Abstract
Background:
Transplantation of mesenchymal stem cells (MSCs) is a promising therapy for degenerative spine conditions. However, cell therapy for painful spine degeneration presently requires use of contrast agents during fluoroscopy-guided injections and the effects of these agents on MSCs represents a gap in knowledge.
Objective:
To investigate the biological effects of contrast media that are co-injected with MSCs.
Design:
Prospective observational study.
Setting:
Academic medical center.
Participants:
Patient-derived clinical-grade culture expanded MSCs.
Interventions:
Iohexol(Omnipaque300) was reduced to 12.5%, 25%, 50% and 100% of the stock solution and incubated with MSCs for 30 minutes, 4 hours and 48 hours. We also used complete media and 12.5%, 25%, 50%, 100% of phosphate buffered saline (PBS) as control group.
Main Outcome Measures:
We examined cytotoxicity of Iohexol at different concentrations and exposure duration, as well as the potential for recovery over time. Cell counts, mitochondrial activity, and quantitative real time reverse-transcriptase polymerase chain reaction (qRT-PCR) of related genes were analyzed immediately after exposure (day 0) and after two days of exposure (day 2).
Results:
Human MSCs exhibit a time- and concentration-dependent cytotoxic response to iodinated CM. A brief 30min exposure did not affect MSCs function and viability. However, extended treatment with iohexol for 4 hours at 50% or higher concentration had a significant impact on both viability and gene expression in MSCs.
Conclusions:
CM (Omnipaque300) is cytotoxic to MSCs in a time-and concentration-dependent manner. Hence, the concentration of CM that accompanies MSC injections should be carefully considered during mesenchymal stem cell therapy for disc degenerative diseases.
Keywords: mesenchymal stem cells, contrast media, cytotoxicity, cell therapy, regenerative medicine, adipose derived stem cells
Introduction
Degenerative musculoskeletal and spine conditions are significant causes of pain and disability [1]. Advances in regenerative medicine and cell-based therapies, particularly the transplantation of mesenchymal stem cells (MSCs), have inspired numerous studies and resulted in clinical trials utilizing these biological therapies to treat degenerative conditions, often reporting favorable outcomes [2]. Recent clinical trial demonstrated that mesenchymal stem cell therapy may be a valid alternative treatment for chronic back pain caused by degenerative disc disease [3]. To validate the safety and utility of MSC therapy for interventional spinal and peripheral joint procedures, it is necessary to address concerns about the potential cytotoxicity of drugs that are co-injected with MSCs.
Among agents that are co-injected with stem cells, iodinated contrast medium (CM) is often used for diagnostic and therapeutic fluoroscopically guided spinal procedures such as endoscopic lumbar discectomy, intradiscal electrothermal therapy, discography, facet joint block, chemonucleolysis, or selective nerve root blockade [4]. Although MSC injections in the peripheral joint are increasingly delivered using ultrasound guidance, contrast media continue to be used in spine procedures and hence the biological effects of contrast agents on stem cells remain to be explored. Iohexol is a non-ionic, monomeric iodinated contrast agent that has been routinely used to ensure accurate needle placement during fluoroscopically guided spine procedures. However, iohexol may affect the biological properties of human MSCs. We addressed this possible concern because of reports of the cytotoxicity of iodinated contrast medium in various cell types such as renal epithelial cells, endothelial cells, mesangial cells, pulmonary mast cells, smooth muscle cells and human disc cells [5, 6, 7, 8]. Several studies found that ionic CM was more toxic than non-ionic CM due to its ionic strength [5, 9, 10]. Because there are concerns with the cytotoxic effects of Iohexol on MSCs, we assessed whether Iohexol is cytotoxic for MSCs. One question that arises is whether short term contact between iohexol and MSCs during injection may negatively affect the long-term viability. Hence, our study investigates the hypothesis that biological effects of short-term treatments (~30 min) may have both acute consequences and may last beyond the initial recovery period.
Methods
General Design
This study was designed to study the effects of different dilutional strengths of iohexol. We note that contrast agents are commercially available in various strengths and it would be clinically useful to study the effects of different premixed concentrations. However, cells in culture cannot be maintained without proper cell culture medium that contains essential physiological nutrients that are required for cell survival. Therefore, to achieve proper biochemical titration of the toxicity of contrast agents on human platelet lysate expanded MSCs in cell culture, it was necessary to perform a dilution series in which agents were mixed with complete cell culture medium at a ratio of 1:4. The experimental arm was exposed to 100%, 50%, 25%, and 12.5% dilutions of Omnipaque 300 (GE Healthcare NDC 0407–1413-86, iohexol injection, iodine content 300mg/ml) in complete media (see table 1). The control arm was exposed to100%, 50%, 25%, and 12.5% dilutions of phosphate buffered saline (PBS) in complete media. We also used complete media (0% Omnipaque and 0% PBS group) as control. Exposure durations are 30 min, 4 hours or 48 hours. Cells were analyzed for viability and function immediately post-exposure (day 0), or after 48 hours recovery (day 2) following Omnipaque/PBS exposure.
Table 1.
Dilution of stock iohexol (Omnipaque300) to working solutions
| Diluted stock concentrations | Final experiment concentration | Final iodine content (mg/ml) |
|---|---|---|
| 100% | 80% | 240 |
| 50% | 40% | 120 |
| 25% | 20% | 60 |
| 12.5% | 10% | 30 |
| 0% | 0% | 0 |
The diluted stock concentrations were 100%, 50%, 25%, 12.5% and 0% dilutions of Omnipaque300 (GE Healthcare NDC 0407–1413–86, iohexol injection, iodine content 300mg/ml) in complete media. Human platelet lysate expanded AMSCs in complete media were mixed with treatment solutions at the ratio of 1:4. Final experimental concentrations are shown as the final percentage of iohexol diluted into complete media.
Cell Isolation
Following IRB approval and informed consent, human MSCs were isolated from lipo-aspirates using previously described methods [11]. In brief, lipo-aspirates were digested with Type I collagenase (Worthington Biochemicals, Lakewood, NJ) for 1.5 hours at 37°C, centrifuged at 400g for 5 min, rinsed with PBS (Life Technologies, Grand Island, NY), and strained using 7μm cell strainers (BD Biosciences, San Jose, CA). Following this, the aspirate was treated with 154 mM NH4Cl, 10 mM KHCO3, and 0.1 mM EDTA for erythrocyte lysis. The remaining MSCs were expanded in advanced minimum essential medium (Life Technologies, Grand Island, NY), supplemented with 5% (vol/vol) human platelet lysate (PLT Max; Mill Creek Life Sciences, Rochester, MN), 2mM Glutamax (Life Technologies, Grand Island, NY), 2 U/mL heparin, and 1% Penn-Strep (100 U/mL penicillin, 100 μg/mL streptomycin; Cellgro, Corning, NY). All MSCs used in experimentation were of passage 7. The donor-derived MSCs selected in this study have previously been shown to express standard MSC markers and are capable of multilineage differentiation [12].
The cells were grown at 37°C in a humidified 5% CO2 incubator until 90% confluent, were trypsinized (TrypLE Express, Life Technologies, Grand Island, NY), and then were seeded onto ninety-six-well plates (for MTS analysis), 24-well plates (for DAPI & Trypan blue staining analysis) and 6-well plates (for RT-qPCR analysis) at a density of 10 000 cells/cm2. We prepared hMSC suspensions with concentrations of 60,000 or 200,000 cells/ml. We added 50ul (60,000 cells/ml), 100ul (200,000 cells/ml) and 500ul (200,000 cells/ml) of human MSC cell suspensions in each well of a 96-well, 24-well and 6-well plate, respectively. The cells were cultured for 24–48 hours until they reached a cell density of 90% confluence prior to experimental treatment.
Treatment Groups
Human MSC cultures were subdivided into triplicate wells per condition and exposed to one of the following dilutions: 100%, 50%, 25%, 12.5% (diluted with complete media) Omnipaque 300 as treatment group and 100%, 50%, 25%, 12.5% (also diluted with complete media) PBS as control group. We also used complete media (0% Omnipaque and 0% PBS group) as control. Volumes of 200 ul, 400 ul and 2000 ul for each group were added to each well in 96-well, 24-well and 6-well plates respectively. Human MSCs were incubated for 30 minutes, 4 hours or 48 hours at 37 °C in a humidified 5% CO2 incubator. After the exposure, the cells were washed in 1 ×PBS solution, and returned to the incubator in fresh culture media. MSC viability was measured immediately postexposure (day 0), and also 48 hours (day 2) following treatment. Expression analysis was performed with the 4 hours exposure group.
Assessment of MSC Viability
To assess cell viability and proliferation potential after contrast exposure, two cell counting methods were used: Trypan Blue (Sigma-Aldrich, St. Louis, MO) and DAPI staining. Twenty microliters of trypsinized cells were mixed with 20 uL of 0.4% Trypan Blue solution and incubated for 3 minutes at room temperature. Ten microliters of that solution were then transferred to a dual-chamber hemocytometer, and the cells were counted using conventional light microscopy. Trypan Blue does not stain living cells because their membranes remain intact. Cell viability was calculated as the fraction of viable cells (number of unstained cells) divided by the total number of cells (number stained and unstained cells) and multiplied by × 100% [13]. We also used DAPI staining (Life Technologies, Grand Island, NY) paired with Image J analysis, an open-source NIH-supported image analysis software program to count the cell number. Images were acquired using an inverted light microscope (Zeiss, Cambridge, MA). DAPI (4',6diamidino-2-phenylindole) is a fluorescent stain that binds strongly to A-T rich regions in DNA. As DAPI can pass through an intact cell membrane, it can be used to stain both live and fixed cells, though it passes through the membrane less efficiently in live cells and therefore the effectiveness of the stain is lower. In our study, we used 0.1% triton to permeabilize the fixed cells, so we also easily stained the lived cells.
To assess the metabolic activity of MSCs after contrast exposure, MTS colorimetric assays (Promega, Madison, WI) were performed at day 0 and 48 hours post-exposure. MTS assays were read using a Spectra Max plus Plate Reader (Molecular Devices, Sunnyvale, CA) at an absorbance wave-length of 490 nm. The assay is based on the reduction of MTS tetrazolium compound by viable cells to generate a colored formazan product that is soluble in cell culture media. This conversion is thought to be carried out by mitochondrial NADPH-dependent dehydrogenase enzymes in metabolically active cells. The formazan dye produced by viable cells can be quantified by measuring the absorbance at 490 nm and reflects cell metabolic activity.
All of the samples were assayed in triplicate.
Expression analysis of human MSCs in response to “Omnipaque exposure”
To assess the effect of Omnipaque exposure on cell activity and behavior, human MSCs were subjected to RT-qPCR analysis, with genes widely recognized as associated with cell proliferation, stress response, and stem cell surface markers (See table 2). Before RT-qPCR, RNA was isolated using the miRNeasy Mini Kit (Qiagen) and total RNA yield was evaluated using the Nanodrop 2000 UV-Vis Spectrophotometer (Thermo Scientific, Inc.). Reverse-transcriptase was then performed to obtain cDNA using Super Script III reverse transcriptase (Invitrogen, Carlsbad, CA). The resulting cDNAs were used in RT-qPCR (CFX384 Real-Time System, BioRad) using SYBR Green detection with primers that detect select biomarkers for stress responses and/or proliferation [early growth response’ transcription factors-1, 2, EGR1, 2], stress responses [activating transcription factor-4, 6, ATF-4, 6] and stem cell surface markers [CD90, CD105]. Primer sequences are listed in Table 2. Gene expression levels were quantified using the 2−Δ Δ Ct method. Values for mRNA expression were normalized to the housekeeping gene GAPDH. Table 2: mRNA primer sequences
Table 2:
mRNA primer sequences
| Gene | Implication | Primers, 5'–3' | |
|---|---|---|---|
| Forward | Reverse | ||
| EGR1 | Stress response &Cell proliferation | ACCCCTCTGTCTACTATTAAGGC | TGGGACTGGTAGCTGGTATTG |
| EGR2 | Stress response &Cell proliferation | ATTCTGAGGCCTCGCAAGTA | GCTTATGCCCAGTGTGGATT |
| ATF4 | Stress response | ATGACCGAAATGAGCTTCCTG | CTGGAGAACCCATGAGGTTTG |
| ATF6 | Stress response | TCCTCGGTCAGTGGACTCTTA | CTTGGGCTGAATTGAAGGTTTTG |
| CD90 | Stem cell surface marker | ATGAAGGTCCTCTACTTATCCGC | GCACTGTGACGTTCTGGGA |
| CD105 | Stem cell surface marker | TGCACTTGGCCTACAATTCCA | AGCTGCCCACTCAAGGATCT |
Statistical Analysis
Descriptive statistics were utilized to demonstrate the effects of contrast exposure on human MSC cell counts (i.e. viability), mitochondrial activity and qPCR on days 0 and 2 after exposure. Results were analyzed by one-way or two-way ANOVA as appropriate, and unpaired Student’s t-test. Statistical significance was set at p < 0.05. Results are presented as the mean ± SD. All statistical analyses were conducted using Stata version 13.1 (StataCorp, college park, tx).
Results
Effects of Omnipaque on Live/Dead Cell Counts and Proliferative Ability of MSCs
Our study addresses the hypothesis that contrast agents may have biological effects on stem cells. Omnipaque is the most common contrast agent for interventions in spine and is available as injection solutions of different strengths (i.e., 180, 240 and 300 mg/ml). Therefore, we tested how different concentration of Omnipaque affects the viability of stem cells at different concentrations.
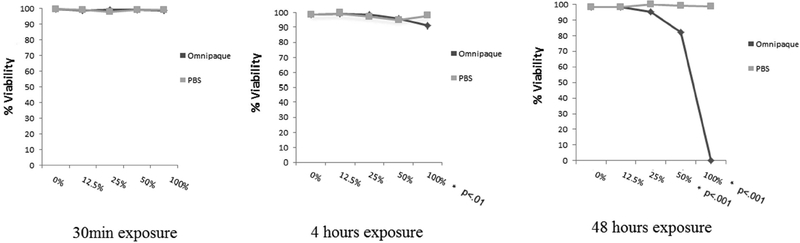
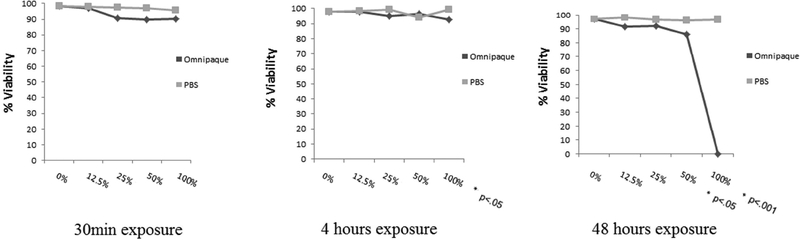
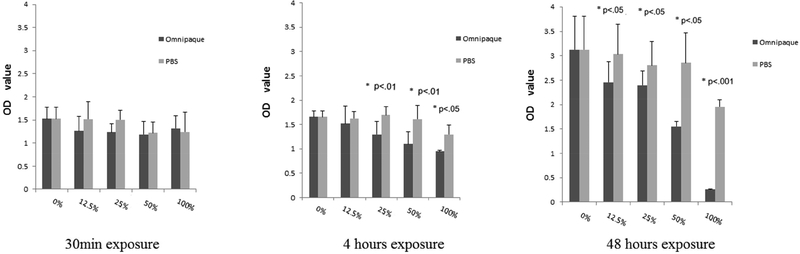
Live/dead cell analysis by Trypan Blue staining is shown in Figures 1A and 1B. MSC viability did not show significant decrease with 30min exposure to any concentration of contrast media, indicating that short exposure of MSCs to contrast media is well tolerated even at high concentration. MSC viability decreased with exposure to 100% Omnipaque after 4-hour exposure, and with exposure to either 50% or 100% Omnipaque after 48 hours exposure (P<.05, Figure 1A, 2B).
Figure 1A.
Percentage of hMSC’s live (Trypan Blue analysis) after 30min, 4 and 48 hours exposure and analyzed immediately.
Figure 1B.
Percentage of hMSC’s live (Trypan Blue analysis) after 30min, 4 and 48 hours exposure and then analyzed after a recovery period of 2 days.
Figure 2B.
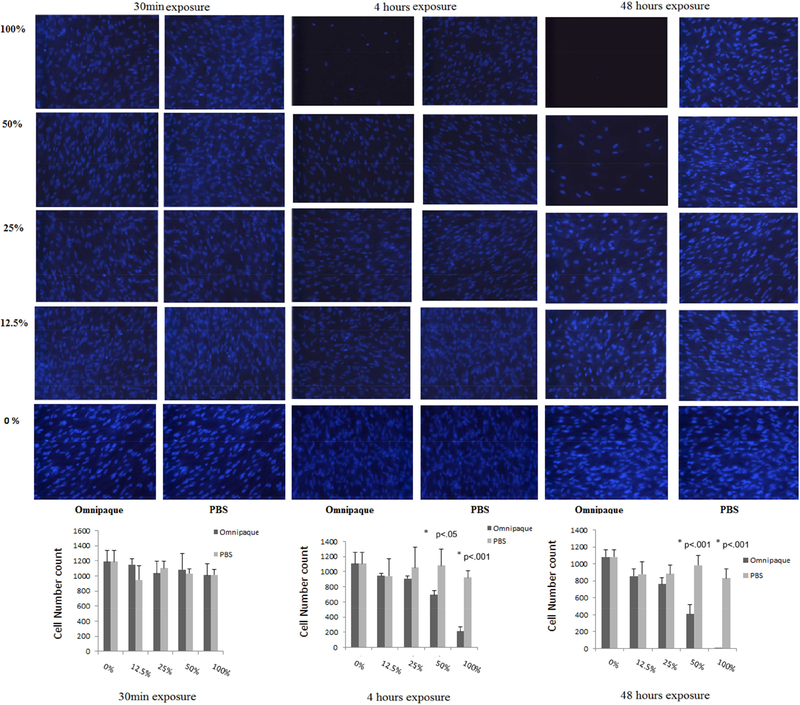
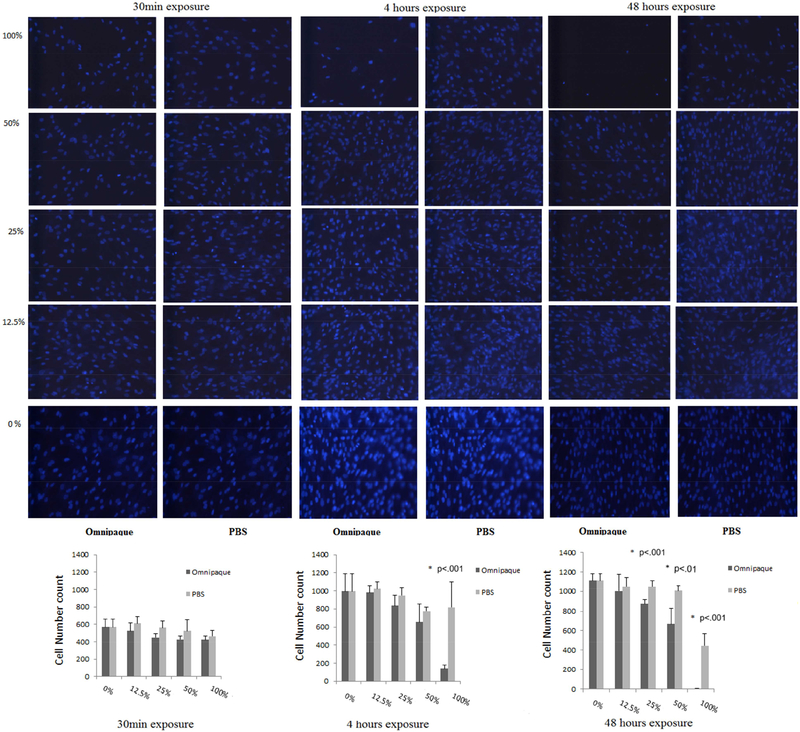
Cell counting of human MSCs as determined by DAPI staining analyzed after two days in culture (day 2) following initial exposure to Omnipaque for 30min, 4 hours and 48 hours.
While our results show that the presence of Omnipaque affects the cellular integrity of MSC, we reasoned that subsequent removal of the Omnipaque may restore the normal biological properties of the cells after a recovery of two days. The results show that cells incubated with contrast media at concentrations of stronger than 50% do not adequately recover.
Proliferative ability was assessed by cell counting following DAPI staining, which showed that viable cell numbers of human MSC’s decreased only in 100% Omnipaque concentration after 4 hours of exposure and in 25%, 50%, 100% Omnipaque concentrations after 48 hours exposure when cells were immediately analyzed without a recovery period (i.e., day 0). Meanwhile, the viability of human MSCs decreased in 50% and 100% Omnipaque concentration after either 4 or 48 hours of exposure groups after recovery for two days in culture (day 2, p<.05, Figure 2A, 2B). Hence, cell viability is compromised immediately after exposure to Omnipaque and does not recover even after two days.
Figure 2A.
Cell counting of human MSCs as determined by DAPI staining analyzed immediately (day 0) after exposure for 30min, 4 hours and 48 hours to Omnipaque.
Effect of Omnipaque on Mitochondrial Activity of MSCs
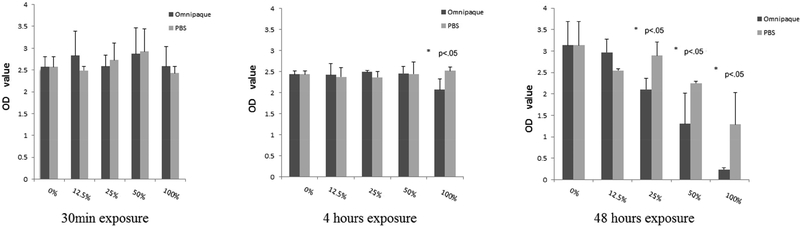
There was no statistically significant effect of 30min Omnipaque exposure on MTS absorbance at either days 0 or 2. This result suggests that 30min treatment does not affect mitochondrial activity as an indicator of both the metabolic activity and viability of human MSCs. When samples were immediately analyzed without recovery time (day 0), MTS absorbance decreased after 4 hours of exposure with Omnipaque concentrations of 25%, 50% and 100%, whereas it decreased after 48 hours with slightly lower Omnipaque concentrations of 12.5%, 25%, 50% and 100%. For comparison, after a two day recovery period (day 2), we observed that MTS absorbance still decreased after 4 hours of exposure in 100% Omnipaque, and decreased at concentrations of 25%, 50% and 100% Omnipaque after 48 hours of exposure (p<.05, Figure 3A, 3B).
Figure 3A.
Mitochondrial activity by MTS assays after 30min, 4 and 48 hours exposure and analyzed immediately.
Figure 3B.
Mitochondrial activity analysis by MTS assays after 30min, 4 and 48 hours exposure and analyzed 2 days later.
Effect of Omnipaque on mRNA Expression in MSCs
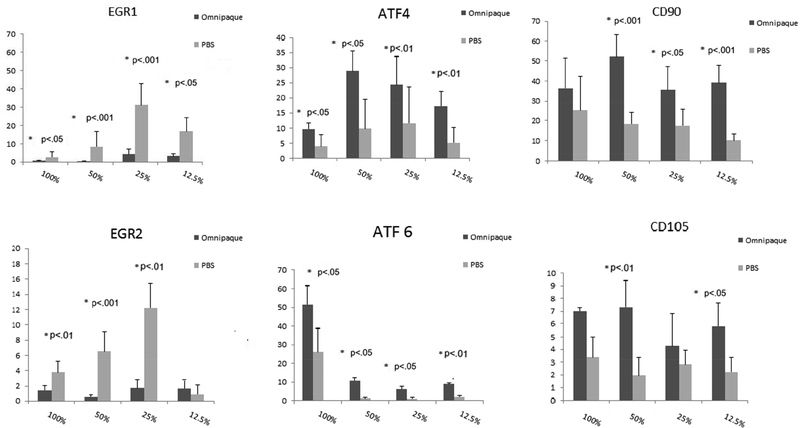
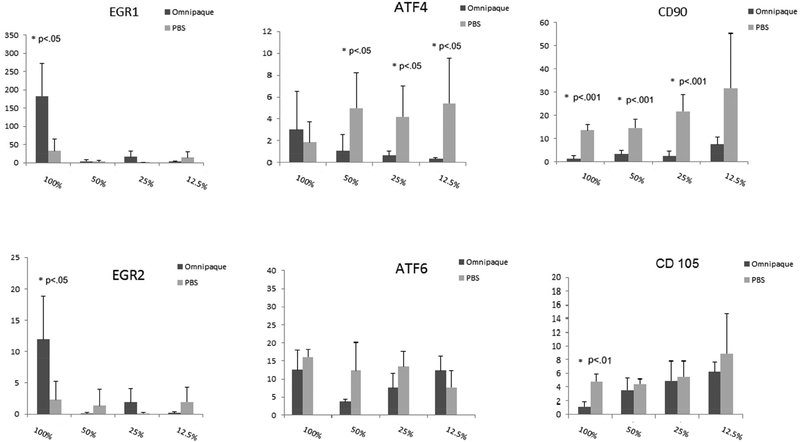
To investigate the molecular events that are triggered by Omnipaque exposure, we measured expression for a number of genes that are indicative of stress responses and/or cell proliferation during a 48-hour time course after initial exposure of 4 hours to Omnipaque exposure. We observed that the early growth responsive transcription factors EGR1 and EGR2 decreased immediately after exposure to a range of concentrations (respectively, 12.5% to 100% and 25% to 100%). We attribute this initial decrease in EGR1 and EGR2 primarily to their roles in cell cycle progression of human MSCs, and thus that their diminished mRNA levels reflect suppression of cell proliferation immediately after Omnipaque exposure. Reassuringly, the levels of EGR1 and EGR2 began to increase even after exposure to 100% Omnipaque and subsequently recovered for two days in normal growth media (Day 2). MSCs subjected to our test conditions expressed very robust levels of genes normally associated with response to endoplasmic reticulum stress and cytoprotective mechanisms, including ATF4 [14] and ATF6 [15]. The robust expression of ATF4 and ATF6 observed at the time of harvest (day 0) subsided after subsequent culture in growth medium (day 2). Examination of mRNA levels for two representative MSC surface markers (CD90 and CD105) revealed an increase in their expression on day 0 and decrease on day 2 after exposure (Figure 4A, 4B). This finding is consistent with a general decrease in expression of cell surface genes when MSCs initiate a phase of active proliferation [11].
Figure 4A.
Gene expression in human MSCs as analyzed by real-time quantitative RT-PCR analysis after exposure to Omnipaque for 4 hours and recovery for 4 hours and analyzed immediately.
Figure 4B.
Gene expression in human MSCs as analyzed by real-time quantitative RT-PCR analysis after exposure to Omnipaque for 4 hours and recovery for 2 days in culture.
Discussion
This is the first study to examine the impact of different concentrations and exposure times of nonionic iodinated contrast Omnipaque on the viability and function of in vitro, culture expanded human MSCs. The most important finding of the current investigation is that the viability, proliferative capacity and metabolic activity of MSCs are compromised following Omnipaque exposure in a concentration and time dependent manner. This cytotoxic effect does not occur after a brief 30min exposure, but is evident after longer exposures to Omnipaque. This time-dependent effect may need to be considered when using Omnipaque in clinical settings where MSCs are used for regenerative cell therapies.
Omnipaque is a nonionic, iodinated contrast medium commonly used for various intradiscal procedures such as in discography and endoscopic spinal surgery. In cell culture studies using kidney [6, 16] and lumbar disc cells [5], ionic contrast agents were significantly more cytotoxic than nonionic contrast dyes. The latter has raised our concerns whether contrast dyes would have similar negative effects on human MSCs. Recently, Ana Chee et al [17] found that iohexol is less cytotoxic as contrast agent than iopamidol that is also a nonionic iodinated contrast agent for intervertebral disc cells. In our studies with human MSCs, we did not test the cytotoxicity of ionic contrast dyes, but restricted our analysis to iohexol (Omnipaque 300), the agent that is widely used in fluoroscopically guided injections into the spine and peripheral joints.
Recently, Centeno et al [18] found that bone marrow-derived MSCs’s viability decreased only for the 80 mg/ml Omnipaque concentration after 4 hours exposure. As we know, it is the first report about the cytotoxicity of contrast medium to human mesenchymal stem cells. But there are several limitations in this study. First, there is little synovial fluid in normal and degenerative intervertebral disc, suggesting that the CM concentration in the local injection site probably very high. Centeno’s “high dose” is not high enough to mimic the situation in disc; second, the evaluation index for cell viability and response to contrast medium exposure is limited.
Omnipaque’s active ingredient is iohexol. Edwards found that iohexol is eliminated from peripheral joints after approximately 3 hours [19]. But there are no published data for Omnipaque clearance from the intervertebral disc. Clinical experience, suggests a longer half-life than for the knee, as patients are routinely imaged several hours after lumbar discography with contrast still visible on CT scan [20]. Meanwhile, there is little synovial fluid in normal and degenerative intervertebral disc, suggesting that the CM concentration in the local injection site probably very high. Importantly, our study used a 100% concentration of Omnipaque that was mixed together with human platelet lysate expanded MSCs in complete media with a volume ratio of 4:1. Thus, the final highest Omnipaque concentration is 80% (with final highest iodine content of 240 mg/ml). Our data indicate that this concentration clearly affects the biological properties of MSCs after 4 hours of exposure.
Specifically, live/dead cell analysis by Trypan Blue staining showed that the viability of human MSCs decreases not only at a 100% concentration (effective concentration 80% due to mixing) after 4 hours exposure, but also at concentrations of 50% and 100% after 48 hours of exposure. Hence, cytotoxicity of Omnipaque is both time and dose-dependent. Interestingly, MTS analysis showed that mitochondrial activity of human MSCs decreases for a range of concentrations (i.e., 25% to 100%) after only 4 hours of exposure when cells are immediately analyzed, and an even broader range when analyzed after 48 hours of exposure (i.e., 12.5% to 100%). Therefore, it appears that the metabolic activity of human MSCs is more sensitive and also concentration and time dependent.
Because metabolic activity is pre-requisite for cell proliferation, the MTS results provide a sensitive index for evaluating cell cytotoxicity that complements live/dead cell analysis. We note that metabolic activity decreased after 4 hours exposure to 50% Omnipaque and returned to normal levels after two-day recovery in culture. These results have two possible ramifications for clinical practice. First, to retain activity and viability human MSCs, the highest dose and exposure to be considered is 50% Omnipaque for 4 hours. Second, it may be beneficial to extend the treatment intervals between discography and stem cell injection therapy for disc degeneration. Although our focus was not on the joint, based on the reported kinematics of Omnipaque washout in joints, Omnipaque used at concentrations of less than 50% would not be expected to appreciably affect MSCs.
Reduced cell viability upon Omnipaque exposure is consistent with our observation that human MSCs mount a cellular stress/cytoprotective response based on expression of several factors (i.e., EGR1, EGR2, ATF4 and ATF6). Expression of EGR1 and EGR2 decreased immediately after Omnipaque exposure (day 0) and increased after recovery in normal cell culture mediam (Day 2). This finding indicates that cell cycle progression of human MSCs is acutely suppressed after Omnipaque exposure, but that cell proliferation can resume within two days in culture. Robust expression of ATF4 and ATF6, which are genes normally associated with response to endoplasmic reticulum stress and cytoprotective mechanisms, was observed at the time of harvest (day 0) and subsided by day 2. Stresses that trigger this response in mammals can include low temperature, hypoxia, ischemia, and oxidative stress [21]. To mitigate potential contributions from endoplasmic reticulum stress that can enhance expression of ATF, future studies may consider using biocompatible protective scaffolding (e.g., alginate) to mitigate the consequences of Omnipaque exposure.
CD90 and CD105 are well-established markers of multi-potent of adult mesenchymal stem cells [22]. Especially during stress conditions (e.g., hypoxia), CD105 gene expression is upregulated and is this may attenuate cell apoptosis [23]. CD90 and CD105 on the cell surface of MSCs are down regulated after differentiation [24,25]. In our study, CD105 expression was upregulated at day 0 culture. One interpretation of this result is that expression is upregulated as part of the stress response. Future studies will be required to confirm whether the stem cell phenotype of MSCs changes after Omnipaque exposure in view of our observation that both CD90 and CD105 were down regulated after short term culture (day 2).
Factors other than osmolality may contribute to the toxic effects of Omnipaque. Ionicity and/or molecular structure (monomeric or dimeric) may be of importance. Giulia found that there was no difference in the extent of cell injury between low-osmolality and iso-osmolality contrast media [26]. Ionic monomeric CM was the most hyperosmolar and was ultimately the most detrimental to cellular viability. Recently Kim et al [5] used mannitol as a non-ionic hyperosmolarity control and found that it had the least toxic influence on human disc cell viability. This indicates that hyperosmolarity alone cannot explain results that ionic monomeric CM is the most detrimental to cellular viability. Rather other physicochemical or biological parameters may dictate sensitivity of MSCs to Omnipaque.
We acknowledge several limitations of the current study that should be considered in the interpretation of the results. First, in vitro cytotoxicity may not adequately reflect in vivo conditions. The cytotoxicity of Omnipaque may differ according to the local environment, including the difference between cultured cells and cells in tissue, the integrated milieu of multiple cell types and tissues, as well as the state of joint or disc degeneration that may alter the pharmacokinetic properties of Omnipaque and/or the cellular response of cells to this contrast agent. Second, we examined in vitro cytotoxicity at stable concentrations over 2 days; although in vivo the Omnipaque concentration may gradually decrease over time, for example, by chemical breakdown or dissipation from the tissue. Although our study conclusively shows that high doses of Iohexol have irreversible cytotoxic effects on MSCs in cell culture after 4 hr of treatment, these effects are very concentration and time dependent. No significant effects are observed at 30 minutes which is a time period more relevant to the clinical setting. Also, dilution of iohexol suffices to mitigate these deleterious effects on MSCs. Furthermore, unlike cell culture studies where iohexol is in continuous contact with cells, it will dilute and dissipate upon injection in patients. Yet, we note that the use of iohexol in intervertebral discs may remain problematic because the compound may neither be diluted nor able to dissipate rapidly. Finally, while stem cell therapies for spine degeneration remain dependent on the use of contrast agents to ensure proper cell delivery, stem cell therapy involving peripheral joints is increasingly performed with ultrasound guidance and thus contrast agents are less of a concern in this practice.
We cultured MSCs with supportive growth media, which may have been more beneficial to them than the adverse environment of a joint or disk into which they might be injected. Hence, the next logical steps in experimentation are studies that examine the effects of human MSCs in explant culture systems ex vivo or in animal models in vivo.
Conclusion
The current in vitro study suggests that iohexol (Omnipaque 300) has direct time- and concentration-dependent cytotoxic effects on human MSCs. The in vitro differences in cytotoxicity study will serve as a foundation for future studies on the biological consequences of contrast media exposure in human MSC, as well as the optimization of CM concentration and treatment intervals between discography and mesenchymal stem cell injection therapy for spine degeneration. While we believe that iohexol could be avoided out of an abundance of caution, it appears safe for clinical procedures that necessitate the use of contrast agents in cell therapy. We recommend that if contrast agents are co-injected, that the lowest concentration will be used that ensures sufficient contrast. We also advise against pre-mixing because lengthy exposure of MSCs to contrast agents is not optimal for cell viability and as such may reduce the active dose of cells delivered during therapy
Acknowledgments
We thank the members of our laboratories, including Rebekah Samsonrah, Janet Denbeigh, Eric Lewallen, Roman Thaler, Emily Camilleri, Scott Riester and Amel Dudakovic, for stimulating discussions, sharing of reagents and/or providing mentorship to our trainees. These studies were supported by the Center of Regenerative Medicine at Mayo Clinic, as well as by the generous philanthropic support of William and Karen Eby, and the charitable foundation in their name.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Vos T, Flaxman AD, Naghavi M et al. Years lived with disability AND (YLDs) AND for 1160 sequelae of 289 diseases and injuries 1990–2010 AND a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380: 2163–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oehme D, Goldschlager T, Ghosh P, Rosenfeld JV, Jenkin G. Cell-Based Therapies Used to Treat Lumbar Degenerative Disc Disease : A Systematic Review of Animal Studiesand Human Clinical Trials. Stem Cells Int 2015; Epub 2015 May 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Orozco L, Soler R, Morera C, Alberca M, Sanchez A. Intervertebral disc repair by autologous mesenchymal bone marrow cells AND a pilot study Transplantation 2011; 92 : 822–828 [DOI] [PubMed] [Google Scholar]
- 4.Discography Stout A.. Phys Med Rehabil Clin N Am 2010; 21: 859–67. [DOI] [PubMed] [Google Scholar]
- 5.Kim KH, Park JY, Park HS, et al. Which iodinated contrast media is the least cytotoxic to human disc cells? Spine J 2015; 15:1021–1027. [DOI] [PubMed] [Google Scholar]
- 6.Haller C, Hizoh I. The cytotoxicity of iodinated radiocontrast agents on renal cells in vitro. Invest Radiol 2004; 39: 149–154. [DOI] [PubMed] [Google Scholar]
- 7.Ten Dam MA, Wetzels JF. Toxicity of contrast media: an update. Neth J Med 2008; 66: 416–422. [PubMed] [Google Scholar]
- 8.Haller C, Schick CS, Zorn M, Kubler W. Cytotoxicity of radiocontrast agents on polarized renal epithelial cell monolayers. Cardiovasc Res 1997;33 : 655–665 [DOI] [PubMed] [Google Scholar]
- 9.Itoh Y, Sendo T, Yano T, Saito M, Kubota T, Oishi R. Comparison of cellular mechanisms underlying histamine release from rat mast cells induced by ionic and nonionic radiographic contrast media. Invest Radiol 2004; 39: 455–461. [DOI] [PubMed] [Google Scholar]
- 10.Hizoh I, Strater J, Schick CS, Kubler W, Haller C. Radiocontrast-induced DNA fragmentation of renal tubular cells in vitro AND role of hypertonicity. Nephrol Dial Transplant 1998; 13: 911–918. [DOI] [PubMed] [Google Scholar]
- 11.Dudakovic A, Camilleri E, Riester SM, et al. High-resolution molecular validation of self-renewal and spontaneous differentiation in clinical-grade adipose-tissue derived human mesenchymal stem cells. J Cell Biochem 2014; 115: 1816–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dudakovic A, Camilleri ET, Lewallen EA, et al. Histone deacetylase inhibition destabilizes the multi-potent state of uncommitted adipose-derived mesenchymal stromal cells. Journal of cellular physiology 2015; 230: 52–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Netti GS, Prattichizzo C, Montemurno E, et al. Exposure to low- vs iso-osmolar contrast agents reduces NADPH-dependent reactive oxygen species generation in a cellular model of renal injury. Free Radic Biol Med 2014; 68: 35–42. [DOI] [PubMed] [Google Scholar]
- 14.Woo SR, Park JE, Kim YH, et al. SIRT1 suppresses activating transcription factor 4 AND (ATF4) AND expression in response to proteasome inhibition. J Microbiol Biotechnol 2013; 23 : 1785–1790. [DOI] [PubMed] [Google Scholar]
- 15.Hetz C The unfolded protein response: controlling cell fate decisions under ER stress and beyond. Nat Rev Mol Cell Biol 2012; 18:13:89–102. [DOI] [PubMed] [Google Scholar]
- 16.Schick CS, Haller C. Comparative cytotoxicity of ionic and non-ionic radiocontrast agents on MDCK cell monolayers in vitro. Nephrol Dial Transplant 1999; 14 : 342–347 [DOI] [PubMed] [Google Scholar]
- 17.Chee AV, Ren J, Lenart BA, Chen EY, Zhang Y, An HS. Cytotoxicity of local anesthetics and nonionic contrast agents on bovine intervertebral disc cells cultured in a three-dimensional culture system. Spine J 2014; 14: 491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Centeno CJ, Schultz JR, Cheever M, et al. Safety and complications reporting update on the re-implantation of culture-expandedmesenchymal stem cells using autologous platelet lysate technique. Curr Stem Cell Res Ther 2011; 6 : 368–378. [DOI] [PubMed] [Google Scholar]
- 19.Edwards SH, Cake MA, Spoelstra G, et al. Biodistribution and clearance of intra-articular liposomes in a large animal model using a radiographic marker. J Liposome Res 2007; 17 : 249–261. [DOI] [PubMed] [Google Scholar]
- 20.Kirkaldy-Willis WH, Hill RJ. A more precise diagnosis for lowback pain. Spine 1979; 4: 102–109. [DOI] [PubMed] [Google Scholar]
- 21.Mamady H, Storey KB. Coping with the stress: expression of ATF4, ATF6, and downstream targets in organs of hibernating ground squirrels.Arch Biochem Biophys 2008; 477:77–85. [DOI] [PubMed] [Google Scholar]
- 22.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for CellularTherapy position statement 2006; 8:315–317. [DOI] [PubMed] [Google Scholar]
- 23.Li C, Issa R, Kumar P, et al. CD105 prevents apoptosis in hypoxic endothelial cells. J Cell Sci 2003; 116:2677–2685. [DOI] [PubMed] [Google Scholar]
- 24.Delorme B, Ringe J, Gallay N, et al Y. Specific plasma membrane protein phenotype of culture-amplified and native human bone marrow mesenchymal stem cells. Blood 2008; 111: 2631–2635. [DOI] [PubMed] [Google Scholar]
- 25.Jin HJ, Park SK, Oh W, Yang YS, Kim SW, Choi SJ.Biochem Biophys Down-regulation of CD105 is associated with multi-lineage differentiation in human umbilical cord blood-derived mesenchymal stem cells. Res Commun 2009; 381: 676–681. [DOI] [PubMed] [Google Scholar]
- 26.Romano G, Briguori C, Quintavalle C, et al. Contrast agents and renal cell apoptosis. Eur Heart J 2008; 29:2569–2576. [DOI] [PubMed] [Google Scholar]