Abstract
Objective
The supramammillary nucleus (SuM) is nestled between the lateral hypothalamus (LH) and the ventral tegmental area (VTA). This neuroanatomical position is consistent with a potential role of this nucleus to regulate ingestive and motivated behavior. Here neuroanatomical, molecular, and behavior approaches are utilized to determine whether SuM contributes to ingestive and food-motivated behavior control.
Methods
Through the application of anterograde and retrograde neural tract tracing with novel designer viral vectors, the current findings show that SuM neurons densely innervate the LH in a sex dimorphic fashion. Glucagon-like peptide-1 (GLP-1) is a clinically targeted neuro-intestinal hormone with a well-established role in regulating energy balance and reward behaviors. Here we determine that GLP-1 receptors (GLP-1R) are expressed throughout the SuM of both sexes, and also directly on SuM LH-projecting neurons and investigate the role of SuM GLP-1R in the regulation of ingestive and motivated behavior in male and female rats.
Results
SuM microinjections of the GLP-1 analogue, exendin-4, reduced ad libitum intake of chow, fat, or sugar solution in both male and female rats, while food-motivated behaviors, measured using the sucrose motivated operant conditioning test, was only reduced in male rats. These data contrasted with the results obtained from a neighboring structure well known for its role in motivation and reward, the VTA, where females displayed a more potent response to GLP-1R activation by exendin-4. In order to determine the physiological role of SuM GLP-1R signaling regulation of energy balance, we utilized an adeno-associated viral vector to site-specifically deliver shRNA for the GLP-1R to the SuM. Surprisingly, and in contrast to previous results for the two SuM neighboring sites, LH and VTA, SuM GLP-1R knockdown increased food seeking and adiposity in obese male rats without altering food intake, body weight or food motivation in lean or obese, female or male rats.
Conclusion
Taken together, these results indicate that SuM potently contributes to ingestive and motivated behavior control; an effect contingent on sex, diet/homeostatic energy balance state and behavior of interest. These data also extend the map of brain sites directly responsive to GLP-1 agonists, and highlight key differences in the role that GLP-1R play in interconnected and neighboring nuclei.
Keywords: GLP-1, Supramammillary, Lateral hypothalamic area, Body weight, Operant conditioning
Highlights
-
•
SuM neurons densely innervate the lateral hypothalamus in a sex dimorphic fashion.
-
•
GLP-1 receptors are expressed throughout the SuM of both sexes.
-
•
Activation of SuM GLP-1R in male or female rats is potently anorexic.
-
•
However, SuM GLP-1R control food reward only in male rats.
-
•
SuM contributions to behavior and metabolism are contingent on sex and diet.
1. Introduction
The prevalence of obesity has continuously increased over the past decades, and already reached global epidemic proportions, yet the underlying mechanisms of this epidemic remain poorly understood [1], [2]. Control of feeding behavior, previously thought to be concentrated in the hypothalamus, is now well established to be distributed throughout the central nervous system (CNS) [3]. Understanding of all nodes and nuclei that contribute to integration of different aspects of feeding control will be necessary for development of novel and effective anti-obesity therapeutics, which to date has been a very elusive task.
Excessive food intake, especially of palatable calorically dense foods, is suggested to be the major culprit behind weight gain [4]. Food reinforcement, or motivation for food, predicts body weight gain and high body mass index in children and adults [5], [6]. The neural circuits underlying motivated behavior for food are partly overlapping but also partly divergent from those regulating homeostatic ingestive behavior [7], [8]. Despite the field's appreciation of these observations, the brain reward circuitry beyond the well-researched mesolimbic nodes, which include the ventral tegmental area (VTA) and nucleus accumbens, is poorly understood.
The supramammillary nucleus (SuM) is suggested to send bidirectional connections with brain regions already known to contribute to feeding regulation, including the hypothalamus, hippocampus, and lateral septum [9], [10]. These neuroanatomical nuclei and connections suggest that the SuM could regulate ingestive and motivated behavior. Yet, very few studies, if any, focus on the SuM. Despite decades old data indicating that the SuM is one of the most sensitive positive reinforcement sites in the brain [11], [12], this nucleus has a long history of being overlooked. In fact, a careful review of the reinforcement literature suggests that the VTA likely emerged as a key reinforcement hotspot at the expense of the SuM. Considering the neuroanatomical proximity of these two nuclei, pharmacological effects in the region, where infusion volumes often likely spanned both nuclei, have been nearly automatically ascribed to the VTA, which if reexamined does not hold up to careful anatomical scrutiny [9].
Glucagon-like peptide-1 (GLP-1) is a potent incretin hormone produced in the intestinal L-cells that potentiates the glucose metabolism-dependent secretion of insulin from pancreatic β cells [13]. GLP-1 is also produced in the brain, primarily by neurons in the nucleus tractus solitarius (NTS) of the hindbrain, which project throughout the central nervous system [14], [15], [16]. Two key functions of brain-acting GLP-1 analogues are reductions of feeding and motivated behavior. We and others have previously demonstrated that both ingestive and motivated behaviors can be suppressed by pharmacological activation of GLP-1R in the VTA [14], [17], [18], [19], [20]. Here we will compare the potential role that VTA and SuM GLP-1R play in food intake and reward in male and female rats.
No literature exists on the role of the SuM in female rats or mice. From the earliest evidence of SuM contribution to positive reinforcement and motivated behavior [12] to the more recent highly publicized findings on the role of SuM in arousal [21], only male rodents were studied. Here the role of the SuM in feeding behavior will be examined for the first time in female and male rats, with a specific aim to determine whether any sex differences exist in SuM-mediated feeding behavior regulation.
Our previous rodent brain imaging data suggest that the SuM is potently activated by peripherally applied GLP-1 agonists in male rats [22]. This activation is synergistically potentiated by conjugation of GLP-1 to estradiol, which allowed for activation of GLP-1 and estrogen receptors simultaneously [23]. These data suggested, but did not test, that activation of SuM in females could be more effective than in males, especially given that it is an estrogen sensitive site expressing both ERα and ERβ in females [24], [25]. Here we will directly test this hypothesis.
In this study we determine whether SuM downstream targets support a potential role in consummatory ingestive or motivated behavior by applying anterograde and retrograde neural tract tracing to detect SuM to lateral hypothalamus (LH) connections utilizing novel designer viral vectors [26]. To understand whether GLP-1-responsive cells are potentially present in the SuM we evaluate GLP-1R expression in the SuM, and also specifically on SuM LH-projecting neurons, using fluorescent in situ hybridization (RNA scope). We then set out to determine if GLP-1R activation in the SuM is necessary and sufficient for motivated and ingestive behavior control using pharmacological and virogenetic (AAV-shRNA) manipulation of SuM GLP-1R signaling in male and female rats. Finally, we determine if the answers to the above questions differ with sex and throughout the estrous cycle.
2. Materials and methods
2.1. Animals
Male and female Sprague–Dawley rats (3 weeks of age at arrival, Charles River, Germany) were housed under a 12-hour light/dark cycle, in individual cages with ad libitum access to chow and water, unless otherwise stated. All studies were carried out with ethical permissions from the Animal Welfare Committee of the University of Gothenburg, in accordance with legal requirements of the European Community (Decree 86/609/EEC). All efforts were made to minimize suffering.
2.2. Brain cannulation
A combination of ketamine (Ketaminol® Vet, Intervet International BV, AN Boxmeer, Holland) (18.75 mg/kg) and xylazine (Rompun® Vet, Bayer Animal Health GmbH, Leverkusen Germany) (2.5 mg/kg) were administered intraperitoneally to achieve surgical anesthesia. For retrograde adeno-associated virus vector (AAV)-assisted neural tract tracing 0.5 μL of a retrograde AAV vector expressing EGFP under the enhanced synapsin promoter; AAV2(Retro)-eSyn-EGFP (1.2 × 10ˆ13 GC/mL) (Vector Biolabs, Malvern, PA, USA) was injected unilaterally to the LH using the following coordinates in relation to the bregma suture; anterior-posterior: −3.3 mm and mediolateral: −1.5 mm and −9.0 mm dorsal to the surface of the skull at a speed of 0.1 uL/min using a Hamilton Neuros 10 μL syringe with a 33 gauge needle (Hamilton Co. Reno, NV, USA). For all neuropharmacology studies, as well as virus injections for the AAV-assisted anterograde mapping [27] (AAV2-hSyn-hM3D(Gq)-mCherry, Addgene, USA), guide cannulae were implanted into the SuM using the following coordinates adapted from [22]: on the midline, 4.7 mm posterior to bregma, and 7.1 mm ventral from the surface of the skull, with injector aimed 9.1 mm ventral to the skull (representative images in Figure 1C and Figure S1). These coordinates were chosen to position the tip of the injector on top of the SuM, yet as far away as possible from other nearby GLP-1R expressing sites like the VTA or interpeduncular nucleus [28]. As a result of this strategy our manipulation may not have reached the most caudal tip of SuM, while consistently reaching the rostral and central SuM. Interestingly the border between the SuM and the mammillary nuclei was largely impermeable to the injection liquid, thus the injection delivered on the midline tended to spill laterally covering both medial and lateral SuM but rarely the ventral, mammillary region; this pattern is clearly visible in Figure 1C. Since SuM overlaps with the VTA on the rostro-caudal axis (VTA is still lateral and dorsal to the SuM) we also inserted cannulae at the level of the VTA in order to compare and differentiate GLP-1R responses in these two neighboring sites (coordinates: ±0.75 mm from midline, 5.7 mm posterior to bregma, and 6.5 mm ventral from the surface of the skull, with injector aimed 8.5 ventral to skull [20]).
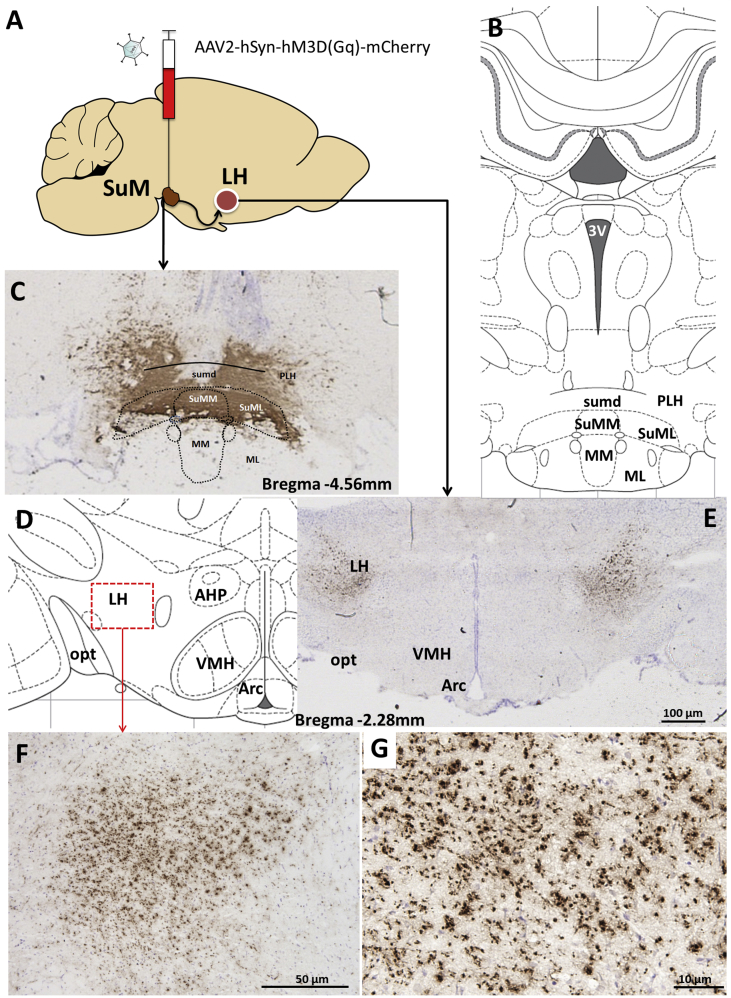
Figure 1.
Neuroanatomical basis for SuM ingestive or motivated behavior control: SuM projections were identified in the lateral hypothalamus (LH) of male rats, a brain area key in energy balance and motivated behavior control. AAV2-mCherry anterograde tracer allowed for detection of dense SuM-originating fibers in the LH (schematic image displayed in A). Rat brain atlas image at bregma level −4.56 mm (B). The viral injection and infection at the level of the SuM encompassed medial (SuMM) and lateral (SuML) SuM as well as large part of dorsal SuM (sumd) with minimal spread to the mammillary region. Neuronal fibers originating in the SuM were detected in the lateral regions of LH in both left and right LH (D–E). Magnification of the LH-detected fibers is shown in F and G. Visualization of the mCherry signal is enhanced by DAB-based immunohistochemistry hence mCherry labeled injection area and fibers in the LH appear brown. Posterior lateral hypothalamus: PLH; 3V: third ventricle; medial mammillary nuclei, comprising pars medialis (MM) and pars lateralis (ML) subdivisions; Arc: arcuate nucleus of the hypothalamus; VMH: ventromedial hypothalamus; AHP: anterior hypothalamic area, posterior; opt: optic tract.
2.3. Neural tract tracing: brain tissue preparation and imaging
Three weeks after viral infusions, animals received a terminal dose of a mixture of ketamine (Ketaminol® Vet, 37.5 mg/kg) and xylazine (Rompun® Vet, 5 mg/kg) prior to the procedure. First, the heart was exposed and prepared with a cannula attached to a perfusion pump (Watson 120S, Watson-Marlow Fluid Technology Group, Wilmington, MA, USA) in order to rinse the circulating blood with filtered saline (speed 15 mL/min) for 5 min and then a fixative (filtered 4% paraformaldehyde-PBS solution) in a volume equal to the body weight at 15 mL/min. Brains were isolated and incubated in 15% Sucrose 4% PFA-PBS solution overnight and placed in 30% sucrose-PBS solution until saturation. Samples were frozen on CO2-ice prior to sample collection with a Leica 3050S cryostat (Leica Biosystems Nussloch GmbH, Nussloch, Germany).
Anterograde: 10 μm sections containing brain regions of interest were stained by immunohistochemistry for mCherry which was targeted to neurons with the use of AAV2-hSyn-hM3D(Gq)-mCherry. Sections were washed with Tris-buffered saline (TBS). Endogenous peroxidases were inhibited by incubating the sections in 3% hydrogen peroxide diluted in methanol for 20 min. Sections were permeabilized for 10 min and non-specific binding was blocked with 10% normal donkey serum (NDS), 1% bovine serum albumin (BSA) and 20% avidin in TBS with 0.1% Tween-20 (TBST) for 60 min. Sections were then incubated in primary antibody overnight at 4 °C (rabbit anti-mCherry, Abcam ab167453) diluted 1/325 in TBST with 10% NDS, 1% BSA and 20% biotin. On the following day, sections were incubated with the Impress anti-rabbit secondary antibody (Vector, MP-7451) for 30 min at RT. The binding was amplified by incubating sections in biotin-tyramide solution for 7 min followed by HRP-conjugated streptavidin for 45 min (both supplied in the Tyramide SuperBoost Kit with AlexaFluor Tyramides, Invitrogen, B40931). The binding sites were revealed by incubating for 3.5 min in 0.05% 3,3‘-diaminobenzidine (DAB; Sigma–Aldrich), 0.05% ammonium II nickel sulphate and 0.05% H2O2. Sections were counterstained with cresyl violet and coverslipped with DPX mounting medium (Sigma–Aldrich). Staining was visualized on a Leica DM600B microscope and images were captured with an Optronics MicroFire Microscope camera.
Retrograde: 20 μm coronal slices were collected on SuperFrost + sample glasses (Thermo scientific, Waltham, MA, USA) and stored at −80 °C. DAPI (4′,6-diamidino-2-phenylindole), a blue-fluorescent DNA stain, was used to visualize cell nuclei. Glass slides with samples were mounted after drying at RT for 20 min using Vectashield H-1200 mounting medium (Vector Labs, Burlingame, CA, USA) and analyzed using a Zeiss LSM700 confocal microscope (Carl Zeiss Microscopy GmbH, Jena, Germany). 10× tile images of three consecutive slices at each coronal level (bregma −4.4, −4.55, and −4.7 mm) were taken in multiple layers of 1–3 μm (5–6 levels) in order to convert the images with the Maximum Projection-software (Zen software, Carl Zeiss Microscopy GmbH, Jena, Germany) for increased depth range.
Cell counting: Coronal brain atlas figures (Paxinos & Watson 5th edition) were superimposed on the fluorescent images of the SuM sections (9 sections were analyzed for each subject, a total of four rats, two males and two females were included in this analysis) according to the corresponding antero-posterior level using GIMP-software (www.gimp.org). After addition of a transparent image layer, red circles were plotted on top of neurons, defined by spherical EGFP expression greater than 70 μm2 [29]. Images containing only the plotted circles in the specific parts of the SuM were exported as jpeg images for analysis. Fiji software [30] was used to automatically count the plotted circles after first conversion of the images into 8-bit gray scale and then by running the particle analysis program.
2.4. In situ hybridization using RNAScope
To localize GLP-1R in the SuM, in situ hybridization for GLP-1R was performed utilizing the RNAscope Multiplex Fluorescent kit (Advanced Cell Diagnostics). Briefly, fresh frozen 12 μm thick SuM-containing brain sections were fixed in 10% formalin (ThermoFisher Scientific, Waltham, MA) for 30 min at 4 °C. Following 2 washes in 1X PBS, brain slices were dehydrated in 50% (5 min), 70% (5 min) and 2 × 100% (5 min each) ethanol and treated with protease solution (pretreatment IV) at RT for 1 h. The protease was washed away with 3 × PBS for a total time of 15 min. Target probe for GLP-1R (Rn-Glp1r-315221) was applied directly on the sections to cover them completely and incubated at 40 °C for 2 h in the HybEZ oven. Next, slides were incubated with preamplifier and amplifier probes (AMP1, 40 °C for 30 min; AMP2, 40 °C for 15 min; AMP3, 40 °C for 30 min), and then with fluorescently labeled probes for GLP-1R (red Alexa-555). Finally, brain sections were incubated for 30 s with DAPI (shown in blue) and mounted with mounting medium for fluorescence (H1000, Vector Laboratories, USA). Fluorescent images of coronal brain sections containing SuM from one male and one female were captured using a confocal microscope (Zeiss LSM700, Germany) and processed with Zen lite software. For co-localization of GLP-1R mRNA and the retrograde EGFP signal 20 μm sections obtained from the neuronal tract tracing experiment (one male and one female rat) were processed as described above for GLP-1R RNAscope. Thus in total RNAscope procedure was performed in two males and two females.
2.5. Real time PCR
Brains were collected from 17-week-old rats, frozen, and placed in the cryostat. SuM, LH, nucleus accumbens (encompassing both the shell and the core regions), and NTS were collected using disposable biopsy punches with plungers (INTEGRA, USA) to assess GLP-1R expression using quantitative real-time PCR (qPCR). RNA was isolated using the RNeasy Lipid Tissue Mini Kit (Quiagen, Germany) and GLP-1R levels were quantified using Taqman gene expression kits from Life Technologies using beta actin as the housekeeping gene (Primer information: GLP-1R: Rn00562406_m1; beta actin: RN00667869_m1). Comparative threshold cycle method [31] was used to quantify relative mRNA expression. For both sexes SuM GLP-1R expression relative to beta actin was set as 1 to visualize the expression in SuM relative to other brain regions.
2.6. Drugs
The GLP-1R agonist exendin-4 (Ex4) and GLP-1R antagonist exendin-9 (Ex9) were purchased from Tocris (Bristol, UK), dissolved in artificial cerebrospinal fluid (aCSF; Tocris; used as vehicle), and stored as aliquots at −20 °C. For testing responses to Ex4 all rats were given 50% of their normal chow intake overnight in order to make sure that sufficient level of ingestion is achieved for it to reveal a hypophagic response. Ex4 was infused into the SuM at two doses, 0.01 and 0.03 μg, in a volume of 0.3 μl early in the light phase and testing was conducted 20 min after. To test the effect of Ex9, the rats were fasted overnight and then given a meal of chow for 20 min. Directly after this meal, the rats received 10 μg of Ex9 (0.3 μl) and were tested 10 min after. All pharmacological treatment experiments were performed within subject, and drug and vehicle injections were applied in a counterbalanced (Latin square) design. Thus for all pharmacological treatment studies, the number of subjects in the vehicle and drug group is the same (always tested within subject). All pharmacological testing, except during obesogenic diet maintenance, was performed on naïve (untreated but trained for the operant condition procedure as describe below) SuM-cannulated rats or VTA-cannulated rats. The Ex4 effect on obesogenic diet was tested in control and knockdown rats (described below).
2.7. Operant conditioning
The operant conditioning procedure is used to assess the motivation to obtain a reward, in this case food reward in the form of a 45 mg sucrose pellet. Training and testing were conducted in rat conditioning chambers (Med-Associates, Georgia, VT, USA) as described previously [18], [32]. All operant response testing was performed under the progressive ratio schedule (PR) in 60 min sessions.
2.8. Locomotor activity
Locomotor activity was measured during the PR testing procedure with infrared sensors located 3 cm from the floor, which allow for detection of movement in the horizontal plane.
2.9. Determination of estrous cycle phases
Estrous cycle was assessed by microscope examination of unstained smear preparations collected from the females each morning or immediately after operant conditioning on days where operant test was performed. Cycle phase was also later confirmed after Papanicolaou smearing and hematoxylin and eosin staining as described previously [33].
2.10. GLP-1R knockdown
To knockdown the expression of the GLP-1R in the SuM, a short hairpin RNA (shRNA) targeting GLP-1R transcripts, was used (for details see [34]). Preliminary in vitro studies demonstrated ∼88% knockdown of GLP-1R expression in a rat neuronal cell line transfected with this shRNA [34]. To knockdown GLP-1R expression in vivo, this shRNA sequence was cloned and packaged into an adeno-associated virus (AAV) (serotype 1; titer = 5.22e12) in collaboration with the Viral Vector Core at the University of Pennsylvania. We have previously shown that this construct reduces GLP-1R expression in vivo, for example in the rat VTA and LH by 50% [34], [35]. A GFP-expressing AAV (titer = 5.0e12) was used as a control. To determine the functional significance of endogenous GLP-1R signaling in the SuM, rats were surgically implanted with SuM-directed guide cannulae as described above for neuropharmacology experiments. Once rats achieved stable sucrose motivated behavior on PR, AAV-expressing GFP (AAV-GFP) or the GLP-1R shRNA (AAV-GLP-1R-shRNA) was infused into the SuM (0.3 μl over 3 min). Microinjectors were left in place for 10 min after infusion to allow for diffusion away from the injection site. Rats chosen for each treatment group were matched for body weight, food intake, and food reward parameters on PR. The averages for each treatment group prior to AAV infusions were as follows: female controls body weight 253 ± standard deviation of 19.4 g (n = 9) and knockdown 253 ± 17.6 g (n = 9) (p = 0.9); male controls body weight 398 ± 45 g (n = 8) and knockdowns 399 ± 37 g (n = 10) (p = 0.9); female controls sucrose pellets earned in PR 10 ± 2 and knockdowns 10 ± 3 (p = 0.9); male controls sucrose pellets earned in PR 10 ± 2 and knockdowns 10 ± 3 (p = 0.9). For PR the average of the last three sessions before the shRNA injection was taken to obtain the most stable value for the group divisions. Body weight and food intake were measured daily after AAV construct infusion for four weeks. In addition, motivated behavior for food (PR operant test) was tested at 21 days after infusion for rats maintained on chow and at day 21 of new diet exposure for rats maintained on the obesogenic diet. All rats were offered the obesogenic diet four weeks after AAV infusion. At the start of the obesogenic diet exposure the body weight of female controls was 308 ± standard deviation of 20 g (n = 9) and knockdowns 301 ± 28 g (n = 10) (p = 0.5). The starting body weight for male controls was 525 ± 33 g (n = 9) and knockdown 537 ± 59 g (n = 9) (p = 0.6).
2.11. HFHS diet
In order to test the contribution of SuM GLP-1R to food intake, food motivation, as well as body weight control under conditions challenging homeostasis, we exposed SuM GLP-1R knockdown rats to a high fat and sugar choice diet (a choice of lard, 30% sucrose solution, and chow). This diet was also used as the obesogenic maintenance diet during the AAV-shRNA experiment described above.
2.12. Adipose tissue collection
Inguinal (subcutaneous) adipose tissue was dissected upon termination of the knockdown experiment by creating a wide skin incision on the abdominal side, removing the skin, and subsequently removing the fat from the underlying muscle layer with the inclusion of the dorsal lumbar pad. After removing the pad, any lymph nodes present among the fat were removed. Dorsal subcutaneous pad was not included in this dissection. The gonadal fat pad was dissected by opening the abdominal wall and extracting the genitals (ovaries or testes, according to the sex) from the abdominal cavity. The fat was then carefully separated from the surrounding tissues.
2.13. Statistical analysis
All the data are presented as mean ± SEM. Statistical significance was analyzed using Student's t test for comparisons of two groups, or one- or two-way ANOVA with post-hoc Holm–Sidak tests when appropriate (GraphPad Prism 7 Software, Inc). P values lower than 0.05 were considered statistically significant.
3. Results
3.1. SuM innervates the lateral hypothalamus in male and female rats
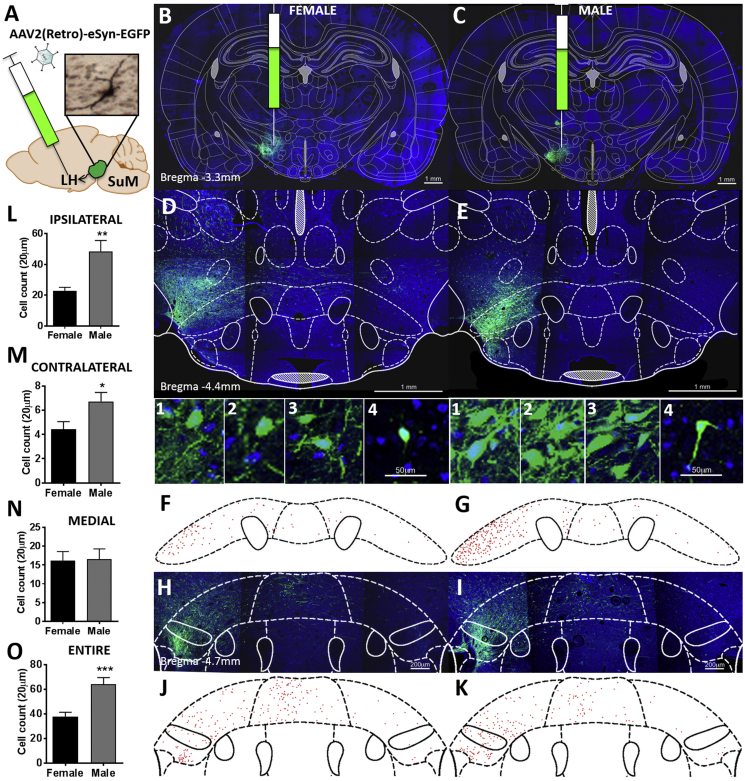
AAV2, a viral vector primarily taken up at the region of dendrites and cell bodies [27], was applied for anterograde mapping originating in the SuM and encompassing both lateral and medial SuM in a male rat. It indicated a dense bundle of fibers specifically in the LH (Figure 1). This strongly suggested that SuM neurons innervate the LH. However it does not unequivocally show that these are terminating fibers; moreover, it does not differentiate the regional distribution of projection neurons within the SuM. In order to confirm these results, determine which region of SuM sends the LH projections, and confirm that these are in fact terminating in the LH, we utilized the novel designer retrograde virus AAV2 (Retro)-eSyn-EGFP (Figure 2A), which permits robust retrograde access to projection neurons with efficiency comparable to classical synthetic retrograde tracers [26]. In this experiment we also included female rats in order to begin to understand potential sex differences in the SuM-LH link. Delivery of the retrograde EGFP AAV-vector was confirmed to localize to the LH by a dense green cloud of fluorescent signal composed of fibers as well as local cellular populations sending input within the LH (Figure 2B,C). Importantly, we observed a robust expression of EGFP in neurons throughout the SuM (Figure 2D–K). While a high number of cell bodies of LH-projecting neurons was found in both male and female brains, the expression profile of single EGFP positive neurons in the target region of the SuM differed between female and male rats. The male rats had a significantly higher number of lateral SuM neurons ipsi- or contra-laterally projecting to the LH (Figure 2L,M). No sex differences were identified in the medial SuM (Figure 2N). When all SuM LH-projecting neurons are analyzed males had significantly more LH-projecting neurons (Figure 2O). We further analyzed rostral (bregma −4.34 to −4.54 mm) and caudal (bregma −4.56 to −4.74 mm) SuM LH-projecting neurons separately (Figure S2), which revealed that the rostral SuM underlies most of the discovered sex difference; however, the caudal SuM had largely similar trends, albeit less pronounced compared to the more rostral sections. Representative images for the rostral SuM are displayed in Figure 2D,E, for male and female respectively, with the corresponding diagrams of cells identified in the rostral region shown in F and G. Similarly, for the caudal SuM representative images are shown in 2H and I, and diagrams in J and K.
Figure 2.
Lateral and medial SuM innervates the LH. Retrograde viral tracer (A) was injected into the LH of female (B) and male (C) rats. Representative confocal images for females (D, H) and males (E, I) in rostral (D,E) and caudal (H,I) SuM, as well as cell body maps corresponding to the confocal images (females: F, J; males: G, K) highlight sex differences in the SuM to LH connectivity. Higher magnification images (1–4) show clear cell bodies with green fluorescent retrogradely carried EGFP. For both sexes, 1–3 are taken from the lateral SuM and 4 from the medial SuM. Comparison of the number of cell bodies of neurons projecting to LH from the ipsilateral (L) SuM of males and females indicates that more LH-projecting neurons are found in males compared to females. Similarly, more LH-projecting neurons are found in the contralateral SuM of males (M). In contrast, medial SuM contained similar numbers of EGFP-labeled neurons in males and females (N). Analysis of all SuM LH-projecting neurons indicates that males have significantly more LH-projecting neurons (O). The representative neuron shown in A was visualized by performing DAB-immunohistochemistry on EGFP retrogradedly carried from LH to lateral SuM in a male coronal section. Cell nuclei are labeled in blue with DAPI. 9 sections were analyzed for each subject, a total of four rats, two males and two females were included in this analysis * p < 0.05, **p < 0.01, ***p < 0.001.
3.2. GLP-1R are present in SuM of male and female rats
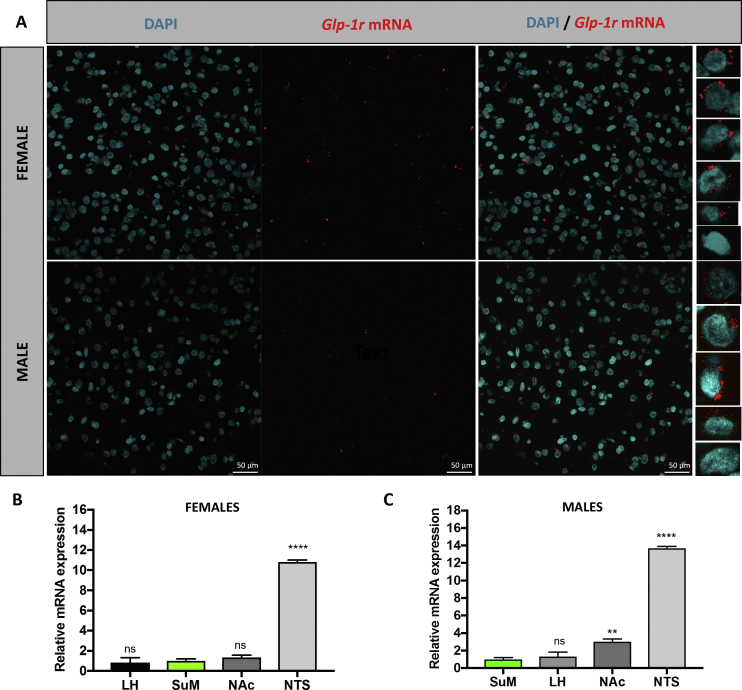
Clear and dense GLP-1R mRNA expression was detected throughout the SuM (Figure 3A) in both male and female rats. Comparison of SuM GLP-1R mRNA levels (n = 7 female, Figure 3B; n = 7 male rats, Figure 3C), with those present in other sites of GLP-1R expression known to have an important contribution to intake and reward processes, reveals that expression of SuM GLP-1R is comparable with the expression in the LH and nucleus accumbens, but lower compared to that found in the nucleus of the solitary tract. Based on quantitative RT-PCR of SuM tissue, no sex differences were readily observed in GLP-1R mRNA expression in SuM (delta Ct values to beta actin in females: 14.8 ± 0.3 (n = 7); and males: 15.1 ± 0.2 (n = 7); p = 0.4). These results highlight that cells in the SuM are wired to respond to GLP-1 or its analogues, in both sexes, and warrant further studies on the potential role that this receptor population may play in energy balance control.
Figure 3.
GLP-1R are expressed in the SuM of male and female rats. A. Clear and dense GLP-1R mRNA expression was detected, using RNAscope in situ hybridization, throughout the SuM in both male and female rats. Representative images, taken at the same level of the medial SuM, from one male and one female are show here. No sex differences were readily observed in GLP-1R mRNA expression in SuM (see PCR-based quantification in the results text). The high magnification individual cell images are displayed on the right panel for both sexes. The last cell for each sex shows an example of a cell not expressing any GLP-1R mRNA. Cell nuclei are shown in blue and labeled with DAPI. For exact SuM location of displayed images see Figure S3. Quantitative comparison of SuM GLP-1R mRNA levels (n = 7 male and n = 7 female rats) with expression of GLP-1R mRNA in other GLP-1R-expressing nuclei in females (B) and males (C). All expression is shown relative to SuM, and the order of presentation is from lowest to highest GLP-1R mRNA levels. ns: not significant, **p < 0.01, ****p < 0.0005 compared to GLP-1R mRNA levels found in SuM (entire SuM included, both medial and lateral wings). NAc: nucleus accumbens, NTS: nucleus of the solitary tract.
3.3. GLP-1R mRNA is present in LH-projecting SuM neurons of male and female rats
We identified neurons in the lateral SuM, an area with the highest number of LH-projecting neurons in both sexes within the SuM, that show GLP-1R expression, as indicated by red fluorescent in situ hybridization signal, in AAV2 (Retro)-eSyn-EGFP labeled neurons (Figure 4). Thus, GLP-1R are present on LH-projecting SuM neurons of both sexes (representative images from male (Figure 4A–F) and female rats (Figure 4G–L)). Of note, both the medial and lateral subdivision of the SuM (in both sexes) also contained GLP-1R not labeled with EGFP, suggesting that the LH may not be the only SuM innervation target impacted by GLP-1R activation. As indicated on the brain atlas sections of Figures S3 and S4, the GLP-1R expression in the medial SuM is displayed in Figure 3 and in the lateral SuM in Figure 4.
Figure 4.
GLP-1R are present on SuM neurons innervating the LH. Representative images, taken at the same level (bregma – 4.36 mm, see Figure S4 for further detail) of the lateral SuM for male and females, are shown here. Cell bodies labeled with green fluorescent protein (EGFP) retrogradely carried from the LH are detected in the lateral SuM of male (A) and female rats (G). GLP-1R mRNA expression, detected by RNAscope in situ hybridization, was found throughout the same area of SuM in both male (B) and female rats (H). High magnification of individual cell images, displayed below the main panels (C–F and I–L) for both sexes, clearly indicates GLP-1R mRNA present on the SuM neurons projecting to the LH. The green serrated line represents a trace of the green EGFP label (to outline the cell body and fibers in the image) and is superimposed on the RNAscope image in order to reveal the signal in the cell that is otherwise made less visible by the strong EGFP label. The scale bar on the magnified panels indicates 10 microns in length.
3.4. Activation of SuM GLP-1R reduces food intake in male and female rats
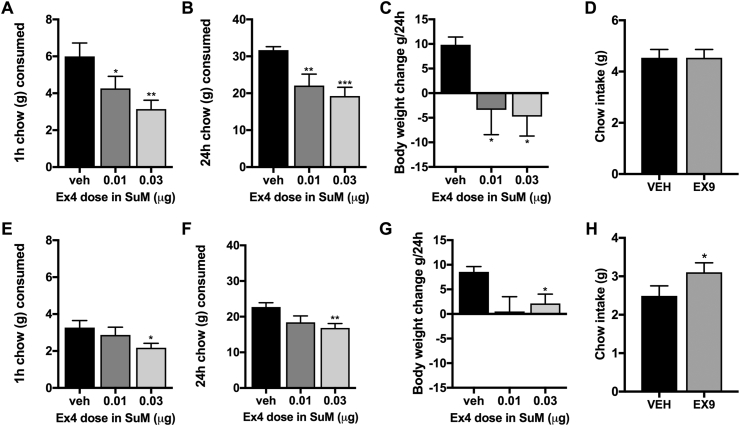
Chow intake at 1 and 24 h was suppressed by both Ex4 doses tested in males (F(2, 24) = 8.03; P = 0.003; Figure 5A and F(2, 24) = 8.24; P = 0.005; Figure 5B). This intake reduction was also associated with weight loss at the 24 h measurement (F(2, 24) = 4.1; P = 0.048; Figure 5C). Blockade of GLP-1R by intra-SuM injection of Ex9, did not alter food intake in male rats (Figure 5D). Female rats reduced their intake at 1 and 24 h (F(2, 26) = 4.24; P = 0.03; Figure 5E and F(2, 26) = 4.53; P = 0.03; Figure 5F), and body weight (F(2, 26) = 3.5; P = 0.05; Figure 5G), with only the higher Ex4 dose exerting a significant influence. In contrast to male rats, blockade of GLP-1R in female rats slightly but significantly increased chow intake (Figure 5H). Considering the high intake of vehicle-injected rats in this experiment we cannot exclude the possibility of a ceiling effect on intake in the male rats as a potential explanation for a lack of effect of Ex9 in males.
Figure 5.
Activation of SuM GLP-1R reduces chow intake and body weight in male and female rats, while blockade of these receptors is only effective in females. Chow ingestion is markedly reduced by both doses of Ex4 at 1 (A) and 24 h (B) measurements in male rats. Similarly a marked reduction in body weight is produced by both doses of SuM-targeted Ex4 (C). However, blockade of SuM GLP-1R in males did not result in hyperphagia (D). In female rats chow intake was also reduced by intra-SuM Ex4 microinjections, at 1 (E) and 24 h after the treatment (F), but only at the higher dose of Ex4. Similarly female rats also lost weight (G). In contrast, to male response, blockade of SuM GLP-1R resulted in mild hyperphagia in females (H). Data are expressed as mean ± SEM. n = 13 for male rats and n = 14 for female rats, tested in a counterbalanced (Latin square) design.*p < 0.05, **p < 0.01,***p < 0.001 compared to vehicle (aCSF).
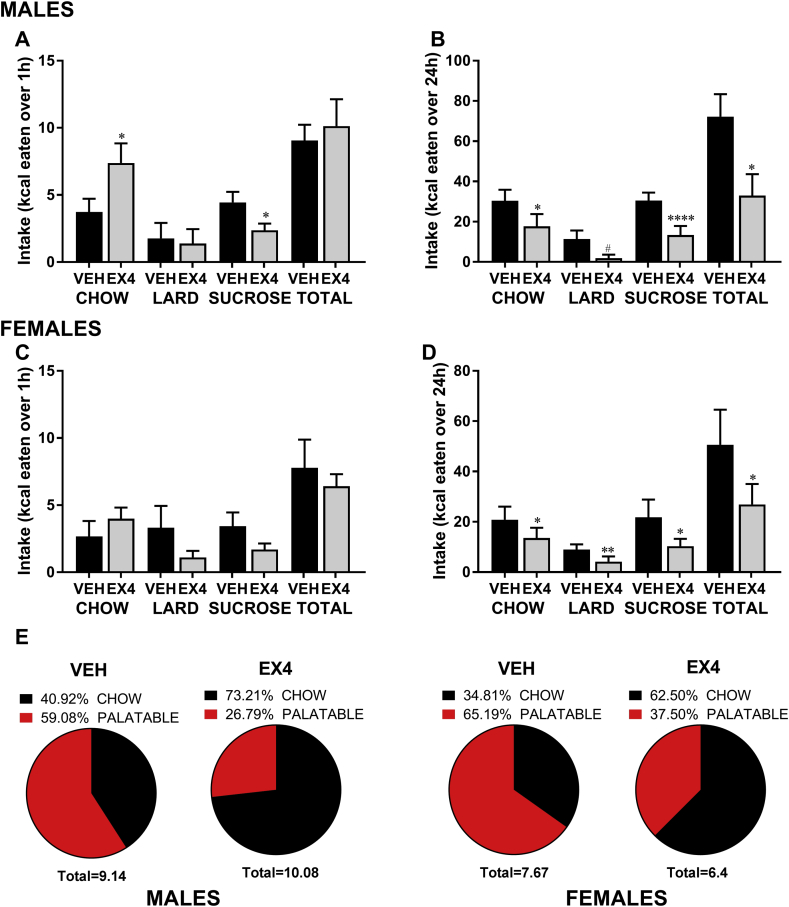
3.5. SuM GLP-1R activation affects ingestive behavior of a palatable food and chow choice diet in male and female rats
Since SuM GLP-1R activation led to a reduction in ingestive behavior for chow, we next set out to understand whether GLP-1R activation in SuM is equally effective at decreasing intake of foods with higher palatability. We chose to do this by offering the rats a choice of chow, lard, and 30% sucrose solution during the SuM Ex4 (0.03 μg) test. These foods constituted the maintenance diet for these rats for 3 weeks before testing. Under this experimental design both male (n = 9) and female (n = 8) rats reduced the intake of high calorie foods offered after Ex4 administration (Figure 6A–D). Even though at 1 h the trends in females did not reach significance, a statistical comparison with the significant effect in male rats revealed that there is no difference between the male and the female intakes at 1 h after Ex4 was applied to the SuM (Figure 6A vs. Figure 6C, two-factor ANOVA for sucrose: interaction F (1, 13) = 0.056, P = 0.8, effect of drug F (1, 13) = 7.03, P < 0.05, effect of sex F (1, 13) = 1.33, P = 0.5; for lard: F (1, 15) = 1.51, P = 0.24, effect of drug F (1, 15) = 1.5, P = 0.24, effect of sex F (1, 15) = 0.2, P = 0.7; for chow: F (1, 15) = 3.031, P = 0.1 effect of drug F (1, 15) = 14.05, P < 0.005, effect of sex F (1, 15) = 2.23, P = 0.16). Surprisingly, at 1 h the intake of chow was significantly increased in males, while palatable food ingestion was simultaneously reduced (Figure 6A,E). Similar trends were present in females (Figure 6C,E). Pie charts illustrate the shift in source of calories, acutely produced by Ex4 in male and female rats (Figure 6E). Interestingly this shift is no longer detectable when 24 h intakes are evaluated. At this time point intake of all foods is significantly reduced in both sexes (two-factor ANOVA for sucrose: interaction F (1, 13) = 1.6, P = 0.23, effect of drug F (1, 13) = 40.7, P < 0.0001, effect of sex F (1, 13) = 1.33, P = 0.4; for lard: interaction F (1, 14) = 1.0, P = 0.34, effect of drug F (1, 14) = 8.7, P < 0.05, effect of sex F (1, 14) = 0.0003, P = 0.9; for chow: interaction F (1, 14) = 0.98, P = 0.34, effect of drug F (1, 14) = 13.7, P < 0.005, effect of sex F (1, 14) = 0.98, P = 0.34). One rat was removed from each sex in sucrose analysis due to spillage. Although, if calculated by % intake of vehicle, chow intake was the least affected by the treatment, compared to the effect on sugar or lard.
Figure 6.
SuM GLP-1R activation affects ingestive behavior of a choice of palatable and chow diet in male and female rats. 1 (A) and 24 h (B) intake of each macronutrient and total intake (in kcal) in males. 1 (C) and 24 h (D) intake of each macronutrient and total intake (in kcal) in females. Pie charts illustrate the shift of energy source selection at 1 h in males and females after intra-SuM vehicle or Ex4 injections, the total below each chart represents the total kcal consumed and included in the chart (E). Data are expressed as mean ± SEM. n = 7–8 for male rats and n = 8–9 for female rats, tested in a counterbalanced (Latin square) design. #p < 0.09, *p < 0.05, **p < 0.01, ****p < 0.0005 compared to vehicle (aCSF).
3.6. Activation of SuM GLP-1R reduces food reward in a sex dimorphic manner
Intra-SuM Ex4 microinjection reduced food reward behavior in male rats, as indicated by reduced number of sucrose rewards earned (F(2, 24) = 13.7; P = 0.0004; Figure 7A) and reduced number of lever presses emitted for the sucrose rewards (F(2, 24) = 10.78; P = 0.0006; Figure 7B), without changes in locomotor activity (F(2, 24) = 0.88; P = 0.39; Figure 7D). Food seeking behavior was also reduced in male rats (F(2, 24) = 10.22; P = 0.0007; Figure 7C). Surprisingly, the same treatment did not change any of the sucrose motivated behavior parameters measured in female rats (Figure 7E–H). Since previous findings indicate that GLP-1R effects are modulated by the estrous cycle phase and estrogens in females [35], it remained possible that the lack of effect of the SuM treatment on motivated behavior in females was due to low estrogen levels at the time of testing, if by chance most of the female rats happened to be in metestrous/diestrous phase. Thus, we next tested the effect of Ex4 in SuM in rats in cycle phases characterized by high estrogen levels or estrogen signaling (proestrous and estrous) or low estrogen levels (metestrous/diestrous). Segregating the effect to these cycle phases did not, however, change the results, and females in all phases remained unresponsive to intra-SuM Ex4 injection (Figure S5).
Figure 7.
Activation of GLP-1R in the supramamillary nucleus (SuM) reduces food reward in a sexually dimorphic manner. Intra-SuM GLP-1 analogue, Ex4, microinjection reduces the amount of sucrose rewards earned (A) and the number of lever presses for the rewards (B) in a progressive ratio (PR) schedule in male rats. Ex4 microinjection also led to a reduction in food seeking for sucrose, without changing locomotor activity (D) in male rats. In contrast to the behavioral changes detected in males, none of the measured parameters were altered in female rats after SuM GLP-1R activation with Ex4 (E–H). Data are expressed as mean ± SEM. n = 13 for male rats and n = 14 for female rats, tested in a counterbalanced (Latin square) design. *p < 0.05, **p < 0.01,***p < 0.001 compared to vehicle (aCSF).
3.7. Motivated, but not ingestive, effects of VTA GLP-1R activation are sex dimorphic
The VTA is a brain region, which harbors cell bodies of dopaminergic neurons. It is widely recognized for its key role in the control of motivated behavior and its dysfunction in diseases of addiction. It is located just dorsally and partly caudally to the SuM. Because of the clear sex dimorphism in motivated behavior control in SuM, and lack of any data on the effect of Ex4 in the VTA of female rats, we decided to ask whether a similarly divergent response is also derived from VTA GLP-1R. In contrast to the results obtained from SuM, GLP-1R activation was effective at reducing motivated behavior and food seeking for food for both sexes (Figure 8A,C,E). Not only was the response clearly present in females but it was also significantly more potent in females, compared to males (Figure 8B,D,F). Interestingly, no effect of estrous cycle on the VTA GLP-1R activation was found (Figure S3). Ingestive behavior on the other hand followed the same pattern as that obtained from the SuM where both sexes were similarly affected by GLP-1R activation in the VTA (Figure 8G,H).
Figure 8.
Motivated and ingestive effects of VTA GLP-1R activation. In contrast to the results obtained from SuM, GLP-1R activation was effective at reducing motivated behavior and food seeking for food for both sexes (A, C, E). Not only was the response clearly present in females but it was also significantly more potent (B, D, F). Interestingly, no effect of estrous cycle on the VTA GLP-1R activation was found (Figure S6). Ingestive behavior on the other hand followed the same pattern as that obtained from the SuM, where both sexes were similarly affected by GLP-1R activation in the VTA (G, H). Data are expressed as mean ± SEM. n = 11 for male rats and n = 12 for female rats, tested in a counterbalanced (Latin square) design.*p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0005 compared to vehicle (aCSF).
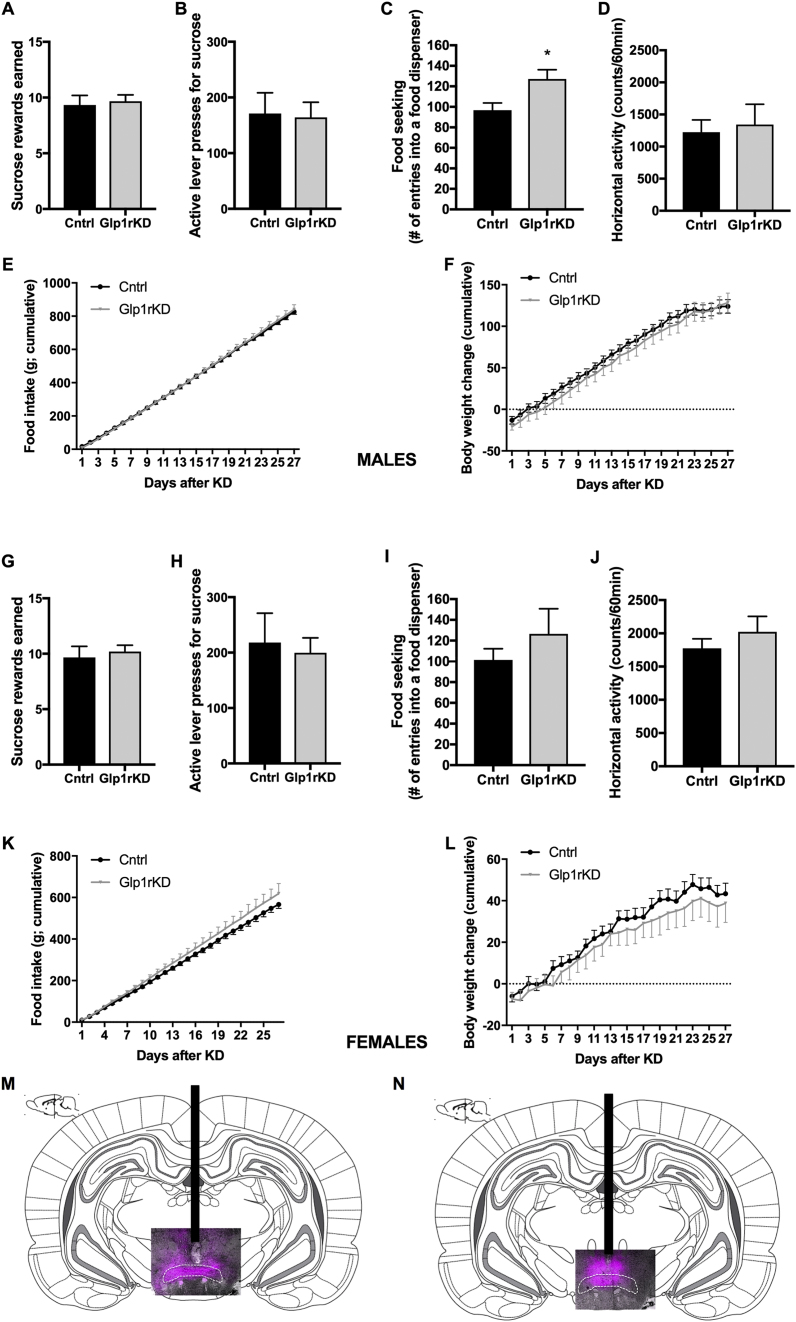
3.8. Chronic SuM-targeted GLP-1R silencing
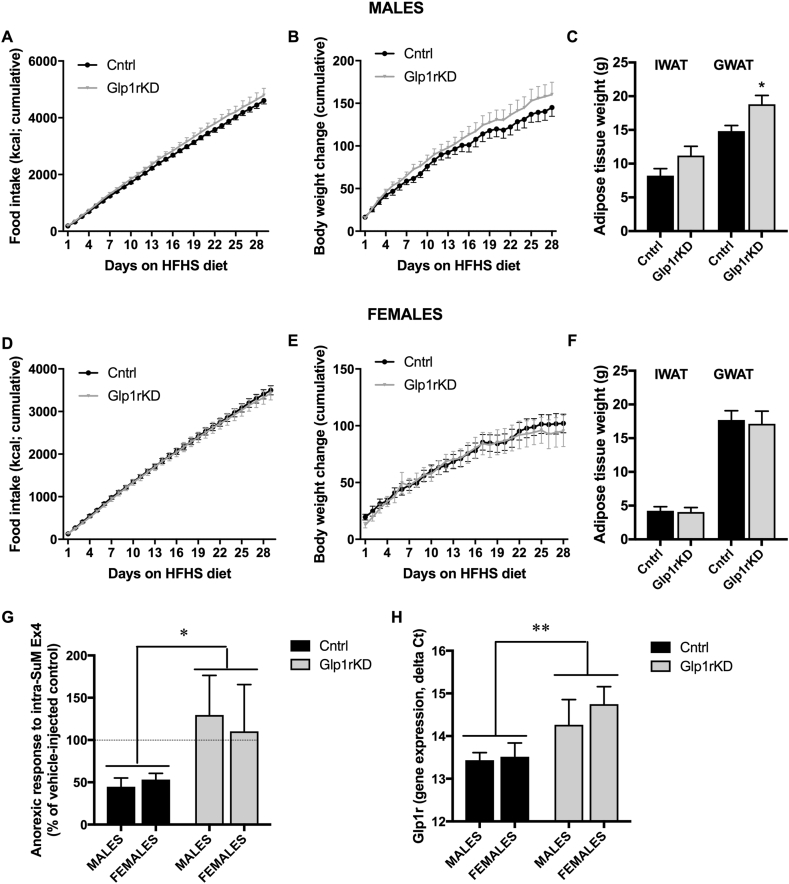
To determine whether endogenous GLP-1R signaling in the SuM is necessary for regulation of body weight, food intake, and motivation for a food reward, AAV-GFP or AAV-GLP-1R-shRNA were administered into the SuM of male and female rats. The efficiency of the knockdown was confirmed by RT-PCR to be identical to that shown by our two previous in vivo studies [34], [35]: ~50%, with no sex differences detected. In contrast to the very potent effect of GLP-1R blockade in the LH on behavior [35], we did not find any significant effect on food motivated behavior (Figure 9A,B) or chow intake and body weight gain over one month period of measurements (Figure 9E,F) in male or female rats (Figure 9G,H,K,L). However, food-seeking behavior was significantly increased in male rats with reduced GLP-1R expression in SuM (Figure 9C, P = 0.01), without an alteration in general locomotor activity (Figure 9D). While there was a trend towards a significant increase in females, this did not reach significance (Figure 9I). Moreover a two-factor ANOVA, analyzing the interaction of sex and knockdown on this parameter did not indicate a significant effect of sex (F (1, 31) = 0.02, P = 0.9), or an interaction (F (1, 31) = 0.03, P = 0.9) on food seeking while indicating a strong trend for an effect of knockdown (F (1, 31) = 3.513, P = 0.07). Since silencing of GLP-1R in SuM led to only minor disruption in food seeking but not other metabolic or behavioral parameters measured, we next set out to challenge the GLP-1R knockdown rats with a high-fat/sugar choice diet to test whether this manipulation would uncover the contribution of GLP-1R in SuM. In contrast to our hypothesis, no changes to weight gain, food intake, or food motivated behavior were detected under the obesogenic diet in SuM knockdown rats compared to controls, males and females alike (Figure 10, Figure S7). Male rats, however, had heavier GWAT after SuM GLP-1R knockdown (Figure 10C). Two outlier values were removed from the GWAT and IWAT data set, as they were both less than 50% of the group average value. Considering the small effect of the knockdown on the parameters measured in this study, the functional efficiency of the knockdown was further confirmed by injection of Ex4 (0.03 μg) directly into the SuM followed by measurement of food intake at 24 h, performed four weeks following the obesogenic diet exposure. Control rats, injected with Ex4 (n = 15, 8 males, 7 females) consumed 47% ± 6% of the total calories consumed over 24 h by vehicle-injected rats (all data broken down by macronutrient and sex presented in Figure 6). In contrast, Ex4-injected GLP-1R knockdown rats (n = 18, 9 males and 9 females) consumed 113% ± 34% of the total calories consumed on vehicle injection day (p < 0.05, Figure 10G). Thus, SuM GLP-1R knockdown was sufficient to block the anorexic response to exogenous GLP-1R agonist. Moreover, SuM GLP-1R gene expression levels were significantly reduced in the same animals (p < 0.01, Figure 10H), and the level of knockdown was similar to that achieved in previous studies [34], [35].
Figure 9.
GLP-1R in the SuM are not necessary for ingestive and motivated behavior regulation, but their loss results in increased food-seeking behavior in lean male rats. In male rats knockdown of GLP-1R in the SuM did not alter sucrose rewards earned (A), and lever presses for the rewards (B) in a progressive ratio (PR) schedule. However, food-seeking (# of entries into a food dispenser) behavior was increased (C) without concurrent changes in locomotor activity (D). Cumulative food intake or weight gain, measured over 4 weeks, was not altered by the SuM GLP-1R knockdown (E, F). In males: n = 9–10 for control (cntrl) and n = 8–9 for GLP-1R knockdown (Glp1rKD). SuM GLP-1R knockdown did not alter the amount of sucrose rewards earned (G), lever presses for the rewards (H) in a progressive ratio (PR) schedule, food-seeking (# of entries into a food dispenser) (I), locomotor activity (J), cumulative food intake (K) or weight gain (L). In females: n = 9 for control and n = 9–10 for GLP-1R knockdown. Representative images of male (M) and female (N) EGFP expression in the SuM fluorescent microscope photo superimposed on an atlas drawing (plate 71, bregma −4.56 mm). White serrated line represents rat atlas SuM border. Data are expressed as mean ± SEM. n = 8–10 for male rats and n = 9–10 for female rats.*p < 0.05, compared to vehicle (aCSF).
Figure 10.
GLP-1R in the SuM are not necessary for food intake or body weight maintenance in high-fat/sugar-fed rats. Knockdown and control male (n = 9 for both treatment groups) and female (n = 9 for controls and n = 10 for knockdown) rats were metabolically challenged with a HFHS diet, and food intake (A, D) and body weight (B, E) were measured daily over 4 weeks. Adipose tissue weight was significantly increased for GWAT in male rats (C; n = 9 for controls and n = 7 for knockdown) without any significant effect of treatment in female rats (F; n = 9 for both treatment groups). Control rats, injected with Ex4 (n = 15, 8 males, 7 females) consumed only half of the total calories consumed over 24 h by vehicle-injected rats (G). In contrast, Ex4-injected GLP-1R knockdown rats (n = 18, 9 males and 9 females) did not reduce their food intake (G). Moreover, SuM GLP-1R gene expression was significantly reduced in the same animals (H). IWAT; inguinal subcutaneous white adipose tissue. GWAT; gonadal white adipose tissue. Data are expressed as mean ± SEM. *p < 0.05, **p < 0.01.
4. Discussion
Almost nothing is known about the role of the SuM in control of consummatory ingestive or motivated behaviors, and, to the best of our knowledge, no previous study has specifically investigated the SuM of females. Here we identify dense and partly sex dimorphic SuM innervation of the LH, a brain area well-known for its potent ability to regulate food intake and body weight. We also show that GLP-1R are expressed throughout the SuM of male and female rats. These receptors were functionally relevant since our behavioral data indicate that they were sufficient, and in select conditions necessary, for ingestive behavior control in males and females. Moreover, SuM GLP-1R control of food reinforcement is sex specific, with SuM GLP-1R control for food-motivated behaviors being present only in males. In obese male rats shRNA-mediated loss of SuM GLP-1R exacerbated visceral fat gain, without affecting any parameters measured in females.
We identified a direct neuroanatomical connection between the SuM and the LH in both male and female rats, suggesting that the SuM is neuroanatomically positioned to control feeding behavior. Many LH-projecting neurons were located in the lateral subdivision of SuM, with male rats showing a significantly higher number of LH-projecting neurons in this subdivision. LH-projecting neurons were also clearly present in the medial SuM, however, in this subdivision of SuM, no sex differences were detected. Furthermore, GLP-1R expression was identified throughout SuM in both males and females. More importantly, GLP-1R were present directly on neuronal cell bodies determined to innervate the LH, suggesting that GLP-1R activation in the SuM may, in part, act by changing neural communication between the SuM and the LH. Expression of total SuM GLP-1R was similar between males and females, and comparable to that found in the LH, and nucleus accumbens, but lower compared to the nucleus of the solitary tract. All three comparator sites have been previously found to be of importance for GLP-1R control of behavior and metabolism [14], [18], [19], [35], [36], [37], [38], [39], [40]. The GLP-1R expression in the SuM is likely complemented by direct delivery of the endogenous brain-produced GLP-1, since a previous study identified preproglucagon immunoreactive fibers in the SuM of a rat, and, interestingly, this immunoreactivity was stronger in the SuM compared to the neighboring VTA [41]. While GLP-1 immunoreactive cells and fibers have been identified in the brain of human [15] and non-human primates [42], with a great degree of overlap indicated between the rodent and primate data, to the best of our knowledge, the SuM has not yet been evaluated in primate studies. However, as recently indicated by the group of Kanoski and colleagues [43] endogenous GLP-1 can also be delivered to sites quite distal of the nucleus of the solitary tract (e.g. hippocampus) via the cerebrospinal fluid (volume transmission). Same delivery route was also recently identified for other neuropeptides (e.g. MCH [44]). This route of delivery is likely relevant for the SuM, as this nucleus is located in proximity of the mammillary recess of the 3rd ventricle.
Activation of SuM GLP-1R suppressed chow intake; this effect lasted throughout the 24 h measurement and led to reduced weight gain in both sexes. These data indicate that satiation signals acting in the SuM can clearly regulate intake of less palatable food. The effect size of intake suppression was substantial, especially in males, with the rats treated with the highest dose of Ex4 eating only half of the chow that vehicle-injected rats ate. While consumption of chow was suppressed in both sexes after SuM Ex4, only male rats responded with a reduced sucrose motivated behavior. This is somewhat surprising since our previous data indicate that adding estrogen to GLP-1 (by conjugation of the two compounds) potently increases sucrose motivated behavior suppression in males compared to vehicle or GLP-1 alone, thus it could follow that females, by virtue of having higher circulation estrogen levels than males may exhibit higher motivated behavior suppression compared to males [22], [23]. Alternatively, it may be that due to the chronically elevated average levels of estrogen in females, there is a degree of tachyphylaxis for estrogen signaling to enhance GLP-1R signaling. Indeed, females in a state of high circulating estrogen levels did not reduce their operant performance for sucrose after Ex4. Since SuM GLP-1R activation led to a reduction in ingestive behavior for chow, but not motivated behavior, for sucrose in female rats we next set out to understand whether the lack of effect in females was driven by increased food palatability or the operant paradigm imposed to obtain the food. We chose to do this by offering the rats a choice of chow, lard, and sucrose solution. In this experiment females, like males, potently reduced their intake of all foods, including sucrose. Thus the lack of effect on motivated behavior in females is unlikely to be driven by the sucrose as the source as reward. It is possible that the differential SuM innervation of, for example the LH shown here, or potentially other reward-relevant brain areas underlies our inability to affect motivated behavior in females after SuM manipulations. This theory, however, is purely speculative at this point and remains to be evaluated.
There is no literature evaluating SuM contribution to motivated behavior of any kind in female rodents, thus it is difficult to speculate whether the lack of GLP-1R activation effect in females can be extended to reinforcers other than food. For males, however, the SuM is a self-stimulation hotspot [11] and disinhibiting SuM neurons, by administration of GABAA antagonist, increases nicotine, amphetamine, and cocaine self-administration [9], [45], [46]. Together with previous data showing that GLP-1R activation in male rats or mice reduces reward derived from nearly all drugs of addiction, including cocaine, nicotine, amphetamine and alcohol [20], [47], it is very likely that SuM GLP-1R activation in males is effective at reducing motivated behaviors for rewarding substances other than food.
Activation of GLP-1R in the VTA suppressed motivated and ingestive behaviors in both sexes, with the effect in males being comparable to previous literature [17], [18]. The VTA-directed drug infusion performed in this study revealed a sex interaction, with respect to motivated behavior that was opposite to that detected for the SuM, with females showing a significantly more potent response. Taking together the motivated behavior effect of VTA and SuM GLP-1R activation, it is also tempting to speculate that while the effect of GLP-1R activation in individual mesolimbic nuclei tested here is sex divergent the net effect of GLP-R activation in both sites may actually be similar. Considering that female rats were previously shown to be more sensitive to intra-ventricular delivery of some anorexic signals, for example leptin, but less sensitive to others, for example insulin [48], [49], it would be of interest to determine whether these differential effects are driven by all or select nuclei already known to drive an anorexic response to these signals.
A wealth of literature shows that GLP-1R in the vast majority of CNS nuclei tested leads to reduced intake of chow in rats and mice (see for example [14] for data review), when chow is offered as the only source of calories. However, we have previously shown that when chow is offered alongside a more palatable option – peanut butter – GLP-1R activation will reduce intake of the palatable food but not chow [40]. Similarly NAc GLP-1R activation reduces high-fat pellet intake but does not change intake of concurrently offered chow [17]. More importantly, intra-VTA Ex4 injections were indicated by one previous report to increase chow intake while reducing intake of high fat [17]. This is in line with the current results, in which intra-SuM Ex4 acutely reduced intake of sucrose but increased intake of chow. Even at the 24 h measurement, chow intake was the least affected of the three foods, without any sex differences. It is thus tempting to speculate that the VTA and SuM do not support a purely anorexic phenotype but instead act in concert to shift macronutrient preference. Since GLP-1-driven reduction in sucrose intake does not seem to require post-ingestive feedback [50], it is further tempting to consider that the macronutrient selective reduction is regulated at the level of the orosensory feedback, an idea that requires further testing. Additionally, the increased intake of chow clearly refutes the idea of a general behavioral inhibition and suggests a selective and refined behavioral regulation by SuM GLP-1R. This is further supported by unaffected locomotor activity, measured during the operant behavior testing.
Obese high fat/sugar-diet fed SuM GLP-1R knockdown male rats gained significantly more body fat compared to controls. This increase in adiposity is likely not due to increased food intake, or motivated behavior based on current data. However, the general activity of the knockdown rats tended to be lower compared to control rats, while this reduction is not significant at the 60 min measurement presented here, if it were to be maintained for multiple hours, it could accumulate to result in a slight increase in adipose and total body weight gain. This is made more probable by recent data identifying SuM as a new key node in the arousal system [21].
The rather small effect of SuM GLP-1R knockdown suggests that while SuM GLP-1R are sufficient to control consummatory ingestive behaviors and motivated behaviors (the latter only in males), they are not necessary for their normal expression. This contrasts with the necessity of GLP-1R for these behaviors shown for both sexes in the LH, or the necessity of VTA and NTS GLP-1R for motivated behavior [34], [35], [37]. This can be especially surprising since SuM is sometimes considered a caudal extension of the LH. Thus, despite neuroanatomical proximity, GLP-1R in these three brain nuclei have a very different contribution to physiology and behavior. As a more minor point, these contrasting results also indicate specificity of injection site.
Collectively our data place the SuM as an important CNS anatomical hub in control of ingestive and motivated behaviors, as well as GLP-1R responses. These observations show partial overlap but also a significant divergence in function compared to its two anatomical neighbors – the VTA and the LH. Importantly, current data for the first time identify neuroanatomical, as well as behavioral sex differences, in the SuM. These results are eye opening considering that to the best of our knowledge no one to date has performed a pharmacological or behavioral evaluation of SuM in female rodents.
Financial disclosures
MRH receives funding from Zealand Pharma and Novo Nordisk that was not used in support of these studies.
Acknowledgements
This research was funded by the Wallenberg Foundation (to KPS via WCMTM), Swedish Research Council (2014–2945 to KPS and 2013–7107 to PR), Novo Nordisk Foundation Excellence project grant (KPS), Ragnar Söderberg Foundation (KPS), Harald Jeanssons Stiftelse and Greta Jeanssons Stiftelse (KPS), and Magnus Bergvalls Stiftelse (KPS), and National Institutes of Health NIH-DK096139 (MRH).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2018.11.005.
Conflicts of interest
All the other authors declare no conflict of interest.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Fig. S1.
Figure S1: Representative spread of injection via the cannula used for neuropharmacology experiments (agonist and antagonist injections) in the female rats (representative image for male rats is shown in Figure 1C). Rat brain atlas slide at bregma level of −4.56 mm (A). Injection of spread of EGFP-expressing viral vector where the virus solution was injected through the neuropharmacology cannula to confirm and label injection reach (B). Depiction (in green) of injection spread on a modified rat brain atlas image (C). Medial SuM: SuMM; lateral SuM: SuML; dorsal SuM: sumd; posterior lateral hypothalamus: PLH; 3V: third ventricle. Coronal brain atlas figures are taken from Paxinos & Watson 5th edition.
Fig. S2.
Figure S2: Cell counts of rostral and caudal SuM LH-projecting neurons, identified by EGFP expression driven by retrograde AAV injected into the LH of male and female rats. Ipsilaterally projecting neurons are found in both sexes at both SuM levels (A-B), with male rat SuM containing higher numbers of LH-projecting neurons compared to female rats. Only few cells were found to project contralaterally in both sexes and levels of SuM (C–D) with no significant sex differences detected. In the medial SuM no sex differences were detected in the rostral (E) or caudal (F) sections. When neurons in all SuM regions are summed up (G–H) sex differences are largely identified only in rostral SuM, driven by a higher number of LH-projecting neurons in the rostral SuM of male rats. 9 sections were analyzed for each subject (5 for rostral SuM and 4 for caudal SuM), a total of four rats, two males and two females were included in this analysis.**p < 0.005,***p < 0.001.
Fig. S3.
Figure S3: High magnification image along with a representative atlas slide indicating the region of RNAscope analysis for GLP-1R mRNA expression in the SuM. Medial SuM: SuMM; lateral SuM: SuML; 3V: third ventricle. Cell nuclei are shown in blue and labeled with DAPI.
Fig. S4.
Figure S4: Confocal microscope images along with a representative atlas slide indicating the location, within the SuM, of higher magnification confocal images displayed in Figure 4. Since, as indicated in Figure 2, the lateral region of the SuM contained the highest number of LH-projecting cells, the colocalization of GLP-1R expression on LH-projecting neurons analysis was performed specifically in the lateral SuM for both males (the red square on the far left) and females the red square in the more medial location of lateral SuM. The precise location of images displayed was selected based on the highest density of EGFP-labeled cell bodies. Medial SuM: SuMM; lateral SuM: SuML; 3V: third ventricle. Cell nuclei are shown in blue and labeled with DAPI.
Fig. S5.
Figure S5: Intra-SuM GLP-1 analogue, Ex4, microinjection does not reduce the amount of sucrose rewards earned (A) or the number of lever presses for the rewards (B) in a progressive ratio (PR) schedule, food seeking for sucrose (C), or locomotor activity (D) in female rats in proestrus or estrus phases of the estrous cycle, i.e. phases in which estrogen levels (proestrus) or effects (estrus) are at their peak. Similarly, none of the measured parameters were altered in female rats in metestrus or diestrus after SuM GLP-1R activation with Ex4 (E–H). Data are expressed as mean ± SEM. n = 14.
Fig. S6.
Figure S6: Motivated and ingestive effects of VTA GLP-1R activation segregated by high and low estrogen estrous cycle phases in female rats. Both MD and PE female rats display a reduction in sucrose rewards earned (A) and reduction in the amount of lever presses emitted for the sucrose rewards (B). Also a reduction in food seeking (C) and 1h chow intake (D) was detected in all female rats, irrespective of their cycle phase. Data are expressed as mean ± SEM. n = 6 for female rats.*p < 0.05, **p < 0.01 compared to vehicle (aCSF). MD: metestrus and diestrus; PE: proestrus and estrus.
Fig. S7.
Figure S7: GLP-1R in the SuM are not necessary for motivated behavior regulation, or food seeking behavior in obese rats. Data are expressed as mean ± SEM. n = 9 for controls and n = 7 for knockdown in male rats and n = 9 for controls and n = 10 for knockdown female rats.
References
- 1.Agha M., Agha R. The rising prevalence of obesity: part A: impact on public health. International Journal of Surgery Oncology. 2017;2:e17. doi: 10.1097/IJ9.0000000000000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hruby A., Hu F.B. The epidemiology of obesity: a big picture. PharmacoEconomics. 2015;33:673–689. doi: 10.1007/s40273-014-0243-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grill H.J. Distributed neural control of energy balance: contributions from hindbrain and hypothalamus. Obesity. 2006;14(Suppl 5):216S–221S. doi: 10.1038/oby.2006.312. [DOI] [PubMed] [Google Scholar]
- 4.Swinburn B., Sacks G., Ravussin E. Increased food energy supply is more than sufficient to explain the US epidemic of obesity. The American Journal of Clinical Nutrition. 2009;90:1453–1456. doi: 10.3945/ajcn.2009.28595. [DOI] [PubMed] [Google Scholar]
- 5.Epstein L.H., Carr K.A., Lin H., Fletcher K.D., Roemmich J.N. Usual energy intake mediates the relationship between food reinforcement and BMI. Obesity (Silver Spring) 2012;20:1815–1819. doi: 10.1038/oby.2012.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hill C., Saxton J., Webber L., Blundell J., Wardle J. The relative reinforcing value of food predicts weight gain in a longitudinal study of 7–10-y-old children. The American Journal of Clinical Nutrition. 2009;90:276–281. doi: 10.3945/ajcn.2009.27479. [DOI] [PubMed] [Google Scholar]
- 7.Berthoud H.R. Metabolic and hedonic drives in the neural control of appetite: who is the boss? Current Opinion in Neurobiology. 2011;21:888–896. doi: 10.1016/j.conb.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Skibicka K.P., Shirazi R.H., Rabasa-Papio C., Alvarez-Crespo M., Neuber C., Vogel H. Divergent circuitry underlying food reward and intake effects of ghrelin: dopaminergic VTA-accumbens projection mediates ghrelin's effect on food reward but not food intake. Neuropharmacology. 2013;73:274–283. doi: 10.1016/j.neuropharm.2013.06.004. [DOI] [PubMed] [Google Scholar]
- 9.Ikemoto S. Brain reward circuitry beyond the mesolimbic dopamine system: a neurobiological theory. Neuroscience & Biobehavioral Reviews. 2010;35:129–150. doi: 10.1016/j.neubiorev.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pan W.X., McNaughton N. The supramammillary area: its organization, functions and relationship to the hippocampus. Progress in Neurobiology. 2004;74:127–166. doi: 10.1016/j.pneurobio.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 11.Olds J. A preliminary mapping of electrical reinforcing effects in the rat brain. Journal of Comparative & Physiological Psychology. 1956;49:281–285. doi: 10.1037/h0041287. [DOI] [PubMed] [Google Scholar]
- 12.Olds M.E., Olds J. Approach-avoidance analysis of rat diencephalon. Journal of Comparative Neurology. 1963;120:259–295. doi: 10.1002/cne.901200206. [DOI] [PubMed] [Google Scholar]
- 13.Holst J.J. The physiology of glucagon-like peptide 1. Physiological Reviews. 2007;87:1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 14.Kanoski S.E., Hayes M.R., Skibicka K.P. GLP-1 and weight loss: unraveling the diverse neural circuitry. American Journal of Physiology, Regulatory, Integrative and Comparative Physiology. 2016;310:R885–R895. doi: 10.1152/ajpregu.00520.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zheng H., Cai L., Rinaman L. Distribution of glucagon-like peptide 1-immunopositive neurons in human caudal medulla. Brain Structure and Function. 2015;220:1213–1219. doi: 10.1007/s00429-014-0714-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng H., Stornetta R.L., Agassandian K., Rinaman L. Glutamatergic phenotype of glucagon-like peptide 1 neurons in the caudal nucleus of the solitary tract in rats. Brain Structure and Function. 2015 Sep;220(5):3011–3022. doi: 10.1007/s00429-014-0841-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alhadeff A.L., Rupprecht L.E., Hayes M.R. GLP-1 neurons in the nucleus of the solitary tract project directly to the ventral tegmental area and nucleus accumbens to control for food intake. Endocrinology. 2012;153:647–658. doi: 10.1210/en.2011-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dickson S.L., Shirazi R.H., Hansson C., Bergquist F., Nissbrandt H., Skibicka K.P. The glucagon-like peptide 1 (GLP-1) analogue, exendin-4, decreases the rewarding value of food: a new role for mesolimbic GLP-1 receptors. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2012;32:4812–4820. doi: 10.1523/JNEUROSCI.6326-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dossat A.M., Diaz R., Gallo L., Panagos A., Kay K., Williams D.L. Nucleus accumbens GLP-1 receptors influence meal size and palatability. American Journal of Physiology. Endocrinology and Metabolism. 2013;304:E1314–E1320. doi: 10.1152/ajpendo.00137.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Skibicka K.P. The central GLP-1: implications for food and drug reward. Frontiers in Neuroscience. 2013;7:181. doi: 10.3389/fnins.2013.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pedersen N.P., Ferrari L., Venner A., Wang J.L., Abbott S.B.G., Vujovic N. Supramammillary glutamate neurons are a key node of the arousal system. Nature Communications. 2017;8:1405. doi: 10.1038/s41467-017-01004-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vogel H., Wolf S., Rabasa C., Rodriguez-Pacheco F., Babaei C.S., Stober F. GLP-1 and estrogen conjugate acts in the supramammillary nucleus to reduce food-reward and body weight. Neuropharmacology. 2016;110:396–406. doi: 10.1016/j.neuropharm.2016.07.039. [DOI] [PubMed] [Google Scholar]
- 23.Finan B., Yang B., Ottaway N., Stemmer K., Muller T.D., Yi C.X. Targeted estrogen delivery reverses the metabolic syndrome. Nature Medicine. 2012;18:1847–1856. doi: 10.1038/nm.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leranth C., Shanabrough M., Horvath T.L. Estrogen receptor-alpha in the raphe serotonergic and supramammillary area calretinin-containing neurons of the female rat. Experimental Brain Research. 1999;128:417–420. doi: 10.1007/s002210050863. [DOI] [PubMed] [Google Scholar]
- 25.Mitra S.W., Hoskin E., Yudkovitz J., Pear L., Wilkinson H.A., Hayashi S. Immunolocalization of estrogen receptor beta in the mouse brain: comparison with estrogen receptor alpha. Endocrinology. 2003;144:2055–2067. doi: 10.1210/en.2002-221069. [DOI] [PubMed] [Google Scholar]
- 26.Tervo D.G., Hwang B.Y., Viswanathan S., Gaj T., Lavzin M., Ritola K.D. A Designer AAV variant permits efficient retrograde access to projection neurons. Neuron. 2016;92:372–382. doi: 10.1016/j.neuron.2016.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ciesielska A., Mittermeyer G., Hadaczek P., Kells A.P., Forsayeth J., Bankiewicz K.S. Anterograde axonal transport of AAV2-GDNF in rat basal ganglia. Molecular Therapy. 2011;19:922–927. doi: 10.1038/mt.2010.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Merchenthaler I., Lane M., Shughrue P. Distribution of pre-pro-glucagon and glucagon-like peptide-1 receptor messenger RNAs in the rat central nervous system. Journal of Comparative Neurology. 1999;403:261–280. doi: 10.1002/(sici)1096-9861(19990111)403:2<261::aid-cne8>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 29.Meitzen J., Pflepsen K.R., Stern C.M., Meisel R.L., Mermelstein P.G. Measurements of neuron soma size and density in rat dorsal striatum, nucleus accumbens core and nucleus accumbens shell: differences between striatal region and brain hemisphere, but not sex. Neuroscience Letters. 2011;487:177–181. doi: 10.1016/j.neulet.2010.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schindelin J., Arganda-Carreras I., Frise E., Kaynig V., Longair M., Pietzsch T. Fiji: an open-source platform for biological-image analysis. Nature Methods. 2012;9:676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(-Delta Delta C) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 32.la Fleur S.E., Vanderschuren L.J., Luijendijk M.C., Kloeze B.M., Tiesjema B., Adan R.A. A reciprocal interaction between food-motivated behavior and diet-induced obesity. International Journal of Obesity (London) 2007;31:1286–1294. doi: 10.1038/sj.ijo.0803570. [DOI] [PubMed] [Google Scholar]
- 33.Chateau D., Geiger J.M., Samama B., Boehm N. Vaginal keratinization during the estrous cycle in rats: a model for evaluating retinoid activity. Skin Pharmacology. 1996;9:9–16. doi: 10.1159/000211385. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt H.D., Mietlicki-Baase E.G., Ige K.Y., Maurer J.J., Reiner D.J., Zimmer D.J. Glucagon-like peptide-1 receptor activation in the ventral tegmental area decreases the reinforcing efficacy of cocaine. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2016;41:1917–1928. doi: 10.1038/npp.2015.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lopez-Ferreras L., Richard J.E., Noble E.E., Eerola K., Anderberg R.H., Olandersson K. Lateral hypothalamic GLP-1 receptors are critical for the control of food reinforcement, ingestive behavior and body weight. Molecular Psychiatry. 2018;23:1157–1168. doi: 10.1038/mp.2017.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alhadeff A.L., Mergler B.D., Zimmer D.J., Turner C.A., Reiner D.J., Schmidt H.D. Endogenous glucagon-like peptide-1 receptor signaling in the nucleus tractus solitarius is required for food intake control. Neuropsychopharmacology. 2017 Jun;42(7):1471–1479. doi: 10.1038/npp.2016.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Alhadeff A.L., Mergler B.D., Zimmer D.J., Turner C.A., Reiner D.J., Schmidt H.D. Endogenous glucagon-like peptide-1 receptor signaling in the nucleus tractus solitarius is required for food intake control. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2017;42:1471–1479. doi: 10.1038/npp.2016.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dossat A.M., Lilly N., Kay K., Williams D.L. Glucagon-like peptide 1 receptors in nucleus accumbens affect food intake. The Journal of neuroscience : The Official Journal of the Society for Neuroscience. 2011;31:14453–14457. doi: 10.1523/JNEUROSCI.3262-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hayes M.R., Skibicka K.P., Grill H.J. Caudal brainstem processing is sufficient for behavioral, sympathetic, and parasympathetic responses driven by peripheral and hindbrain glucagon-like-peptide-1 receptor stimulation. Endocrinology. 2008;149:4059–4068. doi: 10.1210/en.2007-1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richard J.E., Anderberg R.H., Goteson A., Gribble F.M., Reimann F., Skibicka K.P. Activation of the GLP-1 receptors in the nucleus of the solitary tract reduces food reward behavior and targets the mesolimbic system. PLoS One. 2015;10 doi: 10.1371/journal.pone.0119034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jin S.L., Han V.K., Simmons J.G., Towle A.C., Lauder J.M., Lund P.K. Distribution of glucagonlike peptide I (GLP-I), glucagon, and glicentin in the rat brain: an immunocytochemical study. Journal of Comparative Neurology. 1988;271:519–532. doi: 10.1002/cne.902710405. [DOI] [PubMed] [Google Scholar]
- 42.Vrang N., Grove K. The brainstem preproglucagon system in a non-human primate (Macaca mulatta) Brain Research. 2011;1397:28–37. doi: 10.1016/j.brainres.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 43.Hsu T.M., Hahn J.D., Konanur V.R., Lam A., Kanoski S.E. Hippocampal GLP-1 receptors influence food intake, meal size, and effort-based responding for food through volume transmission. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2015;40:327–337. doi: 10.1038/npp.2014.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Noble E.E., Hahn J.D., Konanur V.R., Hsu T.M., Page S.J., Cortella A.M. Control of feeding behavior by cerebral ventricular volume transmission of melanin-concentrating hormone. Cell Metabolism. 2018;28:55–68. doi: 10.1016/j.cmet.2018.05.001. e57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ikemoto S. The supramammillary nucleus mediates primary reinforcement via GABA(A) receptors. Neuropsychopharmacology: Official Publication of the American College of Neuropsychopharmacology. 2005;30:1088–1095. doi: 10.1038/sj.npp.1300660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ikemoto S., Qin M., Liu Z.H. Primary reinforcing effects of nicotine are triggered from multiple regions both inside and outside the ventral tegmental area. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2006;26:723–730. doi: 10.1523/JNEUROSCI.4542-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shirazi R.H., Dickson S.L., Skibicka K.P. Gut peptide GLP-1 and its analogue, Exendin-4, decrease alcohol intake and reward. PLoS One. 2013;8 doi: 10.1371/journal.pone.0061965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Clegg D.J., Brown L.M., Woods S.C., Benoit S.C. Gonadal hormones determine sensitivity to central leptin and insulin. Diabetes. 2006;55:978–987. doi: 10.2337/diabetes.55.04.06.db05-1339. [DOI] [PubMed] [Google Scholar]
- 49.Clegg D.J., Riedy C.A., Smith K.A., Benoit S.C., Woods S.C. Differential sensitivity to central leptin and insulin in male and female rats. Diabetes. 2003;52:682–687. doi: 10.2337/diabetes.52.3.682. [DOI] [PubMed] [Google Scholar]
- 50.Asarian L., Corp E.S., Hrupka B., Geary N. Intracerebroventricular glucagon-like peptide-1 (7-36) amide inhibits sham feeding in rats without eliciting satiety. Physiology & Behavior. 1998;64:367–372. doi: 10.1016/s0031-9384(98)00089-4. [DOI] [PubMed] [Google Scholar]