Abstract
Objective:
The universal genetic testing initiative (UGTI) is a quality improvement effort to increase rates of guideline-based genetic counseling (GC) and genetic testing (GT) of patients with potentially hereditary cancers. The UGTI was disseminated to a county hospital gynecologic oncology clinic that serves a diverse, indigent patient population.
Methods:
Using the Model for Improvement quality improvement framework, interventions including integrated GC, clinic tracking, assisted GC referrals, and provider education were tested over 26 months. A retrospective data review included patients with high-grade, non-mucinous epithelial ovarian, fallopian tube, and primary peritoneal cancers (HGOC) and endometrial cancers (EC) diagnosed between 9/1/12 - 8/31/16. Statistical analyses were performed to describe the population and to evaluate rates of recommendation and use of immunohistochemistry tumor testing (IHC), GC, and GT.
Results:
A cohort of 241 patients (57 HGOC, 184 EC) were included. At the conclusion of the study 84.2% of HGOC patients were referred for GC, 89.6% (43/48) completed GC, and 90.7% (39/43) completed GT. Of EC patients, 81.0% were recommended to have IHC and 62.4% (93/149) completed IHC. Patients with HGOC diagnosed during dissemination of UGTI were significantly more likely to receive a recommendation for GC (p=0.02) and to complete GT (p=0.03) than those diagnosed before UGTI. Patients with EC were significantly more likely to complete IHC if diagnosed after UGTI than those diagnosed prior to dissemination (p<0.001).
Conclusions:
The UGTI can be adapted to increase use of guideline-based cancer genetics services in a diverse, indigent, gynecologic cancer patient population.
Keywords: Genetic counseling, Genetic testing, Ovarian cancer, Endometrial cancer, Quality improvement
Introduction
The National Comprehensive Cancer Network (NCCN) guidelines recommend that all women with invasive, epithelial ovarian, fallopian tube, and primary peritoneal cancers receive genetic counseling (GC) and consider germline genetic testing (GT) for the BRCA1 and BRCA2 genes1. Identification of a BRCA mutation can impact cancer treatment options (such as PARP-inhibitor therapy), guide cancer screening, and can be used for cascade testing to determine family members’ risks. An estimated 15-20% of high-grade, non-mucinous epithelial ovarian, fallopian tube, and primary peritoneal cancers (HGOC) have an underlying BRCA mutation which represents a population with a high mutation prevalence2–4.
In contrast, only 2-3% of patients with endometrial cancer (EC) are estimated to have an underlying germline mutation in the Lynch syndrome genes (MLH1, MSH2/EPCAM, MSH6, and PMS2)5. Patients with EC can have tumor testing for mismatch repair deficiency, assessed through immunohistochemistry (IHC) and/or microsatellite instability (MSI) tests, which can help identify underlying germline Lynch syndrome mutations. The NCCN guidelines support universal tumor testing of all patients with EC, but also allow for the use of stricter criteria (such as restricting testing by age at diagnosis, or meeting Bethesda or Amsterdam criteria)6. Best practice at our institution is to follow universal tumor testing recommendations, given the limitations of restricting tumor testing to subgroups of patients5,7. Tumor test results may guide immunotherapy eligibility determination, and a Lynch syndrome mutation can inform cancer screening recommendations and cascade testing of relatives.
For patients with HGOC or EC, their cancer diagnosis is sufficient to meet NCCN guidelines for genetics assessment, however prior studies have found that in the United States, approximately 12-50% of patients with HGOC receive a recommendation for, or complete, GC and GT and an estimated 13-50% of patients with EC receive tumor testing and appropriate genetics services8–14. Quality improvement is one approach that can be used to address this gap in the receipt of guideline-based care.
We disseminated the previously described quality improvement effort, the Universal Genetic Testing Initiative (UGTI), from a large, academic, tertiary care center gynecologic oncology clinic to a county hospital gynecologic oncology clinic15. The county hospital is part of a fully integrated healthcare system that serves as part of a healthcare safety net for residents of Harris county in Houston, Texas16. We sought to assess the feasibility of UGTI dissemination, and to use quality improvement methods to increase the recommendation for, and use of, genetics services among patients with HGOC and EC in this oncology care setting.
Methods
Approval for the quality improvement project was obtained from The University of Texas MD Anderson Cancer Center’s Quality Improvement Assessment Board and the Harris Health Department of Quality Programs and Accreditation. The retrospective data collection and analysis was approved with a waiver of informed consent from the MD Anderson Cancer Center Institutional Review Board and the Harris Health Institutional Review Board.
Patients diagnosed with HGOC or EC between September 1, 2012 and August 31, 2016 and who received treatment in the outpatient gynecologic oncology clinic at Lyndon B. Johnson Hospital of the Harris Health system were identified from the hospital’s cancer registry. Patients under 18 years of age and those with other cancer histology were excluded. Patients with synchronous HGOC and EC were counted as HGOC cases. The county hospital electronic medical records were used to collect cancer history and clinic encounters through August 31, 2017. All data were collected and stored in a password protected REDCap database17.
The UGTI dissemination was led by a cancer genetic counselor with experience in the original UGTI efforts. The two gynecologic oncologists and the clinic staff (including two nurses, and two advanced practice registered nurses (APRN)) at the county hospital did not participate in the original UGTI. All providers involved in the UGTI dissemination effort at the county hospital, except for one nurse employed by Harris Health, were employed by MD Anderson Cancer Center and provided services to the county hospital through an institutional agreement. Prior to dissemination, a modified version of an environmental scan was used to assess cancer genetics services18. The environmental scan identified that cancer GC clinics were available elsewhere within the hospital (as of 2011) and within the healthcare system at a different hospital, but not within the gynecologic oncology clinic19. The assessment also identified that tumor testing processes were not well defined by the gynecologic oncology clinical team. The tumor testing process throughout this study period required the ordering of IHC on endometrial tumors by a treating physician or APRN. Tumor testing at the county hospital includes IHC analysis without MSI (MSI and MLH1 methylation analyses must be requested by the clinical team based on IHC findings or other clinical indications, and are financially approved by the hospital before they are performed). Processes barriers were primarily related to determination and communication of who would place the order and when, clarifying which patients were eligible for IHC testing, and who would review and triage results.
The Model for Improvement framework, which includes Plan-Do-Study-Act (PDSA) cycles was used to guide UGTI dissemination20,21. Quarterly PDSA cycles were used to evaluate interventions, update clinic data, and to check for opportunities for change or improvement. Clinic interventions were adapted from the original, previously described UGTI interventions: Integrated Genetic Counseling (IGC), Clinic patient tracking, Assisted Genetic Counseling Referral (AGCR), Provider notifications, and Physician education15. A description of each intervention, its measurement, and the adaptations are detailed in Table 1. Adaptations were primarily driven by resource constraints within the county hospital environment. UGTI dissemination was initiated on June 30, 2015 and continued through August 31, 2017, totaling 26 months of active intervention.
Table 1.
UGTI Quality Improvement Interventions
| Name of Intervention | Original UGTI Intervention Description15 | County Hospital UGTI Adaptations | Date Initiated | Intervention Metrics | Duration |
|---|---|---|---|---|---|
| Integrated Genetic Counseling (IGC) | • Integrate genetic counselor in Gyn One clinic • Optimize GC schedule • Standardized urgent appointment request process |
• Optimized scheduling referral criteria: diagnosis of HGOC, diagnosis of EC with abnormal tumor studies, or cascade testing • 4 appointments per GC clinic: 2 morning clinics per month (1st and 3rd Tuesdays) • No urgent appointments • Spanish interpretation provided by telephone |
6/30/2015 | • Frequency of clinic (dates) • Numbers of GC appointments completed, canceled, no-show, or not used • Time between cancer diagnosis and completed GC appointment |
Ongoing |
| Physician education | Physicians attend national meetings and conferences discussing hereditary cancer. Genetic counselors provide education as needed. | • Direct communication by genetic counselor to physicians rather than conference/event • Stakeholder meeting to review tumor testing process, billing, timelines, and expectations with pathology and Gyn One staff prior to launching provider email notifications for patients with EC |
12/1/2015 | • Frequency of meetings for education | Ongoing (as needed) |
| Clinic patient tracking | Research data coordinator collected data from clinic schedules and the medical record to determine whether patients received GC/GT. | • Genetic counselor performed clinic tracking during clinic time when patients canceled/no-showed appointments or when no appointments scheduled. • Clinic tracking included retrospective collection of patients, with first date of new patient presentation to Gyn One clinic of 1/1/2015, then continued prospectively • Included tracking of patients with HGOC and EC |
1/1/2016 | • Frequency of clinic review (quarterly) | Ongoing |
| Assisted Genetic Counseling Referral (AGCR) | Electronic referral to GC drafted for patients who have not had GC/GT. | • Medical record system prohibited drafting of electronic referrals to Gyn One clinic GC • All notifications of patients eligible for GC/GT were sent via email • Notifications sent by genetic counselor to the physician and clinic care team • Notifications were sent for patients with HGOC to have GC, patients with EC to have tumor testing • APRN tracked EC tumor testing requests and triaged patient referrals to GC per results |
1/1/2016 | • Frequency of notifications (quarterly) • Number of cases identified for notification • Outcomes of notifications |
Ongoing |
| Provider email notifications | Research data coordinator and genetic counselor notify physician/care team of upcoming patients not previously referred for GC/GT. |
Abbreviations: Gyn One: gynecologic oncology, GC: Genetic counseling, HGOC: high-grade, non-mucinous epithelial ovarian, fallopian tube, and primary peritoneal cancers, EC: endometrial cancer, GT: genetic testing, APRN: Advanced Practice Registered Nurse
Quality improvement metrics for HGOC patients included measuring the rates of documented recommendation for genetics evaluation, completion of GC, and completion of GT. All HGOC patients completing GT had at least full gene analysis of BRCA1 and BRCA2, consistent with national guidelines. Additional genes were analyzed as clinically indicated based on tumor histology, family history, and/or as multi-gene panels became available. The quality improvement goals were aligned with the original UGTI which were to have at least 80% of HGOC patients receive a recommendation for genetics evaluation and at least 80% complete GC.
Quality improvement metrics for EC patients included measuring the rates of documented recommendation for tumor testing or genetics evaluation, completion of tumor testing, and if results were abnormal (defined as absent MSH2 and/or MSH6, absent MLH1 and/or PMS2 in the setting of absent MLH1 hypermethylation, and/or tumors with MSI-High status) recommendation for GC/GT, completion of GC, and completion of GT. The quality improvement goals for the EC patient population were to have at least 80% of patients receive a recommendation for tumor testing at least 80% complete IHC tumor testing.
Descriptive statistics were used to characterize the study population. Chi-square, Fisher’s exact and Mann-Whitney tests were used to analyze differences between subgroups. Statistical analysis was performed using the IBM SPSS, version 23 (Armonk, NY: IBM Corp.). P-values of <0.05 are considered statistically significant. Charts to assess changes in timeliness were created using Microsoft Excel.
Results
Between September 1, 2012 and August 31, 2016 a total of 241 patients (57 HGOC and 184 EC) were diagnosed and treated at the county hospital gynecologic oncology clinic. Patient demographic, medical, cancer, and family history characteristics are noted in Table 2. The patient population was 63.0% Hispanic/Latina ethnicity, 18.7% non-Hispanic Black, and 18.3% non-Hispanic White or other races. Spanish was the preferred language of 42.0% of patients. Of patients who reported their education, 85.0% reported completing 12 years of school or less. More than half of patients (55.6%) were uninsured and used the county health system’s assistance program.
Table 2.
Demographic and clinical characteristics of study population (N=241)
| Characteristic | HGOC1 (n=57) | EC (n=184) |
|---|---|---|
| Median age at dx (min, max) | 52.5 (23.1, 77.9) | 55.1 (22.5, 73.2) |
| Median BMI at dx (min, max) | 27.7 (19.1, 45.2) | 37.0 (17.1, 68.3) |
| n (%) | n (%) | |
| Marital status | ||
| Single | 20 (35.1) | 56 (30.4) |
| Married | 17 (29.8) | 70 (38.0) |
| Divorced | 6 (10.5) | 21 (11.4) |
| Widowed | 9 (15.8) | 16 (8.7) |
| Separated | 5 (8.8) | 21 (11.4) |
| Race/ethnicity | ||
| White, Hispanic/Latina | 35 (61.4) | 117 (63.6) |
| White, Non-Hispanic | 6 (10.5) | 24 (13.0) |
| Black, Non-Hispanic | 10 (17.5) | 35 (19.0) |
| Other | 6 (10.5) | 8 (4.3) |
| Primary language2 | ||
| English | 34 (60.7) | 104 (56.8) |
| Spanish | 22 (39.3) | 79 (43.2) |
| Years of education3 | ||
| ≤ 8 years | 5 (20.0) | 41 (35.7) |
| 9-12 years | 16 (64.0) | 57 (49.6) |
| > 12 years | 4 (16%) | 17 (14.8) |
| Health insurance | ||
| County Health System (uninsured) | 33 (58.0) | 104 (56.5) |
| Medicare/private insurance | 15 (26.3) | 47 (25.5) |
| Medicaid/managed Medicaid | 9 (15.8) | 33 (17.9) |
| Stage | ||
| I | 11 (19.3) | 109 (59.2) |
| II | 2 (3.5) | 11 (6.0) |
| III | 13 (22.8) | 26 (14.1) |
| IV | 18 (31.6) | 14 (7.6) |
| Unstaged/unknown | 13 (22.8) | 24 (13.0) |
| Grade | ||
| 1 or low | 0 (0.0) | 46 (25.0) |
| 2 | 9 (15.8) | 96 (52.2) |
| 3 or high | 43 (75.4) | 35 (19.0) |
| Unknown | 5 (8.8) | 7 (3.8) |
| Histology | ||
| Serous | 35 (61.4) | 14 (7.6) |
| Endometrioid | 9 (15.8) | 150 (81.5) |
| Other4 | 13 (22.8) | 20 (10.9) |
| Second primary cancer5 | ||
| Yes | 13 (22.8) | 22 (12.0) |
| No | 44 (77.2) | 162 (88.0) |
| Family history of cancer6 | ||
| Yes | 22 (45.8) | 91 (53.5) |
| No | 26 (54.2) | 79 (46.5) |
| Family history included a FDR with cancer | ||
| Yes | 15 (68.2) | 68 (74.7) |
| No | 7 (31.8) | 23 (25.3) |
Six patients had synchronous HGOC and EC and are counted as HGOC
Excludes 2 patients classified as unknown/other (HGOC, n=1; EC, n=1)
Excludes 101 patients whose education was not reported (HGOC, n=32; EC, n=69)
Other HGOC included: clear cell (n=5), Mullerian carcinoma not otherwise specified (n=3), adenocarcinoma (n=3), and mixed epithelial ovarian histology (n=2). Other EC included: adenocarcinoma (n=8), mixed endometrial histology (n=6), carcinosarcoma (n=5), and clear cell (n=1).
HGOC patients were significantly more likely than EC patients to have a second primary cancer (p=0.04). Second primary cancers among the 13 HGOC patients included: synchronous EC (n=6), bilateral breast cancer (n=1), colon cancer (n=1), thyroid cancer (n=1), leukemia (n=1), cervical cancer (n=1), brain cancer/tumor (n=1), stomach cancer/tumor (n=1). Second primary cancers among the 22 EC patients included: cervical or other gynecologic cancer (n=6), colorectal cancer (n=6), breast cancer (n=5), thyroid cancer (n=3), and bladder cancer (n=2).
Family history was not documented at the initial gynecologic oncology visit for 23 patients (HGOC, n=9; EC, n=14). Family history of cancer was not significantly different between HGOC patients and EC patients (p=0.35).
Abbreviations: HGOC = high grade ovarian cancer; EC = endometrial cancer; FDR = first-degree relative
UGTI Results
At the conclusion of the study, 84.2% (48/57) of all patients with HGOC received a recommendation for genetics services, 89.6% (43/48) completed GC, and 90.7% (39/43) completed GT. A total of 20.5% (8/39) of patients tested were found to have a pathogenic variant (6 BRCA1, 1 BRCA2, and 1 MSH2), consistent with previously reported mutation rates in HGOC patient populations. An additional 18.0% (7/39) of HGOC patients tested were found to have a variant of uncertain significance. Patients with HGOC diagnosed during the UGTI dissemination were significantly more likely to receive a recommendation for GC (p=0.02) and to complete genetic testing (p=0.03) than those diagnosed before the UGTI.
For patients with EC, overall 81.0% (149/184) were recommended to have tumor testing but only 62.4% (93/149) completed testing, with 14.0% (13/93) having abnormal results. Of patients with EC who had tumor testing completed, 16.1% (15/93) were recommended for GC including 5 patients with normal tumor testing results, and 76.9% (10/13) of the patients with abnormal results. The 5 patients with normal tumor testing results who were recommended for GC included 2 cases where clinic team was unsure if tumor testing had been done and were requesting review of the case, 1 patient had a family history of cancer, and 2 patients had personal histories of other primary cancer diagnoses. A total of 12 patients with EC were seen for GC (including 10 with abnormal tumor testing results), 8 completed GT (including 7 with abnormal tumor testing results), and 3 had pathogenic variants identified (2 MSH2 and 1 PMS2 mutation, all in patients with abnormal tumor testing results). Patients with EC were significantly more likely to complete tumor testing if diagnosed during the UGTI than those diagnosed prior to dissemination (p<0.001).
Recommendations for GC or tumor testing were not associated with patients’ age at diagnosis, language, ethnicity, educational level, marital status, or type of health insurance coverage. Cancer stage for patients with EC was not associated with recommendation for tumor testing. A higher proportion of Spanish-speaking patients with HGOC completed GT than non-Spanish speaking HGOC patients (p=0.024). Patients with EC who speak Spanish were trending toward higher rates of tumor testing completion, however this was not statistically significant (p=0.06). Hispanic/Latina patients with EC were more likely to complete tumor testing (p = 0.016) than patients of other races/ethnicities.
Figure 1 shows the uptake and the time to GC for patients with HGOC, and the uptake and time to tumor testing for patients with EC, throughout the study period. During the UGTI dissemination, the amount of time between cancer diagnosis and receipt of guideline-based genetics services decreased for patients with HGOC and EC so that most patients diagnosed during the UGTI dissemination received genetics services within 3 months of their cancer diagnosis.
Figure 1.
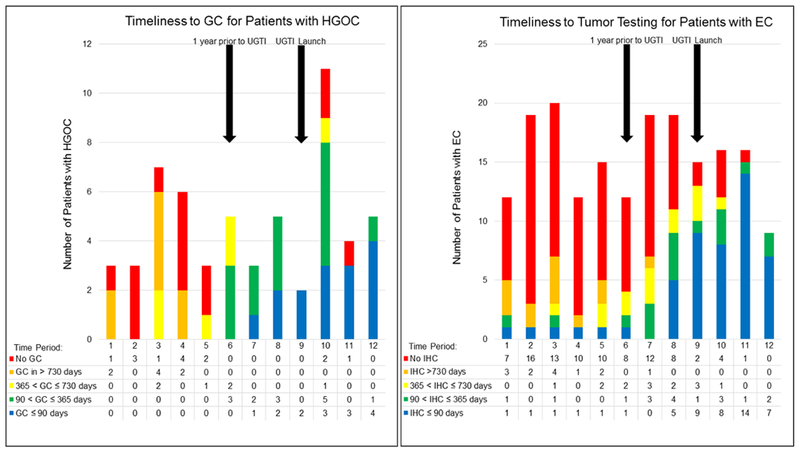
Timeliness Trends in Patient Receipt of Genetics Services
Time is measured in calendar days between cancer diagnosis and event (GC for HGOC and tumor testing for EC). Time periods represent 4 month intervals over the course of the study (9/1/2012 – 8/31/2016). Numbers in the tables represent the number of patients per study time period per timeliness category (time between diagnosis and event). Arrows are used to denote within which time period a UGTI event occurred, with Time Period 9 including the UGTI launch date of 6/30/2015 and Time Period 6 including the one-year prior date of 6/30/2014. The one-year prior date is noted because it captures patients who would be included in the clinic patient tracking intervention and is when an increase in timeliness is expected to improve from baseline due to UGTI activities.
Abbreviations: GC: Genetic Counseling, HGOC: High-grade, non-mucinous epithelial ovarian, fallopian tube, and primary peritoneal cancer, UGTI: Universal Genetic Testing Initiative, EC: endometrial cancer, IHC: immunohistochemistry tumor test.
Interventions
Between June 30, 2015 and August 31, 2017 there were 192 IGC appointments available and 42 patients from the study cohort were seen for GC through the IGC intervention (33 HGOC and 9 EC). Approximately 60% of IGC appointments were used for 84 consults and 31 follow-up visits, serving patients in this study cohort and those diagnosed outside of the study parameters. A total of 40 (20.8%) IGC appointments were scheduled but the patient canceled the appointment or failed to attend. Six of the 26 months of UGTI active intervention had 1 IGC clinic day instead of 2, due to conferences or holidays. Nearly 20% of IGC appointment slots were unused, with no patient scheduled to the appointment slot.
Clinic tracking identified 110 total patients who should have received a recommendation or referral for genetics services, but did not have one documented upon review of the records. Assisted referral notifications were sent to the clinical team for these patients, prompting the recommendation or referral to occur. Of these patients, 14 HGOC patients were noted as “missed” for recommendation and referral to GC. They were included in assisted referral notifications sent in January 2016 (11 patients), April 2016 (3 patients), and July 2016 (0 patients). Eleven (78.6%) of HGOC patients included in notifications were subsequently referred and completed GC. The 3 patients who did not complete GC included 1 patient who was in the process of transferring their care to a different hospital (GC appointment was scheduled but canceled) and 2 patients who were transitioning to hospice care (GC appointment was never scheduled). Nine patients were not recommended or referred for GC and were not included among patients “missed” during clinic tracking, which included 5 patients who were deceased at the time of clinic tracking and 4 patients who had transferred their cancer care prior to the time of clinic tracking.
Clinic tracking identified 96 EC patients as “missed” for recommendation and completion of tumor testing. They were included in notifications in January 2016 (87 patients), April 2016 (7 patients), and July 2016 (1 patient). Of patients included in notifications, 41(42.7%) subsequently underwent tumor testing with 22 having tumor testing performed more than 1 year after diagnosis, representing capture of otherwise missed opportunities. The 55 EC patients who did not complete tumor testing were in the January 2016 notification cohort and the most common barriers to testing included: patient lost to follow-up due to cancelation of appointments, moved to a different city, or insurance coverage issues (27.3%), discharged from oncology follow-up care prior to sending the notification (20%), transitioned to hospice or end-of-life care (9%), discharged at their 2016 visit without additional follow-up visits planned (9%), and future follow-up appointment scheduled outside of the timeframe of this study (7.3%).
Discussion
Dissemination of the UGTI from an academic, tertiary care gynecologic oncology clinic to a county hospital gynecologic oncology clinic successfully increased rates of recommendation and use of guideline-based genetics services in this oncology care setting. Following the 26 months of active intervention, our goal of at least 80% adherence to guideline-based recommendation for genetics services was met, with 84.2% of patients with HGOC recommended to have GC, 81.0% of patients with EC recommended to have tumor testing, and 89.6% of HGOC completed GC and 90.7% completed GT. In total, 11 patients were identified to have a pathogenic germline mutation, including 20.5% of HGOC patients who were tested, which is a mutation rate consistent with previous studies.
The UGTI dissemination expanded from the original UGTI by including EC patients in order to explore the effects of quality improvement efforts on a non-ovarian cancer population and on the complex clinical process of tumor testing. Improving tumor testing rates was challenging and our goal of 80% tumor testing completion was not met. One reason for the lag in improvement of tumor testing was the number of decision points in the testing process, including: identifying the patient with EC before or after surgery, ordering tumor testing during or after surgery, occasionally needing reflexive testing (MLH1 methylation), interpreting the result, and appropriately triaging the patient for genetics evaluation. Each decision point represents where an action may be missed, clinicians may be unsure of their responsibility, or where a patient may discontinue or decline care22. Additionally, the majority of EC patients had Stage I disease, and often after a period of surveillance, these patients are discharged to their primary care provider for follow-up which may reduce opportunities to complete tumor testing if it is not performed at the time of surgery or diagnosis. For patients with advanced stage EC, immunotherapy eligibility determination may provide an incentive to complete tumor testing. There can also be logistical challenges to ordering tumor testing, such as limited amount of specimen available for testing, or poor access to specimens when the patient’s diagnosis occurred at a different hospital. Additionally, IHC testing can occasionally result in patchy or incomplete staining - complicating result interpretation. Despite these challenges, active quality improvement interventions can increase completion of tumor testing (as shown in Figure 1). Disseminating the UGTI was considered feasible because interventions were able to be adapted to the county hospital environment without compromising effectiveness, and active intervention required fewer human resources than the original UGTI as noted in Table 1. Improvements were observed quickly following UGTI dissemination, as shown in Figure 1, likely due to the lower patient volume, smaller and engaged clinical team, and no one-time second-opinion visits for cancer treatment. The county hospital clinic structure and patient volumes may be more similar to gynecologic oncology clinics in rural or community settings, which suggests that UGTI may be feasible in other oncology care environments.
The IGC intervention was effective in the county hospital setting due to the structured referral criteria and limited staff time commitment (8 GC appointments per month). Cost is often cited as a barrier to patients seeking GT, however patients with health insurance met insurance approval guidelines for GT, and those who were uninsured used laboratory financial assistance programs by providing documentation of income. Although cancer GC services were available at the county hospital and within the healthcare system before UGTI dissemination, gynecologic oncology patients were not accessing these services. The improvement seen in the completion of GC and GT among patients with HGOC during UGTI dissemination demonstrates the effect of the IGC intervention, and the integrating or “embedding” genetics services in oncology clinics23,24. The number of HGOC and EC patients identified through clinic tracking as “missed” decreased over time, indicating physician learning and intention to improve, similar to results of audit-and-feedback interventions in other settings25.
The outcomes presented herein are from a single clinic, however strategies used in this single clinic were disseminated from the original UGTI, which had comparable outcomes in patients with HGOC15. Another limitation of our study was that the determination of GC or tumor testing recommendation required documentation in the medical record, which would not capture verbal discussions that occurred during clinic visits. Patients at the county hospital often face barriers to accessing health care (such as transportation issues, financial constraints, lack of health insurance, work and family demands, and differences in language and health literacy status) which were not addressed by the UGTI interventions, but were present in the patient population throughout the study period. Additionally, inclusion of a control arm in quality improvement efforts is often challenging, since excluding patients from receipt of guideline-based care is not recommended or ethical, and randomized systems-level interventions are often not possible. Through retrospective data review of patients diagnosed in September 2012 – June 2015, we captured a pre-dissemination cohort over a sufficient period of time to observe baseline trends in the use of genetics services. The significant and sustained increased use of genetics services during the UGTI dissemination, as shown in Figure 1, suggest that the improvements observed in this study can be attributed to the UGTI interventions, rather than influences from external events or normal variation in practice.
Dissemination of successful single-clinic quality improvement efforts in cancer genetics are limited, especially to undeserved communities, which represents an opportunity for future efforts and research. Further research is needed to assess the costs of applying UGTI in other patient populations and oncology care settings. Resource availability for clinic tracking and data collection and analysis may be a barrier for efficient UGTI dissemination to other settings. Opportunities to automate this process should be explored, as electronic medical records may have increasing capacity to include genetics services (such as receipt of GC and GT) as discrete and searchable fields. Inclusion and prioritization of guideline-based metrics for patient receipt of cancer genetics services in accreditation standards, such as the Commission on Cancer’s Cancer Program Standards, may incentivize hospitals to allocate appropriate resources to collect and track these metrics. Creative approaches to genetics service delivery should be studied in conjunction with quality improvement methods, such as use of telephone GC, telegenetics, and physician-coordinated testing with post-test GC. Dissemination of the UGTI to the county hospital gynecologic oncology clinic required a tailored approach, and accounted for resource availability and clinic volume through use of a genetic counselor employed by a neighboring hospital, and through restricting patient referral eligibility to those with the highest likelihood to have a mutation. Other low-resource clinics may consider further study of this approach to determine if it is efficient and feasible. Our study identified higher rates of tumor testing and GT completion among Hispanic/Latina and Spanish speaking patients. Studies of acculturation, communication, and perception of genetics services in our patient population are needed to evaluate possible reasons for the finding. Additionally, efforts are needed to ensure appropriate genetics services are made available to medically underserved patient populations in order to increase equitable care, decrease missed opportunities for cancer prevention, and improve the understanding of genetic testing and inherited cancer predispositions in diverse patient populations.
Conclusion
The dissemination of the UGTI to a county hospital gynecologic oncology clinic that serves a diverse, indigent patient population was feasible, and successfully increased the use and timeliness of guideline-based genetics services for patients with HGOC and EC over 26 months of active intervention. Tumor testing rates for patients with EC were more resistant to improvement, in part due to the complexity of the clinical process.
Highlights.
Dissemination of genetics quality improvement to a county hospital was feasible
Uptake of recommended genetic services increased to >80% by the end of 26 months
The time between cancer diagnosis and receipt of genetic services decreased
In this diverse, indigent population, 23% of tested patients had germline mutations
Acknowledgements:
We acknowledge the support from The University of Texas MD Anderson Cancer Center Moon Shot™ Program for this project. We thank Grace Tran, Kathleen Doughtie, Tori Thibodeaux, Mariela Nunez, Tracy Powell, and Armando Morin for their support and facilitation of the genetics clinics at Lyndon B. Johnson Hospital. We also thank all of the patients at Lyndon B. Johnson Hospital Gynecologic Oncology clinic for allowing us to participate in their care.
Funding:
This work was supported by The University of Texas MD Anderson Cancer Center Moon Shot™ Program and NIH/NCI award number P30CA016672 (Clinical Trials Support Resource and the Biostatistics Resource Group).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Submission Declaration:
This manuscript is submitted solely to Gynecologic Oncology and is not published elsewhere nor is it under consideration for publication elsewhere. Components of the manuscript were presented at the 2018 Society of Gynecologic Oncology Annual Meeting on Women’s Cancer in New Orleans, LA.
Conflict of Interest:
All authors declare no conflicts of interest. Dr. Sun reports funding from AstraZeneca and Inform Genomics outside the submitted work.
References
- 1.National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Genetic/Familial High-Risk Assessment: Breast and Ovarian, (ed 7/30/2018) www.nccn.org, National Comprehensive Cancer Network, 2018 [Google Scholar]
- 2.Pal T, Permuth-Wey J, Betts JA, et al. : BRCA1 and BRCA2 mutations account for a large proportion of ovarian carcinoma cases. Cancer 104:2807–16, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Risch HA, McLaughlin JR, Cole DE, et al. : Population BRCA1 and BRCA2 mutation frequencies and cancer penetrances: a kin-cohort study in Ontario, Canada. J Natl Cancer Inst 98:1694–706, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Alsop K, Fereday S, Meldrum C, et al. : BRCA mutation frequency and patterns of treatment response in BRCA mutation-positive women with ovarian cancer: a report from the Australian Ovarian Cancer Study Group. J Clin Oncol 30:2654–63, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hampel H, Frankel W, Panescu J, et al. : Screening for Lynch syndrome (hereditary nonpolyposis colorectal cancer) among endometrial cancer patients. Cancer Res 66:7810–7, 2006 [DOI] [PubMed] [Google Scholar]
- 6.National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) Genetic/Familial High-Risk Assessment: Colorectal, (ed 7/12/2018) www.nccn.org, National Comprehensive Cancer Network, 2018 [Google Scholar]
- 7.Watkins JC, Yang EJ, Muto MG, et al. : Universal Screening for Mismatch-Repair Deficiency in Endometrial Cancers to Identify Patients With Lynch Syndrome and Lynch-like Syndrome. Int J Gynecol Pathol 36:115–127, 2017 [DOI] [PubMed] [Google Scholar]
- 8.Febbraro T, Robison K, Wilbur JS, et al. : Adherence patterns to National Comprehensive Cancer Network (NCCN) guidelines for referral to cancer genetic professionals. Gynecol Oncol 138:109–14, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meyer LA, Anderson ME, Lacour RA, et al. : Evaluating women with ovarian cancer for BRCA1 and BRCA2 mutations: Missed opportunities. Obstetrics and Gynecology 115, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Petzel SV, Vogel RI, McNiel J, et al. : Improving referral for genetic risk assessment in ovarian cancer using an electronic medical record system. Int J Gynecol Cancer 24:1003–9, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Powell CB, Littell R, Hoodfar E, et al. : Does the diagnosis of breast or ovarian cancer trigger referral to genetic counseling? Int J Gynecol Cancer 23:431–6, 2013 [DOI] [PubMed] [Google Scholar]
- 12.Childers CP, Childers KK, Maggard-Gibbons M, et al. : National estimates of genetic testing in women with a history of breast or ovarian cancer. J Clin Oncol 35, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Batte BA, Bruegl AS, Daniels MS, et al. : Consequences of universal MSI/IHC in screening ENDOMETRIAL cancer patients for Lynch syndrome. Gynecol Oncol 134:319–25, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee J, Gubernick LR, Brodsky AL, et al. : Missed opportunities: Genetic counseling and testing among an ethnically diverse cohort of women with endometrial cancer. Gynecologic Oncology, 2018 [DOI] [PubMed] [Google Scholar]
- 15.Bednar EM, Oakley HD, Sun CC, et al. : A universal genetic testing initiative for patients with high-grade, non-mucinous epithelial ovarian cancer and the implications for cancer treatment. Gynecol Oncol 146:399–404, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris Health: About Us. www.harrishealth.org, Harris Health [Google Scholar]
- 17.Harris PA, Taylor R, Thielke R, et al. : Research Electronic Data Capture (REDCap) - A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of biomedical informatics 42:377–381, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bednar EM, Walsh MT, Baker E, et al. : Creation and Implementation of an Environmental Scan to Assess Cancer Genetics Services at Three Oncology Care Settings. Journal of Genetic Counseling, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Woodson AH, Profato JL, Park M, et al. : Service Delivery Model and Experiences in a Cancer Genetics Clinic for an Underserved Population. J Health Care Poor Underserved 26:784–91, 2015 [DOI] [PubMed] [Google Scholar]
- 20.Crowl A, Sharma A, Sorge L, et al. : Accelerating quality improvement within your organization: Applying the Model for Improvement. J Am Pharm Assoc (2003) 55:e364–74; quiz e375-6, 2015 [DOI] [PubMed] [Google Scholar]
- 21.Langley GJ, Moen RD, Nolan KM, et al. : The Improvement Guide: A Practical Approach to Enhancing Organizational Performance (ed Second). San Francisco, CA, Jossey-Bass, 2009 [Google Scholar]
- 22.West KM, Burke W, Korngiebel DM: Identifying “ownership” through role descriptions to support implementing universal colorectal cancer tumor screening for Lynch syndrome. Genetics In Medicine 19:1236, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Senter L, O’Malley DM, Backes FJ, et al. : Genetic consultation embedded in a gynecologic oncology clinic improves compliance with guideline-based care. Gynecol Oncol 147:110–114, 2017 [DOI] [PubMed] [Google Scholar]
- 24.Pederson HJ, Hussain N, Noss R, et al. : Impact of an embedded genetic counselor on breast cancer treatment. Breast Cancer Research and Treatment, 2018 [DOI] [PubMed] [Google Scholar]
- 25.Ivers N, Jamtvedt G, Flottorp S, et al. : Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database of Systematic Reviews, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]


