Abstract
Background/Objectives
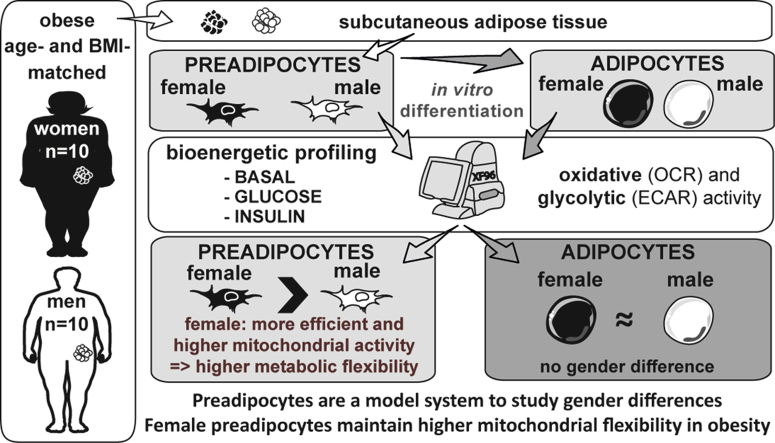
Although the prevalence of obesity and its associated metabolic disorders is increasing in both sexes, the clinical phenotype differs between men and women, highlighting the need for individual treatment options. Mitochondrial dysfunction in various tissues, including white adipose tissue (WAT), has been accepted as a key factor for obesity-associated comorbidities such as diabetes. Given higher expression of mitochondria-related genes in the WAT of women, we hypothesized that gender differences in the bioenergetic profile of white (pre-) adipocytes from obese (age- and BMI-matched) donors must exist.
Subjects/Methods
Using Seahorse technology, we measured oxygen consumption rates (OCR) and extracellular acidification rates (ECAR) of (pre-)adipocytes from male (n = 10) and female (n = 10) deeply-phenotyped obese donors under hypo-, normo- and hyperglycemic (0, 5 and 25 mM glucose) and insulin-stimulated conditions. Additionally, expression levels (mRNA/protein) of mitochondria-related genes (e.g. UQCRC2) and glycolytic enzymes (e.g. PKM2) were determined.
Results
Dissecting cellular OCR and ECAR into different functional modules revealed that preadipocytes from female donors show significantly higher mitochondrial to glycolytic activity (higher OCR/ECAR ratio, p = 0.036), which is supported by a higher ratio of UQCRC2 to PKM2 mRNA levels (p = 0.021). However, no major gender differences are detectable in in vitro differentiated adipocytes (e.g. OCR/ECAR, p = 0.248). Importantly, glucose and insulin suppress mitochondrial activity (i.e. ATP-linked respiration) significantly only in preadipocytes of female donors, reflecting their trends towards higher insulin sensitivity.
Conclusions
Collectively, we show that preadipocytes, but not in vitro differentiated adipocytes, represent a model system to reveal gender differences with clinical importance for metabolic disease status. In particular preadipocytes of females maintain enhanced mitochondrial flexibility, as demonstrated by pronounced responses of ATP-linked respiration to glucose.
Keywords: Oxidative phosphorylation, Glycolysis, Cellular metabolism
Graphical abstract
Highlights
-
•
Preadipocytes may represent a model system to study gender differences.
-
•
Female vs male preadipocytes show higher mitochondrial to glycolytic activity.
-
•
ATP-linked respiration of female preadipocytes is suppressed by glucose and insulin.
-
•
Female vs. male preadipocytes have higher metabolic flexibility via mitochondria.
-
•
Gender differences are not detectable in in vitro differentiated adipocytes.
1. Introduction
Obesity is characterized by reduced mitochondrial biogenesis and activity in several tissues including the white adipose tissue (WAT) [1]. Decreased mitochondrial function in white adipocytes leads to dysfunction of lipid storage and compromised endocrine function of WAT [2], [3]. These observations associate with obesity-induced metabolic complications such as insulin resistance [4]. Several studies demonstrate reduced mitochondrial content and activity of adipocytes from obese donors [5], [6], [7], [8], [9], independent of fat cell size [8], [9]. Furthermore, adipocytes from obese donors show lower oxygen consumption after β-adrenergic stimulation as compared to lean individuals [7]. Of note, gender-differences of lipid metabolism have been described [10], [11], [12], [13], showing that women exhibit higher lipolytic responses than men after epinephrine infusion [14]. Based on these results, it has been suggested that triglyceride synthesis rates in subcutaneous WAT (scWAT) of obese women are higher than in obese men [15]. Notably, these gender differences are reflected at the cellular level. Isolated adipocytes (subcutaneous and visceral) show gender-specific differences in basal and norepinephrine-stimulated lipolysis [13], [16]. Furthermore, the lipolytic capacity of adipocytes appears to be differentially modulated by obesity [16] and weight-reduction [11] in a gender-specific manner. Collectively, these gender-specific differences in lipid metabolism of WAT may be caused by distinct cellular metabolism. Molecular evidence supports the idea of gender differences in the mitochondrial function of fat cells: Higher gene expression related to electron transport chain (ETC) has been observed in the WAT of women, independently of fat distribution and sex hormones [17]. However, a detailed characterization of the cellular bioenergetics of preadipocytes and adipocytes, in particular distinguishing obese men vs. women is still lacking. These studies would reveal whether gender differences of cellular and mitochondrial bioenergetics exist.
The response of adipocyte bioenergetics to substrate supply (such as glucose) and hormonal control (e.g. insulin) may also be gender specific as adipocytes from female mice showed increased insulin sensitivity [10]. Furthermore, the maximally insulin-stimulated glucose uptake is higher in adipocytes from obese women as compared to obese men [18]. In human skeletal muscle, the modulation of bioenergetic parameters by insulin was shown in vitro [19] and in vivo [20], [21], [22], and may relate to clinical parameters such as HOMA-IR and insulin levels in a gender-specific manner [23]. Thus, we hypothesized the existence of gender-specific differences of the bioenergetic machinery in adipocytes, and its differential modulation by insulin. Taking advantage of new technologies simultaneously assessing in real-time mitochondrial and glycolytic activity in intact, undisturbed cell cultures, we determined the bioenergetic profile of preadipocytes and adipocytes from female and male donors. To assess the flexibility of cellular bioenergetics, we apply hypo-, normo- and hyperglycemic conditions, which are modulators of human adipocyte bioenergetics [24]. The responses to insulin are integrated in the experimental setup to determine acute insulin-induced changes in human white adipocyte respiration and glycolytic activity.
2. Material and methods
2.1. Subjects and cell culture
Subcutaneous preadipocytes (stromal vascular fraction, SVF) isolated from 20 metabolically characterized obese patients (10 males, 10 females (6 pre- and 4 post-menopausal), mean age: 41 (range: 26–62) years, mean BMI: 50 (range: 41–70) kg/m2), who underwent bariatric surgery at the University Hospital Tübingen between 2006 and 2010, have been tested for absence of HIV, HBV, HCV, and mycoplasma. Patients had not been on special diet prior to surgery. Details on donors' characteristics (Table S1) and medication (Table S2) can be found in the Appendix A. After expanding the SVF for two generations, cells were frozen in liquid nitrogen until further expansion and experiments. Cells (no visible contamination with epithelial or immune cells, third generation) were seeded and grown until confluency. Then, cells were either subjected to analysis as preadipocytes (d0) or in vitro adipogenic differentiation was induced as described previously [25]. 10 days after induction of differentiation, cells accumulated visible lipid droplets and were analyzed as adipocytes (d10). 24 h before RNA/protein isolation and bioenergetic profiling, cells were cultured in DMEM/F12 containing 0.5% FCS.
2.2. Determination of adipocyte number and DNA quantification
The number of adipocytes was estimated in the monolayers by direct counting using a net micrometer (Leica, Wetzlar, Germany). Cells were counted as adipocytes, when >5 lipid droplets were visible in the cell. DNA content as a surrogate for cell number per XF96-well was determined using picogreen Quanti-iT assay (ThermoFisher).
2.3. Gene expression analysis
RNA was harvested and isolated from preadipocytes and adipocytes using the RNeasy lipid tissue kit or miRNeasy Kit (Qiagen, Hilden, Germany). After reverse transcription (SuperScript, Invitrogen or Transcriptor cDNA Synthesis Kit, Roche), expression of genes together with the housekeeping gene RPS13 was analyzed with Viia realtime PCR or Roche Lightcycler.
2.4. Immunological detection of OXPHOS complexes and glycolytic enzymes
Preadipocytes and adipocytes were lysed (30 min at 4 °C), cleared by centrifugation and protein concentrations were determined using BCA protein assay (Pierce). 15 or 30 μg protein lysate were separated on a 4–12% BisTris gel (Invitrogen) and blotted onto a Nitrocellulose Membrane using iBlot (Invitrogen). Membrane was blocked for 1 h in Odyssey Blocking Buffer (LiCor, Lincoln, NE USA) followed by incubation with primary antibodies. Subsequently, IRDye® or AlexaFluor® secondary antibodies (LiCor or Abcam, Cambridge, England) were used and signals were detected using the Odyssey Sa or classic (LiCor). Following antibodies were use: MitoProfile® Total OXPHOS Human WB Antibody Cocktail (#ab110411, abcam), PFKP (D4B2, Cell Signaling), PKM2 (D78A4, Cell Signaling) and β-tubulin (Santa Cruz, Heidelberg, Germany or Abcam).
2.5. Energetic pathway studies
Preadipocytes (d0) and adipocytes (d10) were washed with XF assay medium containing 0 mM glucose (pH adjusted to 7.5) and incubated with indicated glucose concentrations (0, 5 and 25 mM) for 1 h in a 37 °C air incubator. The XF96 plate (Seahorse Bioscience, Agilent Technologies) was then transferred to a temperature-controlled (37 °C) Seahorse extracellular flux analyzer (Agilent Technologies) and subjected to an equilibration period. One assay cycle comprised a 1-min mix, 2-min wait, and 3-min measure period. Oxygen consumption rates (OCR) were analyzed as follows: after 4 basal assay cycles, medium (0, 5 or 25 mM) or medium (5 mM) with insulin (1 μM) was added by automatic pneumatic injection. After insulin stimulus, OCR and ECAR were recorded for 6 assay cycles (approximately 40 min), before oligomycin (1 μg/ml) injections were made to inhibit the ATP synthase for determination of OCR related to ATP synthesis. After 3 further assay cycles, carbonyl cyanide-4-(trifluoromethoxy)phenylhydrazone (FCCP; 0.5 μM) was injected to stimulate maximal respiration by protonophoric action. After another 3 assay cycles, rotenone (R, 4 μM) plus antimycin A (AA, 2 μM) was added followed by 4 assay cycles to determine the non-mitochondrial OCR. The lowest OCR measurement after addition of R/AA was subtracted from all other rates. To determine extracellular acidification rates (ECARs) deriving from glycolysis, the last injection also contained 2-deoxy-glucose (2DG, 100 mM). Lowest ECAR after last injection was subtracted from all ECAR values to obtain ECAR due to glycolysis. Coupling efficiency (CE) was calculated as the oligomycin-sensitive fraction of mitochondrial respiratory activity. Cellular respiratory control ratio (cRCR) is the ratio of maximal respiration to proton leak respiration. ATP production from OXPHOS and glycolysis was calculated as published previously [24]. OCR to ECAR ratio (OCR/ECAR) is the ATP-linked OCR divided by glycolytic ECAR. After the measurement, cells were lysed and total dsDNA amount per well was determined using Quant-iT PicoGreen dsDNA Assay Kit (Thermo Fisher). All rates were normalized to 50 ng dsDNA (∼mean DNA content/well of all donors) which is approximately 7600 cells assuming 0.0065 ng dsDNA per mammalian cell [26].
2.6. Statistics
For statistical comparison, which served to support the group differences shown in the figures, unpaired t-test (two-tailed) to compare male vs female group was performed or Mann–Whitney test, if data failed to meet assumption of normal distribution (Figure 1, Figure 2, Figure 4). To compare glucose effects and gender (Figure 3), two-way ANOVA (post-hoc: Bonferroni) were performed. To test effects of insulin, one-sample t-test to the value 1 was performed (Figure 4). p < 0.05 was considered statistically significant. Statistical tests were performed using Graph Pad Prism (GraphPad Software, Inc, La Jolla, CA, USA) and Sigma Plot 12.0 (Systat Software, Inc., San Jose, California, USA).
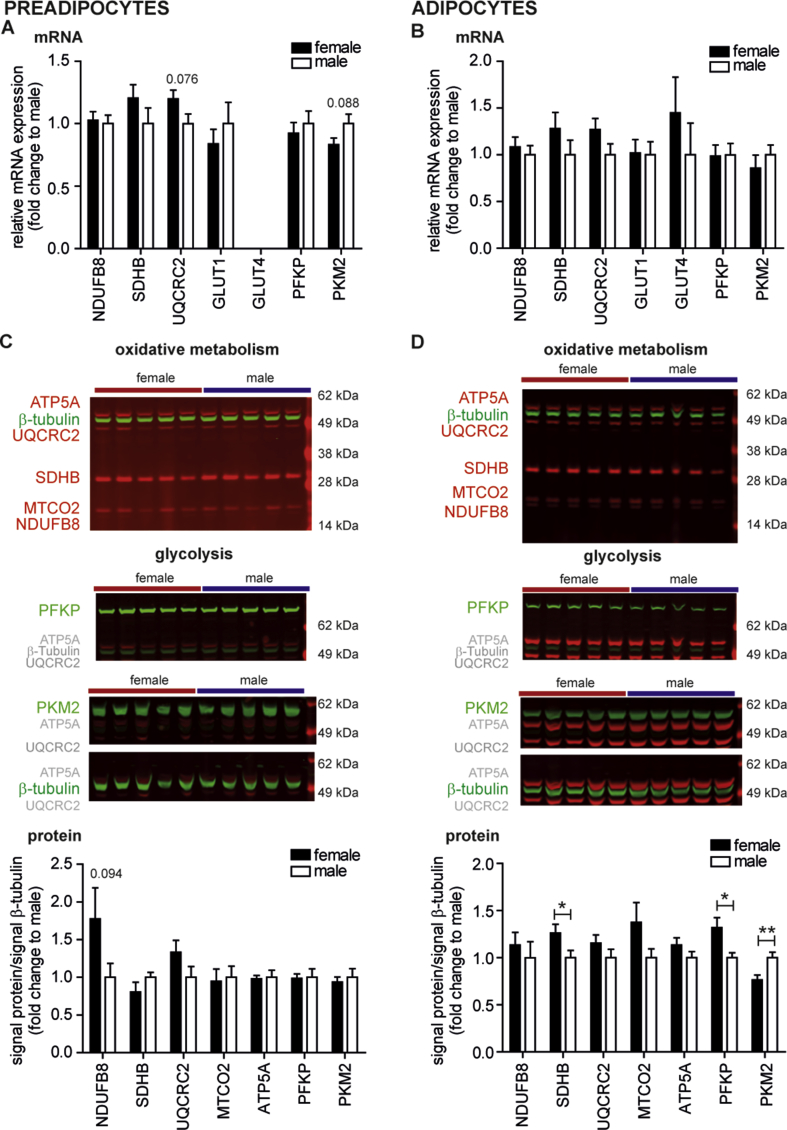
Figure 1.
The expression of genes/proteins involved in oxidative and glucose metabolism of male and female preadipocytes and adipocytes. (A, B) Total mRNA of preadipocytes (d0) and adipocytes (d10) were prepared and analyzed by qPCR. The relative mRNA expression of genes involved in oxidative metabolism (NDUFB8, SDHB, and UQCRC2) and glucose metabolism (GLUT1, GLUT4, PFKP, and PKM2) was normalized by ΔCt to housekeeper RPS13. Data are fold change to men and are the mean + SEM of 10 male and 10 female donors for which bioenergetic pathway analyses were performed. (C, D) Protein lysates of preadipocytes (d0) and adipocytes (d10) were prepared and analyzed by western blot. Representative western blots for preadipocytes and adipocytes of 5 female and 5 male donors using total OXPHOS human antibody cocktail, PFKP, PKM2 and β-tubulin antibody. Quantification of signals for OPXHOS antibodies (NDUFB8, SDHB, UQCRC2, MTCO2 and ATP5A) and glycolytic enzymes (PFKP and PKM2) presented as ratio to β-tubulin and as fold change to the mean of male signal/β-tubulin per membrane. Data are the mean + SEM of 10 male and 10 female donors for which bioenergetic pathway analyses were performed. * < 0.05, **p < 0.01.
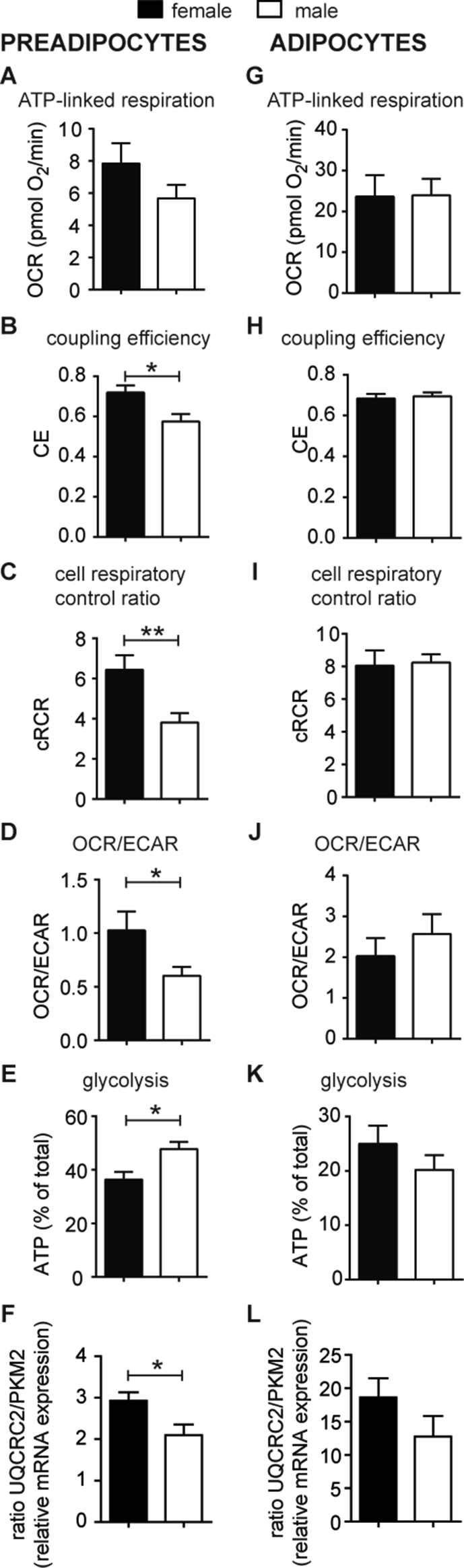
Figure 2.
The cellular metabolism of preadipocytes and adipocytes from obese women and men under normoglycemic conditions. (A–E, G–K) Oxygen consumption (OCR) and extracellular acidification (ECAR) after interference of energetic pathways with specific inhibitors were analyzed under normoglycemic (5 mM) conditions using a XF96 extracellular flux analyzer as described previously [24] and in Materials and Methods. All data were normalized to 50 ng dsDNA per well. OCR and ECAR traces vs time are shown in SI (Figs. S1 and S2). Mitochondrial respiration (Figs. S1G, S2F) was dissected into (A, G) ATP-linked respiration and proton leak respiration (Figs. S1H and S2H). Coupling efficiency (CE, (B, H)) and cell respiratory control ratio (cRCR, (C, I)) were calculated as described in Methods. The OCR/ECAR ratio (D, J) and the percentage of ATP produced by glycolysis (E, K) of male and female preadipocytes and adipocytes. (F, L) The mRNA levels of UQCRC2 and PKM2 mRNA levels were analyzed by qPCR, normalized by ΔCt to RSP13 (c.f. Figure 1) and are presented as ratio of UQCRC2 to PKM2 mRNA levels for preadipocytes and adipoctyes. All data are the mean of 10 men and 10 women + SEM. * <0.05, **p < 0.01.
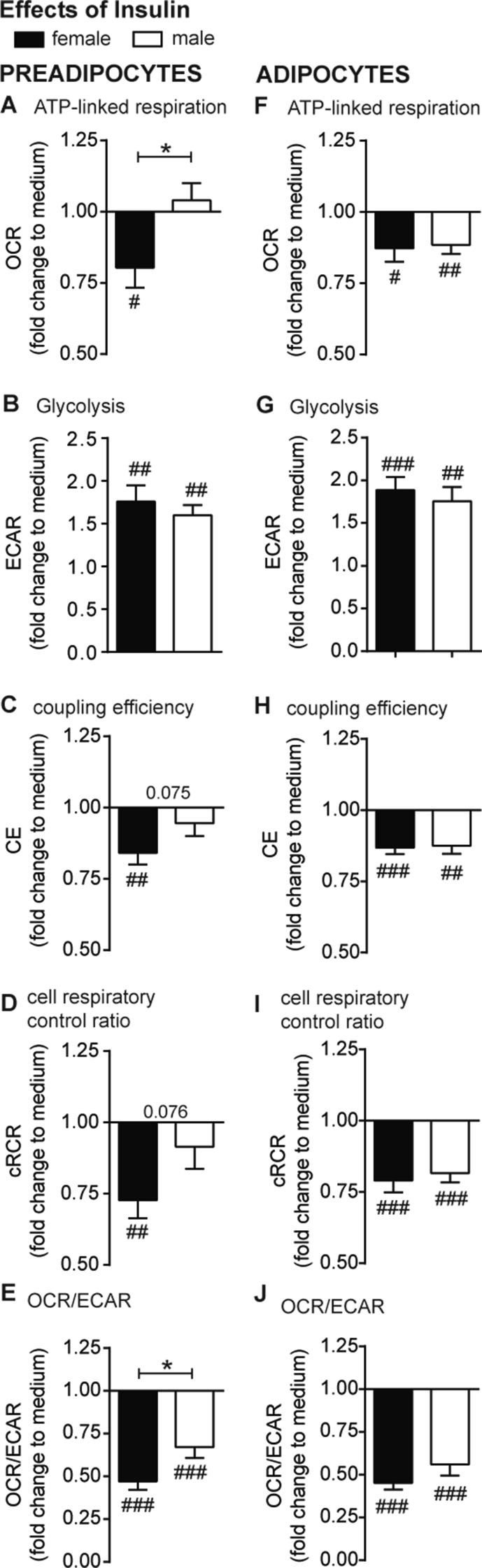
Figure 4.
Acute effects of insulin on the cellular metabolism of preadipocytes and adipocytes from obese men and women. OCR and ECAR were recorded and detailed dissection/analysis was performed as described in Method section. All data are presented as fold change to medium control (normoglycemic condition, c.f. Fig 2) of each donor. Insulin-induced changes in ATP-linked respiration (A, F), glycolysis (B, G), coupling efficiency (CE, (C, H)), cell respiratory control ratio (cRCR, (D, I)) and OCR/ECAR (E, J) are presented for preadipocytes and adipocytes. All data were normalized to 50 ng dsDNA per well and are the mean of 10 men and 10 women + SEM. Gender: *p < 0.05; insulin: #p < 0.05, ##p < 0.01, ###p < 0.001 vs basal.
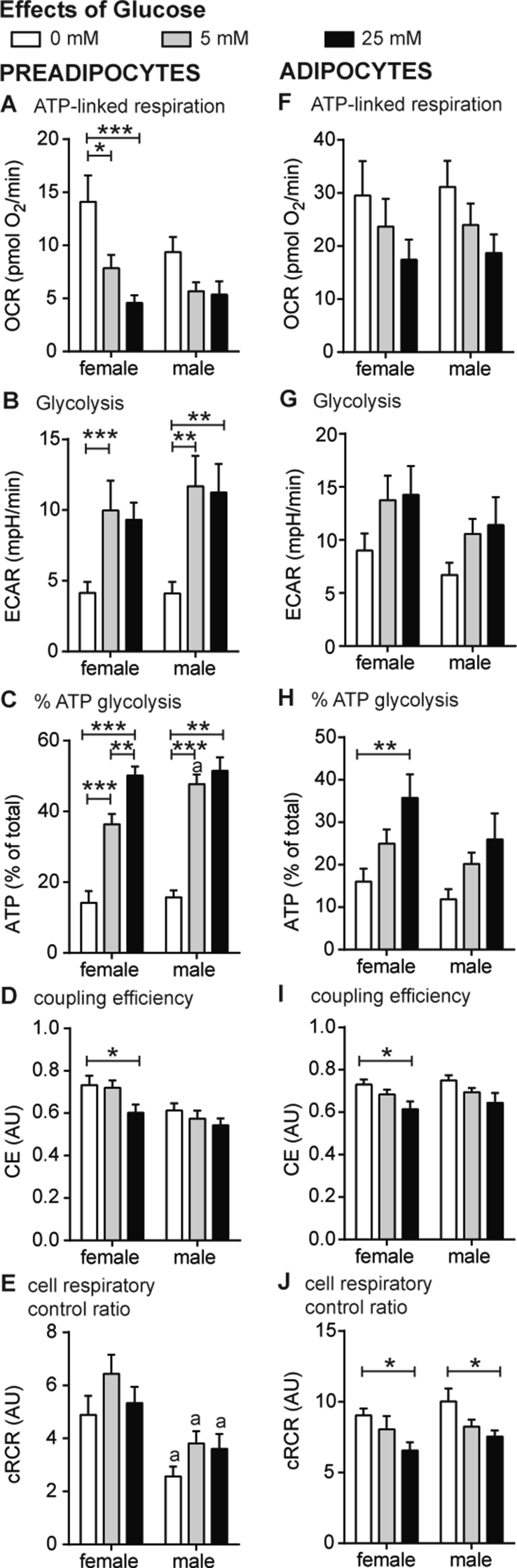
Figure 3.
The cellular metabolism of preadipocytes and adipocytes from obese women and men under hypo- and hyperglycemic conditions. (A–J) Oxygen consumption (OCR) and extracellular acidification (ECAR) after interference of energetic pathways with specific inhibitors were analyzed under hypo- (0 mM) and hyperglycemic (25 mM) conditions together with normoglycemic conditions (c.f. Figure 2) using a XF96 extracellular flux analyzer as described previously [24] and in Materials and Methods. OCR and ECAR traces vs time are shown in SI (Figs. S1 and S2). The effect of glucose on ATP-linked respiration (A, F), glycolysis (B, G), the percentage of ATP produced by glycolysis (C, H), coupling efficiency (D, I) and cRCR (E, J) are shown for preadipocytes and adipocytes. All data were normalized to 50 ng dsDNA per well and are the mean of 10 men and 10 women + SEM. Glucose: * < 0.05, **p < 0.01, ***p < 0.001. Gender: a p < 0.05 vs women.
3. Results
3.1. Electron transport chain (ETC) mRNA and protein expression reveal no major gender differences in preadipocytes and adipocytes
To study gender differences in the bioenergetics of fat cells from obese donors, we analyzed gene expression of subcutaneous preadipocytes (d0) and adipocytes (d10) of 10 obese women and 10 obese men matched for BMI and age (Table S1). We focused on genes encoding ETC components (NDUFB8, UQCRC2, SDHB) and UCP1, and genes involved in glucose uptake and glycolysis (GLUT1, GLUT4, PKM2, and PFKP). No significant differences were detected in ETC component and glycolysis-related mRNA levels between preadipocytes of female vs male donors. In female preadipocytes, UQCRC2 expression trended towards higher expression (p = 0.076) and PKM2 towards lower expression (p = 0.088) as compared to male preadipocytes (Figure 1A). The mRNA levels of ETC components and glycolysis related genes were not significantly different between adipocytes of female donors as compared with male donors (Figure 1B). GLUT4 expression was below detection limit (Ct > 35) in preadipocytes (Figure 1A), but robustly detectable in adipocytes (Figure 1B), confirming previous reports [27]. UCP1 mRNA levels were undetectable in preadipocytes and adipocytes.
The protein abundance of ETC components (NUDFB8, SDHB, UQCRC2, MTCO2, ATP5A) together with two rate-limiting enzymes of glycolysis (PFKP, PKM2) was not significantly different between gender in preadipocytes (Figure 1C). Adipocytes of women displayed higher protein levels of SDHB and PFKP, and lower levels of PKM2 (Figure 1D). Together, the gene expression and protein data of cultured adipocytes suggest no major differences of mitochondrial genes/proteins, contrasting published results on WAT [17].
3.2. Mitochondrial efficiency is higher in preadipocytes from obese women under normoglycemic conditions
To test if there are functional differences in cellular energy metabolism of preadipocytes from obese women compared to men, we analyzed bioenergetic function of preadipocytes under normoglycemic conditions. Total cell number per well was identical, as estimated by DNA content of preadipocytes (Fig. S1A, B). The traces for cellular oxygen consumptions rates (OCR) and acidification rates (ECAR) of preadipocytes (Fig. S1C, D) were analyzed for differences between women and men, as described in Methods and previous publications [24], [28]. No gender differences were found for non-mitochondrial respiration (Fig. S1E), maximal substrate oxidation capacity (Fig. S1F), and ATP-linked respiration (Figure 2A). ECAR values, which report glycolytic activity, were similar between obese women and men (Fig. S1I, J). Next, we calculated coupling efficiency (CE), cellular respiratory control ratio (cRCR) and OCR/ECAR ratio, to internally normalize the traces, enabling us to determine functional changes such as efficiency of energy turnover and switch of metabolic routes with confidence. CE was significantly higher in female vs. male preadipocytes (Figure 2B). Higher mitochondrial efficiency in energy turnover was further supported by significantly higher cRCR (p = 0.012) (Figure 2C). Estimating the total ATP production rates from the Seahorse data with known stoichiometries [24] revealed no differences between preadipocytes from men (50.4 pmol ATP/min ±7.9) and women (56.7 pmol ATP/min ±9.3). However, we observed that the mitochondrial contribution to ATP production was higher in preadipocytes of female donors, which was demonstrated in a significantly higher OCR/ECAR ratio (Figure 2D), and complementary, the proportion of glycolytic ATP production in female was significantly lower (Figure 2E). Notably, the higher ratio of oxidative to glycolytic activity in preadipocytes from women (Figure 2D,E) was also reflected in a higher ratio of UQCRC2 to PKM2 mRNA levels (Figure 2F).
3.3. Adipocytes of obese female and male donors display no differences in cellular energy metabolism
Adipogenic differentiation was induced in the preadipocytes of the identical donors and the differentiated adipocytes subjected to bioenergetic analyses under normoglycemic conditions. No differences in the rate of adipogenic differentiation were detectable between adipocytes from men and women (DNA content and adipocyte number per well (Fig. S2A–C)). As previously performed for preadipocytes, the identical bioenergetic parameters were determined for adipocytes. However, we found no significant gender differences of the bioenergetic parameters under basal, normoglycemic conditions (Fig. S2D–K and Figure 2G–K). Furthermore, differences in the ratio of UQCRC2 to PKM2 mRNA levels disappeared in differentiated adipocytes (Figure 2L).
3.4. Mitochondrial coupling efficiency (CE) and ATP-linked respiration respond to glucose in preadipocytes of obese women
Next, we studied the gender differences of preadipocyte energy metabolism in response to hyperglycemic (25 mM glucose) and hypoglycemic (0 mM glucose) conditions. We previously established the work-flow for in-depth bioenergetics in SGBS adipocytes, which represent a subcutaneous human fat cell model [24]. In this study, we apply this work-flow to compare the bioenergetic profile of preadipocytes from men and women under hypo-, normo- and hyperglycemic conditions (c.f. Figure 2). Preadipocytes from women show a wider scope to adapt mitochondrial energy metabolism to glucose. This was evident in the respiration rates linked to ATP synthesis, where ATP-linked respiration was significantly higher at hypoglycemic conditions (p = 0.034) (Figure 3A). Preadipocytes from both genders increased glycolytic rates with increasing glucose concentration (Figure 3B), which was reflected in significant changes of the OCR/ECAR ratio for preadipocytes from both genders (0 vs 25 mM men: 3.4 vs 0.5; p = 0.008; women 5.9 vs 0.6, p < 0.001). In total, high glucose levels provoked a higher contribution of glycolytic ATP to total ATP production (Figure 3C). Notably, preadipocytes from women significantly decreased CE under hyperglycemic conditions as compared to hypoglycemic condition (Figure 3D). Cellular respiratory control ratio (cRCR) was not significantly changed with increasing glucose concentrations, but preadipocytes from women showed higher cRCRs under all three conditions as compared to men (0 mM: p = 0.006; 5 mM: p = 0.003; 25 mM: p = 0.045) (Figure 3E).
Next, differentiated adipocytes were challenged with hypo-, normo- and hyperglycemic conditions. ATP-linked respiration decreased with increasing glucose concentration (Figure 3F; 0 mM vs 25 mM, p = 0.039), which was paralleled by trends of ECAR in the opposite direction (Figure 3G; 0 mM vs 25 mM, p = 0.057). Both parameters, however, show no gender differences. The relative contribution of glycolysis to ATP production (% ATP from glycolysis, Figure 3H) and CE (Figure 3I) were significantly different between hypo- and hyperglycemic condition in adipocytes of women (p = 0.004 and p = 0.027), but not of men. In contrast, differences of cRCR in response to hypo- and hyperglycemic conditions were found for both genders (Figure 3J).
Taken together, when challenged with hypo- or hyperglycemic conditions, preadipocytes only from women significantly decreased coupling efficiency and reduced ATP-linked respiration, demonstrating gender differences in the adaption of oxidative metabolism to glucose availability. These gender differences were pronounced in preadipocytes and not detectable in ex vivo differentiated adipocytes.
3.5. Distinct bioenergetics responses to insulin are specific for preadipocytes of obese women
Gender-dependent differences have been implicated in an important feature of adipocytes, i.e. insulin-stimulated glucose uptake [10], [18]. Thus, we integrated insulin stimulation during the bioenergetic analysis to investigate gender differences.
Insulin significantly suppressed ATP-linked respiration in preadipocytes of females (Figure 4A). Insulin treatment increased ECAR ∼1.7-fold in preadipocytes, suggesting insulin-induced glycolysis as expected. Interestingly, insulin-dependent glycolytic rates responded irrespective of gender (Figure 4B). Insulin action in preadipocytes from women was reflected in decreased CE (Figure 4C) and cRCR (Figure 4D). Notably, insulin decreased the OCR/ECAR ratio in preadipocytes, thereby indicating the switch from oxidative towards glycolytic metabolism. The OCR/ECAR was significant different between gender, and thus demonstrates increased flexibility of overall metabolism in female preadipocytes (∼−50% for women vs ∼–30% for men) (Figure 4E).
In differentiated adipocytes, we detected insulin-induced suppression of ATP-linked respiration for both genders (Figure 4F). In parallel, the ECAR increased almost 2-fold after insulin treatment independent of gender (Figure 4G). CE (Figure 4H), cRCR (Figure 4I) and OCR/ECAR (Figure 4J) significantly decreased after insulin treatment without gender-specific effects.
Taken together, the responses of mitochondrial bioenergetics to glucose and insulin, in particular ATP-linked respiration and CE, are more pronounced in preadipocytes from obese women, and thus more flexible, allowing higher metabolic plasticity.
Importantly, these gender-dependent differences are no longer detectable in differentiated adipocytes, suggesting that cell intrinsic differences between women and men vanish during adipogenic differentiation, at least in our experimental in vitro setting.
4. Discussion
In the present study we report on gender differences in the cellular energy metabolism of preadipocytes. In particular the differences in insulin-dependent glucose handling may have profound implications for gender-specific treatment of metabolic diseases.
Pronounced gender differences of ATP-linked respiration were found in preadipocytes from obese donors in response to glucose and insulin. The results from female donors suggest that preadipocytes display greater plasticity of oxidative metabolism that may be related to differences in anabolic or catabolic processes. Gender differences have also been found in other progenitor cells, e.g. muscle-derived stem cell (MDSC) and embryonic cells (neurons) from mice and rats, which display sex differences in the susceptibility to stressor-induced cell death [29], [30], [31].
Preadipocytes appear to be a more robust test system to interrogate gender-related hypotheses in relation to metabolism and metabolic diseases, contrasting (in vitro) differentiated adipocytes which showed no, or only minor, bioenergetic gender differences. We cannot exclude that the absence of gender differences may relate to commonly applied experimental conditions, using a hormonal cocktail to induce adipogenic differentiation that putatively overrides existing genetic and epigenetic differences. Thus, the absence of differences in the capacity of in vitro differentiation (Fig. S2A–C) could be due to experimental conditions. However, the differences seen in preadipocytes could potentially impact differentiation in vivo, contributing to gender differences in subcutaneous WAT cellularity/hyperplasia [32]. Vice versa, our observations in preadipocytes in vitro may be primed (e.g. epigenetic changes) by gender-specific differences of the in vivo WAT environment, including sex hormones and nutrients (e.g. glucose, Table S1). For example, whether the menstrual cycle has an impact in this study has not been assessed. Although we cannot formally exclude these confounding factors, it should be noted that the preadipocytes were cultivated for at least three generations in the medium with identical hormone and nutrient concentrations.
Obesity significantly disturbs WAT cellular metabolism [5]. Importantly, this bioenergetic fingerprint is preserved in in vitro differentiated adipocytes, contrasting vanished gender differences. In previous studies, we comprehensively characterized the bioenergetics of human SGBS adipocytes, which represents a “lean”, insulin-sensitive preadipocyte cell strain [24], [33]. Compared to SGBS cells, the ATP-linked respiration of in vitro differentiated adipocytes from obese donors was about 60% lower, suggesting impact of the obesity state on ATP turnover. The depression of ATP metabolism in obesity is further supported by data of Yeo and colleagues, who directly compared SGBS to in vitro differentiated subcutaneous adipocytes from obese donors, the latter showing lower mitochondrial activity and reduced lipid accumulation and insulin-stimulated glucose uptake [34]. Furthermore, isolated mitochondria from human primary (“floating”) adipocytes revealed strong BMI-dependent decreases of mitochondrial activity (measured as ATP-linked respiration) [8], [9]. In line with our observations, a study on basal heat production of primary (“floating”) adipocytes from lean vs obese women and men revealed no gender difference but decreased heat output by obesity of ∼50% [35]. Concerning mitochondrial differences between pre- and mature adipocytes, we calculated for SGBS adipocytes vs preadipocytes from our previously published data ∼4.4-fold higher oxygen consumption [24], which is in a similar range as data from von Heimburg and colleagues (who detected ∼4.8-fold higher respiration in adipocytes vs preadipocytes from lean donors) [36]. In the present study focusing on obese donors, cellular respiration in preadipocytes vs adipocytes increased only ∼3-fold (18 pmol O2/min/50 ng dsDNA vs 53 pmol O2/min/50 ng dsDNA), further supporting the idea that obesity disturbs cellular metabolism. Our data suggest that obesity-induced (epi-)genetic, molecular and metabolic perturbations remain in ex vivo differentiated adipocytes, despite the lack of potential in vivo gender differences. This also confirms previous studies of in vitro differentiated adipocytes, showing molecular differences for metabolic healthy vs unhealthy obese donors [37], together justifying the value of in vitro studies in adipocytes for metabolic disease.
To the best of our knowledge, we report for the first time on significant gender differences of oxidative to glycolytic activity ratios (OCR/ECAR) in preadipocytes from obese donors. This functional difference was also reflected on the gene expression level showing higher levels of UQCRC2 to PKM2 in women (p = 0.021, Figure 2F). However, other molecular bioenergetic markers do not reveal gender difference, contrasting analyses on whole WAT [17], indicating that there is either no robust link of mRNA/protein levels with function, in particular during acute exposure to hormones and nutrients, or that the gender-specific microenvironment created by hormones (e.g. adiponectin [38]) or inflammation (e.g. TNFa [39]) is strongly affecting the expression of bioenergetic markers.
The functional differences in preadipocyte energy metabolism may at least partially provide the basis for well-described systemic gender differences in substrate metabolism [40], [41], [42]. Females display higher net lipid oxidation than males in resting conditions [42]. In particular, when energy demand increases (e.g. during physical activity), women show a higher contribution of fat oxidation to total energy expenditure [40], [41]. For other tissues such as muscle, higher oxidative to glycolytic activity has been suggested for women [43], [44]. In obese and diabetic individuals, increased glycolytic to oxidative muscle metabolism has been reported [45], [46], indicating the link between insulin sensitivity and the balance of oxidative to glycolytic pathways. The over-proportioned reliance on glycolytic pathways, possibly due to compromised oxidative pathways, may be a hallmark of insulin resistance [45]. Our functional studies on adipose cells are comprehensive but would not allow for gender-stratified correlation analyses testing the link between function and clinical parameters at this stage, as the number of 20 donors is too low. Nevertheless, our data indicate that improvement of mitochondrial function and the higher oxidative to glycolytic ratio of preadipocytes from obese women could be beneficial. Improved glucose homeostasis and insulin sensitivity is more frequently observed in obese women as compared to age- and BMI-matched men [47], [48], [49], [50], [51], [52]. Our cohort showed trends towards higher insulin sensitivity in women (Appendix A, Table S1).
The acute insulin stimulus significantly lowered cRCR and coupling efficiency (Figure 4) in human adipocytes from women, contrasting insulin-effects in human myotubes where insulin acutely increases cRCR and coupling efficiency by decreasing proton leak respiration [19]. In adipocytes, neither basal nor FCCP-stimulated respiration rates were robustly altered by insulin (Figure 3A–D); thus, the changes in cRCR and CE are mainly due to changes in proton leak respiration (Fig. S3E,F). Increased proton leak respiration is either caused by changes in the conductance of protons or other ions at the mitochondrial inner membrane. However, similar to human myotubes [19] we found a robust increase of ECAR reporting glycolytic activity in response to insulin, that should also enhance glucose uptake. This is in line with reports on increased glycolytic activities in murine adipocytes [53], [54]. Therefore, extracellular flux analyses are instrumental to monitor insulin sensitivity, and offers simultaneous real-time measurements of both, glycolysis/glucose uptake and mitochondrial oxidation.
5. Conclusions
In summary, preadipocytes retain gender differences in vitro, and cells from obese women possess a higher metabolic flexibility involving oxidative metabolism. Metabolic flexibility may assist to sustain metabolic health better as age- and BMI-matched men. Therapies targeting obesity, adipose tissue and dysfunctional mitochondrial properties must consider gender differences.
Acknowledgments
This work was supported by a grant to M.K., L.B., and H.S. from the German Center for Diabetes Research (DZD) and (in part) by the Helmholtz Alliance ICEMED (Imaging and Curing Environmental Metabolic Diseases) and the Network Fund of the Helmholtz Association. Graphical abstract was created using modified components from Servier Medical Art (https://smart.servier.com).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2018.11.006.
Conflict of interest
The authors have nothing to disclose.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Kim J., Wei Y., Sowers J.R. Role of mitochondrial dysfunction in insulin resistance. Circulation Research. 2008;102(4):401–414. doi: 10.1161/CIRCRESAHA.107.165472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kershaw E.E., Flier J.S. Adipose tissue as an endocrine organ. Journal of Clinical Endocrinology & Metabolism. 2004;89(6):2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 3.Koh E.H., Park J.-Y., Park H.-S., Jeon M.J., Ryu J.W., Kim M. Essential role of mitochondrial function in adiponectin synthesis in adipocytes. Diabetes. 2007;56(12):2973–2981. doi: 10.2337/db07-0510. [DOI] [PubMed] [Google Scholar]
- 4.Guilherme A., Virbasius J.V., Puri V., Czech M.P. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nature Reviews Molecular Cell Biology. 2008;9(5):367–377. doi: 10.1038/nrm2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heinonen S., Buzkova J., Muniandy M., Kaksonen R., Ollikainen M., Ismail K. Impaired mitochondrial biogenesis in adipose tissue in acquired obesity. Diabetes. 2015;64(9):3135–3145. doi: 10.2337/db14-1937. [DOI] [PubMed] [Google Scholar]
- 6.Wilson-Fritch L., Nicoloro S., Chouinard M., Lazar M.A., Chui P.C., Leszyk J. Mitochondrial remodeling in adipose tissue associated with obesity and treatment with rosiglitazone. The Journal of Clinical Investigation. 2004;114(9):1281–1289. doi: 10.1172/JCI21752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yehuda-Shnaidman E., Buehrer B., Pi J., Kumar N., Collins S. Acute stimulation of white adipocyte respiration by PKA-induced lipolysis. Diabetes. 2010;59(10):2474–2483. doi: 10.2337/db10-0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fischer B., Schöttl T., Schempp C., Fromme T., Hauner H., Klingenspor M. Inverse relationship between body mass index and mitochondrial oxidative phosphorylation capacity in human subcutaneous adipocytes. American Journal of Physiology Endocrinology and Metabolism. 2015;309(4):E380–E387. doi: 10.1152/ajpendo.00524.2014. [DOI] [PubMed] [Google Scholar]
- 9.Yin X., Lanza I.R., Swain J.M., Sarr M.G., Nair K.S., Jensen M.D. Adipocyte mitochondrial function is reduced in human obesity independent of fat cell size. The Journal of Clinical Endocrinology and Metabolism. 2014;99(2):E209–E216. doi: 10.1210/jc.2013-3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Macotela Y., Boucher J., Tran T.T., Kahn C.R. Sex and depot differences in adipocyte insulin sensitivity and glucose metabolism. Diabetes. 2009;58(4):803–812. doi: 10.2337/db08-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kolehmainen M., Vidal H., Ohisalo J.J., Pirinen E., Alhava E., Uusitupa M.I.J. Hormone sensitive lipase expression and adipose tissue metabolism show gender difference in obese subjects after weight loss. International Journal of Obesity and Related Metabolic Disorders Journal of the International Association for the Study of Obesity. 2002;26(1):6–16. doi: 10.1038/sj.ijo.0801858. [DOI] [PubMed] [Google Scholar]
- 12.Lundgren M., Burén J., Lindgren P., Myrnäs T., Ruge T., Eriksson J. Sex- and depot-specific lipolysis regulation in human adipocytes: interplay between adrenergic stimulation and glucocorticoids. Hormone and Metabolic Research. 2008;40(12):854–860. doi: 10.1055/s-0028-1087168. [DOI] [PubMed] [Google Scholar]
- 13.Lönnqvist F., Thörne A., Large V., Arner P. Sex differences in visceral fat lipolysis and metabolic complications of obesity. Arteriosclerosis, Thrombosis, and Vascular Biology. 1997;17(7):1472–1480. doi: 10.1161/01.atv.17.7.1472. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt S.L., Bessesen D.H., Stotz S., Peelor F.F., Miller B.F., Horton T.J. Adrenergic control of lipolysis in women compared with men. Journal of Applied Physiology. 2014;117(9):1008–1019. doi: 10.1152/japplphysiol.00003.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edens N.K., Fried S.K., Kral J.G., Hirsch J., Leibel R.L. In vitro lipid synthesis in human adipose tissue from three abdominal sites. American Journal of Physiology Endocrinology and Metabolism. 1993;265(3):E374–E379. doi: 10.1152/ajpendo.1993.265.3.E374. [DOI] [PubMed] [Google Scholar]
- 16.Lofgren P., Hoffstedt J., Ryden M., Thorne A., Holm C., Wahrenberg H. Major gender differences in the lipolytic capacity of abdominal subcutaneous fat cells in obesity observed before and after long-term weight reduction. The Journal of Clinical Endocrinology & Metabolism. 2002;87(2):764–771. doi: 10.1210/jcem.87.2.8254. [DOI] [PubMed] [Google Scholar]
- 17.Nookaew I., Svensson P.-A., Jacobson P., Jernås M., Taube M., Larsson I. Adipose tissue resting energy expenditure and expression of genes involved in mitochondrial function are higher in women than in men. The Journal of Clinical Endocrinology and Metabolism. 2013;98(2):E370–E378. doi: 10.1210/jc.2012-2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Foley J.E., Kashiwagi A., Chang H., Huecksteadt T.P., Lillioja S., Verso M.A. Sex difference in insulin-stimulated glucose transport in rat and human adipocytes. American Journal of Physiology Endocrinology and Metabolism. 1984;246(3):E211–E215. doi: 10.1152/ajpendo.1984.246.3.E211. [DOI] [PubMed] [Google Scholar]
- 19.Nisr R.B., Affourtit C. Insulin acutely improves mitochondrial function of rat and human skeletal muscle by increasing coupling efficiency of oxidative phosphorylation. Biochimica et Biophysica Acta (BBA) Bioenergetics. 2014;1837(2):270–276. doi: 10.1016/j.bbabio.2013.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stump C.S., Short K.R., Bigelow M.L., Schimke J.M., Nair K.S. Effect of insulin on human skeletal muscle mitochondrial ATP production, protein synthesis, and mRNA transcripts. Proceedings of the National Academy of Sciences. 2003;100(13):7996–8001. doi: 10.1073/pnas.1332551100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kacerovsky M., Brehm A., Chmelik M., Schmid A.I., Szendroedi J., Kacerovsky-Bielesz G. Impaired insulin stimulation of muscular ATP production in patients with type 1 diabetes: mitochondria in type 1 diabetes. Journal of Internal Medicine. 2011;269(2):189–199. doi: 10.1111/j.1365-2796.2010.02298.x. [DOI] [PubMed] [Google Scholar]
- 22.Szendroedi J., Schmid A.I., Chmelik M., Toth C., Brehm A., Krssak M. Muscle mitochondrial ATP synthesis and glucose transport/phosphorylation in type 2 diabetes. PLoS Medicine. 2007;4(5):e154. doi: 10.1371/journal.pmed.0040154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bharadwaj M.S., Tyrrell D.J., Leng I., Demons J.L., Lyles M.F., Carr J.J. Relationships between mitochondrial content and bioenergetics with obesity, body composition and fat distribution in healthy older adults. BMC Obesity. 2015;2 doi: 10.1186/s40608-015-0070-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keuper M., Jastroch M., Yi C.-X., Fischer-Posovszky P., Wabitsch M., Tschop M.H. Spare mitochondrial respiratory capacity permits human adipocytes to maintain ATP homeostasis under hypoglycemic conditions. The FASEB Journal. 2014;28(2):761–770. doi: 10.1096/fj.13-238725. [DOI] [PubMed] [Google Scholar]
- 25.Berti L., Irmler M., Zdichavsky M., Meile T., Böhm A., Stefan N. Fibroblast growth factor 21 is elevated in metabolically unhealthy obesity and affects lipid deposition, adipogenesis, and adipokine secretion of human abdominal subcutaneous adipocytes. Molecular Metabolism. 2015;4(7):519–527. doi: 10.1016/j.molmet.2015.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dolezel J., Bartos J., Voglmayr H., Greilhuber J. Letter to the editor. Cytometry. 2003;51A(2):127–128. doi: 10.1002/cyto.a.10013. [DOI] [PubMed] [Google Scholar]
- 27.Hauner H., Röhrig K., Spelleken M., Liu L.S., Eckel J. Development of insulin-responsive glucose uptake and GLUT4 expression in differentiating human adipocyte precursor cells. International Journal of Obesity and Related Metabolic Disorders Journal of the International Association for the Study of Obesity. 1998;22(5):448–453. doi: 10.1038/sj.ijo.0800606. [DOI] [PubMed] [Google Scholar]
- 28.Keuper M., Sachs S., Walheim E., Berti L., Raedle B., Tews D. Activated macrophages control human adipocyte mitochondrial bioenergetics via secreted factors. Molecular Metabolism. 2017;6(10):1226–1239. doi: 10.1016/j.molmet.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Deasy B.M., Lu A., Tebbets J.C., Feduska J.M., Schugar R.C., Pollett J.B. A role for cell sex in stem cell–mediated skeletal muscle regeneration: female cells have higher muscle regeneration efficiency. The Journal of Cell Biology. 2007;177(1):73–86. doi: 10.1083/jcb.200612094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Penaloza C., Estevez B., Orlanski S., Sikorska M., Walker R., Smith C. Sex of the cell dictates its response: differential gene expression and sensitivity to cell death inducing stress in male and female cells. The FASEB Journal. 2009;23(6):1869–1879. doi: 10.1096/fj.08-119388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Du L., Hickey R.W., Bayir H., Watkins S.C., Tyurin V.A., Guo F. Starving neurons show sex difference in autophagy. Journal of Biological Chemistry. 2009;284(4):2383–2396. doi: 10.1074/jbc.M804396200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tchoukalova Y.D., Koutsari C., Karpyak M.V., Votruba S.B., Wendland E., Jensen M.D. Subcutaneous adipocyte size and body fat distribution. The American Journal of Clinical Nutrition. 2008;87(1):56–63. doi: 10.1093/ajcn/87.1.56. [DOI] [PubMed] [Google Scholar]
- 33.Wabitsch M., Brenner R.E., Melzner I., Braun M., Möller P., Heinze E. Characterization of a human preadipocyte cell strain with high capacity for adipose differentiation. International Journal of Obesity. 2001;25(1):8. doi: 10.1038/sj.ijo.0801520. [DOI] [PubMed] [Google Scholar]
- 34.Yeo C.R., Agrawal M., Hoon S., Shabbir A., Shrivastava M.K., Huang S. SGBS cells as a model of human adipocyte browning: a comprehensive comparative study with primary human white subcutaneous adipocytes. Scientific Reports. 2017;7(1):4031. doi: 10.1038/s41598-017-04369-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sörbris R., Nilsson-Ehle P., Monti M., Wadsö I. Differences in heat production between adipocytes from obese and normal weight individuals. FEBS Letters. 1979;101(2):411–414. doi: 10.1016/0014-5793(79)81056-x. [DOI] [PubMed] [Google Scholar]
- 36.von Heimburg D., Hemmrich K., Zachariah S., Staiger H., Pallua N. Oxygen consumption in undifferentiated versus differentiated adipogenic mesenchymal precursor cells. Respiratory Physiology & Neurobiology. 2005;146(2–3):107–116. doi: 10.1016/j.resp.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 37.Böhm A., Halama A., Meile T., Zdichavsky M., Lehmann R., Weigert C. Metabolic signatures of cultured human adipocytes from metabolically healthy versus unhealthy obese individuals. PloS One. 2014;9(4) doi: 10.1371/journal.pone.0093148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kern P.A., Saghizadeh M., Ong J.M., Bosch R.J., Deem R., Simsolo R.B. The expression of tumor necrosis factor in human adipose tissue. Regulation by obesity, weight loss, and relationship to lipoprotein lipase. The Journal of Clinical Investigation. 1995;95(5):2111–2119. doi: 10.1172/JCI117899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cartier A., Côté M., Lemieux I., Pérusse L., Tremblay A., Bouchard C. Sex differences in inflammatory markers: what is the contribution of visceral adiposity? The American Journal of Clinical Nutrition. 2009;89(5):1307–1314. doi: 10.3945/ajcn.2008.27030. [DOI] [PubMed] [Google Scholar]
- 40.Venables M.C. Determinants of fat oxidation during exercise in healthy men and women: a cross-sectional study. Journal of Applied Physiology. 2004;98(1):160–167. doi: 10.1152/japplphysiol.00662.2003. [DOI] [PubMed] [Google Scholar]
- 41.Tarnopolsky M.A. Sex differences in exercise metabolism and the role of 17-beta estradiol. Medicine and Science in Sports and Exercise. 2008;40(4):648–654. doi: 10.1249/MSS.0b013e31816212ff. [DOI] [PubMed] [Google Scholar]
- 42.Tran C., Jacot-Descombes D., Lecoultre V., Fielding B.A., Carrel G., Lê K.-A. Sex differences in lipid and glucose kinetics after ingestion of an acute oral fructose load. British Journal of Nutrition. 2010;104(08):1139–1147. doi: 10.1017/S000711451000190X. [DOI] [PubMed] [Google Scholar]
- 43.Lundsgaard A.-M., Kiens B. Gender differences in skeletal muscle substrate metabolism – molecular mechanisms and insulin sensitivity. Frontiers in Endocrinology. 2014;5 doi: 10.3389/fendo.2014.00195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Green H.J., Fraser I.G., Ranney D.A. Male and female differences in enzyme activities of energy metabolism in vastus lateralis muscle. Journal of the Neurological Sciences. 1984;65(3):323–331. doi: 10.1016/0022-510x(84)90095-9. [DOI] [PubMed] [Google Scholar]
- 45.Simoneau J.-A., Kelley D.E. Altered glycolytic and oxidative capacities of skeletal muscle contribute to insulin resistance in NIDDM. Journal of Applied Physiology. 1997;83(1):166–171. doi: 10.1152/jappl.1997.83.1.166. [DOI] [PubMed] [Google Scholar]
- 46.Simoneau J.A., Colberg S.R., Thaete F.L., Kelley D.E. Skeletal muscle glycolytic and oxidative enzyme capacities are determinants of insulin sensitivity and muscle composition in obese women. FASEB Journal Official Publication of the Federation of American Societies for Experimental Biology. 1995;9(2):273–278. [PubMed] [Google Scholar]
- 47.Kwon S.K. Women are diagnosed with type 2 diabetes at higher body mass indices and older ages than men: Korea national health and nutrition examination survey 2007-2010. Diabetes & Metabolism Journal. 2014;38(1):74–80. doi: 10.4093/dmj.2014.38.1.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gayoso-Diz P., Otero-Gonzalez A., Rodriguez-Alvarez M.X., Gude F., Cadarso-Suarez C., García F. Insulin resistance index (HOMA-IR) levels in a general adult population: curves percentile by gender and age. The EPIRCE study. Diabetes Research and Clinical Practice. 2011;94(1):146–155. doi: 10.1016/j.diabres.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 49.Alemzadeh R., Kichler J. Gender differences in the association of insulin resistance and high-sensitivity c-reactive protein in obese adolescents. Journal of Diabetes and Metabolic Disorders. 2014;13(1):35. doi: 10.1186/2251-6581-13-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walicka M., Bik W., Wolińska-Witort E., Marcinowska-Suchowierska E. Gender dependent dimorphism in adipokines levels and its correlations with insulin resistance in extremely obese patients. Postępy Nauk Medycznych. 2013;1(26):262–266. [Google Scholar]
- 51.Andersson D.P., Arner E., Hogling D.E., Rydén M., Arner P. Abdominal subcutaneous adipose tissue cellularity in men and women. International Journal of Obesity. 2017 doi: 10.1038/ijo.2017.148. [DOI] [PubMed] [Google Scholar]
- 52.Geer E.B., Shen W. Gender differences in insulin resistance, body composition, and energy balance. Gender Medicine. 2009;6:60–75. doi: 10.1016/j.genm.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cai W., Sakaguchi M., Kleinridders A., Pino G.G.-D., Dreyfuss J.M., O'Neill B.T. Domain-dependent effects of insulin and IGF-1 receptors on signalling and gene expression. Nature Communications. 2017;8:14892. doi: 10.1038/ncomms14892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Trefely S., Khoo P.-S., Krycer J.R., Chaudhuri R., Fazakerley D.J., Parker B.L. Kinome screen identifies PFKFB3 and glucose metabolism as important regulators of the insulin/insulin-like growth factor (IGF)-1 signaling pathway. Journal of Biological Chemistry. 2015;290(43):25834–25846. doi: 10.1074/jbc.M115.658815. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.