Abstract
Objective
c-Jun, a prominent member of the activator protein 1 (AP-1) family, is involved in various physiology processes such as cell death and survival. However, a role of hepatic c-Jun in the whole-body metabolism is poorly understood.
Methods
We generated liver-specific c-Jun knock-out (c-jun△li) mice to investigate the effect of hepatic c-Jun on the whole-body physiology, particularly in blood glucose and body temperature. Primary hepatocytes were also used to explore a direct regulation of c-Jun in gluconeogenesis.
Results
c-jun△li mice showed higher hepatic gluconeogenic capacity compared with control mice, and similar results were obtained in vitro. In addition, fibroblast growth factor 21 (FGF21) expression was directly inhibited by c-Jun knockdown and adenovirus-mediated hepatic FGF21 over-expression blocked the effect of c-Jun on gluconeogenesis in c-jun△li mice. Interestingly, c-jun△li mice also exhibited higher body temperature, with induced thermogenesis and uncoupling protein 1 (UCP1) expression in brown adipose tissue (BAT). Furthermore, the body temperature became comparable between c-jun△li and control mice at thermoneutral temperature (30 °C). Moreover, the activity of sympathetic nervous system (SNS) was increased in c-jun△li mice and the higher body temperature was inhibited by beta-adrenergic receptor blocker injection. Finally, the activated SNS and increased body temperature in c-jun△li mice was most likely caused by the signals from the brain and hepatic vagus nerve, as the expression of c-Fos (the molecular marker of neuronal activation) was changed in several brain areas controlling body temperature and body temperature was decreased by selective hepatic vagotomy.
Conclusions
These data demonstrate a novel function of hepatic c-Jun in the regulation of gluconeogenesis and body temperature via FGF21 and neural signals. Our results also provide novel insights into the organ crosstalk in the regulation of the whole-body physiology.
Keywords: Gluconeogenesis, Temperature, Organ crosstalk
Abbreviations: fibroblast growth factor 21, FGF21; uncoupling protein 1, UCP1; brown adipose tissue, BAT; sympathetic nervous system, SNS; tuberous sclerosis complex 1, TSC1; activator protein 1, AP-1; c-Jun N-terminal kinase, JNK; nuclear magnetic resonance, NMR; glucose tolerance tests, GTTs; pyruvate tolerance tests, PTTs; insulin tolerance tests, ITTs; chromatin immunoprecipitation, ChIP; norepinephrine, NE; ventromedial preoptic nucleus, VMPO; dorsomedial hypothalamus, DMH; rostral raphe pallidus nucleus, rRPa; hepatic vagotomy, HV
Highlights
-
•
Liver-specific inactivation of c-Jun increased gluconeogenesis via decreasing FGF21 expression.
-
•
Liver-specific inactivation of c-Jun increased body temperature by promoting thermogenesis in BAT.
-
•
Hepatic c-Jun modulates body temperature via regulating sympathetic nervous system activity and vagus nerve.
1. Introduction
The liver is a vital organ that plays an important role in whole-body physiology, especially in the regulation of glucose homeostasis. It regulates glucose homeostasis by controlling various pathways of glucose metabolism, including glycogenesis, glycogenolysis, glycolysis, and gluconeogenesis [1]. Each process involves many enzymes and it is critical to control the expression of these enzymes to meet the glucose requirement for other organs such as brain. Diminished homeostatic control of glucose metabolism is a common characteristic related to many metabolic diseases [2]. For example, gluconeogenesis, taking place mainly in the liver, is a target of therapy for type 2 diabetes, such as the drug metformin [3]. Key enzymes in this process include glucose 6-phosphatas (G6Pase) and phosphoenolpyruvate carboxykinase (PEPCK) [1]. cAMP-response element binding protein (CREB), forkhead box protein O1 (FoxO1) and some nuclear receptors are important transcription factors for the regulation of hepatic gluconeogenesis [4]. Given the importance of liver in metabolism control in the whole body, it is interesting to use liver-specific knockout mice to explore more regulators of hepatic glucose metabolism to understand the mechanism underlying glucose homeostasis in mammals.
On the other hand, the liver also regulates thermogenesis in brown adipose tissue (BAT) and body temperature [5], [6]. For example, liver-specific tuberous sclerosis complex 1 (TSC1) knockout mice display reduced body temperature [5]; hepatic glucokinase over-expression decreases adaptive thermogenesis by downregulating thermogenesis-related genes in BAT [6]. Over the years, although many regulatory mechanisms regarding these processes have been identified, detailed molecular causes for the hepatic regulation of glucose homeostasis and body temperature remain elusive.
c-Jun, a prominent member of the activator protein 1 (AP-1) family [7], was discovered as a novel oncoprotein encoded by a cellular insert in the genome of avian sarcoma virus 17 [8]. Mice lacking c-Jun in liver display reduced hepatocyte proliferation [9] and impaired liver generation after partial hepatectomy [10]. In addition, c-Jun controls cell survival in liver cancer initiation [11], [12] and acute hepatitis [13]. c-Jun is also a key target of c-Jun N-terminal kinase (JNK) and over-expression of JNK in the liver of wild-type mice decreases insulin sensitivity [14]. Based on the above knowledge, we hypothesized that hepatic c-Jun may have a profound role in the regulation of the whole-body physiology, particularly in blood glucose and body temperature.
Fibroblast growth factor (FGF) 21, a member of the FGF family, binds to FGF receptor and is involved in a variety of biological processes. As a key metabolic regulator, FGF21 is mainly expressed in several metabolically active tissue organ such as the liver [15] and regulates hepatic glucose homeostasis. For example, recent reports have shown that FGF21 improves glucose tolerance and insulin sensitivity [16], [17]. In addition, FGF21 plays role in hepatic gluconeogenesis and glycogenolysis [18]. FGF21 levels are increased under a variety of physiological conditions, including fasting, overfeeding and high-carbohydrate diets [15]. Well known regulators for FGF21 expression include E4-binding protein-4 (E4BP4) [19] and peroxisome proliferator activated receptor alpha (PPARα) [20]. Transcription regulation of FGF21 remains largely unknown.
The aim of our current study was to investigate the possible role of hepatic c-Jun in metabolic control and elucidate the underlying mechanisms. We found that hepatic c-Jun regulates glucose metabolism via FGF21 and modulates body temperature through the neural signals. Because communication between organs is essential for maintaining systemic metabolism homeostasis, our study provides new insights into the crosstalk among different tissues in the regulation of whole-body metabolism and the transcriptional regulation of FGF21.
2. Material and methods
2.1. Animals and treatment
c-junf/f mice were kindly provided by Dr. Erwin F. Wagner (Cancer Cell Biology Program, Spanish National Cancer Research Center). c-junf/f mice were crossed with albumin (Alb)-Cre mice to generate c-Jun liver-specific knockout (c-jun△li) mice. 8- to 12-week-old male mice were maintained on a 12-hr light/dark cycle at 22 °C. Food intake and body weight were measured daily. Mice were given an intraperitoneal (i.p.) injection of propranolol (Sigma, MO, USA) at 5 mg/kg [21] and then temperature was detected. Mice exposed to thermoneutral temperature were placed at 30 °C in an intelligent artificial climate chamber (Ningbo Saifu Experimental Instrument Co., Ltd, Ningbo, China) for 30 h. Mice were intraperitoneally (i.p.) injected with 10 μg/mice FGF21 antibody (Santa Cruz Biotechnology, CA, USA) in a single injection 30 min prior to the experiment [22]. All animal experiments were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee of Shanghai Institute of Nutrition and Health, Chinese Academy of Sciences (CAS).
2.2. Metabolic parameters measurements
Maintained with regular chow, the mice were single-housed in metabolic chamber (Columbus Instruments, OH, USA) to record oxygen consumption, respiratory exchange ratio and locomotor activity [23]. Nuclear magnetic resonance (NMR) imaging system (Bruker, Rheinstetten, GER) was used to measure mice body composition.
2.3. Plasmids construction and cell treatments
The DNA fragment encoding c-Jun was amplified from human HepG2 cells cDNA. Double-stranded siRNA targeting mouse c-Jun was purchased from GenePharma. The siRNA sequence specific for mouse c-Jun: 5′-ggcacagcuuaagcagaa-3’. Mouse primary hepatocytes prepared by collagenase perfusion [24] and 293T cells were maintained in Dulbecco's modified Eagle's medium (DMEM) as described previously [24]. Primary hepatocytes were transfected with c-Jun expressing plasmid by Effectene Transfection Reagent (Qiagen, Hilden, Germany) or c-Jun siRNA by X-tremeGene siRNA Transfection Reagent (Roche Diagnostics, Mannheim, Germany), respectively.
2.4. Generation and administration of recombinant adenoviruses
The adenovirus expressing FGF21 was generated as previously described [16]. Adenoviruses were delivered into mice by tail vein injection using 1 × 109, 2.5 × 108 or 1.25 × 108 pfu/mice as indicated for a single injection.
2.5. Blood glucose, serum insulin, glucose tolerance tests (GTTs), pyruvate tolerance tests (PTTs), insulin tolerance tests (ITTs), HOMA-IR index and glucose-stimulated insulin secretion assays (GSIS)
Blood glucose was measured with a Glucometer Elite monitor. Serum insulin was determined by ELISA assay using Mercodia Ultrasensitive Rat Insulin ELISA kit (ALPCO Diagnostic, NH, USA) in accordance with the manufacturer's instructions. After overnight fasting, GTTs and PTTs were conducted by i.p. injection of 2 g/kg glucose or 2 g/kg pyruvate, respectively [25]. For ITTs, mice were injected with 0.75 units/kg insulin after 4 h fasting. HOMA-IR index was calculated as follows: [fasting glucose levels (mmol/L)] x [fasting serum insulin (μU/ml)]/22.5. Islet isolation and glucose-stimulated insulin secretion assay were performed as previously reported [26].
2.6. Glucose output and glycogen content assays
Glucose output was determined in mouse primary hepatocytes as described previously [27]. We added both lactate and pyruvate as substrates in glucose output experiments. Liver glycogen was measured by a phenol-sulfuric acid colorimetric method [28].
2.7. Measurements of serum norepinephrine (NE) and FGF21 levels
NE and FGF21 levels in serum were measured by ELISA assay using a commercially available NE and FGF21 ELISA kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) in accordance with the manufacturer's instructions.
2.8. Rectal temperature and infrared image
Rectal temperatures of mice were taken at 3:00 P.M. (basal metabolic state) using a digital thermometer provided with a rectal probe (Physitemp Instruments, NJ, USA). BAT temperature was measured using an infrared camera (Magnity Electronics Co., Ltd, Shanghai, China) according to previous publications [29].
2.9. Luciferase assay
Fgf21 promoter (−1911 to +5), its deletion and mutant version were constructed in pGL3-Basic (Promega) utilizing a PCR-based cloning strategy. We found potential AP-1 sites in the Fgf21 promoter using Genomatix Software. HEK293T cells were cotransfected with the internal control vector pRL Renilla (Promega, WI, USA) and plasmids indicated using Lipofectamine 2000. The firefly and Renilla luciferase activities were assayed using Dual-Glo Luciferase Assay System after 24 h (Promega, WI, USA).
2.10. Chromatin immunopricipitation (ChIP) assay
ChIP assays were performed according to the manufacturer's protocol (Millipore, CA, USA) with anti-c-Jun antibody (Cell Signaling Technology, MA, USA) or normal rabbit immunoglobulin G (Santa Cruz Biotechnology, CA, USA) for negative control. Immunoprecipitated Fgf21 promoter was quantified using PCR with primers designed to amplify the 130-bp region encompassing the AP-1 site (forward, 5′-GCCCTTTTCATTCAGACCCC-3’; reverse, 5-TGCCCT CCCCACTCCTG -3′) or a 135-bp upstream region not involved in c-Jun response (forward, 5′- CCTCCCTCAGACCCAAGAGC-3’; reverse, 5′-GTGGCTGGGCTCTGCAGTT-3′).
The annealing temperature of PCR was 52 °C and PCR products were amplified with TaKaRa Ex Taq® DNA polymerase (Takara Bio Inc., Shiga, Japan).
2.11. Selective hepatic vagotomy
Hepatic branch vagotomy or sham surgery (isolation of the nerve without resection) was performed in mice as previously described [30].
2.12. Immunofluorescence (IF) staining
We performed IF staining as previously described [23]. After incubated with anti-c-Fos antibody (Santa Cruz Biotechnology, CA, USA; 1:500) overnight, brain slides were treated with Alexa Fluor 555 and 488–conjugated anti-rabbit antibody (Invitrogen, MA, USA) at room temperature for 1 h. Images were taken by a Zeiss LSM 510 confocal microscope (Carl Zeiss Imaging, Oberkochen, Germany).
2.13. Western blot analysis and measurement of PEPCK and G6PASE activities
Western blot analysis was performed with primary antibodies obtained as follows: anti-c-Jun (Cell Signaling Technology, MA, USA; 1:1000), anti-UCP1, anti-FGF21 and anti-PEPCK (all from Santa Cruz Biotechnology, CA, USA; 1:500 for UCP1 and FGF21, 1:1000 for PEPCK), anti-G6PASE (Abcam, Cambridge, UK; 1:500). PEPCK and G6PASE activities were determined using a PEPCK activity kit (Solarbio Life Sciences, Beijing, China) and a G6PASE activity kit (Solarbio Life Sciences, Beijing, China), respectively. All of these assays were performed according to the manufacturer's instructions.
2.14. RNA isolation and relative quantitative RT-PCR
mRNA levels were examined by RT-PCR with the sequences of primers described as follows: c-Jun: forward, 5′-GCAGAGAGGAAGCGCATGAG-3’; reverse, 5-CCTTTTCCGGCACTTGGA-3’. Fgf21: forward, 5′-ATGGAATGGATG AGATCTAGAGTTGG-3’; reverse, 5-TCTTGGTGGTCATCTGTGTAGAGG-3’. Ucp1: forward, 5′-ACTGCCACACCTCCAGTCATT-3’; reverse, 5′- CTTTGCC TCACTCAGGATTGG-3’. Pepck: forward, 5-‘CTTCTCTGCCAAGGTCATCC-3′, reverse, 5′-TTTTGGGGATGGGCAC-3’; G6pase, forward, 5′- ATGACTTTGGGA TCCAGTCG-3′, reverse, 5′- TGGAACCAGATGGGAAAGAG-3’.
2.15. Statistics
All data are expressed as mean ± SEM. Significant differences were assessed either by two-tailed student t-test or one-way ANOVA followed by the Student-Newman-Keuls (SNK) test, as indicated. For GTTs, PTTs and ITTs, t-test or one-way ANOVA were used to compare the difference between or among different groups of mice at each time point examined. P < 0.05 was considered statistically significant.
3. Results
3.1. c-Jun regulates gluconeogenesis in vivo and in vitro
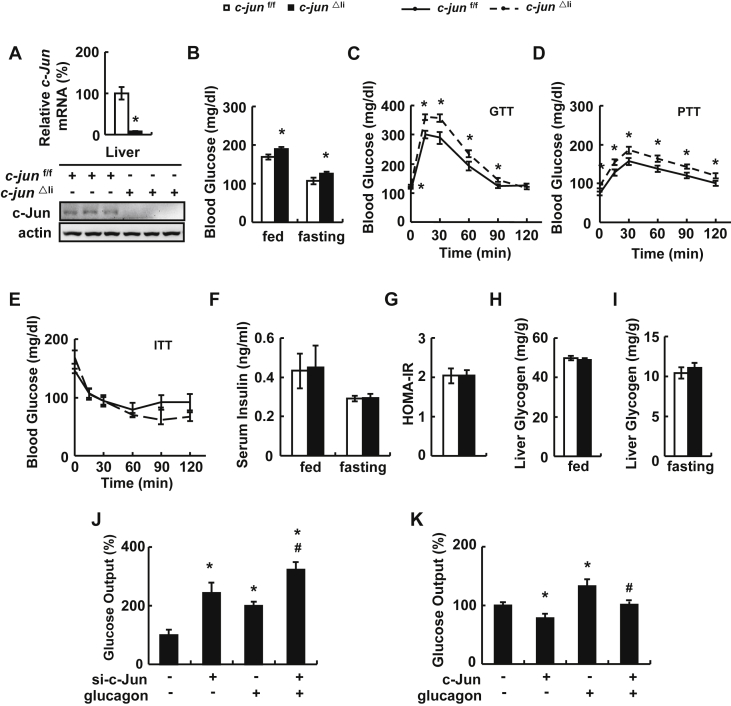
To investigate the role of hepatic c-Jun in glucose metabolism, we generated liver-specific c-Jun knock-out (c-jun△li) mice. The efficiency of c-jun△li mice was demonstrated by the almost completely abolished expression of c-Jun in the liver of c-jun△li mice compared with control mice (Figure 1A). In contrast, the expression of c-Jun was not altered in the hypothalamus and BAT of c-jun△li mice (Supplementary Figure 1A). As described previously [9], apart from the reduction in body size (Supplementary Figure 1B), c-jun△li mice were phenotypically normal. There was no difference in food intake and fat mass relative to body weight as evaluated by nuclear magnetic resonance (NMR) between c-jun△li and control mice (Supplementary Figure 1C). Compared with control mice, the oxygen consumption was increased in c-jun△li mice (Supplementary Figure 1D) with no significant change in the respiratory exchange ratio (RER, VCO2/VO2) and locomotor activity (Supplementary Figure 1E and F). Both fed and fasting blood glucose levels increased significantly in c-jun△li mice compared with the control mice (Figure 1B). Glucose tolerance, glucose generation and insulin tolerance were examined by glucose tolerance tests (GTTs), pyruvate tolerance tests (PTTs) and insulin tolerance tests (ITTs), respectively. The exogenous glucose was cleared slower and gluconeogenesis capacity was higher in the livers of c-jun△li mice than in the control mice (Figure 1C,D). In contrast, there was no difference in the insulin sensitivity between c-jun△li and control mice, as demonstrated by the unaltered ITTs, serum insulin levels and the HOMA-IR index (Figure 1E–G). No difference in serum insulin levels were observed at any time point examined during GTTs (Supplementary Figure 2A). In addition, we isolated primary mouse islets from control and c-jun△li mice and conducted glucose-stimulated insulin secretion assays (GSIS). The glucose-stimulated index (GSI), which reflects the insulin secretion ratio of pancreatic β cells stimulated by high and low glucose [26], remained the same between islets from control and c-jun△li mice (Supplementary Figure 2B).
Figure 1.
c-Jun regulates gluconeogenesis in vivo and in vitro. (A) c-Jun mRNA and protein levels in the liver of c-jun△li mice. (B) Blood glucose levels. (C) glucose tolerance tests (GTTs). (D) pyruvate tolerance tests (PTTs). (E) insulin tolerance tests (ITTs). (F) Serum insulin levels. (G) HOMA-IR index. (H, I) Liver glycogen content under fed and fasting state. (J, K) Glucose output. Primary mouse hepatocytes were transfected with or without c-Jun siRNA for 48 h, or with or without c-Jun over-expressing plasmids for 24 h, and then treated with or without 100 nM glucagon for 10 h, followed by the measurement of glucose production. Data were obtained with mice indicated (n = 6–10 mice per group, 10–12 weeks old) or at least three independent in vitro experiments and presented as means ± SEMs. Statistical significance was calculated using two-tailed student t-test for the effects of c-jun△li mice versus control mice (*: p < 0.05) in A-I or one-way ANOVA followed by the Student-Newman-Keuls (SNK) test in J and K (*: p < 0.05 for the effects of any group versus the control group without glucagon stimulation, #: p < 0.05 c-Jun knockdown or over-expression group versus control group after glucagon stimulation).
Besides gluconeogenesis, glucose metabolism is also affected by glycogenolysis and glycogen synthesis. We therefore examined glycogen content in the livers of c-jun△li mice to evaluate the possible contribution of these processes to the elevated blood glucose levels. However, no differencde was observed in hepatic glycogen contents under fed and fasting conditions between c-jun△li and control mice (Figure 1H,I). Furthermore, we explored the effect of c-Jun on gluconeogenesis in vitro. Inhibition of c-Jun significantly increased the glucose production in primary hepatocytes in the presence or absence of glucagon (Figure 1J). Conversely, the glucose output was lower in primary hepatocytes overexpressing c-Jun (Figure 1K).
We also examined the expression and activity of PEPCK and G6PASE, two key enzymes involved in gluconeogenesis [1], in vivo and in vitro. Compared with control mice, mRNA and protein abundance of PEPCK, but not G6PASE, was increased in the liver of c-jun△li mice (Supplementary Figure 3A). Similar results were observed in primary hepatocytes with c-Jun knockdown (Supplementary Figure 3B). Furthermore, c-Jun over-expression decreased mRNA and protein abundance of PEPCK and didn't change the expression of G6PASE in primary hepatocytes (Supplementary Figure 3C). The activities of PEPCK and G6PASE were changed accordingly under each condition (Supplementary Figure 4).
3.2. Fibroblast growth factor (FGF) 21 is required for c-Jun regulation of glucose metabolism
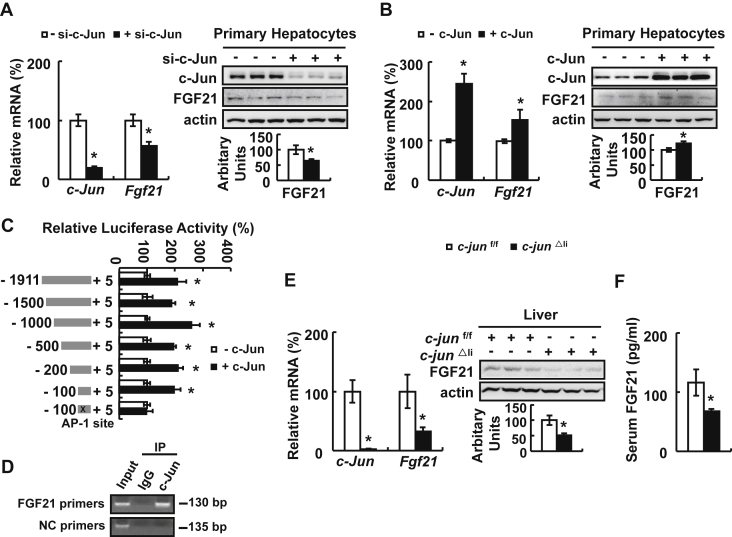
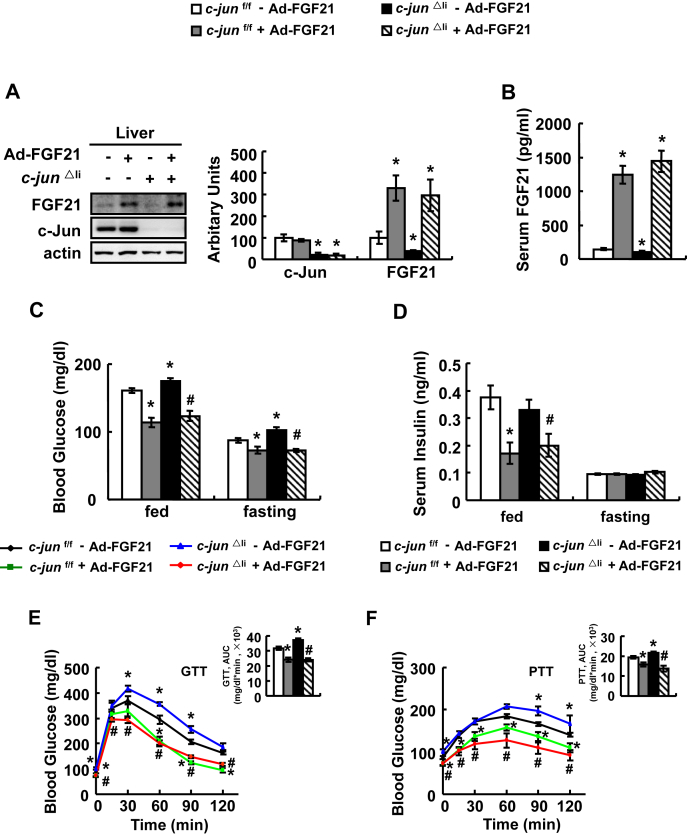
FGF21 is an important regulator for glucose metabolism [18], [31], and we found that the Fgf21 promoter had AP-1 binding sites analyzed by Genomatix Software, which is related to c-Jun [7], suggesting that FGF21 may mediate c-Jun regulation of glucose metabolism. As predicted, FGF21 expression was decreased by c-Jun knockdown and increased by c-Jun over-expression in primary hepatocytes (Figure 2A,B). Further study showed that c-Jun can directly bind to Fgf21 promoter as demonstrated by luciferase assays and chromatin immunoprecipitation (ChIP) assays (Figure 2C,D). As observed in vitro, hepatic and serum FGF21 levels were also decreased significantly in c-jun△li mice (Figure 2E,F). To confirm a role for FGF21 in mediating c-Jun regulation of glucose metabolism, we injected adenoviruses expressing FGF21 (Ad-FGF21) or control adenoviruses expressing green fluorescent protein (Ad-GFP) at a dose of 1 × 109 pfu/mice to c-jun△li and control mice. As expected, hepatic FGF21 expression and serum FGF21 levels were significantly increased by Ad-FGF21 (Figure 3A,B). Ad-FGF21 significantly decreased the levels of fed and fasting blood glucose and serum insulin, but not fasting insulin, in c-jun△li mice (Figure 3C,D). Moreover, the impaired glucose clearance and elevated gluconeogenesis capacity in c-jun△li mice were also markedly reversed by Ad-FGF21 (Figure 3E,F).
Figure 2.
c-Jun regulates FGF21 expression in vitro and in vivo. (A, B) Primary mouse hepatocytes were transfected with or without c-Jun siRNA for 72 h, or with or without c-Jun expressing plasmids for 48 h, followed by the measurement of c-Jun and Fgf21 mRNA and protein expression (top, western blot; bottom, quantitative measurements of FGF21 protein relative to actin). (C) Luciferase activity. Luciferase activity was assessed in HEK293T cells expressing different Fgf21 deletion promoter vectors with or without plasmids expressing c-Jun. (D) ChIP assay was performed in primary mouse hepatocytes. (E) c-Jun and Fgf21 mRNA and protein expression in the liver of c-jun△li mice (top, western blot; bottom, quantitative measurements of FGF21 protein relative to actin). (F) Serum FGF21 levels. Data were obtained with mice indicated (n = 6–10 mice per group, 10 weeks old) or at least three independent in vitro experiments and presented as means ± SEMs. Statistical significance was calculated using two-tailed student t-test for the effects of c-Jun knockdown or over-expression versus the control group (*: p < 0.05) in A-C, E and F.
Figure 3.
FGF21 is required for c-Jun regulation of glucose metabolism. (A–F) c-jun△li mice or control mice were injected with Ad-FGF21 (+Ad-FGF21) or Ad-GFP (- Ad-FGF21), followed by examination of FGF21 and c-Jun expression at day 14 in A, measurement of serum FGF21 levels at day 14 in B, fed blood glucose and serum insulin levels at day 2 or fasting blood glucose and serum insulin levels at day 3 in C and D, performance of GTTs and PTTs at day 3 or 7 in E and F. Data were obtained with mice indicated (n = 6–10 mice per group, 10–12 weeks old) and presented as means ± SEMs. Statistical significance was calculated using the one-way ANOVA followed by the Student-Newman-Keuls (SNK) test in A-F (*: p < 0.05 for the effects of any group of mice versus control mice injected with Ad-GFP; #: p < 0.05 for the effects of Ad-FGF21 versus Ad-GFP in c-jun△li mice).
Because FGF21 expression after virus infection was very strong in the above experiment, we reduced the dose of Ad-FGF21 viruses from 1 × 109 pfu/mice to 2.5 × 108 or 1.25 × 108 pfu/mice, respectively. We found that injection of c-jun△li mice with Ad-FGF21 at the dose of 2.5 × 108 pfu/mice led to a FGF21 expression comparable to the level observed in control mice (Supplementary Figure 5A and B). Under this condition (using Ad-FGF21 at the dose of 2.5 × 108 pfu/mice), Ad-FGF21 also significantly decreased the levels of fed and fasting blood glucose and fed serum insulin, but not fasting serum insulin, in c-jun△li mice (Supplementary Figure 5C and D). Moreover, the impaired glucose clearance and elevated gluconeogenesis capacity in c-jun△li mice were also markedly reversed by Ad-FGF21 (Supplementary Figure 5E and F).
To further confirm a role for FGF21 in mediating c-Jun regulation of glucose metabolism, we used a FGF21-neutralizing antibody to see if it could block the effect of Ad-FGF21. c-jun△li mice were treated with FGF21 antibody after injected with 2.5 × 108 pfu/mice Ad-FGF21. In Ad-FGF21 c-jun△li mice, treatment with FGF21 antibody reduced serum FGF21 levels, increased the levels of fed and fasting blood glucose and fed serum insulin, decreased the glucose clearance and increased gluconeogenesis capacity (Supplementary Figure 6). Theses results confirm an important role of FGF21 in mediating the effects of hepatic c-Jun.
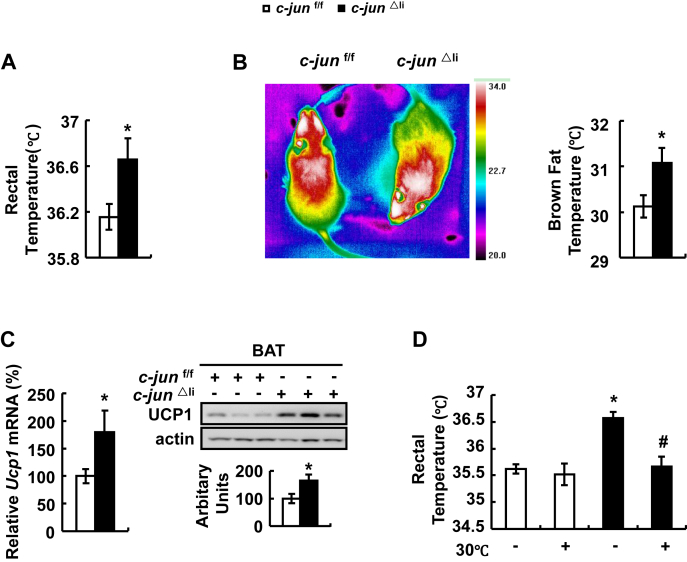
3.3. BAT thermogenesis contributes to the increased body temperature in c-jun△li mice
Besides the important role of c-Jun in regulating glucose metabolism, surprisingly, we found that hepatic c-Jun also modulates body temperature. Rectal temperature was significantly higher in c-jun△li mice compared with control mice (Figure 4A). The higher body temperature observed in c-jun△li mice was possibly caused by increased thermogenesis, which is regulated by uncoupling protein 1 (UCP1) in BAT [32]. Consistently, infrared images analysis showed that thermogenesis was stronger in BAT of c-jun△li compared with control mice (Figure 4B). What's more, BAT UCP1 expression was significantly elevated in c-jun△li mice (Figure 4C). If enhanced thermogenesis in BAT contributes to the increased body temperature of c-jun△li mice, placing them at thermoneutral temperature (30 °C) would reduce the higher body temperature. Indeed, in this condition, c-jun△li and control mice displayed similar body temperature (Figure 4D).
Figure 4.
BAT thermogenesis contributes to the increased body temperature in c-jun△limice. (A) Rectal temperature of mice under normal temperature (22 °C). (B) Infrared images of mice under normal temperature (22 °C). (C) Ucp1 mRNA and protein in BAT (top, western blot; bottom, quantitative measurements of UCP1 protein relative to actin). (D) Rectal temperature of mice under normal temperature (22 °C) or thermoneutral temperature (30 °C). Data were obtained with mice indicated (n = 6–10 mice per group, 10–12 weeks old) and presented as means ± SEMs. Statistical significance was calculated using two-tailed student t-test for the effects of c-jun△li mice versus control mice (*: p < 0.05) in A-C, or using the one-way ANOVA followed by the Student-Newman-Keuls (SNK) test in D (*: p < 0.05 for the effects of any group of mice versus control mice under normal temperature; #: p < 0.05 for the effects of thermoneutral temperature versus normal temperature in c-jun△li mice).
3.4. c-Jun modulates body temperature via regulating sympathetic nervous system (SNS) activity
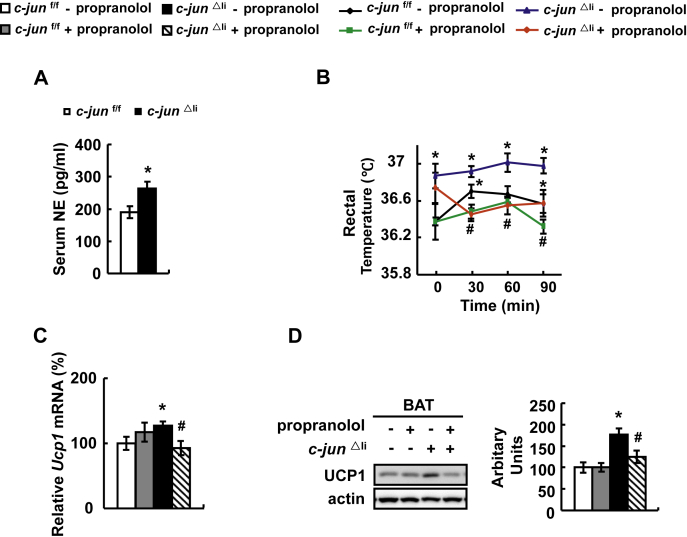
BAT thermogenesis is induced by activation of SNS with the release of norepinephrine (NE) [33]. Not surprisingly, c-jun△li mice had higher serum NE levels, with no change in serum T3 levels, compared with control mice (Figure 5A and Supplementary Figure 7). To determine whether the increased SNS activity contributes to the increased BAT thermogenesis in c-jun△li mice, we blocked SNS signaling by intraperitoneal (i. p.) injection of propranolol, a β-adrenergic receptor antagonist [34], in c-jun△li mice. Propranolol treatment significantly decreased body temperature and BAT UCP1 expression in c-jun△li mice (Figure 5B–D).
Figure 5.
c-Jun modulates body temperature via regulating sympathetic nervous activity. (A) Serum norepinephrine (NE) levels. (B–D) c-jun△li mice or control mice were injected with propranolol (+propranolol) or saline (- propranolol). Then rectal temperature was measured at different time point after injection with propranolol in B, and UCP1 expression was examined in C and D. Data were obtained with mice indicated (n = 6–10 mice per group, 10–12 weeks old) and presented as means ± SEMs. Statistical significance was calculated using two-tailed student t-test for the effects of c-jun△li mice versus the control group (*: p < 0.05) in A, or using one-way ANOVA followed by the Student-Newman-Keuls (SNK) test in B-D. (*: p < 0.05 for the effects of any group of mice versus control mice injected without propranolol; #: p < 0.05 for the effects of propranolol versus saline in c-jun△li mice).
3.5. Selective hepatic vagotomy reverses body temperature in c-jun△li mice
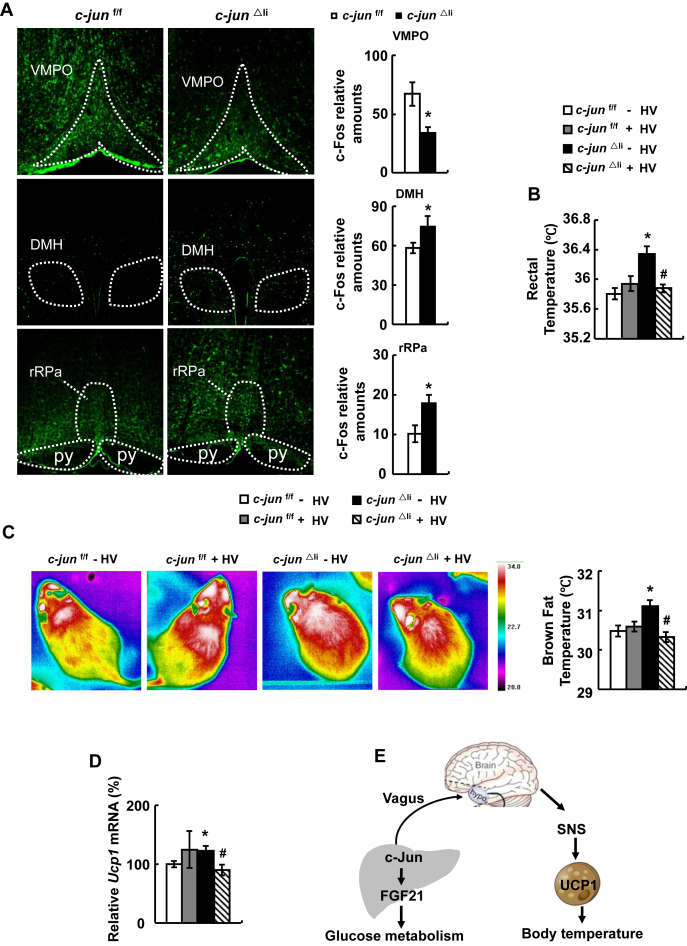
A previous study has shown that SNS activation of BAT thermogenesis is regulated by the afferent signals from the hepatic vagus-brain axis [6], suggesting that hepatic c-Jun may employ a similar mechanism in the regulation of body temperature. To test this possibility, we examined the expression of c-Fos, a molecular marker of neuronal activation [35], in the brain of c-jun△li and control mice. Surprisingly, c-Fos expression was decreased in the ventromedial preoptic nucleus (VMPO), increased in the dorsomedial hypothalamus (DMH) and rostral raphe pallidus nucleus (rRPa), areas that control body temperature [35], [36], [37], of c-jun△li compared with control mice (Figure 6A). To investigate a role of hepatic vagus nerve in the regulation of body temperature in c-jun△li mice, we dissected the hepatic branch of the vagal nerve in c-jun△li and control mice. Food intake and blood glucose levels were not affected by dissecting the hepatic branch of the vagal nerve in both genotypes of mice (Supplementary Figure 8). After selective hepatic vagotomy (HV), the body temperature was significantly decreased in c-jun△li mice (Figure 6B). What's more, infrared images analysis showed that the increased thermogenesis in BAT was reversed by HV in c-jun△li mice (Figure 6C). In addition, increased BAT UCP1 expression observed in c-jun△li mice was also reversed by HV (Figure 6D). In contrast, HV had no effect on the parameters mentioned above in control mice (Figure 6B–D).
Figure 6.
Selective hepatic vagotomy reverses body temperature in c-jun△limice. (A) Immunofluorescence staining detection of c-Fos protein in the VMPO (top), DMH (middle), rRPa (bottom) and the number of c-Fos positive nuclei in these sections. (B–D) c-jun△li mice or control mice were subjected to selective hepatic vagotomy (+HV) or sham surgery (- HV), then rectal temperature (B), infrared images (C), and UCP1 expression (D) were detected after 7 days. (E) Working model. Data were obtained with mice indicated (n = 5–10 mice per group, 10–12 weeks old) and presented as means ± SEMs. Statistical significance was calculated using two-tailed student t-test for the effects of c-jun△li mice versus the control group (*: p < 0.05) in A, or using one-way ANOVA followed by the Student-Newman-Keuls (SNK) test in B-D (*: p < 0.05 for the effects of any group of mice versus control mice without HV; #: p < 0.05 for the effects of HV versus sham surgery in c-jun△li mice).
4. Discussion
The transcriptional regulators of hepatic gluconeogenesis are considered potential therapeutic targets for the treatment of type 2 diabetes [38], [39]. The transcription factor c-Jun is a major regulator of cell death and survival [40]; however, its function in glucose homeostasis remains largely unknown. Null mutations of c-Jun cause embryonic lethality [41], so liver-specific c-Jun knock-out mice models are often used as an experimental system. Here, we described a novel function for hepatic c-Jun in the regulation of blood glucose via affecting gluconeogenesis, as knockdown of hepatic c-Jun expression promotes gluconeogenic capacity in vivo and in vitro.
Furthermore, we found that FGF21 is required for c-Jun regulation of gluconeogenesis. Hepatic gluconeogenesis is tightly controlled by many hormones including glucagon, insulin [42] and the hepatic secreted protein FGF21 [18], [31]. c-Jun is an important member of the AP-1 transcription factor family [7]. AP-1 is a multimeric complex consisting of members of the Jun family forming either homodimers or heterodimers with Fos or activating transcription factor (ATF) proteins [43]. Using Genomatix Software, we found several potential AP-1 sites [44] on the Fgf21 promoter and provided evidence showing that c-Jun can directly regulate FGF21 expression via binding to the Ap-1 site on its promoter. Moreover, FGF21 protein levels were lower in the liver and plasma of c-jun△li mice and adenovirus-mediated hepatic FGF21 over-expression reversed the effects of c-Jun on gluconeogenesis. FGF21-neutralizing antibody could block this effect. These results suggest an important role of FGF21 in mediating hepatic c-Jun effects on gluconeogenesis. Our results also provide new evidence for the transcriptional regulation of FGF21 expression by c-Jun.
Another important observation in this study is that c-jun△li mice displayed an increase in body temperature. In fact, except for the previous work showing that hepatic TSC1 and glucokinase regulates body temperature [5], [6], the molecular mechanisms underlying this inter-organ crosstalk is poorly understood. Thus, our study provides direct evidence showing that hepatic c-Jun contributes to the metabolic alterations on the whole-body level.
Thermogenesis, the production of heat energy, is essential to maintain body temperature, and BAT evolved as a specialized thermogenic organ responsible for adaptive non-shivering thermogenesis [33]. The high mitochondrial mass together with the abundant UCP1 content is crucial for BAT-mediated heat production [33]. In this study, we found the increased thermogenesis and UCP1 expression in BAT of c-jun△li mice. The possible contribution for the increased BAT thermogenesis to the elevated body temperature in c-jun△li mice was supported by the observation that c-jun△li mice displayed no difference in body temperature compared with control mice under thermoneutral (30 °C) temperature (when thermogenesis was inhibited). BAT thermogenesis is induced by the activation of SNS that promotes the release of NE binding to β-adrenergic receptor [32], [45]. In this study, we found that serum NE levels were increased in c-jun△li mice. In addition, suppression of SNS activity using β-adrenergic receptor antagonist decreased body temperature and BAT thermogenesis in c-jun△li mice. These results confirm an important role of SNS in regulating body temperature of c-jun△li mice.
BAT thermogenesis is regulated by neural networks in the central nervous system (CNS) [45]. Accumulating evidence has demonstrated that the vagus nerve plays a crucial role in the brain-liver interaction [46]. The vagus nerve is a mixed nerve composed of 20% efferent and 80% afferent fibers [46]. Previous studies also reported that hepatic peroxisome proliferators-activated receptor γ (PPAR-γ) and glucokinase affects SNS activity through the afferent vagal signals originating in the liver [6], [47]. Therefore, we hypothesized that hepatic c-Jun may interact with the brain via vegus nerve to increase SNS activity, leading to increased thermogenesis in BAT. We examined the c-Fos expression in several brain areas and found that c-Fos expression was decreased in VMPO, increased in DMH and rRPa area. In fact, several experimental results have supported a role for neurons in VMPO, DMH and rRPa in the control of BAT thermogenesis and body temperature [37], [48]. These findings revealed a role for CNS as a critical site where hepatic c-Jun modulates thermogenesis in the BAT and body temperature. Moreover, HV blocked hepatic c-Jun knockout-induced increase of BAT thermogenesis, indicating involvement of a neural route from the liver to hypothalamus.
The understanding of how the liver transmits signals to vagus-brain remains largely unknown. We assume the possible mechanisms by which c-Jun affects the vagus-brain axis include the following possibilities. Some studies show that hepatic alterations in glucose metabolism can affect vagal afferents to brain and sympathetic efferents [6], [49]. Our in vitro results obtained from primary hepatocytes suggest a direct regulation of c-Jun in gluconeogenesis. So hepatic c-Jun may transmit signals to vagus via metabolite from glucose metabolism. Furthermore, it has been shown that hepatic nervous system recognizes changes in ion concentration [50]. An in vitro study found that nerve growth factor (NGF)-inducible c-Jun can transactivate the promoter of neuronal nicotinic acetylcholine receptors (nAChRs) β4 subunit, which are pentameric ligand-gated ion channels important for synaptic transmission in nervous system [51]. Additionally, hepatic secretary factors may affect neuron activity, as shown previously [50]. These possibilities may be involved in c-Jun regulation of vagus-brain axis, which require to be studied in the future.
In addition to the nerve systems, the liver reciprocally interacts with the brain and BAT via secreting hormones [52], [53]. Studies have shown that FGF21 can exert its direct actions on the BAT to increase thermogenesis [54]. FGF21 also acts centrally to induce sympathetic nerve activity [53]. Because FGF21 protein levels were lower in the liver and plasma of c-jun△li mice compared with control mice, it is unlikely that FGF21 contributes to the regulation of thermogenesis in BAT and the body temperature of c-jun△li mice.
Usually, an increase in body temperature has positive consequences on metabolism. However, although c-jun△li mice exhibited higher body temperature, glucose levels increased significantly in c-jun△li mice compared with the control mice. We assume the possible reasons for the incoherence include: 1) the primary cause for both higher body temperature and blood glucose is the deficiency of hepatic c-Jun. Our in vitro results obtained from primary hepatocytes suggest a direct regulation of c-Jun in gluconeogenesis. The effect of hepatic c-Jun on blood glucose is stronger than the effect of higher body temperature; and 2) indeed, there is no difference in food intake between c-jun△li and control mice. However, considering the smaller body weight of c-jun△li mice, the food intake of c-jun△li mice was relative higher than that of control mice that may compensate for the possible increased energy expenditure caused by increased body temperature. Taking the energy intake into account, the increased body temperature and oxygen consumption may not affect total energy homeostasis.
In summary, we showed that liver-specific inactivation of c-Jun increased gluconeogenesis via decreasing FGF21 expression and increased body temperature by promoting thermogenesis in BAT (Figure 6E). Importantly, this liver-BAT system consists of the afferent vagus from the liver and sympathetic efferents from the brain. Our study provides novel insights into the organ crosstalk in the regulation of whole-body metabolism.
Author's contributions
Fei Xiao and Yajie Guo researched data, wrote, reviewed, and edited the manuscript. Jiali Deng, Feixiang Yuan, Yuzhong Xiao, Yuncai Zhou, Kaili, Yan Chen, Hao Ying, Qiwei Zhai, and Shanghai Chen researched data and contributed to discussion. Lijian Hui, Yu Li, Zhimin Hu, Xiao Han, Qichen Fang, and Weiping Jia provided research material. Feifan Guo directed the project, contributed to discussion and wrote, reviewed, and edited the manuscript.
Acknowledgments
This work was supported by grants from the National Natural Science Foundation (31830044, 81870592, 81325005, 81390350, 81471076, 81570777, 81500622, 81600623, 81770852, 81700761, 81700750 and 31630037), Basic Research Project of Shanghai Science and Technology Commission (16JC1404900 and 17XD1404200) and CAS-SAFEA International Partnership Program for Creative Research Teams, CAS Interdisciplinary Innovation Team. F.X. was sponsored by Youth Innovation Promotion Association CAS, Shanghai Rising Star Program and Sanofi-Aventis- SIBS scholarship. Y.G. was supported by China Postdoctoral Science Foundation.
F.G. was also supported by the One Hundred Talents Program of CAS and Shanghai Academic Research Leader.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.molmet.2018.12.003.
Conflict of interest
No potential conflicts of interest relevant to this article were reported.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Han H.S., Kang G., Kim J.S., Choi B.H., Koo S.H. Regulation of glucose metabolism from a liver-centric perspective. Experimental and Molecular Medicine. 2016;48:e218. doi: 10.1038/emm.2015.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bechmann L.P., Hannivoort R.A., Gerken G., Hotamisligil G.S., Trauner M., Canbay A. The interaction of hepatic lipid and glucose metabolism in liver diseases. Journal of Hepatology. 2012;56:952–964. doi: 10.1016/j.jhep.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 3.Hundal R.S., Krssak M., Dufour S., Laurent D., Lebon V., Chandramouli V. Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes. 2000;49:2063–2069. doi: 10.2337/diabetes.49.12.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oh K.J., Han H.S., Kim M.J., Koo S.H. CREB and FoxO1: two transcription factors for the regulation of hepatic gluconeogenesis. BMB Reports. 2013;46:567–574. doi: 10.5483/BMBRep.2013.46.12.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cornu M., Oppliger W., Albert V., Robitaille A.M., Trapani F., Quagliata L. Hepatic mTORC1 controls locomotor activity, body temperature, and lipid metabolism through FGF21. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:11592–11599. doi: 10.1073/pnas.1412047111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsukita S., Yamada T., Uno K., Takahashi K., Kaneko K., Ishigaki Y. Hepatic glucokinase modulates obesity predisposition by regulating BAT thermogenesis via neural signals. Cell Metabolism. 2012;16:825–832. doi: 10.1016/j.cmet.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 7.Raivich G. c-Jun expression, activation and function in neural cell death, inflammation and repair. Journal of Neurochemistry. 2008;107:898–906. doi: 10.1111/j.1471-4159.2008.05684.x. [DOI] [PubMed] [Google Scholar]
- 8.Maki Y., Bos T.J., Davis C., Starbuck M., Vogt P.K. Avian sarcoma virus 17 carries the jun oncogene. Proceedings of the National Academy of Sciences of the United States of America. 1987;84:2848–2852. doi: 10.1073/pnas.84.9.2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Behrens A., Sibilia M., David J.P., Mohle-Steinlein U., Tronche F., Schutz G. Impaired postnatal hepatocyte proliferation and liver regeneration in mice lacking c-jun in the liver. The EMBO Journal. 2002;21:1782–1790. doi: 10.1093/emboj/21.7.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stepniak E., Ricci R., Eferl R., Sumara G., Sumara I., Rath M. c-Jun/AP-1 controls liver regeneration by repressing p53/p21 and p38 MAPK activity. Genes and Development. 2006;20:2306–2314. doi: 10.1101/gad.390506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Min L., Ji Y., Bakiri L., Qiu Z., Cen J., Chen X. Liver cancer initiation is controlled by AP-1 through SIRT6-dependent inhibition of survivin. Nature Cell Biology. 2012;14:1203–1211. doi: 10.1038/ncb2590. [DOI] [PubMed] [Google Scholar]
- 12.Eferl R., Ricci R., Kenner L., Zenz R., David J.P., Rath M. Liver tumor development. c-Jun antagonizes the proapoptotic activity of p53. Cell. 2003;112:181–192. doi: 10.1016/s0092-8674(03)00042-4. [DOI] [PubMed] [Google Scholar]
- 13.Hasselblatt P., Rath M., Komnenovic V., Zatloukal K., Wagner E.F. Hepatocyte survival in acute hepatitis is due to c-Jun/AP-1-dependent expression of inducible nitric oxide synthase. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:17105–17110. doi: 10.1073/pnas.0706272104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakatani Y., Kaneto H., Kawamori D., Hatazaki M., Miyatsuka T., Matsuoka T.A. Modulation of the JNK pathway in liver affects insulin resistance status. The Journal of Biological Chemistry. 2004;279:45803–45809. doi: 10.1074/jbc.M406963200. [DOI] [PubMed] [Google Scholar]
- 15.Xie T., Leung P.S. Fibroblast growth factor 21: a regulator of metabolic disease and health span. American Journal of Physiology Endocrinology and Metabolism. 2017;313:E292–E302. doi: 10.1152/ajpendo.00101.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gong Q., Hu Z., Zhang F., Cui A., Chen X., Jiang H. Fibroblast growth factor 21 improves hepatic insulin sensitivity by inhibiting mammalian target of rapamycin complex 1 in mice. Hepatology. 2016;64:425–438. doi: 10.1002/hep.28523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu J., Lloyd D.J., Hale C., Stanislaus S., Chen M., Sivits G. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes. 2009;58:250–259. doi: 10.2337/db08-0392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang C., Dai J., Yang M., Deng G., Xu S., Jia Y. Silencing of FGF-21 expression promotes hepatic gluconeogenesis and glycogenolysis by regulation of the STAT3-SOCS3 signal. The FEBS Journal. 2014;281:2136–2147. doi: 10.1111/febs.12767. [DOI] [PubMed] [Google Scholar]
- 19.Tong X., Muchnik M., Chen Z., Patel M., Wu N., Joshi S. Transcriptional repressor E4-binding protein 4 (E4BP4) regulates metabolic hormone fibroblast growth factor 21 (FGF21) during circadian cycles and feeding. The Journal of Biological Chemistry. 2010;285:36401–36409. doi: 10.1074/jbc.M110.172866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Badman M.K., Pissios P., Kennedy A.R., Koukos G., Flier J.S., Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell Metabolism. 2007;5:426–437. doi: 10.1016/j.cmet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 21.Alawi K.M., Aubdool A.A., Liang L., Wilde E., Vepa A., Psefteli M.P. The sympathetic nervous system is controlled by transient receptor potential vanilloid 1 in the regulation of body temperature. FASEB Journal. 2015;29:4285–4298. doi: 10.1096/fj.15-272526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Omar B.A., Andersen B., Hald J., Raun K., Nishimura E., Ahren B. Fibroblast growth factor 21 (FGF21) and glucagon-like peptide 1 contribute to diabetes resistance in glucagon receptor-deficient mice. Diabetes. 2014;63:101–110. doi: 10.2337/db13-0710. [DOI] [PubMed] [Google Scholar]
- 23.Xia T., Cheng Y., Zhang Q., Xiao F., Liu B., Chen S. S6K1 in the central nervous system regulates energy expenditure via MC4R/CRH pathways in response to deprivation of an essential amino acid. Diabetes. 2012;61:2461–2471. doi: 10.2337/db11-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Q., Jiang L., Wang J., Li S., Yu Y., You J. Abrogation of hepatic ATP-citrate lyase protects against fatty liver and ameliorates hyperglycemia in leptin receptor-deficient mice. Hepatology. 2009;49:1166–1175. doi: 10.1002/hep.22774. [DOI] [PubMed] [Google Scholar]
- 25.Liang J., Liu C., Qiao A., Cui Y., Zhang H., Cui A. MicroRNA-29a-c decrease fasting blood glucose levels by negatively regulating hepatic gluconeogenesis. Journal of Hepatology. 2013;58:535–542. doi: 10.1016/j.jhep.2012.10.024. [DOI] [PubMed] [Google Scholar]
- 26.Chen F., Sha M., Wang Y., Wu T., Shan W., Liu J. Transcription factor Ets-1 links glucotoxicity to pancreatic beta cell dysfunction through inhibiting PDX-1 expression in rodent models. Diabetologia. 2016;59:316–324. doi: 10.1007/s00125-015-3805-3. [DOI] [PubMed] [Google Scholar]
- 27.Ruan H.B., Han X., Li M.D., Singh J.P., Qian K., Azarhoush S. O-GlcNAc transferase/host cell factor C1 complex regulates gluconeogenesis by modulating PGC-1alpha stability. Cell Metabolism. 2012;16:226–237. doi: 10.1016/j.cmet.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Montgomery R. Determination glycogen. Archives of Biochemistry and Biophysics. 1957;67:378–386. doi: 10.1016/0003-9861(57)90292-8. [DOI] [PubMed] [Google Scholar]
- 29.Warner A., Rahman A., Solsjo P., Gottschling K., Davis B., Vennstrom B. Inappropriate heat dissipation ignites brown fat thermogenesis in mice with a mutant thyroid hormone receptor alpha1. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:16241–16246. doi: 10.1073/pnas.1310300110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lam T.K., Pocai A., Gutierrez-Juarez R., Obici S., Bryan J., Aguilar-Bryan L. Hypothalamic sensing of circulating fatty acids is required for glucose homeostasis. Nature Medicine. 2005;11:320–327. doi: 10.1038/nm1201. [DOI] [PubMed] [Google Scholar]
- 31.Camporez J.P., Asrih M., Zhang D., Kahn M., Samuel V.T., Jurczak M.J. Hepatic insulin resistance and increased hepatic glucose production in mice lacking Fgf21. The Journal of Endocrinology. 2015;226:207–217. doi: 10.1530/JOE-15-0136. [DOI] [PubMed] [Google Scholar]
- 32.Lowell B.B., Spiegelman B.M. Towards a molecular understanding of adaptive thermogenesis. Nature. 2000;404:652–660. doi: 10.1038/35007527. [DOI] [PubMed] [Google Scholar]
- 33.Cannon B., Nedergaard J. Brown adipose tissue: function and physiological significance. Physiological Reviews. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 34.Aupetit J.F., Frassati D., Bui-Xuan B., Freysz M., Faucon G., Timour Q. Efficacy of a beta-adrenergic receptor antagonist, propranolol, in preventing ischaemic ventricular fibrillation: dependence on heart rate and ischaemia duration. Cardiovascular Research. 1998;37:646–655. doi: 10.1016/s0008-6363(97)00304-0. [DOI] [PubMed] [Google Scholar]
- 35.Williams D.L., Schwartz M.W., Bastian L.S., Blevins J.E., Baskin D.G. Immunocytochemistry and laser capture microdissection for real-time quantitative PCR identify hindbrain neurons activated by interaction between leptin and cholecystokinin. The Journal of Histochemistry and Cytochemistry. 2008;56:285–293. doi: 10.1369/jhc.7A7331.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morrison S.F., Nakamura K., Madden C.J. Central control of thermogenesis in mammals. Experimental Physiology. 2008;93:773–797. doi: 10.1113/expphysiol.2007.041848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tan C.L., Cooke E.K., Leib D.E., Lin Y.C., Daly G.E., Zimmerman C.A. Warm-Sensitive neurons that control body temperature. Cell. 2016;167:47–59 e15. doi: 10.1016/j.cell.2016.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoon J.C., Puigserver P., Chen G., Donovan J., Wu Z., Rhee J. Control of hepatic gluconeogenesis through the transcriptional coactivator PGC-1. Nature. 2001;413:131–138. doi: 10.1038/35093050. [DOI] [PubMed] [Google Scholar]
- 39.Puigserver P., Rhee J., Donovan J., Walkey C.J., Yoon J.C., Oriente F. Insulin-regulated hepatic gluconeogenesis through FOXO1-PGC-1alpha interaction. Nature. 2003;423:550–555. doi: 10.1038/nature01667. [DOI] [PubMed] [Google Scholar]
- 40.Kappelmann M., Bosserhoff A., Kuphal S. AP-1/c-Jun transcription factors: regulation and function in malignant melanoma. European Journal of Cell Biology. 2014;93:76–81. doi: 10.1016/j.ejcb.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 41.Johnson R.S., van Lingen B., Papaioannou V.E., Spiegelman B.M. A null mutation at the c-jun locus causes embryonic lethality and retarded cell growth in culture. Genes and Development. 1993;7:1309–1317. doi: 10.1101/gad.7.7b.1309. [DOI] [PubMed] [Google Scholar]
- 42.Parrilla R., Jimenez I., Ayuso-Parrilla M.S. Glucagon and insulin control of gluconeogenesis in the perfused isolated rat liver. Effects on cellular metabolite distribution. European Journal of Biochemistry. 1975;56:375–383. doi: 10.1111/j.1432-1033.1975.tb02243.x. [DOI] [PubMed] [Google Scholar]
- 43.Wisdom R. AP-1: one switch for many signals. Experimental Cell Research. 1999;253:180–185. doi: 10.1006/excr.1999.4685. [DOI] [PubMed] [Google Scholar]
- 44.Nakabeppu Y., Ryder K., Nathans D. DNA binding activities of three murine Jun proteins: stimulation by Fos. Cell. 1988;55:907–915. doi: 10.1016/0092-8674(88)90146-8. [DOI] [PubMed] [Google Scholar]
- 45.Morrison S.F., Madden C.J., Tupone D. Central neural regulation of brown adipose tissue thermogenesis and energy expenditure. Cell Metabolism. 2014;19:741–756. doi: 10.1016/j.cmet.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hiramoto T., Chida Y., Sonoda J., Yoshihara K., Sudo N., Kubo C. The hepatic vagus nerve attenuates Fas-induced apoptosis in the mouse liver via alpha7 nicotinic acetylcholine receptor. Gastroenterology. 2008;134:2122–2131. doi: 10.1053/j.gastro.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 47.Uno K., Katagiri H., Yamada T., Ishigaki Y., Ogihara T., Imai J. Neuronal pathway from the liver modulates energy expenditure and systemic insulin sensitivity. Science. 2006;312:1656–1659. doi: 10.1126/science.1126010. [DOI] [PubMed] [Google Scholar]
- 48.Morrison S.F., Madden C.J. Central nervous system regulation of brown adipose tissue. Comprehensive Physiology. 2014;4:1677–1713. doi: 10.1002/cphy.c140013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Niijima A. The effect of D-glucose on the firing rate of glucose-sensitive vagal afferents in the liver in comparison with the effect of 2-deoxy-D-glucose. Journal of the Autonomic Nervous System. 1984;10:255–260. doi: 10.1016/0165-1838(84)90021-3. [DOI] [PubMed] [Google Scholar]
- 50.Jensen K.J., Alpini G., Glaser S. Hepatic nervous system and neurobiology of the liver. Comprehensive Physiology. 2013;3:655–665. doi: 10.1002/cphy.c120018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Melnikova I.N., Gardner P.D. The signal transduction pathway underlying ion channel gene regulation by SP1-C-Jun interactions. Journal of Biological Chemistry. 2001;276:19040–19045. doi: 10.1074/jbc.M010735200. [DOI] [PubMed] [Google Scholar]
- 52.Scheja L., Heeren J. Metabolic interplay between white, beige, brown adipocytes and the liver. Journal of Hepatology. 2016;64:1176–1186. doi: 10.1016/j.jhep.2016.01.025. [DOI] [PubMed] [Google Scholar]
- 53.Owen B.M., Ding X., Morgan D.A., Coate K.C., Bookout A.L., Rahmouni K. FGF21 acts centrally to induce sympathetic nerve activity, energy expenditure, and weight loss. Cell Metabolism. 2014;20:670–677. doi: 10.1016/j.cmet.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fisher F.M., Kleiner S., Douris N., Fox E.C., Mepani R.J., Verdeguer F. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes and Development. 2012;26:271–281. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.