Abstract
TDP-43 is present in a high proportion of aged brains that do not meet criteria for frontotemporal lobar degeneration (FTLD). We determined whether there are distinct TDP-43 types in non-FTLD brains. From a cohort of 553 brains (Braak neurofibrillary tangle (NFT) stage 0-VI), excluding cases meeting criteria for FTLD, we identified those that had screened positive for TDP-43. We reviewed 14 different brain regions in these TDP-43 positive cases and classified them into those with “typical” TDP-43 immunoreactive inclusions (TDP type-α), and those in which TDP-43 immunoreactivity was adjacent to/associated with NFTs in the same neuron (TDP type-β). We compared pathological, genetic (APOE4, TMEM106B & GRN variants), imaging and clinical data between types, and compared imaging between types and a group of TDP-43 negative cases (n=309). Two-hundred forty-one cases were classified as TDP type-α (n=131, 54%) or TDP type-β (n=110, 46%). Type-α cases were older than type-β at death (median 89 years vs 87; p=0.02). Hippocampal sclerosis was present in 78 type-α cases (60%) and 16 (15%) type-β cases (p<0.001). Type-α cases showed a pattern of widespread TDP-43 deposition commonly extending into temporal, frontal and brainstem regions (84% TDP-43 stage 4–6) while in type-β cases deposition was predominantly limbic, located in amygdala, entorhinal cortex and CA1 region of the hippocampus (84% TDP-43 stages 1–3) (p<0.001). There was a difference in the frequency of TMEM106B protective (GG) and risk (CC) haplotypes (SNP rs3173615 encoding p.T185S) in type-α cases compared to type-β cases (GG/CG/CC: 8%/42%/50% vs 24%/49%/27%; p=0.01).Type-α cases had smaller amygdala (−10.6% [−17.6%, −3.5%]; p=0.003) and hippocampal (−14.4% [−21.6%, −7.3%]; p<0.001) volumes on MRI at death compared to type-β cases, although both types had smaller amygdala and hippocampal volumes compared to TDP-43 negative cases (−7.77%, −21.6%; p <0.001). These findings demonstrate that there is distinct heterogeneity of TDP-43 deposition in non-FTLD brains.
Keywords: TDP-43, frontotemporal lobar degeneration, FTLD, Alzheimer’s disease, TDP-43 type, type-β, MRI, hippocampus, TMEM106B
TAR DNA binding protein of 43 kDa (TDP-43) was first reported to be associated with frontotemporal lobar degeneration (FTLD) and amyotrophic lateral sclerosis in 2006 [2, 41]. It was later shown to be present in 17–74% of brains of aged individuals and those with Alzheimer’s disease [1, 3, 4, 9, 12, 14, 29, 35, 56]. Today, TDP-43 has become important to our understanding of age-related cognitive impairment and Alzheimer’s disease [15, 21]. TDP-43 has been shown to be associated with the presence of dementia, more severe cognitive impairment in general at death and overtime, and loss of episodic memory even after accounting for other pathologies that have also been linked to these clinical features [5, 24–26, 57]. TDP-43 has also been shown to be associated with smaller hippocampal volumes and faster rates of hippocampal atrophy in aging and Alzheimer’s disease [17, 24, 26]. TDP-43 has also been linked to the apolipoprotein epsilon e4 allele (APOE ε4) [15, 53, 58] and the transmembrane protein 106B (TMEM106B) [46] in aging and Alzheimer’s disease.
Over a decade ago we observed some cases with unusual histological characteristics of TDP-43 deposition in aging and Alzheimer’ disease [1]. Most commonly, we observed cases of TDP-43 deposition with morphological features that were “Typical” and similar to those described in one type of frontotemporal lobar degeneration (FTLD) [1]. Specifically, we noted the presence of TDP-43 immunoreactive neuronal cytoplasmic inclusions, neuronal intranuclear inclusions and dystrophic neurites (Figure 1) throughout different brain regions that are characteristic of FTLD-TDP type-A [32]. We did not appreciate characteristic features of any of the other FTLD-TDP types [30, 32] at that time. Interestingly, we also noted cases where TDP-43 deposition appeared distinct. In these cases we observed TDP-43 immunoreactivity that was located adjacent to tau-immunoreactive neurofibrillary tangles (NFTs) (Figure 1) [1]. We showed with confocal microscopy that in such cases there was indeed co-localization of TDP-43 with phosphorylated tau [1]. In addition, with electron microscopy, we showed that TDP-43 immunoreactivity was associated with tightly bundled, paired helical filaments [1]. Interestingly, in these cases, we did not see some of the classic features of TDP-43 that is characteristic of FTLD-TDP type-A, such as neuronal intranuclear inclusions. To date, it remains unknown whether our observations are consistent with true heterogeneity of TDP-43 deposition in non-FTLD brains, i.e. whether there are at least two distinct TDP-43 subtypes; one with typical inclusions, and the other linked to the presence of NFTs.
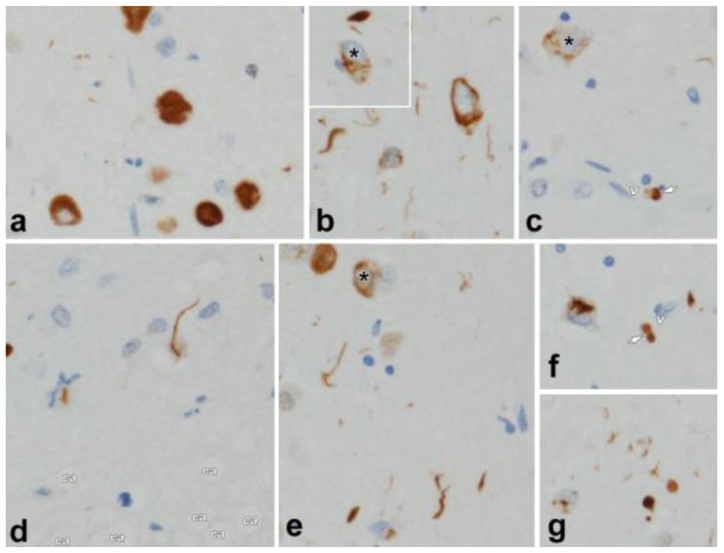
Figure 1.
shows the pathological features of TDP-43 including those of TDP-43 type-β in which TDP-43 immunoreactivity is associated with neurofibrillary tangles.
a. Pick body-like, dense neuronal cytoplasmic inclusions (NCI) in perirhinal cortex, layer II.
b. NCI and neurites in amygdala; inset (asterisk) shows TDP-43 deposits co-mingled with neurofibrillary tangle. (See also cells with asterisks in c. and e.)
c. Perivascular (v = capillary) globular astrocytic inclusion (arrow) in amygdala.
d. Spindly neurites in subpial space (ca = corpora amylacea in glia limitans astrocytes).
e. Pleomorphic TDP-43 pathology in amygdala.
f. Skein-like NCI and perivascular globular inclusion (arrow, v = capillary)
g. Pleomorphic neuronal and neuritic pathology in amygdala.
In this study, we determine whether there is evidence supporting the notion that there may indeed be at least two distinct pathological subtypes of TDP-43 deposition in non-FTLD brains. We hypothesize that there would be evidence supporting the existence of two distinct pathological types.
METHODS
Subjects
We identified all cases in a consecutive autopsy series of prospectively enrolled participants in the Mayo Clinic Alzheimer’s Disease Research Center, Mayo Clinic Alzheimer’s Disease Patient Registry, or the Mayo Clinic Study of Aging, that had a brain autopsy, between Jan 1, 1999, and Dec 31, 2012 and had been screened for the presence of TDP-43 in the amygdala and/or hippocampus. Individuals with a pathological diagnosis of any type of FTLD were excluded [8, 33]. That is, cases where degeneration of the frontal and temporal lobes, either on gross examination or histologically showing microvacuolation, neuronal loss and astrogliosis most prominent in laminar II or transcortical, were the most characteristic feature. From a total of 553 non-FTLD cases that had been screened for TDP-43, a total of 244 cases had screened positive, and 309 (which we will refer to as controls for this study) had screened negative.
Pathological Analysis
All 553 cases and controls had undergone pathological examination according to the recommendations of the Consortium to Establish a Registry for Alzheimer’s disease (CERAD) [37], and each individual had been assigned a Braak NFT stage 0-VI [6] and a CERAD neuritic plaque stage 0–3 [37]. The presence of Lewy bodies in amygdala, brainstem regions or neocortical regions was documented, as was the presence of vascular lesions (micro-infarcts, lacunar infarcts (<1cm), large infarcts (≥ 1cm) and amyloid angiopathy. Hippocampal sclerosis (HpScl) was diagnosed on the basis of neuronal loss in the subiculum and CA1 regions of the hippocampus out of proportion to the observable burden of extracellular neurofibrillary tangle pathology, according to consensus recommendations [10, 43]. The 244 cases that had screened positive for TDP-43 had additional brain sections screened for the presence/absence of TDP-43 and as a result were rendered a TDP-43 stage of 1–6 [19, 20]. To accomplish staging, we screened amygdala, entorhinal cortex, subiculum, dentate gyrus of the hippocampus, occipitotemporal cortex, inferior temporal cortex, basal forebrain, insula, ventral striatum, basal ganglia, superior middle frontal cortex, substantia nigra, midbrain tectum and inferior olive [19, 20]. Staging was rendered as followed: stage 1=TDP-43 deposition was limited to the amygdala; stage 2=TDP-43 extending into the subiculum or entorhinal cortex; stage 3 = extension into the dentate gyrus of the hippocampus or occipitotemporal cortex; stage 4 = extension into inferior temporal cortex, basal forebrain, insula or ventral striatum; stage 5 = extension into brainstem regions (substantia nigra, midbrain tectum or inferior olive) and stage 6 = extension into basal ganglia or frontal cortex. The TDP-43 staging scheme has been validated [48].
This study was approved by the Mayo Clinic Institutional Review Board. Before death, all participants or their proxies had provided written consent for brain autopsy examination.
Determination of TDP-43 type
To determine TDP-43 type we reviewed the morphological features of TDP-43 in all regions where TDP-43 was present (i.e. range: 1–14 regions) in all 244 cases [19,20]. Cases were categorized into those with typical, well described, TDP-43 inclusions such as neuronal cytoplasmic inclusions, neuronal intranuclear inclusions and dystrophic neurites, without NFT associated TDP-43 in any region (designated TDP-43 type-α) and those where TDP-43 was observed adjacent to NFTs in multiple cells in at least 1 brain region screened (designated TDP-43 type-β) (Figure 1). We chose to designate the two variants as type-α and type-β in order to avoid confusion with the TDP-43 type 1, 2, 3 and TDP-43 type A-E typing of FTLD-TDP [8] and to be specific for TDP-43 deposition in non-FTLD brains. Categorization was based on consensus of three experts in degenerative neuropathology (DWD, KAJ, MEM).
Clinical and MRI outcomes
The 244 cases were categorised as cognitively unimpaired, having mild cognitive impairment [42], dementia of the Alzheimer’s type[36], or a non-Alzheimer’s dementia at the last visit before death. For each participant, determination of cognitively normal status was based on consensus agreement between the study coordinator, examining physician, and neuropsychologist who evaluated the participant, taking into account education, prior occupation, visual or hearing deficits, and reviewing all other participant clinical information. Clinical measures that were captured during life included the Mini-Mental State Examination [11], Clinical Dementia Rating Scale Sum of Boxes [34], Wechsler Memory Scale-Revised Logical Memory II [52], Boston Naming Test [28], Control Oral word Association Test [31], Wechsler Adult Intelligence Scale-R Block Design [52], Auditory Verbal Learning Test (AVLT) Delayed Recall [44], and Trail Making Test Parts A and B [47]. Of the 244 cases, 187 had completed a volumetric head MRI during life. If more than one MRI had been performed we analysed the MRI closest to death. Regional volumes were calculated using FreeSurfer version 5.3.0. We assessed volumes of the amygdala, hippocampus, lateral temporal cortex (inferior, middle, superior temporal cortex), parietal (inferior parietal, supramaginal and isthmus cingulate cortex), and lateral superior frontal cortex, and outputted total intracranial volume (TIV) to allow for the correction of head size [54].
Genetic Analyses
Genotypes of the two variants in the TMEM106B and GRN genes were generated by the Taqman method (Invitrogen, Carlsbad, CA, USA). Assays were ordered for rs3173615 (TMEM106B) and rs5848 (GRN) and genotyping was performed following manufacturers protocol on 133 cases with available DNA using a QuantStudio 7 and Taqman Genotyper software 1.4.0. APOE genotyping was performed for all 244 cases as previously described [9].
Statistical analysis
We compared demographic, pathological, clinical, genetic, and neuroimaging variables between TDP-43 type-α and TDP-43 type-β variants using Fisher’s Exact or Wilcoxon rank sum tests where appropriate. P < 0.05 was considered statistically significant. We also fitted a linear regression on log-transformed volume adjusting for age at death, TIV, and Braak stage to assess for relationships between volumes and TDP-43 type (type-α and type-β), as well as between volumes and TDP-43 type versus 309 cases without TDP-43 (i.e. TDP-43 negative cases or TDP-43 stage 0).
In order to predict TDP status we fitted a three-category multinomial logistic regression model to estimate the odds of TDP negative (stage 0) versus type-α versus type-β using four predictors: age at death, total intracranial volume, amygdala volume, and hippocampal volume.
All analyses were performed with R statistical software version 3.1.2 (R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Of the 244 cases with TDP-43, 131 (54%) met criteria for TDP-43 type-α and 110 (46%) met criteria for TDP-43 type-β. Three cases with TDP-43 immunoreactive inclusions were excluded from further analysis as they were found to have predominantly long thick dystrophic neurites in the entorhinal cortex and CA1 region of the hippocampus, reminiscent of FTLD-TDP type-C pathology [32]. There were no significant differences in demographic features between both groups except for age at death where those with TDP-43 type-α were slightly older on average than those with type-β (median [IQR] of 89 [70–104] years vs. 87 [56–105] years) (p=0.02) (Table 1).
Table 1:
Demographic characteristics
| TDP type-α (N =131) | TDP type-β (N = 110) | P-value | |
|---|---|---|---|
| No. Female, n (%) | 86 (66%) | 70 (64%) | 0.79 |
| Age at scan, yrs | 84 (66, 99) | 82 (53, 104) | 0.14 |
| Age at death, yrs | 89 (70, 104) | 87 (56,105) | 0.02 |
Data shown are n (%) or median (range). P-values for categorical variables are from Fisher’s exact tests, and Wilcoxon Rank Sum tests for continuous variables.
Pathological Findings
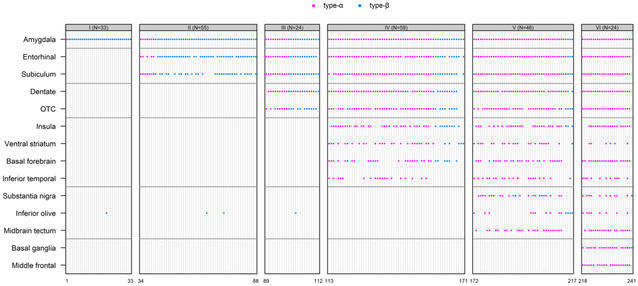
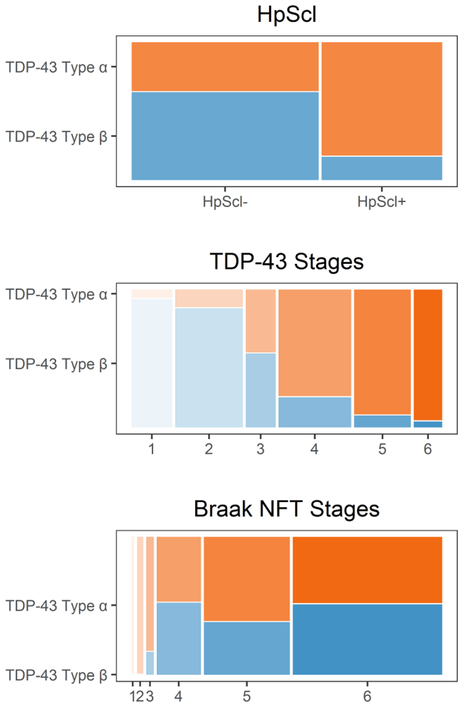
Those designated TDP-43 type-α were much more likely to have HpScl (60%) compared to those designated TDP-43 type-β (15%) (p<0.001) and were more likely to have a significantly higher TDP-43 stage compared to those with TDP-43 type-β (type-α =84% stage 4–6 versus type-β =84% stage 1–3; p<0.001) (Table 2). As an example, there was a 25-fold higher odds of TDP-43 type-β in TDP-43 stage 1 compared to higher stages (95% CI, 7–158) (p<0.0001). In contrast, there was a 22-fold higher odds of TDP-43 type-α in TDP stage 6 versus lower stages (95% CI, 5–400) (p=0.003). There were only two TDP type-α cases with amygdala only TDP-43. At the other end of the staging scheme, there was only one TDP type-β case with frontal lobe or basal ganglia involvement (Figure 2). TDP type-β pathology only involved the inferior temporal lobe in a single case. That is, although type-β cases showed TDP-43 extended to stages 4–6 in 18 cases, only one case (6%) showed TDP-43 involvement of the inferior temporal lobe. This was distinctly different from type-α cases where the inferior temporal lobe was affected in 50% of cases with stages 4–6. We also observed that all five cases that did not conform to the staging scheme, and instead showed “skipped regions” had been classified as TDP type-β (Figure 2). These cases did not conform due to involvement of the inferior olive. Figure 3 shows mosaic plots — graphical representation of two-way tables where the area of each “tile” depends on the number of individuals in a subgroup — that highlight the strong relationships between TDP-43 type and both HpScl and TDP-43 stage; showing that cases with HpScl, and cases with high TDP-43 stages, are more likely to be TDP-43 type-α. Although there was a difference in Braak NFT stage (TDP-43 type-β was associated with higher Braak NFT stage compared to TDP-43 type-α, p=0.03), Figure 3 shows that for Braak stages IV-VI, which accounted for91 % of the data in TDP type-α and 99% in TDP-43 type-β, there was no difference in types. There were no differences in the frequency of Lewy bodies, neuritic plaques or vascular disease between TDP-43 type-α and type-β.
Table 2:
Pathological characteristics
| TDP type-α | TDP type-β | P-value | |
|---|---|---|---|
| (N = 131) | (N = 110) | ||
| HpScI, n (%) | 78 (60%) | 16 (15%) | <0.001 |
| LBD, n (%) | 32 (36%) | 36 (35%) | >0.99 |
| TDP 6 stages, n (%) | <O.001 | ||
| Stage 1 | 2 (2%) | 31 (28%) | |
| Stage 2 | 7 (5%) | 48 (44%) | |
| Stage 3 | 11 (8%) | 13 (12%) | |
| Stage 4 | 46 (35%) | 13 (12%) | |
| Stage 5 | 42 (32%) | 4 (4%) | |
| Stage 6 | 23 (18%) | 1 (1%) | |
| Braak, n (%) | 0.03 | ||
| Braak 1 | 2 (2%) | 0 (0%) | |
| Braak 2 | 5 (4%) | 0 (0%) | |
| Braak 3 | 5 (4%) | 1 (1%) | |
| Braak 4 | 17 (13%) | 19 (17%) | |
| Braak 5 | 43 (33%) | 27 (25%) | |
| Braak 6 | 59 (45%) | 63 (57%) | |
| Amyloid, n (%) | 0.14 | ||
| Amyloid 0 | 11 (9%) | 2 (2%) | |
| Amyloid 1 | 9 (7%) | 9 (8%) | |
| Amyloid 2 | 35 (27%) | 28 (26%) | |
| Amyloid 3 | 74 (57%) | 70 (64%) | |
| Vascular disease, n (%) | 28 (31%) | 27 (26%) | 0.52 |
Data shown are n (%) or median (range). P-values for categorical variables are from Fisher’s exact tests, and Wilcoxon Rank Sum tests for continuous variables.
HpScl = hippocampal sclerosis; LBD = Lewy body disease;
Figure 2:
Patterns of TDP-43 positivity across 14 regions for all 241. The vertical axis indicates regions and the horizontal axis indicates subjects. Red dotes indicates TDP type-α cases and blue dot indicates TDP type-β cases.
Figure 3:
Mosaic plots shows the percentages of hippocampal sclerosis (HpScl), TDP-43, and Braak NFT stages by TDP-43 type. These plots are graphical representations of two-way tables where the area of a “tile” is related to the number of individuals in the table cell. The figure demonstrates the striking association of TDP-43 type-α with HpScl and higher TDP-43 stage. For Braak NFT stages, there is no difference between TDP-43 type-α and type-β at Braak NFT stages IV-VI where > 90% of data exists. Orange color represents TDP-43 type-α; Blue color represents TDP-43 Type-β.
Clinical and Imaging findings
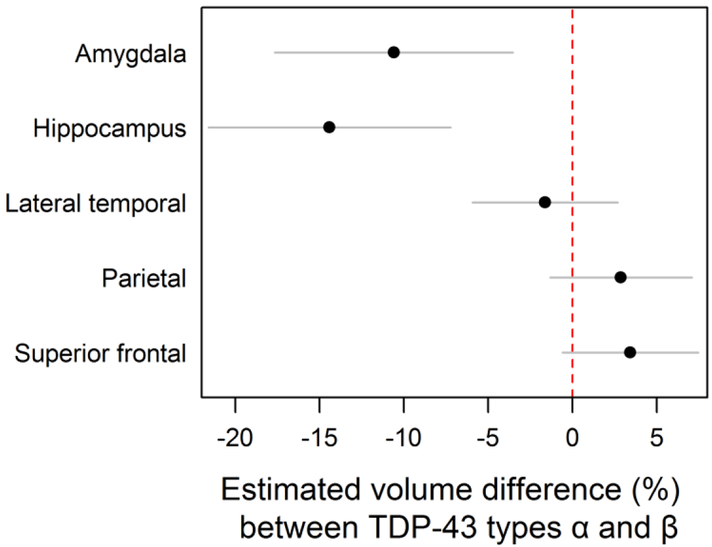
We found no significant differences in the final clinical diagnoses rendered before death, or on clinical measures between TDP-43 type-α and type-β cases, although there was a trend for TDP-43 type-α cases to perform more poorly on the AVLT delayed recall measure and on the Trail Making test B compared to TDP-43 type β (300 seconds vs 240 seconds, p=0.07) (Table 3). On neuroimaging, TDP-43 type-α had significantly smaller hippocampal volumes (4.7[1.6, 8.1] vs 5.2[3.1,7.9]; p=0.005), compared to TDP-43 type β cases, however TDP-43 type β cases had smaller parietal (37.1 [25.7, 52.2] vs 38.9 [23.8;52.6]; p=0.01) and lateral superior frontal (31.8 [23.4, 42.5] vs 32.2 [21.5, 46.4]; p=0.04) volumes (Table 3). However, after adjusting for age at death, TIV, and Braak stage, we found TDP-43 type-α cases to have significantly smaller hippocampal and amygdala volumes compared to TDP-43 type-β cases. Our adjusted analysis showed that cases with TDP-43 type-α had an estimated 10.6% (3.5%, 17.6%) lower amygdala volumes compared to type-β (p=0.003) (Figure 4). For hippocampus, cases with TDP-43 type-α had 14.4%(7.3%, 21.6%) lower volume than TDP-43 type-β (p <0.001).
Table 3:
Clinical and Imaging characteristics
| TDP type-α (N = 131) | TDP type-β (N = 110) | P-value | |
|---|---|---|---|
| Last diagnosis, n (%) | 0.21 | ||
| Cognitively normal | 4 (3%) | 8 (8%) | |
| Mild cognitive impairment | 13 (11%) | 5 (5%) | |
| Alzheimer’s dementia | 92 (78%) | 76 (80%) | |
| Dementia NOS | 9 (8%) | 6 (6%) | |
| Clinical scores | |||
| MMSE | 16 (1,30) | 14 (0, 29) | 0.24 |
| CDRSUM | 12.0 (0.0, 18.0) | 13.0 (0.0, 18.0) | 0.63 |
| Boston Naming Score | 32 (1,58) | 32 (2, 60) | 0.76 |
| DRS total | 97 (23, 144) | 96 (28, 140) | 0.73 |
| AVLT Delayed Recall | 0 (0, 7) | 0 (0, 10) | 0.09 |
| WAIS-R Block Design | 8 (0,31) | 6 (0, 23) | 0.72 |
| WMSR LOG MEM II | 0 (0, 24) | 0 (0, 22) | 0.14 |
| Trail-Making-Test A | 85 (39, 180) | 74 (29, 180) | 0.17 |
| Trail-Making-Test B | 300 (96, 300) | 240 (57, 300) | 0.07 |
| COWAT | 20 (2, 54) | 17 (2,44) | 0.22 |
| Imaging | |||
| Amygdala | 1.8 (0.7,3.1) | 1.9 (1.2,3.0) | 0.14 |
| Hippocampus | 4.7 (1.6,8.1) | 5.2 (3.1,7.9) | 0.005 |
| Lateral temporal | 46.8 (27.9, 68.2) | 47.4 (32.8, 64.3) | 0.68 |
| Parietal | 38.9 (23.8, 52.6) | 37.1 (25.7, 52.2) | 0.01 |
| Superior frontal | 32.2 (21.5,46.4) | 31.8 (23.4,42.5) | 0.04 |
Data shown are median (range). P-values are from Wilcoxon Rank Sum tests. Clinical data are from the last available.
MMSE = Mini-Mental State Examination; CDRSUM = Clinical Dementia Rating Sum of Boxes; DRS = Dementia Rating Scale; AVLT = Auditory Verbal Learning Test; WAIS-R = Wechsler Adult Intelligence Scale revised version; WMS-R LOG MEM = Wechsler Memory Scale Revised Logical memory; COWAT = Control Oral word Association Test
Figure 4.
We investigated the imaging data further by fitting linear regressions adjusting for age at death, total intracranial volume, and Braak stage. Our analysis showed that for cases with TDP-43 type-α had an estimated 11% (4%, 18%, p = 0.003) smaller amygdala volume than TDP-43 type-β. For hippocampi, TDP-43 type-α cases had 14% (7%, 22%, p <0.001) smaller volume than TDP-43 type-β cases. Plotted values are regression estimates and 95% confidence intervals.
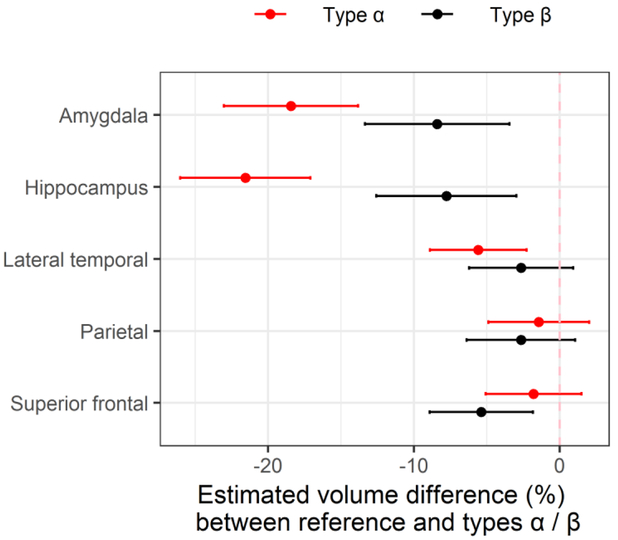
Given that we have previously identified a difference in hippocampal and amygdala volumes between TDP-43 positive and TDP-43 negative cases with TDP-43 positive cases having smaller volumes, and now find TDP-43 type-α cases to have smaller volumes than TDP-43 type-β cases, we compared both TDP-43 types (type-α and type-β) to the group of 309 cases without TDP-43. We found smaller amygdala volumes in both TDP-43 type-α and type-β cases compared to TDP-43 negative controls (type-α = −18.4% [−23.0%,−13.8%] and type-β = −8.40% [−13.4%, - 3.44%]; p<0.001), with similar results for hippocampal volumes for both TDP-43 type-α (−21.6% [−26.0%, −17.1%]; p<0.001) and type-β (−7.77 [−12.6%, −2.96%]; p =0.002) (Figure 5, Table 4). We also found TDP-43 type-α cases to have smaller lateral temporal lobe volumes (−5.59% [−8.90%, −2.28%]; p<0.001) and TDP-43 type-β cases to have smaller superior frontal lobe volumes (−5.37 [−8.91%, −1.83%]; p=0.003) compared to TDP-43 negative controls.
Figure 5.
represents an analysis between 309 TDP-43 negative cases (reference group) and TDP-43 type-α and type-β. After adjusting for age at death, TIV, and Braak stage, we found volume differences in the amygdala and hippocampus between both TDP-43 type-α and type-β cases, lateral temporal for type-α and superior frontal for type-β compared to TDP-43 negative cases. Plotted values are regression estimates and 95% confidence intervals.
Table 4:
Estimated volume differences (%) with 95% CI and p-values between TDP-43 types α/β and controls
| TDP-43 type-α (N = 103) | TDP-43 type-β (N = 84) | |||
|---|---|---|---|---|
| Est (95% Cl) | P-value | Est (95% Cl) | P-value | |
| Amygdala | −18.4% (−23.0%, −13.8%) | <0.001 | −8.40% (−13.4%, −3.44%) | <0.001 |
| Hippocampus | −21.6% (−26.0%, −17.1%) | <0.001 | −7.77% (−12.6%, −2.96%) | 0.002 |
| Lateral temporal | −5.59% (−8.90%, −2.28%) | <0.001 | −2.65% (−6.22%, 0.92%) | 0.15 |
| Parietal | −1.43% (−4.89%, 2.03%) | 0.42 | −2.66% (−6.38%, 1.07%) | 0.16 |
| Superior frontal | −1.79% (−5.07%, 1.50%) | 0.29 | −5.37% (−8.91%, −1.83%) | 0.003 |
Genetic findings
There was no difference in APOE allele frequency or the GRN rs5848 haplotype frequencies between type-α and type-β (Table 5). There was however, a difference in the TMEM106B rs3173615 haplotype frequencies. Specifically, there was a difference in the frequency of TMEM106B protective (GG) and risk (CC) haplotypes (SNP rs3173615 encoding p.T185S) in type-α cases compared to type-β cases (GG/CG/CC: 8%/42%/50% vs 24%/49%/27%; p=0.01)
Table 5:
Genetic characteristics
| TDP type-α | TDP type-β | P-value | |
|---|---|---|---|
| APOE, n (%) | N=131 | N=110 | 0.38 |
| 23 | 7 (5%) | 6 (6%) | |
| 24 | 4 (3%) | 4 (4%) | |
| 33 | 51 (40%) | 34 (31%) | |
| 34 | 46 (36%) | 52 (48%) | |
| 44 | 20 (16%) | 12 (11%) | |
| TMEM106b rs3173615, n (%) | N=62 | N=51 | 0.01 |
| GG | 5 (8%) | 12 (24%) | |
| CG | 26 (42%) | 25 (49%) | |
| CC | 31 (50%) | 14 (27%) | |
| GRN rs5848, n (%) | N=62 | N=51 | 0.96 |
| CC | 26 (42%) | 20 (39%) | |
| CT | 32 (52%) | 27 (53%) | |
| TT | 4 (6%) | 4 (8%) |
Predicting TDP-43 status and type
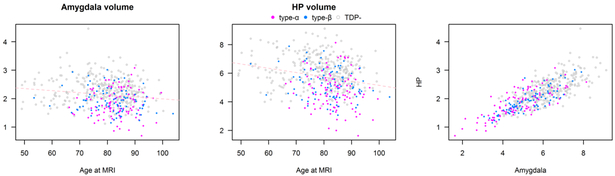
Figure 6 shows the relationships between age, amygdala volume and hippocampal volumes as predictors of TDP-43 status and type. We found that a 10-year increase in age was associated with a 1.8 fold increase in the odds of type-α relative to being TDP negative (95% CI 1.3–2.5, p<0.001) and a 1.6 fold increase in the odds of type-α relative to being type-β (95% CI 1.1—2.3, p=0.02). Relative to those who were TDP-43 negative, lower amygdala and lower hippocampal volumes were associated with increased odds of both types-α and type-β. Specifically, a 0.5 cm3 decrease in amygdala volume was associated with 1.7 fold increase in the odds of type-α versus TDP negative (95% CI 1.0–2.8; p=0.04) and a 1.9 fold increase in the odds of type-β versus TDP negative (95% CI 1.2–3.1; p=0.01). In terms of hippocampal volume, a 1 cm3 decrease in hippocampal volume was associated with a 2.7 fold increase in the odds of type-α relative to TDP negative (95% CI 1.8–4.0; p<0.001) and a 1.5 fold increase in the odds of type-β versus TDP negative (95% CI 1.0–2.1; p=0.03). Further, the same decrease of 1 cm3 in the hippocampus was associated with a 1.8 fold increase in the odds of type-α relative to type-β (95% CI 1.2–2.9; p=0.008).
Figure 6.
shows the relationships between age at MRI scan and amygdala volume (left plot), age at MRI scan and hippocampal volume (middle plot) and hippocampal volume and amygdala volume (right plot). TDP negative cases are shaded grey, TDP type-α cases are shaded red and TDP type-β cases are shaded blue. The plots show that in general for a given age those with the smallest amygdala and hippocampal volumes are TDP type-α. It also shows that there is strong correlation between amygdala and hippocampal volumes.
DISCUSSION
In this study we assess whether there is heterogeneity in TDP-43 deposition in non-FTLD brains. We found significant pathological differences in HpScl and TDP-43 stage, and significant genetic difference in TMEM106B haplotype frequencies, between TDP-43 type-α and type-β cases. We also found type-α cases to be associated with smaller hippocampi and amygdala volumes compared to TDP-43 type-β cases and both types to have smaller volumes (amygdala and hippocampi), compared to TDP-43 negative cases.
The FTLDs are classified first by abnormal protein deposition and then subclassified within protein category. FTLD-TDP has been shown to have five different types (type A-E) [30, 32], each with distinct histological features and with some types having distinct clinical and neuroimaging characteristics. We now show that, similar to FTLD-TDP, non-FTLD TDP-43 deposition also has distinct types. TDP-43 type-αis pathologically characterized by the presence of neuronal cytoplasmic inclusions, dystrophic neurites, neuronal intranuclear inclusions, and more widespread TDP-43 deposition with a majority of cases having frontotemporal neocortex involvement. Furthermore, HpScl is a feature of TDP-43 type-α. Therefore, TDP type-α is pathologically similar to FTLD-TDP type A [32]; although the burden of TDP-43 deposition is distinctly less in non-FTLD cases (personal observation). TDP-43 type-β is pathologically distinct from type-α with the main pathological characteristic being TDP-43 co-localized with NFTs in the same neuron. It is, therefore, not surprising that TDP-43 type-β was uncommon in cases of low Braak stage. Out of 110 type-β cases, 109 (99%) occurred in cases with Braak stage IV-VI. Therefore, TDP-43 type-β is much more likely to be identified in cases of high probability Alzheimer’s disease [13] where there is extensive NFT distribution. With-that-said, there is no evidence that TDP-43 type-β is more strongly associated with Alzheimer’s disease. In fact, both types were equally associated with a high frequency of the APOE ε4 genotype, and there was no difference in the CERAD neuritic plaque stage between them. It is certainly possible that type-α and type-β lie on a spectrum, but it appears less likely to be the situation that one type is a precursor to the other type, particularly given that we have also identified genetic differences between them.
Single nucleotide polymorphisms in TMEM106B are associated with an increased risk of FTLD-TDP [50] and SNP rs3173615, which is in linkage disequilibrium with SNP rs1990622, dictates a change from threonine to serine at position 185 (p.T185S). It has been shown that subjects with FTLD-TDP are less likely to have two copies of the minor TMEM106B allele (GG haplotype) compared to cognitively normal controls (6% GG in FTLD-TDP versus 19% GG in normal controls) [49]. Hence, the GG haplotype is considered protective. In our aged non-FTLD cohort, we found the TMEM106B GG haplotype frequency to be 8% in type-α cases, similar to the frequency reported in FTLD-TDP, and 24% in type-β cases, similar to the frequency reported in normal controls. This finding further supports TDP type-α being related to FTLD-TDP, and TDP type-α and β being distinct. Future genetic studies are needed to compare TMEM106B haplotype frequency across the FTLD-TDP types and TDP type-α and type-β.
We found three cases with TDP-43 that did not conform to either type-α or type-β designation. In these three cases, TDP-43 deposition was characterized by the presence of long thick dystrophic neurites in the neocortex and Pick body like inclusions in the dentate gyrus of the hippocampus. These features were identical to the features that characterizes FTLD -TDP type C [22, 32] which is strongly associated with a clinical diagnosis of semantic dementia [40]. None of our three cases were clinically diagnosed with frontotemporal dementia, including semantic dementia. Therefore, whether such cases represent a third variant of TDP-43 in non-FTLD brains or whether they are cases of FTLD-TDP type C remains to be determined. We did not come across cases with TDP-43 pathological features similar to those described in FTLD-TDP type B, D or E pathology. FTLD-TDP type B has many TDP-43 immunoreactive pre-inclusions and is strongly associated with clinical motor neuron disease [18, 22, 32] and genetically with C9ORF72 mutations [38]. FTLD-TDP type-D is associated TDP-43 immunoreactive neuronal intranuclear inclusions only, inclusion body myopathy, Paget disease of the bone and mutations in the valosin-containing protein gene [51]. FTLD-TDP type E is associated with TDP-43 immunoreactive granulofilamentous neuronal inclusions, abundant grains, and oligodendroglia inclusions and a very rapid clinical course [30]. Both FTLD-TDP type D and type E are extremely rare entities and none of the accompanying features of FTLD-TDP type B, D, E (motor neuron disease, myopathy, Paget’s disease of the bone and rapid course) were a feature of any of our cases. Hence, it is no surprise that we did not identify any such cases in our cohort.
One of the most striking differences that we observed between TDP-43 type-α and type-β was in the distribution of TDP-43. We observed that 85% of the type-α cases had widespread TDP-43 deposition beyond limbic areas which was significantly different from in type-β were approximately 85% of the cases showed a limbic-predominant pattern. In fact, we found a difference even when both types were stage 4. Specifically, while stage 4 type-α cases commonly involved the inferior temporal lobe, the inferior temporal lobe was never involved in any stage 4 cases. In fact, the inferior temporal lobe was only one involved in 1 stage 4–6 type-β case. This is strong evidence for type-β to truly have an association with limbic regions while type-α shows more of an association with the fronto-temporal neocortices.
Another difference we observed was with HpScl that was found to be a feature of TDP-43 type-α, not so much type-β. This is a significant finding given that HpScl has been intricately linked to TDP-43 [1, 24, 26, 39]. Interestingly, HpScl is most common in FTLD-TDP type A compared to FTLD type B and FTLD-TDP type C [22] although it does occur in cases with frontotemporal dementia with motor neuron disease[16] which typically shows FTLD-TDP type B pathology [22]. Many studies have struggled with separating the effects of TDP-43 from the effects of HpScl and have had to perform mediation analyses to try to do so [17, 26, 39, 58], similar to comparing TDP-43 cases with and without HpScl. The findings from this study supports an argument for all these prior studies basically separating the effect of TDP type-α from the effect of TDP-43 type-β. We therefore propose that future studies conduct TDP-43 typing, and assess for the effect of each type, instead of, or in addition to, conducting mediation analyses. While it is likely that HpScl is playing a role in explaining why TDP-43 type-α cases have smaller hippocampal volumes than TDP-43 type-β cases, it does not explain the difference observed in amygdala volume. It is possible that the more severe medial temporal volume loss in TDP-43 type-α could also be due to differences in TDP-43 burden or biology. For the latter, it is possible that there is a difference in the ratio of TDP-43 species (TDP-43 fragments vs full-length TDP-43) in type-α versus type-β [59].
We present evidence that the regional volumetric differences observed in this study could be useful to help predict TDP-43 status and type during life. We found that older age, and smaller volumes of the amygdala and hippocampus all helped predict TDP-43 positivity, although hippocampal volumes performed the best when also predicting TDP type and, therefore, could prove to be the most useful biomarker. In our cohort, cases with 1cm3 smaller hippocampi were almost twice as likely to be type-α as type-β. Given our TMEM106B haplotype findings, it is possible that prediction could be further improved when considering both hippocampal volumes and TMEM106B haplotype.
In our previous reports demonstrating a pattern of possible TDP-43 spread, i.e. TDP-43 staging scheme, we observed some cases that did not fit the TDP-43 staging scheme [19, 20]. In these non-conforming cases we noted that there were skipped regions (i.e. there were no involvement of regions from stages 3 or 4, yet there was TDP-43 deposition in the inferior olive but no other region from stage 5). We have now identified such cases as being TDP-43 type-β. The reason for involvement of the inferior olive without involvement other stage3–5 regions is unclear but it does bring forth the discussion of whether some TDP type-β cases could be mixed type-α/type-β cases. We cannot refute or confirm this possibility. However, this possibility would help to explain why we did not observe many TDP type-α cases that were stages 1–3 as such cases would have been classified as TDP type-β once there were NFT associated TDP-43.
There was a trend for TDP-43 type-α cases to perform poorer on the Trail-Making-Test B, a test of executive function, which would suggest that TDP-43 type-α may be having a greater effect on frontal systems. However, this observation requires further analysis since it was only trend level significance and since TDP-43 type-β cases, not type-α cases, showed a stronger association with smaller frontal lobe volumes compared to controls. We only found a trend for difference in one of our measures of loss of episodic memory between TDP-43 type-α and type-β cases. It likely did not reach significance since cases with TDP-43 type-α and type-β performed at floor level, with average test scores of 0 on both measures of episodic memory loss. This would support the notion that although the presence of TDP-43, independent of type, is strongly associated with loss of episodic memory, TDP-43 type-α may have a stronger association with episodic memory loss.
For years the field has struggled with the question of whether TDP-43 depositing in the brains of old-age individuals, particularly those with high TDP stage, should be considered FTLD-TDP. A diagnosis of FTLD requires evidence of a pathological process targeting the frontal and temporal lobes out of proportion to the rest of the brain. In fact, FTLD is pathologically diagnosed when there is gross and histological evidence of focal/distinct degeneration of the frontal and/or temporal lobes [8, 33]. We did not find gross or histological evidence of a lobar degeneration of the frontal and temporal lobes in these cases, and the deposition of TDP-43 (type-α and type-β) was much less than what is typically observed in FTLD-TDP. We did find TDP-43 type-α and type-β to be association with frontal and temporal lobe atrophy, but this was not striking, and amygdala and hippocampal atrophy was much more striking. We have also previously shown that even cases with high TDP-43 stage do not show clinical features of FTLD [20, 27] or much frontal and temporal atrophy on MRI [20] which is in sharp contrast to the striking degree of frontotemporal atrophy observed in FTLD-TDP cases [45, 55]. All these findings would not support TDP-43 type-α and type-β being an FTLD. With-that said, we are aware of FTLD cases that are not associated with striking and predominant frontal and temporal lobe atrophy or degeneration on pathology [23]. Furthermore, mutations in the C9ORF72 gene, for example, can be associated with FTLD-TDP pathology with little-to-no frontotemporal atrophy [40], and mutations in the progranulin gene can be associated with FTLD-TDP with prominent parietal lobe atrophy [40]. Alzheimer’s disease has been shown to be associated with prominent frontal lobar degeneration [7]. Therefore, maybe the real issue is that the phenotype of FTLD needs to be readdressed?
Another question the field has struggled with is whether TDP-43 in non-FTLD-TDP brains is biologically similar to, or different from the TDP-43 in FTLDTDP. This study was not designed to, and hence cannot answer this biological question. What we can say, is that there were morphological similarities between FTLD-TDP type-A and TDP-43 type-α that are not obviously distinguishable, at least at the light microscopic level. What is next needed to address this question are molecular, biochemical and additional genetic analyses of TDP-43 type α, type β and the FTLD-TDP types.
In summary, in this study we show that there are distinct TDP-43 variants in non-FTLD brains including those with Alzheimer’s disease. Further investigation is now needed to determine whether there are biochemical and genetic differences between both types, and how these two types are associated with FTLD-TDP.
ACKNOWLEDGEMENT
This study was funded by the following grants from the US National Institutes of Health (National Institute on Aging): R01 AG037491, P50 AG16574, U01 AG006786 and R35 NS097261. We thank the families of the patients who donated their brains to science and thus allowed completion of this study.
References
- 1.Amador-Ortiz C, Lin WL, Ahmed Z, Personett D, Davies P, Duara R, Graff-Radford NR, Hutton ML, Dickson DW (2007) TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer’s disease. Ann Neurol 61: 435–445 Doi 10.1002/ana.21154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arai T, Hasegawa M, Akiyama H, Ikeda K, Nonaka T, Mori H, Mann D, Tsuchiya K, Yoshida M, Hashizume Y et al. (2006) TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Biochem Biophys Res Commun 351: 602–611 Doi 10.1016/j.bbrc.2006.10.093 [DOI] [PubMed] [Google Scholar]
- 3.Arai T, Mackenzie IR, Hasegawa M, Nonoka T, Niizato K, Tsuchiya K, Iritani S, Onaya M, Akiyama H (2009) Phosphorylated TDP-43 in Alzheimer’s disease and dementia with Lewy bodies. Acta Neuropathol 117: 125–136 Doi 10.1007/s00401-008-0480-1 [DOI] [PubMed] [Google Scholar]
- 4.Arnold SJ, Dugger BN, Beach TG (2013) TDP-43 deposition in prospectively followed, cognitively normal elderly individuals: correlation with argyrophilic grains but not other concomitant pathologies. Acta Neuropathol 126: 51–57 Doi 10.1007/s00401-013-1110-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyle PA, Yang J, Yu L, Leurgans SE, Capuano AW, Schneider JA, Wilson RS, Bennett DA (2017) Varied effects of age-related neuropathologies on the trajectory of late life cognitive decline. Brain 140: 804–812 Doi 10.1093/brain/aww341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Braak H, Braak E (1991) Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol 82: 239–259 [DOI] [PubMed] [Google Scholar]
- 7.Brun A (1987) Frontal lobe degeneration of non-Alzheimer type. I. Neuropathology . Arch Gerontol Geriatr 6: 193–208 [DOI] [PubMed] [Google Scholar]
- 8.Cairns NJ, Bigio EH, Mackenzie IR, Neumann M, Lee VM, Hatanpaa KJ, White CL, 3rd, Schneider JA, Grinberg LT, Halliday G et al. (2007) Neuropathologic diagnostic and nosologic criteria for frontotem poral lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol 114: 5–22 Doi 10.1007/s00401-007-0237-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davidson YS, Raby S, Foulds PG, Robinson A, Thompson JC, Sikkink S, Yusuf I, Amin H, DuPlessis D, Troakes C et al. (2011) TDP-43 pathological changes in early onset familial and sporadic Alzheimer’s disease, late onset Alzheimer’s disease and Down’s syndrome: association with age, hippocampal sclerosis and clinical phenotype. Acta Neuropathol 122: 703–713 Doi 10.1007/s00401-011-0879-y [DOI] [PubMed] [Google Scholar]
- 10.Dickson DW, Davies P, Bevona C, Van Hoeven KH, Factor SM, Grober E, Aronson MK, Crystal HA (1994) Hippocampal sclerosis: a common pathological feature of dementia in very old (> or = 80 years of age) humans. Acta Neuropathol 88: 212–221 [DOI] [PubMed] [Google Scholar]
- 11.Folstein MF, Robins LN, Helzer JE (1983) The Mini-Mental State Examination. Arch Gen Psychiatry 40: 812. [DOI] [PubMed] [Google Scholar]
- 12.Geser F, Robinson JL, Malunda JA, Xie SX, Clark CM, Kwong LK, Moberg PJ, Moore EM, Van Deerlin VM, Lee VM et al. (2010) Pathological 43-kDa transactivation response DNA-binding protein in older adults with and w ithout severe mental illness. Arch Neurol 67: 1238–1250 Doi 10.1001/archneurol.2010.254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Group W (1998) Consensus report of the Working Group on: “Molecular and Biochemical Markers of Alzheimer’s Disease”. The Ronald and Nancy Reagan Research Institute of the Alzheimer’s Association and the National Institute on Aging Working Group. Neurobiol Aging 19:109–116 [PubMed] [Google Scholar]
- 14.Hu WT, Josephs KA, Knopman DS, Boeve BF, Dickson DW, Petersen RC, Parisi JE (2008) Temporal lobar predominance of TDP-43 neuronal cytoplasmic inclusions in Alzheimer disease. Acta Neuropathol 116: 215–220 Doi 10.1007/s00401-008-0400-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Josephs KA (2018) Fitting TDP-43 into the APOE epsilon4 and neurodegeneration story. Lancet Neurol 17: 735–737 Doi 10.1016/S1474-4422(18)30288-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Josephs KA, Dickson DW (2007) Hippocampal sclerosis in tau-negative frontotemporal lobar degeneration. Neurobiol Aging 28: 1718–1722 Doi 10.1016/j.neurobiolaging.2006.07.010 [DOI] [PubMed] [Google Scholar]
- 17.Josephs KA, Dickson DW, Tosakulwong N, Weigand SD, Murray ME, Petrucelli L, Liesinger AM, Senjem ML, Spychalla AJ, Knopman DS et al. (2017) Rates of hippocampal atrophy and presence of post-mortem TDP-43 in patients with Alzheimer’s disease: a longitudinal retrospective study. Lancet Neurol 16: 917–924 Doi 10.1016/S1474-4422(17)30284-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Josephs KA, Hodges JR, Snowden JS, Mackenzie IR, Neumann M, Mann DM, Dickson DW (2011) Neuropathological background of phenotypical variability in frontotemporal dementia. Acta Neuropathol 122: 137–153 Doi 10.1007/s00401-011-0839-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Josephs KA, Murray ME, W hitwell JL, Parisi JE, Petrucelli L, Jack CR, Petersen RC, Dickson DW (2014) Staging TDP-43 pathology in Alzheimer’s disease. Acta Neuropathol 127: 441–450 Doi 10.1007/s00401-013-1211-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Josephs KA, Murray ME, W hitwell JL, Tosakulwong N, Weigand SD, Petrucelli L, Liesinger AM, Petersen RC, Parisi JE, Dickson DW (2016) Updated TDP-43 in Alzheimer’s disease staging scheme. Acta Neuropathol 131: 571–585 Doi 10.1007/s00401-016-1537-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Josephs KA, Nelson PT (2015) Unlocking the mysteries of TDP-43. Neurology 84: 870–871 Doi 10.1212/WNL.0000000000001322 [DOI] [PubMed] [Google Scholar]
- 22.Josephs KA, Stroh A, Dugger B, Dickson DW (2009) Evaluation of subcortical pathology and clinical correlations in FTLD-U subtypes. Acta Neuropathol 118: 349–358 Doi 10.1007/s00401-009-0547-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Josephs KA W hitwell JL, Jack CR, Parisi JE, Dickson DW (2006) Frontotemporal lobar degeneration w ithout lobar atrophy. Arch Neurol 63: 1632–1638 Doi 10.1001/archneur.63.11.1632 [DOI] [PubMed] [Google Scholar]
- 24.Josephs KA W hitwell JL, Knopman DS, Hu WT, Stroh DA, Bake M, ademakers R, Boeve BF, Parisi JE, Smith GE et al. (2008) Abnormal TDP-43 immunoreactivity in AD modifies clinicopathologic and radiologic phenotype. Neurology 70: 1850–1857 Doi 10.1212/01.wnl.0000304041.09418.b1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Josephs KA W hitwell JL, Tosakulwong N, Weigand SD, Murray ME, Liesinger AM, Petrucelli L, Senjem ML, Ivnik RJ, Parisi JE et al. (2015) TAR DNA-binding protein 43 and pathological subtype of Alzheimer’s disease impact clinical features. Ann Neurol 78: 697–709 Doi 10.1002/ana.24493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Josephs KA W hitwell JL, Weigand SD, Murray ME, Tosakulwong N, Liesinger AM, Petrucelli L, Senjem ML, Knopman DS, Boeve BF et al. (2014) TDP-43 is a key player in the clinical features associated with Alzheimer’s disease. Acta Neuropathol 127: 811–824 Doi 10.1007/s00401-014-1269-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jung Y, Dickson DW, Murray ME, W hitwell JL, Knopman DS, Boeve BF, Jack CR, Jr, Parisi JE, Petersen RC, Josephs KA (2014) TDP-43 in Alzheimer’s disease is not associated with clinical FTLD or Parkinsonism. J Neurol 261: 1344–1348 Doi 10.1007/s00415-014-7352-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaplan E, Goodglass H, Weintraub S (1978) The Boston Naming Test. Veterans Administration Medical Center, City [Google Scholar]
- 29.Kwong LK, Uryu K, Trojanowski JQ, Lee VM (2008) TDP-43 proteinopathies: neurodegenerative protein misfolding diseases w ithout amyloidosis. Neurosignals 16: 41–51 Doi 10.1159/000109758 [DOI] [PubMed] [Google Scholar]
- 30.Lee EB, Porta S, Michael Baer G, Xu Y, Suh E, Kwong LK, Elman L, Grossman M, Lee VM, Irwin DJ et al. (2017) Expansion of the classification of FTLD-TDP: distinct pathology associated with rapidly progressive frontotem poral degeneration. Acta Neuropathol 134: 65–78 Doi 10.1007/s00401-017-1679-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lezak M (1995) Neuropsychological Assessment Oxford University Press, City [Google Scholar]
- 32.Mackenzie IR, Neumann M, Baborie A, Sampathu DM, Du Plessis D, Jaros E, Perry RH, Trojanowski JQ, Mann DM, Lee VM (2011) A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol 122: 111–113 Doi 10.1007/s00401-011-0845-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mackenzie IR, Neumann M, Bigio EH, Cairns NJ, Alafuzoff I, Kril J, Kovacs GG, Ghetti B, Halliday G, Holm IE et al. (2009) Nomenclature for neuropathologic subtypes of frontotemporal lobar degeneration: consensus recommendations. Acta Neuropathol 117: 15–18 Doi 10.1007/s00401-008-0460-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mattis S (1988) Dementia rating scale: Professional Manual. Psychological Assessment Resources, City [Google Scholar]
- 35.McAleese KE, Walker L, Erskine D, Thomas AJ, McKeith IG, Attems J (2017) TDP-43 pathology in Alzheimer’s disease, dementia with Lewy bodies and ageing. Brain Pathol 27: 472–479 Doi 10.1111/bpa.12424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR, Jr., Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R et al. (2011) The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7: 263–269 Doi 10.1016/j.jalz.2011.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L (1991) The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology 41: 479–486 [DOI] [PubMed] [Google Scholar]
- 38.Murray ME, Bieniek KF, Banks Greenberg M, DeJesus-Hernandez M, Rutherford NJ, van Blitterswijk M, Niemantsverdriet E, Ash PE, Gendron TF, Kouri N et al. (2013) Progressive amnestic dementia, hippocampal sclerosis, and mutation in C9ORF72. Acta Neuropathol 126: 545–554 Doi 10.1007/s00401-013-1161-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nag S, Yu L, Capuano AW, Wilson RS, Leurgans SE, Bennett DA, Schneider JA (2015) Hippocampal sclerosis and TDP-43 pathology in aging and Alzheimer disease. Ann Neurol 77: 942–952 Doi 10.1002/ana.24388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neary D, Snowden JS, Gustafson L, Passant U, Stuss D, Black S, Freedman M, Kertesz A, Robert PH, Albert M et al. (1998) Frontotemporal lobar degeneration: a consensus on clinical diagnostic criteria. Neurology 51: 1546–1554 [DOI] [PubMed] [Google Scholar]
- 41.Neumann M, Sampathu DM, Kwong LK, Truax AC, Micsenyi MC, Chou TT, Bruce J, Schuck T, Grossman M, Clark CM et al. (2006) Ubiquitinated TDP-43 in frontotem poral lobar degeneration and amyotrophic lateral sclerosis. Science 314: 130–133 Doi 10.1126/science.1134108 [DOI] [PubMed] [Google Scholar]
- 42.Petersen RC (2004) Mild cognitive impairment as a diagnostic entity. J Intern Med 256: 183–194 Doi 10.1111/j.1365-2796.2004.01388.x [DOI] [PubMed] [Google Scholar]
- 43.Rauramaa T, Pikkarainen M, Englund E, Ince PG, Jellinger K, Paetau A, Alafuzoff I (2013) Consensus recommendations on pathologic changes in the hippocampus: a postmortem multicenter inter-rater study. J Neuropathol Exp Neurol 72: 452–461 Doi 10.1097/NEN.0b013e318292492a [DOI] [PubMed] [Google Scholar]
- 44.Rey A (1964) L’examen clinique en psychologie. Presses Universitaires de France, City [Google Scholar]
- 45.Rohrer JD, Geser F, Zhou J, Gennatas ED, Sidhu M, Trojanowski JQ, Dearmond SJ, Miller BL, Seeley WW (2010) TDP-43 subtypes are associated w ith distinct atrophy patterns in frontotemporal dementia. Neurology 75: 2204–2211 Doi 10.1212/WNL.0b013e318202038c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rutherford NJ, Carrasquillo MM, Li M, Bisceglio G, Menke J, Josephs KA, Parisi JE, Petersen RC, Graff-Radford NR, Younkin SG et al. (2012) TMEM106B risk variant is implicated in the pathologic presentation of Alzheimer disease. Neurology 79: 717–718 Doi 10.1212/WNL.0b013e318264e3ac [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spreen O, Strauss E (1998) Compendium of Neuropsychological tests, second edition: administration, norms and commentary. Oxford University Press, City [Google Scholar]
- 48.Tan RH, Kril JJ, Fatima M, McGeachie A, McCann H, Shepherd C, Forrest SL, Affleck A, Kwok JB, Hodges JR et al. (2015) TDP-43 proteinopathies: pathological identification of brain regions differentiating clinical phenotypes. Brain 138: 3110–3122 Doi 10.1093/brain/awv220 [DOI] [PubMed] [Google Scholar]
- 49.van Blitterswijk M, Mullen B, Nicholson AM, Bieniek KF, Heckman MG, Baker MC, DeJesus- Hernandez M, Finch NA, Brown PH, Murray ME et al. (2014) TMEM106B protects C9ORF72 expansion carriers against frontotem poral dementia. Acta Neuropathol 127: 397–406 Doi 10.1007/s00401-013-1240-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van Deerlin VM, Sleiman PM, Martinez-Lage M, Chen-Plotkin A, Wang LS, Graff-Radford NR, Dickson DW, Rademakers R, Boeve BF, Grossman M et al. (2010) Common variants at 7p21 are associated with frontotemporal lobar degeneration with TDP-43 inclusions. Nat Genet 42: 234–239 Doi 10.1038/ng.536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Watts GD W ymer J, Kovach MJ, Mehta SG, Mumm S, Darvish D, Pestronk A, Whyte MP, Kimonis VE (2004) Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosin-containing protein. Nat Genet 36: 377–381 Doi 10.1038/ng1332 [DOI] [PubMed] [Google Scholar]
- 52.Wechsler D (1987) Wechsler Memory Scale-Revised (Manual). Psychological Corporation, City [Google Scholar]
- 53.Wennberg A, Tosalkulwong N, Lesnick T, Murray ME, W hitwell JL, Liesinger AM, Petrucelli L, Boeve B, Parisi JE, Knopman D et al. (2018) Association of apolipoprotein E epsilon 4 with transcative response DNA binding protein 43. JAMA Neurol: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Whitwell JL, Crum WR, Watt HC, Fox NC (2001) Normalization of cerebral volumes by use of intracranial volume: implications for longitudinal quantitative MR imaging. AJNR American journal of neuroradiology 22: 1483–1489 [PMC free article] [PubMed] [Google Scholar]
- 55.Whitwell JL, Jack CR,, Parisi JE, Senjem ML, Knopman DS, Boeve BF, Rademakers R, Baker M, Petersen RC, Dickson DW et al. (2010) Does TDP-43 type confer a distinct pattern of atrophy in frontotemporal lobar degeneration? Neurology 75: 2212–2220 Doi 10.1212/WNL.0b013e31820203c2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wilson AC, Dugger BN, Dickson DW, Wang DS (2011) TDP-43 in aging and Alzheimer’s disease - a review. Int J Clin Exp Pathol 4: 147–155 [PMC free article] [PubMed] [Google Scholar]
- 57.Wilson RS, Yu L, Trojanowski JQ, Chen EY, Boyle PA, Bennett DA, Schneider JA (2013) TDP-43 pathology, cognitive decline, and dementia in old age. JAMA Neurol 70: 1418–1424 Doi 10.1001/jamaneurol.2013.3961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang HS, Yu L, White CC, Chibnik LB, Chhatwal JP, Sperling RA, Bennett DA, Schneider JA, De Jager PL (2018) Evaluation of TDP-43 proteinopathy and hippocampal sclerosis in relation to APOE epsilon4 haplotype status: a community-based cohort study. Lancet Neurol 17: 773–781 Doi 10.1016/S1474-4422(18)30251-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang YJ, Xu YF, Cook C, Gendron TF, Roettges P, Link CD, Lin WL, Tong J, Castanedes-Casey M, Ash P et al. (2009) Aberrant cleavage of TDP-43 enhances aggregation and cellular toxicity. Proc Natl Acad Sci U S A 106: 7607–7612 Doi 10.1073/pnas.0900688106 [DOI] [PMC free article] [PubMed] [Google Scholar]