Abstract
Published studies have shown great variability in response when aerosolized furosemide has been tested as a palliative treatment for dyspnea. We hypothesized that a higher furosemide dose with controlled aerosol administration would produce consistent dyspnea relief. We optimized deposition by controlling inspiratory flow (300–500 ml/s) and tidal volume (15% predicted vital capacity) while delivering 3.4 micron aerosol from either saline or 80 mg of furosemide. We induced dyspnea in healthy subjects by varying inspired PCO2 while restricting minute ventilation. Subjects rated “Breathing Discomfort” on a Visual Analog Scale (BDVAS, 100% Full Scale ≡ intolerable). At the PETCO2 producing 60% BDVAS pre-treatment, furosemide produced a clinically meaningful reduction of BDVAS (i.e., > 20%FS) in 5/11 subjects; saline reduced dyspnea in 3/11 subjects; neither treatment worsened dyspnea in any subject. Furosemide and saline treatment effects were not statistically different. There were no significant adverse events. Higher furosemide dose and controlled delivery did not improve consistency of treatment effect compared with prior studies.
Keywords: Dyspnea, Furosemide, Palliative care, Symptom management
1. INTRODUCTION
Non-opioid treatments for refractory dyspnea are eagerly sought – among the possibilities is aerosol furosemide. Furosemide, a chloride channel blocker commonly used as a diuretic, also acts on pulmonary mechanoreceptors in rats when administered as an aerosol; slowly adapting pulmonary stretch receptors are profoundly sensitized and rapidly adapting stretch receptors (which also respond to lung collapse) are desensitized (Sudo et al., 2000). Increased tidal volume in humans relieves air hunger, the most uncomfortable form of dyspnea, via pulmonary mechanoreceptors (Banzett et al., 2008; Evans et al., 2002; Manning et al., 1992). Furosemide is postulated to act on dyspnea by providing an illusory report of larger tidal volume to the brain.
Prior to our most recent study (Morelot-Panzini et al., 2017), those investigators that reported aerosol furosemide administration methods did not control the inspiratory flow rate or inspired volume during aerosol administration. Inspiratory flow is a key parameter in deposition; if flow is high, particles will deposit preferentially in the oropharynx and upper airways, not deeper in the lung where most of the slowly adapting stretch receptors are found (Bennett et al., 2002; Brain and Valberg, 1979; Li et al., 2009; Moren et al., 1994; Sant’Ambrogio and Sant’Ambrogio, 1982). Inspiratory volume is also an important parameter, as shallow breathing will preferentially deposit aerosol in central airways (Bennett et al., 2002; Brain and Valberg, 1979; Moren et al., 1994). In addition, prior studies that did report methodology used a bi-directional breathe-through nebulizer, so that aerosol generated during expiration was lost into the room; actual inhaled dose would vary depending on the ratio of inspiratory to expiratory time.
A number of laboratory and clinical studies have shown furosemide to provide substantial relief of dyspnea in some, but not all, subjects (Jensen et al., 2008; Kohara et al., 2003; Moosavi et al., 2007; Morelot-Panzini et al., 2017; Nishino et al., 2000; Ong et al., 2004). (No study has reported appreciable worsening of dyspnea following furosemide.) The source of this wide response variability is unknown. We hypothesized several reasons that the effect of furosemide might have failed in some subjects:
Inadequate Dose? Most studies have used a nebulized dose of 20 or 40 mg furosemide (much less is actually deposited in the lung); our most recent study employed a 40mg aerosolized dose. We hypothesized that a higher dose would produce more consistent dyspnea relief – In the present study we administered 80 mg nebulized furosemide, at least twice the dose used in previous studies.
Sub-optimal deposition of drug? Apart from our most recent study (Morelot-Panzini et al., 2017), published studies did not control the inspiratory flow rate or inspired volume during furosemide aerosol administration, nor did they control aerosol lost to the room during expiration. We hypothesized that better control over aerosol administration would produce more consistent dyspnea relief. Our first study in this series controlled inspiratory flow and volume and minimized loss of aerosol generated during expiration; however, we did not find a more uniform treatment effect. The present study uses the same methods to control inspiratory flow and volume and minimize aerosol loss and tests the effect of doubling the dose.
Innate insensitivity to tidal volume relief. The presumed mechanism of furosemide relief relies on the vagal pulmonary stretch receptor afferent pathway inhibiting dyspnea (“tidal volume relief”). The potency of this pathway may vary among individuals due to differences in afferent input and CNS processing. We hypothesized that furosemide works poorly in those individuals who have weak tidal volume relief. We tested the potency of the tidal volume relief pathway by comparing breathing discomfort during free breathing with breathing discomfort during constrained breathing.
2. METHODS.
These studies were approved by the BIDMC IRB. Administration of aerosol furosemide was performed under IND 108667 from the US FDA. The IND permitted only one aerosol furosemide administration to any given individual subject.
2.1. Measurements
Psychophysical Measurements. Subjects rated “Breathing Discomfort” (BD) on a Visual Analog Scale (VAS). End points of the BDVAS scale were 0% Full Scale (%FS) labeled ‘none’ (i.e., no breathing discomfort) and 100%FS labeled ‘extreme’ breathing discomfort. Subjects were informed that rating ‘extreme’ would cause the experimenter to immediately reduce the stimulus. Following each run, subjects also completed a Multidimensional Dyspnea Profile (MDP) (Banzett et al., 2015).
Physiological Measurements. We recorded tidal PCO2 and tidal flow, and pressure at the airway opening. Pulse rate, tidal PO2, oxygen saturation, and non-invasive blood pressure (2 min intervals) and ECG were monitored for safety. Details of instrumentation can be found in the online supplement. Key variables derived for analysis were end-tidal PCO2 (PETCO2, tidal volume (VT), respiratory rate (fR) and minute ventilation () (derived from integration of inspiratory and expiratory flow).
2.2. Dyspnea model
We used a model of laboratory dyspnea in healthy subjects employed previously in a number of studies (Banzett et al., 1996; Banzett et al., 1990; Banzett et al., 2000; Evans et al., 2002; Manning et al., 1992). Graded levels of dyspnea were induced by changing inspired PCO2 while holding minute ventilation constant slightly above resting levels. We manipulated inspired PCO2 to alter PETCO2 in steps, with initial over- or under-shoot of inspired PCO2 to achieve steady state as rapidly as possible. Step duration was approximately 3 min, allowing about 2.5 min of steady PETCO2.
Minute ventilation was held constant at approximately 0.14 liters•min−1•kg−1 with a mean respiratory rate of 11.8, range 10.6–12.4 breaths per minute. (Slight adjustments of each subject’s tidal volume and rate were made during runup sessions to ensure that all subjects were comfortable at baseline settings). Tidal volume, respiratory rate, inspiratory flow, and expiratory time were controlled by a Siemens 900C clinical ventilator set on volume control with triggering set to minimum sensitivity (20 cmH2O below current PEEP setting). Inspired oxygen fraction was constant at 30%. Subjects were coached to relax as much as possible: they were trained to avoid negative inspiratory pressures greater than −20cmH2O, and were trained to relax to normal end-expiratory volume with each breath; those who could not adhere to these guidelines during run-up days were not studied further. Because the ventilator controlled flow and timing precisely, and because triggered breaths or stacked breaths are more obvious, this method provides tighter control over ventilatory variables than the bag-limit method used in our recent study (Morelot-Panzini et al., 2017).
The two essential components of the dyspnea challenge are controlled hypercapnia and controlled ventilation. In the present experiment, PETCO2 was the independent variable, thus is explicitly entered in the analysis. We were successful in keeping ventilation constant throughout in each individual. On furosemide test days, ventilation, averaged across subjects, was 8.41 L•min−1 pre-treatment and 8.34 L•min−1 post-treatment (p=0.36, paired T test); individual differences were less than 0.4 L•min-1 (i.e., < 5%, within measurement precision) in all but one case (AF88, 0.63 L•min−1 decrease post-treatment). On saline test days, ventilation was 8.40 L•min−1 pre-treatment and 8.35 L•min−1 post-treatment (p=0.21, paired T test); all individual differences were less than 0.4 L•min−1. Supplement Fig S-3 depicts ventilation matching in each subject. (Ventilation was measured at the benchmark PETCO2; used for assessment of dyspnea treatment effect – see below).
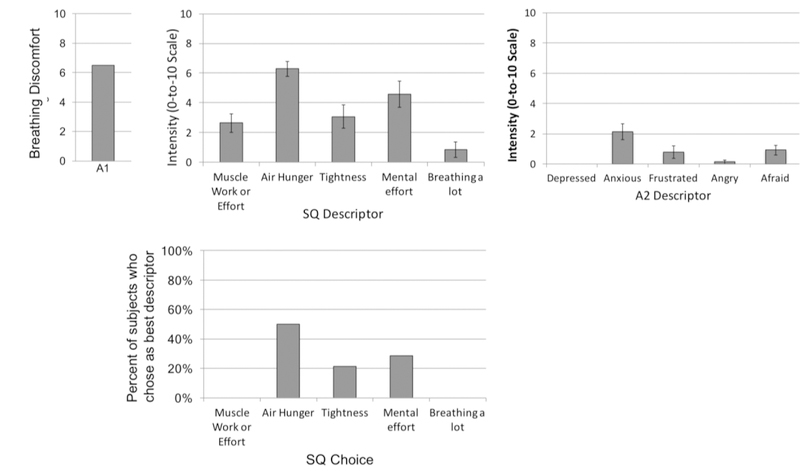
Subjects most often categorized the discomfort during this test as ‘air hunger’, although mental effort and tightness were also sometimes chosen (see profile of subjective experiences in Fig. 1.
Fig 1.
Sensory qualities and emotional responses to the dyspnea model used for treatment testing. Upper panels depict mean rating of each item; lower panel depicts the percent of subjects who chose each descriptor as most accurately describing the experience. Data were obtained using the ‘Multidimensional Dyspna Profile’ (Banzett et al., 2015). Data are from a pre-treatment test on the first drug or placebo treatment day for the 11 subjects used in the main analysis. Subjects were asked to complete the MDP with reference to the last 30 sec of each run. To weight subjects equally, we selected one run from each subject: the first run that terminated in a rating of overall breathing discomfort (A1) of 50 to 80 % of full scale. Full Sensory Quality (SQ) descriptor clusters as follows: My breathing requires muscle work or effort; I am not getting enough air or I am smothering or I feel hunger for air; My chest and lungs feel tight or constricted; My breathing requires mental effort or concentration; I am breathing a lot.
2.3. Aerosol Delivery
To ensure uniformity and efficiency of aerosol deposition, we used a clinical ventilator to control inspiratory flow (300–500ml/sec) and tidal volume (15% of predicted vital capacity). Three ultrasonic screen nebulizers (Aeroneb) fed 3.4 micron aerosol to a manifold in series with the inspiratory line (See Supplement Fig S-1). Apart from aerosolized dose, the methodology of aerosol delivery was identical to that used in our prior study (Morelot-Panzini et al., 2017).
2.4. Subjects & Protocol
We studied 2 healthy men and 10 healthy women not familiar with pulmonary research. Median subject age was 24, range 18–32. Subjects participated on 3 treatment test days and were informed that they would receive furosemide, saline, and albuterol on separate days in random order; they actually received furosemide once and saline twice (the albuterol deception was done to balance expectation in case subjects detected side effects of furosemide). More complete information on subjects included and excluded are given in Supplement section “Subjects: Selection and Characteristics”. Resting PETCO2 was measured at the beginning of each visit.
2.4.1. ‘Run-up’ Sessions in advance of treatment testing.
Each subject visited the laboratory on 2 or 3 “run-up days” prior to treatment testing to familiarize them with laboratory procedures and to characterize their responses to the dyspnea stimulus. We screened for subjects who were unable to consistently report their breathing discomfort as VAS ratings (‘poor raters’) as described in Supplement Section “Protocol”. During one run-up day, we included a graded CO2 stimulus during free breathing.
2.4.2. Treatment test sessions.
Subjects participated on one furosemide and 2 saline test days. Order of presentation was randomized and balanced among subjects. Treatment sessions are described in more detail in Supplement section “Protocol”.
Before treatment the subject underwent trials with the standard dyspnea model to determine the pre-treatment response. Following treatment, the Operator (the experimenter controlling the stimulus) administered a similar sequence and range of PETCO2 levels as before treatment. The Operator could view the subject’s BDVAS ratings before treatment, but was blinded to BDVAS data post-treatment and until all subjects completed the study; he was also blinded to the actual aerosol administration and to the subject’s frequency of urination. Volume urinated was replaced by an equal volume of electrolyte and carbohydrate mix.
2.5. Analysis
2.5.1. Data Reduction
Measurements of physiological variables and BDVAS were collected in the last 90 sec of each 3-min PETCO2 step (details in Supplement Section “Data Reduction”). Each trial provided 4–6 data points (CO2 steps with associated BDVAS ratings) before quality control review of data described below.
As per our a priori analysis plan we determined a single number representing ‘treatment effect’, change in dyspnea at the PETCO2 that caused breathing discomfort (BDVAS) equal to 60%FS (termed the ‘benchmark PETCO2’). All results are expressed in absolute terms (%FS), rather than a percentage of the pre-treatment value. The process for determining treatment effect is detailed in Supplement Section “Determination of Treatment Effect”.
2.5.2. Data Quality Control
2.5.2.1. Primary analysis - (A Priori Criteria)
Before the primary analysis we removed data points if the physiological recordings showed departure from controlled stimulus conditions, as detailed in Supplement section “Primary analysis - Stimulus Physiology Screening”. Stimulus quality control usually resulted in the loss of only a few data points each day; however, no experiment in AF68 survived the stimulus physiology quality control. Thus, 11 furosemide and 21 saline tests in 11 subjects are presented in this analysis.
2.5.2.2. Secondary analysis - High Quality Regressions (Post-Hoc Criteria)
We performed a second analysis with added post-hoc quality criteria to reduce the possibility of reporting spurious results as described in Supplement Section “Secondary analysis - High Quality Regressions”. There were 7 subjects who had high quality data on the furosemide test day and at least one saline test day. These are the individual data in which we have highest confidence, but the results lead to similar conclusions.
2.5.3. Statistical Comparisons
We planned a priori to compare each subject’s furosemide response to the mean of all subjects’ responses to saline, predicated on the supposition that placebo responses would be small and random. Because this turned out not to be the case, we present other analyses as well; all lead to the same conclusions.
Using an alternate approach, we categorized subjects as “responders” or “anti-responders” if they showed a treatment effect of at least 20% of full scale, a criterion set well before the experiments were conducted. Since then, a meta-analysis of dyspnea treatment trials has suggested that a treatment effect equivalent to 18.2% FS is a “large change” relative to variance, and that one can statistically detect patient preference for treatments that produce as little as 9% FS improvement.
3. RESULTS
3.1. Subjects
We enrolled 56 subjects; 44 subjects dropped out or were eliminated before treatment studies for various reasons detailed in Supplement Table S-1, among these were 11 subjects eliminated because they were ‘poor raters’. Twelve subjects were tested with both aerosol furosemide and aerosol saline. Post experiment analysis of physiological data revealed that one Subject, AF68, failed to adequately comply with the stimulus task and his data were eliminated from further analysis.
3.2. Primary analysis results (a priori quality control)
This primary analysis includes all data that met a priori physiological criteria; the individual results are presented graphically in Fig 2. Changes in ratings are represented as %FS (not as percent of baseline dyspnea); therefore, they provide a measure of the absolute change in rating.
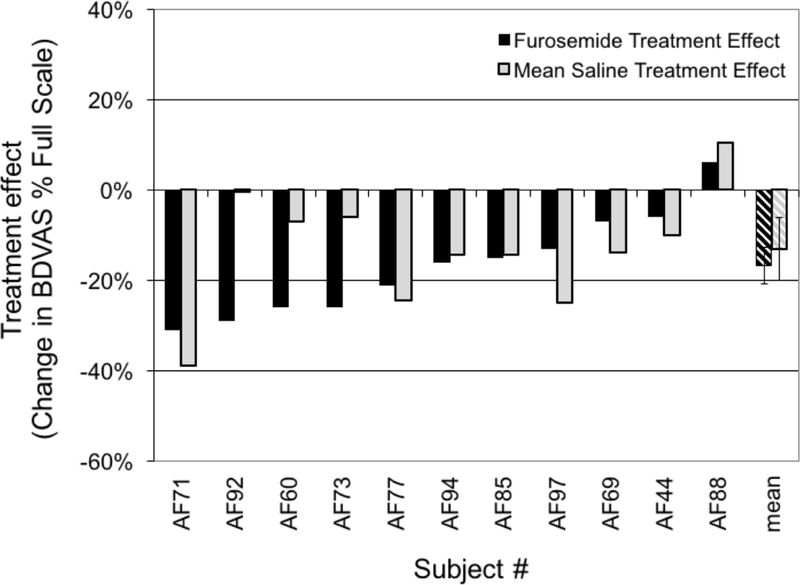
Figure 2.
Primary analysis: change in Breathing Discomfort at benchmark PETCO2. Black bars depict treatment effect following 80 mg furosemide aerosol; shaded bars indicate treatment effect following an equal volume of saline aerosol. Subjects are arranged in order of furosemide response. Crosshatched bars indicate mean ±SE. Treatment effects of both furosemide and saline were significantly different from zero, but were not significantly different from each other. Treatment effects are represented as %FS and, therefore, provide a measure of the absolute change in rating.
3.2.1. Furosemide response
Aerosol furosemide resulted in significant reduction of breathing discomfort compared to the pre-drug baseline condition on that day. The mean treatment effect was a reduction in BDVAS of 17%FS (±3% SE, P=0.001). Although there was wide variation in individual responses, nearly all tests resulted in reduced breathing discomfort; slight worsening was observed on only 1 of 11 aerosol furosemide test days. Taking a dyspnea reduction of 20%FS as clinically meaningful, we found 5/11 meaningful responses to furosemide (45% of 11 tests in 11 subjects).
3.2.2. Saline response
Aerosol saline also resulted in significant reduction of Breathing Discomfort of 13%FS ±4% (mean±SE, P=0.008; average of 2 saline days per subject, except only one day available in AF44). There was again a wide variation in individual response, but nearly all tests resulted in reduced breathing discomfort. Taking a dyspnea reduction of 20%FS as clinically meaningful, we found meaningful responses to saline on 7/21 individual test days (33%); when the two test days for subjects were averaged, we found meaningful responses to saline in 3/11 subjects (27%). Responses for the two saline days were not well correlated across individuals (r2=0.16).
The effect of aerosol furosemide was not different from the mean effect of aerosol saline, our a priori hypothesis. Individual responses to furosemide were not correlated to saline responses across individuals (r2=0.19). Although the response to furosemide was slightly larger, it was not significantly different from the saline response whether analyzed as a difference from mean saline response as originally intended (P=0.32) or as paired comparison (P=0.39).
3.3. Secondary analysis results (post hoc stringent quality control)
The secondary analysis (removal of downsteps and rejection of trials having low stimulus-response correlation) produced higher quality data, but data in 4 subjects did not meet stringent criteria. The resulting average r2 for stimulus-response correlation in 7 subjects was 0.78 (range 0.50 to .99). Thus, higher confidence in individual results comes at the cost of reduced sample size (7 vs 11 subjects). Results following stringent quality control are shown in Fig 3.
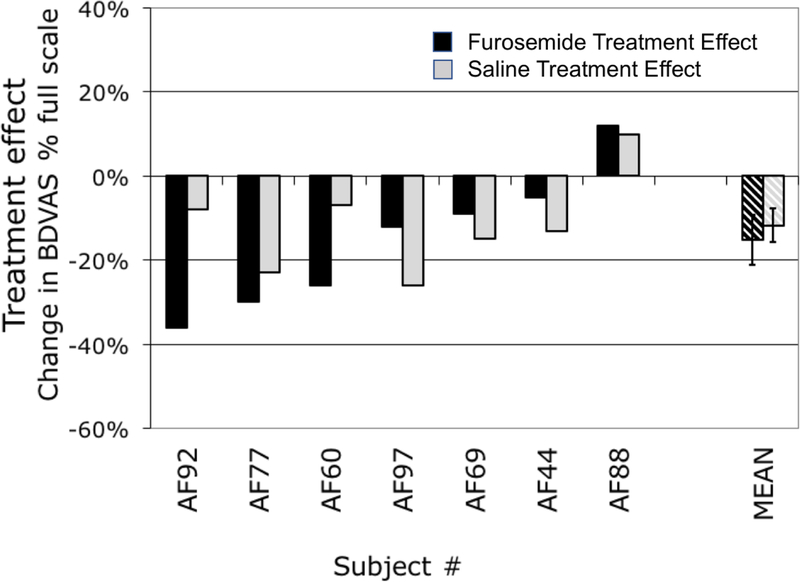
Fig 3.
Treatment effect of furosemide (blackbars) and saline (shaded bars) in the seven subjects who provided high quality data for aerosol furosemide treatment. Bars indicate the change in Breathing Discomfort at benchmark PETCO2 following 80 mg furosemide aerosol or an equal volume of saline. Crosshatched bars indicate mean ±SE. AF68, AF71, AF73, AF85, & AF94 contributed no data that survived stringent quality control.
3.3.1. Furosemide response
The mean reduction of Breathing Discomfort following aerosol furosemide was 15% (±6% SE, P=.053). Wide variation in individual response remained in the seven subjects whose data passed stringent quality control: Taking a dyspnea reduction of 20%FS as clinically meaningful, we found meaningful responses to furosemide in 3 of 7 subjects (i.e., 43% of trials).
3.3.2. Saline response
All seven subjects who provided high quality furosemide data provided at least one set of high quality data for aerosol saline; three provided high quality data on two aerosol saline test days – these were averaged to provide the graphed data. The mean reduction of BDVAS after saline was 10% FS (±4%SE, P=.042). The response to aerosol saline was not significantly different from the response to aerosol furosemide (P=0.7, Paired T Test). As with Furosemide, there was wide variation in response. We observed meaningful responses to saline on 4 of 10 saline test days that passed quality control (40% of trials); when the two test days for subjects were averaged, we found meaningful responses to saline in 2/7 subjects (29%). Although there were insufficient points in 3 subjects to test the regression of the 2 saline days, it was evident that the response to saline was not consistent from day to day.
The responses to furosemide were not correlated to mean saline responses across individuals (r2=0.13). Although the response to furosemide was slightly larger than the saline mean saline response, the difference was not significant (P=0.58, paired comparison).
3.4. Temporal effects
It is possible that the effect of furosemide takes time to develop, and would not yet be apparent at the time of the first post-treatment run (12 minutes on average after finishing aerosol administration) or that the effect is brief enough to be diminished by the time of our second post-treatment run (started 49 minutes on average after aerosol administration). We therefore compared the first post-treatment run to the second post-treatment run. In this analysis, some individual post-drug regression lines contained fewer than 4 points, thus we present only the group average result. The first post-furosemide run showed a mean reduction in BDVAS of 14.2%FS (±10.2%SE) while the second run showed a reduction of 10.9%FS (±7.9%), and the order effect varied among subjects, thus was not statistically significant (p=0.68, paired T Test). Similarly, after aerosol saline the first run showed a slightly larger response (9.5 vs 6.7%FS, p=0.62)
3.5. Tidal Volume relief sensitivity
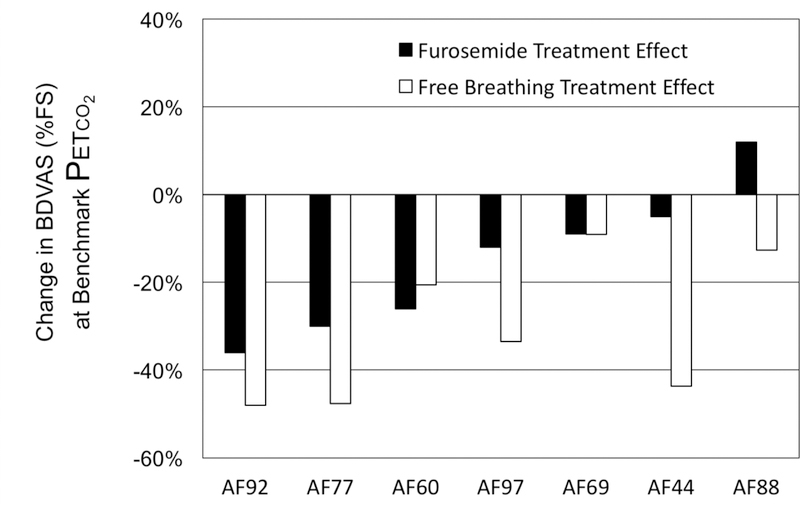
When subjects were allowed to breathe freely, breathing discomfort fell by an average of 31% at the benchmark PETCO2; individual variation was high (SD±17%). The two subjects who showed little relief with free breathing (AF69 & AF88) also showed little relief with furosemide (both subjects tripled ventilation during free breathing). Conversely, the three subjects (AF92, AF77, and AF60) who showed clinically relevant relief after furosemide also showed clinically relevant relief when allowed to breath freely. However, there were subjects who showed strong relief with free breathing but not with furosemide treatment. Furosemide response was therefore only modestly correlated to free breathing response (r2=0.35). See Figure 4.
Figure 4.
Responses to aerosol furosemide (black bars) and free breathing (shaded bars) in the 7 subjects passing stringent quality control. Subjects are arranged in order of furosemide response.
3.6. Side effects
As expected, inhaled furosemide absorbed through the respiratory tract caused some diuresis (see below). Some subjects reported a mild bitter taste and/or irritation with furosemide inhalation. Although a few subjects reported mild urge to cough, it was not strong enough to preclude drug administration. Changes in blood pressure were small and insignificant (see Supplement Table S-3). There were no significant adverse events.
3.7. Exploration of explanatory factors
We examined two other characteristics of individuals to explore possible reasons that individual responses to furosemide and saline varied widely. These post hoc explorations of individual characteristics were carried out only in the 7 subjects who passed stringent quality control because confidence in individual results is much higher. Due to the small sample, and post hoc nature of these hypothesis-generating explorations we present only a brief summary here; more details will be found in the Supplement.
3.7.1. Diuresis-systemic action of absorbed furosemide
We calculated the cumulative urine output one hour following aerosol administration, interpolating between adjacent measurements as necessary. (mean 1320ml ±394SD following furosemide vs 474ml ±146SD following saline, p<0.0001 paired T test). There was substantial variation among individuals; urine output at one hour after aerosol furosemide ranged from 201% to 548% of output following aerosol saline; there was, however, no correlation between furosemide-induced diuresis and the effect of furosemide on breathing discomfort (r2=0.13; Supplement Fig S-5), which suggests that variation in renal action of the absorbed drug cannot explain variation in treatment effect.
3.7.2. Expectations
Subjects (except AF44 & AF71) were debriefed following the last test on the final day. They were asked to categorize their expectations that one or both drugs would reduce discomfort. Three subjects averred no expectation at the outset (AF69, AF88, and AF97). The remainder of the subjects had somewhat high (AF60, AF73, AF77, AF85) to very high (AF68, AF92, AF94) expectations that one or both drugs would be effective. The weak relationship of treatment effects to prior expectation for the 7 subjects with data surviving post hoc quality control is shown in Supplement Fig S-6.
4. DISCUSSION
4.1. Overview
As in most prior studies of aerosol furosemide, there was great variability of the response among individuals. Our study shows doubling the maximum dose used in prior studies does not appreciably increase the proportion of subjects who respond, even with careful control of factors influencing aerosol deposition.
Because the relief of dyspnea by large breaths and the relief of dyspnea by furosemide are both thought to depend on pulmonary stretch receptor afferents (Manning et al., 1992; Sudo et al., 2000), we hypothesized a priori that those individuals having little dyspnea relief larger tidal volumes might also have weaker relief from aerosol furosemide. Our findings in the present work and the companion paper (Morelot-Panzini et al., 2017) support this idea, in that subjects who showed little relief with free breathing also showed little relief with furosemide. However, low tidal volume relief cannot be the sole explanation for lack of response to furosemide; in both studies there were subjects who did not respond to furosemide despite showing brisk volume relief. Strong tidal volume relief may be a necessary, but not sufficient, characteristic for effective furosemide action, but other factors may limit effective treatment. For instance, the companion study (Morelot-Panzini et al., 2017) showed that subjects having greater efficiency of furosemide absorption into the blood showed less treatment response, suggesting that rapid removal from stretch receptor sites by the blood may limit drug action.
Systemic furosemide is known to relieve dyspnea in congestive heart failure, and the presumptive mechanism is through diuresis leading to lessening of pulmonary vascular congestion and edema. The dyspnea relief experienced after aerosol furosemide treatment in the present study was not related to the diuretic effect, which is unsurprising as our subjects did not have congestive heart failure.
Apart from responsiveness to lung inflation, no other individual characteristics stood out in our examinations. In contrast to our most recent study, the response to furosemide was not correlated with the response to saline, as might be expected in subjects susceptible to placebo effect.
Set against the effectiveness of aerosol furosemide is the fact that aerosol saline, having even fewer side effects, produced nearly as much dyspnea relief in a similar portion of subjects. Saline response was also a problem in interpreting the results of the first study in this series (Morelot-Panzini et al., 2017). In a study designed to examine the effect of expectation on response, we informed subjects that they would only receive saline placebo, and that it was not expected to improve their dyspnea – there was still one instance of a substantial treatment effect (O’Donnell et al., 2016). In the present study, we sought to reduce the opportunity for spurious saline response in several ways: 1) We attempted to minimize the prior expectations of the subjects and make drug administration less predictable; 2) we utilized a slightly different laboratory model of dyspnea that allows the subject less latitude to alter breathing pattern; 3) we tested the response to saline twice in each individual (due to FDA restrictions, we were unable to test furosemide twice); 4) we queried subjects about their expectations following the study, 5) we administered stimuli in both up and down steps to make the experience less predictable. These precautions did not eliminate responses to saline.
4.2. Limitations
We found a number of limitations to the present approach.
Recruitment and retention were problematic in this study. We made several alterations to our earlier protocol (Morelot-Panzini et al., 2017) in order to tire subjects less during testing days and reduce the number of laboratory visits. We eliminated venipuncture for sampling systemic furosemide levels (but we did measure the systemic side effect of clinical interest, diuresis). We made fewer measurements on run-up days, we eliminated free-breathing tests on treatment test days. In addition, we made the stimulus less predictable by introducing downsteps on treatment days (however, we the mistake of allowing insufficient time for downsteps to reach steady state in some subjects). We also made fewer measurements before and after treatment and minimized the duration of each stimulus step. Together, these reduced the number of data points per subject. Some of these problems may have been rectified had we analyzed data immediately, but we avoided data analysis until all studies were complete to maintain blinding in all members of the team who could influence changes in design.
No study has incremented aerosol furosemide doses to determine if non-responders could be converted into responders. The terms of the IND issued by the FDA allowed only one administration of aerosol furosemide to a given individual, we could not test the reliability of response or non-response with repeated treatment, nor could we test the effect of increasing dose in non-responders. Although the IND did not allow multiple administration to an individual, it did allow larger doses to be given to a different cohort of subjects. We therefore have no dose-response information within subject. Although the comparison of 40 and 80 mg doses using the same aerosol methodology in different subject groups suggests no improvement with escalating dose, it is possible that even higher doses of aerosol furosemide might prove more effective in those subjects, and our results suggest that higher doses will be safe in healthy volunteers.
Personal psychological characteristics (e.g., optimism or persuadability) and situational expectations are independently associated with the strength of placebo effect (Corsi and Colloca, 2017; Del Re et al., 2013). The poor correlation between furosemide treatment effect and saline treatment effect, as well as poor correlation between repeated saline effects, argue that the placebo-susceptibility of individual subjects are unlikely to explain our results. Despite our efforts to avoid creating expectations of successful treatment, most subjects reported having expectations that treatment would be effective. It is possible that such expectations are unavoidable with today’s procedures of recruitment and informed consent, and widespread pharmaceutical marketing – factors that may relate to the observation that placebo effects are increasing in drug treatment trials (Del Re et al., 2013; Silberman, 2009; Tuttle et al., 2015). We saw a lack of strong correlation between subject-reported expectation and treatment effect, but to avoid subjects ‘over-thinking’ the experiment, we did not debrief them until after the final test, thus we depended on accurate recall. Although our results do not point to a specific correlate of placebo effect, the possibility cannot be ignored.
4.3. Conclusions.
We disproved two of the hypotheses under test, resolving at least some of the uncertainty about furosemide response variance: Neither 1) well-controlled aerosol delivery, nor 2) doubling the dose produced a larger proportion of responders in this study. In addition, we showed that systemic absorption and diuresis cannot explain the result in these healthy subjects, and provided information suggesting that individuals who lack strong tidal volume relief will not respond to aerosol furosemide. Furthermore, we observed no adverse effect beyond the expected modest diuresis, even with the highest dose used thus far.
Aerosol administration of both saline and furosemide produced a clinically relevant reduction of Breathing Discomfort (≥20% full scale) although not in the same subjects. The effects of both treatments were statistically significant compared to the pre-drug condition (p<.05), and the effect of furosemide was slightly greater than saline (NS). Increase in Breathing Discomfort following either treatment was rare and small. The most obvious inference is that psychological placebo effects occur at random times, and explains all findings. This may be true, however, there may be reason to believe that aerosol furosemide has a pharmacological effect. 1) The average dyspnea relief in furosemide “responders” in both present studies was larger than the response to saline; 2) It may be that saline is not the best control, as it, too, may have some pharmacological effect. 3) The response of pulmonary stretch receptors to furosemide provides a sound mechanistic basis for the action of furosemide; 4) Some of the furosemide response failures may be explained by a weak stretch receptor relief pathway (non-responders to free breathing in both studies) or to variation of drug deposition and absorption in the lung (inverse correlation to absorption efficiency in the 40 mg study).
Variable improvement of dyspnea following aerosol furosemide is consistent with most prior clinical and laboratory studies, as is the rarity of worsening dyspnea. Despite this variation, a treatment that can provide substantial relief of dyspnea to 45% of individuals with little expense, minimal side effects, and no sedation could be an asset; if these results were reproduced in larger clinical studies, furosemide could be added to our complement of general approaches to palliate dyspnea.
Supplementary Material
HIGHLIGHTS.
We tested hypotheses that might explain failure of aerosol furosemide to alleviate experimental dyspnea.
Control of aerosol delivery did not increase the proportion of subjects who responded.
Doubling furosemide dose did not increase the proportion of subjects who responded..
Response variation was partially explained by variation of response to large breaths.
Acknowledgments
Supported by NIH-NR12009
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest: none declared
References
- 1.Banzett R, Lansing R, Evans K, Shea S, 1996. Stimulus-response characteristics of CO2-induced air hunger in normal subjects. Respiration Physiology 103, 19–31. [DOI] [PubMed] [Google Scholar]
- 2.Banzett RB, Lansing RW, Brown R, Topulos GP, Yager D, Steele SM, Londono B, Loring SH, Reid MB, Adams L, et al. , 1990. ‘Air hunger’ from increased PCO2 persists after complete neuromuscular block in humans. Respir Physiol 81, 1–17. [DOI] [PubMed] [Google Scholar]
- 3.Banzett RB, Mulnier HE, Murphy K, Rosen SD, Wise RJ, Adams L, 2000. Breathlessness in humans activates insular cortex. Neuroreport 11, 2117–2120. [DOI] [PubMed] [Google Scholar]
- 4.Banzett RB, O’Donnell CR, Guilfoyle TE, Parshall MB, Schwartzstein RM, Meek PM, Gracely RH, Lansing RW, 2015. Multidimensional Dyspnea Profile: an instrument for clinical and laboratory research. Eur Respir J 45, 1681–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banzett RB, Pedersen SH, Schwartzstein RM, Lansing RW, 2008. The affective dimension of laboratory dyspnea: air hunger is more unpleasant than work/effort. Am J Respir Crit Care Med 177, 1384–1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bennett WD, Brown JS, Zeman KL, Hu SC, Scheuch G, Sommerer K, 2002. Targeting delivery of aerosols to different lung regions. J Aerosol Med 15, 179–188. [DOI] [PubMed] [Google Scholar]
- 7.Brain JD, Valberg PA, 1979. Deposition of aerosol in the respiratory tract. Am Rev Respir Dis 120, 1325–1373. [DOI] [PubMed] [Google Scholar]
- 8.Corsi N, Colloca L, 2017. Placebo and Nocebo Effects: The Advantage of Measuring Expectations and Psychological Factors. Frontiers in Psychology 8, 308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Del Re AC, Maisel N, Blodgett JC, Wilbourne P, Finney JW, 2013. Placebo group improvement in trials of pharmacotherapies for alcohol use disorders: a multivariate meta-analysis examining change over time. Journal of clinical psychopharmacology 33, 649–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evans KC, Banzett RB, Adams L, McKay L, Frackowiak RS, Corfield DR, 2002. BOLD fMRI identifies limbic, paralimbic, and cerebellar activation during air hunger. J Neurophysiol 88, 1500–1511. [DOI] [PubMed] [Google Scholar]
- 11.Jensen D, Amjadi K, Harris-McAllister V, Webb KA, O’Donnell DE, 2008. Mechanisms of dyspnoea relief and improved exercise endurance after furosemide inhalation in COPD. Thorax 63, 606–613. [DOI] [PubMed] [Google Scholar]
- 12.Kohara H, Ueoka H, Aoe K, Maeda T, Takeyama H, Saito R, Shima Y, Uchitomi Y, 2003. Effect of nebulized furosemide in terminally ill cancer patients with dyspnea. J Pain Symptom Manage 26, 962–967. [DOI] [PubMed] [Google Scholar]
- 13.Li H, Du L, Otmishi P, He Y, Guardiola J, Yu J, 2009. Opposite responses to lidocaine between intrapulmonary mechanical and chemical sensors. Am J Physiol Regul Integr Comp Physiol 297, R853–858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manning HL, Shea SA, Schwartzstein RM, Lansing RW, Brown R, Banzett RB, 1992. Reduced tidal volume increases ‘air hunger’ at fixed PCO2 in ventilated quadriplegics. Respiration Physiology 90, 19–30. [DOI] [PubMed] [Google Scholar]
- 15.Moosavi SH, Binks AP, Lansing RW, Topulos GP, Banzett RB, Schwartzstein RM, 2007. Effect of inhaled furosemide on air hunger induced in healthy humans. Respir Physiol Neurobiol 156, 1–8. [DOI] [PubMed] [Google Scholar]
- 16.Morelot-Panzini C, O’Donnell CR, Lansing RW, Schwartzstein RM, Banzett RB, 2017. Aerosol furosemide for dyspnea: Controlled delivery does not improve effectiveness. Respir Physiol Neurobiol Submitted [DOI] [PMC free article] [PubMed]
- 17.Moren F, Dolovich M, Newhouse M, Newman S, (1994). Aerosols in Medicine: Principles, Diagnosis, and Therapy, 2nd ed. Elsevier, Amsterdam. [Google Scholar]
- 18.Nishino T, Ide T, Sudo T, Sato J, 2000. Inhaled furosemide greatly alleviates the sensation of experimentally induced dyspnea. Am J Respir Crit Care Med 161, 1963–1967. [DOI] [PubMed] [Google Scholar]
- 19.O’Donnell CR, Lansing RW, Schwartzstein RM, Banzett R, 2016. The Effect of Aerosol Saline on Laboratory-Induced Dyspnea. Lung [DOI] [PMC free article] [PubMed]
- 20.Ong KC, Kor AC, Chong WF, Earnest A, Wang YT, 2004. Effects of inhaled furosemide on exertional dyspnea in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 169, 1028–1033. [DOI] [PubMed] [Google Scholar]
- 21.Sant’Ambrogio FB, Sant’Ambrogio G, 1982. Circulatory accessibility of nervous receptors localized in the tracheobronchial tree. Respiration Physiology 49, 49–73. [DOI] [PubMed] [Google Scholar]
- 22.Silberman S, (2009). Placebos Are Getting More Effective. Drugmakers Are Desperate to Know Why, Wired Magazine. Condé Nast, Accessed online
- 23.Sudo T, Hayashi F, Nishino T, 2000. Responses of tracheobronchial receptors to inhaled furosemide in anesthetized rats. Am J Respir Crit Care Med 162, 971–975. [DOI] [PubMed] [Google Scholar]
- 24.Tuttle AH, Tohyama S, Ramsay T, Kimmelman J, Schweinhardt P, Bennett GJ, Mogil JS, 2015. Increasing placebo responses over time in U.S. clinical trials of neuropathic pain. Pain 156, 2616–2626. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.