Abstract
Community-based HIV testing and counseling (HTC) programs have become an important part of the healthcare system in South Africa and other low- and middle-income countries with a high HIV prevalence and strained primary healthcare system. Current HTC programs excel at identifying people living with HIV (PLH) but leave gaps in linkage to care and antiretroviral therapy (ART) as most HTC programs do not have the capacity to ensure that linkage has occurred. This article presents the protocol for an mHealth study that is pilot testing a mobile platform in KwaZulu-Natal (KZN), South Africa, to improve linkage to care and ART adherence after home-based HTC. Testing data are shared with designated clinics. PLH are identified using fingerprint scans, mobile numbers, or South African IDs. If PLH do not present at a designated clinic after testing HIV positive, study field staff are sent SMS alerts to prompt follow-up visits. Similarly, if PLH do not refill ART prescriptions after their initial 1-month dose runs out, SMS alerts that are sent to field staff. This paper presents the mHealth study protocol and baseline sample characteristics (N = 101 PLH). Analyses will summarize rates of linkage to care and ART prescription refills. Cost-effectiveness analyses will examine the costs and benefits of linkage and ART adherence using our mHealth system. Linkage to care rates will be compared between our study and a historical control, that is, provided by a prior HTC program that was conducted in KZN without our mHealth system (n = 615).
Keywords: HIV, ARTadherence, mHealth, Home visiting, Linkage to care
Introduction
Community-Based HIV Testing and Counseling
Community-based HIV testing and counseling (HTC) programs deploy trained paraprofessional medical staff to conduct HIV testing in high-risk communities and provide clinic referral for individuals who test positive. The approach is an integral part of healthcare systems in South Africa and other low- and middle-income countries (LMIC). South Africa has the largest number of people living with HIV (PLH) and the national HIV prevalence is estimated to be 12%, ranging from 9.2 to 27.6% (Shisana 2013). It is well known that early HIV testing and engagement with antiretroviral therapy (ART) reduces morbidity, mortality, and infectiousness of the virus (Anderson et al. 2014; Coetzee et al. 2004; Cohen et al. 2011; Eaton et al. 2014; Mills et al. 2011; Smith et al. 2013; Wouters et al. 2007). Yet, many South Africans do not visit health facilities for regular checkups (McLaren et al. 2014). Thus, community-based HTC has become an important strategy adopted by the South African government as it seeks to attain the first 90 target (diagnosing 90% of people with HIV who do not know their HIV status) (South African Department of Health 2015; World Health Organization 2015). One specific community-based strategy that has shown real promise is home visitation by health workers to test and counsel individuals in their homes. Home-based HTC is an effective approach to identifying new HIV cases (Barnabas et al. 2016; Kimaiyo et al. 2010; Molesworth et al. 2010; Naik et al. 2012; Tumwesigye et al. 2010; van Rooyen et al. 2013). However, a significant challenge with community-based HTC is linkage to care. Large numbers of PLH identified through these community-based strategies delay or never initiate ART (Rosen and Fox 2011).
mHealth and the HIV Care Continuum
Mobile phone accessibility is high among South Africans, as the country has the highest proportion of mobile phone users per population. In the context of a rural population with poor access to health services and without funds to easily travel, this introduces an opportunity to deliver certain health interventions directly into people’s homes (Goel et al. 2013; Catalani et al. 2013). mHealth programs have capitalized on mobile phone infrastructure to support several steps along the HIV care continuum, including the identification of HIV cases, retention in care, and ART adherence. SMS (text messaging) is a favored approach in South Africa and low- and middle-income countries (LMIC) due to its low cost and flexibility across mobile systems relative to other mHealth tools, such as mobile apps (Forrest et al. 2015; Lester et al. 2010; Mukund and Murray 2010). For example, SMS has been used to promote HIV testing in communities and has served as medical appointment and ART reminders for PLH who are linked to care. The current study addresses two gaps in the care continuum that pertain to HTC programs. First, this study addresses challenges associated with identifying and keeping track of clients who live in informal housing, which often lacks physical addresses. As part of the study’s formative phase, focus groups were conducted with research field staff, hereafter referred to as field staff, which identified a need for mobile mapping tools that could be used to better coordinate and navigate visitation routes (van Heerden et al. 2017). In response, our research team developed a mobile mapping application that can be used by field staff to plan and record home visits, which may improve efficiency and prevent duplication.
Second, this study developed an mHealth platform, hereafter referred to as the Biolink platform, that addresses gaps between current field and clinic systems by sharing patient information to improve linkage to care after home-based HTC. In South Africa, field and clinic systems are fragmented and staff-conducting HTC does not know if PLH follow through on clinic referrals after testing positive for HIV infection because there is no universal patient identifier that uniquely identifies patients across field and clinic systems. One possible solution is an identity document that has been proposed by the Department of Home Affairs and provides an identification number, hereafter referred to as a South African ID, to all South African citizens and permanent residents who are at least 16 years old (Department of Home Affairs 2016). A limitation is that not all eligible individuals have a South African ID. Another potential solution is provided by biometric systems. Fingerprint scans have been used to verify the identity of patients enrolled in community- and clinic-based HTC programs in South Africa and Uganda (Chamie et al. 2014; Nglazi et al. 2012). Next steps call for biometric systems to be tested as identification tools that could, in turn, be used to provide patient identifiers. In addition to testing, the acceptability of biometric identification systems by PLH and other community members needs to be evaluated in the context of HTC and linkage to care. Focus group discussions with field staff introduced above raised concerns around acceptability. For example, discussants felt that older PLH may be less trusting of biometric systems and would be more hesitant to have their fingers scanned relative to younger PLH (van Heerden et al. 2017).
The Biolink Platform
The Biolink platform is based on prior HTC programs where field staff enter home visitation data, including HIV test results and PLH contact information, into an Android™ smartphone app that was developed by Mobenzi (http://www.mobenzi.com/) (Barnabas et al. 2016; van Rooyen et al. 2013). Data entered into the smartphone app are encrypted and uploaded to a password-protected cloud-based storage system and is accessible to the research team. Clinic staff can access the data through the Mobenzi app to record when PLH present at a clinic after referral and when they pick up their first prescription refill for ART. PLH who do not link to care and who do not pick up prescription refills within a specified time after testing positive during home-based HTC generate automated SMS alerts that are sent to field staff mobile phones. Field staff then follow-up with their assigned PLH to facilitate linkage and/or medication adherence.
This article describes how the Biolink platform is being implemented through a one-arm study to test the feasibility, acceptability, and cost-effectiveness of the platform to improve linkage to care and initial ART adherence after HIV identification during home-based HTC. A baseline sample of PLH was recruited to pilot test the platform (n = 101) in three communities in KwaZulu-Natal (KZN), South Africa, hereafter referred to as mHealth communities. PLH will be re-evaluated at 3 and 6 months after baseline on a primary outcome for the fraction of PLH who are linked to care within a month of testing positive during home-based HTC. The outcome will be compared between mHealth communities and communities in KZN from a prior home-based HTC study (i.e., a historical control; Barnabas et al. 2016), hereafter referred to as control communities. The one-month window is based on the target period for linkage in control communities. The study will also examine rates of ART prescription refills after the initial prescription runs out and the feasibility and acceptability of using several patient identifiers to link field and clinic data in mHealth communities. Identifiers include South African IDs and fingerprint scans. Lastly, we will conduct cost-effectiveness analyses that have largely been lacking for mHealth studies and are crucial to evaluate mHealth scalability in LMIC (Krah and de Kruijf 2016; Leon et al. 2012). Cost-effectiveness analyses will improve resource allocation when developing future home-based HTC and mHealth programs. The focus of this article is on the study protocol and lessons learned from the baseline data. We do so to provide a basis for HIV clinicians and researchers in their own work as they leverage mHealth technology to target HIV care continuum outcomes.
Methods
Recruitment
Recruitment took place between November 2016 and April 2017 in three mHealth communities in the Greater Edendale Area in KZN. Each community was selected based on the presence of a public health clinic that (1) offered free HIV healthcare services, including ART medications, (2) was within walking distance of community households, and (3) employed clinic staff who were able and willing to use the Biolink platform.
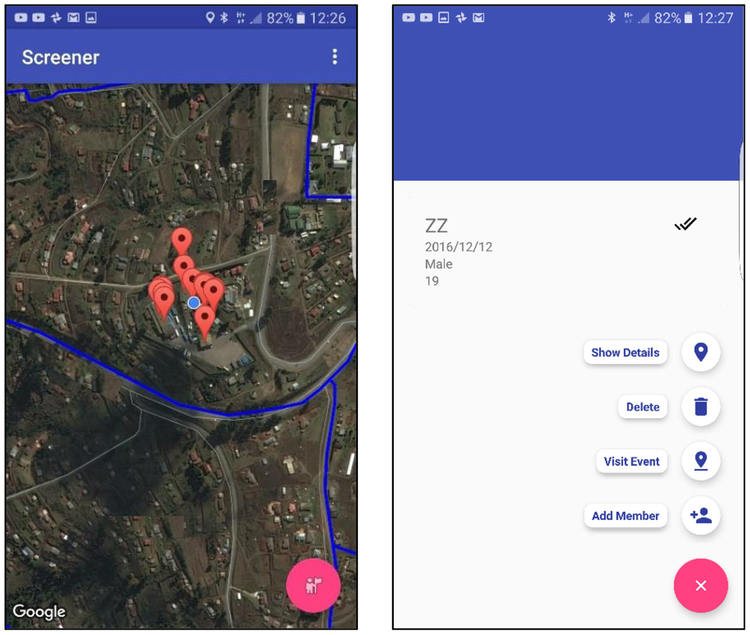
Blocks of households in each community were enumerated from aerial maps and randomly selected for home visitation. Home visitation occurred in two waves. Research staff, referred to as community mobilizers, visited homes to introduce the study and determine likely participation. Households with interested residents were recorded in a mobile mapping app; screenshots of the app are shown in Fig. 1. The mapping app was developed by the last author (van Heerden) to streamline paper-based community screening protocols that were developed to fully enumerate all structures within a given study area, collect geo-coordinates from each structure and ensure that it is a household dwelling. Our mapping app does not require pre-location and coding of structures. This addresses a key limitation in traditional web-based mapping tools, as the entry of a physical address is not required. Moreover, all enumerated households immediately appear on other fieldworker phones running the app. This improves fieldworker coordination and reduces duplicate visitation.
Fig. 1.
Screenshots from the community mapping and screening app showing map overview with community enumeration area boundaries and location of visited homes (left) and options available when enrolling a new household and recording the visit (right). Street and clinic names have been removed to mask locations
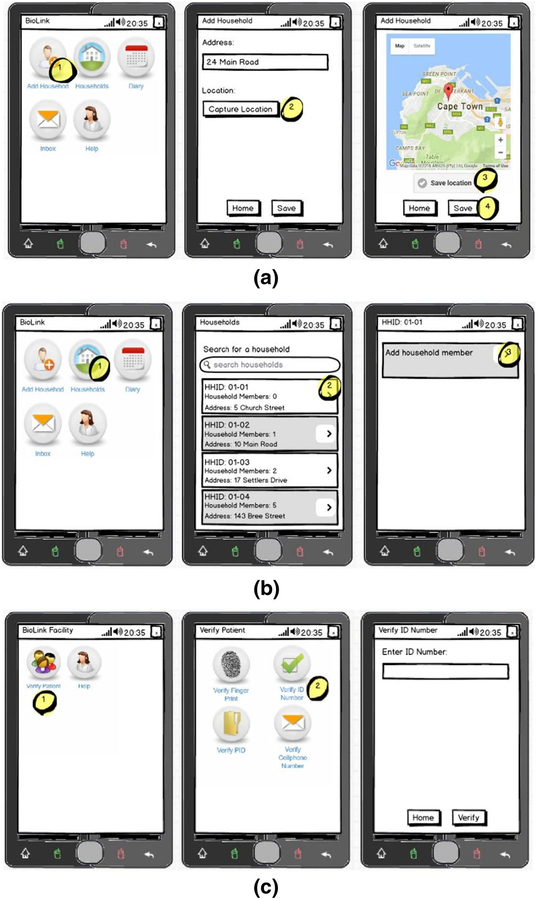
Field staff returned to the homes with interested household members between 2 and 5 days later to conduct home-based HTC. Field staff are trained in multiple roles to administer HIV rapid diagnostic tests, provide HIV counseling, enroll study participants, and collect data. Household and screening data were entered into the Mobenzi mobile app illustrated in Fig. 2. The flow of potential study participants through screening procedures is summarized in Fig. 3. We screened 962 individuals. Individuals were assessed on age, HIV status, and pregnancy, if female gender was reported. Individuals were first asked if they were at least 18 years old; younger individuals were screened out (n = 133). Individuals were then asked if they had previously been diagnosed with HIV; individuals reporting a prior diagnosis were screened out (n = 90). Remaining individuals who gave oral consent were administered an HIV test. Men and non-pregnant women who tested positive for HIV were invited to join the study after signing a consent form (n = 101). Pregnant women could opt to be tested for HIV, but were excluded from study participation. Study incentives were given as food parcels.
Fig. 2.
Renderings of screenshots to illustrate functionality of Mobenzi app to a enter households; b household members into the Mobenzi system by field staff; and c verify the identity of enrolled household members (PLH) upon presentation at a clinic by clinic staff. Fictitious names and addresses are shown for illustration
Fig. 3.
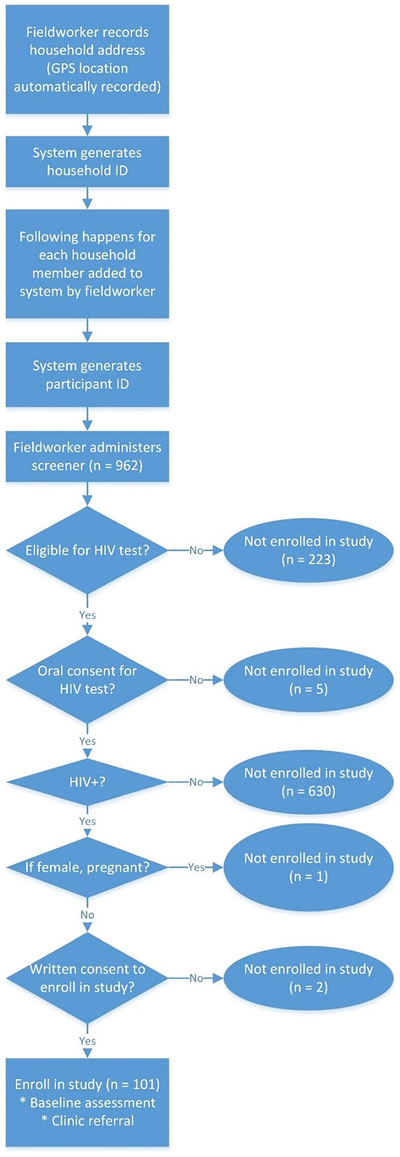
Screening and recruitment procedures that begin when fieldworkers approach households for home-based HTC and end with the enrollment of participants in the study
After enrollment, study participants were administered a baseline assessment through the Mobenzi mobile app and given a referral card to one of three health clinics based on proximity to the nearest clinic. During the baseline assessment, participants were asked to provide their name, surname, and at least one additional piece of identifying information: a phone number, a South African ID number, or a fingerprint scan that was collected through a Futronic scanner (http://futronic-tech.com/) that connects to field staff mobile phones (Samsung Galaxy J1 Ace Neo).
Follow-up Procedures
When a participant presents at their assigned health clinic after enrollment, DOH clinic staff record their visit using the Mobenzi mobile app (Fig. 2). The participant is identified using a South African ID, fingerprint, or cell phone number. Initiation of HIV care then begins by starting a clinic file for the patient.
If a participant does not present at the assigned clinic within 3 weeks of enrolling in the study, an automated SMS is sent by the Biolink platform to the fieldworker assigned to the participant to notify them that their participant has not linked to care. Field staff then attempt to contact the participant to assist with linkage. A second SMS is sent to field staff if PLH are still not linked to care after 6 weeks of study enrollment.
During the initial clinic visit, PLH are given a 1-month supply of ART and are expected to return to the clinic within a month to refill their ART prescription. If their ART prescription is refilled, this is recorded by clinic staff through the Mobenzi mobile app. Otherwise, an automated SMS is sent to the fieldworker phone to follow-up with the PLH to assist with the prescription refill.
After the baseline assessment, fieldworkers return for home visitation at 3 and 6 months to administer follow-up assessments to ascertain self-reported health and healthcare utilization. In addition to scheduled assessments, participants are encouraged to contact study staff any time they experience social harm because of their HIV-positive test result, including verbal, physical, or economic abuse. In addition, all field staff have field experience on prior studies and training to identify signs of distress and social harm during home visits. If social harm is identified or reported by PLH, staff make referrals to one of the local non-governmental organizations that offer counseling and support. Field staff also alert their supervisor. Cases deemed serious are referred to a “study” psychologist who is available for counseling across multiple research projects.
Data Collection
Field staff enter data into one of the following forms through the Mobenzi mobile app:
Baseline form to conduct a baseline assessment during the initial home visit.
Follow-up form to conduct assessments at 3 and 6-month follow-up visits.
Contact-attempt form to record the number and mode of contacts, e.g., SMS or a phone call, that are made to schedule follow-up visits and respond to SMSs that assigned participants have not linked to care or refilled their ART prescription.
Social harm form to record social harms that are reported by study participants because of their HIV-positive serostatus, including what type of social harm they experienced.
DOH clinic staff fill out a facility form to record participant visit dates and reasons for visits, either as a referral visit after home-based HTC or to refill their initial 1-month ART prescription. Details on measures that are contained in the baseline and follow-up forms follow.
Baseline Measures
Demographic and Background Characteristics
Participants are asked about their age, gender, and if they are the head of household and if not, their relationship to the head of household. “Head of household” is a familiar concept in South Africa described as the person who makes household decisions. Participants are then asked if they slept at home at least two nights a week.
Mobile technology usage includes the number of different mobile numbers used, how often a mobile phone is used for texting, the preferred mode of phone contact, such as voice, SMS, or Whatsapp, and if they would be “happy to use text messages or phone calls to communicate with health providers about their care”.
Health
Participants are asked how many times they tested for HIV before the current home visit, and if they have been diagnosed with chronic illnesses, such as diabetes, hypertension, and asthma. Participants are also asked if they ever tested positive for TB and if so, if they received treatment.
Follow-up Measures
Clinic Visitation
Participants are asked if they visited a health clinic for HIV care since their last home visit. If participants indicate having visited a clinic, they are then asked the name of the clinic, what challenges they encountered during visitation (e.g., “Long wait times”), and CD4 count and viral load test results. Participants who do not indicate visiting a clinic since their last home visit are asked why (e.g., “Not due for next appointment”, “Too busy”, “Don’t know where to go”) and if they plan to visit a clinic to receive HIV care in the next month.
HIV Medication
Participants are asked if they are taking HIV medications (ART). Participants who indicate taking ART are asked when they started, which medications they are taking, how much of their prescribed ART they took in the last 30 days, how many days of medication they missed in the last 30 days, and why they are not taking ART, if missed days were indicated (e.g., “ART were not available at the clinic”, “I did not feel sick”).
Control Community Data
Historical control data is provided by a prior HTC study that conducted home- and community-based HTC between June 2013 and March 2015 in two communities in the uMgungundlovu District of KZN (Barnabas et al. 2016). Individuals who tested positive were randomized to one of two groups including: (1) A lay counselor follow-up group (n = 312) where PLH received visits at home at 1, 3, and 6 months after they tested HIV positive until PLH reported having visited a clinic. (2) A clinic facilitation group (n = 303) where lay counselors coordinated with PLH to meet them at the clinic and explain the steps of engagement in care. Linkage rates from our study are compared to those from two arms in the control communities that collected linkage data with enough granularity to enable comparisons (N = 615). ART adherence is also an important HIV care continuum but not comparable between mHealth and control community data. In South Africa, universal test and treat policies were introduced in September 2016 and implemented in mHealth communities. Prior ART initiation during the control community study period was conditional on a CD4 count less than 500.
Measuring Costs
Economic and financial costs associated with the delivery of home-based HTC, mHealth, clinic facilitation for unlinked participants, field staff follow-up visits, and social harm counseling are being collected from the perspective of the healthcare system using a cost data collection spreadsheet and manual. Expenditures are further classified into one of four categories: (1) training and start-up costs; (2) personnel, including fringe benefits; (3) recurring costs, including supplies and services; (4) capital (items valued at $100 or more and deemed to last a year or more); and (5) facilities. Personnel include fieldworkers, clinic staff, and managers associated with the intervention. Study staff salaries (e.g., field staff) are captured through programmatic expenditures and clinic staff salaries are captured provided by DOH. Capital includes phones and portable scanners; these costs are annualized over their expected useful life using a discount rate of 3% (WHO 2008). Supplies include HIV test kits, ART, and referral cards. Facility costs are valued based on total monthly rental cost or market value and multiplied by the proportion utilized for the intervention. Services include usage of the Biolink platform such as cloud storage and support. Research and evaluation costs (e.g., costs associated with follow-up evaluations), and product development are separated out and excluded from analyses.
Costs associated with the control communities are estimated based on the costs of clinic facilitation and fieldworker follow-up visits identified in the intervention.
Data Analysis Plan
Summary statistics will be presented for the number of PLH linked to care within a month of testing positive, and the number of prescription refills for ART medications. Social harm information, general health characteristics, and healthcare utilization data from the baseline and follow-up assessments will also be reported. Logistic regression will be used to compare the proportion of PLH who link to care within a month of testing positive for HIV between mHealth and control communities.
Cost Analysis
Cost analyses will be based on standard practices for evaluating HIV interventions (Marseille et al. 2011; Holtgrave et al. 1998) and cost-effectiveness analyses of mHealth interventions in LMIC that are starting to emerge in the literature (e.g., Prinja et al. 2016). Total and unit costs of the intervention will be calculated and reported in accordance with the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) (Husereau et al. 2013). Cost categories will be compared to understand the primary drivers of the intervention. Total costs will be compared to the number of participants served, HIV infections identified, PLH linked to care within 1 month, and ART prescription refills.
Additionally, using an incremental cost-effectiveness ratio (ICER), we will compare the costs and benefits of the mHealth intervention to two arms of the control group: the lay counselor follow-up group and the clinic facilitation group. We assume that participants in the intervention and control groups had access to similar home-based HTC services and community resources. Compared to the control group’s lay counselor follow-up arm, the costs related to mHealth, clinic facilitation, and social harm counseling will be the source of incremental cost of the intervention. Compared to the control group’s facilitation arm, the costs of the intervention will be mHealth, fieldworker follow-up visits, and social harm counseling. Additionally, since only non-linked participants in the intervention received clinic facilitation, we will subtract the per participant costs of clinic facilitation from the intervention for those that did not receive this service.
Risk difference estimates will be derived from the logistic regression described above to identify the number of additional PLH linked to care by 1 month in the intervention communities due to the mHealth program. The ICER will reflect the incremental costs of the intervention described above divided by the number of additional linkages in the intervention.
Results
Among those screened for enrollment into the study, we found an HIV prevalence of 23% (n = 90 with a prior diagnosis and 101 newly diagnosed out of 829). Table 1 shows baseline characteristics of PLH in the study (N = 101). The median age of PLH was 35 years old (range = 18 to 75). Most PLH were female (79%) and reported sleeping at home at least two nights a week (100%). A little less than half of the PLH reported a prior HIV test (48.5%); the remaining PLH reported not knowing if they had previously been tested (27%), declined to answer (20%), or had not been tested (5%). Most PLH reported not being diagnosed with other illnesses (82%); seven PLH reported being diagnosed with hypertension, seven with diabetes, two with hypertension and diabetes, two with asthma, and one with ulcers. Half of the PLH had been tested for tuberculosis (TB) in their lifetime (52%); out of those testing positive for TB, all had received treatment and one was currently taking TB medication.
Table 1.
Baseline characteristics of study sample (N = 101)
| Sociodemographics | % | n |
|---|---|---|
| Female gender | 79% | 80 |
| Relationship to head of household | ||
| Child | 36% | 36 |
| Self | 30% | 30 |
| Parent | 16% | 16 |
| Sibling | 8% | 8 |
| Relative | 6% | 6 |
| Sexual partner/wife/husband | 5% | 5 |
| General health | ||
| Prior HIV test | ||
| Yes | 48.5% | 49 |
| No | 5% | 5 |
| Do not know | 27% | 27 |
| Decline to answer | 20% | 20 |
| Currently diagnosed with chronic illness | ||
| None | 82% | 83 |
| Hypertension or diabetes | 13% | 13 |
| Asthma | 2% | 2 |
| Ulcers | 1% | 1 |
| Decline to answer | 2% | 2 |
| Ever tested for tuberculosis (TB) | 52% | 53 |
| Tested positive for TB (out of those tested; N = 53 | 38% | 20 |
| Use of mobile technology | ||
| Number of mobile numbers currently using | ||
| None | 4% | 4 |
| One | 90% | 91 |
| Two or more | 6% | 6 |
| Frequency of texting on a mobile phone | ||
| At least once a day | 40.5% | 41 |
| More than once a week (but less than daily) | 29% | 29 |
| A few times a month | 9% | 9 |
| A few times during lifetime | 13% | 13 |
| Never | 8% | 8 |
| Decline to answer | 1% | 1 |
| Preferred mode of communication | ||
| Voice call | 49.5% | 50 |
| Text (SMS) | 47.5% | 48 |
| WhatsApp message | 3% | 3 |
| Interest in mobile communication with health provider | ||
| Yes | 96% | 97 |
| No | 2% | 2 |
| Decline to answer | 2% | 2 |
Almost all PLH reported using at least one mobile phone number (96%) and expressed interest in communicating with a healthcare provider about their care through voice communication or SMS (96%). A little less than half of the PLH reported texting at least once a day (41%). Given the degree of mobile phone usage, it was not surprising that 98% of the PLH submitted a phone number as a participant identifier during enrollment into the study. Almost three quarters of the PLH supplied a South African ID number (72%; N = 73). All PLH were willing to have their fingerprints scanned but we were only able to scan fingerprints for 35 PLH (35%) due to faulty scanners or software glitches. Investigators debriefed field staff to verify that missing fingerprint scans were due to technical difficulties and not due to PLH who refused to have their fingerprints scanned.
Discussion
Efforts to monitor the linking of newly diagnosed PLH to care after home-based HTC have traditionally fallen outside the reach of mHealth technologies. PLH are given referral cards or accompanied to a clinic as a “warm hand-off” (Barnabas et al. 2016). Our study is innovative in its design by alerting field staff via SMS when linkage has not occurred. Field staff can then focus efforts to link PLH to those in greatest need of assistance. Based on an assumption that many PLH will link to care on their own, our design has the potential to reduce the number of personnel needed to successfully link PLH to care by focusing resources on those clients most in need of assistance.
We found a high HIV prevalence that matches a population prevalence estimate of 23% from a prior study in KZN (Alsallaq et al. 2013). This underscores the need for programs to support linkage and retention in care. Most of our study samples are women, which is not surprising since we conducted a home-based HTC program during the day when men are more likely to be out of the home than women. The high proportion of women in our study is a reminder of a continuing need to address the gender gap by reaching more men for HIV testing (Sharma et al. 2017) and to evaluate potential innovative outreach strategies, such as HIV self-testing kits (Makusha et al. 2015).
Our study is too early in its implementation to examine linkage rates and other outcomes, but several lessons have emerged. The mobile mapping tool we developed is successfully being used by field staff and will continue to be developed with new features. Nhavoto and Grönlund (2013) used GPS location traces to track the movement of patients who were linked to three clinics in Mozambique. In this vein, one direction for future app development could combine information from static locations, such as homes, and patients’ current locations to better offer home-based healthcare to a patient’s current location.
Biometric fingerprint identification was acceptable to all study participants despite focus group caveats about the wide-scale acceptability of biometric identification systems in our population (van Heerden et al. 2017). A limitation in drawing conclusions about acceptability is that our sample was relatively small. However, the acceptability of biometric systems on a larger scale has been demonstrated through successful implementations of fingerprint verification systems in health facilities located in South Africa, Kenya, and Uganda (Chamie et al. 2014; Nglazi et al. 2012; Serwaa-Bonsu et al. 2010). A lack of robustness in our fingerprint scanning system was unexpected; scans failed for two thirds of the sample. The high proportion of failures was due to a combination of faulty scanners, delays in troubleshooting as there was a time lag in relaying scanner issues from field staff to the main research office so that scanners could be replaced, and software issues in configuring the biometric system for participant identification. As noted above, HTC biometric systems have been used for the simpler task of participant verification. Fingerprint identification issues that we encountered underscore the importance of building flexibility into the identification system as recommended by our focus groups (van Heerden et al. 2017), especially for fieldwork in resource-poor settings. Luckily, we followed the advice of our focus groups as our study allowed identification through fingerprints, South African IDs, and cell phone numbers.
Eventually, reliance on a single method of identification may become more practical in South Africa. Almost all PLH reported using mobile phones and interest in mobile communication with a healthcare provider. This is in line with prior studies that have reported on the ubiquity of mobile phones in South Africa (Cargo 2013). Currently, biometric scans are high-end smartphone features, such as iris identification, that is, available on the Samsung S8. In a few years, it is reasonable to surmise that these biometric features will trickle down to less costly phones and may make biometric scanning a more robust identification option in the field. Moreover, the South African government initiated the development of an Automated Biometric Identification System in 2016 (ABIS; Ihekwoaba 2018). Additional infrastructure for a universal identification system is being provided. It was also encouraging to note that many PLH supplied a South African ID number for identification. The feasibility of the South African ID as a primary patient identifier will likely increase with a continued expansion in ID programs and may eventually be more practical as means of identification than biometric solutions in South Africa. Amid countrywide identification system expansions, a remaining challenge will be the need to identify non-residents when they present for care at health clinics, as well as residents who cannot be identified through the system for any number of reasons (e.g., a resident who arrives at a clinic without a national ID card). System flexibility to allow for multiple identifiers as we did in our study will likely remain.
Rates of self-reported hypertension and diabetes among PLH in our study were lower than expected based on hypertension and diabetes screening results from PLH in a prior study conducted in KZN (van Heerden et al. 2016), highlighting the need for screening of both communicable and noncommunicable diseases. Integrated screening programs are needed to make the best use of limited healthcare resources in South Africa and other resource-poor settings (Govindasamy et al. 2013). Current health screening practices typically target single diseases and have largely been funded by donors, which is not sustainable long term (Rotheram-Borus et al. 2011).
To the best of our knowledge, cost-effectiveness analysis using data related to mHealth interventions from South Africa and other LMIC has largely been lacking in the literature, with Prinja et al. (2016) as a rare exception. This is especially true for data on how mHealth programs can improve resource allocation and, in turn, lead to cost benefits, such as a potential reduction in personnel that are needed to link PLH to care when SMS alert systems are implemented. Therefore, costing information from this study will provide a valuable contribution to the literature.
We acknowledge a limitation in being unable to report common cost-effectiveness indicators for HIV programs that call for simulation studies, such as measures that incorporate infections averted and quality-adjusted life years. Our sample is relatively small as a basis for simulation studies. Moreover, our study was focused on the feasibility of linkage and did not collect requisite measures that such calculations call for. For example, we did not collect sexual behavior data to estimate infections averted nor the length of HIV infection to estimate quality-adjusted life years. Further, ART adherence is also an important HIV care continuum outcome; however, we are not be able to compare mHealth and control communities on this outcome because current universal ART administration differ from policies that were in place during implementation of the interventions in the control communities. Previously, ART was only administered to PLH when CD4 cell counts dropped below a threshold. There are limitations in using historical data as a comparison for our study data regarding changes in the number of HIV testing and treatment clinics since the time the historical data was collected. Cultural and social changes regarding HIV stigma can also present a potential bias as social views on HIV may have changed since South Africa has increased its efforts to scale-up HIV treatment (Bor et al. 2013). Lastly, there is a limitation in generalizing our findings to PLH due to the over representativeness of women in the sample. This finding is evident in other similar community-based HIV screening studies and highlights how concepts of masculinity shape health seeking behavior of males in this context (Hawkes and Buse 2013; Higgins et al. 2010). Nonetheless, data that are being collected in this study will inform the development of future home-based HTC programs that incorporate mHealth tools to enhance the work of field staff. By examining the feasibility and acceptability of our current program, we hope to get a better sense of the optimal balance of what procedures can be automated through mHealth protocols and what requires direct human intervention to aid PLH in linking to care and initiating ART.
Acknowledgments
Funding This research was supported by the National Institute of Mental Health (R21MH106351; P30MH58107).
Compliance with Ethical Standards
Conflict of Interest The authors declare that they have no conflict of interest.
Ethical Approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The UCLA Institutional Review Board (IRB# 16–001207) and the HSRC Research Ethics Committee (REC 7/15/06/16) has approved and monitors the study protocol over time.
Informed Consent Informed consent was obtained from all individual participants included in the study.
References
- Alsallaq RA, Baeten JM, Celum CI, Hughes JP, Abu-Raddad L, Barnabas RV, & Hallett TB (2013). Understanding the potential impact of a combination HIV prevention intervention in a hyper-endemic community. PLoS One, 8, e54575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S-J, Cherutich P, Kilonzo N, Cremin I, Fecht D, Kimanga D, et al. (2014). Maximising the effect of combination HIV prevention through prioritisation of the people and places in greatest need: a modelling study. Lancet, 384, 249–256. [DOI] [PubMed] [Google Scholar]
- Barnabas RV, van Rooyen H, Tumwesigye E, Brantley J, Baeten JM, van Heerden A, et al. (2016). Uptake of antiretroviral therapy and male circumcision after community-based HIV testing and strategies for linkage to care versus standard clinic referral: a multisite, open-label, randomised controlled trial in South Africa and Uganda. Lancet HIV, 3, e212–e220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bor J, Herbst AJ, Newell ML, & Bärnighausen T (2013). Increases in adult life expectancy in rural South Africa: valuing the scale-up of HIV treatment. Science, 339, 961–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cargo M (2013). South Africa mHealth Landscape. https://www.gsma.com/mea/wp-content/uploads/2014/09/South-Africa-mHealth-Landscape_June-2013-1.pdf.
- Catalani C, Philbrick W, Fraser H, Mechael P, & Israelski DM (2013). mHealth for HIV treatment & prevention: a systematic review of the literature. The Open AIDS Journal, 7, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamie G, Kwarisiima D, Clark TD, Kabami J, Jain V, Geng E, et al. (2014). Uptake of community-based HIV testing during a multi-disease health campaign in rural Uganda. PLoS One, 9, e84317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coetzee D, Hildebrand K, Boulle A, Maartens G, Louis F, Labatala V, et al. (2004). Outcomes after two years of providing antiretroviral treatment in Khayelitsha, South Africa. AIDS, 18, 887–895. [DOI] [PubMed] [Google Scholar]
- Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, et al. (2011). Prevention of HIV-1 infection with early antiretroviral therapy. New England Journal of Medicine, 365, 493–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department of Home Affairs. (2016). http://www.dha.gov.za/index.php/identity-documents2.
- Eaton JW, Menzies NA, Stover J, Cambiano V, Chindelevitch L, Cori A, et al. (2014). Health benefits, costs, and cost-effectiveness of earlier eligibility for adult antiretroviral therapy and expanded treatment coverage: a combined analysis of 12 mathematical models. The Lancet Global Health, 2, e23–e24. [DOI] [PubMed] [Google Scholar]
- Forrest JI, Wiens M, Kanters S, Nsanzimana S, Lester RT, & Mills EJ (2015). Mobile health applications for HIV prevention and care in Africa. Current Opinion in HIVand AIDS, 10, 464–471. [DOI] [PubMed] [Google Scholar]
- Goel S, Bhatnagar N, Sharma D, & Singh A (2013). Bridging the human resource gap in primary health care delivery systems of developing countries with mHealth: narrative literature review. JMIR mHealth and uHealth, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grovindasamy D, Kranzer K, van Schaik N, Noubary F, Wood R, Walensky RP, et al. (2013). Linkage to HIV, TB and noncommunicable disease care from a mobile testing unit in Cape Town, South Africa. PLoS One, 8, e80017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkes S, & Buse K (2013). Gender and global health: evidence, policy, and inconvenient truths. The Lancet, 381, 1783–1787. [DOI] [PubMed] [Google Scholar]
- Higgins JA, Hoffman S, & Dworkin SL (2010). Rethinking gender, heterosexual men, and women’s vulnerability to HIV/AIDS. American Journal of Public Health, 100, 435–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtgrave DR, Pinkerton SD, Jones TS, Lurie P, & Vlahov D (1998). Cost and cost-effectiveness of increasing access to sterile syringes and needles as an HIV prevention intervention in the United States. Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology, 18, S133–S138. [DOI] [PubMed] [Google Scholar]
- Husereau D Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, Augustovski F, Briggs AH, Mauskopf J, & Loder E (2013). Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. BMC Medicine, 11, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihekwoaba C (2018). South Africa launches new automated biometric identification system. TheNerve Africa. Retrieved from https://thenerveafrica.com/18665/south-africa-launches-new-automated-biometric-identification-system/. [Google Scholar]
- Kimaiyo S, Were MC, Shen C, & Mamlin J (2010). Home-based HIV counselling and testing in western Kenya. East African Medical Journal, 87, 100–108. [DOI] [PubMed] [Google Scholar]
- Krah EFM, & de Kruijf JG (2016). Exploring the ambivalent evidence base of mobile health (mHealth): a systematic literature review on the use of mobile phones for the improvement of community health in Africa. Digital Health, 2, 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon N, Schneider H, & Daviaud E (2012). Applying a framework for assessing the health system challenges to scaling up mHealth in South Africa. BMC Medical Informatics and Decision Making, 12, 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester RT, Ritvo P, Mills EJ, Kariri A, Karanja S, Chung MH, et al. (2010). Effects of a mobile phone short message service on antiretroviral treatment adherence in Kenya (WelTel Kenya1): a randomized trial. Lancet, 376, 1838–1845. [DOI] [PubMed] [Google Scholar]
- Makusha T, Knight L, Taegtmeyer M, Tulloch O, Davids A, Lim J, et al. (2015). HIV self-testing could “revolutionize testing in South Africa, but it has got to be done properly”: perceptions of key stakeholders. PLoS One, 10, e0122783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marseille E, Shade SB, Myers J, & Morin S (2011). The cost-effectiveness of HIV prevention interventions for HIV-infected patients seen in clinical settings. JAIDS, 56, e87–e94. [DOI] [PubMed] [Google Scholar]
- McLaren Z, Ardington C, & Leibbrandt M (2014). Distance decay and persistent health care disparities in South Africa. BMC Health Services Research, 14, 541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills EJ, Bakanda C, Birungi J, Chan K, Ford N, Cooper CL, et al. (2011). Life expectancy of persons receiving combination antiretroviral therapy in low-income countries: a cohort analysis from Uganda. Annals of Internal Medicine, 155, 209–216. [DOI] [PubMed] [Google Scholar]
- Molesworth AM, Ndhlovu R, Banda E, Saul J, Ngwira B, Glynn JR, et al. (2010). High accuracy of home-based community rapid HIV testing in rural Malawi. JAIDS, 55, 625–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukund BKC, & Murray PJ (2010). Cell phone short messaging service (SMS) for HIV/AIDS in South Africa: a literature review. Studies in Health Technology and Informatics, 160, 530–534. [PubMed] [Google Scholar]
- Naik R, Tabana H, Doherty T, Zembe W, & Jackson D (2012). Client characteristics and acceptability of a homebased HIV counselling and testing intervention in rural South Africa. BMC Public Health, 12, 824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nglazi MD, van Schaik N, Kranzer K, Lawn SD, Wood R, & Bekker L-G (2012). An incentivized HIV counselling and testing program targeting hard-to-reach unemployed men in Cape Town, South Africa. JAIDS, 59, e28–e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nhavoto JA, & Grönlund Å (2013). Design and implementation of a web-based application for patient management and decision support using mobile phones and geographic information system. 8th Health Informatics in Africa Conference (HELINA 2013) http://jhia-online.org/index.php/jhia/article/view/39/9. [Google Scholar]
- Prinja S, Nimesh R, Gupta A, Bahuguna P, Thakur JS, Gupta M, & Singh T (2016). Impact assessment and cost-effectiveness of m-health application used by community health workers for maternal, newborn and child health care services in rural Uttar Pradesh, India: a study protocol. Global Health Action, 9, 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen S, & Fox MP (2011). Retention in HIV care between testing and treatment in sub-Saharan Africa: a systematic review. PLoS Medicine, 8, e1001056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotheram-Borus MJ, le Roux IM, Tomlinson M, Mbewub N, Comulada WS, le Roux K, et al. (2011). Philani plus (+): a mentor mother community health worker home visiting program to improve maternal and infants’ outcomes. Prevention Science, 12, 372–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MK, Rutstein SE, Powers KA, Fidler S, Miller WC, Eron JJ, & Cohen MS (2013). The detection and management of early HIV infection: A clinical and public health emergency. Journal of Acquired Immune Deficiency Syndromes, 63, S187–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- South African Department of Health. (2015). National HIV testing services: policy and guidelines 2015. http://www.nacosa.org.za/wp-content/uploads/2016/05/HTS-Policy-guideline.pdf.
- Sharma M, Barnabas RV, & Celum C (2017). Community-based strategies to strengthen men’s engagement in the HIV care cascade in sub-Saharan Africa. PLoS Medicine, 14, e1002262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serwaa-Bonsu A, Herbst A, Reniers G, Ijaa W, Clark B, Kabudula C, & Sankoh O (2010). First experiences in the implementation of biometric technology to link data from health and demographic surveillance systems with health facility data. Global Health Action, 3, 2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shisana O (2013). HIV/AIDS in South Africa: at last the glass is half full. In: 6th South African AIDS Conference Durban, South Africa: Human Sciences Research Council. [Google Scholar]
- Tumwesigye E, Wana G, Kasasa S, Muganzi E, & Nuwaha F (2010). High uptake of home-based, district-wide, HIV counseling and testing in Uganda. AIDS Patient Care and STDs, 24, 735–741. [DOI] [PubMed] [Google Scholar]
- van Heerden A, Barnabas R, van Rooyen H, & Celum C (2016). Integrating non-communicable disease (NCD) screening and referral to care into home HIV testing and counselling in rural KwaZulu-Nata, South Africa: burden of disease. http://www.hsrc.ac.za/en/research-outputs/view/8240.
- van Heerden A, Harris DM, van Rooyen H, Barnabas RV, Ramanathan N, Ngcobo N, et al. (2017). Perceived mHealth barriers and benefits for home-based HIV testing and counselling and other care: qualitative findings from health officials, community health workers, and persons living with HIV in South Africa. Social Science & Medicine, 183, 97–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooyen H, Barnabas RV, Baeten JM, Phakathi Z, Joseph P, Krows M, et al. (2013). High HIV testing uptake and linkage to care in a novel program of home-based HIV counselling and testing with facilitated referral in KwaZulu-Natal, South Africa. JAIDS, 64, e1–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization. (2008). WHO guide for standardization of economic evaluations of immunization programmes. http://apps.who.int/iris/bitstream/10665/69981/1/WHO_IVB_08.14_eng.pdf.
- World Health Organization. (2015). Consolidated guidelines on HIV testing services 2015. http://apps.who.int/iris/bitstream/10665/179870/1/9789241508926_eng.pdf?ua=1&ua=1.
- Wouters E, Meulemans H, Van Rensburg HCJ, Heunis JC, & Mortelmans D (2007). Short-term physical and emotional health outcomes of public sector ART in the free state province of South Africa. Quality of Life Research, 16, 1461–1471. [DOI] [PubMed] [Google Scholar]