions. The “in vial” concentrations of MDA in the quality control samples were 3.12 and 15.6 nM, respectively.
Fig. 4.
Stability of malondialdehyde-2,4-dinitrophenylhydrazine derivatives in an autosampler at room temperature.
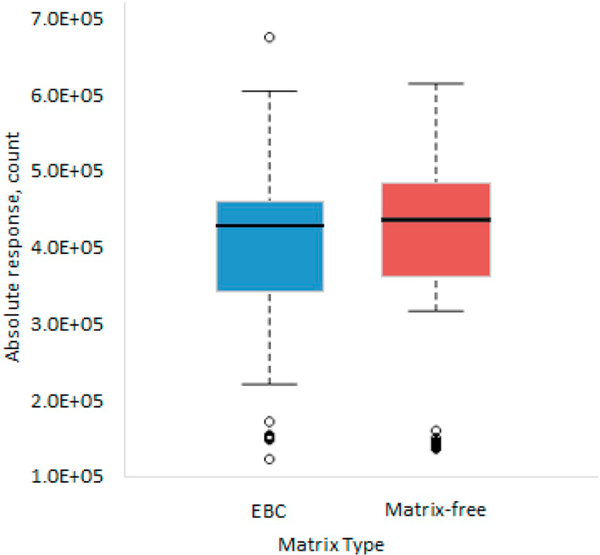
For the matrix effect study, the results (Fig. 5) shows that the average peak area responses of labeled IS derivatives detected in EBC and matrix-free samples were similar. A Wilcoxon rank sum test did not reveal a statistically significant difference in the responses of labeled IS derivatives (p-value = 0.236) detected between the matrix-free and EBC samples.
Fig. 5.
Comparison of the internal standard response between exhaled breath condensate (EBC) samples and matrix-free samples (blank, calibration curve, and quality control samples).
To demonstrate its applicability, the developed method was used to measure MDA in 205 individual EBC samples. After the final concentrations were calculated, approximately 95% had MDA concentrations greater than 1.0 nM. The concentrations ranged from 1.2 nM to 150 nM. About 4% of the analyzed samples had MDA concentrations below the LOD. About 1% of the analyzed samples had levels higher than the LOD but lower than 1.0 nM. For all the EBC samples that had MDA concentrations above 1.0 nM, the median was 6.82 nM (IQR 4.08–9.88).
4. Discussion
We successfully developed a sensitive and selective method capable of quantifying levels of MDA in EBC samples collected from human subjects in an epidemiologic investigation. Our method employs an ultra-high performance liquid chromatograph coupled with UPLC-MS. Our method is the first reported to be capable of measuring MDA using this type of instrument. HPLC-MS instruments are not commonly used for the measurement of trace levels of low molecular weight compounds in biological samples due to their mass-to-charge ratios lying in a mass range with significant chemical noise. However, single quadrupole instruments are typically 1/3 to 1/2 the cost of triple quadrupole instruments, making them more accessible to laboratories with limited resources. We showed that, although triple quadrupole mass spectrometry is usually used to analyze these types of samples, the combination and optimization of separation mechanisms may solve the problem in a more economical way.
Our method deployed chemical derivatization using DNPH, mainly to enable effective, reversed-phase, chromatographic separation of the target MDA derivative. Optimal separation of the derivatives from the background noise was achieved through pseudo, two-dimensional chromatographic separation, combining Agilent Eclipse Plus C18 RRHD and Agilent Eclipse Plus Phenyl-Hexyl analytical columns. Combining two different reversed-phase analytical columns enabled the introduction of differential hydrophobic interaction mechanisms, offering increased selectivity. In the C18 analytical column, the target derivatives were separated primarily using hydrophobic interactions as well as van der Waal forces. When they passed through the Phenyl-Hexyl analytical column, π–π interactions occurred between the aromatic functional groups of the stationary phase and the target derivatives. The change in the retention mechanism, likely, played a fundamental role in effective isolation of the target and labeled IS derivatives from interfering compounds or chemical noise. This resulted in an increase in the signalto-noise ratio of the peak of target derivatives. This subsequently improved the sensitivity and the ability to detect MDA in EBC samples.
The pseudo, two-dimensional chromatographic separation configuration was executed via the pre-existing 2P/6P valve located on the mass spectrometer. This valve is typically used to divert the HPLC flow to a waste reservoir or to the nebulizer for electrospray ionization. Use of this pre-existing switching valve required no additional cost. In addition, the method did not require an extraction, further reducing any costs associated with sample preparation.
The method was validated and demonstrated satisfactory performance. The method accuracies and precisions are shown to be in compliance with FDA guidance [27]. The method has minimal matrix effects. Regardless, we recommended that the isotopically labeled MDA be used as an IS to account for unexpected or random matrix effects, mainly due to the variation in biochemical compositions of the EBC samples collected from different individuals. In addition, although the derivatives were found to be stable inside the autosampler for at least 48 h after derivatization, a minor increase in MDA levels beyond 48 h may result from the release of the bound form of MDA [28]. As such, analysis of the derivatized samples after 48 h is not recommended. The developed method has an LOD of 0.58 nM and is able to cover a quantification range of 1.0 to 200 nM per EBC sample. The low LOD achieved from the current method settings allow for the analysis of human EBC samples. As noted, the method was able to detect MDA in about 96% of the analyzed samples. As shown in Table 3, where a brief overview of previous studies on MDA concentrations measured in EBC samples is provided, our method was able to produce comparable values. Our LOD is as low as those reported from methods using more sophisticated and costly instrumentation, such as tandem mass spectrometry.
Table 3.
Summary of previous studies with reported concentrations of malondialdehyde in exhaled breath condensate samples.
| LOD [nM] | Value [nM] | Derivatizing agent | Detector/mass analyzer | Subject characteristics for shown values | Reference |
|---|---|---|---|---|---|
| 1.07 | 19.4 ± 1.9 | DNPH | APCI+ MS/MS | Healthy non-smoking children | Corradi et al. [16] |
| 1.07 | 12.1 ± 1.8 | DNPH | APCI+ MS/MS | Healthy non-smoking children | Corradi et al. [30] |
| N/A | 4.11 ± 1.29 | DNPH | APCI+ MS/MS | Workers who manufacture multi-walled carbon nanotubes | Lee et al. [31] |
| 1.00 | 11.2 (1.07–23.4) | DNPH | APCI+ MS/MS | Healthy controls | Andreoli et al. [32] |
| 1.00 | 24 (11.8–32.6) | DNPH | APCI+ MS/MS | Chronic obstructive pulmonary disease (COPD) patients | Corradi et al. [33] |
| 0.1 | 1.57 (1.17–2.9) | DNPH | APCI+ MS/MS | Cleaners chronically exposed to chlorinated compounds | Casimirri et al. [34] |
| 4.1 | 15.2 (4–79) | TBA | Fluorescence | Patients with idiopathic pulmonary fibrosis | Bartoli et al. [6] |
| 1.8 | 15.2 (12–15.5) | TBA | Fluorescence | Non-smoking adults aged 45 to 88 years old | Cui et al. [28] |
| 10 | 96.1 ± 11.6 | TBA | Fluorescence | Acute exacerbation of COPD patients | Antus et al. [35] |
| 1.8 | 16.0 ± 1.9 | TBA | Fluorescence | Non-smoking participants during the Olympics in Beijing | Gong et al. [5] |
Data are mean ± SD or median (IQRs: 25th–75th percentile). Note: APCI+ =atmospheric pressure chemical ionization operated in positive ionization mode, MS/ MS = tandem mass spectrometer, ESI= electrospray ionization operated in negative ionization mode, TBA= thiobarbituric acid, and DPNH= 2,4-dinitrophenylhydrazine.
Chemical derivatization of MDA has its limitations. The use of strong acids or bases during derivatization can induce further release of MDA from biological components found in the samples [28]. This results in a higher MDA concentration than the biologically produced concentration following endogenous lipid peroxidation processes. Therefore, it is impractical to compare results across individuals or from studies using different chemical derivatization conditions. In addition, we suggest that chemical derivatization of MDA only be used in epidemiological studies seeking to investigate the change in individual MDA production. This includes any studies employing a pair-wise sample collection or longitudinal sample collection across multiple times from individuals. With these study designs, an artificial release of MDA during derivatization would be unlikely to bias the overall outcomes.
When collecting EBC samples, it is important to note the collection conditions. Some researchers recommend that the measured MDA concentrations be corrected for total EBC volume and condensation temperature to enable an accurate comparison between studies [29]. However, these concerns are reduced when assessing an acute within-subject change in MDA concentration from EBC samples collected under identical conditions and analyzed concurrently.
Also, because the current m/z ratios of the derivatives we generate in our study still lie within the noise region of the mass spectrometer, we observe relatively high intra-day variation of MDA concentrations in blank samples. At this region, the sensitivity of the target derivatives is likely suppressed, resulting in a higher degree of signal variation across different samples. As such, the LOD for each sample will vary. If derivatizing agents with higher molecular weights are used, the m/z ratios of the derivatives will shift to a higher mass region that is known to have less chemical noise, likely increasing the sensitivity of the method. Thus, other potential derivatizing reagents should be tested in future studies.
5. Conclusion
We developed a sensitive, pseudo two-dimensional UPLC analytical method that is capable of quantifying biological concentrations of endogenous MDA in EBC samples. The developed method is validated, and its performance complies with the FDA guideline. The method was implemented on 205 unknown EBC samples and more than 500 injections were performed on a single configuration of columns, which confirmed the method’s robustness and applicability to epidemiological studies. The sensitivity of the method is improved over fluorescence-based methods and is comparable to other mass spectrometric based-methods, including those using expensive tandem mass spectrometric techniques.
Acknowledgments
Funding
This work was financially supported by National Institutes of Health grants 5UM1HL134590–03, P50ES026071, 83615301, P30ES019776, K25ES020355, and the Laney Graduate School of Emory University.
Footnotes
Declarations of interest
None.
References
- [1].Ayala A, et al. , Lipid peroxidation: production, metabolism, and signaling mechanisms of malondialdehyde and 4-hydroxy-2-nonenal, Oxidative Med. Cell. Longev 2014 (2014) 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Khoubnasabjafari M, Ansarin K, Jouyban A, Reliability of malondialdehyde as a biomarker of oxidative stress in psychological disorders, BioImpacts: BI 5 (3) (2015) 123–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Del Rio D, Stewart AJ, Pellegrini N, A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress, Nutr. Metab. Cardiovasc. Dis 15 (4) (2005) 316–328. [DOI] [PubMed] [Google Scholar]
- [4].Rahman I, Kelly F, Review biomarkers in breath condensate: a promising new noninvasive technique in free radical research, Free Radic. Res 37 (12) (2003) 1253–1266. [DOI] [PubMed] [Google Scholar]
- [5].Gong J, et al. , Malondialdehyde in exhaled breath condensate and urine as a biomarker of air pollution induced oxidative stress, J. Expo. Sci. Environ. Epidemiol 23 (3) (2013) 322–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bartoli ML, et al. , Malondialdehyde in exhaled breath condensate as a marker of oxidative stress in different pulmonary diseases, Mediat. Inflamm 2011 (2011) 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Grob N, Aytekin M, Dweik R, Biomarkers in exhaled breath condensate: a review of collection, processing and analysis, J. Breath Res 2 (3) (2008) 037004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Il’yasova D, Scarbrough P, Spasojevic I, Urinary biomarkers of oxidative status, Clin. Chim. Acta 413 (19) (2012) 1446–1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Khoubnasabjafari M, Ansarin K, Jouyban A, Critical review of malondialdehyde analysis in biological samples, Curr. Pharm. Anal 12 (1) (2016) 4–17. [Google Scholar]
- [10].Liang Y, Yeligar SM, Brown LAS, Exhaled breath condensate: a promising source for biomarkers of lung disease, Sci. World J 2012 (2012) 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Ahmadzai H, et al. , Exhaled breath condensate: A comprehensive update, Clinical Chemistry and Laboratory Medicine, 2013, p. 1343. [DOI] [PubMed] [Google Scholar]
- [12].Pelletier G, et al. , Associations between urinary biomarkers of oxidative stress and air pollutants observed in a randomized crossover exposure to steel mill emissions, Int. J. Hyg. Environ. Health 220 (2, Part B) (2017) 387–394. [DOI] [PubMed] [Google Scholar]
- [13].Pelclova D, et al. , Oxidative stress markers are elevated in exhaled breath condensate of workers exposed to nanoparticles during iron oxide pigment production, J. Breath Res 10 (1) (2016) 016004. [DOI] [PubMed] [Google Scholar]
- [14].Pelclova D, et al. , Markers of lipid oxidative damage in the exhaled breath condensate of nano TiO2 production workers, Nanotoxicology 11 (1) (2017) 52–63. [DOI] [PubMed] [Google Scholar]
- [15].Romieu I, et al. , Exhaled breath malondialdehyde as a marker of effect of exposure to air pollution in children with asthma, J. Allergy Clin. Immunol 121 (4) (2008) 903–909.e6. [DOI] [PubMed] [Google Scholar]
- [16].Corradi M, et al. , Aldehydes and glutathione in exhaled breath condensate of children with asthma exacerbation, Am. J. Respir. Crit. Care Med 167 (3) (2003) 395–399. [DOI] [PubMed] [Google Scholar]
- [17].Giera M, Lingeman H, Niessen WMA, Recent advancements in the LC- and GC-based analysis of malondialdehyde (MDA): a brief overview, Chromatographia 75 (9) (2012) 433–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Rezaei Z, Jamshidzadeh A, Sanati E, A rapid and sensitive method for the determination of malondialdehyde as its hydralazine derivative in human plasma using high performance liquid chromatography, Anal. Methods 5 (12) (2013) 2995–2999. [Google Scholar]
- [19].Lord HL, et al. , Determination of malondialdehyde in human plasma by fully automated solid phase analytical derivatization, J. Chromatogr. B 877 (13) (2009) 1292–1298. [DOI] [PubMed] [Google Scholar]
- [20].Syslová K, et al. , Rapid and easy method for monitoring oxidative stress markers in body fluids of patients with asbestos or silica-induced lung diseases, J. Chromatogr. B 877 (24) (2009) 2477–2486. [DOI] [PubMed] [Google Scholar]
- [21].Chen J-L, et al. , Determination of urinary malondialdehyde by isotope dilution LCMS/MS with automated solid-phase extraction: a cautionary note on derivatization optimization, Free Radic. Biol. Med 51 (9) (2011) 1823–1829. [DOI] [PubMed] [Google Scholar]
- [22].Tsikas D, et al. , Development, validation and biomedical applications of stable-isotope dilution GC–MS and GC–MS/MS techniques for circulating malondialdehyde (MDA) after pentafluorobenzyl bromide derivatization: MDA as a biomarker of oxidative stress and its relation to 15(S)-8-iso-prostaglandin F2α and nitric oxide (NO), J. Chromatogr. B 1019 (2016) 95–111. [DOI] [PubMed] [Google Scholar]
- [23].Panuwet P, et al. , Biological matrix effects in quantitative tandem mass spectrometry-based analytical methods: advancing biomonitoring, Crit. Rev. Anal. Chem 46 (2) (2016) 93–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Pilz J, Meineke I, Gleiter CH, Measurement of free and bound malondialdehyde in plasma by high-performance liquid chromatography as the 2,4-dinitrophenylhydrazine derivative, J. Chromatogr. B Biomed. Sci. Appl 742 (2) (2000) 315–325. [DOI] [PubMed] [Google Scholar]
- [25].Armbruster DA, Pry T, Limit of blank, limit of detection and limit of quantitation, Clin. Biochem. Rev 29 (Suppl. 1) (2008) S49–S52. [PMC free article] [PubMed] [Google Scholar]
- [26].Chesher D, Evaluating assay precision, Clin. Biochem. Rev 29 (Suppl. 1) (2008) S23–S26. [PMC free article] [PubMed] [Google Scholar]
- [27].Health, U.D.O,H. Services, Guidance for Industry, Bioanalytical Method Validation, http://www.fda.gov/cder/guidance/index.htm, (2001).
- [28].Cui X, et al. , Relationship between free and total malondialdehyde, a well-established marker of oxidative stress, in various types of human biospecimens, J. Thorac. Dis 10 (5) (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Goldoni M, et al. , Influence of condensation temperature on selected exhaled breath parameters, BMC Pulm. Med 5 (1) (2005) 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Corradi M, et al. , Aldehydes in exhaled breath condensate of patients with chronic obstructive pulmonary disease, Am. J. Respir. Crit. Care Med 167 (10) (2003) 1380–1386. [DOI] [PubMed] [Google Scholar]
- [31].Lee JS, et al. , Health surveillance study of workers who manufacture multi-walled carbon nanotubes, Nanotoxicology 9 (6) (2015) 802–811. [DOI] [PubMed] [Google Scholar]
- [32].Roberta A, et al. , Determination of patterns of biologically relevant aldehydes in exhaled breath condensate of healthy subjects by liquid chromatography/atmospheric chemical ionization tandem mass spectrometry, Rapid Commun. Mass Spectrom 17 (7) (2003) 637–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Corradi M, et al. , Comparison between exhaled and sputum oxidative stress biomarkers in chronic airway inflammation, Eur. Respir. J 24 (6) (2004) 1011–1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Casimirri E, et al. , Biomarkers of oxidative-stress and inflammation in exhaled breath condensate from hospital cleaners, Biomarkers 21 (2) (2016) 115–122. [DOI] [PubMed] [Google Scholar]
- [35].Balazs A, et al. , Monitoring oxidative stress during chronic obstructive pulmonary disease exacerbations using malondialdehyde, Respirology 19 (1) (2014) 74–79. [DOI] [PubMed] [Google Scholar]