Significance
The presence of a familiar nonfearful conspecific during the extinction training session inhibits the retrieval but not the consolidation of the extinction of contextual fear conditioning. This effect relies on ventromedial prefrontal cortex rather than hippocampal gene expression and on ribosomal- and mTOR-dependent protein synthesis. These results provide knowledge about the cellular mechanisms and brain structures involved on the effect of social support in changing behavior and fear extinction memory.
Keywords: contextual fear conditioning, extinction, prefrontal cortex, hippocampus, social support
Abstract
Extinction of contextual fear conditioning (CFC) in the presence of a familiar nonfearful conspecific (social support), such as that of others tasks, can occur regardless of whether the original memory is retrieved during the extinction training. Extinction with social support is blocked by the protein synthesis inhibitors anisomycin and rapamycin and by the inhibitor of gene expression 5,6-dichloro-1-β-d-ribofuranosylbenzimidazole infused immediately after extinction training into the ventromedial prefrontal cortex (vmPFC) but unlike regular CFC extinction not in the CA1 region of the dorsal hippocampus. So social support generates a form of learning that differs from extinction acquired without social support in terms of the brain structures involved. This finding may lead to a better understanding of the brain mechanisms involved in the social support of memories and in therapies for disorders related to dysfunctional fear memories. Thus, here we show that the consolidation of extinction memory with social support relies on vmPFC rather than hippocampus gene expression and ribosomal- and mammalian target of rapamycin-dependent protein synthesis. These results provide additional knowledge about the cellular mechanisms and brain structures involved on the effect of social support in changing behavior and fear extinction memory.
Fear memories are essential for survival, however, their overexpression and/or generalization to other than the original stimulus, may lead to fear- and anxiety-related disorders, such as phobias and post-traumatic stress disorder (1–7). Currently, the first-line treatment for these disorders is the extinction-based exposure therapies (2, 3, 8, 9), which suppresses fear response by repeatedly exposing the subjects to the fear-inducing stimulus without harmful consequences (10).
Pavlovian fear conditioning (FC) is a widely used experimental model to study fear learning and extinction (8, 11–13). In this paradigm, a neutral conditioned stimulus (CS) is paired with an aversive unconditioned stimulus (US). Subsequent presentations of the CS alone elicit a conditioned fear response (2, 7, 12). Multiple presentations of the CS in the absence of the US will eventually induce extinction memory, which decreases the fear response to the CS (2, 7, 11). Fear extinction memory requires, at the time of consolidation, protein synthesis in several brain regions, such as hippocampus (14–19), ventromedial prefrontal cortex (8), and basolateral amigdala (20).
The expression of a fear response can be modulated by many factors including by social presence (21–24). It has been reported that the presence of a conspecific reduces stress and fear responses to threat situations (25–30). Such reduction in fear responses can be greater when the conspecific is familiar and/or nonfearful (15). This social support (S) effect is known as social buffering and seems to involve direct physical interaction, visual observation, and/or olfaction (25, 26, 31–34). In addition, the presence of a conspecific blocks the fear response to an auditory CS (35) and facilitated fear extinction.
In the current paper, we first examined the effect of S by a familiar nonfearful conspecific during an unreinforced retrieval on the extinction memory of contextual FC (CFC). Then, we study the effects of a ribosomal protein synthesis inhibitor, anisomycin (Ani); a mammalian target of rapamycin- (Rapa-) (mTOR-) dependent protein synthesis inhibitor, Rapa; and a gene expression inhibitor, 5,6-dichloro-1-β-d-ribofuranosylbenzimidazole (DRB) on fear extincion memory of CFC with S when infused into the CA1 region of the dorsal hippocampus or ventromedial prefrontal cortex (vmPFC).
Results
Effect of S by a Familiar Conspecific on the Extinction of CFC.
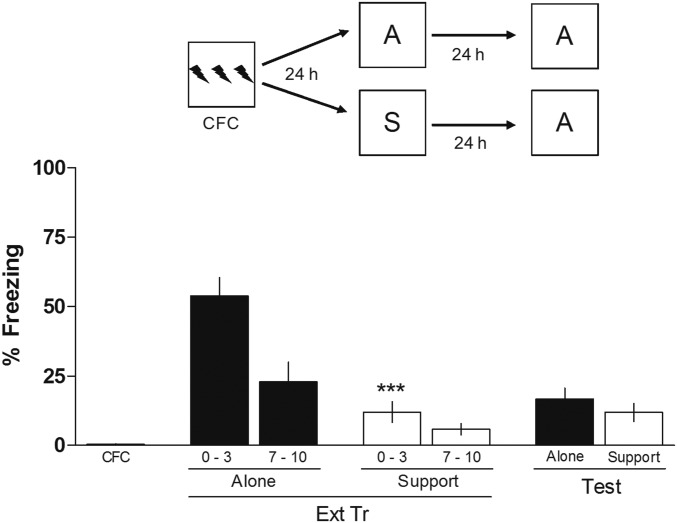
To verify the effect of S on the extinction memory of CFC, animals were submitted to a training session (CFC) alone. After 24 h, they were submitted to a 10-min extinction training (Ext Tr) session either Alone (A) or in the presence of a familiar conspecific, Support; and after another 24 h, the animals underwent a 3-min extinction retention test (Test) always alone. As can be observed in Fig. 1, animals whose Ext Tr occurred with S expressed less freezing behavior than animals submitted to the Ext Tr alone. One-way ANOVA showed significant differences between groups (F3,28 = 16.40; P < 0.0001), and the Newman–Keuls test revealed significant differences between the first 3 min of Ext Tr support and the first 3 min of Ext Tr A. However, both groups (A and Support) exhibited similar levels of freezing during the Test, indicating that, even in the absence of retrieval, animals submitted to the Ext Tr with a familiar conspecific were able to learn the extinction of CFC. Clearly, S adds a degree of complexity to the task under study (one more stimulus and its consequences to be analyzed besides the regular CS and US).
Fig. 1.
Effect of S by a familiar conspecific on the extinction of CFC. Animals were trained in CFC (three 2-s, 0.5-mA scramble foot shocks separated by 30-s intervals). After 24 h, animals were submitted to an Ext Tr session either A or with S. Twenty-four hours later, the animals underwent a Test in which they were alone. The figure shows the percentage of time spent freezing in the first 2 min of the Tr, in the first 3 min and the last 3 min of the Ext Tr, and in the Ext Test. Data are expressed as mean ± SEM (n = 8 animals per group). ***P < 0.0001 vs. group A in the first 3 min of the Ext Tr, the Newman–Keuls test after one-way ANOVA. (Upper) Schematic of the behavioral protocol used.
Effect of Ani, Rapa, and DRB Given into the vmPFC on the Consolidation of the Extinction of CFC with S.
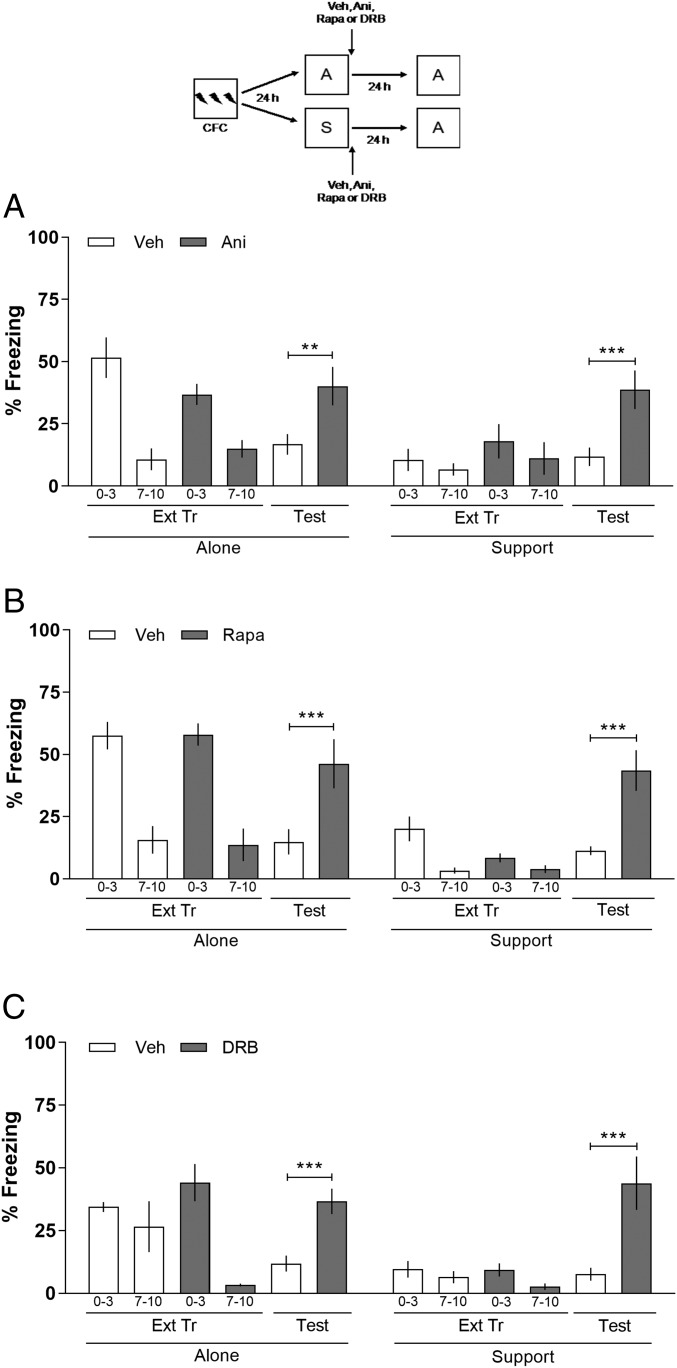
A time-honored way of assessing whether a given brain structure participates in a given behavioral task is to study the effect of inhibition of ribosomal or mTOR-mediated protein synthesis and of gene expression in that structure. For these purposes, the effect of the localized infusion of well-known inhibitors of these processes, the most widely used of which are Ani, Rapa, and DRB, respectively (7, 14, 15). To verify the participation of vmPFC on the extinction of CFC with S, animals were submitted to a training session (CFC) alone. After 24 h, they were submitted to an Ext Tr either A or Support. Immediately after the Ext Tr session, animals received intra-vmPFC infusions of vehicle (Veh), anisomycin (Ani, 80 μg per side; inhibitor of ribosomal protein synthesis), rapamycin (Rapa, 5 μg per side; inhibitor of mTOR-mediated protein synthesis) or DRB (8 ng per side; inhibitor of gene expression). The doses were taken from the literature (7, 14, 15). Twenty-four hours later, the animals underwent a Test always alone. In Fig. 2A, on the Test, two-way ANOVA revealed a significant difference among the variables: interaction (F7,85 = 3.62; P = 0.0018; Fig. 2A), treatment (F7,85 = 15.47; P < 0.0001), and groups (F1,85 = 12.81; P = 0.0006). In Fig. 2B, interaction (F7,76 = 8.65; P < 0.0001), treatment (F7,76 = 30.56; P < 0.0001), and groups (F1,76 = 39.91; P < 0.0001) and in Fig. 2C, interaction (F7,76 = 5.55; P < 0.0001), treatment (F7,76 = 20.12, P < 0.001), and groups (F1,76 = 18.25; P < 0.0001). During the Test, animals of both A and Support groups that received intra-vmPFC infusions of Ani (A), Rapa (B), or DRB (C) showed an impairment of extinction memory compared with their control groups. Boferroni’s post hoc revealed significant differences among Veh vs. Ani (P < 0.01; Fig. 2A), Veh vs. Rapa (P < 0.001; Fig. 2B), and Veh vs. DRB (P < 0.001; Fig. 2C) groups A. Similar results were observed on the Test support groups: Veh vs. Ani (P < 0.001; Fig. 2A), Veh vs. Rapa (P < 0.001; Fig. 2B), and Veh vs. DRB (P < 0.001; Fig. 2C). The results obtained using Ani, Rapa, and DRB infusions suggest that the vmPFC is involved in the consolidation of the extinction of CFC with S.
Fig. 2.
Effect of Ani, Rapa, and DRB given into the vmPFC on the consolidation of the extinction of CFC with S. Animals with infusion cannulae implanted in the vmPFC were trained in CFC (three 2-s, 0.5-mA scramble foot shocks separated by 30-s intervals). After 24 h, the animals were submitted to an Ext Tr either A or with S. Immediately after the Ext Tr session, the animals were bilaterally infused intra-vmPFC with Veh, Ani (80 μg per side) (A), Rapa (5 μg per side) (B), or DRB (8 ng per side) (C). Twenty-four hours later, the animals underwent an Ext Test alone. When given into the vmPFC, Ani, Rapa, and DRB blocked the consolidation of the extinction of CFC. The figure shows the percentage of time spent freezing in the first 2 min of the Tr, in the first 3 min and the last 3 min of the Ext Tr, and in the Test. Data are expressed as mean ± SEM (n = 5–7 animals per group). **P < 0.01 and ***P < 0.001 vs. control groups in the retention Test, Bonferroni’s post hoc after two-way ANOVA. (Upper) Schematic of the behavioral protocol used.
Effect of Ani, Rapa, and DRB Given into the CA1 on the Consolidation of the Extinction of CFC with S.
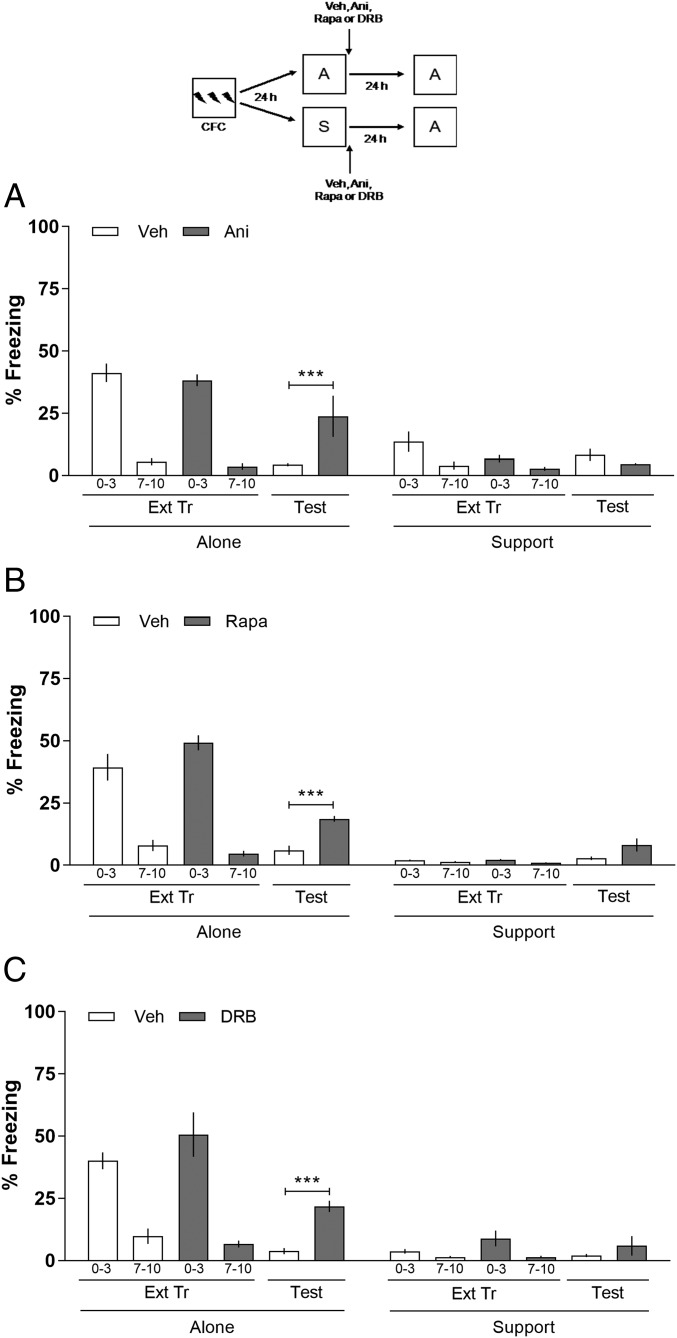
To verify the participation of the CA1 region of the hippocampus on the extinction of CFC with S, the protocol described above was repeated, except that now, animals received intra-CA1 infusions of Veh, Ani (80 μg per side), Rapa (5 μg per side), or DRB (8 ng per side) immediately after the Ext Tr. In Fig. 3A, on the Test, two-way ANOVA revealed a significant difference among the variables: interaction (F7,68 = 13.30; P < 0.001), treatment (F7,68 = 28.26; P < 0.001), and groups (F1,68 = 49.53; P < 0.001). In Fig. 3B, interaction (F7,80 = 47.09; P < 0.0001), treatment (F7,80 = 52.81; P < 0.001), and groups (F1,80 = 208.87; P < 0.0001) and in Fig. 3C, interaction (F7,80 = 16.09; P < 0.0001), treatment (F7,80 = 28.33; P < 0.0001), and groups (F1,80 = 89.66; P < 0.0001). As shown in Fig. 3, animals whose Ext Tr occurred A and received intra-CA1 infusions of Ani (A), Rapa (B), or DRB (C) exhibit an impairment on extinction memory compared with their control groups on the Test. Boferroni’s post hoc revealed significant differences among Veh vs. Ani (P < 0.001; Fig. 3A), Veh vs. Rapa (P < 0.001; Fig. 3B), and Veh vs. DRB (P < 0.001; Fig. 3C) groups A. Whereas, the animals whose Ext Tr occurred with S and received intra-CA1 infusions of Ani (A), Rapa (B), or DRB (C) were able to extinguish the memory as well as the control group on the Test. The results obtained using Ani, Rapa, and DRB infusions suggest that the CA1 region of the hippocampus is not involved in the consolidation of the extinction of CFC with S.
Fig. 3.
Effect of Ani, Rapa, and DRB given into the CA1 on the consolidation of the extinction of CFC with S. Animals with infusion cannulae implanted in the CA1 were trained in CFC. After 24 h, the animals were submitted to an Ext Tr either A or with S. Immediately after the Ext Tr session, the animals were bilaterally infused intra-CA1 with Veh, Ani (80 μg per side) (A), Rapa (5 μg per side) (B), or DRB (8 ng per side) (C). Twenty-four hours later, the animals underwent an Ext Test alone. When given into the CA1, Ani, Rapa, and DRB blocked the consolidation of the extinction of CFC of group A but not with S. The figure shows the percentage of time spent freezing in the first 2 min of the Tr, in the first 3 min and the last 3 min of the Ext Tr, and in the Test. Data are expressed as mean ± SEM (n = 5–7 animals per group). ***P < 0.001 vs. control groups in the retention Test, Bonferroni’s post hoc after two-way ANOVA. (Upper) Schematic of the behavioral protocol used.
Discussion
Here, we show that the presence of a familiar nonfearful conspecific during the Ext Tr session inhibits the retrieval but not the consolidation of the extinction of CFC. Concerning whether the vmPFC and CA1 region of the dorsal hippocampus play a role in the extinction of CFC with S, our findings show that Ani, Rapa, and DRB given into the vmPFC but not into the CA1 impairs the consolidation of the extinction of CFC. So S generates a form of learning that differs from extinction acquired without S in terms of the brain structures involved.
Stress and fear responses induced by exposure to stressful stimuli can be attenuated when an animal is exposed in the presence of a conspecific (25, 35). This phenomenon is known as social buffering and has been demonstrated as an important strategy of S in humans (36) and other species, including pigs (37), guinea pigs (38), cats (39), sheep (40), rhesus monkeys (41), zebrafish (42), and rodents (21, 25, 27, 31, 35, 43).
The effect of social buffering on fear memory in rodents demonstrates that the presence of a conspecific decreases escape, avoidance, and freezing behavior (25, 35, 44, 45) and can occur either by pair housing after a stressful traumatic event or by pair exposure to an acute stressor or FC with an unfamiliar conspecific animal (28, 45), however, the effect is more prominent when the conspecific is a familiar animal (46).
Studies investigating the neural pathways that underlie the social buffering of conditioned fear responses indicate that pair exposure to a contextual CS attenuates the c-Fos expression in the paraventricular nucleus (PVN) of the hypothalamus, lateral amygdala (LA), and central amygdala (28, 35, 47). Also, the presence of a conspecific suppressed the behavioral responses and hypothalamic-pituitary-adrenal axis activation to the CS, leading the corticosterone levels equal to a nonconditioned group (46). The pharmacological antagonism and genetic down-regulation of oxytocin receptors in the lateral septum but not in the hippocampus suppressed, whereas oxytocin administration facilitated, the reduction of FC behavior induced by preexposure to nonfearful conspecifics (48).
The effect of S also seems to occur during the extinction process. Animals submitted to an Ext Tr that is unable by itself to induce extinction of fear memory when in the presence of an unfamiliar conspecific exhibited inhibition of freezing responses on the Test session that was followed by a decreased c-Fos expression in the PVN and LA, indicating a facilitation of extinction (22). Bredy and Barad (49) reported that exposing mice to a recently FC familiar conspecific or to a urinary chemosignal from shocked conspecifics facilitates extinction learning but not the retention of extinction memory. Moreover, the presence of another animal in the Ext Tr facilitates extinction memory consolidation, and this effect is mediated by oxytocin in mPFC, once the intra-mPFC infusions of an oxytocin selective agonist enhanced, whereas the infusion of an antagonist, blocked the facilitation of extinction induced by a conspecific (21).
Here, we verified that the presence of a familiar conspecific on the Ext Tr session was capable of inhibiting the retrieval of the fear memory but not the consolidation of the extinction of CFC. This is in agreement with other results demonstrating that the social presence facilitates the extinction of fear memories (21, 22, 31, 49) and with recent data showing that retrieval performance is not necessary for the initiation, maintenance, or spontaneous recovery of extinction (16). That is, the results suggest that, when extinction occurs in pairs especially in the presence of a familiar conspecific, it provides the inhibition of the original fear association. This effect could be caused by physical contact or social interaction, although these variables were not measured in this paper.
The involvement of the vmPFC (50–54) and the CA1 region of the hippocampus (17, 54, 55) together with the baso-LA and other brain structures (7, 8, 18, 55, 56) in the extinction learning has been extensively described. The manipulation with protein synthesis inhibitors and signaling pathways indicates that these brain structures are crucial for the consolidation of extinction (57).
The present paper shows that intra-vmPFC infusions of Ani, Rapa, or DRB immediately after the Ext Tr session inhibits CFC extinction in animals trained, extinguished, and Tested alone as amply described before (7, 8, 56, 58). More importantly, we verified that, in animals whose extinction occurred in the presence of a familiar conspecific, the consolidation of the extinction of CFC with S was abolished when protein synthesis was blocked in the vmPFC. This suggested that vmPFC participates in the consolidation of the extinction of CFC with S and requires ribosomal- and mTOR-dependent protein synthesis and gene expression. When infused intra-CA1 immediately after the extinction training session, Ani, Rapa, or DRB also inhibited the extinction of CFC in animals trained, extinguished, and Tested alone demonstrating that, as previous described (7, 8, 17, 59), extinction requires ribosomal- and mTOR-dependent protein syntheses and gene expression in the hippocampus, however, had no effect on the extinction with S.
The involvement of the vmPFC in learning with S suggests that it may be more complex than learning without S. In a recent report, the enhanced retrieval of humans with highly superior memories correlates with the increased medial PFC activity measured by fMRI (60); that area appears to be related to the processing of more complex memories than those that take place without its intervention.
Thus, here, we show that consolidation of the extinction memory with S relies on vmPFC rather than hippocampal gene expression and ribosomal- and mTOR-dependent protein synthesis. These results provide additional knowledge about the cellular mechanisms and brain structures involved in the effect of S in changing behavior and fear extinction memory.
Materials and Methods
Animals.
Male Wistar rats (CrlCembe:WI; 3 mo old, 300–330 g) from Centro de Modelos Biologicos e Experimentais (CeMBE) of the Pontifical Catholic University of Rio Grande do Sul, Porto Alegre, Brazil were housed and maintained in groups of four per housing box with free access to food and water under a 12-h light/dark cycle (lights on at 7:00 AM) and the room’s temperature maintained at 22 to 23 °C. Each animal was randomly assigned to the group A, S (the group of subjects submitted to the extinction learning in the presence of a familiar nonfearful conspecific), or the animal used as S (rat placed with the subject during Ext Tr). Cage mates were assigned to the group of subjects submitted to the presence of a conspecific or to the S group to maintain the familiarity among them. All experimental procedures were approved by the Animal Committee on Ethics in the Care and Use of Laboratory Animals of the Pontifical Catholic University of Rio Grande do Sul, Brazil.
Surgery.
Animals were deeply anesthetized with i.p. injections of ketamine (75 mg/kg) and xylazine (10 mg/kg) and implanted with a 22-gauge bilateral guide cannula 1 mm above the CA1 region of the dorsal hippocampus (anterior −4.2 mm, lateral ± 3.0 mm, ventral −1.8 mm; from Bregma) or the ventromedial prefrontal cortex (anterior +3.2 mm, lateral ± 0.8 mm, ventral −4.1 mm; from Bregma) according to the coordinates of the Atlas by Paxinos and Watson (61). Dental acrylic cement was used to fix the guide cannulae to the skull. After surgery, animals were allowed 7 d for recovery before behavioral procedures and were handled daily for 3 d before the behavioral experiments.
Extinction of CFC.
For the CFC, animals were individually placed into the conditioning chamber (a 35 × 35 × 35 cm aluminum box with acrylic walls, a floor of stainless-steel grid bars connected to a device to deliver the foot-shock presentations, and placed inside a sound-attenuating box with a ventilating fan) and after 2 min, three electrical foot shocks (0.5 mA, 2 s) were delivered with a 30-s interval between them. Animals were removed from the conditioning chamber 30 s after the last foot shock and placed back in their home cages. After 24 h, animals were placed in the same conditioning chamber, Alone (A) or in the presence of a familiar nonfearful conspecific (Social Support, S) for a 10-min extinction training of CFC with no foot shocks. Twenty-four hours later, all animals were placed again in the same apparatus alone for a 3-min extinction retention Test again with no foot shocks. After each use, the apparatus was cleaned with 70% ethanol. The percentage of time that the animals spent freezing (i.e., no visible movement except for respiration) in the apparatus was measured (8, 14–16).
Pharmacological Interventions.
Animals received intravmCPF or intra-CA1 infusions of 0.9% saline (Veh), Anisomycin (Ani, 80 μg per side; inhibitor of protein synthesis), Rapamycin (Rapa, 5 μg per side; mTOR-dependent protein synthesis inhibitor) and DRB (8 ng per side; inhibitor of gene expression) immediately after the extinction training session.
The doses used were chosen based on previous studies reporting their efficacy (14, 15, 62, 63). For the drug infusions, a 10-μL Hamilton syringe was connected through a polyethylene tube to an infusion needle, and 1 μL (at a rate of 0.5 μL/30 s) was bilaterally infused into the CA1 region of the dorsal hippocampus or into the vmPFC. Control groups received equal volumes of sterile saline (0.9%). At the end, the infusion needle was left in place for an additional 60 s to prevent backflow and was then withdrawn, placed on the other side, and the procedure was repeated.
Statistical Analysis.
Statistical analysis was performed using GraphPad Prism software. Data were analyzed by one-way ANOVA followed by the Newman–Keuls test or by two-way ANOVA followed by the Bonferroni test and presented as mean ± SEM. For all data, the values of P < 0.05 were considered statistically significant.
Acknowledgments
This work was supported by research grants from the National Council of Research of Brazil (CNPq), the Brazilian Agency for Graduate Studies (CAPES), and the State Foundation for Science Support (FAPERGS).
Footnotes
The authors declare no conflict of interest.
References
- 1.Heim C, Nemeroff CB. Neurobiology of posttraumatic stress disorder. CNS Spectr. 2009;14(Suppl 1):13–24. [PubMed] [Google Scholar]
- 2.Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: Ten years of progress. Annu Rev Psychol. 2012;63:129–151. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Milad MR, Rosenbaum BL, Simon NM. Neuroscience of fear extinction: Implications for assessment and treatment of fear-based and anxiety related disorders. Behav Res Ther. 2014;62:17–23. doi: 10.1016/j.brat.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Goshen I, et al. Dynamics of retrieval strategies for remote memories. Cell. 2011;147:678–689. doi: 10.1016/j.cell.2011.09.033. [DOI] [PubMed] [Google Scholar]
- 5.Cowansage KK, et al. Direct reactivation of a coherent neocortical memory of context. Neuron. 2014;84:432–441. doi: 10.1016/j.neuron.2014.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tanaka KZ, et al. Cortical representations are reinstated by the hippocampus during memory retrieval. Neuron. 2014;84:347–354. doi: 10.1016/j.neuron.2014.09.037. [DOI] [PubMed] [Google Scholar]
- 7.Izquierdo I, Furini CRG, Myskiw JC. Fear memory. Physiol Rev. 2016;96:695–750. doi: 10.1152/physrev.00018.2015. [DOI] [PubMed] [Google Scholar]
- 8.Fiorenza NG, Rosa J, Izquierdo I, Myskiw JC. Modulation of the extinction of two different fear-motivated tasks in three distinct brain areas. Behav Brain Res. 2012;232:210–216. doi: 10.1016/j.bbr.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 9.Maren S, Holmes A. Stress and fear extinction. Neuropsychopharmacology. 2016;41:58–79. doi: 10.1038/npp.2015.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McLean CP, Foa EB. Prolonged exposure therapy for post-traumatic stress disorder: A review of evidence and dissemination. Expert Rev Neurother. 2011;11:1151–1163. doi: 10.1586/ern.11.94. [DOI] [PubMed] [Google Scholar]
- 11.Milad MR, Rauch SL, Pitman RK, Quirk GJ. Fear extinction in rats: Implications for human brain imaging and anxiety disorders. Biol Psychol. 2006;73:61–71. doi: 10.1016/j.biopsycho.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 12.Chang C, et al. Fear extinction in rodents. Curr Protoc Neurosci. 2009;Chapter 8:Unit8.23. doi: 10.1002/0471142301.ns0823s47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LeDoux JE. Coming to terms with fear. Proc Natl Acad Sci USA. 2014;111:2871–2878. doi: 10.1073/pnas.1400335111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de Carvalho Myskiw J, Furini CRG, Benetti F, Izquierdo I. Hippocampal molecular mechanisms involved in the enhancement of fear extinction caused by exposure to novelty. Proc Natl Acad Sci USA. 2014;111:4572–4577. doi: 10.1073/pnas.1400423111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Carvalho Myskiw J, Benetti F, Izquierdo I. Behavioral tagging of extinction learning. Proc Natl Acad Sci USA. 2013;110:1071–1076. doi: 10.1073/pnas.1220875110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Carvalho Myskiw J, Furini CRG, Schmidt B, Ferreira F, Izquierdo I. Extinction learning, which consists of the inhibition of retrieval, can be learned without retrieval. Proc Natl Acad Sci USA. 2015;112:E230–E233. doi: 10.1073/pnas.1423465112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vianna MR, Szapiro G, McGaugh JL, Medina JH, Izquierdo I. Retrieval of memory for fear-motivated training initiates extinction requiring protein synthesis in the rat hippocampus. Proc Natl Acad Sci USA. 2001;98:12251–12254. doi: 10.1073/pnas.211433298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kitamura T, et al. Engrams and circuits crucial for systems consolidation of a memory. Science. 2017;356:73–78. doi: 10.1126/science.aam6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nader K, Schafe GE, Le Doux JE. Fear memories require protein synthesis in the amygdala for reconsolidation after retrieval. Nature. 2000;406:722–726. doi: 10.1038/35021052. [DOI] [PubMed] [Google Scholar]
- 20.Lin C-H, et al. Identification of calcineurin as a key signal in the extinction of fear memory. J Neurosci. 2003;23:1574–1579. doi: 10.1523/JNEUROSCI.23-05-01574.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brill-Maoz N, Maroun M. Extinction of fear is facilitated by social presence: Synergism with prefrontal oxytocin. Psychoneuroendocrinology. 2016;66:75–81. doi: 10.1016/j.psyneuen.2016.01.003. [DOI] [PubMed] [Google Scholar]
- 22.Mikami K, Kiyokawa Y, Takeuchi Y, Mori Y. Social buffering enhances extinction of conditioned fear responses in male rats. Physiol Behav. 2016;163:123–128. doi: 10.1016/j.physbeh.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Hall DA. The reaction between elastase and elastic tissue. 1. The substrate. Biochem J. 1955;59:459–465. doi: 10.1042/bj0590459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baum WM, Rachlin HC. Choice as time allocation. J Exp Anal Behav. 1969;12:861–874. doi: 10.1901/jeab.1969.12-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davitz JR, Mason DJ. Socially facilitated reduction of a fear response in rats. J Comp Physiol Psychol. 1955;48:149–151. doi: 10.1037/h0046411. [DOI] [PubMed] [Google Scholar]
- 26.Kiyokawa Y, Takeuchi Y, Nishihara M, Mori Y. Main olfactory system mediates social buffering of conditioned fear responses in male rats. Eur J Neurosci. 2009;29:777–785. doi: 10.1111/j.1460-9568.2009.06618.x. [DOI] [PubMed] [Google Scholar]
- 27.Kiyokawa Y, Wakabayashi Y, Takeuchi Y, Mori Y. The neural pathway underlying social buffering of conditioned fear responses in male rats. Eur J Neurosci. 2012;36:3429–3437. doi: 10.1111/j.1460-9568.2012.08257.x. [DOI] [PubMed] [Google Scholar]
- 28.Kiyokawa Y, Takeuchi Y, Mori Y. Two types of social buffering differentially mitigate conditioned fear responses. Eur J Neurosci. 2007;26:3606–3613. doi: 10.1111/j.1460-9568.2007.05969.x. [DOI] [PubMed] [Google Scholar]
- 29.DeVries AC, Glasper ER, Detillion CE. Social modulation of stress responses. Physiol Behav. 2003;79:399–407. doi: 10.1016/s0031-9384(03)00152-5. [DOI] [PubMed] [Google Scholar]
- 30.Nakayasu T, Kato K. Is full physical contact necessary for buffering effects of pair housing on social stress in rats? Behav Processes. 2011;86:230–235. doi: 10.1016/j.beproc.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 31.Kiyokawa Y, Kikusui T, Takeuchi Y, Mori Y. Partner’s stress status influences social buffering effects in rats. Behav Neurosci. 2004;118:798–804. doi: 10.1037/0735-7044.118.4.798. [DOI] [PubMed] [Google Scholar]
- 32.Guzmán YF, Tronson NC, Guedea A, Huh KH, Gao C, Radulovic J. Social modeling of conditioned fear in mice by non-fearful conspecifics. Behav Brain Res. 2009;201:173–178. doi: 10.1016/j.bbr.2009.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cohen S, Wills TA. Stress, social support, and the buffering hypothesis. Psychol Bull. 1985;98:310–357. [PubMed] [Google Scholar]
- 34.Liu H, Yuan T-F. Physical interaction is required in social buffering induced by a familiar conspecific. Sci Rep. 2016;6:39788. doi: 10.1038/srep39788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kiyokawa Y, Takeuchi Y. Social buffering ameliorates conditioned fear responses in the presence of an auditory conditioned stimulus. Physiol Behav. 2017;168:34–40. doi: 10.1016/j.physbeh.2016.10.020. [DOI] [PubMed] [Google Scholar]
- 36.Beck JG, Grant DM, Clapp JD, Palyo SA. Understanding the interpersonal impact of trauma: Contributions of PTSD and depression. J Anxiety Disord. 2009;23:443–450. doi: 10.1016/j.janxdis.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kanitz E, Hameister T, Tuchscherer M, Tuchscherer A, Puppe B. Social support attenuates the adverse consequences of social deprivation stress in domestic piglets. Horm Behav. 2014;65:203–210. doi: 10.1016/j.yhbeh.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 38.Hennessy MB, Zate R, Maken DS. Social buffering of the cortisol response of adult female Guinea pigs. Physiol Behav. 2008;93:883–888. doi: 10.1016/j.physbeh.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 39.John ER, Chesler P, Bartlett F, Victor I. Observation learning in cats. Science. 1968;159:1489–1491. doi: 10.1126/science.159.3822.1489. [DOI] [PubMed] [Google Scholar]
- 40.Lyons DM, Price EO, Moberg GP. Social grouping tendencies and separation-induced distress in juvenile sheep and goats. Dev Psychobiol. 1993;26:251–259. doi: 10.1002/dev.420260503. [DOI] [PubMed] [Google Scholar]
- 41.Winslow JT, Noble PL, Lyons CK, Sterk SM, Insel TR. Rearing effects on cerebrospinal fluid oxytocin concentration and social buffering in rhesus monkeys. Neuropsychopharmacology. 2003;28:910–918. doi: 10.1038/sj.npp.1300128. [DOI] [PubMed] [Google Scholar]
- 42.Faustino AI, Tacão-Monteiro A, Oliveira RF. Mechanisms of social buffering of fear in zebrafish. Sci Rep. 2017;7:44329. doi: 10.1038/srep44329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klein B, et al. Activation of the mouse odorant receptor 37 subsystem coincides with a reduction of novel environment-induced activity within the paraventricular nucleus of the hypothalamus. Eur J Neurosci. 2015;41:793–801. doi: 10.1111/ejn.12838. [DOI] [PubMed] [Google Scholar]
- 44.Ishii A, Kiyokawa Y, Takeuchi Y, Mori Y. Social buffering ameliorates conditioned fear responses in female rats. Horm Behav. 2016;81:53–58. doi: 10.1016/j.yhbeh.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 45.Lee H, Noh J. Pair exposure with conspecific during fear conditioning induces the link between freezing and passive avoidance behaviors in rats. Neurosci Res. 2016;108:40–45. doi: 10.1016/j.neures.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 46.Kiyokawa Y, Honda A, Takeuchi Y, Mori Y. A familiar conspecific is more effective than an unfamiliar conspecific for social buffering of conditioned fear responses in male rats. Behav Brain Res. 2014;267:189–193. doi: 10.1016/j.bbr.2014.03.043. [DOI] [PubMed] [Google Scholar]
- 47.Fuzzo F, et al. Social buffering suppresses fear-associated activation of the lateral amygdala in male rats: Behavioral and neurophysiological evidence. Front Neurosci. 2015;9:99. doi: 10.3389/fnins.2015.00099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guzmán YF, et al. Role of oxytocin receptors in modulation of fear by social memory. Psychopharmacology (Berl) 2014;231:2097–2105. doi: 10.1007/s00213-013-3356-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bredy TW, Barad M. Social modulation of associative fear learning by pheromone communication. Learn Mem. 2008;16:12–18. doi: 10.1101/lm.1226009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Santini E, Muller RU, Quirk GJ. Consolidation of extinction learning involves transfer from NMDA-independent to NMDA-dependent memory. J Neurosci. 2001;21:9009–9017. doi: 10.1523/JNEUROSCI.21-22-09009.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Santini E, Quirk GJ, Porter JT. Fear conditioning and extinction differentially modify the intrinsic excitability of infralimbic neurons. J Neurosci. 2008;28:4028–4036. doi: 10.1523/JNEUROSCI.2623-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Santini E, Sepulveda-Orengo M, Porter JT. Muscarinic receptors modulate the intrinsic excitability of infralimbic neurons and consolidation of fear extinction. Neuropsychopharmacology. 2012;37:2047–2056. doi: 10.1038/npp.2012.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Do-Monte FH, Manzano-Nieves G, Quiñones-Laracuente K, Ramos-Medina L, Quirk GJ. Revisiting the role of infralimbic cortex in fear extinction with optogenetics. J Neurosci. 2015;35:3607–3615. doi: 10.1523/JNEUROSCI.3137-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology. 2011;36:529–538. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vianna MR, Igaz LM, Coitinho AS, Medina JH, Izquierdo I. Memory extinction requires gene expression in rat hippocampus. Neurobiol Learn Mem. 2003;79:199–203. doi: 10.1016/s1074-7427(03)00003-0. [DOI] [PubMed] [Google Scholar]
- 56.Mamiya N, et al. Brain region-specific gene expression activation required for reconsolidation and extinction of contextual fear memory. J Neurosci. 2009;29:402–413. doi: 10.1523/JNEUROSCI.4639-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- 58.Santini E, Ge H, Ren K, Peña de Ortiz S, Quirk GJ. Consolidation of fear extinction requires protein synthesis in the medial prefrontal cortex. J Neurosci. 2004;24:5704–5710. doi: 10.1523/JNEUROSCI.0786-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vianna MR, Coitinho AS, Izquierdo I. Role of the hippocampus and amygdala in the extinction of fear-motivated learning. Curr Neurovasc Res. 2004;1:55–60. doi: 10.2174/1567202043480170. [DOI] [PubMed] [Google Scholar]
- 60.Santangelo V, et al. Enhanced brain activity associated with memory access in highly superior autobiographical memory. Proc Natl Acad Sci USA. 2018;115:7795–7800. doi: 10.1073/pnas.1802730115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; New York: 1998. [DOI] [PubMed] [Google Scholar]
- 62.Igaz LM, Vianna MRM, Medina JH, Izquierdo I. Two time periods of hippocampal mRNA synthesis are required for memory consolidation of fear-motivated learning. J Neurosci. 2002;22:6781–6789. doi: 10.1523/JNEUROSCI.22-15-06781.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Myskiw JC, et al. On the participation of mTOR in recognition memory. Neurobiol Learn Mem. 2008;89:338–351. doi: 10.1016/j.nlm.2007.10.002. [DOI] [PubMed] [Google Scholar]