Abstract
Background:
Vasovagal syncope (VVS) patients have a reduced health−related quality of life (HRQoL). There are limited data comparing HRQoL and psychological profile in VVS patients and healthy individuals. We tested the hypothesis that VVS patients have greater impairment in both HRQoL and psychological profile compared to healthy non−fainting individuals, and that both outcome measures are negatively correlated for VVS patients.
Methods:
The RAND 36−Item Health Survey (RAND36), global health visual analogue scale (VAS), Hospital Anxiety and Depression Scale, Anxiety Sensitivity Index, and Positive and Negative Affect Schedule – Expanded Form were completed by healthy individuals and at baseline by VVS patients enrolled in the Second Prevention of Syncope Trial, a randomized, placebo−controlled trial of fludrocortisone for VVS.
Results:
Data were available on 76 VVS patients (34±14 years; 68% F) and 85 healthy participants (35±11 years; 80% F). Compared to healthy participants, VVS patients reported poorer HRQoL on all scales of the RAND36 and the VAS. VVS patients had significantly greater anxiety, depression, and anxiety sensitivity (each p<0.001). VVS patients had more negative affect (p<0.001) and less positive affect (p=0.003) compared to healthy participants. Anxiety, depression, and anxiety sensitivity were negatively correlated with HRQoL for VVS patients, but not for healthy participants.
Conclusion:
In this first direct comparison, VVS patients have a significantly reduced HRQoL and more anxiety and depression compared to healthy non−fainting individuals. For VVS patients, there is a relationship between psychological distress and HRQoL, suggesting a potential benefit from more comprehensive assessment and treatment.
Keywords: vasovagal syncope, anxiety, depression, quality of life
INTRODUCTION
Vasovagal Syncope (VVS) is the most common form of syncope, and has a high lifetime cumulative incidence in the general population. Approximately 35% of people between 35−60 years old have had at least 1 VVS episode.1 Syncope is also highly recurrent, with overall 1−year recurrence rates of approximately 25−35%.2 Patients with frequently recurring VVS experience an impaired health−related quality of life (HRQoL) compared to general population norms3, although it is not associated with an increased risk of mortality.2 There is also significant psychological distress observed within the VVS population itself4, compared to patients with unexplained syncope5, 6 and the general population.7 Despite knowing that both the physical and psychosocial functioning of syncope patients are negatively impacted, there are few data comparing these outcomes with those of closely matched healthy individuals.
Comparing the patient population to a defined control group is needed to isolate and confirm the impact of VVS on patients and to identify specific treatment targets. Prior data have compared VVS patients to a general reference population, which may not be demographically well matched. Moreover, as a significant proportion of the general population experience VVS episodes, these analyses are also likely to miss this confounding clinical factor. Prospectively collected data from a contemporary sample of healthy non−fainting individuals can strengthen the comparisons through close matching, and by ensuring that underlying comorbidities that may affect outcomes are not present.
In this study, we sought to examine the differences in HRQoL and psychological profile between VVS patients and a group of well−matched non−fainting healthy subjects, and secondarily to examine the relationship between HRQoL and psychological distress in VVS patients and healthy individuals. We tested the hypothesis that VVS patients have greater impairment in HRQoL and increased psychological distress compared to healthy individuals, and that there is a negative correlation between HRQoL and psychological distress for VVS patients.
METHODS
Participants
This cross−sectional study was a sub−study of the Second Prevention of Syncope Trial (POST2), which was a multi−center, longitudinal, randomized, placebo−controlled trial of fludrocortisone for VVS patients.8, 9 Patients were recruited from syncope and arrhythmia clinics, and were eligible for inclusion into the study if they were ≥14 years, had a score of ≥−2 on the Calgary Syncope Symptom Score, and had ≥3 lifetime syncopal spells before enrollment. Patients were excluded if they could not give informed consent; had other causes of syncope; had other significant non−cardiovascular or cardiovascular diseases, or a permanent pacemaker, glaucoma, diabetes mellitus, hepatic or renal disease, a seizure disorder, or hypertension; had a contraindication to, another clinical indication for fludrocortisone for the treatment of syncope. They were also excluded if they had postural tachycardia (heart rate increase ≥30 beats/min from lying to standing) or orthostatic hypotension (BP decrease ≥20/10 mmHg) during a 5−minute stand test.
Healthy individuals were recruited from volunteers known to Vanderbilt University’s Autonomic Dysfunction Center, through the Vanderbilt Clinical Research Volunteer database, and through advertisements in the Vanderbilt community using an email distribution system. They were consecutive volunteers and eligible for inclusion into the study if they were between 18−60 years (inclusive). Their “healthy” status was self−reported. They were excluded if they could not give informed consent; experienced ≥1 prior episodes of syncope; had a history of psychiatric illness; had current or prior use of psychiatric medications; had cardiovascular disease or a permanent pacemaker. They were also excluded if they had a major chronic non−cardiovascular disease, seizure disorder, hypertension (blood pressure ≥ 130/85 on 2 occasions), renal dysfunction, diabetes mellitus, hepatic disease or glaucoma. Healthy individuals were not matched to individual patients. Healthy subjects were administered the tools online, through the Vanderbilt University Research Data Capture (ReDCap) Survey tool.10 They were compensated $25 for their time involved in participating in the study. Data from a subset of the healthy individuals were previously used to compare sleep disturbances and HRQoL to patients with Postural Tachycardia Syndrome (POTS).11
POST2 was approved by the Conjoint Health Research Ethics Board for the Calgary site and the coordinating center, and by the local Ethics Boards for each site. Data collection for the healthy volunteers was approved by the Vanderbilt University Institutional Review Board. Each individual has given their written informed consent.
Surveys
All study participants were administered the RAND 36−Item Health Survey (RAND36), a global health visual analogue scale (VAS), Hospital Anxiety and Depression Scale (HADS), Anxiety Sensitivity Index (ASI), and Positive and Negative Affect Schedule – Expanded Form (PANAS−X) at baseline.
RAND36
The RAND36 is a widely used HRQoL survey composed of 36 items from the Medical Outcomes Study.12 It assesses 8 health concepts, including physical functioning, role limitations due to physical health, role limitations due to emotional problems, social functioning, emotional well−being, energy or fatigue, pain, and general health. Role limitations refer to limitations in the ability to work, perform housework, schoolwork, involvement in community, etc. The scores range from 0−100, with higher scores indicating greater levels of functioning and a more favourable health state.13 Summary physical health and mental health composite scores can be calculated from the 8 scales.
Global Health VAS
The VAS was administered as a horizontal sliding scale in ReDCap Survey with anchors at 0 (worst imaginable health state) to 100 (best imaginable health state).
HADS
The HADS is a self−rating instrument that screens for clinically significant anxiety and depression in patients attending a general medical clinic.14 The tool consists of 14 items scored between 0 and 3. Higher scores indicate greater experiences of anxiety and depression. There are also cut−off scores for clinically probable levels of anxiety and depression. For both subscales, individual scores ≥11 indicates probable anxiety or depression, 8−10 indicates borderline anxiety or depression, and ≤7 are negative for anxiety or depression.
ASI
Anxiety sensitivity is defined as the fear of anxiety−related sensations, arising from beliefs that these sensations have negative somatic, psychological, or social consequences.15 The ASI is a well−validated scale consisting of 16 items, each specifying a potential negative consequence associated with the experience of anxiety.15 The total score is the sum of the scores of all items, and ranges from 0 to 64. Higher scores indicate greater anxiety sensitivity. A score of ≥33 is considered a positive result for having clinically significant anxiety sensitivity.15, 16
PANAS−X
The PANAS−X is a widely used and validated psychometric measure.17 The primary units of measure are two general dimensions known as “positive affect” and “negative affect”. In addition, the PANAS−X provides scores for 11 specific emotional states categorized into basic negative emotions (fear, hostility, guilt, and sadness), basic positive emotions (joviality, self−assurance, and attentiveness) and other affective states (shyness, fatigue, serenity, and surprise). There are 60 items, which include words or phrases reflecting the dimensions. Participants are asked to rate the extent to which they experienced each feeling in the past few weeks. Higher scores indicate greater feelings of that emotional state.
Statistical Analysis
Demographic and clinical information are expressed as percentages for categorical data, mean±standard deviation (SD) for normally distributed continuous variables, and median (interquartile range [IQR], 25th percentile, 75th percentile) for non−normally distributed continuous variables. Categorical demographic variables were compared using the chi−squared test, unless the assumption of having a minimum expected count of 5 per cell was violated, in which case the Fisher’s exact test was used. Continuous demographic variables were assessed with the Student’s t test (normally distributed) or Mann−Whitney U test (non−normally distributed).
Baseline assessments were used in the analysis for VVS patients. Scores for the RAND36, VAS, HADS, ASI, and PANAS−X were calculated according to their respective scoring guidelines, and expressed as mean±SD. There were incomplete surveys from 10 VVS patients and 1 healthy participant for the PANAS−X, as well as 3 VVS patients and 1 healthy participant for the HADS and ASI. VVS patients with incomplete surveys were not different in their demographics or number of prior faints compared to patients with complete surveys. Scale and total scores for the individuals’ surveys with missing data were not calculated.
Differences between mean survey scores of VVS patients and healthy individuals were examined using the Student’s t−test and verified with the Mann−Whitney U test. A secondary analysis involved 1:1 matching of VVS patients and healthy individuals by both age and sex. Due to the 1:1 matching, differences in mean survey scores were examined using the paired t−test and verified with the Wilcoxon Signed Rank test.
Individual anxiety and depression scores in the HADS were categorized according to the pre−defined cut−off scores, as indicating “probable anxiety or depression”, “borderline anxiety or depression”, and “negative for anxiety or depression”. Scores from the ASI were also dichotomized for positive or negative anxiety sensitivity. Differences between groups were analyzed using the Fisher’s exact test. Simple linear regression and Pearson correlations were used to identify the relationship between psychological distress (anxiety, depression, and anxiety sensitivity) and HRQoL (RAND36) in VVS patients and healthy participants.
All p−values were two−tailed and the statistical significance was set at p< 0.05. The analyses were performed using STATA 14 (StataCorp, College Station, TX, USA). Figures were made using GraphPad Prism version 7.01 (GraphPad Software, La Jolla, CA, USA).
RESULTS
Demographics
Baseline data were available for 161 study participants, including 76 VVS patients and 85 healthy non−fainting individuals. Demographic and clinical information for VVS patients and healthy individuals are presented in Table 1. There were no significant differences in age (34±14 years vs. 35±11 years, p=0.5) or sex (68% F vs. 80% F, p=0.09) between groups. Prior to study enrollment, VVS patients had experienced a median of 16 (IQR 7, 50) lifetime syncope episodes over a median of 13 (IQR 3, 25) years. They reported a median of 4 (IQR 2, 20) syncopal episodes in the year prior to enrollment. Additionally, matching of VVS patients and healthy individuals by age and sex yielded 28 pairs.
TABLE 1.
Demographic and baseline information of VVS patients and healthy individuals
| VVS Patients (n=76) |
Healthy Individuals (n=85) |
|
|---|---|---|
| Female, n (%) | 51 (68%) | 67 (80%) |
| Age at study enrollment, mean±SD | 34±14 | 35±11 |
| VVS Onset age, mean±SD | 17±12 | - |
| VVS Symptom duration, median (IQR) | 13 (3, 25) | - |
| Number of Faints – lifetime, median (IQR) | 16 (7, 50) | - |
| Number of Faints – past year, median (IQR) | 4 (2, 20) | - |
VVS – Vasovagal syncope; SD – standard deviation; IQR – interquartile range (25th percentile, 75th percentile)
Health−Related Quality of Life
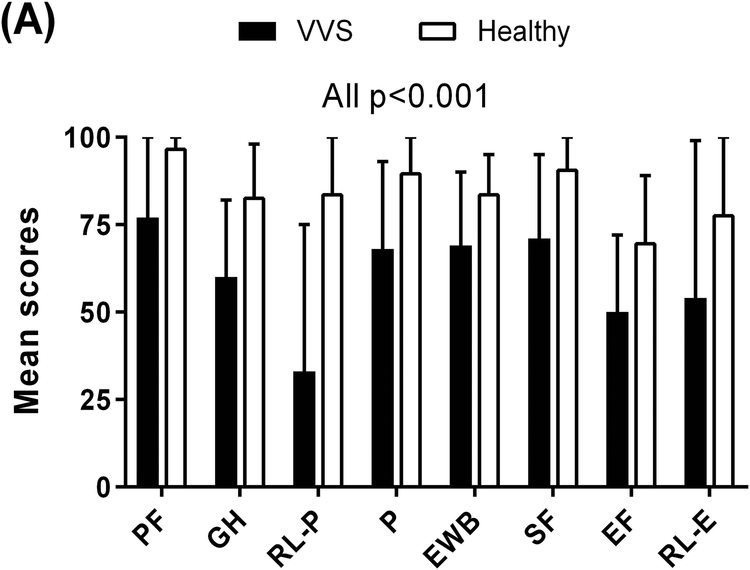
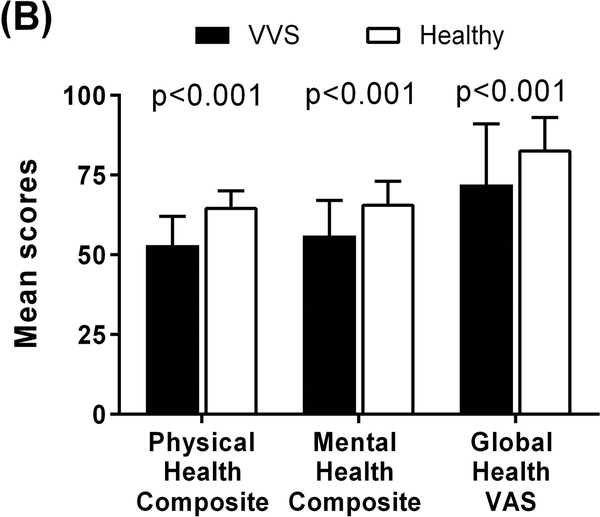
The HRQoL data are summarized in Supplementary Table S1. Compared to healthy non−fainting individuals, VVS patients reported significantly poorer scores in all 8 of the health dimensions measured by the RAND36 (Figure 1A). Specifically, of the scales relating to physical health, VVS patients reported poorer physical functioning (77±24 vs. 97±6; p<0.001) and general health perception (60±22 vs. 83±15; p<0.001). They had greater role limitations due to physical health (33±42 vs. 84±29; p<0.001) and experienced more pain (68±25 vs. 90±16; p<0.001) compared to healthy participants. They also scored lower on the physical health composite score (53±9 vs. 65±5; p<0.001) (Figure 1B). Similarly, of the scales relating to mental health, VVS patients indicated poorer emotional well−being (69±21 vs. 84±11; p<0.001) and social functioning (71±24 vs. 91±15; p<0.001). They had less energy (50±22 vs. 70±19; p<0.001) and greater role limitations due to emotional problems (54±45 vs. 78±37; p<0.001). In addition, VVS patients reported lower mental health composite scores compared to healthy individuals (56±11 vs. 66±7; p<0.001).
Figure 1. HRQoL Scores of VVS Patients and Healthy Individuals.
Higher scores in each scale indicate greater levels of functioning and a more favourable health state. Panel A: Compared to healthy individuals, VVS patients reported poorer scores in all 8 of the health dimensions of the RAND36. Panel B: VVS patients also scored lower in the global assessments of HRQoL, measured by the RAND36 physical and mental health composite scores and global health visual analogue scale. PF – physical functioning; GH – general health; RL−P – role limitations due to physical health; P – pain; EWB – emotional well−being; SF – social functioning; EF – energy/fatigue; RL−E – role limitations to due emotional health; PHC – physical health composite; MHC – mental health composite; VAS – visual analogue scale.
Using the global health VAS, VVS patients reported a markedly reduced global HRQoL compared to healthy participants (72±19 vs. 83±10; p<0.001) (Figure 1B).
In the analyses of matched pairs, VVS patients reported significantly lower scores in all of the health dimensions and composite scores measured by the RAND36 (Table 2). They also reported poorer global HRQoL in the VAS compared to healthy individuals.
TABLE 2.
HRQoL and psychological profile scores of VVS patients compared to age and sex matched healthy individuals
| HRQoL | VVS Patients (n=28) |
Healthy Individuals (n=28) |
P value |
|---|---|---|---|
| RAND36 | |||
| Physical functioning | 76±22 | 98±3 | <0.001 |
| General health | 53±21 | 87±10 | <0.001 |
| Role limitations (physical health) | 15±33 | 89±28 | <0.001 |
| Pain | 65±23 | 88±24 | 0.004 |
| Emotional well−being | 69±17 | 85±12 | 0.001 |
| Social functioning | 62±22 | 93±15 | <0.001 |
| Energy/Fatigue | 47±21 | 72±19 | <0.001 |
| Role limitations (emotional problems) | 49±43 | 88±30 | 0.001 |
| Physical health composite | 50±7 | 66±4 | <0.001 |
| Mental health composite | 54±10 | 67±7 | <0.001 |
| Global Health VAS | 69±18 | 84±10 | 0.001 |
| Psychological Profile | |||
| HADS | |||
| Anxiety | 7.4±3.8 | 5.6±4.3 | 0.127 |
| Depression | 3.7±3.2 | 1.9±2.0 | 0.007 |
| ASI | 41±12 | 28±8 | <0.001 |
| PANAS−X | |||
| General Dimension Scales | |||
| Negative Affect | 20±6 | 16±6 | 0.028 |
| Positive Affect | 28±7 | 32±8 | 0.054 |
HRQoL – Health−related quality of life; VAS – visual analogue scale
Psychological Profile
Anxiety and Depression
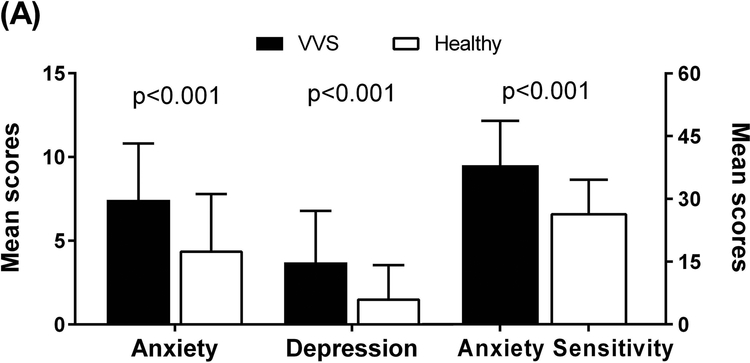
The data pertaining to the HADS and ASI are summarized in Table S1. Using the HADS, VVS patients scored significantly higher (worse) in the HADS anxiety subscale compared to healthy non−fainting participants (7.4±3.4 vs. 4.4±3.4; p<0.001) (Figure 2A). VVS patients also reported significantly higher scores for the HADS depression subscale (3.7±3.1 vs. 1.6±2.0; p<0.001). There was greater anxiety sensitivity (from ASI) in VVS patients than healthy participants (38±11 vs. 27±8; p<0.001).
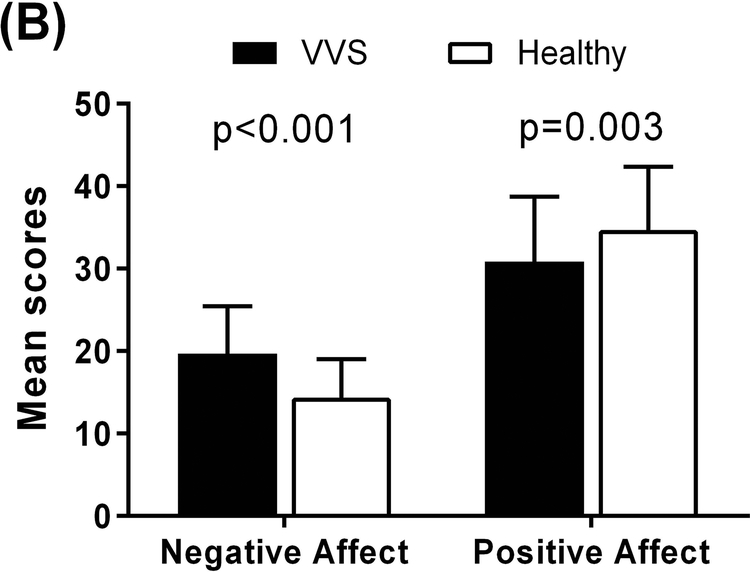
Figure 2. Psychological Profile Scores of VVS Patients and Healthy Individuals.
Higher scores indicate greater experiences of anxiety, depression, and anxiety sensitivity. Panel A: Compared to healthy individuals, VVS patients reported greater anxiety and depression using the HADS (left axis) and greater anxiety sensitivity using the ASI (right axis). Panel B: In the PANAS−X, VVS patients reported more negative affect and less positive affect compared to healthy individuals.
When the anxiety and depression scores from the HADS were categorized as “probable”, “borderline”, or “absent”, there were significant differences between groups in the number of individuals in each category of anxiety (p<0.001) and depression (p=0.001). Significantly more VVS patients had “borderline” or “probable” anxiety overall than healthy individuals (40% vs. 14%; p<0.001). VVS patients also reported more “borderline” or “probable” cases of depression compared to healthy individuals (14% vs. 2%; p=0.013). Additionally, 10% of VVS patients had both “borderline” or “probable” anxiety and depression compared to only 1% of healthy individuals (p=0.025). From the ASI, more VVS patients reported a positive result for having clinically significant anxiety sensitivity compared to healthy individuals (65% vs. 19%; p<0.001).
In the analyses comparing VVS patients to age and sex matched healthy individuals, VVS patients reported significantly higher scores in the HADS depression subscale, and no differences in the HADS anxiety subscale (Table 2). They also had greater anxiety sensitivity than healthy individuals.
Positive and Negative Affect
In the PANAS−X, VVS patients reported more negative affect (20±6 vs. 14±5; p<0.001) and less positive affect (31±8 vs. 35±8; p=0.003) compared to healthy non−fainting individuals (Table S1) (Figure 2B). They also reported higher levels of basic negative emotions, including fear (12±4 vs. 8±3; p<0.001), hostility (11±4 vs. 9±4; p=0.003), guilt (10±4 vs. 8±3; p=0.002), and sadness (9±4 vs. 8±3; p=0.002). Of the basic positive emotions, patients reported being less attentive compared to healthy individuals (13±3 vs. 15±3; p<0.001). VVS patients were also more shy (7±3 vs. 6±3; p=0.023), had greater fatigue (12±4 vs. 8±3; p<0.001), and were less serene (9±2 vs. 10±3; p=0.016).
VVS patients had greater negative affect, and no differences in positive affect, compared to age and sex matched healthy individuals (Table 2).
Relationship between Psychological Distress and HRQoL
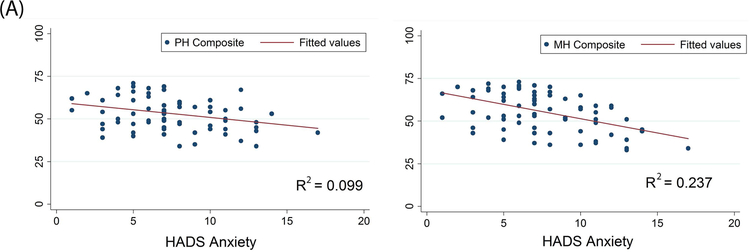
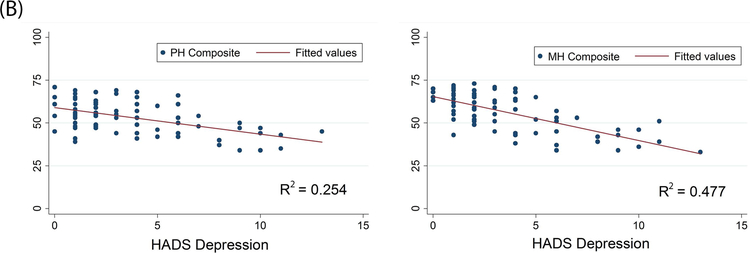
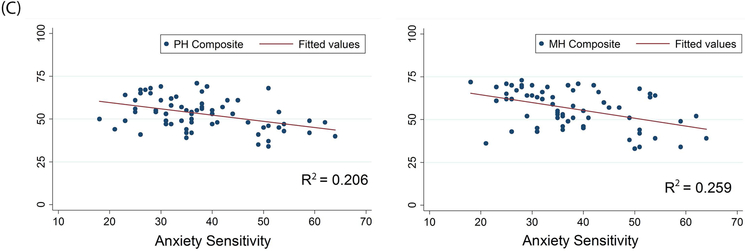
For VVS patients, there was a weak, but statistically significant, negative correlation between the HADS anxiety subscale and 7 of the 8 health dimensions of the RAND36 (Table 3). The HADS depression subscale was negatively correlated with all 8 of the health dimensions of the RAND36. There was also a negative relationship between anxiety sensitivity (from the ASI) and 7 of the 8 health dimensions of the RAND36. Moreover, anxiety, depression, and anxiety sensitivity all showed a significant negative relationship with the physical and mental health composite scores of the RAND36 (p<0.001) (Figure 3).
TABLE 3.
Correlations between psychological distress and HRQoL scores in VVS patients
| R−squared | Pearson’s r | P value | |
|---|---|---|---|
| Anxiety (HADS) | |||
| Physical functioning | 0.03 | −0.162 | 0.169 |
| General health | 0.05 | −0.239 | 0.04 |
| Role limitations (physical health) | 0.10 | −0.328 | 0.004 |
| Pain | 0.06 | −0.26 | 0.026 |
| Emotional well−being | 0.29 | −0.543 | <0.001 |
| Social functioning | 0.13 | −0.369 | 0.001 |
| Energy/Fatigue | 0.10 | −0.332 | 0.004 |
| Role limitations (emotional problems) | 0.14 | −0.382 | 0.001 |
| Depression (HADS) | |||
| Physical functioning | 0.14 | −0.354 | 0.002 |
| General health | 0.15 | −0.395 | 0.001 |
| Role limitations (physical health) | 0.14 | −0.389 | 0.001 |
| Pain | 0.17 | −0.443 | <0.001 |
| Emotional well−being | 0.46 | −0.691 | <0.001 |
| Social functioning | 0.27 | −0.534 | <0.001 |
| Energy/Fatigue | 0.36 | −0.617 | <0.001 |
| Role limitations (emotional problems) | 0.30 | −0.559 | <0.001 |
| Anxiety Sensitivity (ASI) | |||
| Physical functioning | 0.14 | −0.371 | 0.002 |
| General health | 0.22 | −0.437 | <0.001 |
| Role limitations (physical health) | 0.12 | −0.312 | 0.01 |
| Pain | 0.04 | −0.138 | 0.264 |
| Emotional well−being | 0.19 | −0.367 | 0.003 |
| Social functioning | 0.19 | −0.387 | 0.001 |
| Energy/Fatigue | 0.19 | −0.375 | 0.002 |
| Role limitations (emotional problems) | 0.21 | −0.411 | 0.001 |
Figure 3. Correlations between Psychological Distress and HRQoL in VVS Patients.
Panel A: Anxiety, as measured by the HADS, is negatively correlated with the physical and mental health composite scores of the RAND36. Panel B: Depression, as measured by the HADS, is negatively correlated with the physical and mental health composite scores of the RAND36. Panel C: Increases in anxiety sensitivity (in the ASI) are associated with a decrease in both physical and mental health (in the RAND36). PH Composite – physical health composite; MH Composite – mental health composite.
In contrast, healthy participants only reported statistically significant negative correlations between HADS anxiety and pain (RAND36) (r=−0.24, p=0.031), as well as HADS depression and role limitations due to emotional health (RAND36) (r=−0.276, p=0.013). They also indicated a negative relationship between anxiety sensitivity and emotional well−being (RAND36) (r=−0.297, p=0.007). There were no significant correlations between the psychological distress scores and the RAND36 physical and mental health composite scores for the healthy participants.
DISCUSSION
The findings from this study support a negative relationship between VVS and a patient’s quality of life and psychological profile. VVS patients report significant impairment across multiple HRQoL and psychological subscales compared to well−matched non−fainting healthy individuals. Furthermore, there is a negative correlation between psychological distress and HRQoL for VVS patients, whereas the same relationship is less prominent in healthy participants.
VVS and Quality of Life
Compared to healthy individuals, VVS patients reported a poorer HRQoL in all eight of the health dimensions measured by the RAND36, as well as the global health VAS. To our knowledge, only one other study has used a healthy control group in comparing HRQoL with tilt−induced VVS patients.18 Our findings are consistent with the previous study, which found a marked reduction in all of the HRQoL scales of the SF36 in patients compared to controls. However, our study used a more diverse group of healthy volunteers, while the control group in the previous study consisted of nurses from the cardiovascular department.
Other studies have found substantially impaired HRQoL in syncope patients compared to UK population norms in all health dimensions of the EQ−5D3, and lower scores on all scales of the SF−36 compared to the Dutch national population.19 These data have several limitations. One of the aforementioned studies compared to all−cause syncope and not just vasovagal syncope.19 Compared to VVS, cardiac syncope is associated with an increased risk of major adverse outcomes, including mortality.20 It is therefore possible that HRQoL also varies between different types of syncope. Our study eliminates these potential confounding variables by comparing with a healthy participant group and ensuring only patients with a VVS diagnosis are involved. Further, other studies compared patients with the general population, meaning that the age ranges were less closely matched and that underlying comorbidities in the population could not be controlled; for example, a significant number of individuals in the general population experience syncope. These factors may potentially influence reported outcomes. Our cohort included healthy non−fainting individuals of similar ages to the VVS patients, thereby further strengthening the comparison. Lastly, the population norms used in previous comparisons may be representative of data from the remote past, whereas our study provided a contemporary sample for comparison.
VVS and Psychological Distress
VVS patients were significantly more anxious and depressed than healthy participants. They also reported more negative affect and less positive affect. This is concordant with previous findings that VVS patients have higher levels of general psychological complaints compared to a general reference population.7 One limitation of prior studies is that psychological distress varies with age and gender, so a general reference population may not be well matched. Our study used a closely sex and age−matched group of individuals with no previous diagnosis of psychiatric disorders or other comorbidities. Additionally, a greater percentage of VVS patients reported borderline or clinically significant levels of anxiety and depression compared to healthy individuals. This aligns with previous findings that have shown a greater prevalence of anxiety (28% vs. 5%, p<0.001) and mood disorders (18% vs. 3%; p<0.001) in VVS patients compared to control subjects.18 However, this prior study had a smaller sample size and used the Minnesota Multiphasic Personality Inventory−2. The larger sample size in our study, along with the use of a different assessment tool, provide further support for the differences observed between groups.
Anxiety sensitivity is a specific personality trait that has previously been observed in a proportion of patients with VVS and patients with unexplained syncope.16 Our results indicate that VVS patients also report having greater anxiety sensitivity compared to healthy individuals. This trait is related to the perception that anxiety is linked to negative effects.15 Individuals with high levels of anxiety sensitivity have a tendency towards showing exaggerated and prolonged reactions to anxiety−provoking stimuli. A recent study examined whether fluoxetine, a serotonin−reuptake inhibitor (SSRI) and effective therapy for anxiety and panic disorders, was able to prevent VVS in patients with elevated anxiety sensitivity (60/106 patients assessed).21 The study found that patients receiving fluoxetine reported a significant decrease in the incidence of syncope and presyncope during follow up compared to patients receiving placebo (p<0.05). SSRIs have also been suggested in the treatment of VVS due to the effect of serotonin on the modulation of HR and BP, although results from previous studies have been mixed.22, 23
Another study observed that in syncope patients with a psychiatric diagnosis, those patients who were willing to undergo tailored psychotherapy sessions and adjunctive medical treatment for their specific condition experienced no recurrent syncope during follow up, whereas those who refused therapy continued to experience frequent episodes.24 It is possible that the early identification of a patient’s psychological profile will enable clinicians to provide better treatment, both to improve psychological well−being and prevent VVS recurrence. Moreover, our results show that increased anxiety, depression, and anxiety sensitivity are related to a poorer HRQoL in VVS patients, but not for healthy participants. This suggests that psychological treatment may be beneficial in helping to improve QoL for VVS patients in particular.
Clinical Implications
Identifying the differences in quality of life and psychological well−being between VVS patients and healthy individuals confirms the impact of VVS, and promotes greater awareness amongst both health care professionals and the patient population. It has been suggested that distress experienced by patients may influence the natural history of VVS. This distress may be more relevant to the patient than the number of actual faints that they are experiencing. Previous findings have also reported that the VVS patients who do not respond effectively to conventional interventions have higher levels of psychological distress at baseline.4 Acknowledging these factors will potentially instigate the development of targeted interventions, such as more comprehensive assessments by the treating physician during presentation and follow up, and improving physical and mental health outcomes through multidisciplinary approaches. This may help to supplement current therapies, which mainly target the pathophysiological aspects of VVS.
Study Limitations
A main limitation of the study is that the data were collected using subjective, self−reported measures of HRQoL and psychological distress. These measures may be open to bias and misinterpretation. However, a countervailing strength is that this study used common and validated patient−reported outcome tools, which would increase the reliability of these data.
In addition, while the results show that VVS patients have significant psychological distress at or around the time of enrollment into a clinical trial, it does not provide confirmation as to whether psychological distress is a cause or consequence of VVS. This is further limited by the lack of information regarding previously diagnosed psychiatric disorders or psychological distress experienced by the VVS patients. This study evaluated symptomatic patients with recurrent episodes who had been referred to syncope or arrhythmia clinics. It is therefore possible that these patients are not fully representative of all individuals with syncope.
Conclusions
VVS patients have poorer HRQoL and greater psychological distress compared to healthy non−fainting individuals. Further, there is a negative relationship between psychological distress and HRQoL for VVS patients, but not for healthy individuals. Patients may therefore benefit from a more comprehensive assessment of HRQoL, anxiety and depression during presentation and during subsequent follow up visits. Moreover, for patients with an indication of psychological distress, targeted interventions addressing these outcomes may also help to improve HRQoL. To provide further understanding of the impact of VVS, future studies should aim to identify how HRQoL and psychological distress in VVS patients changes over the course of their treatment, as well as determining other factors that are important in predicting or ensuring improvement.
Supplementary Material
Acknowledgments
FUNDING SOURCES
The Second Prevention of Syncope Trial was funded by an operating grant from the Canadian Institutes of Health Research (CIHR; Ottawa, ON, Canada) to RSS. The healthy participants were assessed through funding provided to SRR by the Vanderbilt Institute for Clinical and Translational Research funded by a Clinical and Translational Science Award from the National Center for Advancing Translational Science from the National Institutes of Health (UL1 TR000445). SRR receives research support from the Canadian Institutes of Health Research (CIHR; Ottawa, ON, Canada) grant MOP142426 and the Cardiac Arrhythmia Network of Canada (CANet; London, ON, Canada) grants SRG−15−P01−001 and SRG−17−P27−001.
NIH Grant number: UL1 TR000445
Footnotes
DISCLOSURES
SRR is a consultant for Lundbeck NA Ltd., GE Healthcare, Abbott Laboratories, and Boston Scientific Corporation. Other authors have no conflicts to declare.
REFERENCES
- 1.Ganzeboom KS, Mairuhu G, Reitsma JB, Linzer M, Wieling W and van Dijk N. Lifetime cumulative incidence of syncope in the general population: a study of 549 Dutch subjects aged 35–60 years. J Cardiovasc Electrophysiol. 2006;17:1172–6. [DOI] [PubMed] [Google Scholar]
- 2.Sheldon RS, Grubb BP 2nd, Olshansky B, Shen WK, Calkins H, Brignole M, Raj SR, Krahn AD, Morillo CA, Stewart JM, Sutton R, Sandroni P, Friday KJ, Hachul DT, Cohen MI, Lau DH, Mayuga KA, Moak JP, Sandhu RK and Kanjwal K. 2015 heart rhythm society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm. 2015;12:e41–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rose MS, Koshman ML, Spreng S and Sheldon R. The relationship between health–related quality of life and frequency of spells in patients with syncope. J Clin Epidemiol. 2000;53:1209–16. [DOI] [PubMed] [Google Scholar]
- 4.Gracie J, Newton JL, Norton M, Baker C and Freeston M. The role of psychological factors in response to treatment in neurocardiogenic (vasovagal) syncope. Europace. 2006;8:636–43. [DOI] [PubMed] [Google Scholar]
- 5.Cohen TJ, Thayapran N, Ibrahim B, Quan C, Quan W and von zur Muhlen F. An association between anxiety and neurocardiogenic syncope during head–up tilt table testing. Pacing Clin Electrophysiol. 2000;23:837–41. [DOI] [PubMed] [Google Scholar]
- 6.McGrady A, Kern–Buell C, Bush E, Khuder S and Grubb BP. Psychological and physiological factors associated with tilt table testing for neurally mediated syncopal syndromes. Pacing Clin Electrophysiol. 2001;24:296–301. [DOI] [PubMed] [Google Scholar]
- 7.Romme JJ, van Dijk N, Go–Schon IK, Casteelen G, Wieling W and Reitsma JB. Association between psychological complaints and recurrence of vasovagal syncope. Clin Auton Res. 2011;21:373–80. [DOI] [PubMed] [Google Scholar]
- 8.Sheldon R, Raj SR, Rose MS, Morillo CA, Krahn AD, Medina E, Talajic M, Kus T, Seifer CM, Lelonek M, Klingenheben T, Parkash R, Ritchie D, McRae M and Investigators P. Fludrocortisone for the Prevention of Vasovagal Syncope: A Randomized, Placebo–Controlled Trial. J Am Coll Cardiol. 2016;68:1–9. [DOI] [PubMed] [Google Scholar]
- 9.Raj SR, Rose S, Ritchie D, Sheldon RS and Investigators PI. The Second Prevention of Syncope Trial (POST II)––a randomized clinical trial of fludrocortisone for the prevention of neurally mediated syncope: rationale and study design. Am Heart J. 2006;151:1186 e11–7. [DOI] [PubMed] [Google Scholar]
- 10.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N and Conde JG. Research electronic data capture (REDCap)––a metadata–driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bagai K, Song Y, Ling JF, Malow B, Black BK, Biaggioni I, Robertson D and Raj SR. Sleep disturbances and diminished quality of life in postural tachycardia syndrome. J Clin Sleep Med. 2011;7:204–10. [PMC free article] [PubMed] [Google Scholar]
- 12.Hays RD and Morales LS. The RAND–36 measure of health–related quality of life. Ann Med. 2001;33:350–7. [DOI] [PubMed] [Google Scholar]
- 13.Hays RD, Sherbourne CD and Mazel RM. The RAND 36–Item Health Survey 1.0. Health Econ. 1993;2:217–27. [DOI] [PubMed] [Google Scholar]
- 14.Zigmond AS and Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67:361–70. [DOI] [PubMed] [Google Scholar]
- 15.Reiss S, Peterson RA, Gursky DM and McNally RJ. Anxiety sensitivity, anxiety frequency and the prediction of fearfulness. Behav Res Ther. 1986;24:1–8. [DOI] [PubMed] [Google Scholar]
- 16.D’Antono B, Dupuis G, St–Jean K, Levesque K, Nadeau R, Guerra P, Thibault B and Kus T. Prospective evaluation of psychological distress and psychiatric morbidity in recurrent vasovagal and unexplained syncope. J Psychosom Res. 2009;67:213–22. [DOI] [PubMed] [Google Scholar]
- 17.Watson D and Clark LA. Manual for the Positive and Negative Affect Schedule – Expanded Form. 1994.
- 18.Giada F, Silvestri I, Rossillo A, Nicotera PG, Manzillo GF and Raviele A. Psychiatric profile, quality of life and risk of syncopal recurrence in patients with tilt–induced vasovagal syncope. Europace. 2005;7:465–71. [DOI] [PubMed] [Google Scholar]
- 19.van Dijk N, Sprangers MA, Colman N, Boer KR, Wieling W and Linzer M. Clinical factors associated with quality of life in patients with transient loss of consciousness. J Cardiovasc Electrophysiol. 2006;17:998–1003. [DOI] [PubMed] [Google Scholar]
- 20.Soteriades ES, Evans JC, Larson MG, Chen MH, Chen L, Benjamin EJ and Levy D. Incidence and prognosis of syncope. N Engl J Med. 2002;347:878–85. [DOI] [PubMed] [Google Scholar]
- 21.Flevari P, Leftheriotis D, Repasos E, Katsaras D, Katsimardos A and Lekakis J. Fluoxetine vs. placebo for the treatment of recurrent vasovagal syncope with anxiety sensitivity. Europace. 2017;19:127–131. [DOI] [PubMed] [Google Scholar]
- 22.Di Girolamo E, Di Iorio C, Sabatini P, Leonzio L, Barbone C and Barsotti A. Effects of paroxetine hydrochloride, a selective serotonin reuptake inhibitor, on refractory vasovagal syncope: a randomized, double–blind, placebo–controlled study. J Am Coll Cardiol. 1999;33:1227–30. [DOI] [PubMed] [Google Scholar]
- 23.Theodorakis GN, Leftheriotis D, Livanis EG, Flevari P, Karabela G, Aggelopoulou N and Kremastinos DT. Fluoxetine vs. propranolol in the treatment of vasovagal syncope: a prospective, randomized, placebo–controlled study. Europace. 2006;8:193–8. [DOI] [PubMed] [Google Scholar]
- 24.Ventura R, Maas R, Ruppel R, Stuhr U, Schuchert A, Meinertz T and Nienaber CA. Psychiatric conditions in patients with recurrent unexplained syncope. Europace. 2001;3:311–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.