Abstract
Objective
Structurally-improved GIP analogs were developed to determine precisely whether GIP receptor (GIPR) agonism or antagonism lowers body weight in obese mice.
Methods
A series of peptide-based GIP analogs, including structurally diverse agonists and a long-acting antagonist, were generated and characterized in vitro using functional assays in cell systems overexpressing human and mouse derived receptors. These analogs were characterized in vivo in DIO mice following acute dosing for effects on glycemic control, and following chronic dosing for effects on body weight and food intake. Pair-feeding studies and indirect calorimetry were used to survey the mechanism for body weight lowering. Congenital Gipr−/− and Glp1r−/− DIO mice were used to investigate the selectivity of the agonists and to ascribe the pharmacology to effects mediated by the GIPR.
Results
Non-acylated, Aib2 substituted analogs derived from human GIP sequence showed full in vitro potency at human GIPR and subtly reduced in vitro potency at mouse GIPR without cross-reactivity at GLP-1R. These GIPR agonists lowered acute blood glucose in wild-type and Glp1r−/− mice, and this effect was absent in Gipr−/− mice, which confirmed selectivity towards GIPR. Chronic treatment of DIO mice resulted in modest yet consistent, dose-dependent decreased body weight across many studies with diverse analogs. The mechanism for body weight lowering is due to reductions in food intake, not energy expenditure, as suggested by pair-feeding studies and indirect calorimetry assessment. The weight lowering effect was preserved in DIO Glp-1r−/− mice and absent in DIO Gipr−/− mice. The body weight lowering efficacy of GIPR agonists was enhanced with analogs that exhibit higher mouse GIPR potency, with increased frequency of administration, and with fatty-acylated peptides of extended duration of action. Additionally, a fatty-acylated, N-terminally truncated GIP analog was shown to have high in vitro antagonism potency for human and mouse GIPR without cross-reactive activity at mouse GLP-1R or mouse glucagon receptor (GcgR). This acylated antagonist sufficiently inhibited the acute effects of GIP to improve glucose tolerance in DIO mice. Chronic treatment of DIO mice with high doses of this acylated GIPR antagonist did not result in body weight change. Further, co-treatment of this acylated GIPR antagonist with liraglutide, an acylated GLP-1R agonist, to DIO mice did not result in increased body weight lowering relative to liraglutide-treated mice. Enhanced body weight lowering in DIO mice was evident however following co-treatment of long-acting selective individual agonists for GLP-1R and GIPR, consistent with previous data.
Conclusions
We conclude that peptide-based GIPR agonists, not peptide-based GIPR antagonists, that are suitably optimized for receptor selectivity, cross-species activity, and duration of action consistently lower body weight in DIO mice, although with moderate efficacy relative to GLP-1R agonists. These preclinical rodent pharmacology results, in accordance with recent clinical results, provide definitive proof that systemic GIPR agonism, not antagonism, is beneficial for body weight loss.
Keywords: Glucose-dependent insulinotropic polypeptide (GIP), Agonism, Obesity, Diet-induced obese (DIO) mice, Pharmacology
Highlights
-
•
Structurally-refined and diverse GIP receptor agonists consistently lower body weight in DIO mice.
-
•
Body weight lowering of GIP receptor agonists is maintained in GLP-1R−/− mice yet abolished in GIPR−/− mice.
-
•
GIP receptor agonism promotes body weight lowering via food intake mechanism.
-
•
A fully potent long-acting GIP receptor antagonist does not lower body weight in DIO mice.
1. Introduction
Structurally optimized analogs of glucagon-like peptide 1 (GLP-1) have provided profound therapeutic improvements in management of glucose and body weight to lessen adverse cardiovascular events in T2D patients [1], [2]. In contrast, GIP, despite its role as a physiological incretin and partner with GLP-1, has failed to advance as a therapeutic agent. Notably the insulinotropic effect of GIP receptor agonism is diminished in modest hyperglycemia [3] but purportedly reversed with improved glycemic control [4]. This latter observation served as a catalyst to integrating coagonism at GIP and GLP-1 receptors (GIPR and GLP-1R, respectively) as a strategy in which the latter activity primes the former to achieve a more optimal reversal of the metabolic syndrome [4]. Two different peptides, NNC0090-2746 (MAR709) and LY3298176 (Tirzepatide), with high potency dual incretin agonism have advanced to multi-dose clinical studies. The preclinical and clinical results have demonstrated improvements in glycemic control and body weight that exceeds what is achieved with comparable dosing of benchmark GLP-1R specific agonists [5], [6], [7], [8]. Despite these beneficial clinical results of combinatorial therapy, the pharmacological effect of GIP alone to lower body weight has not been adequately addressed.
The role of GIP to regulate systemic metabolism beyond its direct effect at the endocrine pancreas remains controversial and confusing, particularly as it relates to GIP action to promote gain in fat mass (reviewed in [9]). Mouse models of diminished GIP activity, including GIPR knockout mice [10], immunization against GIP [11], [12], enteroendocrine K cell ablation [13], and chemical antagonism [14], [15] have shown reduced or delayed body weight gain in rodents exposed to obesogenic diets. Furthermore, elevated levels of GIP are associated with visceral fat deposition [16], and acute GIP infusion increased adipose tissue vasoactivity and adipogenesis in humans [17]. These rodent loss-of-function studies and experimental human physiology results have fostered beliefs that GIPR antagonism can pharmacologically improve body weight upon chronic administration. On the contrary, there is experimental evidence that amplified GIP action is also beneficial to body weight, including transgenic overexpression of GIP [18], single [19] and combinatorial preclinical pharmacology [5], [20], [21], [22], and genome-wide association studies [23]. However, it has yet to be definitively shown whether chronic pharmacological manipulation of the GIPR system in either direction is beneficial or detrimental for body weight in obese mice, which is instrumental to study the molecular underpinnings of GIP-mediated pharmacological effects. At a minimum, it has been shown that GIPR mono-agonists improve glucose metabolism and clearly do not promote further weight gain in mice with established obesity [5], [21], [24], [25], [26].
To specifically address this dichotomy in whether GIPR agonism or antagonism provides body weight lowering pharmacology, we systematically investigated the impact of acute and chronic treatment of non-diabetic, genetically wild-type, diet-induced obese (DIO) mice with GIPR agonists optimized for sustained duration of action and activity at mouse GIPR. In all cases, peripheral injections of defined, selective GIPR agonists clearly promoted modest body weight loss when administered chronically to DIO mice. These effects are maintained in mice lacking GLP-1R, and absent in mice lacking GIPR. Furthermore, similar study with a selective, long-acting mouse GIPR antagonist at high dose did not lower body weight in obese mice with chronic therapy. The collective use of peptide agonists, peptide antagonists, and mice lacking GIPR or GLP-1R substantiates GIPR agonism and not antagonism to promote body weight loss in obese rodents.
2. Materials and methods
2.1. Peptide synthesis, purification and analysis
Peptides were prepared by automated Fmoc/t-Bu solid-phase methodology employing a Symphony peptide synthesizer (Peptide Technology, Tucson, AZ) starting with Wang resin (AAPPtec, Louisville, KY) and 6-Cl-HOBt/DIC activation. All conventional residues were purchased from Midwest Biotech (Fisher, IN), 6-Cl-HOBt and DIC was obtained from AAPPtec (Louisville, KY). Peptides were cleaved from the resin and de-protected by treatment with TFA containing 2.5% TIS, 2.5% H2O, 1.5% methanol, 2.5% phenol, 0.5% DODT and 0.5% of dimethylsulfoxide. Peptide was precipitated with cold ethyl ether from a filtered TFA solution according to standard procedure. Peptides were purified by preparative RP-HPLC on an Inspire-C8 (manually self-packed, 21.2 × 250 mm, 10 μm, DIKMA) and/or a Kinetex C8 (AXIA supplied, 21.2 × 250 mm, 5 μm, Phenomenex) column with 0.05% TFA/H2O and 0.05% TFA/CH3CN as elution buffers. Purified peptides were characterized by LC-MS (1260 Infinity-6120 Quadrupole LC-MS, Agilent) on Kinetex C8 (4.6 × 75 mm, 2.6 μm, Phenomenex) column with 0.05% TFA/H2O buffer employing a 5%–70% linear increase in CH3CN over 15 min with 2.5 min delay, UV detection of absorbance at λ = 214 nm. All peptides were ≥95% pure as determined by analytical HPLC. Aqueous peptide concentrations were assessed by UV absorption at λ = 280 nm as measured by a NanoDrop 1000 spectrophotometer (Thermo Scientific, Wilmington, DE). Extinction coefficients at λ = 280 nm were calculated using online Peptide Property Calculator (Innovagen, PepCalc.com).
We prepared a fatty-acylated GIP analog with selective antagonism at mGIPR; NαAc, K10[γEγE-C16], Arg18, hGIP(5–42). To do so the N-terminal four amino acids of native GIP were excluded from the synthesis and the peptide assembly was terminated by acetylation. Acetylation was performed on resin in the presence of tenfold excess of acetic anhydride/DIEA in DCM for 1 h. This peptide was fatty-acylated on the side-chain of the lysine at residue 10 (native GIP numbering) with a repeat γ-glutamate, γ-glutamate (γE,γE) spacer which was terminated with a saturated palmitic acid (C16:0). Acylation was performed on resin with tenfold excess of Fmoc-Glu-OtBu/DEPBT/DIEA, repeated for double coupling, followed by tenfold excess of palmitic acid/DEPBT/DIEA. Arginine was used as residue 18 (native GIP numbering).
2.2. In vitro receptor activity
Each analog was tested for its ability to activate or inhibit the receptor of interest through a cell-based luciferase reporter gene assay that indirectly measures cAMP production. Unacylated analogs were tested in the presence of 0.5% bovine serum albumin (BSA). Acylated analogs were tested in the presence of 1% ovalbumin. HEK293 cells (ATCC, CRL-1573) or BHK cells independently over-expressing the human or mouse receptors and firefly luciferase reporter gene linked to cAMP response element were used for the assays. The cells were serum deprived for 16 h and then incubated with serial dilutions of standard hormones or peptide analogs for 5 h at 37 °C, 5% CO2 and 90% humidity in 96 well poly-d-Lysine-coated “Biocoat” plates (BD Biosciences, San Jose, CA). To measure GIPR receptor antagonism, the cells were co-incubated with the native GIP at a constant concentration equal to the EC50 of the respective native ligand and serial dilutions of the analogs. GLP-1 and glucagon receptor antagonism was similarly performed at their respective cells using native hormone at a constant concentration equal to EC90. At the end of the incubation period 50 μl SteadyLite luciferase substrate was added to each well. The plate was sealed, shaken 10 min at 800 rpm in the dark and the luminescence signal was measured using an EnSight multimode reader (Perkin–Elmer, Waltham, MA), or a DTX-880 multimode detector (Beckman Coulter, Brea, CA). Each peptide concentration was tested in duplicate within the plate. GraphPad Prism 6 or Origin software was used to plot signal versus peptide concentration graphs and EC50 for each individual experiment was determined using non-linear three parameter regression analysis or sigmoidal fit with logistic function, Levenberg-Marguardt integration algorithm and statistical weight assigned to each data point. Reported EC50 and standard deviation (S.D.) values represent averages from at least three independent experiments.
2.3. Mouse pharmacology studies
Energy metabolism studies were conducted in DIO male C57Bl/6j mice (Jackson Laboratories) fed an obesogenic diet (D12331; Research Diets Inc., New Brunswick, NJ; 58% fat, 5.56 kcal/g). All dietary challenges began at 8 weeks of age. Mice were group housed on a 12:12 h light–dark cycle at 22 °C, with free access to food and water. Mice were maintained on the obesogenic diet for at least 16 weeks prior to initiation of pharmacological studies. All injections and challenge tests were performed in the light cycle. Peptides were administered in phosphate buffer and as subcutaneous injections at the indicated doses and frequency, at a volume of 5 μL per gram of measured body weight. Mice were randomized and evenly distributed into test groups (n = 8) according to body weight.
2.4. Glucose tolerance tests
For the analysis of glucose tolerance, mice had restricted access to food for 6 h prior to the challenge test. Mice were intraperitoneally injected with 2.0 g glucose per kg of BW [20% (wt/v) D glucose (Sigma–Aldrich) in 0.9% (wt/v) saline]. Tail blood glucose concentrations (mg/dl) were measured using a handheld glucometer (TheraSense Freestyle, Abbott). To measure the acute effects of the agonists to improve glucose tolerance, mice were subcutaneously injected with the test peptides 15 min prior to the glucose challenge. To measure the acute effects of the fatty-acylated antagonist to inhibit the effects of a bolus of a GIPR agonist on glucose tolerance, the peptide was subcutaneously administered 120 min prior to the glucose challenge while the fatty-acylated agonist was administered 60 min prior to the glucose challenge. To measure the effects on glucose tolerance following chronic treatment, the glucose tolerance test was performed 24 h after previous subcutaneous injection of the test article.
2.5. Indirect calorimetry
Energy expenditure and respiratory exchange ratio were assessed using a combined indirect calorimetry system (TSE Systems, Chesterfield, MO, USA). O2 consumption and CO2 production were measured every 15 min for a total of up to 120 h (after 48 h of adaptation).
2.6. Genetically modified animals
Glp1r−/− [27] and Gipr−/− [28] were generated as previously described.
2.7. Statistics
Statistical analyses were performed using Graph Prism 6 (GraphPad Software, San Diego, CA USA). Results are expressed as mean ± SEM unless otherwise specified. For multiple group comparison analysis, one- or two-way ANOVA was used, as appropriate, followed by Dunnett or Sidak post hoc adjustment. Repeated-measures ANOVA were also used whenever appropriate. Differences with P values less than 0.05 were considered significant.
3. Results
3.1. Structures and in vitro receptor activity of GIPR agonists
We first designed a GIP analog derived from the human GIP (hGIP) sequence and proceeded to explore the in vitro pharmacology at mouse and human GIPR (abbreviated sequences in Table 1; analytical data in Table S1). Whereas mGIP and hGIP were equally potent at human GIPR (hGIPR), the potency of hGIP at mouse GIPR (mGIPR) was reduced by nearly fivefold relative to the potency of mGIP (Table 1). 2-aminoisobutyric acid (Aib) was introduced at the second position to enhance in vivo pharmacokinetic properties through proteolytic resistance to DPP-IV. Substitution with Aib2 also increased potency of hGIP at both human and mouse GIPR by approximately twofold. No cross-reactivity was observed at mouse-derived GLP-1 or glucagon receptors (data not shown).
Table 1.
In vitro potency profiles of GIPR agonists at human and mouse GIPR. Data reported as mean ± standard deviation with at least three independent experiments.
| Modifications | Abbreviation | hGIPR |
mGIPR |
|---|---|---|---|
| EC50 [pM] | EC50 [pM] | ||
| hGIP | 5.8 ± 2.6 | 35.6 ± 16.1 | |
| Aib2 | hGIP Aib2 | 3.9 ± 1.7 | 11.7 ± 9.0 |
| Arg18, Arg30, Ser34 | mGIP | 11.5 ± 9.9 | 6.2 ± 2.2 |
| Aib2, Arg18, Arg30, Ser34 | mGIP Aib2 | 4.3 ± 1.9 | 3.9 ± 1.2 |
| Aib2, Arg18 | hGIP Aib2 Arg18 | 3.0 ± 1.1 | 3.4 ± 0.8 |
| Aib2, Arg18 | hGIP Aib2 Arg18 | 3.0 ± 1.1 | 3.4 ± 0.8 |
| Aib2, Arg16, Cex | hGIP Aib2 Cex* | 3.8 ± 1.4 | |
| Aib2, Cex | hGIP Aib2 Cex | 3.6 ± 1.2 | |
| Aib2, Lys43(γEγEγE-C16) | Acyl hGIP Aib2 | 1.6 ± 0.9 | 8.4 ± 1.5 |
3.2. Confirmation of in vivo mGIPR activity and selectivity
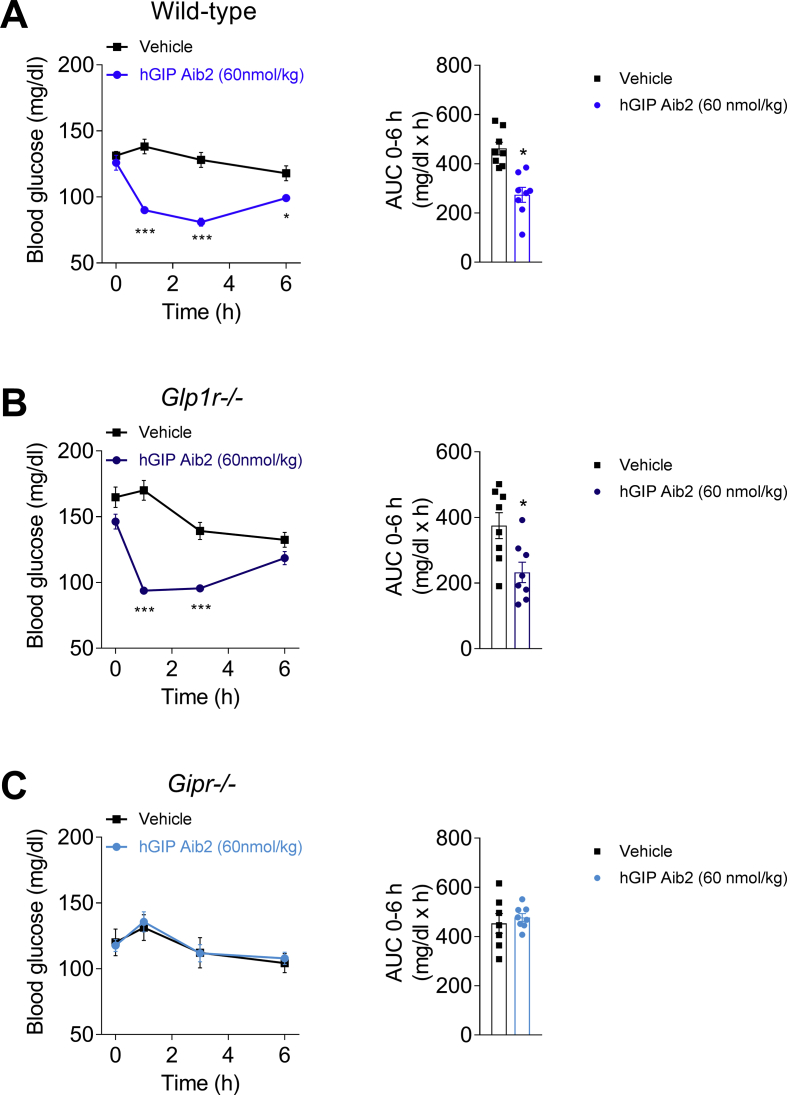
Translation of in vitro to in vivo mGIPR potency was assessed by change in blood glucose response to a bolus injection of GIP analogs. The human derived, Aib2 substituted GIP analog (hGIP Aib2) transiently lowered ad libitum-fed blood glucose in wild-type mice (Figure 1A), consistent with results following treatment with other DPP-IV protected analogs [26]. This property was still evident in Glp1r−/− mice (Figure 1B); however, hGIP Aib2 did not lower blood glucose in Gipr−/− mice (Figure 1C), collectively demonstrating the in vivo selectivity of this peptide to mGIPR.
Figure 1.
Acute blood glucose effects of GIPR agonism in mice. Effects on blood glucose over 2 h and AUC following a single injection of vehicle or hGIP Aib2 at 60 nmol/kg in lean (A) wild-type mice (blue circles), (B) Glp1r−/− mice (dark blue circles), and (C) Gipr−/− mice (light blue circles). Animals (N = 8) were fasted for 2 h prior to a subcutaneous injection of hGIP Aib2. AUC was calculated based on the change in blood glucose from time point t = 0 within each group. Data are presented as mean ± SEM. *P < 0.05 compared with vehicle treated controls within each genotype and calculated using regular one-way or two-way ANOVA with Dunnett's multiple comparisons test.
3.3. GIPR mono-agonists lower body weight in DIO mice via food intake mechanisms
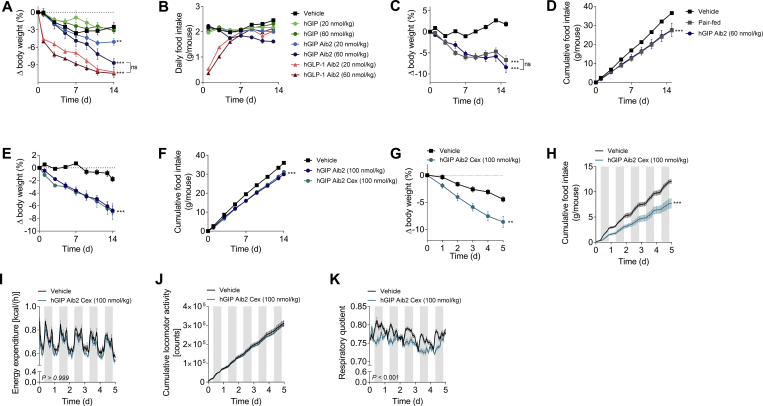
The primary objective of this report was to explore body weight regulation in non-diabetic rodents; as such, we assessed the weight-lowering efficacy of chronic GIPR agonism. Wild-type DIO mice were treated with two doses (20 and 60 nmol/kg) of native hGIP or hGIP Aib2 via daily subcutaneous injections for 14 days. For comparison purposes, we tested a DPP-IV protected GLP-1 analog (GLP-1 Aib2 Cex) [29]. Where native hGIP had negligible effect on body weight, likely the result of rapid in vivo inactivation by DPP-IV processing, hGIP Aib2 dose-dependently decreased body weight (Figure 2A). The higher dose treatment with hGIP Aib2 caused 8.7% body weight loss after two weeks, whereas the equivalent dose of the GLP-1 analog resulted in a 10.5% body weight loss. Interestingly, the temporal dynamics of the body weight loss was markedly different between the GLP-1 and GIP analogs. Most of the weight loss induced by the GLP-1 analog was achieved within the first three days of treatment and the daily loss in body weight gradually diminished as treatment progressed, in part due to the dissipation of its anorectic effects (Figure 2B). When compared to the GLP-1 analog, the body weight loss induced by hGIP Aib2 was slower in onset but became evident with repeat administration over time. The reduction in food intake following hGIP Aib2 administration was a critical component to the body weight loss, since comparable body weight loss was induced with pair-feeding as with two weeks of daily treatment (Figure 2C,D).
Figure 2.
GIPR agonism lowers body weight by food intake mechanisms in DIO mice. Effects in DIO mice (baseline body weight of 54.8 g) on (A) body weight change and (B) cumulative food intake following daily subcutaneous injections of vehicle (black squares), native hGIP (green circles), hGIP Aib2 (blue circles), and GLP-1 Aib2 Cex (red circles) at doses of 20 nmol/kg (light shading) or 60 nmol/kg (dark shading). Effects in DIO mice (baseline body weight of 44.5 g) on (C) body weight change and (D) cumulative food intake following daily subcutaneous injections of vehicle (black squares), hGIP Aib2 at a dose of 60 nmol/kg (blue circles), and pair-fed vehicle treated mice (gray squares). Effects in DIO mice (baseline body weight of 63.5 g) on (E) body weight change and (F) cumulative food intake following daily subcutaneous injections of vehicle (black squares), hGIP Aib2 (blue circles), and hGIP Aib2 Cex* (teal circles) at a dose of 100 nmol/kg. Effects in DIO mice (baseline body weight of 59.4 g) on (G) body weight change, (H) cumulative food intake, (I) energy expenditure, (J) locomotor activity, and (K) respiratory quotient, as measured by indirect calorimetry, following daily injections of vehicle (black squares) and hGIP Aib2 Cex (teal circles) at a dose of 100 nmol/kg. Group sizes are N = 8 and data are presented as mean ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with vehicle treated controls within each study unless otherwise indicated. Statistical analysis was calculated using regular two-way ANOVA with Dunnett's multiple comparisons test when necessary (A–F).
The C-terminus of native GIP is prone to proteolytic cleavage to yield an N-terminal fragment of 31-residues. We substituted it with the C-terminal peptide derived from exendin-4 (Cex), as has been done previously [5], [25]. This substitution had minimal impact on human or mouse GIPR in vitro potency relative to native hormones (Table 1), and in DIO mice, hGIP Aib2 Cex was equally efficacious in lowering body weight and reducing food intake as the hGIP analog without Cex (Figure 2E,F). In a separate experiment aimed at assessing mechanisms that support GIPR agonism-mediated weight loss, daily treatment with hGIP Aib2 Cex induced an 8.6% body weight loss after five days of treatment (Figure 2G) and significantly lowered food intake (Figure 2H) in DIO mice, which aligns well with the first results. As shown by indirect calorimetry, GIPR agonism did not increase energy expenditure (Figure 2I), consistent with the results from pair-feeding (Figure 2C,D), and did not change ambulatory activity (Figure 2J). However, respiratory exchange ratio (RER) was decreased with hGIP Aib2 Cex treatment relative to vehicle (Figure 2K), suggesting that GIP partitions fuel utilization towards fat oxidation.
3.4. GIPR mono-agonists require GIPR, not GLP-1R, for body weight lowering benefit in DIO mice
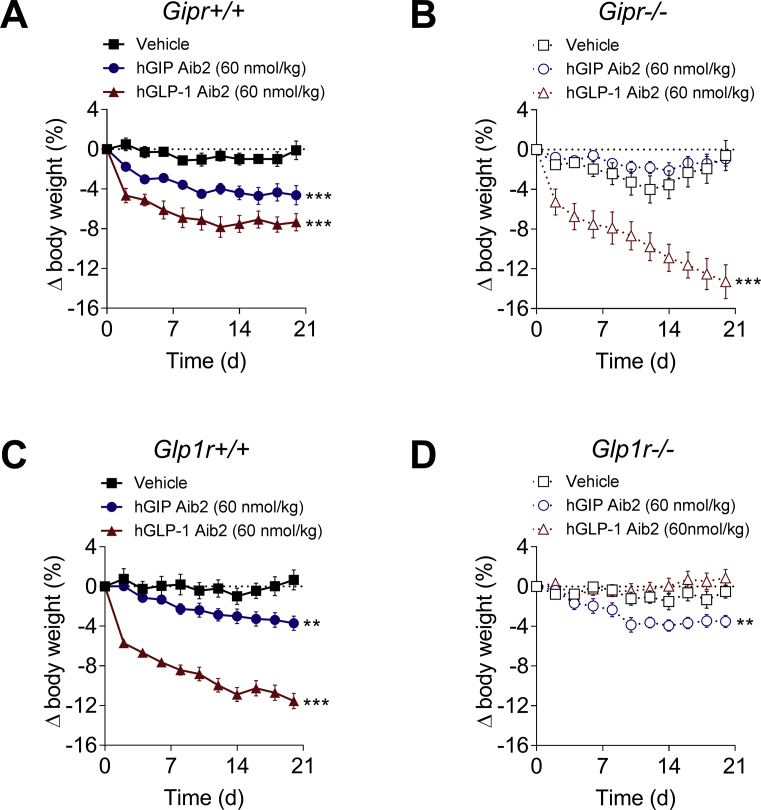
Despite the specificity demonstrated by in vitro receptor profiling, the effect on feeding observed with mGIPR mono-agonism raised the possibility of cross-reactive in vivo pharmacological action at GLP-1R. To address specificity in weight-lowering activity, hGIP Aib2 was chronically administered to DIO mice that were deficient in Gipr (Gipr−/−) or Glp1r (Glp1r−/−). Chronic treatment failed to lower body weight in DIO Gipr−/− mice relative to wild-type littermates, while a GLP-1 analog control promoted body weight loss regardless of Gipr expression (Figure 3A,B). As expected, the GLP-1 analog failed to reduce body weight in DIO Glp1r−/− mice (Figure 3C,D). In contrast, hGIP Aib2 lowered body weight to the same magnitude in DIO Glp1r−/− mice relative to wild-type littermates (Figure 3C,D), indicating the effect to be specific to GIPR activity.
Figure 3.
hGIP Aib2 lowers body weight in DIO mice via GIPR. Effects on body weight change following daily subcutaneous injections of vehicle (black squares), hGIP Aib2 (blue circles) and GLP-1 Aib2 Cex (red triangles) at a dose of 60 nmol/kg in DIO (A) Gipr+/+ (solid shapes with solid lines; baseline body weight of 53.2 g) and (B) Gipr−/− (open shapes with dashed lines; baseline body weight of 45.9 g) littermates. Effects on body weight change following daily subcutaneous injections of vehicle (black squares), hGIP Aib2 (blue circles) and GLP-1 Aib2 Cex (red triangles) at a dose of 60 nmol/kg in DIO (C) Glp1r+/+ (solid shapes with solid lines; baseline body weight of 46.3 g) and (D) Glp1r−/− (open shapes with dashed lines; baseline body weight of 39.5 g) littermates. Group sizes are N = 8 and data are presented as mean ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with vehicle treated controls within each genotype. Statistical analysis was calculated using regular two-way ANOVA with Dunnett's multiple comparisons test.
3.5. Mouse derived GIPR agonists are more potent to lower body weight in DIO mice
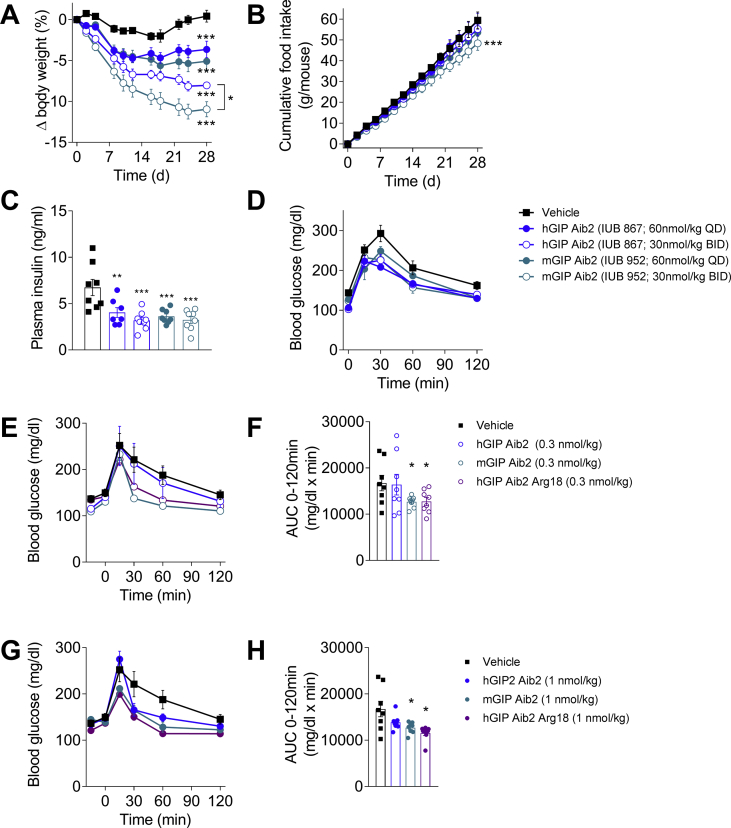
The translational impact of in vitro potency differences between mGIP and hGIP (Table 1) was assessed by examining the effect of Aib2 protected analogs on body weight lowering over four weeks of administration in DIO mice. Simultaneously, we determined whether sustained daily exposure could enhance efficacy through comparison of once (QD) and twice daily (BID) injections at equivalent total dose. In general, BID administration led to increased body weight lowering compared to QD dosing (Figure 4A). Furthermore, BID dosing of the mGIP Aib2 analog caused more body weight loss (11.0% from baseline) than identical treatment with hGIP Aib2 (8.0% from baseline) (Figure 4A), which corresponds with the food intake effect (Figure 4B). These results are consistent with the enhanced in vitro potency of mGIP peptide at mGIPR (Table 1). Both analogs led to comparable reductions in circulating insulin levels and improved glucose tolerance at end of treatment (Figure 4C,D).
Figure 4.
Mouse derived GIP is more potent at lowering body weight than human derived GIP. Effects in DIO mice (baseline body weight of 49.8 g) on (A) body weight change, (B) cumulative food intake, (C) plasma insulin, and (D) glucose tolerance following daily (solid shapes) or twice-daily (open shapes) subcutaneous injections of vehicle (black squares), hGIP Aib2 (blue circles) and mGIP Aib2 (teal circles) at equivalent daily dose of 60 nmol/kg. The BID treatment groups received two injections at a dose level of 30 nmol/kg each separated by 8 h between injections. Effects in DIO mice on (E–H) acute glucose tolerance following a single subcutaneous injection of vehicle (black squares), hGIP Aib2 (blue circles), mGIP Aib2 (teal circles) and hGIP Aib2 Arg18 (purple circles) at doses of (E, F) 0.3 nmol/kg (open shapes) or (G, H) 1 nmol/kg (solid shapes) given 15 min prior to the glucose challenge. Group sizes are N = 8, and data are presented as mean ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with vehicle treated controls within each study unless otherwise indicated. Statistical analysis was calculated using regular one-way or two-way ANOVA with Dunnett's multiple comparisons test.
The pivotal mutation that governs the enhanced potency of mGIP was reduced to His18Arg. Introduction of Arg18 into the hGIP Aib2 sequence increased in vitro potency at mGIPR such that it matched mGIP Aib2 potency (Table 1). It represents a “murinized” analog of a human peptide that has equal potency at mGIPR and hGIPR. The improved mGIPR potency translated to an enhanced in vivo effect in mice as an acute injection of mGIP Aib2 improved acute glucose tolerance in DIO mice to greater extent than the comparable human peptide when studied at two different doses (Figure 4E,H). Acute injection of the “murinized” analog improved glucose tolerance to the same extent as mGIP Aib2, which was also superior to the human derived Aib2 analog (Figure 4E,H).
3.6. GIPR antagonism does not lower body weight in DIO mice
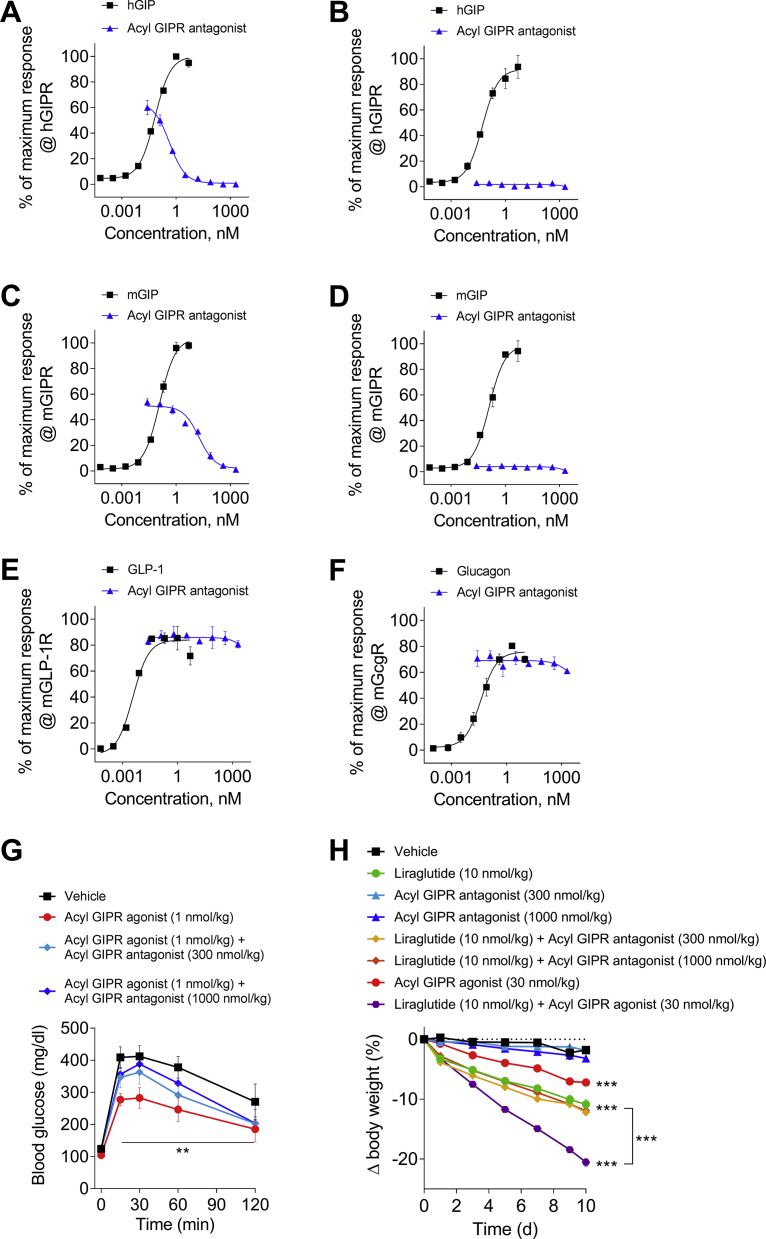
As a final, direct confirmation that GIPR agonism and not antagonism lowers body weight in mice, we tested a selective, prolonged-acting and potent GIP-based peptide antagonist in DIO mice. It is an N-terminally shortened [30] and acetylated peptide that has been site-specifically fatty acylated to support exposure allowing for daily subcutaneous dosing, and substituted with Arg18 to enhance potency at mGIPR. This peptide antagonist hGIP(5–42) NαAc K10[γEγE-C16] Arg18 is herein referred to as “acyl GIPR antagonist.” It showed potent antagonism at hGIPR (Figure 5A) and mGIPR (Figure 5C), albeit with less relative potency at the latter. Importantly, this peptide did not exhibit any degree of partial agonism at hGIPR (Figure 5B) or mGIPR (Figure 5D) as was subsequently reported for the now mischaracterized GIPR antagonist, Pro3 hGIP [31]. This antagonist also did not demonstrate any cross-reactive antagonism to mouse GLP-1R (Figure 5E) or mouse glucagon receptor (GcgR) (Figure 5E). When administered to DIO mice two hours prior to a glucose tolerance test, this antagonist lessened the improved glucose tolerance elicited by a fatty acylated GIPR agonist; hGIP Aib2,K43[γEγEγE-C16], herein referred to as “acyl GIPR agonist” (Figure 5G). This in vivo demonstration corroborates the in vitro characterization of this acyl GIPR antagonist as a selective inhibitor of GIPR agonism.
Figure 5.
Antagonism of mGIPR does not cause weight loss in DIO mice. In vitro receptor potency of hGIP(5–42) NαAc K10[γEγE-C16] Arg18 tested for (A) hGIPR antagonism, (B) hGIPR agonism, (C) mGIPR antagonism, (D) mGIPR agonism, (E) mGLP-1R antagonism, and (F) mGcgR antagonism. (G) Effects in DIO mice on acute glucose tolerance following a single subcutaneous injection of vehicle (black squares), hGIP Aib2 K43[γEγEγE-C16] at 1 nmol/kg (red circles), or the co-administration of hGIP Aib2 K43[γEγEγE-C16] at 1 nmol/kg with hGIP(5–42) NαAc K10[γEγE-C16] Arg18 at doses of 300 nmol/kg (light blue diamonds) and 1000 nmol/kg (blue diamonds). The acyl GIPR antagonist, hGIP(5–42) NαAc K10[γEγE-C16] Arg18, was administered 2 h before the glucose challenge. The acyl GIPR agonist, hGIP Aib2 K43[γEγEγE-C16], was administered 1 h before the glucose challenge. (H) Effects in DIO mice (baseline body weight of 57.2 g) on body weight change following daily subcutaneous injections of vehicle (black squares), liraglutide at 10 nmol/kg (green circles), hGIP(5–42) NαAc K10[γEγE-C16] Arg18 at doses of 300 nmol/kg (light blue triangles) and 1000 nmol/kg (blue triangles), co-administration of liraglutide (10 nmol/kg) with hGIP(5–42) NαAc K10[γEγE-C16] Arg18 at 300 nmol/kg (yellow diamonds) and 1000 nmol/kg (orange diamonds), hGIP Aib2 K43[γEγEγE-C16] at 30 nmol/kg (red circles), or co-administration of liraglutide (10 nmol/kg) with hGIP Aib2 K43[γEγEγE-C16] at 30 nmol/kg (purple hexagons). Group sizes are N = 8 and data are presented as mean ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001 compared with vehicle treated controls within each study unless otherwise indicated. Statistical analysis was calculated using regular two-way ANOVA with Dunnett's multiple comparisons test.
When chronically administered to DIO mice as a daily subcutaneous injection at doses of 300 nmol/kg or 1 μmol/kg for ten days, the acyl GIPR antagonist did not cause any measurable change in body weight relative to vehicle controls (Figure 5H). These results clearly demonstrate that reduction of endogenous GIP action via mGIPR antagonism alone does not lower body weight in obese mice. Comparative treatment with 30 nmol/kg of the acyl GIPR agonist or with the fatty acylated GLP-1R agonist, liraglutide, respectively lowered body weight by 7.2% and 10.9%. Co-treatment resulted in a reduction of 20.5% (Figure 5H), supportive of the beneficial virtues of dual incretin agonism in lowering body weight [5]. In contrast, co-administration of acyl GIPR antagonist with liraglutide resulted in body weight lowering effects indistinguishable from liraglutide alone (Figure 5H).
4. Discussion
Employment of GIPR signaling, specifically the direction of this manipulation, as a strategy to improve body weight remains a contentious subject of intense scientific investigation [22], [32], [33]. Despite the physiological benefits of GIP on glucose control, the initial direction in drug design was directed towards attenuating GIP action with peptide antagonists for the treatment of patients with T2D and obesity [34], and that assumption currently continues in scientific research [35]. However, it has yet to be shown that an elevated level of circulating GIP is a pathophysiological driver of T2D. Furthermore, inhibiting an endogenous insulinotropic response seems counterintuitive for pharmacotherapies aimed to improve glucose control. Despite these incongruences, it is important to note that the studies comprising this report were not designed to address the question of which direction to manipulate GIPR signaling for glycemic benefit in diabetic rodent models, but rather to specifically address the question of which direction to manipulate GIPR signaling for body weight benefit in non-diabetic, genetically wild-type obese mice.
The proposition for GIPR antagonists as opposed to GIPR agonists for the treatment of obesity largely originates from the report that congenital global Gipr−/− mice are protected from diet-induced and genetic-induced obesity [10]. Importantly and seemingly forgotten is the comparable phenotype displayed by congenital Glp1r−/− mice [36]. Furthermore, the [Pro3] GIP analog was shown to decrease body weight in obese mice [37], and at the time of its original report, the body weight lowering effect was believed to be the product of GIPR antagonism. However, this peptide has since been thoroughly tested and is now re-characterized as a weak partial agonist at mGIPR [31]. Thus, those historical results on body weight should be interpreted with caution but support mGIPR agonism in lowering body weight. Therefore, to directly address this dichotomy as to which direction to manipulate the GIP system for therapeutic benefits in obesity, a series of precise chemical reagents were developed and studied for their pharmacological effects in obese rodents.
There are appreciable opportunities to improve the pharmacological profile of GIP by chemical optimization. As such, the GIP analogs reported herein were specifically advanced to address the question of how GIPR agonism affects body weight in DIO mice with chronic dosing. Through a series of diagnostic studies measuring the acute effects on basal glucose levels in wild-type, Gipr−/−, and Glp1r−/− mice, we demonstrate that these GIP analogs are selective for mouse GIPR and suitable for studies to specifically address the effects of GIPR mono-agonism on body weight in obese mice. Chronic administration to DIO mice caused meaningful body weight loss in numerous DIO mouse studies using several structurally different analogs, but relatively high doses were required. This latter notion is important as chronic, twice daily administration of D-Ala2 GIP analogs to DIO mice did not result in significant body weight change [26], likely due to using subthreshold doses, which notably were lower than doses used here with the Aib2 GIP analogs.
Our results also indicate that the human and mouse sequences demonstrate differential potency at mGIPR, but not hGIPR. This largely recapitulates previous results [31], and the pivotal amino acid change influencing potency among the three differences between human and mouse GIP is His18Arg. Protection against DDP-IV cleavage by incorporation of Aib2 also increased in vitro and in vivo potency. Noteworthy is our discovery that Aib2 enhances in vitro potency at mGIPR when incorporated into the human GIP sequence. One potential explanation for the lack of body weight lowering effects of other reported GIPR agonists [21], [25] could be due to this species divergence and subsequent reduced potency at mGIPR as that pharmacological aspect was not previously studied in those reports.
Extending time action is another strategy to increase in vivo efficacy. Twice daily administration of the unacylated analogs herein caused greater body weight loss in DIO mice than the equivalent dose administered as a single daily injection, suggesting that protracted time action should yield more effective therapy. As such, we report a fatty acylated, Aib2 substituted analog of hGIP lowered body weight in DIO mice following chronic administration. However, the pharmacokinetic profile for this analog was not studied but is expected to be similar to comparably acylated peptides such as liraglutide. This effect size on body weight is consistent with that achieved with chronic BID administration of another equivalent acylated analog, although statistical significance was not reached in that study [21]. However, another long-acting GIP analog, presumably lipidated with a diacid-based fatty acid although the structure was not reported, failed to significantly lower body weight in DIO mice at high doses; however, potency at mGIPR was not reported for that analog either [8].
Native, full-length GIP has limited aqueous solubility and is prone to aggregation in liquid formulations. Since the C-terminal tail of GIP is dispensable for activity at the receptor [38], we chose to explore more radical modifications to this region. The C-terminal tail of native GIP can be replaced with the C-terminal extension of exendin-4 (Cex) to improve biophysical properties and physiological stability, and notably has been utilized in other GIPR agonists [5], [25]. Our Cex-substituted analogs proved similarly efficacious on body weight as GIP analogs with a native C-terminal tail, however C-terminally truncated molecules were not studied for effects on body weight.
Recent commercialization attempts have focused on attenuating GIP action for metabolic benefits. An intellectual property filing (WO2017/112824) shows that treatment with an anti-GIPR antibody alone slows body weight gain in growing DIO mice and obese monkeys, and potentiates the body weight benefits of GLP-1R agonists. Sufficient peer review is required to verify the results, particularly the selectivity profile towards GIPR since antibodies directed to G protein-coupled receptors can be promiscuous. It has been speculated, albeit not rigorously tested, that the acute effects of GIP on adipose tissue vasoactivity and fatty acid re-esterification in humans would eventually progress to increased adiposity with continuous exposure [39]. However, it is important to note that the contributing effects of insulin cannot be excluded, and GLP-1 has also been shown to increase adipose tissue blood flow [40] yet GLP-1 pharmacology does not promote weight gain. In several recent reports detailing the acute administration of peptide-based GIPR antagonists optimized for clinical use, it has been postulated that chronic GIPR antagonism can be utilized as a potential therapeutic strategy for the treatment of obesity [32], [35]. These peptide-based GIPR antagonists have been rationally designed through a series of structure-activity relationships and characterized in vitro at the family of receptors [41]. In healthy human volunteers, one such antagonist (hGIP (3–30)-NH2) blocked a majority of the insulinotropic response as a result of the GIP co-infusion [42] and blocked the vasoactivity induced by an acute bolus infusion of GIP [32]. At minimum, these studies clearly demonstrate the ability of this antagonist to block physiological responses attributed to GIP but stop short in demonstrating an effect on body weight. Based on these results, it was speculated that antagonizing GIPR would be of value to improve body weight in humans and is a basis for recent commercialization efforts (WO2016/034186). However, none of these reports study whether chronic therapy with a peptide-based GIPR antagonist induces body weight loss, promotes weight gain or exacerbates HFD-induced weight gain in chronic preclinical pharmacology studies. Such chronic studies are likely hindered because an insufficiently short half-life and limited solubility to support chronic infusion studies. Additionally, species-specific differences in the receptor pharmacological profile also complicate the preclinical study of this analog. This antagonist [hGIP (3–30)-NH2] shows a substantially reduced affinity for the rat GIPR relative to hGIPR [33], [43]. A rat-derived GIP (3–30)NH2 sequence is also limited by a partial agonistic profile at GIPR [31], again highlighting the difficulties in translational research particularly evident with the GIP system.
To directly address whether pharmacological attenuation of physiological GIP provides therapeutic benefits in obese mice, we pursued a selective long-acting antagonist to complement the work we report on GIPR agonism. We report a GIP analog of sufficient aqueous solubility that employs a shortened N-terminus [30], the Arg18 substitution, and a site-specific fatty-acylation to achieve selective, high potency antagonism at mouse and human GIPR. While this acyl GIPR antagonist is of lesser potency at mGIPR relative to hGIPR and could benefit from further chemical refinement if it were to be advanced to clinical study, it is suitable for preclinical pharmacology study in mice. This acylated GIPR antagonist blocked GIPR agonist-induced improvements in glucose tolerance in DIO mice, and had no apparent effect on body weight in DIO mice following high dose chronic therapy, demonstrating that inhibition of endogenous GIP does not promote body weight loss. Co-treatment of liraglutide with this acyl GIPR antagonist also did not result in additional body weight lowering benefit, which importantly was evident upon co-treatment with GIPR agonists. These collective results once again illustrate the amplification of GLP-1R agonist-induced weight lowering with GIPR agonism [5], [8], and not with GIPR antagonism. The pharmacokinetic profile of this antagonist was not studied but is expected to be comparable to the acylated agonist that we utilized. These results of synergistic body weight lowering with co-agonism and no evidence of anti-obesity activity in GIPR antagonism when administered alone or in concert with GLP-1 agonism convincingly addresses the primary objective of this report.
The mechanism of action that drives the body weight lowering benefits of GIPR agonism has yet to be fully elucidated, and is not the primary purpose of this report. The body weight loss induced by selective mGIPR agonism appears to be predominantly driven by food intake, as interpreted from pair-feeding and indirect calorimetry studies, which is consistent with previous studies of co-agonists [5], [8]. Although not studied here, this suggests the engagement of neural circuits in the brain involved in feeding regulation, which is consistent with central food intake suppression observed after central administration of GIP and GLP-1 [44]. Whether the engagement is directly mediated by GIPR signaling in the brain is a question that remains to be investigated, but the lack of weight-lowering effects of other GIPR agonists in leptin-deficient mice [25] suggests a peripheral to central connection. As GIPR is expressed in adipose tissues and involved in various aspects of lipid homeostasis [45], GIPR-mediated body weight lowering could be the consequence of improved insulin sensitivity, thus lessening the dysregulated lipogenesis observed in hyperinsulinemic obesity, which could explain the lack of body weight lowering observed in insulinopenic obese mice when other GIP analogs were used [25]. GIPR mediated effects on immunometabolism can also contribute to improved systemic metabolism and adipocyte function [46]. It also cannot be excluded that the preferential direction to manipulate the GIPR system can differ based on tissue and site of action such that analogs that favor more central nervous system biodistribution or analogs that have tissue-specific receptor modulator function, such as selective antagonism at adipose GIPR and functional agonism at islet, myeloid, and brain GIPR, would be beneficial. We expect that the use of quality pharmacological tools as reported here, in combination with precise GIPR expression mapping, conditional Gipr−/− mice, and more sophisticated pharmacology studies will be valuable in delineating the mechanisms driving the therapeutic benefits of GIP action on body weight control.
5. Conclusion
The benefits of GIP to improve glucose homeostasis have been classically counteracted by historical observations about its potential obesogenic activity, which have reduced the interest in its development as therapy for the treatment of metabolic disease. In fact, some recent literature and drug development programs still advocate for GIPR antagonism, as opposed to GIPR agonism, to elicit therapeutic benefits in cardiometabolic diseases despite mounting clinical evidence suggesting GIPR agonism is the preferred direction, at least when in combination with GLP-1R agonism. Here, we combine refined biological and chemical tools to show that chronic GIPR agonism alone, not antagonism, lowers body weight in obese mice. Collectively, these results herein with chemically refined GIPR agonists of protracted time action that possess high potency, selective activity at mouse receptors have repeatedly improved body weight in DIO mice, and structural optimization to improve potency, time action, and biophysical properties can enhance the pharmacological profile of GIP analogs. These preclinical results, together with emerging clinical combinatorial pharmacology results, provide a clear answer to the longstanding scientific debate on whether to amplify or attenuate GIP action for body weight benefit.
Author contributions
P.A.M. conceptualized the project, synthesized agonists, performed in vitro assays, and interpreted data. B.F. conceptualized the project, performed in vivo pharmacology experiments, interpreted data, and co-wrote the manuscript. V.G. performed in vitro assays and interpreted data. B.Y. synthesized antagonists and interpreted data. M.H.T interpreted data and edited the manuscript. R.D.D. conceptualized the project, interpreted data, co-wrote the manuscript, and is the guarantor of the work. D.P.-T. conceptualized the project, interpreted data, and co-wrote the manuscript.
Acknowledgments
We would like to acknowledge Jenna Hall, Marita Rivir, Kathleen Smith, Joyce Sorrell, and Emily Yates for assistance with in vivo pharmacology studies. We would like to acknowledge Joe Chabenne and Steph Mowery for assistance with in vitro cell-based assays, and Patrick J. Knerr for assistance with chemical synthesis. This work was partially supported by funds of the University of Cincinnati-College of Medicine (F102150) to D.P.-T.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2018.12.001.
Contributor Information
Brian Finan, Email: bfin@novonordisk.com.
Diego Perez-Tilve, Email: pereztdo@ucmail.uc.edu.
Conflicts of interest
B.F., V.G., B.Y., and R.D.D. are current employees of Novo Nordisk. M.H.T. and D.P-T. individually maintain a research collaboration and receive funding from Novo Nordisk. The experimental results were obtained prior to the collaborations with Novo Nordisk. The opinions expressed in this article are those of the authors, not Novo Nordisk.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Marso S.P., Bain S.C., Consoli A., Eliaschewitz F.G., Jodar E., Leiter L.A. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. New England Journal of Medicine. 2016;375(19):1834–1844. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 2.Marso S.P., Daniels G.H., Brown-Frandsen K., Kristensen P., Mann J.F., Nauck M.A. Liraglutide and cardiovascular outcomes in type 2 diabetes. New England Journal of Medicine. 2016;375(4):311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nauck M.A., Heimesaat M.M., Orskov C., Holst J.J., Ebert R., Creutzfeldt W. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. Journal of Clinical Investigation. 1993;91(1):301–307. doi: 10.1172/JCI116186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hojberg P.V., Vilsboll T., Rabol R., Knop F.K., Bache M., Krarup T. Four weeks of near-normalisation of blood glucose improves the insulin response to glucagon-like peptide-1 and glucose-dependent insulinotropic polypeptide in patients with type 2 diabetes. Diabetologia. 2009;52(2):199–207. doi: 10.1007/s00125-008-1195-5. [DOI] [PubMed] [Google Scholar]
- 5.Finan B., Ma T., Ottaway N., Muller T.D., Habegger K.M., Heppner K.M. Unimolecular dual incretins maximize metabolic benefits in rodents, monkeys, and humans. Science Translational Medicine. 2013;5(209):209ra151. doi: 10.1126/scitranslmed.3007218. [DOI] [PubMed] [Google Scholar]
- 6.Frias J.P., Bastyr E.J., 3rd, Vignati L., Tschop M.H., Schmitt C., Owen K. The sustained effects of a dual GIP/GLP-1 receptor agonist, NNC0090-2746, in patients with type 2 diabetes. Cell Metabolism. 2017;26(2):343–352 e2. doi: 10.1016/j.cmet.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 7.Frias J.P., Nauck M.A., Van J., Kutner M.E., Cui X., Benson C. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet. 2018;392(10160):2180–2193. doi: 10.1016/S0140-6736(18)32260-8. [DOI] [PubMed] [Google Scholar]
- 8.Coskun T., Sloop K.W., Loghin C., Alsina-Fernandez J., Urva S., Bokvist K.B. LY3298176, a novel dual GIP and GLP-1 receptor agonist for the treatment of type 2 diabetes mellitus: from discovery to clinical proof of concept. Mol Metab. 2018;18:3–14. doi: 10.1016/j.molmet.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finan B., Muller T.D., Clemmensen C., Perez-Tilve D., DiMarchi R.D., Tschop M.H. Reappraisal of GIP pharmacology for metabolic diseases. Trends in Molecular Medicine. 2016;22(5):359–376. doi: 10.1016/j.molmed.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 10.Miyawaki K., Yamada Y., Ban N., Ihara Y., Tsukiyama K., Zhou H. Inhibition of gastric inhibitory polypeptide signaling prevents obesity. Nature Medicine. 2002;8(7):738–742. doi: 10.1038/nm727. [DOI] [PubMed] [Google Scholar]
- 11.Boylan M.O., Glazebrook P.A., Tatalovic M., Wolfe M.M. Gastric inhibitory polypeptide immunoneutralization attenuates development of obesity in mice. American Journal of Physiology. Endocrinology and Metabolism. 2015;309(12):E1008–E1018. doi: 10.1152/ajpendo.00345.2015. [DOI] [PubMed] [Google Scholar]
- 12.Fulurija A., Lutz T.A., Sladko K., Osto M., Wielinga P.Y., Bachmann M.F. Vaccination against GIP for the treatment of obesity. PLoS One. 2008;3(9) doi: 10.1371/journal.pone.0003163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Althage M.C., Ford E.L., Wang S., Tso P., Polonsky K.S., Wice B.M. Targeted ablation of glucose-dependent insulinotropic polypeptide-producing cells in transgenic mice reduces obesity and insulin resistance induced by a high fat diet. Journal of Biological Chemistry. 2008;283(26):18365–18376. doi: 10.1074/jbc.M710466200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakamura T., Tanimoto H., Mizuno Y., Okamoto M., Takeuchi M., Tsubamoto Y. Gastric inhibitory polypeptide receptor antagonist, SKL-14959, suppressed body weight gain on diet-induced obesity mice. Obes Sci Pract. 2018;4(2):194–203. doi: 10.1002/osp4.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pathak V., Gault V.A., Flatt P.R., Irwin N. Antagonism of gastric inhibitory polypeptide (GIP) by palmitoylation of GIP analogues with N- and C-terminal modifications improves obesity and metabolic control in high fat fed mice. Molecular and Cellular Endocrinology. 2015;401:120–129. doi: 10.1016/j.mce.2014.10.025. [DOI] [PubMed] [Google Scholar]
- 16.Moller C.L., Vistisen D., Faerch K., Johansen N.B., Witte D.R., Jonsson A. Glucose-Dependent insulinotropic polypeptide is associated with lower low-density lipoprotein but unhealthy fat distribution, independent of insulin: the ADDITION-PRO study. The Journal of Cinical Endocrinology and Metabolism. 2016;101(2):485–493. doi: 10.1210/jc.2015-3133. [DOI] [PubMed] [Google Scholar]
- 17.Asmar M., Simonsen L., Madsbad S., Stallknecht B., Holst J.J., Bulow J. Glucose-dependent insulinotropic polypeptide may enhance fatty acid re-esterification in subcutaneous abdominal adipose tissue in lean humans. Diabetes. 2010;59(9):2160–2163. doi: 10.2337/db10-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim S.J., Nian C., Karunakaran S., Clee S.M., Isales C.M., McIntosh C.H. GIP-overexpressing mice demonstrate reduced diet-induced obesity and steatosis, and improved glucose homeostasis. PLoS One. 2012;7(7) doi: 10.1371/journal.pone.0040156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szalowska E., Meijer K., Kloosterhuis N., Razaee F., Priebe M., Vonk R.J. Sub-chronic administration of stable GIP analog in mice decreases serum LPL activity and body weight. Peptides. 2011;32(5):938–945. doi: 10.1016/j.peptides.2011.02.011. [DOI] [PubMed] [Google Scholar]
- 20.Gault V.A., Kerr B.D., Harriott P., Flatt P.R. Administration of an acylated GLP-1 and GIP preparation provides added beneficial glucose-lowering and insulinotropic actions over single incretins in mice with Type 2 diabetes and obesity. Clinical Science. 2011;121(3):107–117. doi: 10.1042/CS20110006. [DOI] [PubMed] [Google Scholar]
- 21.Norregaard P.K., Deryabina M.A., Tofteng Shelton P., Fog J.U., Daugaard J.R., Eriksson P.O. A novel GIP analogue, ZP4165, enhances glucagon-like peptide-1-induced body weight loss and improves glycaemic control in rodents. Diabetes, Obesity and Metabolism. 2018;20(1):60–68. doi: 10.1111/dom.13034. [DOI] [PubMed] [Google Scholar]
- 22.Millar P.J., Pathak V., Moffett R.C., Pathak N.M., Bjourson A.J., O'Kane M.J. Beneficial metabolic actions of a stable GIP agonist following pre-treatment with a SGLT2 inhibitor in high fat fed diabetic mice. Molecular and Cellular Endocrinology. 2016;420:37–45. doi: 10.1016/j.mce.2015.11.019. [DOI] [PubMed] [Google Scholar]
- 23.Speliotes E.K., Willer C.J., Berndt S.I., Monda K.L., Thorleifsson G., Jackson A.U. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nature Genetics. 2010;42(11):937–948. doi: 10.1038/ng.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kerr B.D., Irwin N., Flatt P.R., Gault V.A. Prolonged GIP receptor activation using stable mini-PEGylated GIP improves glucose homeostasis and beta-cell function in age-related glucose intolerance. Peptides. 2009;30(2):219–225. doi: 10.1016/j.peptides.2008.10.017. [DOI] [PubMed] [Google Scholar]
- 25.Tatarkiewicz K., Hargrove D.M., Jodka C.M., Gedulin B.R., Smith P.A., Hoyt J.A. A novel long-acting glucose-dependent insulinotropic peptide analogue: enhanced efficacy in normal and diabetic rodents. Diabetes, Obesity and Metabolism. 2014;16(1):75–85. doi: 10.1111/dom.12181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gault V.A., Porter D.W., Irwin N., Flatt P.R. Comparison of sub-chronic metabolic effects of stable forms of naturally occurring GIP(1-30) and GIP(1-42) in high-fat fed mice. Journal of Endocrinology. 2011;208(3):265–271. doi: 10.1530/JOE-10-0419. [DOI] [PubMed] [Google Scholar]
- 27.Scrocchi L.A., Brown T.J., MaClusky N., Brubaker P.L., Auerbach A.B., Joyner A.L. Glucose intolerance but normal satiety in mice with a null mutation in the glucagon-like peptide 1 receptor gene. Nature Medicine. 1996;2(11):1254–1258. doi: 10.1038/nm1196-1254. [DOI] [PubMed] [Google Scholar]
- 28.Miyawaki K., Yamada Y., Yano H., Niwa H., Ban N., Ihara Y. Glucose intolerance caused by a defect in the entero-insular axis: a study in gastric inhibitory polypeptide receptor knockout mice. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(26):14843–14847. doi: 10.1073/pnas.96.26.14843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Finan B., Yang B., Ottaway N., Stemmer K., Muller T.D., Yi C.X. Targeted estrogen delivery reverses the metabolic syndrome. Nature Medicine. 2012;18(12):1847–1856. doi: 10.1038/nm.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kerr B.D., Flatt A.J., Flatt P.R., Gault V.A. Characterization and biological actions of N-terminal truncated forms of glucose-dependent insulinotropic polypeptide. Biochemical and Biophysical Research Communications. 2011;404(3):870–876. doi: 10.1016/j.bbrc.2010.12.077. [DOI] [PubMed] [Google Scholar]
- 31.Sparre-Ulrich A.H., Hansen L.S., Svendsen B., Christensen M., Knop F.K., Hartmann B. Species-specific action of (Pro3)GIP - a full agonist at human GIP receptors, but a partial agonist and competitive antagonist at rat and mouse GIP receptors. British Journal of Pharmacology. 2016;173(1):27–38. doi: 10.1111/bph.13323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Asmar M., Asmar A., Simonsen L., Gasbjerg L.S., Sparre-Ulrich A.H., Rosenkilde M.M. The gluco- and liporegulatory and vasodilatory effects of glucose-dependent insulinotropic polypeptide (GIP) are abolished by an antagonist of the human GIP receptor. Diabetes. 2017;66(9):2363–2371. doi: 10.2337/db17-0480. [DOI] [PubMed] [Google Scholar]
- 33.Sparre-Ulrich A.H., Gabe M.N., Gasbjerg L.S., Christiansen C.B., Svendsen B., Hartmann B. GIP(3-30)NH2 is a potent competitive antagonist of the GIP receptor and effectively inhibits GIP-mediated insulin, glucagon, and somatostatin release. Biochemical Pharmacology. 2017;131:78–88. doi: 10.1016/j.bcp.2017.02.012. [DOI] [PubMed] [Google Scholar]
- 34.Gault V.A., O'Harte F.P., Harriott P., Mooney M.H., Green B.D., Flatt P.R. Effects of the novel (Pro3)GIP antagonist and exendin(9-39)amide on GIP- and GLP-1-induced cyclic AMP generation, insulin secretion and postprandial insulin release in obese diabetic (ob/ob) mice: evidence that GIP is the major physiological incretin. Diabetologia. 2003;46(2):222–230. doi: 10.1007/s00125-002-1028-x. [DOI] [PubMed] [Google Scholar]
- 35.Gasbjerg L.S., Gabe M.B.N., Hartmann B., Christensen M.B., Knop F.K., Holst J.J. Glucose-dependent insulinotropic polypeptide (GIP) receptor antagonists as anti-diabetic agents. Peptides. 2018;100:173–181. doi: 10.1016/j.peptides.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 36.Scrocchi L.A., Drucker D.J. Effects of aging and a high fat diet on body weight and glucose tolerance in glucagon-like peptide-1 receptor -/- mice. Endocrinology. 1998;139(7):3127–3132. doi: 10.1210/endo.139.7.6092. [DOI] [PubMed] [Google Scholar]
- 37.Gault V.A., McClean P.L., Cassidy R.S., Irwin N., Flatt P.R. Chemical gastric inhibitory polypeptide receptor antagonism protects against obesity, insulin resistance, glucose intolerance and associated disturbances in mice fed high-fat and cafeteria diets. Diabetologia. 2007;50(8):1752–1762. doi: 10.1007/s00125-007-0710-4. [DOI] [PubMed] [Google Scholar]
- 38.Hinke S.A., Manhart S., Pamir N., Demuth H., Gelling W.R., Pederson R.A. Identification of a bioactive domain in the amino-terminus of glucose-dependent insulinotropic polypeptide (GIP) Biochimica et Biophysica Acta. 2001;1547(1):143–155. doi: 10.1016/s0167-4838(01)00181-9. [DOI] [PubMed] [Google Scholar]
- 39.Asmar M., Asmar A., Simonsen L., Holst J.J., Bulow J. Glucose-dependent insulinotropic polypeptide increases blood flow in adipose tissue of humans by recruiting capillaries. The Journal of Cinical Endocrinology and Metabolism. 2018 doi: 10.1210/jc.2018-00389. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 40.Asmar A., Asmar M., Simonsen L., Madsbad S., Holst J.J., Hartmann B. Glucagon-like peptide-1 elicits vasodilation in adipose tissue and skeletal muscle in healthy men. Physiological reports. 2017;5(3) doi: 10.14814/phy2.13073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hansen L.S., Sparre-Ulrich A.H., Christensen M., Knop F.K., Hartmann B., Holst J.J. N-terminally and C-terminally truncated forms of glucose-dependent insulinotropic polypeptide are high-affinity competitive antagonists of the human GIP receptor. British Journal of Pharmacology. 2016;173(5):826–838. doi: 10.1111/bph.13384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gasbjerg L.S., Christensen M.B., Hartmann B., Lanng A.R., Sparre-Ulrich A.H., Gabe M.B.N. GIP(3-30)NH2 is an efficacious GIP receptor antagonist in humans: a randomised, double-blinded, placebo-controlled, crossover study. Diabetologia. 2018;61(2):413–423. doi: 10.1007/s00125-017-4447-4. [DOI] [PubMed] [Google Scholar]
- 43.Gabe M.B.N., Sparre-Ulrich A.H., Pedersen M.F., Gasbjerg L.S., Inoue A., Brauner-Osborne H. Human GIP(3-30)NH2 inhibits G protein-dependent as well as G protein-independent signaling and is selective for the GIP receptor with high-affinity binding to primate but not rodent GIP receptors. Biochemical Pharmacology. 2018;150:97–107. doi: 10.1016/j.bcp.2018.01.040. [DOI] [PubMed] [Google Scholar]
- 44.NamKoong C., Kim M.S., Jang B.T., Lee Y.H., Cho Y.M., Choi H.J. Central administration of GLP-1 and GIP decreases feeding in mice. Biochemical and Biophysical Research Communications. 2017;490(2):247–252. doi: 10.1016/j.bbrc.2017.06.031. [DOI] [PubMed] [Google Scholar]
- 45.Getty-Kaushik L., Song D.H., Boylan M.O., Corkey B.E., Wolfe M.M. Glucose-dependent insulinotropic polypeptide modulates adipocyte lipolysis and reesterification. Obesity. 2006;14(7):1124–1131. doi: 10.1038/oby.2006.129. [DOI] [PubMed] [Google Scholar]
- 46.Mantelmacher F.D., Zvibel I., Cohen K., Epshtein A., Pasmanik-Chor M., Vogl T. GIP regulates inflammation and body weight by restraining myeloid-cell-derived S100A8/A9. Nature Metabolism. 2018 doi: 10.1038/s42255-018-0001-z. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.