Abstract
Lung cancer is the leading cause of cancer mortality around the world. The lack of detailed understanding of the cellular and molecular mechanisms participating in the lung tumor progression restrains the development of efficient treatments. Recently, by using state-of-art technologies, including in vivo sophisticated Cre/loxP technologies in combination with lung tumor models, it was revealed that osteoblasts activate neutrophils that promote tumor growth in the lung. Strikingly, genetic ablation of osteoblasts abolished lung tumor progression via interruption of SiglecFhigh–expressing neutrophils supply to the tumor microenvironment. Interestingly, SiglecFhigh neutrophil signature was associated with worse lung adenocarcinoma patients outcome. This study identifies novel cellular targets for lung cancer treatment. Here, we summarize and evaluate recent advances in our understanding of lung tumor microenvironment.
Keywords: osteoblasts, neutrophils, lung, tumor microenvironment
INTRODUCTION
Lung cancer is the major cause of death worldwide [1]. Although meaningful increment has been made in our knowledge of disease pathogenesis, lung cancer is still a fatal disease [2]. The initiation and progression of lung cancer are attributed to genetic and certain environmental factors, such as air pollution and exposure to tobacco smoke. Despite several improvements, treatments like surgery, radiotherapy, and chemotherapy can rarely control completely this disease. Patients with lung cancer have a limited long-term survival, mainly due to the escape of tumor initiating cells of the initial treatment [3]. Since these escaped cells are more resistant to treatments, adjuvant therapies that could effectively destroy these remaining cancer cells would have a considerable impact on tumor treatment.
In the past few decades, research groups have focused their concentration mostly on cancer cells [4]. Nevertheless, emerging evidence demonstrates that the surroundings where these malignant cells are located play key roles in tumor development [5]. Tumor microenvironment is the local environment where tumorigenesis and tumor growth occur, composed of blood vessels, innervations, extracellular matrix, signaling molecules, growth factors, and other non-malignant cells, including immune cells, mesenchymal stem cells, fibroblasts, adipocytes, and pericytes [6–35]. The tumor microenvironment plays important roles in tumor initiation, development, invasion, and metastasis [36]. The constituents of the tumor microenvironment may cross-talk with cancer cells as well as between them.
Lung tumors are also comprised of neoplastic cells and their surrounding microenvironment, which affects the proliferation, survival, migration and drug resistance of lung cancer cells [37]. Although enormous advancement has been accomplished in our understanding of the importance of the tumor microenvironment, each new discovery informs us how complex is the control of tumor growth. The relationship between the lung cancer cells and the microenvironment in which they reside plays a pivotal role in determining whether and how these malignant cells grow. The capacity to prevent, limit, or reverse lung tumor growth in cancer patients unfortunately has not progressed significantly, due to the complexity of the mechanisms underlying this process. Uncovering the cellular and molecular mechanisms by which the tumor growth is controlled is crucial for the success of clinical applications.
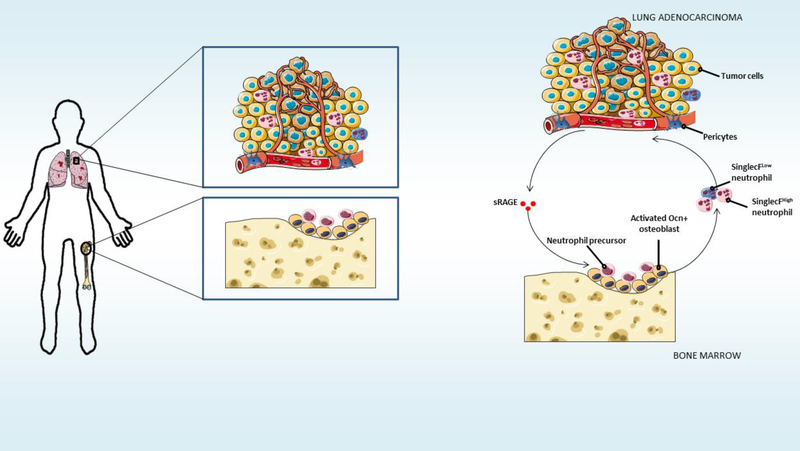
Neutrophils are the most abundant circulating cells in the blood, being constantly produced in the bone marrow due to their short lifespan [38]. After inflammatory stimuli, they migrate to tissues where they perform their functions [39]. Interestingly, growing evidence suggests that neutrophils may regulate tumor development [40]. Although neutrophils may present pro- or anti-tumoral activity, depending on specific tumor microenvironments [41], we are still far from fully understanding their function in the lung tumor microenvironment, and how this role can be regulated. Now, in an article in Science, Engblom and colleagues reveal the heterogeneity of neutrophils in the lung cancer microenvironment, and that only SiglecFhigh– expressing neutrophils promote tumor growth [42]. The authors investigated the systemic crosstalk between lung tumor and the bone that regulates the recruitment of pro-tumoral neutrophils by using elegant state-of-the-art techniques, including in vivo sophisticated Cre/loxP technologies in combination with several lung tumor models. Engblom and colleagues revealed, by using fluorescence-molecular tomography and Ocn-YFP mice, that lung tumors disrupt bone homeostatic activity, by increasing the number of osteocalcin (Ocn)-expressing osteoblasts through tumor-secreted factors [42]. Strikingly, these osteoblasts, in turn, promote lung tumor growth through the priming of pro-tumoral neutrophils (Figure 1). The authors demonstrated that genetic ablation of osteoblasts, by using Ocn-Cre/iDTR mice, abolished lung tumor progression, via the interruption of SiglecFhigh–expressing neutrophils supply to the tumor microenvironment [42]. Moreover, Engblom and colleagues showed that SiglecFhigh–expressing neutrophils expand tumor growth in vivo. Importantly, for translation into clinics, the authors found an association of SiglecFhigh neutrophil signature with worse lung adenocarcinoma patients outcome. This study identifies a systemic communication between lung tumor and bone, even in the absence of metastasis. These results also offer novel therapeutic targets for lung tumor treatments.
Figure 1. Osteoblasts prime neutrophils that promote tumor growth in the lung.
The relationship between the lung tumor and its surroundings plays a pivotal role in determining whether and how malignant cancer cells grow. The study of Engblom and colleagues now reveals a novel very important function of systemic cross-talk between lung tumor and the bone that regulates cancer progression [42]. Lung tumors increase the number of osteocalcin (Ocn)expressing osteoblasts through tumor-secreted factors. These osteoblasts prime SiglecFhigh– expressing neutrophils, which in turn expand lung tumor growth in vivo. With the appearance of state-of-art techniques, future studies will reveal in detail the cellular and molecular components that regulate the lung tumor microenvironment.
Here, we discuss the findings from this work, and evaluate recent advances in our understanding of the lung tumor microenvironment.
PERSPECTIVES / FUTURE DIRECTIONS
TUMOR REGULATION OF THE IMMUNE SYSTEM
Deciphering how exactly malignant cancer cells regulate the immune system promises to bring improvements in the way we treat cancer [43]. Immune cells within the tumor may occupy as much as half of its mass in some tumors [44]. Thus, cancer malignant growth does not rely exclusively on its intrinsic genetic and epigenetic alterations. Besides its capacity to spread to secondary tissues as metastasis, the primary tumor can also interact with distant organs by tumor-induced systemic factors [45]. Such factors, e.g. CXCL12, osteopontin, VEGF, TGFβ and G-CSF, may affect hematopoiesis in the bone marrow microenvironment, altering the formation of other immune cells, in addition to neutrophils, including monocytes, macrophages, and lymphocytes [46,47]. The recruitment of these cells may comprise the tumor microenvironment, influencing tumor angiogenesis, invasion, and immune suppression [47–49]. It will be important to explore how these other immune cells influence neutrophil mobilization to the lung tumor microenvironment, and whether tumor-driven inflammatory signaling to other immune cell populations is involved in this process. Interestingly, neutrophils, together with other immune cells, also participate in the preparation of the pre-metastatic niche for the initial seeding of cancer cells in the lung [50–52,48,53–56]. These immune cells are recruited to the pre-metastatic lung, enhancing pulmonary metastasis by production of tumor growth-promoting factors. The role of other cells from outside the lung in the establishment of the pulmonary tumor niche is still not completely determined. Also, the details about the cross-talk between distinct immune cell subsets involved in the arrival of tumor cells to the lung remains to be examined. Further studies are required to evaluate the importance of neutrophils’ interactions with other immune cells in the pulmonary tumor growth. Importantly, future studies should also explore whether tumor location is important for its immune-modulatory roles.
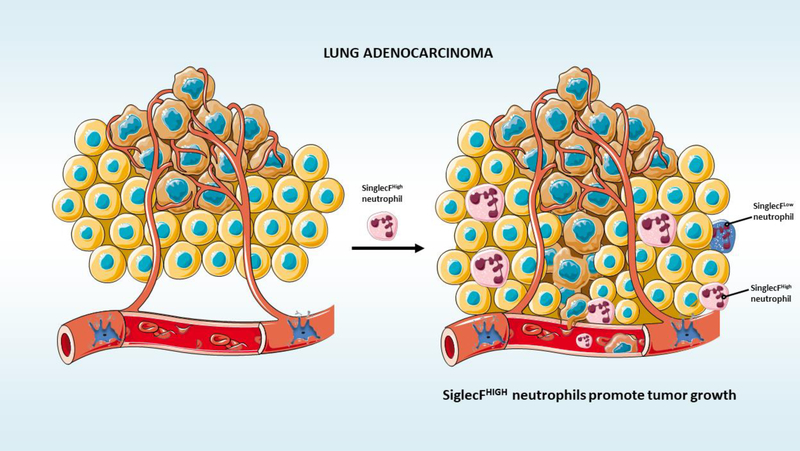
The lung neoplasic cells actively interact with the immune system. Lung adenocarcinoma microenvironment presents infiltration from several immune cells, including lymphocytes, macrophages, dendritic cells, and others [57–60]. These interactions may be essential for the shaping of lung cancer growth, and development. For instance, the presence of macrophages is associated with poor survival [58], while dendritic cells are linked to prolonged survival [59]. Despite this knowledge, how exactly different immune subsets interact with SiglecFhigh– expressing neutrophils and affect lung adenocarcinoma progression remains to be elucidated (Figure 2). Understanding the degree to which immune cells are modulated in the lung adenocarcinoma microenvironment during cancer progression will provide insights into how the lung tumor and immune cells shape each other as they evolve.
Figure 2. SiglecFhigh–expressing neutrophils expand tumor growth in vivo.
SiglecFhigh–expressing neutrophils promote lung adenocarcinoma.
TUMOR-ACTIVATED OSTEOBLASTS
It is known from previous studies that metastatic tumor cells promote increased bone formation via osteoblasts activation [61]. Increased bone growth originates mineralized tissue where malignant cancer cells reside, causing more osteoblastic lesions, stimulating cancer growth [62]. A recent study showed that the newly formed osteoblasts derive from bone marrow endothelial cells when those are stimulated by metastatic cancer cells [63,64]. Engblom and colleagues introduce a new concept: osteoblasts can be remotely activated by tumor cells remotely even when those are not present in the bone [42]. Future studies will reveal what is the origin of newly formed osteoblasts activated by lung tumor, and whether they are also derived from endothelial cells. Also, it will be important to explore whether, in osteoblastic lesions caused by metastatic malignant cells, Siglechigh–expressing tumor promoting neutrophils are also being generated.
Osteoblasts have been shown to be heterogeneous. Nonetheless, Engblom and colleagues consider osteoblasts as a homogeneous cell population in their study [42]. Osteoblasts from distinct bones differ in their embryonic origins. For instance, craniofacial bones osteoblasts derive from the neural crest, while long bones osteoblasts derive from the mesoderm. Osteoblasts also differ in the way they form the bone. In the clavicle and in the craniofacial skeleton, it happens via intramembranous ossification, while in the remaining skeleton, through endochondral ossification [65–67]. Osteoblasts from varying bones utilize distinct molecules for their activities. For instance, 1,25-dihydroxyvitamin D(3) and parathyroid hormone affect differently osteoblasts from distinct sites [68]. Long bone metaphysis is highly sensitive to parathyroid hormone, while calvaria is not [69]. Also, hypoxia-inducible factor α and Indian hedgehog are essential for osteoblastic activity during endochondral ossification but not during intramembranous ossification [70,71]. Inorganic and organic matrix composition produced by calvaria and long bones osteoblasts also differ [72,73]. Due to the crucial role played by osteoblasts discovered by Engblom et al. (2017), the question arises as to whether the osteoblast subpopulations from different bones differ in their capacity to promote lung tumor growth.
Moreover, the main findings from Engbom et al. (2017) study are based on the data obtained from Ocn-Cre mice [42]. It is known that osteocalcin gene is expressed by mature osteoblasts [74]. Note however that expression of osteocalcin is not restricted to mature osteoblasts. Other osteoblast lineage cells, including hypertrophic chondrocytes, pre-osteoblasts, and osteocytes may also express osteocalcin [75–78]. In addition, osteocalcin is expressed in megakaryocytes [79], platelets [79], and brain [80]. Furthermore, a recent study shows, by using high-resolution microscopy of bone sections and flow cytometry, that, additionally to mature osteoblasts, Ocn-Cre mice exhibit Cre recombinase activity in the majority of CXCL12-abundant reticular (CAR) cells and arteriolar pericytes [81]. Thus, it is possible that the effect on Siglechigh–expressing tumor promoting neutrophils could be due to a different cell type, other than osteoblasts. To perform osteoblasts-specific targeting, a more specific mouse model should be used in future studies, i.e. mouse 2.3 kb Col1α1-Cre mice [82].
Engblom and colleagues reveal in their work that osteoblasts prime SiglecFhigh– expressing neutrophils to promote lung tumor growth [42]. However, it remains unclear whether this priming needs direct contact between osteoblasts and neutrophils, or whether it depends on molecules secreted by osteoblasts to the bone marrow milieu or even to the circulation that will induce the formation of SiglecFhigh–expressing neutrophils. Interestingly, a recent study showed that the lung microenvironment serves as a niche to a subtype of hematopoietic stem cells that produce all hematopoietic lineages, including neutrophils [83,84]. It remains unknown whether the neutrophils produced in the lung are also primed by the bones through osteoblasts-derived molecules present in the circulation, and what percentage of tumor promoting SiglecFhigh– expressing neutrophils originate from the lung. Also, it will be important to analyze whether some cells in the lung microenvironment are targeted in Ocn-Cre mice as discussed above [81].
BONE MARROW NICHE FOR TUMOR-ACTIVATED NEUTROPHILS
The hematopoietic stem cells, which give rise to all blood and immune cells throughout life, reside in a specific complex niche in the bone marrow, that supports the homeostasis of these cells, composed by osteoblasts, osteocytes, osteoclasts, endothelial cells, adipocytes, smooth muscle cells, pericytes, fibroblasts, macrophages, megakaryocytes, lymphocytes, hematopoietic progenitors, neutrophils, peripheral innervations, and Schwann cells [18,85,64,86–89,19,9,10,90,93]. Recent studies have revealed distinct contributions of several cellular components of the bone marrow to different functions of hematopoietic stem cells regulation. Nothing is known about the needed niche for the production of tumor-promoting SiglecFhigh–expressing neutrophils in the bone marrow. It remains to be revealed whether there is a specific niche in the bone marrow where those SiglecFhigh–expressing neutrophils are being formed.
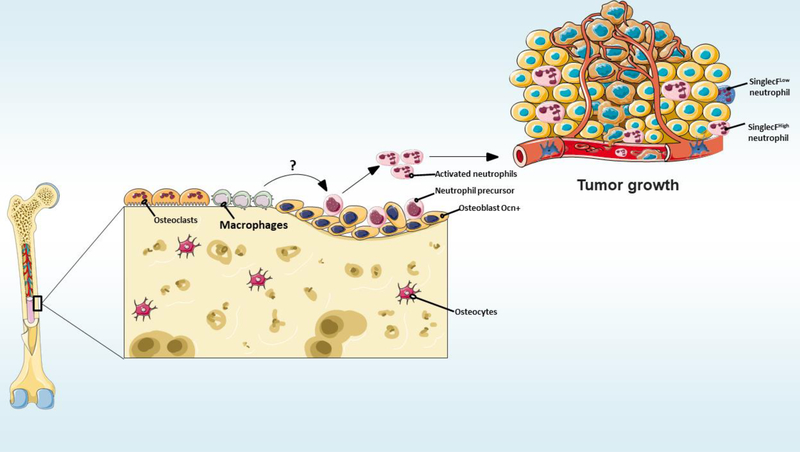
Bone marrow neutrophils cooperate with macrophages in several important pathophysiologic functions, including immunomodulatory, inflammatory, and phagocytic activities [94,95]. Although it is known that bone marrow macrophages enhance lung metastasis [96], the details of these mechanisms remain poorly understood. Future studies should explore whether the macrophage is an important niche cell for neutrophil priming by osteoblasts (Figure 3). Engblom and colleagues revealed that genetic ablation of osteoblasts, by using Ocn-CreiDTR mice, is sufficient to interrupt lung tumor progression. Their findings also indicate that SiglecFhigh neutrophils support cancer progression by promoting the differentiation of tumorassociated macrophages [42]. Surprisingly, their own results also show that genetic ablation of macrophages, by using CD169-DTR mice, did not suppress lung tumor growth. As previous data demonstrates that macrophages drive lung tumor growth [97], future studies will clarify this issue, which possibly could be methodological.
Figure 3. Do bone marrow macrophages modulate neutrophil activation?
The details of the mechanisms by which bone marrow macrophages enhance lung metastasis remain poorly understood. Future studies should explore whether macrophages are important niche cells for neutrophil priming by osteoblasts.
ROLE OF SiglecF EXPRESSION IN NEUTORPHILS DURING TUMOR
PROGRESSION
Engblom and colleagues show that neutrophils expressing SiglecF induce tumor growth [42]. Siglecs (sialic acid binding immunoglobulin-like lectins) are well characterized (immunoglobulin-type) lectins identified by an amino-terminal V-set immunoglobulin domain that mediates sialic acid residues binding on glycoproteins and glycolipids, followed by varying numbers of C2-set immunoglobulin domains [98–100]. These proteins were first identified as receptors important for: tolerance induction, pathogen recognition and uptake, and regulation of cell activation [100]. Siglecs are expressed in a variety of immune and non-immune cells in the central nervous system, prostate, kidney, placenta, amniotic epithelium, and others [100–105].
SiglecF is a component of Siglecs family, and was first described in eosinophils [100]. Binding of SiglecF to a specific glycan present in the asthmatic airway potentiates eosinophil apoptosis in this condition [106]. Although this suggest a suppressive role for SiglecF on eosinophils, the function of SiglecF in other cells, in which its expression has also been detected, such as activated T cells [107], macrophages [108], and neutrophils [109,107,42], remains unclear. As multiple cell types in the tumor microenvironment express SiglecF, conditional deletion of this antigen specifically from tumoral neutrophils, by the creation of SiglecF floxed mice crossed with neutorphil-specific inducible CreER drivers, will reveal the exact role of SiglecF in neutrophils during tumor development.
The knowledge on Siglecs biology is based mainly on experiments with mouse models due to the limitations associated with mechanistic studies in humans. Human Siglec8 was identified as the murine functional paralog of SiglecF [110,111]. Findings in mice imply the possible value of Siglecs as therapeutic targets in humans. Further investigation of these proteins is required to supply proof of concept for targeting Siglecs in cancer.
LUNG TUMOR MICROENVIRONMENT
The capacity to eliminate single genes in specific cellular populations in adult mice has allowed us to answer specific questions regarding the roles of different cell types in the regulation of several physiopathologic conditions. In the lung tumor microenvironment, the exact contribution of non-malignant cells that may play important roles in stimulating osteoblasts remains uncertain. It also remains unclear whether lung cancer cells signal directly to osteoblasts, or indirectly via other cells from the lung microenvironment. Engblom and colleagues suggest, by in vitro experiments, that tumor-derived soluble RAGE stimulates osteoblasts to regulate protumoral neutrophil formation [42]. Nevertheless, several cell types in the lung tumor microenvironment can produce soluble RAGE and other tumor-associated molecules [112]. RAGE has not been conditionally deleted from tumor cells, so there is no direct evidence that cancer cells are the only/main functionally important source of this other osteoblasts-stimulating molecules. The generation of RAGE floxed mice to be crossed with cell type-specific inducible CreER drivers will allow us to specifically delete RAGE in several cells from the lung tumor niche. In addition to studies in genetic mouse models, transcriptomic and single cell analysis of various cells in the lung tumor microenvironment represent fundamental tools that will help us understand the roles of different cells from the lung tumor microenvironment in the communication with osteoblasts.
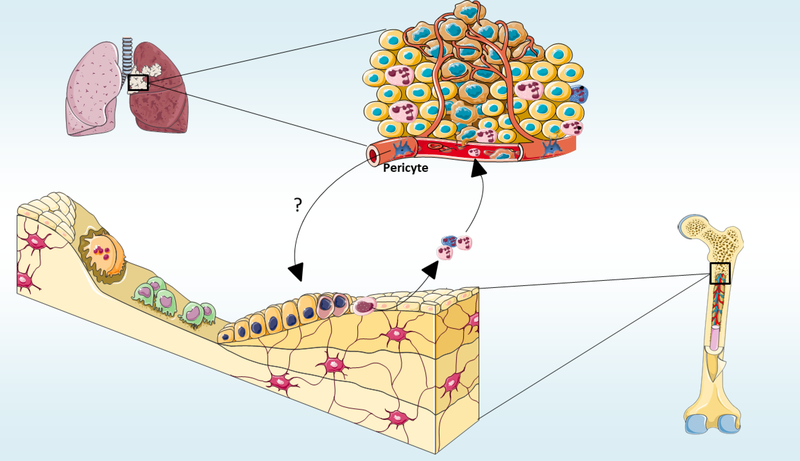
Lungs are a prevalent location to where metastatic cancer cells home, and several premetastatic modifications have been described in this organ [113]. The pre-metastatic lung niches include both resident cells and cells recruited from other organs [114,115]. It will be interesting to examine whether the cells that form the pre-metastatic lung niches communicate with osteoblasts before the tumor cells seeding. Also, it is still unknown whether cancer cells from other organs that metastasize to the lung also stimulate osteoblasts. Investigation and characterization of cells from the lung microenvironment that play important roles in setting the pre-metastatic niche is necessary to develop targeted therapies to revert alterations of local microenvironment, inhibiting metastatic establishment [114]. As recent studies showed that lung pericytes are essential components of the lung pre-metastatic niche [116], exploring whether lung pericytes communicate with osteoblasts in the bones seems promising (Figure 4). In addition to promoting existent lung tumor growth, it should be explored whether SiglecFhigh– expressing neutrophils are also able to form the pre-metastatic niche in the lung. Moreover, several malignant cancer cells have a predilection to metastasize also to the bones, which comprise fertile ground for the accommodation and growth of malignant metastatic cells [117]. Future studies should elucidate whether this activation of osteoblasts activity by lung tumor cells also creates a pre-metastatic niche in the bone.
Figure 4. Do pulmonary pericytes communicate with osteoblasts in the bones to induce tumor development?
Lung pericytes are essential components of the lung pre-metastatic niche. Whether adenocarcinoma pericytes communication is important for osteoblasts to activate Siglechigh– expressing neutrophils remains unknown.
CLINICAL RELEVANCE
Lung cancer, like other oncological disorders, is heterogeneous. Thus, this disease has various histological types. The two main lung cancer categories have been defined: non-small cell lung cancer and small cell lung cancer. Around four fifth of lung cancers are non-small cell lung cancers, which have distinct molecular profiles and clinical course forms of the disease [118,119]: lung adenocarcinoma, squamous cell carcinoma, large cell neuroendocrine carcinoma, adenosquamous carcinoma, and large cell carcinoma. These cancer types arise from diverse locations within the lung [120,121]. Engblom and colleagues analyzed in their studies lung adenocarcinomas [42]. It remains to be explored whether the communication with the bone is a common feature to all types of lung tumors, or it is specific to adenocarcinoma. Furthermore, future studies should explore whether other types of solid tumors also communicate with osteoblasts, even in the absence of bone metastasis.
Several pulmonary disorders, characterized by deregulated inflammation in the lung microenvironment, have a big risk of developing into lung cancer [122–124]. Common inflammatory mediators, including TGFβ, TNFα, PGE2, HGF, and IL1β, drive chronic obstructive pulmonary disease, such as pulmonary fibrosis, and emphysema. These mediators also play essential roles in the regulation of bone marrow homeostasis, affecting immune cell survival, migration, proliferation, and differentiation [125–130]. Thus, it is possible that these molecules, altered in these pulmonary disorders, participate in bone marrow alterations that will affect lung cancer development. As the neutrophils that promote lung tumor growth are primed by osteoblasts in the bone [42], it will be interesting to explore at what stage of lung cancer development the cross-talk with the bone starts. Also, it remains unknown whether the lungs in these pre-neoplasic conditions are already receiving Siglechigh–expressing neutrophils primed in the bone marrow. If yes, targeting these neutrophils should be explored as a potential way to inhibit the evolution of these inflammatory disorders into lung cancer.
Age is one of the main risks factors for lung cancer [131]. During aging of the hematopoietic system, myeloid cells increase in number, and their properties are affected; for instance, aged neutrophils migrate less in response to stimuli [132]. It will be interesting to explore whether this increase in number corresponds to the appearance of tumor-promoting SiglecFhigh–expressing neutrophils.
To translate animal research on tumor-promoting SiglecFhigh–expressing neutrophils to humans, specific markers selectively expressed in cells equivalent to these ones must be validated in human tissues. Engblom and colleagues performed RNA-sequencing in SiglecFhigh– expressing neutrophils isolated from mice [42]. A detailed analysis of this data may provide new specific surface markers to develop therapies that will target these receptors in patients with lung cancer.
FUTURE DIRECTIONS AND CONCLUSIONS
In conclusion, the study by Engblom and colleagues reveals a novel unexpected role of osteoblasts in lung tumor progression. However, our understanding of lung tumor biology still remains limited, and the complexity and interactions of different cellular components and molecules of the lung microenvironment during tumor progression should be elucidated in future works. A great challenge for the future will be to translate the research from mouse models into patients. Whether cancer cells at an early stage of human lung adenocarcinoma promote neutrophil priming via osteoblasts remains to be determined. Improving the availability of human lung and bone marrow samples will be essential to reach this goal.
ACKNOWLEDGMENTS
Alexander Birbrair is supported by a grant from Instituto Serrapilheira/Serra-1708–15285, a grant from Pró-reitoria de Pesquisa/Universidade Federal de Minas Gerais (PRPq/UFMG) (Edital 05/2016); a grant from FAPEMIG [Rede Mineira de Engenharia de Tecidos e Terapia Celular (REMETTEC, RED-00570–16)], and a grant from FAPEMIG [Rede De Pesquisa Em Doenças Infecciosas Humanas E Animais Do Estado De Minas Gerais (RED-00313–16)]; Akiva Mintz is supported by the National Institute of Health (1R01CA179072–01A1) and by the American Cancer Society Mentored Research Scholar grant (124443-MRSG-13–121-01-CDD).
Footnotes
CONFLICT OF INTEREST
The authors indicate no potential conflicts of interest.
REFERENCES
- 1.Mendes F, Antunes C, Abrantes AM, Goncalves AC, Nobre-Gois I, Sarmento AB, et al. (2015). Lung cancer: the immune system and radiation. Br J Biomed Sci, 72(2), 7884. [DOI] [PubMed] [Google Scholar]
- 2.Chapman AM, Sun KY, Ruestow P, Cowan DM, & Madl AK (2016). Lung cancer mutation profile of EGFR, ALK, and KRAS: Meta-analysis and comparison of never and ever smokers. Lung Cancer, 102, 122–134, doi: 10.1016/j.lungcan.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 3.Skowronek J (2015). Brachytherapy in the treatment of lung cancer - a valuable solution. J Contemp Brachytherapy, 7(4), 297–311, doi: 10.5114/jcb.2015.54038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Birbrair A, Sattiraju A, Zhu D, Zulato G, Batista I, Nguyen VT, et al. (2017). Novel Peripherally Derived Neural-Like Stem Cells as Therapeutic Carriers for Treating Glioblastomas. Stem Cells Transl Med, 6(2), 471–481, doi: 10.5966/sctm.2016-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vannucci L (2015). Stroma as an Active Player in the Development of the Tumor Microenvironment. Cancer Microenviron, 8(3), 159–166, doi: 10.1007/s12307-014-0150x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Junttila MR, & de Sauvage FJ (2013). Influence of tumour micro-environment heterogeneity on therapeutic response. Nature, 501(7467), 346–354, doi: 10.1038/nature12626. [DOI] [PubMed] [Google Scholar]
- 7.Birbrair A (2017). Stem Cell Microenvironments and Beyond. Adv Exp Med Biol, 1041, 1–3, doi: 10.1007/978-3-319-69194-7_1. [DOI] [PubMed] [Google Scholar]
- 8.Birbrair A, Zhang T, Wang ZM, Messi ML, Olson JD, Mintz A, et al. (2014). Type-2 pericytes participate in normal and tumoral angiogenesis. Am J Physiol Cell Physiol, 307(1), C25–38, doi: 10.1152/ajpcell.00084.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Birbrair A, Zhang T, Wang ZM, Messi ML, Mintz A, & Delbono O (2015). Pericytes at the intersection between tissue regeneration and pathology. Clin Sci (Lond), 128(2), 81–93, doi: 10.1042/CS20140278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Birbrair A, Zhang T, Wang ZM, Messi ML, Mintz A, & Delbono O (2014). Pericytes: multitasking cells in the regeneration of injured, diseased, and aged skeletal muscle. Front Aging Neurosci, 6, 245, doi: 10.3389/fnagi.2014.00245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Birbrair A, Borges IDT, Gilson Sena IF, Almeida GG, da Silva Meirelles L, Goncalves R, et al. (2017). How Plastic Are Pericytes? Stem Cells Dev, 26(14), 10131019, doi: 10.1089/scd.2017.0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Birbrair A, & Delbono O (2015). Pericytes are Essential for Skeletal Muscle Formation. Stem Cell Rev, 11(4), 547–548, doi: 10.1007/s12015-015-9588-6. [DOI] [PubMed] [Google Scholar]
- 13.Birbrair A, Zhang T, Files DC, Mannava S, Smith T, Wang ZM, et al. (2014). Type-1 pericytes accumulate after tissue injury and produce collagen in an organdependent manner. Stem Cell Res Ther, 5(6), 122, doi: 10.1186/scrt512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, et al. (2013). Skeletal muscle pericyte subtypes differ in their differentiation potential. Stem Cell Res, 10(1), 67–84, doi: 10.1016/j.scr.2012.09.003 S1873–5061(12)00089-X [pii]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, et al. (2013). Role of pericytes in skeletal muscle regeneration and fat accumulation. Stem Cells Dev, 22(16), 2298–2314, doi: 10.1089/scd.2012.0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Birbrair A, Zhang T, Wang ZM, Messi ML, Mintz A, & Delbono O (2013). Type-1 pericytes participate in fibrous tissue deposition in aged skeletal muscle. Am J Physiol Cell Physiol, 305(11), C1098–1113, doi: 10.1152/ajpcell.00171.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Almeida VM, Paiva AE, Sena IFG, Mintz A, Magno LAV, & Birbrair A (2017). Pericytes Make Spinal Cord Breathless after Injury. Neuroscientist, doi: 10.1177/1073858417731522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prazeres P, Almeida VM, Lousado L, Andreotti JP, Paiva AE, Santos GSP, et al. (2017). Macrophages Generate Pericytes in the Developing Brain. Cell Mol Neurobiol, doi: 10.1007/s10571-017-0549-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dias Moura Prazeres PH, Sena IFG, Borges IDT, de Azevedo PO, Andreotti JP, de Paiva AE, et al. (2017). Pericytes are heterogeneous in their origin within the same tissue. Dev Biol, 427(1), 6–11, doi: 10.1016/j.ydbio.2017.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guerra DAP, Paiva AE, Sena IFG, Azevedo PO, Batista ML Jr., Mintz A, et al. (2017). Adipocytes role in the bone marrow niche. Cytometry A, doi: 10.1002/cyto.a.23301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Costa MA, Paiva AE, Andreotti JP, Cardoso MV, Cardoso CD, Mintz A, et al. (2018). Pericytes constrict blood vessels after myocardial ischemia. J Mol Cell Cardiol, doi: 10.1016/j.yjmcc.2018.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Azevedo PO, Sena IFG, Andreotti JP, Carvalho-Tavares J, Alves-Filho JC, Cunha TM, et al. (2017). Pericytes modulate myelination in the central nervous system. J Cell Physiol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Santos GSP, Prazeres P, Mintz A, & Birbrair A (2017). Role of pericytes in the retina. Eye (Lond), doi: 10.1038/eye.2017.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Asada N, Kunisaki Y, Pierce H, Wang Z, Fernandez NF, Birbrair A, et al. (2017). Differential cytokine contributions of perivascular haematopoietic stem cell niches. Nat Cell Biol, 19(3), 214–223, doi: 10.1038/ncb3475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khan JA, Mendelson A, Kunisaki Y, Birbrair A, Kou Y, Arnal-Estape A, et al. (2016). Fetal liver hematopoietic stem cell niches associate with portal vessels. Science, 351(6269), 176–180, doi: 10.1126/science.aad0084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Birbrair A, Wang ZM, Messi ML, Enikolopov GN, & Delbono O (2011). NestinGFP transgene reveals neural precursor cells in adult skeletal muscle. PLoS One, 6(2), e16816, doi: 10.1371/journal.pone.0016816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Birbrair A, Zhang T, Wang ZM, Messi ML, Enikolopov GN, Mintz A, et al. (2013). Skeletal muscle neural progenitor cells exhibit properties of NG2-glia. Exp Cell Res, 319(1), 45–63, doi:S0014-4827(12)00400-4[pii] 10.1016/j.yexcr.2012.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prazeres PHDM, Turquetti AOM, Azevedo PO, Barreto RSN, Miglino MA, Mintz A, et al. (2018). Perivascular cell αv integrins as a target to treat skeletal muscle fibrosis. Int J Biochem Cell Biol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Andreotti JP, Paiva AE, Prazeres P, Guerra DAP, Silva WN, Vaz RS, et al. (2018). The role of natural killer cells in the uterine microenvironment during pregnancy. Cell Mol Immunol, doi: 10.1038/s41423-018-0023-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andreotti JP, Prazeres PHDM, Magno LAV, Romano-Silva MA, Mintz A, & Birbrair A (2018). Neurogenesis in the postnatal cerebellum after injury. Int J Dev Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guerra DAP, Paiva AE, Sena IFG, Azevedo PO, Silva WN, Mintz A, et al. (2018). Targeting glioblastoma-derived pericytes improves chemotherapeutic outcome. Angiogenesis, doi: 10.1007/s10456-018-9621-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guerra DAP, Paiva AE, Sena IFG, Azevedo PO, Batista ML Jr., Mintz A, et al. (2018). Adipocytes role in the bone marrow niche. Cytometry A, 93(2), 167–171, doi: 10.1002/cyto.a.23301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sena IFG, Paiva AE, Prazeres P, Azevedo PO, Lousado L, Bhutia SK, et al. (2018). Glioblastoma-activated pericytes support tumor growth via immunosuppression. Cancer Med, doi: 10.1002/cam4.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coatti GC, Frangini M, Valadares MC, Gomes JP, Lima NO, Cavacana N, et al. (2017). Pericytes Extend Survival of ALS SOD1 Mice and Induce the Expression of Antioxidant Enzymes in the Murine Model and in IPSCs Derived Neuronal Cells from an ALS Patient. Stem Cell Rev, doi: 10.1007/s12015-017-9752-2. [DOI] [PubMed] [Google Scholar]
- 35.Pereira LX, Viana CTR, Orellano LAA, Almeida SA, Vasconcelos AC, Goes AM, et al. (2017). Synthetic matrix of polyether-polyurethane as a biological platform for pancreatic regeneration. Life Sci, 176, 67–74, doi: 10.1016/j.lfs.2017.03.015. [DOI] [PubMed] [Google Scholar]
- 36.Quail DF, & Joyce JA (2013). Microenvironmental regulation of tumor progression and metastasis. Nat Med, 19(11), 1423–1437, doi: 10.1038/nm.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Egeblad M, Nakasone ES, & Werb Z (2010). Tumors as organs: complex tissues that interface with the entire organism. Dev Cell, 18(6), 884–901, doi: 10.1016/j.devcel.2010.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zilio S, & Serafini P (2016). Neutrophils and Granulocytic MDSC: The Janus God of Cancer Immunotherapy. Vaccines (Basel), 4(3), doi: 10.3390/vaccines4030031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aulakh GK (2017). Neutrophils in the lung: “the first responders”. Cell Tissue Res, doi: 10.1007/s00441-017-2748-z. [DOI] [PubMed] [Google Scholar]
- 40.Nicolas-Avila JA, Adrover JM, & Hidalgo A (2017). Neutrophils in Homeostasis, Immunity, and Cancer. Immunity, 46(1), 15–28, doi: 10.1016/j.immuni.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 41.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, et al. (2009). Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell, 16(3), 183–194, doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Engblom C, Pfirschke C, Zilionis R, Da Silva Martins J, Bos SA, Courties G, et al. (2017). Osteoblasts remotely supply lung tumors with cancer-promoting SiglecF(high) neutrophils. Science, 358(6367), doi: 10.1126/science.aal5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vanneman M, & Dranoff G (2012). Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer, 12(4), 237–251, doi: 10.1038/nrc3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lewis CE, Leek R, Harris A, & McGee JO (1995). Cytokine regulation of angiogenesis in breast cancer: the role of tumor-associated macrophages. J Leukoc Biol, 57(5), 747–751. [DOI] [PubMed] [Google Scholar]
- 45.Hanahan D, & Coussens LM (2012). Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell, 21(3), 309–322, doi: 10.1016/j.ccr.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 46.Smyth MJ, Cretney E, Kershaw MH, & Hayakawa Y (2004). Cytokines in cancer immunity and immunotherapy. Immunol Rev, 202, 275–293, doi: 10.1111/j.01052896.2004.00199.x. [DOI] [PubMed] [Google Scholar]
- 47.McAllister SS, & Weinberg RA (2014). The tumour-induced systemic environment as a critical regulator of cancer progression and metastasis. Nat Cell Biol, 16(8), 717–727, doi: 10.1038/ncb3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wculek SK, & Malanchi I (2015). Neutrophils support lung colonization of metastasisinitiating breast cancer cells. Nature, 528(7582), 413–417, doi: 10.1038/nature16140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liotta LA, & Kohn EC (2001). The microenvironment of the tumour-host interface. Nature, 411(6835), 375–379, doi: 10.1038/35077241. [DOI] [PubMed] [Google Scholar]
- 50.Headley MB, Bins A, Nip A, Roberts EW, Looney MR, Gerard A, et al. (2016). Visualization of immediate immune responses to pioneer metastatic cells in the lung. Nature, 531(7595), 513–517, doi: 10.1038/nature16985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Erler JT, Bennewith KL, Cox TR, Lang G, Bird D, Koong A, et al. (2009). Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell, 15(1), 35–44, doi: 10.1016/j.ccr.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, et al. (2005). VEGFR1-positive haematopoietic bone marrow progenitors initiate the premetastatic niche. Nature, 438(7069), 820–827, doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Coffelt SB, Kersten K, Doornebal CW, Weiden J, Vrijland K, Hau CS, et al. (2015). IL-17-producing gammadelta T cells and neutrophils conspire to promote breast cancer metastasis. Nature, 522(7556), 345–348, doi: 10.1038/nature14282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chow A, Zhou W, Liu L, Fong MY, Champer J, Van Haute D, et al. (2014). Macrophage immunomodulation by breast cancer-derived exosomes requires Toll-like receptor 2-mediated activation of NF-kappaB. Sci Rep, 4, 5750, doi: 10.1038/srep05750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nielsen SR, Quaranta V, Linford A, Emeagi P, Rainer C, Santos A, et al. (2016). Macrophage-secreted granulin supports pancreatic cancer metastasis by inducing liver fibrosis. Nat Cell Biol, 18(5), 549–560, doi: 10.1038/ncb3340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.van Deventer HW, Palmieri DA, Wu QP, McCook EC, & Serody JS (2013). Circulating fibrocytes prepare the lung for cancer metastasis by recruiting Ly-6C+ monocytes via CCL2. J Immunol, 190(9), 4861–4867, doi: 10.4049/jimmunol.1202857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Brambilla E, Le Teuff G, Marguet S, Lantuejoul S, Dunant A, Graziano S, et al. (2016). Prognostic Effect of Tumor Lymphocytic Infiltration in Resectable Non-SmallCell Lung Cancer. J Clin Oncol, 34(11), 1223–1230, doi: 10.1200/JCO.2015.63.0970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Welsh TJ, Green RH, Richardson D, Waller DA, O’Byrne KJ, & Bradding P (2005). Macrophage and mast-cell invasion of tumor cell islets confers a marked survival advantage in non-small-cell lung cancer. J Clin Oncol, 23(35), 8959–8967, doi: 10.1200/JCO.2005.01.4910. [DOI] [PubMed] [Google Scholar]
- 59.Dieu-Nosjean MC, Antoine M, Danel C, Heudes D, Wislez M, Poulot V, et al. (2008). Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol, 26(27), 4410–4417, doi: 10.1200/JCO.2007.15.0284. [DOI] [PubMed] [Google Scholar]
- 60.Lavin Y, Kobayashi S, Leader A, Amir ED, Elefant N, Bigenwald C, et al. (2017). Innate Immune Landscape in Early Lung Adenocarcinoma by Paired Single-Cell Analyses. Cell, 169(4), 750–765 e717, doi: 10.1016/j.cell.2017.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Boyle WJ, Simonet WS, & Lacey DL (2003). Osteoclast differentiation and activation. Nature, 423(6937), 337–342, doi: 10.1038/nature01658. [DOI] [PubMed] [Google Scholar]
- 62.Kingsley LA, Fournier PG, Chirgwin JM, & Guise TA (2007). Molecular biology of bone metastasis. Mol Cancer Ther, 6(10), 2609–2617, doi: 10.1158/1535-7163.MCT07-0234. [DOI] [PubMed] [Google Scholar]
- 63.Lin SC, Lee YC, Yu G, Cheng CJ, Zhou X, Chu K, et al. (2017). Endothelial-toOsteoblast Conversion Generates Osteoblastic Metastasis of Prostate Cancer. Dev Cell, 41(5), 467–480 e463, doi: 10.1016/j.devcel.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Paiva AE, Lousado L, Almeida VM, Andreotti JP, Santos GSP, Azevedo PO, et al. (2017). Endothelial Cells as Precursors for Osteoblasts in the Metastatic Prostate Cancer Bone. Neoplasia, 19(11), 928–931, doi: 10.1016/j.neo.2017.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Long F (2011). Building strong bones: molecular regulation of the osteoblast lineage. Nat Rev Mol Cell Biol, 13(1), 27–38, doi: 10.1038/nrm3254. [DOI] [PubMed] [Google Scholar]
- 66.Olsen BR, Reginato AM, & Wang W (2000). Bone development. Annu Rev Cell Dev Biol, 16, 191–220, doi: 10.1146/annurev.cellbio.16.1.191. [DOI] [PubMed] [Google Scholar]
- 67.Helms JA, & Schneider RA (2003). Cranial skeletal biology. Nature, 423(6937), 326331, doi: 10.1038/nature01656. [DOI] [PubMed] [Google Scholar]
- 68.Liu H, Guo J, Wang L, Chen N, Karaplis A, Goltzman D, et al. (2009). Distinctive anabolic roles of 1,25-dihydroxyvitamin D(3) and parathyroid hormone in teeth and mandible versus long bones. J Endocrinol, 203(2), 203–213, doi: 10.1677/JOE-09-0247. [DOI] [PubMed] [Google Scholar]
- 69.Kishi T, Hagino H, Kishimoto H, & Nagashima H (1998). Bone responses at various skeletal sites to human parathyroid hormone in ovariectomized rats: effects of long-term administration, withdrawal, and readministration. Bone, 22(5), 515–522. [DOI] [PubMed] [Google Scholar]
- 70.Long F, Chung UI, Ohba S, McMahon J, Kronenberg HM, & McMahon AP (2004). Ihh signaling is directly required for the osteoblast lineage in the endochondral skeleton. Development, 131(6), 1309–1318, doi: 10.1242/dev.01006. [DOI] [PubMed] [Google Scholar]
- 71.Wang Y, Wan C, Deng L, Liu X, Cao X, Gilbert SR, et al. (2007). The hypoxiainducible factor alpha pathway couples angiogenesis to osteogenesis during skeletal development. J Clin Invest, 117(6), 1616–1626, doi: 10.1172/JCI31581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sodek KL, Tupy JH, Sodek J, & Grynpas MD (2000). Relationships between bone protein and mineral in developing porcine long bone and calvaria. Bone, 26(2), 189–198. [DOI] [PubMed] [Google Scholar]
- 73.van den Bos T, Speijer D, Bank RA, Bromme D, & Everts V (2008). Differences in matrix composition between calvaria and long bone in mice suggest differences in biomechanical properties and resorption: Special emphasis on collagen. Bone, 43(3), 459468, doi: 10.1016/j.bone.2008.05.009. [DOI] [PubMed] [Google Scholar]
- 74.Komori T (2008). Regulation of bone development and maintenance by Runx2. Front Biosci, 13, 898–903. [DOI] [PubMed] [Google Scholar]
- 75.Lian JB, McKee MD, Todd AM, & Gerstenfeld LC (1993). Induction of bonerelated proteins, osteocalcin and osteopontin, and their matrix ultrastructural localization with development of chondrocyte hypertrophy in vitro. J Cell Biochem, 52(2), 206–219, doi: 10.1002/jcb.240520212. [DOI] [PubMed] [Google Scholar]
- 76.Pockwinse SM, Lawrence JB, Singer RH, Stein JL, Lian JB, & Stein GS (1993). Gene expression at single cell resolution associated with development of the bone cell phenotype: ultrastructural and in situ hybridization analysis. Bone, 14(3), 347–352. [DOI] [PubMed] [Google Scholar]
- 77.Nakase T, Takaoka K, Hirakawa K, Hirota S, Takemura T, Onoue H, et al. (1994). Alterations in the expression of osteonectin, osteopontin and osteocalcin mRNAs during the development of skeletal tissues in vivo. Bone Miner, 26(2), 109–122. [DOI] [PubMed] [Google Scholar]
- 78.Ikeda T, Nomura S, Yamaguchi A, Suda T, & Yoshiki S (1992). In situ hybridization of bone matrix proteins in undecalcified adult rat bone sections. J Histochem Cytochem, 40(8), 1079–1088, doi: 10.1177/40.8.1619274. [DOI] [PubMed] [Google Scholar]
- 79.Thiede MA, Smock SL, Petersen DN, Grasser WA, Thompson DD, & Nishimoto SK (1994). Presence of messenger ribonucleic acid encoding osteocalcin, a marker of bone turnover, in bone marrow megakaryocytes and peripheral blood platelets. Endocrinology, 135(3), 929–937, doi: 10.1210/endo.135.3.8070388. [DOI] [PubMed] [Google Scholar]
- 80.Fleet JC, & Hock JM (1994). Identification of osteocalcin mRNA in nonosteoid tissue of rats and humans by reverse transcription-polymerase chain reaction. J Bone Miner Res, 9(10), 1565–1573, doi: 10.1002/jbmr.5650091009. [DOI] [PubMed] [Google Scholar]
- 81.Zhang J, & Link DC (2016). Targeting of Mesenchymal Stromal Cells by CreRecombinase Transgenes Commonly Used to Target Osteoblast Lineage Cells. J Bone Miner Res, 31(11), 2001–2007, doi: 10.1002/jbmr.2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Dacquin R, Starbuck M, Schinke T, & Karsenty G (2002). Mouse alpha1(I)-collagen promoter is the best known promoter to drive efficient Cre recombinase expression in osteoblast. Dev Dyn, 224(2), 245–251, doi: 10.1002/dvdy.10100. [DOI] [PubMed] [Google Scholar]
- 83.Lefrancais E, Ortiz-Munoz G, Caudrillier A, Mallavia B, Liu F, Sayah DM, et al. (2017). The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature, 544(7648), 105–109, doi: 10.1038/nature21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Borges I, Sena I, Azevedo P, Andreotti J, Almeida V, Paiva A, et al. (2017). Lung as a Niche for Hematopoietic Progenitors. Stem Cell Rev, 13(5), 567–574, doi: 10.1007/s12015-017-9747-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lousado L, Prazeres P, Andreotti JP, Paiva AE, Azevedo PO, Santos GSP, et al. (2017). Schwann cell precursors as a source for adrenal gland chromaffin cells. Cell Death Dis, 8(10), e3072, doi: 10.1038/cddis.2017.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Azevedo PO, Lousado L, Paiva AE, Andreotti JP, Santos GSP, Sena IFG, et al. (2017). Endothelial cells maintain neural stem cells quiescent in their niche. Neuroscience, 363, 62–65, doi: 10.1016/j.neuroscience.2017.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schofield R (1978). The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells, 4(1–2), 7–25. [PubMed] [Google Scholar]
- 88.Birbrair A, & Frenette PS (2016). Niche heterogeneity in the bone marrow. Ann N Y Acad Sci, 1370(1), 82–96, doi: 10.1111/nyas.13016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Andreotti JP, Lousado L, Magno LAV, & Birbrair A (2017). Hypothalamic Neurons Take Center Stage in the Neural Stem Cell Niche. Cell Stem Cell, 21(3), 293–294, doi: 10.1016/j.stem.2017.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sena IFG, Prazeres P, Santos GSP, Borges IT, Azevedo PO, Andreotti JP, et al. (2017). Identity of Gli1+ cells in the bone marrow. Exp Hematol, 54, 12–16, doi: 10.1016/j.exphem.2017.06.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Sena IFG, Borges IT, Lousado L, Azevedo PO, Andreotti JP, Almeida VM, et al. (2017). LepR+ cells dispute hegemony with Gli1+ cells in bone marrow fibrosis. Cell Cycle, 1–5, doi: 10.1080/15384101.2017.1367072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Alvarenga EC, Silva WN, Vasconcellos R, Paredes-Gamero EJ, Mintz A, & Birbrair A (2018). Promyelocytic leukemia protein in mesenchymal stem cells is essential for leukemia progression. Annals of Hematology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Silva WN, Leonel C, Prazeres PHDM, Sena IFG, Guerra DAP, Diniz IMA, et al. (2018). Role of Schwann cells in cutaneous wound healing. Wound Repair Regen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Silva MT, & Correia-Neves M (2012). Neutrophils and macrophages: the main partners of phagocyte cell systems. Front Immunol, 3, 174, doi: 10.3389/fimmu.2012.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Silva WN, Prazeres P, Paiva AE, Lousado L, Turquetti AOM, Barreto RSN, et al. (2018). Macrophage-derived GPNMB accelerates skin healing. Exp Dermatol, doi: 10.1111/exd.13524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cho HJ, Jung JI, Lim DY, Kwon GT, Her S, Park JH, et al. (2012). Bone marrow-derived, alternatively activated macrophages enhance solid tumor growth and lung metastasis of mammary carcinoma cells in a Balb/C mouse orthotopic model. Breast Cancer Res, 14(3), R81, doi: 10.1186/bcr3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cortez-Retamozo V, Etzrodt M, Newton A, Rauch PJ, Chudnovskiy A, Berger C, et al. (2012). Origins of tumor-associated macrophages and neutrophils. Proc Natl Acad Sci U S A, 109(7), 2491–2496, doi: 10.1073/pnas.1113744109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Crocker PR, Clark EA, Filbin M, Gordon S, Jones Y, Kehrl JH, et al. (1998). Siglecs: a family of sialic-acid binding lectins. Glycobiology, 8(2), v. [DOI] [PubMed] [Google Scholar]
- 99.Kelm S, Pelz A, Schauer R, Filbin MT, Tang S, de Bellard ME, et al. (1994). Sialoadhesin, myelin-associated glycoprotein and CD22 define a new family of sialic acid-dependent adhesion molecules of the immunoglobulin superfamily. Curr Biol, 4(11), 965–972. [DOI] [PubMed] [Google Scholar]
- 100.Macauley MS, Crocker PR, & Paulson JC (2014). Siglec-mediated regulation of immune cell function in disease. Nat Rev Immunol, 14(10), 653–666, doi: 10.1038/nri3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Brinkman-Van der Linden EC, Hurtado-Ziola N, Hayakawa T, Wiggleton L, Benirschke K, Varki A, et al. (2007). Human-specific expression of Siglec-6 in the placenta. Glycobiology, 17(9), 922–931, doi: 10.1093/glycob/cwm065. [DOI] [PubMed] [Google Scholar]
- 102.Mitra N, Banda K, Altheide TK, Schaffer L, Johnson-Pais TL, Beuten J, et al. (2011). SIGLEC12, a human-specific segregating (pseudo)gene, encodes a signaling molecule expressed in prostate carcinomas. J Biol Chem, 286(26), 23003–23011, doi: 10.1074/jbc.M111.244152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ali SR, Fong JJ, Carlin AF, Busch TD, Linden R, Angata T, et al. (2014). Siglec-5 and Siglec-14 are polymorphic paired receptors that modulate neutrophil and amnion signaling responses to group B Streptococcus. J Exp Med, 211(6), 1231–1242, doi: 10.1084/jem.20131853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rochereau N, Drocourt D, Perouzel E, Pavot V, Redelinghuys P, Brown GD, et al. (2013). Dectin-1 is essential for reverse transcytosis of glycosylated SIgA-antigen complexes by intestinal M cells. PLoS Biol, 11(9), e1001658, doi: 10.1371/journal.pbio.1001658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Angata T, Nycholat CM, & Macauley MS (2015). Therapeutic Targeting of Siglecs using Antibody- and Glycan-Based Approaches. Trends Pharmacol Sci, 36(10), 645–660, doi: 10.1016/j.tips.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kiwamoto T, Katoh T, Evans CM, Janssen WJ, Brummet ME, Hudson SA, et al. (2015). Endogenous airway mucins carry glycans that bind Siglec-F and induce eosinophil apoptosis. J Allergy Clin Immunol, 135(5), 1329–1340 e1329, doi: 10.1016/j.jaci.2014.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang M, Angata T, Cho JY, Miller M, Broide DH, & Varki A (2007). Defining the in vivo function of Siglec-F, a CD33-related Siglec expressed on mouse eosinophils. Blood, 109(10), 4280–4287, doi: 10.1182/blood-2006-08-039255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kirby AC, Coles MC, & Kaye PM (2009). Alveolar macrophages transport pathogens to lung draining lymph nodes. J Immunol, 183(3), 1983–1989, doi: 10.4049/jimmunol.0901089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Suzukawa M, Miller M, Rosenthal P, Cho JY, Doherty TA, Varki A, et al. (2013). Sialyltransferase ST3Gal-III regulates Siglec-F ligand formation and eosinophilic lung inflammation in mice. J Immunol, 190(12), 5939–5948, doi: 10.4049/jimmunol.1203455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tateno H, Crocker PR, & Paulson JC (2005). Mouse Siglec-F and human Siglec-8 are functionally convergent paralogs that are selectively expressed on eosinophils and recognize 6’-sulfo-sialyl Lewis X as a preferred glycan ligand. Glycobiology, 15(11), 1125–1135, doi: 10.1093/glycob/cwi097. [DOI] [PubMed] [Google Scholar]
- 111.Bochner BS (2009). Siglec-8 on human eosinophils and mast cells, and Siglec-F on murine eosinophils, are functionally related inhibitory receptors. Clin Exp Allergy, 39(3), 317–324, doi: 10.1111/j.1365-2222.2008.03173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tam XH, Shiu SW, Leng L, Bucala R, Betteridge DJ, & Tan KC (2011). Enhanced expression of receptor for advanced glycation end-products is associated with low circulating soluble isoforms of the receptor in Type 2 diabetes. Clin Sci (Lond), 120(2), 81–89, doi: 10.1042/CS20100256. [DOI] [PubMed] [Google Scholar]
- 113.Weidle UH, Birzele F, Kollmorgen G, & Ruger R (2016). Molecular Basis of Lung Tropism of Metastasis. Cancer Genomics Proteomics, 13(2), 129–139. [PubMed] [Google Scholar]
- 114.Liu Y, & Cao X (2016). Characteristics and Significance of the Pre-metastatic Niche. Cancer Cell, 30(5), 668–681, doi: 10.1016/j.ccell.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 115.Paiva AE, Lousado L, Guerra DAP, Azevedo PO, Sena IFG, Andreotti JP, et al. (2018). Pericytes in the Premetastatic Niche. Cancer Res, doi: 10.1158/00085472.CAN-17-3883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Murgai M, Ju W, Eason M, Kline J, Beury DW, Kaczanowska S, et al. (2017). KLF4-dependent perivascular cell plasticity mediates pre-metastatic niche formation and metastasis. Nat Med, 23(10), 1176–1190, doi: 10.1038/nm.4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gartrell BA, & Saad F (2014). Managing bone metastases and reducing skeletal related events in prostate cancer. Nat Rev Clin Oncol, 11(6), 335–345, doi: 10.1038/nrclinonc.2014.70. [DOI] [PubMed] [Google Scholar]
- 118.Travis WD (2012). Update on small cell carcinoma and its differentiation from squamous cell carcinoma and other non-small cell carcinomas. Mod Pathol, 25 Suppl 1, S18–30, doi: 10.1038/modpathol.2011.150. [DOI] [PubMed] [Google Scholar]
- 119.Schnabel PA, & Junker K (2015). [Pulmonary neuroendocrine tumors in the new WHO 2015 classification: Start of breaking new grounds?]. Pathologe, 36(3), 283–292, doi: 10.1007/s00292-015-0030-2. [DOI] [PubMed] [Google Scholar]
- 120.Hanna JM, & Onaitis MW (2013). Cell of origin of lung cancer. J Carcinog, 12, 6, doi: 10.4103/1477-3163.109033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Muller KM (1984). Histological classification and histogenesis of lung cancer. Eur J Respir Dis, 65(1), 4–19. [PubMed] [Google Scholar]
- 122.O’Byrne KJ, & Dalgleish AG (2001). Chronic immune activation and inflammation as the cause of malignancy. Br J Cancer, 85(4), 473–483, doi: 10.1054/bjoc.2001.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kim V, Rogers TJ, & Criner GJ (2008). New concepts in the pathobiology of chronic obstructive pulmonary disease. Proc Am Thorac Soc, 5(4), 478–485, doi: 10.1513/pats.200802-014ET. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Samet JM (2000). Does idiopathic pulmonary fibrosis increase lung cancer risk? Am J Respir Crit Care Med, 161(1), 1–2, doi: 10.1164/ajrccm.161.1.ed14-99. [DOI] [PubMed] [Google Scholar]
- 125.Kasagi S, & Chen W (2013). TGF-beta1 on osteoimmunology and the bone component cells. Cell Biosci, 3(1), 4, doi: 10.1186/2045-3701-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Osta B, Benedetti G, & Miossec P (2014). Classical and Paradoxical Effects of TNFalpha on Bone Homeostasis. Front Immunol, 5, 48, doi: 10.3389/fimmu.2014.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Nakanishi M, & Rosenberg DW (2013). Multifaceted roles of PGE2 in inflammation and cancer. Semin Immunopathol, 35(2), 123–137, doi: 10.1007/s00281-012-0342-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Coudriet GM, He J, Trucco M, Mars WM, & Piganelli JD (2010). Hepatocyte growth factor modulates interleukin-6 production in bone marrow derived macrophages: implications for inflammatory mediated diseases. PLoS One, 5(11), e15384, doi: 10.1371/journal.pone.0015384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kennedy DE, & Knight KL (2017). Inflammatory Changes in Bone Marrow Microenvironment Associated with Declining B Lymphopoiesis. J Immunol, 198(9), 3471–3479, doi: 10.4049/jimmunol.1601643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wong J, Tran LT, Magun EA, Magun BE, & Wood LJ (2014). Production of IL1beta by bone marrow-derived macrophages in response to chemotherapeutic drugs: synergistic effects of doxorubicin and vincristine. Cancer Biol Ther, 15(10), 1395–1403, doi: 10.4161/cbt.29922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Niccoli T, & Partridge L (2012). Ageing as a risk factor for disease. Curr Biol, 22(17), R741–752, doi: 10.1016/j.cub.2012.07.024. [DOI] [PubMed] [Google Scholar]
- 132.Kuranda K, Vargaftig J, de la Rochere P, Dosquet C, Charron D, Bardin F, et al. (2011). Age-related changes in human hematopoietic stem/progenitor cells. Aging Cell, 10(3), 542–546, doi: 10.1111/j.1474-9726.2011.00675.x. [DOI] [PubMed] [Google Scholar]