Abstract
Background.
Surgical site infections (SSIs) are a major cause of morbidity, mortality, and healthcare costs, and patients undergoing simultaneous colorectal/liver resections are at an especially high SSI risk.
Methods.
Data were collected on all patients undergoing synchronous colorectal/liver resection from 2011 to 2016 (n=424). The intervention, implemented in 2013, included 13 multidisciplinary perioperative components. The primary endpoints were superficial/deep and organ space SSIs. Secondary endpoints were hospital length of stay (LOS) and 30-day readmission rate. To control for changes in SSI rates independent of the intervention, interrupted time series analysis was conducted.
Results.
Overall, superficial/deep, and organ space SSIs decreased by 60.5% (p<0.001), 80.6% (p<0.001), and 47.6% (p<0.008), respectively. In the pre-intervention cohort (n=231), there were 79 (34.2%), 31 (13.4%), and 48 (20.8%) total, superficial/deep, and organs space SSIs, respectively. In the post-intervention cohort (n=193), there were 26 (13.5%), 5 (2.6%), and 21 (10.9%) total, superficial/deep, and organs space SSIs, respectively. Median LOS decreased from 9 to 8 days (p<0.001). Readmission rates did not change (p=0.6). Interrupted time series analysis found no significant trends in SSI rate within the pre-intervention (p=0.35) and post-intervention (p=0.55) periods.
Discussion.
In combined colorectal/liver resection patients, implementation of a multidisciplinary care bundle was associated with a 61% reduction in SSIs, with the greatest impact on superficial/deep SSI, and modest reduction in LOS. The absence of trends within each time period indicated that the intervention was likely responsible for SSI reduction. Future efforts should target further reduction in organ space SSI.
Introduction
Surgical site infection (SSI) is the most common type of healthcare-associated infection, occurring at a rate of more than 150,000 cases per year 1-2. They are a major cause of morbidity and mortality, leading to prolonged hospitalization 2-4, increased rates of readmission 3, 5, additional invasive procedures 6, and reduced survival 7-8. SSIs are also a considerable cause of increased healthcare costs2. High rates of SSI are associated with colorectal surgery 1, 7, which has been the subject of many performance improvement efforts 9-10. To reduce rates of SSI, a perioperative care bundle was implemented in 2013.
Internal review at Memorial Sloan Kettering Cancer Center (MSK) identified combined colorectal/liver resection as among the operations associated with the highest rates of SSI. This approach, used to eliminate both colorectal cancer and metastases to the liver (found at diagnosis in15% of patients 11-12), is favored over metachronous resection by many centers because it limits exposure to general anesthesia, shortens total hospital length of stay, and reduces overall complications 13-14.
The aim of this study was to evaluate the effects of the SSI reduction intervention in an expanded and consecutive series of patients undergoing combined colorectal/liver resection.
Methods
All patients undergoing synchronous intestinal and liver resection at MSK from January 1, 2011, to December 31, 2016, were identified from a prospective database and included in this study. Superficial (skin and subcutaneous space), deep (fascia and muscle), and organ space SSI within 30 days of surgery were defined according to CDC guidelines 15. SSI data were collected by review of a prospective complication database, as well as review of inpatient and outpatient electronic medical records. SSI was categorized either as superficial/deep or organ space. Demographic, surgical, and pathology information was collected, including method of wound closure (primary or modified closure). Modified wound closure method included temporary packing of a portion of the closed incision, placing subcutaneous drains, or applying negative pressure surface vacuum dressings over closed incisions.
The intervention, implemented on November 1, 2013, consisted of a multidisciplinary bundle of 13 perioperative components (Table 1), one of which was a preoperative estimate of SSI risk based on an MSK SSI calculator. The bundle was developed following literature review and discussion by representatives from the Departments of Surgery, Medicine, Anesthesia, Nursing, Infection Control, Administration, and Quality and Safety (Table S1). Components were chosen if there were high levels of supporting evidence or if they were considered reasonable, associated with minimal risk, and potentially beneficial. They include measures advised by the Joint Commission’s Surgical Care Improvement Project (SCIP) related to perioperative use of antibiotics, maintenance of normothermia, and appropriate method of hair removal at the surgical site,16 which were advised prior to 2013. Other components include consultation for elevated hemoglobin A1C, mechanical bowel preparation with oral antibiotics including 500 mg metronidazole (Flagyl) and 1000 mg neomycin at 7 pm and 10 pm the night prior to surgery, preoperative 4% chlorhexidine shower the night before and morning of surgery, timely intraoperative re-dosing of antibiotics, use of clean instruments for incision closure (closing tray), and a shower on postoperative day 2. A novel intervention was to provide surgeons an estimated SSI risk prior to surgery. SSI risk was estimated using an internally developed SSI risk calculator as previously described 17 and electronically delivered the day prior to surgery. Surgeons utilized this estimate at their own discretion in selecting the method of wound closure.
Table 1.
Components of the perioperative bundle.
| Preoperative | • Appropriate oral antibiotic selection • Appropriate oral antibiotic administration the night before surgery • Mechanical bowel preparation • Medical evaluation for elevated hemoglobin A1C • Skin cleansing with chlorhexidine the night before and the morning of surgerya • Surgeon notification of SSI risk using MSK SSI prediction tool |
| Intraoperative | • Antibiotic administration before initial incisionb • Appropriate method of hair removal (electronic clippers or no hair removal) • Maintenance of normothermia • Intraoperative antibiotic re-dosing • Separate surgical closing tray for open procedures |
| Postoperative | • Discontinuation of antibiotics at 24 h • Patient shower on postoperative day 2 |
Patients were given a 4-oz (118-mL) bottle of 4% chlorhexidine gluconate (Hibiclens) during preoperative teaching and instructed to use it during a shower the night before and the morning of surgery. Written instructions were as follows. “To use Hibiclens, open the bottle and pour some solution into your hand or a washcloth. Move away from the shower stream to avoid rinsing off the Hibiclens too soon. Rub it gently over your body from your neck to your waist and rinse. Don’t let the solution get into your eyes, ears, mouth, or genital area. Don’t use any other soap. Dry yourself off with a clean towel after your shower.”
Preoperative intravenous antibiotics included 2 gm cefotetan, re-dosed every 6 h intraoperatively. Patients with penicillin allergy received clindamycin and gentamicin.
The primary endpoints were overall, superficial/deep, and organ space SSI. Secondary endpoints were hospital length of stay (LOS) and 30-day readmission rate. Statistical analysis was performed using R statistical software (version 3.4.2). Continuous variables were analyzed by Wilcoxon rank sum test and categorical variables by Fisher’s exact test. To control for the possibility that differences in SSI rates between the pre- and post-intervention cohorts were the result of ongoing trends over time rather than implementation of the care bundle, we conducted an interrupted time series analysis, after placing patients into quintiles, using a linear time trend in a logistic regression model, separately for the pre- and post-intervention time periods 18.
Results
A total of 424 consecutively treated patients were identified and classified as preintervention or post-intervention according to surgery date relative to care bundle rollout on November 1, 2013. Clinicopathologic characteristics of the overall, pre-intervention (n=231), and post-intervention (n=193) cohorts are shown in Table 2. The pre- and post-intervention cohorts were similar in regard to age, sex, body mass index, presence of diabetes, American Society of Anesthesiologists classification, wound classification, operation duration, type of intestinal procedure, extent of liver resection, and EBL.
Table 2.
Characteristics of the study population.
| Characteristic | All patients n = 424 | Pre-intervention n = 231 | Post-intervention n = 193 | p value |
|---|---|---|---|---|
| Age, years | 56 (21, 87) | 56 (21, 87) | 55 (28, 85) | 0.658 |
| Female gender | 205 (48.3%) | 107 (46.3%) | 98 (50.8%) | 0.381 |
| BMI | 27.03 (16.3, 50.04 | 27.03 (16.3, 50.04) | 27.05 (16.3, 48.1) | 0.742 |
| History of smoking | 179 (42.2%) | 109 (47.2%) | 70 (36.3%) | 0.03 |
| History of diabetes | 39 (9.2%) | 17 (7.4%) | 22 (11.4%) | 0.178 |
| CCI | 10 (3, 17) | 10 (4, 17) | 10 (3, 15) | 0.009 |
| ASA classification | 0.356 | |||
| 1 | 3 (0.7%) | 2 (0.9%) | 1 (0.5%) | |
| 2 | 138 (32.5%) | 83 (35.9%) | 55 (28.5%) | |
| 3 | 276 (65.1%) | 143 (61.9%) | 133 (68.9%) | |
| 4 | 7 (1.7%) | 3 (1.3%) | 4 (2.1%) | |
| Wound classification | 0.512 | |||
| CC | 368 (86.8%) | 203 (87.9%) | 165 (85.5%) | |
| CO | 55 (13%) | 27 (11.7%) | 28 (14.5%) | |
| Dirty | 1 (0.2%) | 1 (0.4%) | 0 (0%) | |
| Operation duration, hours | 5.01 (1.27, 13.4) | 5.05 (1.27, 13.4) | 4.97 (1.3, 12.15) | 0.654 |
| Hospital LOS, days | 9 (4, 60) | 9 (4, 60) | 8 (4, 48) | <.001 |
| Laparoscopy | 20 (4.7%) | 6 (2.6%) | 14 (7.3%) | 0.036 |
| Stoma creation | 68 (16%) | 39 (16.9%) | 29 (15%) | 0.69 |
| Modified wound closure | 99 (23.3%) | 21 (9.1%) | 78 (40.4%) | <.001 |
| Intestinal procedure | 0.454 | |||
| Rectum | 164 (38.7%) | 94 (40.7%) | 70 (36.2%) | |
| Colon | 237 (55.9%) | 123 (53.2%) | 114 (59.1%) | |
| Ostomy closure | 23 (5.4%) | 14 (6.1%) | 9 (4.7%) | |
| Major liver resection | 88 (20.8%) | 50 (21.6%) | 38 (19.7%) | 0.633 |
| EBL | 350 (25, 5000) | 400mL (25, 3,450) | 350mL (25, 5,000) | 0.050 |
Continuous variables are presented as median (range), and categorical variables as n (%). BMI, body mass index; CCI, Charlson comorbidity index; ASA, American Society of Anesthesiologists; CC, clean-contaminated; CO, contaminated; LOS, length of stay; LAR, low abdominal resection; APR, abdominoperineal resection. EBL, estimated blood loss.
The two cohorts differed in terms of a few demographic, clinical, and surgical characteristics. Though the median Charlson comorbidity index (CCI) was the same between the two cohorts, the range limits were lower in the post-intervention cohort. The post-intervention cohort also had fewer smokers and a higher rate of laparoscopic procedures. Lastly, modified wound closure was performed in a higher percentage of patients in the post-intervention cohort. In the post-intervention cohort, 115 (59.6%) incisions were treated with primary closure, 64 (33.2%) were closed with a surface vacuum dressing, 8 (4.2%) were closed with subcutaneous drains, and 6 (3.1%) were packed open.
The results for the primary and secondary endpoints are outlined in Table 3. The overall SSI rate decreased by 60.5% (34.2% vs. 13.5%, p<0.001) after implementation of the perioperative bundle. Analyzing by type of infection, superficial/deep SSIs decreased by 80.6% (13.4% v. 2.6%, p<0.001), and organ space SSIs decreased by 47.6% (20.8% v. 10.9%, p<0.008) after implementation. In the pre-intervention cohort, there were 43 patients with organ space infection, 8 of whom showed signs of sepsis. Of the 43 patients, 38 had percutaneous drains placed by interventional radiology and 3 required reoperation. In the post-intervention group, there were 15 patients with organ space infection, 4 of whom showed signs of sepsis. Of the 15 patients, 12 had percutaneous drains placed by interventional radiology and 2 required reoperation.
Table 3.
Summary of primary and secondary study endpoints.
| Pre-Intervention n (%) | Post-Intervention n (%) | Absolute Reduction (95% CI) | p value | |
|---|---|---|---|---|
| Overall SSI | 79 (34.2%) | 26 (13.5%) | 20.7% (12–29) | <0.001 |
| Superficial/Deep Infection | 31 (13.4%) | 5 (2.6%) | 10.8% (5–16) | <0.001 |
| Organ Space Infection | 48 (20.8%) | 21 (10.9%) | 9.9% (3–13) | 0.008 |
| LOS (days, median, range) | 9 (4–60) | 8 (4–48) | 1 (0.8–2.2) | <0.001 |
| 30 day readmission rate | 43 (18.6%) | 40 (20.7%) | 3 (−6–10) | 0.62 |
SSI, surgical site infection; LOS, length of stay; IQR, interquartile range; CI, confidence interval.
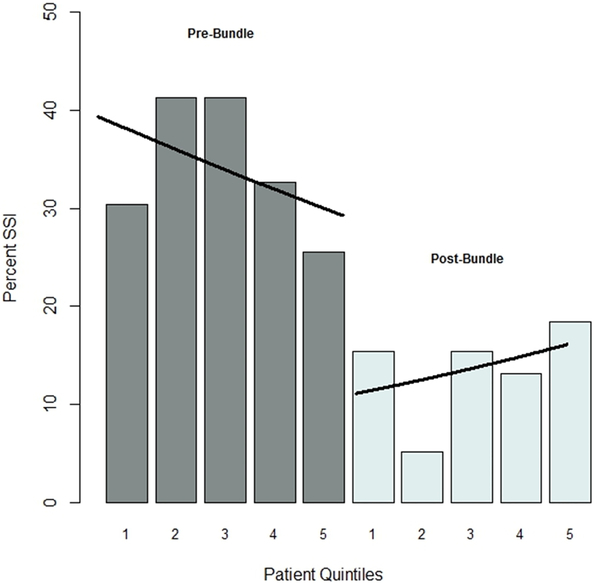
We have also examined whether there were time trends within the pre- and postintervention cohorts that might explain the observed differences in SSI rates, using interrupted time series analysis. The analysis revealed no significant time trends in SSI rates during the pre-intervention (OR=1, 95% confidence interval (0.99–1), p=0.35) or post-intervention (OR=1, 95% confidence interval (0.99–1.01), p=0.55) period, indicating that the differences between the pre- and post-intervention cohorts cannot be explained by time trends extraneous to the intervention (Figure 1).
Figure 1.
Interrupted time series analysis of SSI rates with overlying logistic regression curves for the pre-intervention (OR=1, 95% confidence interval (0.99 – 1), p=0.35) and post-intervention (OR=1, 95% confidence interval (0.99 – 1.01), p=0.55) time periods.
The median hospital LOS decreased by 1 day in the post-intervention cohort, without change in 30-day readmission. from 9 days (interquartile range [IQR] 4–60) in the pre-intervention cohort to 8 days (IQR 4–48) post-intervention (p<0.001). There was no significant change in 30-day readmission rates (18.6% v. 20.7%, p=0.60).
Discussion
In this study, we show that a 13-component perioperative care bundle developed at MSK was associated with a reduced rate of SSI for combined colorectal and liver resection by 61%, including 81% and 48% reductions in superficial/deep and organ space SSI, respectively. These reductions are especially significant given the frequency of SSI following colorectal and hepatic surgeries, which have both been the subject of previous SSI reduction programs 9-10, 19-20. We are confident that the intervention was responsible for the change in SSI rates, as an interrupted time series analysis found no significant trends in SSI rates pre- and post-intervention.
Although compliance with bundle components could not be reliably measured in this retrospective study of patients undergoing combined resection, it was likely similar to that found by a prospective SSI reduction study conducted at MSK, in which compliance rates exceeded 85%.17 That analysis, which included all colorectal cancer patients reported to the Hospital-Acquired Infection Reporting Program of the state of New York, covered an overlapping period.
Other SSI reduction projects have similarly reported a smaller reduction in organ space SSI compared to superficial/deep SSI, which suggests differing etiologies. A meta-analysis by Zywot et al. tracked SSI outcomes both pre- and post-bundle implementation in 7,304 colorectal surgery patients in 11 studies, and found that superficial SSI decreased by 44% (p<0.001), while organ space SSI decreased 34% (p=0.048) 10. Organ space SSI is likely related to anastomotic dehiscence and/or infection of post-hepatectomy ascites, which may not be fully addressed with current SSI reduction interventions 10, 21.
The care bundle was associated with a small decrease in hospital LOS, but did not affect 30-day readmission rate. LOS decreased from 9 days to 8 days, similar to previous reports on SSI-reducing interventions in hepatic 19 and colorectal 22-26 surgery. However, a confounding factor in many of these studies as well as our own is concurrent implementation of enhanced recovery pathways that also decrease hospital LOS. The lack of change in 30-day readmission rates was somewhat surprising; however, this is consistent with other studies 19, 24-25 and likely is due to infections being identified during the index admission or during the follow-up period and being adequately dealt with by visiting nursing services. In addition, readmission following combined colorectal and liver resection is multifactorial and only partially related to SSI.
Since our study design lacked randomization, it is important to rule out the possibility that the observed differences can be explained either by changes in the distribution of factors from the pre-intervention period to the post-intervention period or by exogenous time trends. We found that only a few factors (history of smoking, CCI, laparoscopy, and modified wound closure) differed significantly between the two cohorts (Table 2), and an interrupted time series analysis (Figure 1) revealed no discernible time trend in either of the cohorts that could point to an extraneous decrease in SSI rates independent of the intervention. We therefore conclude that the intervention was responsible for the reduction in SSIs.
Differences between the pre- and post-intervention cohorts included modified wound closure rates, smoking history (47.2% v. 36.3%, p=0.03), CCI scores (median 10, range 4–17 v. 10, 3–15, p=0.009), and rate of laparoscopic procedures (2.6% v. 7.3%, p=0.036). Wound closure method, which was left to surgeon discretion, may have been influenced by one of the interventions, electronically providing surgeons an estimated risk of SSI the day prior to surgery. Indeed, modified closure was more common in the post-intervention cohort (40.4%) compared to the pre-intervention cohort (9.1%, p<0.001). The most frequent modified closure method was negative pressure surface vacuum dressing, which has been reported to reduce SSI 27. Although a recent study did not show benefit, possibly because dressings remained in place for a shorter period and only patients at high risk for SSIs were included 28, a meta-analysis of 10 randomized controlled trials concluded that negative pressure wound therapy was effective at decreasing SSI compared to standard postoperative practices 29.
The other differences between cohorts are unlikely to account for the change in SSI rates following the intervention. Smoking, which was less prevalent in the post-bundle cohort, has previously been associated with increased SSI risk 21, 30. However, the difference in self-reported tobacco use (which tends to be under-reported) appeared too small to affect SSI rates. CCI scores, a marker of baseline comorbid severity, only differed in terms of range; the median CCI score was the same in both patient cohorts. Finally, though laparoscopy has been clearly shown to reduce SSI 31-34 and there were more minimally invasive procedures in the post-intervention cohort compared to pre-intervention (7.3% versus 2.6%, respectively), the proportions were small. Subgroup analysis based on smoking history and wound closure confirmed the post-intervention reduction in SSIs (data not shown). Further statistical investigations to identify independent risk factors of SSI using multivariable modeling and propensity score analysis were not feasible due to the small number of SSIs in the post-intervention cohort.
There are limitations to our study. First, we grouped superficial and deep SSIs into a single category because they probably have similar etiologies and are grouped together in our institutional complication database. Second, although MSK is a high-volume tertiary referral cancer, the findings of this single institution study may not be readily applicable to all hospital systems. Lastly, as all components of the bundle were implemented together, it is not possible to measure the relative impact on SSI reduction.
In conclusion, implementation of a multidisciplinary SSI reduction program was associated with a dramatic reduction in SSIs following synchronous colorectal and liver resection, a procedure associated with particularly high risk of these complications. Specifically, our 13-point intervention was associated with a reduction in SSIs by over 60%, with the greatest reductions in superficial/deep SSIs. Future interventions should be directed toward reducing organ space infections.
Supplementary Material
Acknowledgment
We gratefully acknowledge Jessica L. Moore and Arthur Gelmis for editing the manuscript.
Funding
This work was supported by NCI grant P30 CA008748.
Footnotes
Previous communication: The findings of this study were presented at the AHPBA Annual Meeting in Miami Beach, March 7-11, 2018. The data for 53 of the patients in this study are included in a study published in the British Journal of Surgery.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, et al. Multistate point-prevalence survey of health care-associated infections. N Engl J Med. 2014. March 27;370(13):1198–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zimlichman E, Henderson D, Tamir O, Franz C, Song P, Yamin CK, et al. Health care-associated infections: a meta-analysis of costs and financial impact on the US health care system. JAMA Intern Med. 2013. December 9-23;173(22):2039–46. [DOI] [PubMed] [Google Scholar]
- 3.de Lissovoy G, Fraeman K, Hutchins V, Murphy D, Song D, Vaughn BB. Surgical site infection: incidence and impact on hospital utilization and treatment costs. Am J Infect Control. 2009. June;37(5):387–97. [DOI] [PubMed] [Google Scholar]
- 4.Dimick JB, Chen SL, Taheri PA, Henderson WG, Khuri SF, Campbell DA Jr. Hospital costs associated with surgical complications: a report from the privatesector National Surgical Quality Improvement Program. J Am Coll Surg 2004;199:531–7. [DOI] [PubMed] [Google Scholar]
- 5.Merkow RP, Ju MH, Chung JW, Hall BL, Cohen ME, Williams MV, et al. Underlying reasons associated with hospital readmission following surgery in the United States. JAMA. 2015. February 3;313(5):483–95. [DOI] [PubMed] [Google Scholar]
- 6.Astagneau P, Rious C, Golliot F, Brucker G. Morbidity and mortality associated with surgical site infections: results from the 1997–1999 INCISO surveillance. J Hosp Infect. 2001;48:267–274. [DOI] [PubMed] [Google Scholar]
- 7.Khuri SF, Henderson WG, DePalma RG, Mosca C, Healey NA, Kumbhani DJ; Participants in the VA National Surgical Quality Improvement Program. Determinants of long-term survival after major surgery and the adverse effect of postoperative complications. Ann Surg. 2005. September;242(3):326–41; discussion 341–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Artinyan A, Orcutt ST, Anaya DA, Richardson P, Chen GJ, Berger DH. Infectious postoperative complications decrease long-term survival in patients undergoing curative surgery for colorectal cancer: a study of 12,075 patients. Ann Surg. 2015. March;261(3):497–505. [DOI] [PubMed] [Google Scholar]
- 9.Tanner J, Padley W, Assadian O, Leaper D, Kiernan M, Edmiston C. Do surgical care bundles reduce the risk of surgical site infections in patients undergoing colorectal surgery? A systematic review and cohort meta-analysis of 8,515 patients. Surgery. 2015. July;158(1):66–77. [DOI] [PubMed] [Google Scholar]
- 10.Zywot A, Lau CSM, Stephen Fletcher H, Paul S. Bundles Prevent Surgical Site Infections After Colorectal Surgery: Meta-analysis and Systematic Review. J Gastrointest Surg. 2017. June 15. [DOI] [PubMed] [Google Scholar]
- 11.Manfredi S, Lepage C, Hatem C, Coatmeur O, Faivre J, Bouvier AM. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg. 2006. August;244(2):254–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomassen I, van Gestel YR, Lemmens VE, de Hingh IH. Incidence, prognosis, and treatment options for patients with synchronous peritoneal carcinomatosis and liver metastases from colorectal origin. Dis Colon Rectum. 2013. December;56(12):1373–80. [DOI] [PubMed] [Google Scholar]
- 13.Silberhumer GR, Paty PB, Temple LK, Araujo RL, Denton B, Gonen M, et al. Simultaneous resection for rectal cancer with synchronous liver metastasis is a safe procedure. Am J Surg. 2015. June;209(6):935–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fahy BN and Fischer CP. Synchronous resection of colorectal primary and hepatic metastasis. J Gastrointest Oncol. 2012. March;3(1):48–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Centers for Disease Control and Prevention. Surgical Site Infection (SSI) Event. 2017. (Accessed February 7, 2017, at https://www.cdc.gov/nhsn/pdfs/pscmanual/9pscssicurrent.pdf).
- 16.Joint Commission. Specifications manual for Joint Commission national quality core measures: Surgical Care Improvement Project. 2010. (Accessed November 22, 2017, at https://manual.jointcommission.org/releases/archive/TJC2010B/SurgicalCareImprovementProject.html
- 17.Weiser MR, Gonen M, Usiak S, Pottinger T, Samedy P, Patel D, et al. Effectiveness of a multidisciplinary patient care bundle for reducing surgical-site infections. Br J Surg., in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bernal JL, Cummins S, Gasparrini A. Interrupted time series regression for the evaluation of public health interventions: a tutorial. Int J Epidemiol. 2017, 46:348–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hill MV, Holubar SD, Garfield Legare CI, Luurtsema CM, Barth RJ Jr. Perioperative Bundle Decreases Postoperative Hepatic Surgery Infections. Ann Surg Oncol. 2015. December;22 Suppl 3:S1140–6. [DOI] [PubMed] [Google Scholar]
- 20.Ceppa EP, Pitt HA, House MG, Kilbane EM, Nakeeb A, Schmidt CM, et al. Reducing surgical site infections in hepatopancreatobiliary surgery. HPB (Oxford). 2013. May;15(5):384–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lawson EH, Hall BL, Ko CY. Risk factors for superficial vs deep/organ-space surgical site infections: implications for quality improvement initiatives. JAMA Surg. 2013. September;148(9):849–58. [DOI] [PubMed] [Google Scholar]
- 22.DeHaas D, Aufderheide S, Gano J, Weigandt J, Ries J, Faust B. Colorectal surgical site infection reduction strategies. Am J Surg. 2016. July;212(1):175–7. [DOI] [PubMed] [Google Scholar]
- 23.Hedrick TL, Heckman JA, Smith RL, Sawyer RG, Friel CM, Foley EF. Efficacy of protocol implementation on incidence of wound infection in colorectal operations. J Am Coll Surg. 2007. September;205(3):432–8. [DOI] [PubMed] [Google Scholar]
- 24.Keenan JE, Speicher PJ, Thacker JK, Walter M, Kuchibhatla M, Mantyh CR. The preventive surgical site infection bundle in colorectal surgery: an effective approach to surgical site infection reduction and health care cost savings. JAMA Surg. 2014. October;149(10):1045–52. [DOI] [PubMed] [Google Scholar]
- 25.Keenan JE, Speicher PJ, Nussbaum DP, Adam MA, Miller TE, Mantyh CR, Thacker JK. Improving Outcomes in Colorectal Surgery by Sequential Implementation of Multiple Standardized Care Programs. J Am Coll Surg. 2015. August;221(2):404–14.e1. [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto T, Morimoto T, Kita R, Masui H, Kinoshita H, Sakamoto Y, et al. The preventive surgical site infection bundle in patients with colorectal perforation. BMC Surg. 2015. December 18;15:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blackham AU, Farrah JP, McCoy TP, Schmidt BS, Shen P. Prevention of surgical site infections in high-risk patients with laparotomy incisions using negative-pressure therapy. Am J Surg. 2013. June;205(6):647–54. [DOI] [PubMed] [Google Scholar]
- 28.Shen P, Blackham AU, Lewis S, Clark CJ, Howerton R, Mogal HD, et al. Phase II Randomized Trial of Negative-Pressure Wound Therapy to Decrease Surgical Site Infection in Patients Undergoing Laparotomy for Gastrointestinal, Pancreatic, and Peritoneal Surface Malignancies. J Am Coll Surg. 2017. April;224(4):726–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hyldig N, Birke-Sorensen H, Kruse M, Vinter C, Joergensen JS, Sorensen JA, et al. Meta-analysis of negative-pressure wound therapy for closed surgical incisions. Br J Surg. 2016. April;103(5):477–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Moreno Elola-Olaso A, Davenport DL, Hundley JC, Daily MF, Gedaly R. Predictors of surgical site infection after liver resection: a multicentre analysis using National Surgical Quality Improvement Program data. HPB (Oxford). 2012. February;14(2):136–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Aimaq R, Akopian G, Kaufman HS. Surgical site infection rates in laparoscopic versus open colorectal surgery. Am Surg 2011;77:1290–4. [DOI] [PubMed] [Google Scholar]
- 32.Cerdán Santacruz C, Frasson M, Flor-Lorente B, Ramos Rodríguez JL, Trallero Anoro M, Millán Scheiding M, et al. Laparoscopy may decrease morbidity and length of stay after elective colon cancer resection, especially in frail patients: results from an observational real-life study. Surg Endosc. 2017. December;31(12):5032–5042. [DOI] [PubMed] [Google Scholar]
- 33.Abraham NS, Young JM, Solomon MJ. Meta-analysis of short-term outcomes after laparoscopic resection for colorectal cancer. Br J Surg. 2004. September;91(9):1111–24. [DOI] [PubMed] [Google Scholar]
- 34.Schwenk W, Haase O, Neudecker J, Müller JM. Short term benefits for laparoscopic colorectal resection. Cochrane Database Syst Rev. 2005. July 20;(3):CD003145. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.