Abstract
A growing literature ties in utero conditions to life course outcomes, including education, earnings, and adult health and mortality. A smaller literature has begun to examine the intergenerational impacts of in utero conditions. A link between these two literatures—the impacts of in utero conditions on family formation—has had few examinations but offers a potential set of mechanisms for the intergenerational reach of early conditions. This paper draws from the 1960 US Decennial Census to examine whether individuals exposed in utero to the 1918/19 influenza pandemic had different family formation patterns than adjacent unexposed cohorts. The findings suggest small overall effects on marriage rates, number of children, and several measures of “type” of spouse for men, but moderate effects for women. For example, women with in utero exposure during their first trimester marry men with 0.2 fewer years of schooling than those not exposed. The findings show that exposed individuals have spouses with lower schooling than unexposed counterparts, this effect is particularly large for women, and it increases the likelihood of marrying spouses with very low levels of schooling.
Keywords: In utero exposure, Life course analysis, Family formation, 1918 influenza pandemic
1. Introduction and back ground literature
A large and growing literature has shown evidence of life course effects of poor in utero conditions (Almond and Currie, 2011 for review). A key set of studies have used the 1918/1919 influenza pandemic as an in utero shock that was largely unanticipated and short lived in order to estimate causal effects on life course outcomes. Almond (2006) estimated that in utero exposure to the influenza pandemic in the US resulted in lower educational attainments as well as increased the likelihood of disability and lower incomes fifty or more years later. Related research (Mazumder et al., 2010; Almond and Mazumder, 2005; Fletcher, 2014) has linked this exposure to additional measures of adult health and mortality. Much of this work has been supportive of the importance of the fetal origin hypothesis—that early exposures shape long term health and socio economic trajectories, possibly through the early programming of critical body systems and epigenetic signatures that persistent throughout the lifecourse.
A smaller literature has taken findings from animal models that point to the possibility that insults to one generation may impact future generations to begin to examine the intergenerational effects of the influenza pandemic. Richter and Ol of Robling (2013) were the first to identify an effect of prenatal exposure to the 1918 flu p and emic on the outcomes of the subsequent generation. The authors use historical influenza morbidity data matched to birth information and find that maternal in utero exposure in the second trimester lowers educational attainment for female offspring but not for male offspring. They also find an analogous result for paternal exposure and male out comes: second trimester r exposure lowers educational attainment for male offspring.1 Cook et al. (2016) use a sample from Wiscons in and find that children of exposed individuals had lower educational attainment, as did grandchildren.
An important question raised by the emerging evidence of intergenerational effects of in utero exposure is whether the mechanism is biological or social (or both). A possible biological mechanism is through epigenetic markers—where the environment “turns off/on/up/down” levels of genetic expression—that may be able to pass between parents and children. While there is currently limited evidence of this mechanism in humans2, many studies using animal models, particularly mice, have shown these effects (Stewart et al., 1980). An alternative mechanism linking in utero exposure to an insult to the outcomes of the next generation is through social mechanisms. For example, exposed individuals could suffer in the marriage market and thus the offspring would have “lower quality” parents. While there has been much investigation of the impacts of the influenza pandemic in the US, investigations of f amily formation have not been undertaken.
Indeed, much of the evidence in the literature that focuses on impacts of in utero exposures on marriage and fertility outcomes is from less developed settings3 or from earlier historical periods.4 Marital status was found to be negatively impacted by early childhood exposure to the 1959–61 China famine (Almond et al., 2007; Brandt et al., 2016). Lee (2014) found increases in the number of children born and reductions in spousal education for women in utero during the harshest part of the Korean War.5 This paper extends the literature by estimating the impacts of in utero exposure to the 1918/1919 influenza pandemic on marriage and fertility out comes using data from the 1960 Census. Results suggest that this domain of outcomes were affected in exposed females but not exposed males. The results suggest a need for further investigation for the sources of these gender differences.
A potential implication of these results suggests a difficulty of interpreting intergenerational effect estimates in other work. Specifically, models of transgenerational inheritance of epigenetic signatures often rely on a sex-difference between the number of generations between the exposure and the outcome to obtain evidence for inheritance (e.g. Richter and Ol of Robling, 2013). Because pregnant females carry two generations (fetus and sex cells of the fetus) during an environmental exposure, researchers often seek a fourth generation’s outcomes (exposed mother, daughter, child-of-daughter, grandchild-of-daughter) to confirm transgenerational effects. Since an exposed male only carries a single generation of sex cells, researchers can examine impacts on the (not directly exposed) third generation to confirm trans generational effects. The findings of this paper, that in utero shocks may have sex-specific social effects, further cloud the ability of n prior work to take advantage of sex-specific effects across generations to confirm trans generational transmission.
2. Empirical methods
The empirical analysis largely follows Almond (2006) and subsequent work that focuses the data on a narrow window of birth cohorts around the 1918/1919 influenza pandemic. The key assumption is that the pandemic arrived quickly, exposed a narrow set of birth quarter-cohorts, and then vanished quickly. The speed of the pandemic and that it was unanticipated would suggest that birth cohorts surrounding the 1918/1919 “treated” group can serve as appropriate counter factual controls to allow a causal interpretation of the findings. Since the marriage and fertility outcomes of these birth cohorts are also affected by the Great Depression (when the exposed cohorts were approximately 15–20 years old) (e.g. Hill, 2015), having these control groups who were also affected by the Great Depression can potentially allow a separation between exposure to the pandemic and the Great Depression.
The main analysis uses a 10-year window on each side of the pandemic, but additional analyses are shown that narrows and widens this windows to assess the robustness of the main results. The key controls are for birth year and birth quarter, and the analysis is first pooled and also stratified by gender. A key estimating equation from the literature is given by the following form:
The primary focus is on the coefficient 𝛽1, which measures the effect of influenza exposure on a number of outcomes for i individuals. Year of birth time trends and their squar are denoted by γTi, and ϵi is representative of a clustered error term (on birth year).
More specifically, we will further separate the YOB indicator into five quarter of birth indicators, as the influenza pandemic began in the final quarter of 1918 and lasted into the second quarter of 1919. Thus, potentially exposed individuals were born between the fourth quarter of 1918 (only exposed during the third trimester) and the fourth quarter of 1919 (mainly exposed in the first trimester). The estimating equation is thus:
Where birth quarte r fixed effects (Qi) are also added to the equation, as Buckles and Hungerman (2013) have shown that the composition of births differ by quarter of birth.
3. Data
The data for this study are drawn from the public use version of the 1% US 1960 Decennial Census available at IPUMS (Ruggles et al., 2015).6 Alternative datasets that were considered were the 1940, 1950, and 1970 Census files. The 1940 Census is now complete count, though it only allows a snapshot of outcomes in a n early stage of the life course (i.e. the respondents who were exposed to the 1918/19 influenza pandemic are surveyed when they are 21/22 years old). Since the average age-at-first marriage for women during this time is 21 (see Table 1 below), the fertility and marriage outcomes would be severely censored. A limitation with the 1950 Census is that every household has one “sample-line” person who answered questions, so that the analys is that explores impacts on spousal characteristics would be unavailable; in addition, quarter of cbirth is unavailable for individuals over the age of one. The 1970 Census has the limitation that the exposed cohort is 51/52 years old, so that the number of children in the house hold at the survey may not fully represent total fertility.7
Table 1.
Descriptive Statistics 1960 Census, Birth Cohorts.1908–1927.
| Variable | Obs | Mean | Std Dev | Min | Max |
|---|---|---|---|---|---|
| Educational Attainment | 442,907 | 10.70 | 3.02 | 0 | 17 |
| Any Children | 442,907 | 0.72 | 0.45 | 0 | 1 |
| Number of Children | 442,907 | 1.85 | 1.74 | 0 | 9 |
| Married | 442,907 | 0.85 | 0.36 | 0 | 1 |
| Age at First Marriage | 410,672 | 23.11 | 5.06 | 14 | 50 |
| Male | 442,907 | 0.49 | 0.50 | 0 | 1 |
| Non White | 442,907 | 0.10 | 0.31 | 0 | 1 |
| Age | 442,907 | 40.91 | 5.43 | 32 | 51 |
| Born 1918 Q4 | 442,907 | 0.01 | 0.12 | 0 | 1 |
| Born 1919 Q1 | 442,907 | 0.01 | 0.11 | 0 | 1 |
| Born 1919 Q2 | 442,907 | 0.01 | 0.11 | 0 | 1 |
| Born 1919 Q3 | 442,907 | 0.01 | 0.11 | 0 | 1 |
| Born 1919 Q4 | 442,907 | 0.01 | 0.12 | 0 | 1 |
| Born 2Q | 442,907 | 0.25 | 0.43 | 0 | 1 |
| Born 3Q | 442,907 | 0.26 | 0.44 | 0 | 1 |
| Born 4Q | 442,907 | 0.24 | 0.43 | 0 | 1 |
| Birth Year | 442,907 | 1918 | 5.41 | 1909 | 1927 |
The analysis focuses on marriage and fertility outcomes in the 1960 Census. To anchor the analysis for comparison with Almond (2006), I also analyze educational attainment information. Marriage outcomes include whether the individual is currently married (in 1960), the age at first marriage, and the age and educational attainment of the spouse (using the relationship variables in the Census to match husbands and wives). Fertility outcomes include whether there are any children in the household and the number of children. In addition, I examine whether exposure to the influenza epidemic created “missing” demographic cells by estimating the impacts of exposure on both the count of respondents in each birth quarter/year and also whether the individuals represented in the census are more/less likely to be female or nonwhite.8
Table 1 presents summary statistics for individuals in the Census who were born between 1909–1927. The average educational attainment is nearly 11 years, 72% of the sample has children in their household with an average of 1.85 children, 85% of the respondents are married with an average age of first marriage of 23.11. Nearly 50% of the sample are male and only 10% are not white. Approximately 1% of the sample was born in each of the five quarters of influenza exposure, though the large Census samples will allow precise estimates of effects below. Appendix A Table A1 stratifies the descriptive statistics based on gender; the main difference that is uncovered is that the average age at first marriage is less than 22 for women and over 24.5 for men. Appendix A Table A2 reports the sub-sample for the second analysis of spouse characteristics, which is smaller because only the married individuals are eligible to be included in the table. This subsample is slightly more educated and has slightly more children than the full sample that is not conditioned on being married.
Table A1.
Descriptive Statistics 1960 Census, Birth Cohorts 1908–1927 By Gender.
| Females | Males | |||||
|---|---|---|---|---|---|---|
| Variable | Obs | Mean | Std. | Obs | Mean | Std. |
| Educational Attainment | 226,692 | 10.71 | 2.79 | 216,215 | 10.69 | 3.24 |
| Any Children | 226,692 | 0.72 | 0.45 | 216,215 | 0.72 | 0.45 |
| Number of Children | 226,692 | 1.82 | 1.73 | 216,215 | 1.89 | 1.75 |
| Married | 226,692 | 0.83 | 0.37 | 216,215 | 0.86 | 0.34 |
| Age at First Marriage | 212,546 | 21.80 | 4.85 | 198,126 | 24.51 | 4.89 |
| Male | 226,692 | 0.00 | 0.00 | 216,215 | 1.00 | 0.00 |
| Non White | 226,692 | 0.11 | 0.31 | 216,215 | 0.10 | 0.30 |
| Age | 226,692 | 40.88 | 5.42 | 216,215 | 40.95 | 5.43 |
| Born 1918 Q4 | 226,692 | 0.01 | 0.12 | 216,215 | 0.01 | 0.11 |
| Born 1919 Q1 | 226,692 | 0.01 | 0.11 | 216,215 | 0.01 | 0.11 |
| Born 1919 Q2 | 226,692 | 0.01 | 0.11 | 216,215 | 0.01 | 0.11 |
| Born 1919 Q3 | 226,692 | 0.01 | 0.11 | 216,215 | 0.01 | 0.11 |
| Born 1919 Q4 | 226,692 | 0.01 | 0.12 | 216,215 | 0.01 | 0.12 |
| Born 2Q | 226,692 | 0.25 | 0.43 | 216,215 | 0.25 | 0.43 |
| Born 3Q | 226,692 | 0.26 | 0.44 | 216,215 | 0.26 | 0.44 |
| Born 4Q | 226,692 | 0.24 | 0.43 | 216,215 | 0.24 | 0.43 |
| Birth Year | 226,692 | 1918.37 | 5.40 | 216,215 | 1918.30 | 5.42 |
Table A2.
Descriptive Statistics 1960 Census, Birth Cohorts 1908–1927 Couples Sample (2 Observations Per Couple).
| Variable | Obs | Mean | Std Dev | Min | Max |
|---|---|---|---|---|---|
| Educational Attainment | 360,300 | 10.83 | 2.91 | 0 | 17 |
| Any Children | 360,300 | 0.81 | 0.39 | 0 | 1 |
| Number of Children | 360,300 | 2.12 | 1.71 | 0 | 9 |
| Married | 360,300 | 1.00 | 0.00 | 1 | 1 |
| Age at First Marriage | 360,300 | 23.18 | 5.00 | 14 | 50 |
| Spouse Education | 360,300 | 10.78 | 2.93 | 0 | 17 |
| Spouse Age | 360,300 | 41.17 | 7.79 | 14 | 100 |
| Male | 360,300 | 0.50 | 0.50 | 0 | 1 |
| Non White | 360,300 | 0.08 | 0.28 | 0 | 1 |
| Age | 360,300 | 40.82 | 5.39 | 32 | 51 |
| Born 1918 Q4 | 360,300 | 0.01 | 0.12 | 0 | 1 |
| Born 1919 Q1 | 360,300 | 0.01 | 0.11 | 0 | 1 |
| Born 1919 Q2 | 360,300 | 0.01 | 0.11 | 0 | 1 |
| Born 1919 Q3 | 360,300 | 0.01 | 0.11 | 0 | 1 |
| Born 1919 Q4 | 360,300 | 0.01 | 0.12 | 0 | 1 |
| Born 2Q | 360,300 | 0.25 | 0.43 | 0 | 1 |
| Born 3Q | 360,300 | 0.26 | 0.44 | 0 | 1 |
| Born 4Q | 360,300 | 0.24 | 0.43 | 0 | 1 |
| Birth Year | 360,300 | 1918.43 | 5.38 | 1909 | 1927 |
4. Results
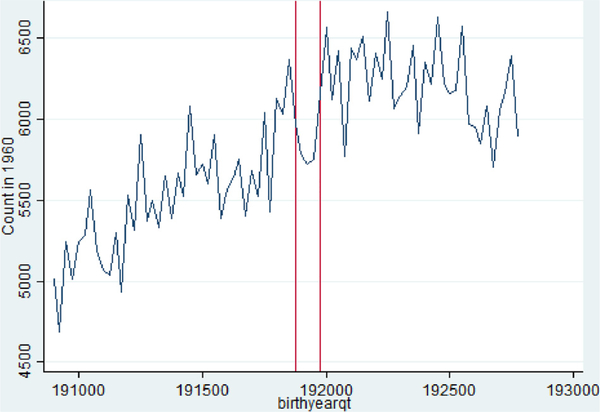
Before estimating the impacts of in utero exposure to the influenza pandemic on adult outcomes, I first explore how the influenza pandemic may have shaped the cohorts through mortality selection. There are wide estimates of the mortality effects of the pandemic, some sources suggest 675,000 in the US (Johnson and Mueller, 2002). While the largest effects were among the youngest and oldest in the nation, the pandemic was unique in having large mortality burdens for young and middle age adults (25–45). Fig. 1 displays evidence that there are “missing” people in this cohort due to the pandemic.9
Fig 1.
“Missing” People by Birth Quarter in the 1960 Census from the 1918/1919 Pandemic Birth Cohorts.1908–1927.
Notes: Vertical Red Lines Indicate 1918 Q4 and 1919 Q4 Birth Cohorts.
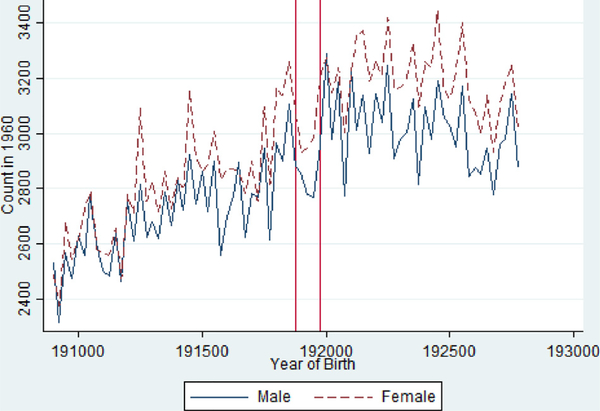
A next question is whether the pandemic shaped the composition of the surviving cohort in measurable ways. One metric that could suggest that the pandemic culled the “weakest” babies is the male/female ratio, as there is substantial evidence that male fetuses are more sensitive to environmental stressors than female fetuses (Hamoudi and Nobles, 2014; Noymer and Garenne, 2000). Fig. 2 stratifies Fig. 1 by gender and shows remarkably similar findings. Table 2 presents regression analysis that also confirms relatively large reductions in the size of the cohorts but reductions that are nearly identical in magnitude (and not statistically different) by gender—the middle three quarters experienced a 5–10% reduction in size while the first and last quarter show 2–5% increases. This evidence is suggestive that the pandemic did not shape the composition of births but could have left lingering disadvantages for those who survived.10
Fig 2.
“Missing” People by Birth Quarter in the 1960 Census from the 1918/1919 Pandemic Birth Cohorts 1908–1927, Stratified by Gender.
Notes: Vertical Red Lines Indicate 1918 Q4 and 1919 Q4 Birth Cohorts.
Table 2.
Associations Between In Utero Influenza Exposure and Surviving Population.
| Sample | Log (Count) Males | Log (Count) Females |
|---|---|---|
| Fixed Effects | None | None |
| Birth Cohorts | 1919 +/− 10 | 1919 +/− 10 |
| Born 1918, Q4 | 0.028** | 0.033** |
| (0.013) | (0.013) | |
| Born 1919, Q1 | −0.046*** | −0.060*** |
| (0.016) | (0.013) | |
| Born 1919, Q2 | −0.045*** | −0.048*** |
| (0.010) | (0.014) | |
| Born 1919, Q3 | −0.107*** | −0.087*** |
| (0.010) | (0.014) | |
| Born 1919, Q4 | 0.046*** | 0.058*** |
| (0.013) | (0.013) | |
| 2nd Birth Quarter | −0.029*** | −0.006 |
| (0.008) | (0.007) | |
| 3rd Birth Quarter | 0.030*** | 0.045*** |
| (0.008) | (0.008) | |
| 4th Birth Quarter | −0.056*** | −0.029*** |
| (0.011) | (0.007) | |
| Birth Year (centered) | −0.010*** | −0.013*** |
| (0.001) | (0.002) | |
| Birth Year Squared | −0.001*** | −0.001*** |
| (0.000) | (0.000) | |
| Constant | 7.995*** | 8.031*** |
| (0.015) | (0.013) | |
| Observations | 76 | 76 |
| R-squared | 0.793 | 0.819 |
Robust standard errors in parentheses.
Level of Analysis: Quarter of Birth Counts from the Census.
p < 0.1.
p < 0.05.
p < 0.01.
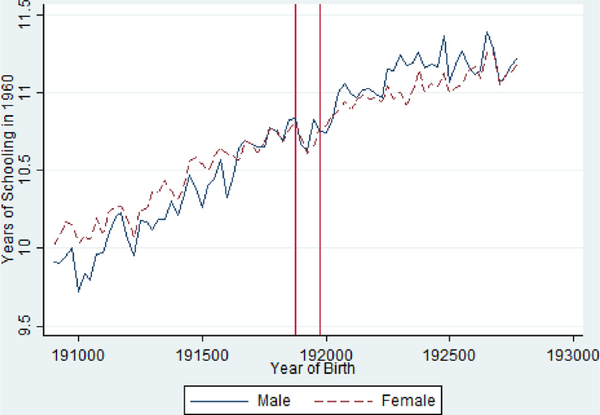
Table 3 reports associations between birth cohort exposure and socio economic outcomes measured in 1960 (i.e. approximately age 41–42 for the exposed cohorts). Each column uses an analytic sample centered at the 1919 birth cohort and adding 10 years of birth cohorts on each side. Appendix A Table A3 shows the same analysis with alternative window lengths. To compare the results to the past literature, Column 1 reports estimates of the impact of in utero exposure on years of schooling and shows approximately 0.1 year reductions, which is very similar to the findings from Almond (2006) (Fig. 3).
Table 3.
Associations Between In Utero Influenza Exposure and Adult Outcomes.
| Education | Married | Age at First Marriage | Any Children | Number of Children | |
|---|---|---|---|---|---|
| Fixed Effects? | State | State | State | State | State |
| Cohorts | 1919 +/− 10 | 1919 +/− 10 | 1919 +/− 10 | 1919 +/− 10 | 1919 +/− 10 |
| Born 1918, Q4 | −0.020* | −0.001 | −0.079*** | 0.006*** | 0.014 |
| (0.010) | (0.001) | (0.027) | (0.002) | (0.014) | |
| Born 1919, Q1 | −0.068*** | −0.002 | −0.033 | −0.001 | 0.002 |
| (0.015) | (0.002) | (0.020) | (0.002) | (0.019) | |
| Born 1919, Q2 | −0.135*** | −0.004*** | −0.047** | −0.006*** | −0.030 |
| (0.010) | (0.001) | (0.021) | (0.001) | (0.017) | |
| Born 1919, Q3 | −0.084*** | −0.002 | −0.033 | −0.013*** | −0.036** |
| (0.011) | (0.001) | (0.019) | (0.002) | (0.014) | |
| Born 1919, Q4 | −0.118*** | −0.002** | −0.126*** | 0.001 | 0.004 |
| (0.010) | (0.001) | (0.027) | (0.002) | (0.014) | |
| 2ndBirth Quarter | 0.022 | −0.003* | 0.178*** | −0.003* | −0.003 |
| (0.015) | (0.001) | (0.023) | (0.002) | (0.007) | |
| 3rd Birth Quarter | 0.101*** | -0.002 | 0.076*** | 0.001 | 0.001 |
| (0.015) | (0.002) | (0.023) | (0.003) | (0.012) | |
| 4th Birth Quarter | 0.136*** | −0.002 | −0.166*** | 0.006* | 0.016 |
| (0.013) | (0.002) | (0.021) | (0.003) | (0.014) | |
| Birth Year (centered) | −0.076*** | −0.003*** | 0.160*** | −0.016*** | −0.073*** |
| (0.002) | (0.000) | (0.001) | (0.000) | (0.002) | |
| Birth Year Squared | −0.003*** | −0.000*** | −0.002*** | −0.001*** | −0.005*** |
| (0.000) | (0.000) | (0.000) | (0.000) | (0.000) | |
| Male | −0.016 | 0.025*** | 2.646*** | −0.011 | 0.032 |
| (0.027) | (0.007) | (0.068) | (0.014) | (0.063) | |
| Non White | −1.879*** | −0.154*** | 0.209*** | −0.161 | 0.051*** |
| (0.061) | (0.002) | (0.027) | (0.005) | (0.017) | |
| Constant | 10.151*** | 0.882*** | 20.773*** | 0.803*** | 2.114*** |
| (0.032) | (0.006) | (0.119) | (0.011) | (0.050) | |
| Observations | 446,642 | 446,642 | 413,525 | 446,642 | 446,642 |
| R-squared | 0.084 | 0.023 | 0.128 | 0.046 | 0.053 |
Additional Controls: State Fixed Effects. Robust standard errors in parentheses.
p < 0.1.
p < 0.05.
p < 0.01.
Table A3.
Associations Between In Utero Influenza Exposure and Adult Outcomes Robustness to Birth Cohort Window.
| Education | Education | Education | Education | Education | |
|---|---|---|---|---|---|
| State Fixed Effects | State Fixed Effects | State Fixed Effects | State Fixed Effects | State Fixed Effects | |
| Cohorts | 1919 +/− 2 | 1919 +/− 6 | 1919 +/− 10 | 1919 +/− 14 | 1919 +/− 18 |
| Born 1918, Q4 | −0.083*** | −0.016 | −0.020* | 0.016 | 0.011 |
| (0.002) | (0.012) | (0.010) | (0.016) | (0.015) | |
| Born 1919, Q1 | −0.064 | −0.066*** | −0.068*** | −0.022 | −0.024 |
| (0.036) | (0.018) | (0.015) | (0.018) | (0.015) | |
| Born 1919, Q2 | −0.106** | −0.132*** | −0.135*** | −0.100*** | −0.099*** |
| (0.021) | (0.007) | (0.010) | (0.016) | (0.014) | |
| Born 1919, Q3 | −0.076** | −0.071*** | −0.084*** | −0.045*** | −0.055*** |
| (0.016) | (0.011) | (0.011) | (0.014) | (0.013) | |
| Born 1919, Q4 | −0.165*** | −0.118*** | −0.118*** | −0.077*** | −0.082*** |
| (0.001) | (0.012) | (0.010) | (0.016) | (0.015) | |
| Constant | 10.195*** | 10.103*** | 10.151*** | 10.163*** | 10.192*** |
| (0.056) | (0.049) | (0.032) | (0.033) | (0.030) | |
| Observations | 72,805 | 264,354 | 446,642 | 614,800 | 770,617 |
| R-squared | 0.078 | 0.076 | 0.084 | 0.091 | 0.103 |
| Married | Married | Married | Married | Married | |
| State Fixed Effects | State Fixed Effects | State Fixed Effects | State Fixed Effects | State Fixed Effects | |
| Cohorts | 1919 +/− 2 | 1919 +/− 6 | 1919 +/− 10 | 1919 +/− 14 | 1919 +/− 18 |
| Born 1918, Q4 | 0.003*** | 0.000 | −0.001 | −0.002* | −0.008** |
| (0.000) | (0.001) | (0.001) | (0.001) | (0.003) | |
| Born 1919, Q1 | 0.001** | −0.001 | −0.002 | −0.002 | −0.010** |
| (0.000) | (0.002) | (0.002) | (0.002) | (0.005 | |
| Born 1919, Q2 | 0.000 | −0.002 | −0.004*** | −0.004** | −0.011*** |
| (0.001) | (0.001) | (0.001) | (0.001) | (0.004) | |
| Born 1919, Q3 | 0.002 | 0.000 | −0.002 | −0.003* | −0.008** |
| (0.001) | (0.002) | (0.001) | (0.001) | (0.004) | |
| Born 1919, Q4 | 0.001*** | −0.001 | −0.002** | −0.003** | −0.008** |
| (0.000) | (0.001) | (0.001) | (0.001) | (0.003) | |
| Constant | 0.872*** | 0.875*** | 0.882*** | 0.889*** | 0.903*** |
| (0.011) | (0.007) | (0.006) | (0.007) | (0.011) | |
| Observations | 72,805 | 264,354 | 446,642 | 614,800 | 770,617 |
| R-square | 0.026 | 0.023 | 0.023 | 0.024 | 0.030 |
| Age at First Marriage | Age at First Marriage | Age at First Marriage | Age at First Marriage | Age at First Marriage | |
| State Fixed Effects | State Fixed Effects | State Fixed Effects | State Fixed Effects | State Fixed Effects | |
| Cohorts | 1919 +/− 2 | 1919 +/− 6 | 1919 +/− 10 | 1919 +/− 14 | 1919 +/− 18 |
| Born 1918, Q4 | −0.235*** | −0.061 | −0.079*** | −0.074** | −0.093** |
| (0.006) | (0.040) | (0.027) | (0.033) | (0.034) | |
| Born 1919, Q1 | −0.090* | −0.054** | −0.033 | −0.044 | −0.058** |
| (0.030) | (0.024) | (0.020) | (0.026) | (0.028) | |
| Born 1919, Q2 | −0.027 | −0.070** | −0.047** | −0.070*** | −0.088*** |
| (0.013) | (0.025) | (0.021) | (0.022) | (0.024) | |
| Born 1919, Q3 | −0.082 | −0.022 | −0.033 | −0.049** | −0.070** |
| (0.046) | (0.032) | (0.019) | (0.024) | (0.026) | |
| Born 1919, Q4 | −0.266*** | −0.105** | −0.126*** | −0.136*** | −0.161*** |
| (0.002) | (0.040) | (0.027) | (0.033) | (0.035) | |
| Constant | 20.808*** | 20.860*** | 20.773*** | 20.737*** | 20.792*** |
| (0.107) | (0.122) | (0.119) | (0.100) | (0.101) | |
| Observations | 67,799 | 245,894 | 413,525 | 564,522 | 695,931 |
| R-squared | 0.104 | 0.110 | 0.128 | 0.143 | 0.163 |
| Any Child | Any Child | Any Child | Any Child | Any Child | |
| State Fixed Effects | State Fixed Effects | State Fixed Effects | State Fixed Effects | State Fixed Effects | |
| Cohorts | 1919 +/− 2 | 1919 +/− 6 | 1919 +/− 10 | 1919 +/− 14 | 1919 +/− 18 |
| Born 1918, Q4 | 0.015*** | 0.005 | 0.006*** | 0.008*** | 0.007 |
| (0.000) | (0.003) | (0.002) | (0.002) | (0.004) | |
| Born 1919, Q1 | 0.004 | −0.002 | −0.001 | 0.001 | −0.002 |
| (0.005) | (0.002) | (0.002) | (0.003) | (0.007) | |
| Born 1919, Q2 | −0.004*** | −0.007*** | −0.006*** | −0.004** | −0.007 |
| (0.000) | (0.002) | (0.001) | (0.002) | (0.006) | |
| Born 1919, Q3 | −0.011 | −0.013*** | −0.013*** | −0.011*** | −0.011** |
| (0.005) | (0.003) | (0.002) | (0.002) | (0.005) | |
| Born 1919, Q4 | 0.012*** | −0.000 | 0.001 | 0.004* | 0.004 |
| (0.000) | (0.003) | (0.002) | (0.002) | (0.005) | |
| Constant | 0.801*** | 0.791*** | 0.803*** | 0.803*** | 0.816*** |
| (0.013) | (0.007) | (0.011) | (0.013) | (0.016) | |
| Observations | 72,805 | 264,354 | 446,642 | 614,800 | 770,617 |
| R-squared | 0.024 | 0.030 | 0.046 | 0.071 | 0.099 |
| Number of Children | Number of Children | Number of Children | Number of Children | Number of Children | |
| State Fixed Effects | State Fixed Effects | State Fixed Effects | State Fixed Effects | State Fixed Effects | |
| Cohorts | 1919 +/− 2 | 1919 +/− 6 | 1919 +/− 10 | 1919 +/− 14 | 1919 +/− 18 |
| Born 1918, Q4 | 0.046*** | 0.016 | 0.014 | 0.016 | 0.020 |
| (0.001) | (0.009) | (0.014) | (0.026) | (0.041) | |
| Born 1919, Q1 | 0.022 | 0.006 | 0.002 | 0.006 | 0.011 |
| (0.009) | (0.011) | (0.019) | (0.030) | (0.047) | |
| Born 1919, Q2 | −0.034 | −0.039*** | −0.030 | −0.023 | −0.016 |
| (0.022) | (0.009) | (0.017) | (0.028) | (0.044) | |
| Born 1919, Q3 | −0.036 | −0.041*** | −0.036** | −0.028 | −0.014 |
| (0.030) | (0.009) | (0.014) | (0.025) | (0.042) | |
| Born 1919, Q4 | 0.032*** | −0.005 | 0.004 | 0.019 | 0.037 |
| (0.002) | (0.010) | (0.014) | (0.027) | (0.042) | |
| Constant | 2.088*** | 2.073*** | 2.114*** | 2.129*** | 2.153*** |
| (0.058) | (0.043) | (0.050) | (0.058) | (0.072) | |
| Observations | 72,805 | 264,354 | 446,642 | 614,800 | 770,617 |
| R-squared | 0.014 | 0.029 | 0.053 | 0.079 | 0.108 |
Additional Controls: Same as Table 3. Robust standard errors in parentheses.
p < 0.1.
p < 0.05.
p <0.01.
Fig 3.
Educational Attainment by Birth Quarter in the 1960 Census from the 1918/ 1919 Pandemic Birth Cohorts 1908–1927, Stratified by Gender.
Notes: Vertical Red Lines Include 1918 Q4 and 1919 Q4 Birth Cohorts.
Having established the comparability of estimated impacts of inutero exposure to the influenza pandemic on educational attainment as previous research, the next set of estimates in Table 3 explores whether these in utero exposures also affected marital outcomes in the sample. Perhaps surprisingly, the estimates for marriage and fertility are much more muted. The likelihood of marriage exposed cohorts, and the standard errors are small enough to rule out modest effects. For those who married, the age at first marriage is slightly lower, by about a month or less. Column 4 and 5 show small reductions in the likelihood of having children and the number of children. Altogether, the gender-pooled estimates suggest quite moderate impacts on a range of marriage and fertility outcomes for those exposed in utero to the influenza pandemic, a next question is whether there is evidence of heterogeneity based on gender.
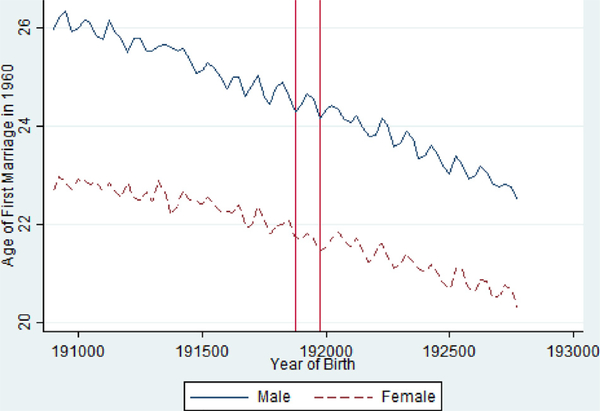
Table 4 stratifies the last set of results by gender to explore the possibilities of differential impacts. While the educational 11 impacts were somewhat similar (also found in Almond, 2006), the results do suggest differences in marriage and fertility outcomes. Exposed females are slightly less likely to be married and get married at younger ages, while males experience no impact on marriage and get married at slightly older ages. Fig. 4 shows unadjusted evidence of a larger and uninterrupted reduction of marriage likelihoods for females across the five exposed birth quarters whereas the male time series shows limited evidence of consistent effects across the quarters of birth. Fig. 5 again shows a relatively stable decline and osculation in the average age of first marriage for men but some evidence of marrying at younger ages than would be expected for the exposed cohorts.
Table 4.
Associations Between In Utero Influenza Exposure and Adult Outcomes Stratified By Gender.
| Education | Education | Married | Married | Age at First Marriage | Age at First Marriage | Any Children | Any Children | # Children | # Children | |
|---|---|---|---|---|---|---|---|---|---|---|
| Sample | Male | Female | Male | Female | Male | Female | Male | Female | Male | Female |
| Fixed Effects? | State | State | State | State | State | State | State | State | State | State |
| Born 1918, Q4 | −0.029 | −0.014 | −0.006*** | 0.004*** | −0.111*** | −0.051 | 0.010*** | 0.003 | 0.030** | 0.001 |
| (0.018) | (0.010) | (0.002) | (0.001) | (0.034) | (0.034) | (0.002) | (0.002) | (0.012) | (0.019) | |
| Born 1919, Q1 | −0.095*** | −0.043*** | 0.001 | −0.005** | 0.013 | −0.074*** | 0.004 | −0.005*** | 0.050*** | −0.044* |
| (0.027 | (0.011) | (0.002) | (0.002) | (0.038) | (0.023) | (0.003) | (0.002) | (0.017) | (0.023) | |
| Born 1919, Q2 | −0.141*** | −0.129*** | −0.003 | −0.005*** | 0.064** | −0.149*** | 0.000 | −0.011*** | −0.008 | −0.048** |
| (0.016) | (0.011) | (0.002) | (0.002) | (0.030) | (0.024) | (0.002) | (0.002) | (0.015) | (0.021) | |
| Born 1919, Q3 | −0.022 | −0.144*** | −0.002 | −0.002* | 0.096*** | −0.150*** | −0.011*** | −0.014*** | −0.049*** | −0.024 |
| (0.015) | (0.013) | (0.002) | (0.001) | (0.030) | (0.024) | (0.003) | (0.002) | (0.013) | (0.018) | |
| Born 1919, Q4 | −0.166*** | −0.075*** | −0.003 | −0.002 | −0.048 | −0.199*** | 0.002 | 0.000 | 0.050*** | −0.037* |
| (0.018) | (0.010) | (0.002) | (0.001) | (0.033) | (0.034) | (0.002) | (0.002) | (0.013) | (0.019) | |
| Constant | 10.096*** | 10.183*** | 0.917*** | 0.872*** | 23.597*** | 20.614*** | 0.805*** | 0.790*** | 2.204*** | 2.056*** |
| (0.081) | (0.041) | (0.006) | (0.007) | (0.107) | (0.115) | (0.007) | (0.008) | (0.033) | (0.037) | |
| Observations | 217,863 | 228,779 | 217,863 | 228,779 | 199,033 | 214,492 | 217,863 | 228,779 | 217,863 | 228,779 |
| R-squared | 0.094 | 0.076 | 0.016 | 0.033 | 0.067 | 0.053 | 0.034 | 0.069 | 0.031 | 0.085 |
Cohorts: 1919 +/− 10 years. Additional Controls: Same as Table 3. Robust standard errors in parentheses.
p <0.1.
p <0.05.
p < 0.01.
Fig 4.
Marriage Rates by Birth Quarter in the 1960 Census from the 1918/1919 Pandemic Birth Cohorts 1908–1927, Stratified by Gender.
Notes: Vertical Red Lines Include 1918 Q4 and 1919 Q4 Birth Cohorts.
Fig 5.
Average Age at First Marriage by Birth Quarter in the 1960 Census from the 1918/1919 Pandemic Birth Cohorts 1908–1927, Stratified by Gender.
Notes: Vertical Red Lines Include 1918 Q4 and 1919 Q4 Birth Cohorts.
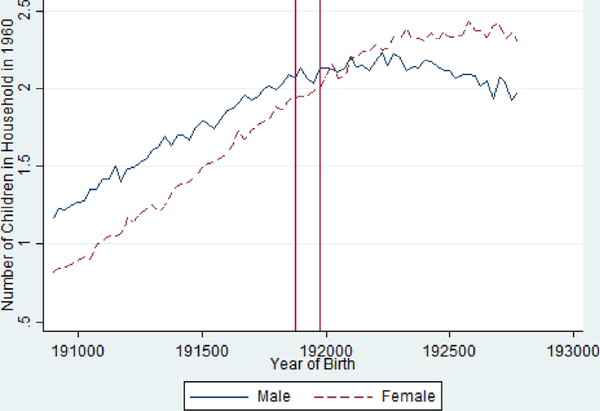
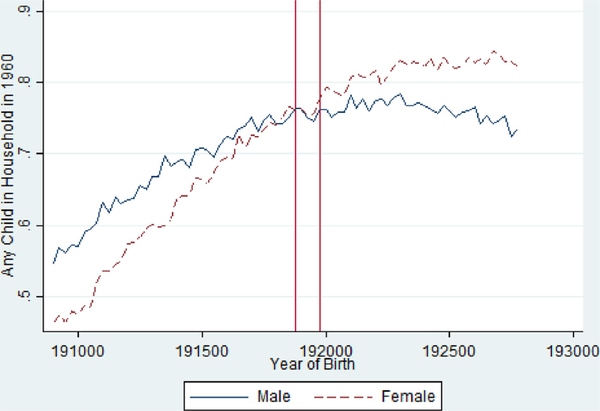
Table 4 also shows that exposed females are less likely to have children and have fewer children (Fig. 7 ), where as the effects for males are smaller and more mixed. Fig. 6 suggests limited effects for the likelihood of having any children for men but somewhat clearer evidence of reductions for women. Again, the estimated impacts are quite small in all cases.
Fig 7.
Average Number of Children by Birth Quarter in the 1960 Census from the 1918/1919 Pandemic Birth Cohorts 1908–1927, Stratified by Gender.
Notes: Vertical Red Lines Include 1918 Q4 and 1919 Q4 Birth Cohorts.
Fig 6.
Proportion with A Child by Birth Quarter in the 1960 Census from the 1918/ 1919 Pandemic Birth Cohorts 1908–1927, Stratified by Gender.
Notes: Vertical Red Lines Include 1918 Q4 and 1919 Q4 Birth Cohorts.
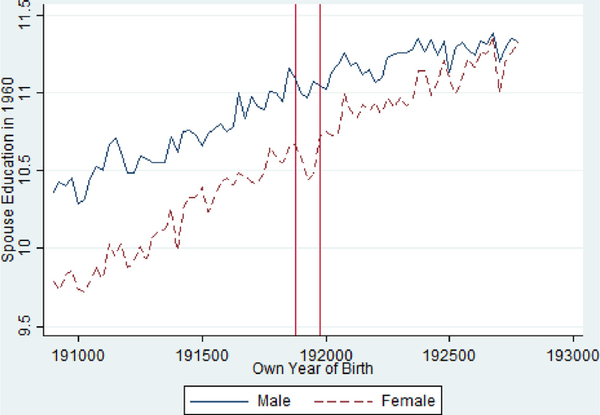
To further examine marital outcomes, Table 5 examines spousal characteristics of individuals who were exposed to the pandemic in utero. Like previous results, the findings suggest differences by gender. Exposed females who marry have less educated spouses and unexposed females; indeed, the effect sizes are generally larger than the main effects on own education. In contrast, exposed males face little to no reduction in spousal education. Fig. 8 plots these unadjusted relationships and again points to visual evidence of a large reduction in spousal education for the exposed cohorts of females but limited visual evidence for males. Table 6 shows that the spousal education effects for exposed females are concentrated among spouses with very low levels of schooling.
Table 5.
Associations Between In Utero Influenza Exposure and Spousal Characteristics Stratified by Gender.
| Spouse Education | Spouse Education | Spouse | Spouse | |
|---|---|---|---|---|
| Age | Age | |||
| Sample | Females | Males | Females | Males |
| Fixed Effects? | State | State | State | State |
| Birth Cohorts | 1919 +/− 10 | 1919 +/− 10 | 1919 +/− 10 | 1919 +/− 10 |
| Born 1918, Q4 | −0.062*** | 0.038* | 0.010 | 0.070* |
| (0.019) | (0.020) | (0.046) | (0.035) | |
| Born 1919, Q1 | −0.017 | 0.014 | 0.215*** | 0.038 |
| (0.020) | (0.018) | (0.037) | (0.042) | |
| Born 1919, Q2 | −0.134*** | −0.074*** | 0.244*** | −0.028 |
| (0.012) | (0.018) | (0.042) | (0.027) | |
| Born 1919, Q3 | −0.214*** | 0.013 | 0.043 | −0.031 |
| (0.014) | (0.018) | (0.043) | (0.039) | |
| Born 1919, Q4 | −0.056*** | −0.023 | 0.081* | −0.024 |
| (0.018) | (0.021) | (0.046) | (0.036) | |
| 2nd Birth Quarter | −0.015 | 0.046*** | −0.112*** | −0.197*** |
| (0.024) | (0.013) | (0.025) | (0.032) | |
| 3rd Birth Quarter | 0.039* | 0.097*** | −0.350*** | −0.365*** |
| (0.022) | (0.020) | (0.040) | (0.043) | |
| 4th Birth Quarter | 0.090*** | 0.104*** | −0.573*** | −0.585*** |
| (0.026) | (0.016) | (0.036) | (0.037) | |
| Constant | 9.785*** | 10.116*** | 45.493*** | 38.217*** |
| (0.064) | (0.048) | (0.093) | (0.081) | |
| Observations | 183,900 | 180,085 | 183,900 | 180,085 |
| R-squared | 0.114 | 0.085 | 0.505 | 0.540 |
Additional Controls: State Fixed Effects, Birth year and birth year squared (for columns 1 and 2). Robust standard errors in parentheses.
p <0.05.
p <0.1.
p < 0.01.
Fig 8.
Average Spousal Education Level by Birth Quarter in the 1960 Census from the 1918/1919 Pandemic Birth Cohorts 1908–1927, Stratified by Gender.
Notes: Vertical Red Lines Include 1918 Q4 and 1919 Q4 Birth Cohorts.
Table 6.
Associations Between In Utero Influenza Exposure and Spousal Education Examination Across Education Margins.
| Outcome: | Education =<4 | Education =<8 | Education =<10 | Education =<12 | Education =<14 | Education =<16 |
|---|---|---|---|---|---|---|
| Born 1918, Q4 | 0.886*** | 1.018 | 0.999 | 0.949*** | 0.942* | 0.746*** |
| (0.028) | (0.017) | (0.019) | (0.018) | (0.029) | (0.037) | |
| Born 1919, Q1 | 1.212*** | 1.044*** | 1.002 | 0.907*** | 0.835*** | 0.875** |
| (0.028) | (0.017) | (0.019) | (0.020) | (0.019) | (0.054) | |
| Born 1919, Q2 | 1.225*** | 1.027** | 1.062*** | 1.080*** | 1.004 | 0.972 |
| (0.044) | (0.014) | (0.016) | (0.018) | (0.016) | (0.043) | |
| Born 1919, Q3 | 1.098*** | 1.052*** | 0.990 | 0.949** | 0.994 | 0.981 |
| (0.037 | (0.017) | (0.017) | (0.020) | (0.025) | (0.040) | |
| Born 1919, Q4 | 1.261*** | 0.992 | 1.057*** | 0.994 | 1.005 | 1.257*** |
| (0.041) | (0.017) | (0.020) | (0.018) | (0.031) | (0.065) | |
| Observations | 179,017 | 179,018 | 179,020 | 179,022 | 179,024 | 179,026 |
Additional Controls: Same as Table 5. Robust standard errors in parentheses.
Odds ratios reported. The outcome is measured as a binary indicator of whether spousal education is less than or equal to the value provided.
p <0.1.
p <0.05.
p < 0.01.
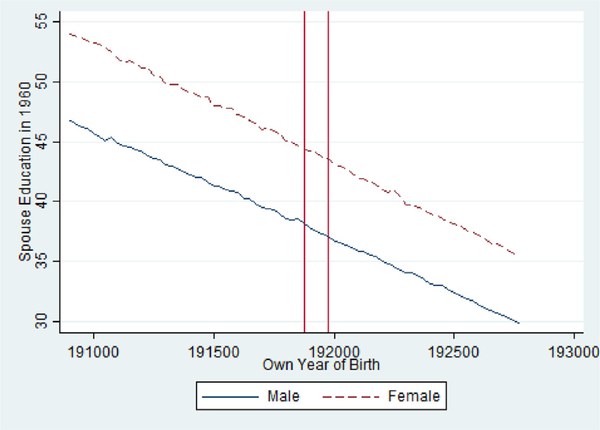
For spousal age, while Table 5 suggests that exposed females marry older men than unexposed females, Fig. 9 suggests no differences compared to the trend line, which implies this regression may be misspecified by its inclusion of a quadratic birth year term and birth quarter controls.
Fig 9.
Average Spousal Age by Birth Quarter in the 1960 Census from the 1918/1919. Table 1 Pandemic Birth Cohorts 1908–1927, Stratified by Gender.
Notes: Vertical Red Lines Include 1918 Q4 and 1919 Q4 Birth Cohorts.
5. Conclusions
This paper is among a small number of investigations that focus on the marriage and fertility implications of exposure to in utero insults. While there is growing evidence that exposure to in utero famine conditions impact later household formation, especially using datasets from China, less is known about a broader set of in utero exposures, such as severe strains of influenza. Additionally, while the effects of the 1918/1919 pandemic have been established along a number of domains, including human capital and health capital outcomes, no current work has examined the pandemic’s influence on household formation outcomes.
The main results of the paper are that women exposed in utero to the 1918/1919 influenza pandemic have patterns of marriage and fertility that differ than their unexposed counterparts. While I find relatively small effects of whether women are married, there is evidence that they get married earlier and marry less educated men. In contrast, while men’s own education is shaped by their in utero exposure, their family formation outcomes do not seem to be affected. There are limitations to the analysis that should be considered when interpreting these results. First, the outcome measures are taken in 1960, over 40 years following the exposure. These measures are unable to capture some of the richness of marriage and fertility events that occur over the forty year time period. In particular, children who have exited the household are not represented in the measures, as are spouses who exited through divorce or death; it is important to recall, though, that in order for these issues to bias the estimates, the measurement error would need to be different for the affected vs. unaffected cohorts. Additionally, while there was important unmeasured geographic variation in the reach of the pandemic, this variation is eliminated through the use of state-of-birth fixed effects rather than leveraged to explore dose-response relationships. Related, birth year and quarter is used as the “treatment” variable, making the interpretation of the results “intent to treat” estimates rather than the effects of actual influenza exposure, which is not measured in the data. These limitations should be viewed in light of the advantages of the data, which include large sample sizes that allow precise estimates and the ability to rule out modest effect sizes, population representativeness, and the ability to assign “treatment” based on quarter of birth rather than age or birth year.
Evidence on the effects of in utero exposures to disease for family formation outcomes is important both to understand the full effects of these exposures as well as in understanding and interpreting a second vein of the literature, which has found intergenerational consequences to in utero exposures. One possibility of this link is biological and occurs through epigenetic transmission, where the environmental imprint of the parent is passed to the child through some (yet unknown) epigenetic mechanism, as has been shown in many animal models. A second possibility is that the intergenerational effects are mediated through social processes, like the marriage market outcomes of the first generation, that then shapes the next generation. Indeed, the results from this paper suggest some evidence of this social mechanism, as exposed females attain both lower education and also marry husbands with lower education than similar unexposed females.12 Because this social mechanism appears to be largely absent for males, we might expect intergenerational effects to be larger for children of exposed females than for children of exposed males if the social mechanism is the leading pathway. Indeed, results in Cook et al. (2016) find exactly this pattern on the second generation’s educational attainment and other outcomes. Under- standing additional mechanisms that link in utero exposure to family formation outcomes as well as to intergenerational processes should be the subject of future work.
Acknowledgments
I thank Angela Forgues for helpful comments. This research was supported grant by core grants to the Center for Demography and Ecology at the University of Wisconsin-Madison (P2C HD047873) and to the Center for Demography of Health and Aging at the University of Wisconsin-Madison (P30 AG017266).
Appendix A
Footnotes
van den Berg and Pinger (2016) examine multigenerational effects of f amine during pre-puberty in Germany during World War I.
Sen et al. (2015) presents supporting evidence from 35 mother-infant pairs in Detroit.
Donaldson and Keniston (2014) find evidence of investments in child quality in India due to the influenza pandemic.
Bengtsson and Dribe (2006) examine the impacts of exposure to small pox on fertility in the 18th and 19th century.
A larger literature has explored the impacts of childhood exposure (rather than a focus on in utero exposure) on later marriage market outcomes and have shown sex differences in the effects (e.g. Van den Berg and Gupta, 2015).
An alternative measure to consider is the “number of children ever born”, which is available in both the 1960 and 1970 census. In the 1960 census, only married women were asked this question. In the 1970 census, only women were asked the question. Because the analysis focuses on potential sex differences in the impacts of the influenza pandemic and family outcomes, the lack of data for men requires the use of the “children in the household” measure rather than the “number of children ever born” measure. A limitation of the “children in the household” measure is that some children may have already left the household at the time of the survey and other children may not yet be born. An advantage of the research design is that these issues of measurement would need to be discontinuous for the 1918/1919 birth quarters to bias the analysis.
This analysis is related to the recent work by Boberg-Fazlic et al. (2017) who explore the fertility dynamics of the influenza pandemic in Sweden.
While it would be useful to leverage geographic variation in the influenza pandemic, Almond (2006, p 686) notes “Influenza infection was not made a reportable disease in the United
These results are in contrast to the large female/male ratio changes from the famine in China documented in Brandt, Siow, and Vogel (2016).
.See also Fig. 3, which presents unadjusted averages of years of schooling by birth cohort quarter.
The pattern of results also suggests the possibility of a social mechanism in parent-of-origin effects in genetics, which is the phenomenon that the effects of genotype on phenotype differ based on whether the genotype was inherited from the father or mother (Lawson et al., 2013 for overview).
References
- Almond Douglas., 2006. Is the 1918 influenza pandemi c over? Long-term effects of in utero influenza exposure in the post-1940 US population. J. Polit. Econ 114 (4), 672–712. [Google Scholar]
- Almond Douglas, Currie Janet, 2011. Killing me softly: the fetal origins hypothesis. J.Econ. Perspect 25 (3), 153–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almond Douglas, Mazumder Bhashkar, 2005. The 1918 influenza pandemic and subsequent health outcomes: an analysis of SIPP data. Am. Econ. Rev 258–262. [DOI] [PubMed] [Google Scholar]
- Almond Douglas, Edlund Lena, Li Hongbin, Zhang Junsen, 2007. Long-Term Effects of the 1959–1961 China Famine: Mainland China and Hong Kong: No. w13384.National Bureau of Economic Research. [Google Scholar]
- Bengtsson and Dribe, 2006. Deliberate control in a natural fertility population: Southern Sweden, 1766–1864. Demography 43. [DOI] [PubMed] [Google Scholar]
- Boberg-Fazlic Nina, Ivets Maryna, Karlsson Martin, Nilsson Therese, 2017. Disease and Fertility: Evidence from the 1918 Influenza Pandemic in Sweden. No.10834.Institute for the Study of Labor (IZA). [DOI] [PubMed] [Google Scholar]
- Brandt Loren, Siow Aloysius, Vogel Carl, 2016. Large demographic shocks and small changes in the marriage Market. J. Eur. Econ. Assoc 14 (6), 1437–1468. [Google Scholar]
- Buckles Kasey S., Hungerman Daniel M., 2013. Season of birth and later outcomes: Old questions, new answers. Rev. Econ. Stat 95 (3), 711–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook C Justin Fletcher, Jason M, Forgues Angela, 2016. Multigenerational effects of early life health shocks. Presented at the 2016 PAA Meetings,. [Google Scholar]
- Donaldson Dave, Keniston Daniel, 2014. How Positive Was the Positive Check? Investment and Fertility in the After math of the 1918 Influenza in India.. [Google Scholar]
- Fletcher Jason M., 2014. Examining the long term mortality effects of early health shocks. U.S. Census Bureau Working Paper,. https://papers.ssrn.com/sol3/papers.cfm?abstract_id=2423544. [Google Scholar]
- Hamoudi Amar, Nobles Jenna, 2014. Do daughters really cause divorce? stress, pregnancy, and family composition. Demography 51 (4), 1423–1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill Matthew J., 2015. Love in the time of the depression: the effect of economic conditions on marriage in the great depression. J. Econ. Hist 75 (01), 163–189. [Google Scholar]
- Johnson Niall P.A.S., Mueller Juergen, 2002. Updating the accounts: global mortality of the 1918–1920 “ Spanish” influenza pandemic. Bull. Hist. Med 76 (1), 105–115. [DOI] [PubMed] [Google Scholar]
- Lawson Heather A., Cheverud James M., Wolf Jason B., 2013. Genomic imprinting and parent-of-origin effects on complex traits. Nat. Rev. Genet 14 (9), 609–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Chulhee., 2014. In utero exposure to the Korean War and its long-term effects on socio economic and health outcomes. J. Health Econ 33, 76–93. [DOI] [PubMed] [Google Scholar]
- Mazumder Bhashkar, Almond Douglas, Park Kyung, Crimmins Eileen M., Finch Caleb E., 2010. Lingering prenatal effects of the 1918 influenza pandemic on cardiovascular disease. J. Dev. Origins Health Dis 1 (01), 26–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noymer Andrew, Garenne Michel, 2000. The 1918 influenza epidemic’s effects on sex differentials in mortality in the United States. Popul. Dev. Rev 26 (3), 565–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter André, Olof Robling Per, 2013. Multigenerational effects of the 1918–19 influenza pandemic in Sweden. Swed. Inst. Soc. Res 5. [Google Scholar]
- Ruggles Stevenm, Genadek Katie, Goeken Ronald, Grover Josiah, Sobek Matthew, 2015. Integrated Public Use Micro data Series: Version 6.0 [Machine-Readable Database]. University of Minnesota, Minneapolis. [Google Scholar]
- Sen Arko, Heredia Nicole, Senut Marie-Claude, Land Susan, Hollocher Kurt, Lu Xiangyi, Dereski Mary O., Ruden Douglas M., 2015. Multigenerational epigenetic inheritance in humans: DNA methylation changes associated with maternal exposure to lead can be transmitted to the grandchildren. Sci. Rep 5, 14466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart RJC, Sheppard Hilda G., Preece RF, Waterlow John C., 1980. The effect of rehabilitation at different stages of development of rats marginally malnourished for ten to twelve generations. Br. J. Nutr 43 (3), 403–412. [DOI] [PubMed] [Google Scholar]
- van den Berg Gerard J., Pinger Pia R., 2016. Transgenerational effects of childhood conditions on third generation health and education outcomes. Econ. Hum. Biol 23, 103–120. [DOI] [PubMed] [Google Scholar]
- Van den Berg Gerard J., Gupta Sumedha, 2015. The role of marriage in the causal path way from economic conditions early in life to mortality. J. Health Econ 40, 141–158. [DOI] [PubMed] [Google Scholar]