Abstract
Background
Cancer cells favor the use of less efficient glycolysis rather than mitochondrial oxidative phosphorylation to metabolize glucose, even in oxygen-rich conditions, a distinct metabolic alteration named the Warburg effect or aerobic glycolysis. In adult cells, bifunctional 6-phosphofructo-2-kinase/fructose-2, 6-bisphosphatase (PFKFB) family members are responsible for controlling the steady-state cytoplasmic levels of fructose-2,6-bisphosphate, which allosterically activates 6-phosphofructo-1-kinase, the key enzyme catalyzing the rate-limiting reaction of glycolysis. PFKFB3 and PFKFB4 are the two main isoenzymes overexpressed in various human cancers.
Scope of review
In this review, we summarize recent findings on the glycolytic and extraglycolytic roles of PFKFB3 and PFKFB4 in cancer progression and discuss potential therapies for targeting of PFKFB3 and PFKFB4.
Major conclusions
PFKFB3 has the highest kinase activity to shunt glucose toward glycolysis, whereas PFKFB4 has more FBPase-2 activity, redirecting glucose toward the pentose phosphate pathway, providing reducing power for lipid biosynthesis and scavenging reactive oxygen species. Co-expression of PFKFB3 and PFKFB4 provides sufficient glucose metabolism to satisfy the bioenergetics demand and redox homeostasis requirements of cancer cells. Various reversible post-translational modifications of PFKFB3 enable cancer cells to flexibly adapt glucose metabolism in response to diverse stress conditions. In addition to playing important roles in tumor cell glucose metabolism, PFKFB3 and PFKFB4 are widely involved in multiple biological processes, such as cell cycle regulation, autophagy, and transcriptional regulation in a non-glycolysis-dependent manner.
Keywords: Warburg effect; Aerobic glycolysis; 6-Phosphofructo-2-kinase/fructose-2,6-bisphosphatase; Pentose phosphate pathway; Metabolic reprogramming
1. Introduction
Tumor cells take up and consume large amounts of glucose. However, tumor cells prefer to metabolize glucose through glycolysis rather than mitochondrial oxidative phosphorylation (OXPHOS), even in oxygen-rich conditions, producing large amounts of lactic acid; this phenomenon is called the Warburg effect or aerobic glycolysis [1], [2]. The complexity of metabolism in tumor cells far exceeds Warburg's initial anticipation [3]. In addition to glycolysis, some tumors cells rely on glutaminolysis, the pentose phosphate pathway (PPP), de novo fatty acid biogenesis, lipid droplet formation, and fatty acid oxidation [4], [5], [6]. As a hallmark of cancer metabolic reprogramming, active aerobic glycolysis not only allows rapid production of adenosine triphosphate (ATP) to support cell survival in energetic stress, but also satisfies anabolic metabolism by providing intermediate metabolites for the biosynthesis of nucleic acids, proteins, and lipids, which are important building blocks required for proliferating cells [7]. For example, carbon units derived from glucose can be used for de novo synthesis of nucleic acids, fatty acids, and amino acids [8]. It is hypothesized that aerobic metabolism may induce the selection of tumor cells able to survive under acidic and hypoxic conditions. The shift from mitochondrial OXPHOS to glycolytic metabolism also benefits tumor cells to reduce intracellular reactive oxygen species (ROS) levels, thereby enabling tumor cells to maintain cellular redox homeostasis and cell survival under nutrient limitation and prolonged hypoxia conditions [9], [10], [11]. Previous studies have deepened our understanding of the contribution of aerobic glycolysis to epigenetic modifications, transcriptional regulation, and signal transduction, which all are fundamental in cancer development [12].
The conversion of fructose 6-phosphate (F6P) to fructose 1, 6-diphosphate (FBP) catalyzed by phosphofructokinase (PFK) 1 is the committed step to determine glycolytic flux. PFK1 is allosterically regulated by several metabolites, including ATP and fructose 2,6-bisphosphate (F-2,6-BP), a shunt product converted from F6P by PFK2 (Figure 1). PFK2 is a well-known bifunctional enzyme that simultaneously exerts kinase and phosphatase activities, indicating that this enzyme catalyzes both the formation and degradation of F-2,6-BP. In humans, there are four isozymes of PFK2, i.e., PFKFB1, PFKFB2, PFKFB3, and PFKFB4, that exert tissue-specific expression patterns and distinct kinase-to-phosphatase activity [13] (Table 1). All four isozymes are induced by hypoxia in vivo; however, the hypoxia responsiveness varies in different organs [14].
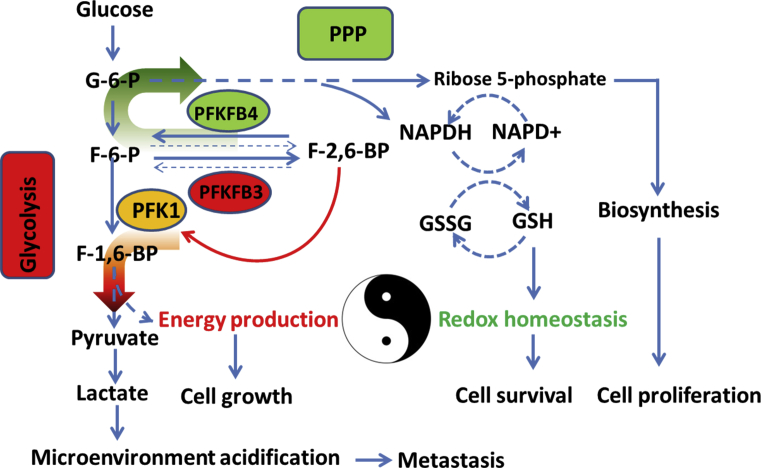
Figure 1.
PFKFB3 and PFKFB4 coordinate glycolysis and the PPP in cancer cells. PFKFB3 has the highest kinase:phosphatase ratio (710:1), whereas PFKFB4 has more phosphatase activity within a kinase:phosphatase ratio of 4.6:1. Both PFKFB3 and PFKFB4 are hypoxia inducible and contribute to hypoxia-induced glycolysis; however, their functions and biochemical properties are distinct. PFKFB3 strongly increases F-2,6-BP levels and promotes glucose utilization through glycolysis, but reduces glucose entry into the PPP. In contrast, PFKFB4 redirects more glucose through the PPP to produce NAPDH and ribose 5-phosphate, supporting ROS detoxification and lipid and nucleotide synthesis. Co-expression of PFKFB3 and PFKFB4 fine-tunes glucose metabolism in cancer cells, ensuring continued energy supply and redox homeostasis.
Table 1.
Properties of PFK2 isoenzymes.
| Gene | Tissue expression pattern | Kinase/bisphosphatase ratio | Expression in cancers |
|---|---|---|---|
| PFKFB1 | Liver, muscle, white adipose tissue, fetal, hypoxia inducible | 2.5 (rat liver) 1.2 (bovine liver) 0.4 (rat muscle) |
Not detected |
| PFKFB2 | Heart, hypoxia inducible | 1.8 (bovine heart) | Melanoma [133], osteosarcoma [134], HCC [135], prostate cancer [136] |
| PFKFB3 | Ubiquitous, hypoxia inducible | 710:1 (human placenta) | Breast cancer, colon cancer, HCC, astrocytoma, glioma |
| PFKFB4 | Testis, hypoxia inducible | 4.6:1 (recombinant human PFKFB4), 0.9 (human testis) |
SCNC, glioblastoma, bladder cancer, gastric, pancreatic cancer, prostate cancer, AML |
In this review, we summarize the roles of PFK2 members, particularly PFKFB3 and PFKFB4, in the regulation of glucose metabolism and extra-glycolytic functions in cancer development.
2. Regulation of glycolytic flux in cancer cells by PFK2
The glycolytic flux in tumor cells is regulated by fine-tuning of different enzymatic reactions, enabling glucose-derived carbon units to enter other anabolic pathways, such as biosynthesis of nucleotides and proteins. Tumor cells exploit PFK2 to stimulate glycolysis [15]. Activation of oncogenic signaling, including Myc overexpression, estrogen receptor (ER) signaling, activation of Ras, and loss of p53, has been found to stimulate glycolysis in part by activating PFK2 isozymes [16], [17]. Among the four isoenzymes, PFKFB3 is frequently overexpressed in cancer, including breast cancer [18], colon cancer [18], [19], hepatocellular carcinoma (HCC) [20], and high-grade astrocytoma [21]. PFKFB4 is also overexpressed in various human cancers, including prostatic small cell neuroendocrine carcinoma (SCNC) [22], glioblastoma [23], bladder cancer [24], gastric cancer, and pancreatic cancer [25]]. Overexpression of PFKFB3 is associated with poor survival in patients with HCC [19], whereas high PFKFB4 expression predicts unfavorable prognosis in patients with primary glioblastoma and bladder cancer [23], [24].
PFKFB3 is an essential glycolytic mediator required for the oncogenicity of H-rasV12. Loss of PFKFB3 suppresses anchorage-independent growth and tumorigenicity of H-rasV12-transformed lung fibroblasts [26]. Silencing of PFKFB3 decreases cellular levels of F-2,6-BP, lactate, and ATP, suppressing anchorage-independent growth and inducing tumor cell apoptosis. Additionally, PFKFB3 stimulates glycolytic flux to support cancer cell growth [27], [28], [29], [30], [31]. Selective inhibition of PFKFB4 expression reduces F-2,6-BP, glucose uptake and tumor growth in vivo [32], [33]. Furthermore, silencing either PFKFB3 or PFKFB4 in lung adenocarcinoma H460 cells attenuates hypoxia-induced glycolytic flux and suppresses cancer cell proliferation and survival under hypoxia [32], [33]. PFKFB4 has been shown to be directly regulated by peroxisome proliferator-activating receptor γ (PPARγ) and is required for PPARγ-stimulated glycolysis in HCC [34]. Depletion of PFKFB4 in brain cancer stem-like cells (CSCs) reduces lactate secretion and ATP levels but induces massive cell death, suggesting that PFKFB4 may be essential for maintaining the stemness of brain CSCs [23]. In response to mitophagy-dependent removal of mitochondria and energetic deficit during prolonged mitotic arrest, AMP-activated protein kinase (AMPK) signaling is activated, resulting in phosphorylation and activation of PFKFB3. AMPK positively controls glycolytic flux via mitotic-specific promotion of PFKFB3 translation and phosphorylation of PFKFB3 at Ser461. Silencing or pharmacological inhibition of PFKFB3 reduces F-2,6-BP levels, glucose consumption, and lactate production, leading to precocious mitotic cell death [35]. Similarly, depletion of PFKFB4 in mitotically arrested ovarian cancer cells causes a similar phenotype [36], suggesting that the metabolic switch from OXPHOS to glycolysis represents a crucial mechanism utilized by cancer cells to escape from death during mitotic arrest. These studies indicate that replacement of oxidative respiration by PFK2-stimulated glycolysis may be involved in tumor refractoriness to microtubule poisons [37]. All four PFK2 members have simultaneous kinase and phosphatase activities. Although some studies have shown that the kinase domain of PFKFB4 is required for stimulating glycolysis and xenograft tumor growth [33], the activities of PFKFB4 are more balanced, with the bisphosphatase activity being relative higher. Several independent studies have demonstrated the crucial role of PFKFB4 in managing ROS accumulation through diverting glucose metabolic intermediates to the oxidative arm of the pentose phosphate pathway (PPP) in various cancer cells [38], [39], [40], [41]. These phenomena could be explained by the observation that the enzymes are also post-translationally regulated; therefore, their relative kinase and bisphosphatase activities could adapt to the changing cellular needs. For example, ROS-induced S-glutathionylation of PFKFB3 inactivates its kinase activity and causes a metabolic shift from glycolysis to PPP, facilitating NAPDH generation and ROS detoxification in cancer cells [42].
3. PFKFB3 contributes to pathological neovascularization
Pathological angiogenesis and vascular malformations are common in solid tumors. Tumor vessels are irregularly shaped, within gaps between cells that cause vessel leakiness. Leaky, twisted blood vessels not only reduce the delivery of chemotherapy drugs but also accelerate tumor metastasis [43]. Tumor endothelial cells (TECs) exhibit active proliferation and migration abilities compared with normal endothelial cells (ECs). ECs, particularly TECs, primarily use glycolysis rather than OXPHOS for ATP generation [44]. The expression level of PFKFB3 is much higher in TECs than in normal ECs. Additionally, overexpression of PFKFB3 promotes vessel branching via inhibition of the pro-stalk activity of Notch signaling [44]. Upon hypoxia and human vascular endothelial growth factor stimulation, PFKFB3 expression is upregulated in ECs and promotes filopodia/lamellipodia formation and directional migration in ECs. Depletion of PFKFB3 in ECs suppresses xenograft tumor growth due to insufficient blood vessels [45], and pharmacological inhibition of PFKFB3 by the small molecule 3-(3-pyridinyl)-1-(4-pyridinyl)-2-propen-1-one (3PO) inhibits EC proliferation and migration, leading to reduced vessel sprouting in EC spheroids, zebrafish embryos, and postnatal mouse retinas [46].
A recent study revealed that genetic or pharmacological inhibition of PFKFB3 in TECs tightens the vascular barrier by reducing VE-cadherin endocytosis in TECs and promoting pericyte coverage on TECs, leading to improved vessel maturation and perfusion, thereby reducing tumor cell intravasation and metastasis without affecting cell proliferation or tumor volume [47]. Furthermore, PFKFB3 inhibition downregulates vascular cell adhesion protein-1, E-selectin, and intracellular adhesion molecule-1 expression in TECs through inhibition of nuclear factor-κB signaling, leading to impairment of tumor extravasation [47]. Traditional antivascular treatments aim to reduce angiogenesis and subsequently inhibit primary tumor growth. However, reducing angiogenesis hinders the delivery of chemotherapy drugs and may exacerbate internal hypoxia, creating a more hostile milieu and allowing tumor cells to escape via invasion and metastasis [48], [49], [50], [51], [52]. Given that traditional anti-angiogenic therapies have shown limited success, tumor vessel normalization by targeting PFKFB3-mediated glycolysis is emerging as a promising strategy for those “difficult-to-cure” diseases associated with vascular dysregulation, such as cancer [53], [54], [55], [56].
4. Coordination of glycolysis and the PPP in tumor cells by PFKFB3 and PFKFB4
In addition to glycolysis, metabolic reprogramming also enhances the PPP in tumors to support cancer cell proliferation and metastasis [57], [58], [59], [60]. The PPP is a glucose metabolic pathway parallel to glycolysis and consists of two distinct arms. The first is the oxidative arm, in which glucose-6-phosphate (G6P) is converted to ribulose 5-phosphate coupled with generation of nicotinamide adenine dinucleotide phosphate (NADPH). The second arm is the nonoxidative synthesis of 5-carbon sugars as well as ribose 5-phosphate used for de novo nucleotide biosynthesis. Ribulose-5-phosphate from the oxidative PPP promotes acetyl-CoA carboxylase 1 activation and lipogenesis by inhibiting activation of liver kinase B1/AMPK signaling [61]. The PPP generates approximately 60% of NADPH in humans, which not only provides reducing power for the reductive biosynthesis of macromolecules such as lipids but also prevents oxidative stress by scavenging ROS [62]. Many NAPDH-requiring processes are essential for cell survival. Suppression of glucose 6-phosphate dehydrogenase, the rate-limiting enzyme of the oxidative PPP arm, leads to reduction of ribulose 5-phosphate but elevated ROS levels, thereby decreasing lipogenesis and RNA biosynthesis in cancer cells and attenuating cell proliferation and tumor growth [61].
Because glucose in cancer cells is primarily utilized through either glycolysis or the PPP, it should be shunted into these two pathways to balance anabolic metabolism and redox homeostasis, ensuring unlimited cell proliferation and escaping cell death. Both PFKFB3 and PFKFB4 are induced by hypoxia and are required for the glycolytic response to hypoxia [32]; however, their biochemical characteristics are distinctly different. PFKFB3 has the highest kinase activity among the four isozymes and preferentially shunts glucose toward glycolysis. Inhibition of PFKFB3 reroutes glucose metabolism from glycolytic flux to PPP [42], [63], [64], [65], [66]. In contrast, silencing of PFKFB4 causes increases in F-2,6-BP level in glucose-dependent prostate cancer cells, which, in turn, siphons more glucose into glycolytic flux at the expense of PPP impairment and reduced NAPDH production, leading to apoptosis of prostate cancer cells and attenuating tumor growth. However, co-silencing of PFKFB3 reduces F-2,6-BP levels and rescues PC cells from cell death triggered by PFKFB4 deletion [38], emphasizing the importance of an appropriate balance between glycolysis and the PPP for cancer cell survival. Interestingly, silencing of PFKFB4 impairs the PPP in prostate cancer cells but not in nonmalignant RWPE1 prostate cells [38]. An unbiased RNAi-based screen also showed that knockdown of PFKFB4 in prostate cancer cells impairs the PPP and causes ROS accumulation and induction of autophagy [40]. In a recent study, PFKFB4 was characterized as a binding partner of the oncogenic transcription factor SRC-3 and was found to promote SRC-3-driven transcription of target genes to participate in nonoxidative PPP and purine anabolism [41]. Similarly, depletion of PFKFB4 in p53-deficient cancer cells impairs the oxidative PPP, resulting in disruption of cellular biosynthetic activity and increasing ROS; this effect is not observed in cells expressing wild-type p53 [39]. This result could be explained by the observation that malignant cells, such as p53-mutant cells, require more pentose phosphate and NAPDH than non-transformed cells for nucleic acid synthesis and to maintain redox homeostasis; thus, the high phosphatase activity of PFKFB4 safeguards cancer cells from excessive oxidative stress. Moreover, wild-type p53 represses PFKFB3 transcription and reroutes glucose into the PPP to increase nucleotide production, thereby facilitating DNA damage repair [65]. Accordingly, tumor cells with different p53 genetic backgrounds are able to exploit different PFK2 isoenzymes to control the dynamic balance of glycolysis and PPP, ensuring cell survival. The functional differences between PFKFB3 and PFKFB4 allow tumor cells to exploit these enzymes as a pair of valves shunting glucose utilization through either glycolysis or the PPP [67], altering the balance of bioenergetics and cellular redox homeostasis (Figure 1).
5. Post-translational regulation of PFKFB3 confers metabolic flexibility to tumor cells
The transcription of all four PFK2 family members can be induced by hypoxia. Hypoxic induction of PFKFB isozymes is dependent on hypoxia-inducible factor-1α (HIF-1α) activation [14]. Moreover, activation of mammalian target of rapamycin (mTOR) signaling also upregulates PFKFB3 transcription in a HIF-1α-dependent manner [68]. In addition, PFKFB3 mRNA transcription is also directly regulated by ER. Estradiol (E2) stimulates glucose uptake and glycolysis in ER (+) breast cancer cells through induction of PFKFB3 [69]. Steroid receptor coactivator (SRC)-2 along with progesterone receptor have been shown to bind to a progesterone-responsive element within the PFKFB3 promoter and activate its transcription in human endometrial stromal cells [70]. Transcription of PFKFB3 is also regulated by various stress stimuli (NaCl, H2O2, ultraviolet radiation, and anisomycin) through serum-response factor binding to a serum-response element in the PFKFB3 promoter [71]. A recent study revealed that the Ets transcription factor PU.1 promotes PFKFB3 transcription in tyrosine kinase inhibitor (TKI)-resistant chronic myeloid leukemia (CML) cells [72]. In addition to transcription control, translation of PFKFB3 mRNA is promoted by AMPK signaling during mitosis via involvement of a cytoplasmic polyadenylation element in the 3′-untranslated region of PFKFB3 mRNA [35]. PFKFB4 is also transcriptionally regulated by several oncogenic signaling pathways. For example, PFKFB4 is positively regulated by CD44 in prostate small cell neuroendocrine carcinoma cells [73]. Additionally, fibroblast growth factor-2 promotes PFKFB4 transcription by activating the binding of the transcription factor CREB to a putative CRE-binding sequence in the PFKFB4 promoter [74]. Otherwise, both promoters of PFKFB3 and PFKFB4 have p53 response elements, and the transcription of these genes is directly repressed by wild-type p53 [39], [65].
In addition to transcriptional control of enzymes, reversible modifications of existing metabolic enzymes provide a more flexible and economical way to quickly respond to environmental changes at the metabolic level. Among the four PFK2 isozymes, PFKFB3 is the most intensively studied. PFKFB3 protein stability, subcellular localization, and kinase activity are reversely regulated by various post-translational modifications in response to stress stimuli, which allows tumor cells to make rapid adaptive changes to metabolic stress.
5.1. Regulation of PFKFB3 activity by phosphorylation
Tumor cells, vascular ECs, and smooth muscle cells express highly phosphorylated PFKFB3 proteins. Phosphorylated PFKFB3 has higher kinase activity than its unphosphorylated form [75]. AMPK is a central cellular energy sensor that promotes or suppresses cancer cell survival in a context-dependent manner. An increase in the AMP/ATP ratio leads to activation of AMPK. Upon activation, AMPK stimulates catabolic metabolism and inhibits anabolic processes [76]. AMPK is activated under ATP deprivation conditions, such as hypoxia [77], [78]. Subsequently, activation of AMPK promotes the survival of hypoxic tumors through induction of autophagy [79], [80] and promotion of fatty acid oxidation [81]. Increase of AMP would not only activate AMPK to promote survival in hypoxic tumors but would also be expected to allosterically stimulate PFK-1 activity to enhance glycolysis [82]. AMPK phosphorylates PFKFB3 at Ser461, enhances the glycolytic activity of PFKFB3, and promotes the proliferation of cancer cells [19], [83] (Figure 2). Phosphorylation of PFKFB3 at Ser461 by AMPK rapidly provides sufficient ATP, which alleviates the bioenergetic crisis resulting from mitophagy-mediated removal of mitochondria and prevents mitotic cell death during prolonged mitotic arrest [35] (Figure 2). It needs to be clarified that under hypoxic conditions, PFKFB3 activation by AMPK-induced phosphorylation of Ser461 would increase glycolytic flux via a rise in F-2,6-BP which allosterically stimulates PFK1 activity. AMPK also phosphorylates heart PFKFB2 at Ser466 (equivalent to Ser461 of PFKFB3) and promotes heart glycolysis during ischemia [74], suggesting that Ser461 in PFK2 members is a pivotal regulatory site for determining glycolytic flux. However, the phosphorylation of PFKFB4 has not been extensively studied to date, which might be due to the fact that sites in the N- and C-terminal regulatory domains of the other PFKFB isoenzymes are not conserved. In addition to AMPK, p38/mitogen-activated protein kinase-activated protein kinase 2 also phosphorylates PFKFB3 at Ser461 upon exposure to stress stimuli [71] (Figure 2). Phosphorylation of PFKFB3 protein at Thr463 and Ser467 by cyclin-dependent kinase (CDK) 6 also leads to enhanced glycolysis and breast cancer cell proliferation [84] (Figure 2). In contrast, phosphorylation at Ser269 of PFKFB3 by inhibitor of nuclear factor kappa-B kinase subunit beta (IKKβ) in response to low glutamine conditions inhibits its activity [85] (Figure 2). Reversible phosphorylation of PFKFB3 protein allows tumor cells to rapidly adjust their bioenergetics to cope with the complexity of the tumor environment and variable stress states within tumors.
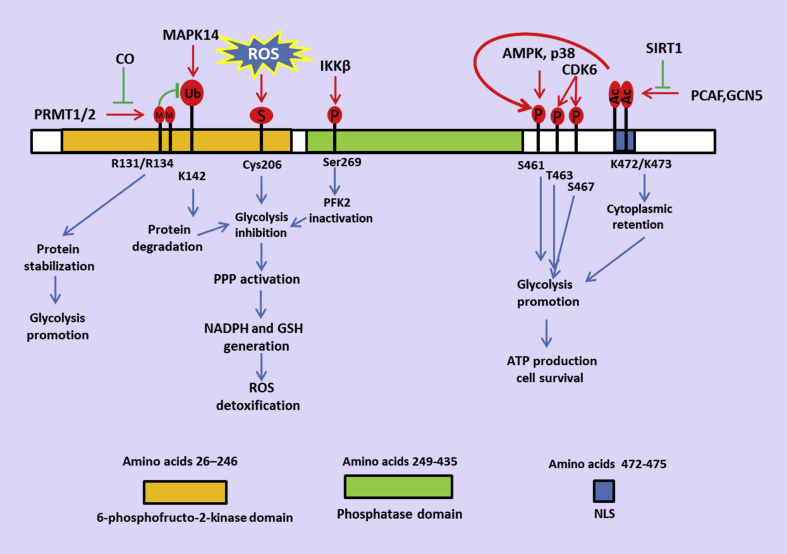
Figure 2.
Multiple post-translational modifications of PFKFB3 differentially regulate glucose utilization. PFKFB3 protein levels, subcellular localization, and PFK2 activity are largely affected by post-translational modifications. Phosphorylation of Ser461 by AMPK and Thr463 and Ser467 by CDK6 activates PFK2 activity and promotes glycolysis. PCAF- and GCN5-mediated acetylation of Lys472/473 disrupts the NLS and sequesters PFKFB3 in the cytoplasm, facilitating its phosphorylation on Ser461 by AMPK. Polyubiquitination on Lys142 of PFKFB3 leads to proteasomal degradation of PFKFB3, shunting glucose metabolism from glycolysis to the PPP. Asymmetrical dimethylation on Arg131 and Arg134 stabilizes PFKFB3. Reduced methylation of PFKFB3 reroutes flux into the oxidative arm of PPP.
5.2. ROS-dependent S-glutathionylation of PFKFB3
Persistent glycolysis and redox regulation are two intertwined processes. Glycolytic flux largely affects intracellular ROS levels. For example, enhanced activity of PFKFB3 in T lymphocytes increases ROS generation as an end product of glycolysis [86]. Excessive oxidative stress in turn induces failure of glycolysis by inactivating a number of glycolytic enzymes, such as glyceraldehyde 3-phosphate dehydrogenase, pyruvate kinase M2 (PKM2), and PFK1 [87]. Because excessive ROS accumulation disrupts cell survival, cancer cells must maintain redox homeostasis through metabolic redirection based on intracellular ROS levels [87], [88]. In the presence of excessive ROS, PFKFB3, rather than other PFKFB isoenzymes, is S-glutathionylated on Cys206, which is a critical residue near the entrance to the 6-phosphofructo-2-kinase catalytic pocket, resulting in decreased catalytic activity for synthesis of F-2,6-BP [42] (Figure 2). S-glutathionylation on Cys206 induced by high ROS levels inactivates PFKFB3 and leads to shifting glucose utilization from glycolysis to the PPP, ensuring NAPDH production, regeneration of glutathione (GSH), and ROS detoxification in cancer cells (Figure 2). ROS-sensitive regulation of PFKFB3 activity allows tumor cells to use glucose, thereby permitting the synthesis of antioxidants, such as NAPDH and GSH, at the expense of glycolytic energy management. This process also enables cells to escape the detrimental effects of excessive oxidative stress. Thus, S-glutathionylation of PFKFB3 provides cancer cells with a reversible and faster thiol-based switch independent of transcriptional or signaling pathways to cope with oxidative damage through metabolic rewiring.
5.3. Regulation of PFKFB3 protein stability by polyubiquitination and methylation
Ubiquitin-mediated proteasomal degradation of PFKFB3 protein reroutes glucose metabolism from glycolysis to the PPP, thereby promoting NAPDH production and maintenance of the proper intracellular redox environment under conditions of nutrient deprivation. Two distinct degradation sequences of PFKFB3 protein, i.e., the KEN box and DSG motif Ser273, are required for recognition by APC/CFZR1 and SCF-BTRC E3 ligase complexes, respectively (Figure 2) [63]. In contrast, activation of the N-methyl-d-aspartate subtype of glutamate receptors prevents APC/CDH1-mediated proteasomal degradation of PFKFB3 protein in cortical neurons, leading to oxidative stress and apoptosis of neurons due to an insufficient PPP [66], [89].
Asymmetrical dimethylation at Arg131 and Arg134 of the N-terminal fragment of PFKFB3 leads to stabilization of PFKFB3 protein in human leukemia U937 cells (Figure 2). Protein arginine methyltransferase (PRMT) 1 and PRMT4 are responsible for dimethylation at these two sites of PFKFB3 in vitro. Carbon monoxide (CO), a byproduct of heme oxygenase-1 (HO-1), catalyzes heme degradation processes and reduces methylation of PFKFB3 by inhibiting heme-containing cystathionine b-synthase (CBS) activity, which plays a crucial role in protein arginine methylation [90]. Reduced methylation leads to K142 polyubiquitination of PFKFB3 followed by proteasomal degradation, shifting glucose utilization from glycolysis toward the PPP [64] (Figure 2). The HO-1/CO system is upregulated in tumor tissues upon exposure to anticancer reagents, radiation therapy, oxidative stress, or prolonged hypoxia, resulting in promotion of tumor neovascularization and drug resistance [91]. CBS knockdown suppresses PFKFB3 methylation and causes metabolic rewiring toward the PPP and acquisition of chemoresistance [59], consistent with the observation that metabolic remodeling from glycolysis to the PPP contributes to drug resistance by increasing NADPH and reducing glutathione used for drug detoxification [92].
5.4. Regulation of cytoplasmic/nuclear shuttling of PFKFB3 by acetylation
Various metabolic enzymes can translocate from the cytoplasm to the nucleus and exert nonmetabolic functions [93]. One example is PKM2, an alternative splicing isoform of the PKM gene. Upon stimulation of epidermal growth factor receptor signaling, PKM2 is phosphorylated at Ser37 in the cytoplasm, leading to a conformational change in PKM2 from a tetramer to a monomer and inducing translocation into the nucleus [94], [95]. PFKFB3 protein also shuttles from the cytoplasm to the nucleus in transformed cells. Nuclear PFKFB3 protein stimulates cancer cell proliferation via an extraglycolytic mechanism [96]. A nuclear localization signal (NLS) sequence, KKPR (amino acid residues 472–475), at the COOH-terminal of PFKFB3 is required for trafficking of PFKFB3 protein to the nucleus; Lys472 and Lys473 are key residues in this sequence [96] (Figure 2). Continued excessive glycolysis can reduce glucose utilization through the PPP, resulting in insufficient NAPDH production and oxidative stress. Considering the inadequate blood supply inside tumor tissues and the limited glucose source, nuclear PFKFB3 protein allows cancer cells to proliferate without weakening the PPP and disrupting intracellular homeostasis.
Acetylation at Lys472 of PFKFB3 protein disrupts the NLS motif and promotes cytosolic retention of PFKFB3 by impairing the interaction between PFKFB3 and importin α5 [83]. p300/CBP-associated factor (PCAF) and GCN5 are primarily responsible for catalyzing Lys472 acetylation in PFKFB3, and sirtuin 1 is the major deacetylase [83] (Figure 2). Although acetylation of PFKFB3 does not affect its intrinsic 6-phosphofructo-2-kinase activities, cytosolic retention of PFKFB3 by Lys472 acetylation increases the phosphorylation of PFKFB3 at Ser461 (Figure 2), which is known to be associated with enhanced glycolysis activity. DNA damage induced by cisplatin stimulates PFKFB3 acetylation on Lys472 in an ATM-dependent manner, which then promotes glycolysis and protects cells from apoptosis. Notably, the protective effects of cytoplasmic PFKFB3 are highly dependent on its catalytic activity [83], suggesting that increased glycolysis is important for survival of cancer cells in the presence of cisplatin. Increased glycolytic activity rapidly provides ATP for cell survival during a bioenergetic crisis, consistent with the hypothesis that increased intracellular ATP enables tumor cells to use ATP-dependent transporters, such as P-glycoprotein (MDR1 protein), to pump cytotoxic drugs out and hence increase cell survival [97]. Another explanation is that ATP production from glycolysis facilitates the phosphorylation of BAD, a pro-apoptotic BH3 domain only protein. Deprivation of ATP generated by glycolysis induces rapid dephosphorylation of BAD at Ser112, allowing replacement of Bcl-2 or Bcl-xL associated with BAX and then activation of intrinsic mitochondrial apoptosis pathway [98].
6. Extra-glycolytic functions of PFKFB3 and PFKFB4 in cancer
Growing evidence has suggested that glycolytic enzymes exert non-glycolytic roles in the regulation of various physiological or pathological processes [12]. In addition to well-recognized roles in aerobic glycolysis, biosynthesis, and redox homeostasis, PFKFB3 and PFKFB4 have also been implicated in transcriptional regulation, cell cycle progression, and autophagy.
6.1. Regulation of the cell cycle by nuclear PFKFB3
PFKFB3 protein translocates to the nucleus in transformed cells [20], [96]. Overexpression of nuclear PFKFB3 markedly accelerates cell proliferation without increasing glycolytic activity, indicating that nuclear PFKFB3 exhibits an extraglycolytic role to promote cell proliferation [96]. Recent studies have unveiled the association of PFKFB3 with CDKs and established the roles of nuclear PFKFB3 in cell cycle regulation. The expression of nuclear PFKFB3 (variant 5) promotes cell growth by upregulating CDK1, CDC25C, and CCND3 while downregulating p27 protein levels in HeLa cells (Figure 3). This function of PFKFB3 in promoting proliferation is dependent on both its kinase activity and nuclear localization because both kinase-inactive (Arg75/Ala76) and cytoplasmic (Lys472/473Ala) mutants fail to stimulate cell proliferation, suggesting that nuclear delivery of F-2,6-BP is required for cell cycle regulatory functions [96]. Further research has indicated that nuclear F-2,6-BP activates CDK1, leading to CDK-mediated phosphorylation of the Cip/Kip protein p27 at Thr187, which in turn results in ubiquitination and proteasomal degradation of p27 (Figure 3). Silencing of PFKFB3 in HeLa cells delays G1/S phase progression of the cell cycle and promotes apoptosis [99]. A recent study demonstrated that PFKFB3 binds directly to CDK4 and prevents CDK4 from undergoing proteasomal degradation mediated by the heat shock protein (HSP) 90/Cdc37/CDK4 complex. Loss of PFKFB3 disrupts the HSP90/Cdc37/CDK4 complex, resulting in rapid ubiquitination and degradation of CDK4 (Figure 3). Lys147 of PFKFB3 is crucial for binding with CDK4, and mutation of this lysine residue to alanine abolishes this interaction and fails to stabilize CDK4 protein [100]. Interestingly, PFKFB3 is associated with both CDK6 and F-Box and WD repeat domain containing 7. Association with CDK6 increases PFKFB3 phosphorylation on Thr463 and Ser467 in breast cancer cells [84].
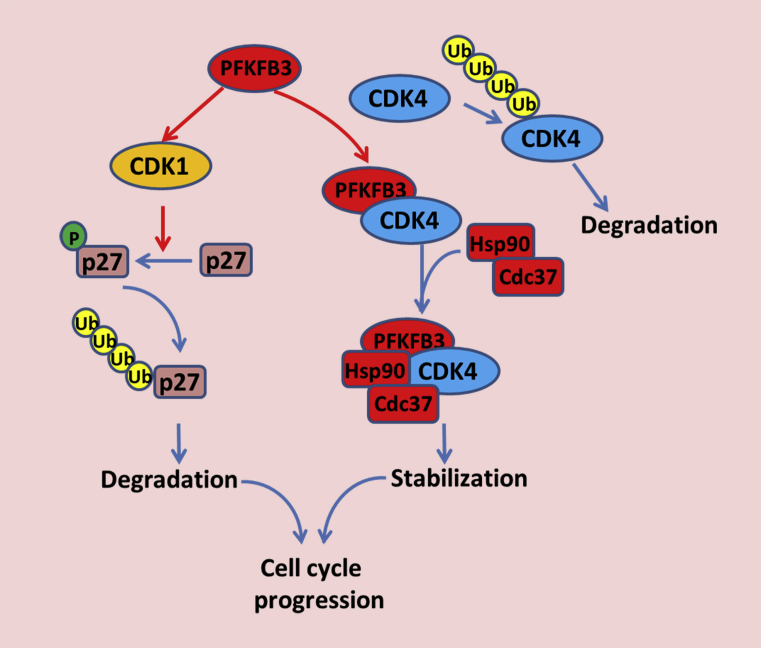
Figure 3.
Nuclear PFKFB3 promotes cell cycle progression via modulating CDK activities. Wild-type PFKFB3 is mainly localized in the nucleus. Nuclear PFKFB3 activates CDK1 in the presence of F-2,6-BP, resulting in CDK1-mediated phosphorylation of p27 at Thr187 and subsequent proteasomal degradation. Nuclear PFKFB3 also directly binds to CDK4 and promotes the formation of the HSP90/Cdc37/CDK4 complex, which stabilizes CDK4 protein and accelerates G1/S phase cell cycle progression.
6.2. PFKFB 4 acts as a protein kinase and is involved in transcriptional regulation
A major discovery in recent years is that some metabolic enzymes can act as protein kinases, as exemplified by PKM2. Nuclear PKM2 phosphorylates histone H3 at Thr11 to regulate gene transcription [101]. In addition, PKM2 also phosphorylates a number of non-histone proteins, including Bub3, myosin light chain 2 [102], and PAK2 [103], to regulate mitosis and invasiveness in cancer. A recent study uncovered a previously unrecognized role of PFKFB4 as a protein kinase; PFKFB4 phosphorylates the CBP-interacting domain of SRC-3 on Ser857 by transferring a phosphate group from ATP, thereby increasing SRC-3 activity. Phosphorylation at Ser857 promotes the association of SRC-3 with ATF4 and recruitment to the target gene transketolase (TKT; Figure 4), which is a major metabolic enzyme mediating the nonoxidative PPP and purine synthesis. In addition, SRC-3 also upregulates two other enzymes that directly or indirectly participate in purine anabolism, i.e., adenosine monophosphate deaminase-1 (AMPD1) and xanthine dehydrogenase (XDH; Figure 4). Silencing of either PFKFB4 or SRC-3 reduces not only the intracellular pools of ribose-5P but also the concentration of purine nucleotides and intermediates, including adenosine, xanthine, and guanine, in high glycolytic breast cancer cells [41]. These results are consistent with previous reports demonstrating that PFKFB4 is essential for the PPP in cancer cells [38], [39]. Thus, PFKFB4 promotes glucose utilization through the PPP via both metabolic and transcriptional regulation. Furthermore, PFKFB4-dependent SRC-3 phosphorylation at Ser857 also promotes ER activity in the presence of E2 and glucose (Figure 4). However, the possibility that PFKFB4 acts as a protein kinase is still a preliminary hypothesis, and additional studies are needed to confirm the protein kinase function of PFKFB4 and to clarify how PFKFB4 phosphorylates SRC-3 protein. Future studies should also evaluate whether SRC-3 phosphorylation on Ser857 is inhibited by the PFKFB4 6-phosphofructo-2-kinase inhibitor 5MPN. Moreover, it is still unclear whether PFKFB4 directly phosphorylates SRC-3 or an insect protein kinase bound to PFKFB4 mediates the phosphorylation of SRC-3 [104].
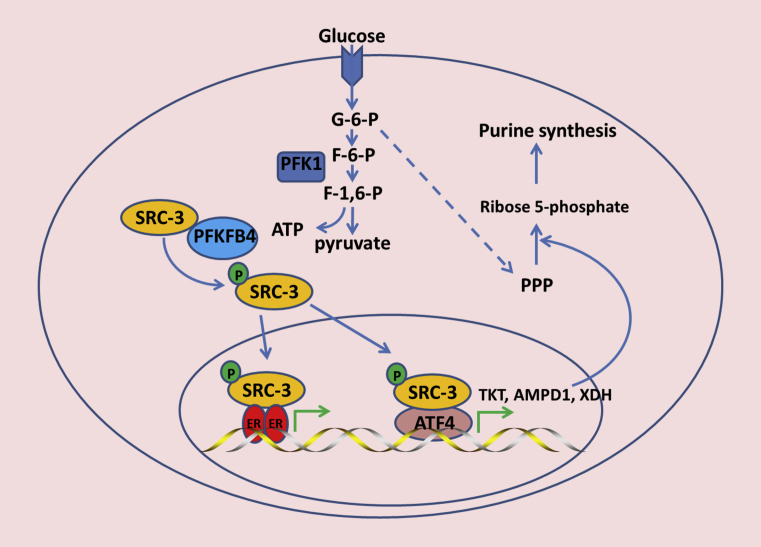
Figure 4.
PFKFB4 regulates SRC-3 activity. In addition to its canonical function in glucose metabolism, PFKFB4 binds to and phosphorylates the co-activator SRC-3 at Ser857 in glycolytic breast cancer cells, Phosphorylation at Ser857 enhances SRC-3 activity and facilitates SRC-3 binding with the ER and recruitment of ATF4 to activate the transcription of TKT, AMPD1, and XDH, which directly or indirectly promotes pyrimidine synthesis.
6.3. Regulation of autophagy by PFKFB3 and PFKFB4
Degradation of cytoplasmic contents by active autophagy is required for efficient metabolic adaptation to fulfill biosynthetic demand [86], [105]. Atg5-independent mitophagy contributes to metabolic reprogramming from mitochondrial oxidative phosphorylation to glycolysis during prolonged mitotic arrest [106]. In contrast, autophagy is essential for cancer cell survival by providing the metabolites required for the maintenance of mitochondrial respiration and redox homeostasis when glycolysis is blocked [107]]. Autophagy is regulated by various stress stimuli, including nutrient deprivation, oxidative stress, and hypoxia, and is also associated with drug resistance [108], [109], [110].
Because PFKFB3 and PFKFB4 differentially implicated in maintaining energy production and redox homeostasis, they can either promote or suppress autophagy in a cell context- or stimulus type-dependent manner. A cytoplasmic PFKFB3 Lys472/473Ala mutant was found to strongly stimulate glycolytic ATP generation, thereby inhibiting autophagy in renal cell carcinoma (RCC) cells [111]. Similarly, loss of PFKFB3 function in HCT-116 colon adenocarcinoma cells by either siRNA transfection or 3PO treatment triggers autophagy due to reduction of glycolytic ATP generation [30]. However, PFKFB3 also exhibits autophagy-promoting functions in RCC cells. These activities of PFKFB3 are dependent on its nuclear localization and are related to redox homeostasis [111]. Reduction of PFKFB3 expression shunts glucose metabolism into the PPP and produces excessive NAPDH, resulting in reductive stress and limited utilization of autophagy, as demonstrated in rheumatoid arthritis (RA) CD4+ T cells. Silencing of PFKFB3 in T-cell receptor-stimulated T cell blasts suppresses autophagy, and forced expression of PFKFB3 accelerates autophagic flux [112]. However, PFKFB3 is not required for rapamycin-induced autophagy flux in RCC cells, indicating that PFKFB3 is likely to regulate autophagy in a stimulus type-dependent manner [111].
PFKFB4 can also positively or negatively regulate autophagy. Depletion of PFKFB4 decreases flux into the PPP, which impairs NADPH generation and enhances ROS levels to induce autophagy [40]. A recent study showed that PFKFB4 is positively regulated by endothelial tyrosine kinase (Etk) and directly binds with Etk in small cell lung cancer (SCLC) cells. Depletion of either Etk or PFKFB4 in SCLC cells decreases autophagic activity. Conversely, high expression of PFKFB4 contributes to acquisition of chemoresistance and is correlated with poor chemotherapy responses and prognosis in patients with SCLC [113]. However, the mechanisms through which PFKFB4 regulates autophagy are still unclear, and further studies are needed to determine how the interaction between Etk and PFKFB4 regulates autophagy induction in chemoresistant SCLC cells.
7. Targeting PFKFB3 and PFKFB4 as a therapeutic strategy in patients with cancer
The reliance of cancer cells on glycolysis for ATP generation may represent an effective method for killing cancer cells, particularly for cells with mitochondrial respiratory defects. Furthermore, increased glycolysis in tumor cells is frequently associated with drug resistance, suggesting that drug resistance may be overcome by inhibition of glycolysis in cancer cells [98], [114]. PFKFB3 and PFKFB4 are the major PFK2 isozymes overexpressed in human cancers and therefore attracted more attention owing to their potential applications as therapeutic targets [115].
The first small molecule antagonist of PFKFB3 is 3PO. 3PO inhibits recombinant PFKFB3 activity and exerts antitumor effects in tumor-bearing mice. Ectopic expression of PFKFB3 suppresses the cytostatic activity of 3PO in Jurkat T cell leukemia cells and confers resistance to 3PO, accompanied by increased F-2,6-BP levels, supporting that 3PO functions in part by inhibiting the PFK2 activity of PFKFB3 [116]. Moreover, 3PO sensitizes breast cancer cells to microtubule poisons [35], suggesting that inhibition of PFKFB activity in combination with conventional chemotherapeutic drugs may have synergistic effects on cancer cells. Furthermore, inhibition of PFKFB3 by 3PO leads to tumor vessel normalization, thereby reducing metastasis and enhancing the chemotherapy efficacy of cisplatin [47]. These findings highlight the potential therapeutic opportunities for normalizing tumor vessels in malignant disease.
Due to its poor water solubility and the relatively high dose required to achieve sufficient efficacy, 3PO has not been evaluated in clinical trials in patients with cancer. Additionally, 3PO displays no selectivity against PFKFB3 and shows similar half-maximal inhibitory concentrations for the four PFKFB isoenzymes [117]. PFK15 (1-(4-pyridinyl)-3-(2-quinolinyl)-2-propen-1-one) is a 3PO derivative in which a pyridinyl ring is substituted with a quinolinyl ring. Computational modeling suggests that both 3PO and PFK15 dock in the substrate-binding domain of PFKFB3. However, the quinolinyl ring of PFK15 closely interacts with residues from the ADP/ATP binding site of PFKFB3, conferring PFK15 with approximately 100-fold more activity against PFKFB3 than 3PO. Furthermore, PFK15 is more specific than 3PO, with no obvious effects on the activities of purified PFK1, hexokinase, phosphoglucose isomerase, PFKFB4, or any of the tested 96 kinases, and displays improved pharmacokinetic properties compared with 3PO [118]. PFK15 suppresses glucose uptake by tumors in vivo and exhibits antitumor efficacy comparable to that of commonly used chemotherapeutic drugs (e.g., irinotecan and gemcitabine) [118]. Additionally, PFK15 exerts potent antitumor effects in multiple human cancer models, including HCC, head and neck squamous cell carcinoma, and gastric cancer cells [20], [119], [120]. Combined treatment with PFK15 and other compounds affecting glucose metabolism results in synergistic antitumor effects [121], [122]. Studies have also shown that inhibition of glycolysis restores the sensitivities of resistant cancer cells to chemotherapy. For example, PFKFB3 silencing or inhibition by 3PO or PFK15 markedly enhances the antitumor effects of TKIs such as imatinib in CML cells and prolongs the survival of allo-transplanted and xeno-transplanted CML model mice [72].
To date, only one PFK15 derivative, PFK-158, has entered a phase 1 clinical trial (clinicaltrials.gov #NCT02044861). Preclinical studies have indicated that PFK158 is a potent and specific PFKFB3 inhibitor, suppressing glycolysis and tumor growth in a mouse model. Importantly, PFK-158 showed improved tolerance in vivo than the prior two generations of PFKFB3 inhibitors. A dose escalation, multicenter phase 1 study demonstrated that PFK-158 is safe, with no significant dose-limiting toxicities or drug-related adverse events. One patient with advanced cancer enrolled in the phase 1 trial, which showed a marked reduction of overall hepatic metastatic tumor burden [123].
Using structure-based virtual computational screening, Chesney et al. discovered a first-in-class PFKFB4 inhibitor, 5-(n-(8-methoxy-4-quinolyl) amino) pentyl nitrate (5MPN), which showed potent antitumor effects and high oral bioavailability in a preclinical mouse model [124]. 5MPN appears to competitively bind to the F6P binding site of PFKFB4 and suppresses its kinase activity, reducing intracellular F-2,6-BP, glycolysis, and ATP content, which, in turn, suppresses lung adenocarcinoma cell proliferation. Notably, however, 5MPN does not affect PFK1 or PFKFB3 activity and does not alter the activity of 97 protein kinases at 10 μM, suggesting that 5MPN has selectivity for PFKFB4. Moreover, 5MPN selectively inhibits RAS-transformed cell proliferation and tumor growth but exerts little effect on normal cells, consistent with the hypothesis that malignant transformed cells rely more heavily on glycolysis [124]. Importantly, the FBPase-2 activity of PFKFB4 is crucial for maintaining redox balance and promoting prostate cancer cell survival [38]. Additionally, depletion of PFKFB4 leads to accumulation of ROS and cell death in p53-deficient cells [39]. However, no strategies to selectively inhibit FBPase-2 activity have been developed. Table 2 summarizes small molecule antagonists of PFKFB3 and PFKFB4.
Table 2.
PFKFB inhibitors.
| Inhibitors | Targets | Diseases | Developmental stage | References |
|---|---|---|---|---|
| 3PO | PFKFB3 | Jurkat T cell leukemia cells, breast cancer cells | In vitro study | [116], [137] |
| PFK-15 | PFKFB3 | Liver cancer, colon cancer, bladder cancer cells, CML | In vitro and preclinical evaluation | [118], [122] |
| PFK-158 | PFKFB3 | Hepatic metastatic tumor | Pharmacokinetic profiling, phase 1 clinical trial | [123] |
| N4A | PFKFB3 | HeLa cells | In vitro study | [138] |
| YN1 | PFKFB3 | HeLa cells | In vitro study | [138] |
| YZ9 | PFKFB3 | Not tested | Preliminary in vitro characterization | [138] |
| BrAcNHEtOP | Liver PFK2, testis PFK2 | Not tested | In vitro study | [139] |
| PQP | PFKFB3 | Colonic and bladder cancer cells | In vitro study; less growth inhibitory effect than PFK-15 | [122] |
| Compound 26 | PFKFB3 | Not tested | In vivo pharmacokinetic characterization | [140] |
| 5MPN | PFKFB4 | Lung adenocarcinoma cells | In vitro study, xenograft tumor | [124] |
Abbreviations: BrAcNHEtOP: N-bromoacetylethanolamine phosphate; 5MPN: 5-(n-(8-methoxy-4-quinolyl) amino) pentyl nitrate.
8. Perspectives
Although increased glycolysis is fundamental to cancer development, due to the complexity of metabolic reprogramming, pharmacologic disruption of glycolysis has not been shown to have clinical benefits. Excessive glucose consumption via glycolysis disrupts NADPH homeostasis, sensitizing cancer cells to oxidative cell death [125]. Thus, rather than attempting to inhibit glycolysis in the clinic, this high dependence on glycolysis may be exploited as a metabolic weakness that sensitizes tumor cells to oxidative death. For example, IKKβ-deficient cancer cells exhibit constitutive activation of PFKFB3, making these cells highly sensitive to glutamine depletion-induced cell death [85]. Ferroptosis is an iron-dependent mechanisms of programmed cell death triggered by peroxidation of unsaturated phospholipids. Blocking of cysteine-dependent GSH synthesis by inhibition of cysteine/glutamate antiporter (system Xc−) or loss of glutathione peroxidase 4 activity disrupts the antioxidant defenses of the cell, leading to cancer cell death by ferroptosis [126], [127]. Additionally, loss of PFKFB4 disrupts the NAPDH-generating PPP and leads to toxic ROS accumulation, ultimately resulting cancer cell death [38], [39]. Thus, inhibition of PFKFB4 may offer a metabolic vulnerability point to ferroptosis-inducing therapeutics.
In cancer biology, metabolic heterogeneity, which not only presents across human tumors [128], [129] but also exists within different regions of the same tumor [130], has become a major challenge. Although intratumor heterogeneity is well documented at the genetic level, the interactions between intratumor metabolic differences and genetic variations are less clear [131]. Although several technologies for characterization of intratumor heterogeneity at the genetic and epigenetic levels have been developed [132], methods for measurement of intratumor metabolic differences are urgently needed. Moreover, several topics should still be studied. For example, PFKFB4 has been shown to act as a protein kinase; however, it is still unclear whether specific inhibitor against the protein kinase activity of PFKFB4 can be developed without affecting its metabolite kinase activity. Surprisingly, although PFKFB3 activity is regulated by multiple forms of covalent modification, this is not the case for PFKFB4. Additional studies are needed to explore the possibility that post-translational modifications controls PFKFB4 activity under various stressful conditions during cancer progression. PFKFB3 has the highest kinase activity of all PFK isoforms and is primarily localized in the nucleus, suggesting that this protein may also act as protein kinase. Therefore, it is necessary to test this hypothesis and identify the substrates of this protein.
Authors' contributions
MY, YYB, and BX wrote the manuscript. YXT, WX, and GYL revised and corrected the manuscript.
Acknowledgments
This study was supported in part by Grants from The National Natural Science Foundation of China (81572667, 81772902, 81872278, 81703131), the National “111” Project (Project #111-2-12), The Natural Science Foundation of Hunan Province, China (2018JJ1040, 2017JJ3440), and Hunan Provincial Key Research and Development Program (2018SK2130, 2018SK2131). We apologize to those researchers whose work in the field could not be cited in the present review due to space constrains.
Conflicts of interest statement
The authors declare no potential conflicts of interest.
References
- 1.Warburg O. On the origin of cancer cells. Science. 1956;123:309–314. doi: 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- 2.Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269–270. [PubMed] [Google Scholar]
- 3.Ward P.S., Thompson C.B. Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer Cell. 2012;21:297–308. doi: 10.1016/j.ccr.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wise D.R., Thompson C.B. Glutamine addiction: a new therapeutic target in cancer. Trends in Biochemical Sciences. 2010;35:427–433. doi: 10.1016/j.tibs.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yi M., Li J., Chen S., Cai J., Ban Y., Peng Q. Emerging role of lipid metabolism alterations in Cancer stem cells. Journal of Experimental & Clinical Cancer Research: CR. 2018;37:118. doi: 10.1186/s13046-018-0784-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang L., Wei F., Wu Y., He Y., Shi L., Xiong F. Role of metabolism in cancer cell radioresistance and radiosensitization methods. Journal of Experimental & Clinical Cancer Research: CR. 2018;37:87. doi: 10.1186/s13046-018-0758-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cerella C., Dicato M., Diederich M. Modulatory roles of glycolytic enzymes in cell death. Biochemical Pharmacology. 2014;92:22–30. doi: 10.1016/j.bcp.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Kovacevic Z. The pathway of glutamine and glutamate oxidation in isolated mitochondria from mammalian cells. The Biochemical Journal. 1971;125:757–763. doi: 10.1042/bj1250757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim J.W., Tchernyshyov I., Semenza G.L., Dang C.V. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metabolism. 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Papandreou I., Cairns R.A., Fontana L., Lim A.L., Denko N.C. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metabolism. 2006;3:187–197. doi: 10.1016/j.cmet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 11.Semenza G.L. HIF-1: upstream and downstream of cancer metabolism. Current Opinion in Genetics & Development. 2010;20:51–56. doi: 10.1016/j.gde.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lu Z., Hunter T. Metabolic kinases moonlighting as protein kinases. Trends in Biochemical Sciences. 2018;43:301–310. doi: 10.1016/j.tibs.2018.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rider M.H., Bertrand L., Vertommen D., Michels P.A., Rousseau G.G., Hue L. 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase: head-to-head with a bifunctional enzyme that controls glycolysis. The Biochemical Journal. 2004;381:561–579. doi: 10.1042/BJ20040752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Minchenko O., Opentanova I., Caro J. Hypoxic regulation of the 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase gene family (PFKFB-1-4) expression in vivo. FEBS Letters. 2003;554:264–270. doi: 10.1016/s0014-5793(03)01179-7. [DOI] [PubMed] [Google Scholar]
- 15.Chesney J. 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase and tumor cell glycolysis. Current Opinion in Clinical Nutrition and Metabolic Care. 2006;9:535–539. doi: 10.1097/01.mco.0000241661.15514.fb. [DOI] [PubMed] [Google Scholar]
- 16.Yalcin A., Telang S., Clem B., Chesney J. Regulation of glucose metabolism by 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatases in cancer. Experimental and Molecular Pathology. 2009;86:174–179. doi: 10.1016/j.yexmp.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Trenti A., Tedesco S., Boscaro C., Ferri N., Cignarella A., Trevisi L. The glycolytic enzyme PFKFB3 is involved in estrogen-mediated angiogenesis via GPER1. The Journal of Pharmacology and Experimental Therapeutics. 2017;361:398–407. doi: 10.1124/jpet.116.238212. [DOI] [PubMed] [Google Scholar]
- 18.Minchenko O.H., Ochiai A., Opentanova I.L., Ogura T., Minchenko D.O., Caro J. Overexpression of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase-4 in the human breast and colon malignant tumors. Biochimie. 2005;87:1005–1010. doi: 10.1016/j.biochi.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 19.Bando H., Atsumi T., Nishio T., Niwa H., Mishima S., Shimizu C. Phosphorylation of the 6-phosphofructo-2-kinase/fructose 2,6-bisphosphatase/PFKFB3 family of glycolytic regulators in human cancer. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research. 2005;11:5784–5792. doi: 10.1158/1078-0432.CCR-05-0149. [DOI] [PubMed] [Google Scholar]
- 20.Shi W.K., Zhu X.D., Wang C.H., Zhang Y.Y., Cai H., Li X.L. PFKFB3 blockade inhibits hepatocellular carcinoma growth by impairing DNA repair through AKT. Cell Death & Disease. 2018;9:428. doi: 10.1038/s41419-018-0435-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kessler R., Bleichert F., Warnke J.P., Eschrich K. 6-Phosphofructo-2-kinase/fructose-2,6-bisphosphatase (PFKFB3) is up-regulated in high-grade astrocytomas. Journal of Neuro-oncology. 2008;86:257–264. doi: 10.1007/s11060-007-9471-7. [DOI] [PubMed] [Google Scholar]
- 22.Li W., Cohen A., Sun Y., Squires J., Braas D., Graeber T.G. The Role of CD44 in glucose metabolism in prostatic small cell neuroendocrine carcinoma. Molecular Cancer Research: MCR. 2016;14:344–353. doi: 10.1158/1541-7786.MCR-15-0466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goidts V., Bageritz J., Puccio L., Nakata S., Zapatka M., Barbus S. RNAi screening in glioma stem-like cells identifies PFKFB4 as a key molecule important for cancer cell survival. Oncogene. 2012;31:3235–3243. doi: 10.1038/onc.2011.490. [DOI] [PubMed] [Google Scholar]
- 24.Yun S.J., Jo S.W., Ha Y.S., Lee O.J., Kim W.T., Kim Y.J. PFKFB4 as a prognostic marker in non-muscle-invasive bladder cancer. Urologic Oncology. 2012;30:893–899. doi: 10.1016/j.urolonc.2010.08.018. [DOI] [PubMed] [Google Scholar]
- 25.Bobarykina A.Y., Minchenko D.O., Opentanova I.L., Moenner M., Caro J., Esumi H. Hypoxic regulation of PFKFB-3 and PFKFB-4 gene expression in gastric and pancreatic cancer cell lines and expression of PFKFB genes in gastric cancers. Acta Biochimica Polonica. 2006;53:789–799. [PubMed] [Google Scholar]
- 26.Telang S., Yalcin A., Clem A.L., Bucala R., Lane A.N., Eaton J.W. Ras transformation requires metabolic control by 6-phosphofructo-2-kinase. Oncogene. 2006;25:7225–7234. doi: 10.1038/sj.onc.1209709. [DOI] [PubMed] [Google Scholar]
- 27.O'Neal J., Clem A., Reynolds L., Dougherty S., Imbert-Fernandez Y., Telang S. Inhibition of 6-phosphofructo-2-kinase (PFKFB3) suppresses glucose metabolism and the growth of HER2+ breast cancer. Breast Cancer Research and Treatment. 2016;160:29–40. doi: 10.1007/s10549-016-3968-8. [DOI] [PubMed] [Google Scholar]
- 28.Hirata T., Watanabe M., Miura S., Ijichi K., Fukasawa M., Sakakibara R. Inhibition of tumor cell growth by a specific 6-phosphofructo-2-kinase inhibitor, N-bromoacetylethanolamine phosphate, and its analogues. Bioscience, Biotechnology, and Biochemistry. 2000;64:2047–2052. doi: 10.1271/bbb.64.2047. [DOI] [PubMed] [Google Scholar]
- 29.Jeon Y.K., Yoo D.R., Jang Y.H., Jang S.Y., Nam M.J. Sulforaphane induces apoptosis in human hepatic cancer cells through inhibition of 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase4, mediated by hypoxia inducible factor-1-dependent pathway. Biochimica et Biophysica Acta. 2011;1814:1340–1348. doi: 10.1016/j.bbapap.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 30.Klarer A.C., O'Neal J., Imbert-Fernandez Y., Clem A., Ellis S.R., Clark J. Inhibition of 6-phosphofructo-2-kinase (PFKFB3) induces autophagy as a survival mechanism. Cancer & Metabolism. 2014;2:2. doi: 10.1186/2049-3002-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Calvo M.N., Bartrons R., Castano E., Perales J.C., Navarro-Sabate A., Manzano A. PFKFB3 gene silencing decreases glycolysis, induces cell-cycle delay and inhibits anchorage-independent growth in HeLa cells. FEBS Letters. 2006;580:3308–3314. doi: 10.1016/j.febslet.2006.04.093. [DOI] [PubMed] [Google Scholar]
- 32.Chesney J., Clark J., Klarer A.C., Imbert-Fernandez Y., Lane A.N., Telang S. Fructose-2,6-bisphosphate synthesis by 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 4 (PFKFB4) is required for the glycolytic response to hypoxia and tumor growth. Oncotarget. 2014;5:6670–6686. doi: 10.18632/oncotarget.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.J Clark Y.I.-F., Chesney J., Telang S. The kinase domain of PFKFB4 is required to stimulate the glucose metabolism and growth of H460 xenografts. Cancer & Metabolism. 2014;2(Suppl. 1):P74. [Google Scholar]
- 34.Shu Y., Lu Y., Pang X., Zheng W., Huang Y., Li J. Phosphorylation of PPARgamma at Ser84 promotes glycolysis and cell proliferation in hepatocellular carcinoma by targeting PFKFB4. Oncotarget. 2016;7:76984–76994. doi: 10.18632/oncotarget.12764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Domenech E., Maestre C., Esteban-Martinez L., Partida D., Pascual R., Fernandez-Miranda G. AMPK and PFKFB3 mediate glycolysis and survival in response to mitophagy during mitotic arrest. Nature Cell Biology. 2015;17:1304–1316. doi: 10.1038/ncb3231. [DOI] [PubMed] [Google Scholar]
- 36.Taylor C., Mannion D., Miranda F., Karaminejadranjbar M., Herrero-Gonzalez S., Hellner K. Loss of PFKFB4 induces cell death in mitotically arrested ovarian cancer cells. Oncotarget. 2017;8:17960–17980. doi: 10.18632/oncotarget.14910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Esteban-Martinez L., Domenech E., Boya P., Salazar-Roa M., Malumbres M. Mitophagy in mitosis: more than a myth. Autophagy. 2015;11:2379–2380. doi: 10.1080/15548627.2015.1108509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ros S., Santos C.R., Moco S., Baenke F., Kelly G., Howell M. Functional metabolic screen identifies 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 4 as an important regulator of prostate cancer cell survival. Cancer Discovery. 2012;2:328–343. doi: 10.1158/2159-8290.CD-11-0234. [DOI] [PubMed] [Google Scholar]
- 39.Ros S., Floter J., Kaymak I., Da Costa C., Houddane A., Dubuis S. 6-Phosphofructo-2-kinase/fructose-2,6-biphosphatase 4 is essential for p53-null cancer cells. Oncogene. 2017;36:3287–3299. doi: 10.1038/onc.2016.477. [DOI] [PubMed] [Google Scholar]
- 40.Strohecker A.M., Joshi S., Possemato R., Abraham R.T., Sabatini D.M., White E. Identification of 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase as a novel autophagy regulator by high content shRNA screening. Oncogene. 2015;34:5662–5676. doi: 10.1038/onc.2015.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dasgupta S., Rajapakshe K., Zhu B., Nikolai B.C., Yi P., Putluri N. Metabolic enzyme PFKFB4 activates transcriptional coactivator SRC-3 to drive breast cancer. Nature. 2018;556:249–254. doi: 10.1038/s41586-018-0018-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Seo M., Lee Y.H. PFKFB3 regulates oxidative stress homeostasis via its S-glutathionylation in cancer. Journal of Molecular Biology. 2014;426:830–842. doi: 10.1016/j.jmb.2013.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ruoslahti E. Specialization of tumour vasculature. Nature Reviews Cancer. 2002;2:83–90. doi: 10.1038/nrc724. [DOI] [PubMed] [Google Scholar]
- 44.De Bock K., Georgiadou M., Schoors S., Kuchnio A., Wong B.W., Cantelmo A.R. Role of PFKFB3-driven glycolysis in vessel sprouting. Cell. 2013;154:651–663. doi: 10.1016/j.cell.2013.06.037. [DOI] [PubMed] [Google Scholar]
- 45.Xu Y., An X., Guo X., Habtetsion T.G., Wang Y., Xu X. Endothelial PFKFB3 plays a critical role in angiogenesis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2014;34:1231–1239. doi: 10.1161/ATVBAHA.113.303041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schoors S., De Bock K., Cantelmo A.R., Georgiadou M., Ghesquiere B., Cauwenberghs S. Partial and transient reduction of glycolysis by PFKFB3 blockade reduces pathological angiogenesis. Cell Metabolism. 2014;19:37–48. doi: 10.1016/j.cmet.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 47.Cantelmo A.R., Conradi L.C., Brajic A., Goveia J., Kalucka J., Pircher A. Inhibition of the glycolytic activator pfkfb3 in endothelium induces tumor vessel normalization, impairs metastasis, and improves chemotherapy. Cancer Cell. 2016;30:968–985. doi: 10.1016/j.ccell.2016.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ebos J.M., Lee C.R., Cruz-Munoz W., Bjarnason G.A., Christensen J.G., Kerbel R.S. Accelerated metastasis after short-term treatment with a potent inhibitor of tumor angiogenesis. Cancer Cell. 2009;15:232–239. doi: 10.1016/j.ccr.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paez-Ribes M., Allen E., Hudock J., Takeda T., Okuyama H., Vinals F. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell. 2009;15:220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Loges S., Mazzone M., Hohensinner P., Carmeliet P. Silencing or fueling metastasis with VEGF inhibitors: antiangiogenesis revisited. Cancer Cell. 2009;15:167–170. doi: 10.1016/j.ccr.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 51.Yi M., Wang W., Chen S., Peng Y., Li J., Cai J. Dual-functionality of RASSF1A overexpression in A375 cells is mediated by activation of IL-6/STAT3 regulatory loop. Molecular Biology Reports. 2018;45:1277–1287. doi: 10.1007/s11033-018-4288-3. [DOI] [PubMed] [Google Scholar]
- 52.Li J., Wang W., Chen S., Cai J., Ban Y., Peng Q. FOXA1 reprograms the TGF-beta-stimulated transcriptional program from a metastasis promoter to a tumor suppressor in nasopharyngeal carcinoma. Cancer Letters. 2018;442:1–14. doi: 10.1016/j.canlet.2018.10.036. [DOI] [PubMed] [Google Scholar]
- 53.Yang Q., Hou P. Targeting PFKFB3 in the endothelium for cancer therapy. Trends in Molecular Medicine. 2017;23:197–200. doi: 10.1016/j.molmed.2017.01.008. [DOI] [PubMed] [Google Scholar]
- 54.Teuwen L.A., Draoui N., Dubois C., Carmeliet P. Endothelial cell metabolism: an update anno 2017. Current Opinion in Hematology. 2017;24:240–247. doi: 10.1097/MOH.0000000000000335. [DOI] [PubMed] [Google Scholar]
- 55.Draoui N., de Zeeuw P., Carmeliet P. Angiogenesis revisited from a metabolic perspective: role and therapeutic implications of endothelial cell metabolism. Open Biology. 2017;7 doi: 10.1098/rsob.170219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gee M.S., Procopio W.N., Makonnen S., Feldman M.D., Yeilding N.M., Lee W.M. Tumor vessel development and maturation impose limits on the effectiveness of anti-vascular therapy. American Journal Of Pathology. 2003;162:183–193. doi: 10.1016/S0002-9440(10)63809-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bechard M.E., Word A.E., Tran A.V., Liu X., Locasale J.W., McDonald O.G. Pentose conversions support the tumorigenesis of pancreatic cancer distant metastases. Oncogene. 2018;37:5248–5256. doi: 10.1038/s41388-018-0346-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mele L., Paino F., Papaccio F., Regad T., Boocock D., Stiuso P. A new inhibitor of glucose-6-phosphate dehydrogenase blocks pentose phosphate pathway and suppresses malignant proliferation and metastasis in vivo. Cell Death & Disease. 2018;9:572. doi: 10.1038/s41419-018-0635-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wu S., Wang H., Li Y., Xie Y., Huang C., Zhao H. Transcription factor YY1 promotes cell proliferation by directly activating the pentose phosphate pathway. Cancer Research. 2018;78:4549–4562. doi: 10.1158/0008-5472.CAN-17-4047. [DOI] [PubMed] [Google Scholar]
- 60.Kowalik M.A., Columbano A., Perra A. Emerging role of the pentose phosphate pathway in hepatocellular carcinoma. Frontiers in Oncology. 2017;7:87. doi: 10.3389/fonc.2017.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lin R., Elf S., Shan C., Kang H.B., Ji Q., Zhou L. 6-Phosphogluconate dehydrogenase links oxidative PPP, lipogenesis and tumour growth by inhibiting LKB1-AMPK signalling. Nature Cell Biology. 2015;17:1484–1496. doi: 10.1038/ncb3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kroemer G., Pouyssegur J. Tumor cell metabolism: cancer's Achilles' heel. Cancer Cell. 2008;13:472–482. doi: 10.1016/j.ccr.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 63.Desideri E., Vegliante R., Cardaci S., Nepravishta R., Paci M., Ciriolo M.R. MAPK14/p38alpha-dependent modulation of glucose metabolism affects ROS levels and autophagy during starvation. Autophagy. 2014;10:1652–1665. doi: 10.4161/auto.29456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yamamoto T., Takano N., Ishiwata K., Ohmura M., Nagahata Y., Matsuura T. Reduced methylation of PFKFB3 in cancer cells shunts glucose towards the pentose phosphate pathway. Nature Communications. 2014;5:3480. doi: 10.1038/ncomms4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Franklin D.A., He Y., Leslie P.L., Tikunov A.P., Fenger N., Macdonald J.M. p53 coordinates DNA repair with nucleotide synthesis by suppressing PFKFB3 expression and promoting the pentose phosphate pathway. Scientific Reports. 2016;6:38067. doi: 10.1038/srep38067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Herrero-Mendez A., Almeida A., Fernandez E., Maestre C., Moncada S., Bolanos J.P. The bioenergetic and antioxidant status of neurons is controlled by continuous degradation of a key glycolytic enzyme by APC/C-Cdh1. Nature Cell Biology. 2009;11:747–752. doi: 10.1038/ncb1881. [DOI] [PubMed] [Google Scholar]
- 67.Dang C.V. Cancer cell metabolism: there is no ROS for the weary. Cancer Discovery. 2012;2:304–307. doi: 10.1158/2159-8290.CD-12-0069. [DOI] [PubMed] [Google Scholar]
- 68.Feng Y., Wu L. mTOR up-regulation of PFKFB3 is essential for acute myeloid leukemia cell survival. Biochemical and Biophysical Research Communications. 2017;483:897–903. doi: 10.1016/j.bbrc.2017.01.031. [DOI] [PubMed] [Google Scholar]
- 69.Imbert-Fernandez Y., Clem B.F., O'Neal J., Kerr D.A., Spaulding R., Lanceta L. Estradiol stimulates glucose metabolism via 6-phosphofructo-2-kinase (PFKFB3) Journal of Biological Chemistry. 2014;289:9440–9448. doi: 10.1074/jbc.M113.529990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kommagani R., Szwarc M.M., Kovanci E., Gibbons W.E., Putluri N., Maity S. Acceleration of the glycolytic flux by steroid receptor coactivator-2 is essential for endometrial decidualization. PLoS Genetics. 2013;9:e1003900. doi: 10.1371/journal.pgen.1003900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Novellasdemunt L., Bultot L., Manzano A., Ventura F., Rosa J.L., Vertommen D. PFKFB3 activation in cancer cells by the p38/MK2 pathway in response to stress stimuli. The Biochemical Journal. 2013;452:531–543. doi: 10.1042/BJ20121886. [DOI] [PubMed] [Google Scholar]
- 72.Zhu Y., Lu L., Qiao C., Shan Y., Li H., Qian S. Targeting PFKFB3 sensitizes chronic myelogenous leukemia cells to tyrosine kinase inhibitor. Oncogene. 2018;37:2837–2849. doi: 10.1038/s41388-018-0157-8. [DOI] [PubMed] [Google Scholar]
- 73.Li W., Qian L., Lin J., Huang G., Hao N., Wei X. CD44 regulates prostate cancer proliferation, invasion and migration via PDK1 and PFKFB4. Oncotarget. 2017;8:65143–65151. doi: 10.18632/oncotarget.17821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gomez M., Manzano A., Figueras A., Vinals F., Ventura F., Rosa J.L. Sertoli-secreted FGF-2 induces PFKFB4 isozyme expression in mouse spermatogenic cells by activation of the MEK/ERK/CREB pathway American journal of physiology. Endocrinology and Metabolism. 2012;303:E695–E707. doi: 10.1152/ajpendo.00381.2011. [DOI] [PubMed] [Google Scholar]
- 75.Marsin A.S., Bouzin C., Bertrand L., Hue L. The stimulation of glycolysis by hypoxia in activated monocytes is mediated by AMP-activated protein kinase and inducible 6-phosphofructo-2-kinase. The Journal of Biological Chemistry. 2002;277:30778–30783. doi: 10.1074/jbc.M205213200. [DOI] [PubMed] [Google Scholar]
- 76.Faubert B., Vincent E.E., Poffenberger M.C., Jones R.G. The AMP-activated protein kinase (AMPK) and cancer: many faces of a metabolic regulator. Cancer Letters. 2015;356:165–170. doi: 10.1016/j.canlet.2014.01.018. [DOI] [PubMed] [Google Scholar]
- 77.Sanchez-Martinez C., Estevez A.M., Aragon J.J. Phosphofructokinase C isozyme from ascites tumor cells: cloning, expression, and properties. Biochemical and Biophysical Research Communications. 2000;271:635–640. doi: 10.1006/bbrc.2000.2681. [DOI] [PubMed] [Google Scholar]
- 78.Moral-Sanz J., Lewis S.A., MacMillan S., Ross F.A., Thomson A., Viollet B. The LKB1-AMPK-alpha1 signaling pathway triggers hypoxic pulmonary vasoconstriction downstream of mitochondria. Science Signaling. 2018;11 doi: 10.1126/scisignal.aau0296. [DOI] [PubMed] [Google Scholar]
- 79.Lee M., Hwang J.T., Lee H.J., Jung S.N., Kang I., Chi S.G. AMP-activated protein kinase activity is critical for hypoxia-inducible factor-1 transcriptional activity and its target gene expression under hypoxic conditions in DU145 cells. The Journal of Biological Chemistry. 2003;278:39653–39661. doi: 10.1074/jbc.M306104200. [DOI] [PubMed] [Google Scholar]
- 80.Egan D.F., Shackelford D.B., Mihaylova M.M., Gelino S., Kohnz R.A., Mair W. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science. 2011;331:456–461. doi: 10.1126/science.1196371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim J., Kundu M., Viollet B., Guan K.L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nature Cell Biology. 2011;13:132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.McFadden J.W., Corl B.A. Activation of AMP-activated protein kinase (AMPK) inhibits fatty acid synthesis in bovine mammary epithelial cells. Biochemical and Biophysical Research Communications. 2009;390:388–393. doi: 10.1016/j.bbrc.2009.09.017. [DOI] [PubMed] [Google Scholar]
- 83.Li F.L., Liu J.P., Bao R.X., Yan G., Feng X., Xu Y.P. Acetylation accumulates PFKFB3 in cytoplasm to promote glycolysis and protects cells from cisplatin-induced apoptosis. Nature Communications. 2018;9:508. doi: 10.1038/s41467-018-02950-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xing Z., Zhang Y., Liang K., Yan L., Xiang Y., Li C. Expression of long non-coding RNA YIYA promotes glycolysis in breast cancer. Cancer Research. 2018 doi: 10.1158/0008-5472.CAN-17-0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Reid M.A., Lowman X.H., Pan M., Tran T.Q., Warmoes M.O., Ishak Gabra M.B. IKKbeta promotes metabolic adaptation to glutamine deprivation via phosphorylation and inhibition of PFKFB3. Genes & Development. 2016;30:1837–1851. doi: 10.1101/gad.287235.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yang Z., Goronzy J.J., Weyand C.M. The glycolytic enzyme PFKFB3/phosphofructokinase regulates autophagy. Autophagy. 2014;10:382–383. doi: 10.4161/auto.27345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mullarky E., Cantley L.C. Diverting glycolysis to combat oxidative stress. In: Nakao K., Minato N., Uemoto S., editors. Innovative medicine: basic research and development, Tokyo. 2015. pp. 3–23. [Google Scholar]
- 88.Toyokuni S., Okamoto K., Yodoi J., Hiai H. Persistent oxidative stress in cancer. FEBS Letters. 1995;358:1–3. doi: 10.1016/0014-5793(94)01368-b. [DOI] [PubMed] [Google Scholar]
- 89.Rodriguez-Rodriguez P., Fernandez E., Almeida A., Bolanos J.P. Excitotoxic stimulus stabilizes PFKFB3 causing pentose-phosphate pathway to glycolysis switch and neurodegeneration. Cell Death & Differentiation. 2012;19:1582–1589. doi: 10.1038/cdd.2012.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yamamoto T., Takano N., Ishiwata K., Suematsu M. Carbon monoxide stimulates global protein methylation via its inhibitory action on cystathionine beta-synthase. Journal of Clinical Biochemistry & Nutrition. 2011;48:96–100. doi: 10.3164/jcbn.11-011FR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Loboda A., Jozkowicz A., Dulak J. HO-1/CO system in tumor growth, angiogenesis and metabolism – targeting HO-1 as an anti-tumor therapy. Vascular Pharmacology. 2015;74:11–22. doi: 10.1016/j.vph.2015.09.004. [DOI] [PubMed] [Google Scholar]
- 92.Suematsu M., Nakamura T., Tokumoto Y., Yamamoto T., Kajimura M., Kabe Y. CO-CBS-H2 S Axis: from vascular mediator to cancer regulator. Microcirculation. 2016;23:183–190. doi: 10.1111/micc.12253. [DOI] [PubMed] [Google Scholar]
- 93.He Y., Gao M., Cao Y., Tang H., Liu S., Tao Y. Nuclear localization of metabolic enzymes in immunity and metastasis. Biochimica et Biophysica Acta Reviews on Cancer. 2017;1868:359–371. doi: 10.1016/j.bbcan.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 94.Yang W., Xia Y., Ji H., Zheng Y., Liang J., Huang W. Nuclear PKM2 regulates beta-catenin transactivation upon EGFR activation. Nature. 2011;480:118–122. doi: 10.1038/nature10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Wang H.J., Hsieh Y.J., Cheng W.C., Lin C.P., Lin Y.S., Yang S.F. JMJD5 regulates PKM2 nuclear translocation and reprograms HIF-1alpha-mediated glucose metabolism. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:279–284. doi: 10.1073/pnas.1311249111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yalcin A., Clem B.F., Simmons A., Lane A., Nelson K., Clem A.L. Nuclear targeting of 6-phosphofructo-2-kinase (PFKFB3) increases proliferation via cyclin-dependent kinases. Journal of Biological Chemistry. 2009;284:24223–24232. doi: 10.1074/jbc.M109.016816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Loar P., Wahl H., Kshirsagar M., Gossner G., Griffith K., Liu J.R. Inhibition of glycolysis enhances cisplatin-induced apoptosis in ovarian cancer cells. American Journal of Obstetrics and Gynecology. 2010;202 doi: 10.1016/j.ajog.2009.10.883. 371 e371-378. [DOI] [PubMed] [Google Scholar]
- 98.Xu R.H., Pelicano H., Zhou Y., Carew J.S., Feng L., Bhalla K.N. Inhibition of glycolysis in cancer cells: a novel strategy to overcome drug resistance associated with mitochondrial respiratory defect and hypoxia. Cancer Research. 2005;65:613–621. [PubMed] [Google Scholar]
- 99.Yalcin A., Clem B.F., Imbert-Fernandez Y., Ozcan S.C., Peker S., O'Neal J. 6-Phosphofructo-2-kinase (PFKFB3) promotes cell cycle progression and suppresses apoptosis via Cdk1-mediated phosphorylation of p27. Cell Death & Disease. 2014;5:e1337. doi: 10.1038/cddis.2014.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jia W., Zhao X., Zhao L., Yan H., Li J., Yang H. Non-canonical roles of PFKFB3 in regulation of cell cycle through binding to CDK4. Oncogene. 2018;37:1685–1698. doi: 10.1038/s41388-017-0072-4. [DOI] [PubMed] [Google Scholar]
- 101.Yang W., Lu Z. Nuclear PKM2 regulates the Warburg effect. Cell Cycle. 2013;12:3154–3158. doi: 10.4161/cc.26182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Jiang Y., Li X., Yang W., Hawke D.H., Zheng Y., Xia Y. PKM2 regulates chromosome segregation and mitosis progression of tumor cells. Molecular Cell. 2014;53:75–87. doi: 10.1016/j.molcel.2013.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cheng T.Y., Yang Y.C., Wang H.P., Tien Y.W., Shun C.T., Huang H.Y. Pyruvate kinase M2 promotes pancreatic ductal adenocarcinoma invasion and metastasis through phosphorylation and stabilization of PAK2 protein. Oncogene. 2018;37:1730–1742. doi: 10.1038/s41388-017-0086-y. [DOI] [PubMed] [Google Scholar]
- 104.Goncalves M.D., Cantley L.C. A glycolysis outsider steps into the cancer spotlight. Cell Metabolism. 2018;28:3–4. doi: 10.1016/j.cmet.2018.06.017. [DOI] [PubMed] [Google Scholar]
- 105.Ohsumi Y. Historical landmarks of autophagy research. Cell Research. 2014;24:9–23. doi: 10.1038/cr.2013.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ma T., Li J., Xu Y., Yu C., Xu T., Wang H. Atg5-independent autophagy regulates mitochondrial clearance and is essential for iPSC reprogramming. Nature Cell Biology. 2015;17:1379–1387. doi: 10.1038/ncb3256. [DOI] [PubMed] [Google Scholar]
- 107.Lue H.W., Podolak J., Kolahi K., Cheng L., Rao S., Garg D. Metabolic reprogramming ensures cancer cell survival despite oncogenic signaling blockade. Genes & Development. 2017;31:2067–2084. doi: 10.1101/gad.305292.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Webster B.R., Scott I., Traba J., Han K., Sack M.N. Regulation of autophagy and mitophagy by nutrient availability and acetylation. Biochimica et Biophysica Acta. 2014;1841:525–534. doi: 10.1016/j.bbalip.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Lee J.M., Wagner M., Xiao R., Kim K.H., Feng D., Lazar M.A. Nutrient-sensing nuclear receptors coordinate autophagy. Nature. 2014;516:112–115. doi: 10.1038/nature13961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Inoki K., Kim J., Guan K.L. AMPK and mTOR in cellular energy homeostasis and drug targets. Annual Review of Pharmacology and Toxicology. 2012;52:381–400. doi: 10.1146/annurev-pharmtox-010611-134537. [DOI] [PubMed] [Google Scholar]
- 111.Yan S., Wei X., Xu S., Sun H., Wang W., Liu L. 6-Phosphofructo-2-kinase/fructose-2,6-bisphosphatase isoform 3 spatially mediates autophagy through the AMPK signaling pathway. Oncotarget. 2017;8:80909–80922. doi: 10.18632/oncotarget.20757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yang Z., Fujii H., Mohan S.V., Goronzy J.J., Weyand C.M. Phosphofructokinase deficiency impairs ATP generation, autophagy, and redox balance in rheumatoid arthritis T cells. The Journal of Experimental Medicine. 2013;210:2119–2134. doi: 10.1084/jem.20130252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang Q., Zeng F., Sun Y., Qiu Q., Zhang J., Huang W. Etk Interaction with PFKFB4 modulates chemoresistance of small-cell lung cancer by regulating autophagy. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research. 2018;24:950–962. doi: 10.1158/1078-0432.CCR-17-1475. [DOI] [PubMed] [Google Scholar]
- 114.Zhao Y., Butler E.B., Tan M. Targeting cellular metabolism to improve cancer therapeutics. Cell Death & Disease. 2013;4:e532. doi: 10.1038/cddis.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bartrons R., Rodriguez-Garcia A., Simon-Molas H., Castano E., Manzano A., Navarro-Sabate A. The potential utility of PFKFB3 as a therapeutic target. Expert Opinion on Therapeutic Targets. 2018;22:659–674. doi: 10.1080/14728222.2018.1498082. [DOI] [PubMed] [Google Scholar]
- 116.Clem B., Telang S., Clem A., Yalcin A., Meier J., Simmons A. Small-molecule inhibition of 6-phosphofructo-2-kinase activity suppresses glycolytic flux and tumor growth. Molecular Cancer Therapeutics. 2008;7:110–120. doi: 10.1158/1535-7163.MCT-07-0482. [DOI] [PubMed] [Google Scholar]
- 117.Houddane A., Bultot L., Novellasdemunt L., Johanns M., Gueuning M.A., Vertommen D. Role of Akt/PKB and PFKFB isoenzymes in the control of glycolysis, cell proliferation and protein synthesis in mitogen-stimulated thymocytes. Cellular Signalling. 2017;34:23–37. doi: 10.1016/j.cellsig.2017.02.019. [DOI] [PubMed] [Google Scholar]
- 118.Clem B.F., O'Neal J., Tapolsky G., Clem A.L., Imbert-Fernandez Y., Kerr D.A., 2nd Targeting 6-phosphofructo-2-kinase (PFKFB3) as a therapeutic strategy against cancer. Molecular Cancer Therapeutics. 2013;12:1461–1470. doi: 10.1158/1535-7163.MCT-13-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li H.M., Yang J.G., Liu Z.J., Wang W.M., Yu Z.L., Ren J.G. Blockage of glycolysis by targeting PFKFB3 suppresses tumor growth and metastasis in head and neck squamous cell carcinoma. Journal of Experimental & Clinical Cancer Research: CR. 2017;36:7. doi: 10.1186/s13046-016-0481-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhu W., Ye L., Zhang J., Yu P., Wang H., Ye Z. PFK15, a small molecule inhibitor of PFKFB3, induces cell cycle arrest, apoptosis and inhibits invasion in gastric cancer. PLoS One. 2016;11:e0163768. doi: 10.1371/journal.pone.0163768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Lea M.A., Altayyar M., desBordes C. Inhibition of growth of bladder cancer cells by 3-(3-pyridinyl)-1-(4-pyridinyl)-2-propen-1-one in combination with other compounds affecting glucose metabolism. Anticancer Research. 2015;35:5889–5899. [PubMed] [Google Scholar]
- 122.Lea M.A., Guzman Y., Desbordes C. Inhibition of growth by combined treatment with inhibitors of lactate dehydrogenase and either phenformin or inhibitors of 6-Phosphofructo-2-kinase/Fructose-2,6-bisphosphatase 3. Anticancer Research. 2016;36:1479–1488. [PubMed] [Google Scholar]
- 123.Redman R., Pohlmann P., Kurman M., Tapolsky G.H., Chesney J. PFK-158, first-in-man and first-in-class inhibitor of PFKFB3/glycolysis: a phase I, dose escalation, multi-center study in patients with advanced solid malignancies. Cancer Research. 2015;75 [Google Scholar]
- 124.Chesney J., Clark J., Lanceta L., Trent J.O., Telang S. Targeting the sugar metabolism of tumors with a first-in-class 6-phosphofructo-2-kinase (PFKFB4) inhibitor. Oncotarget. 2015;6:18001–18011. doi: 10.18632/oncotarget.4534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gelman S.J., Naser F., Mahieu N.G., McKenzie L.D., Dunn G.P., Chheda M.G. Consumption of NADPH for 2-HG synthesis increases pentose phosphate pathway flux and sensitizes cells to oxidative stress. Cell Reports. 2018;22:512–522. doi: 10.1016/j.celrep.2017.12.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dixon S.J., Lemberg K.M., Lamprecht M.R., Skouta R., Zaitsev E.M., Gleason C.E. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149:1060–1072. doi: 10.1016/j.cell.2012.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yang W.S., Stockwell B.R. Ferroptosis: death by lipid peroxidation. Trends in Cell Biology. 2016;26:165–176. doi: 10.1016/j.tcb.2015.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hakimi A.A., Reznik E., Lee C.H., Creighton C.J., Brannon A.R., Luna A. An integrated metabolic atlas of clear cell renal cell carcinoma. Cancer Cell. 2016;29:104–116. doi: 10.1016/j.ccell.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tu C., Zeng Z., Qi P., Li X., Guo C., Xiong F. Identification of genomic alterations in nasopharyngeal carcinoma and nasopharyngeal carcinoma-derived Epstein-Barr virus by whole genome sequencing. Carcinogenesis. 2018 doi: 10.1093/carcin/bgy108. [DOI] [PubMed] [Google Scholar]
- 130.Okegawa T., Morimoto M., Nishizawa S., Kitazawa S., Honda K., Araki H. Intratumor heterogeneity in primary kidney cancer revealed by metabolic profiling of multiple spatially separated samples within tumors. EBioMedicine. 2017;19:31–38. doi: 10.1016/j.ebiom.2017.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Liu X., Locasale J.W. Metabolomics reveals intratumor heterogeneity – implications for precision medicine. EBioMedicine. 2017;19:4–5. doi: 10.1016/j.ebiom.2017.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Beca F., Polyak K. Intratumor heterogeneity in breast cancer. Advances in Experimental Medicine & Biology. 2016;882:169–189. doi: 10.1007/978-3-319-22909-6_7. [DOI] [PubMed] [Google Scholar]
- 133.Houles T., Gravel S.P., Lavoie G., Shin S., Savall M., Meant A. RSK regulates PFK-2 activity to promote metabolic rewiring in melanoma. Cancer Research. 2018;78:2191–2204. doi: 10.1158/0008-5472.CAN-17-2215. [DOI] [PubMed] [Google Scholar]
- 134.Zhao S.J., Shen Y.F., Li Q., He Y.J., Zhang Y.K., Hu L.P. SLIT2/ROBO1 axis contributes to the Warburg effect in osteosarcoma through activation of SRC/ERK/c-MYC/PFKFB2 pathway. Cell Death & Disease. 2018;9:390. doi: 10.1038/s41419-018-0419-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Ji D., Lu Z.T., Li Y.Q., Liang Z.Y., Zhang P.F., Li C. MACC1 expression correlates with PFKFB2 and survival in hepatocellular carcinoma. Asian Pacific Journal of Cancer Prevention: APJCP. 2014;15:999–1003. doi: 10.7314/apjcp.2014.15.2.999. [DOI] [PubMed] [Google Scholar]
- 136.Moon J.S., Jin W.J., Kwak J.H., Kim H.J., Yun M.J., Kim J.W. Androgen stimulates glycolysis for de novo lipid synthesis by increasing the activities of hexokinase 2 and 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 2 in prostate cancer cells. The Biochemical Journal. 2011;433:225–233. doi: 10.1042/BJ20101104. [DOI] [PubMed] [Google Scholar]
- 137.Akter S., Clem B.F., Lee H.J., Chesney J., Bae Y. Block copolymer micelles for controlled delivery of glycolytic enzyme inhibitors. Pharmaceutical Research. 2012;29:847–855. doi: 10.1007/s11095-011-0613-4. [DOI] [PubMed] [Google Scholar]
- 138.Seo M., Kim J.D., Neau D., Sehgal I., Lee Y.H. Structure-based development of small molecule PFKFB3 inhibitors: a framework for potential cancer therapeutic agents targeting the Warburg effect. PLoS One. 2011;6:e24179. doi: 10.1371/journal.pone.0024179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Harada Y., Tominaga N., Watanabe M., Shimokawa R., Ishiguro M., Sakakibara R. Inhibition of fructose-6-phosphate,2-kinase by N-bromoacetylethanolamine phosphate in vitro and in vivo. Journal of Biochemistry. 1997;121:724–730. doi: 10.1093/oxfordjournals.jbchem.a021646. [DOI] [PubMed] [Google Scholar]
- 140.Boyd S., Brookfield J.L., Critchlow S.E., Cumming I.A., Curtis N.J., Debreczeni J. Structure-based design of potent and selective inhibitors of the metabolic kinase PFKFB3. Journal of Medicinal Chemistry. 2015;58:3611–3625. doi: 10.1021/acs.jmedchem.5b00352. [DOI] [PubMed] [Google Scholar]