The ability to store surplus calories as fat for use during fasting or periods of increased energy demands is a fundamental process required for survival of all species. Fat consumed in the diet is incorporated into triglyceride-rich lipoproteins (chylomicrons) in the intestine for delivery to peripheral tissues. The liver also secretes triglyceride-rich very low-density lipoproteins (VLDLs). Chylomicrons and VLDL triglycerides are hydrolyzed within capillaries by lipoprotein lipase (LPL) (1), releasing lipids for storage and utilization by vital tissues. The processing of lipoproteins by LPL has been studied intensively for more than a half century and has proved highly relevant to clinical medicine. A complete deficiency of LPL results in an inability to hydrolyze the triglycerides in chylomicrons and VLDLs, resulting in severe hypertriglyceridemia (chylomicronemia) and a substantial risk of acute pancreatitis (2). Partial deficiencies in LPL cause mild to moderate hypertriglyceridemia and increased risk for coronary artery disease (3, 4).
The ability of LPL to hydrolyze plasma triglycerides is absolutely dependent on glycosylphosphatidylinositol (GPI)-anchored high-density lipoprotein–binding protein 1 (GPIHBP1), a GPI-anchored endothelial cell protein that binds LPL and tethers it to the luminal surface of capillaries (1). GPIHBP1 deficiency severely impairs triglyceride processing and causes severe hypertriglyceridemia (indistinguishable from that caused by LPL deficiency) (5, 6). Despite intensive research, structures for LPL and GPIHBP1 have been elusive. However, in PNAS, Birrane et al. (7) report the crystal structure of the LPL–GPIHBP1 complex. This accomplishment culminates a decade of collaborative studies by Stephen G. Young and collaborators that have focused on defining GPIHBP1 function in triglyceride metabolism.
LPL activity was first reported by Hahn (8) in 1943, who described the appearance of a lipemia clearing factor in dogs after an injection of heparin. The activity of LPL is regulated by apolipoproteins on the surface of lipoproteins (most notably apo-CII and apo-AV) and by angiopoietin-like protein (ANGPTL)3, ANGPTL4, and ANGPTL8 (9). LPL is synthesized and secreted primarily by adipocytes and myocytes, and LPL secretion is widely assumed to require the formation of LPL homodimers, with two LPL monomers oriented in a head-to-tail fashion (1). While adipocytes and myocytes are the main source of LPL, the vast majority of LPL activity resides on capillary endothelial cells. How LPL reaches the capillary lumen represented a fundamental issue in LPL physiology for many years but was largely ignored. Ultimately, however, the mechanism by which LPL reaches the capillary lumen was clarified by examining the function of GPIHBP1.
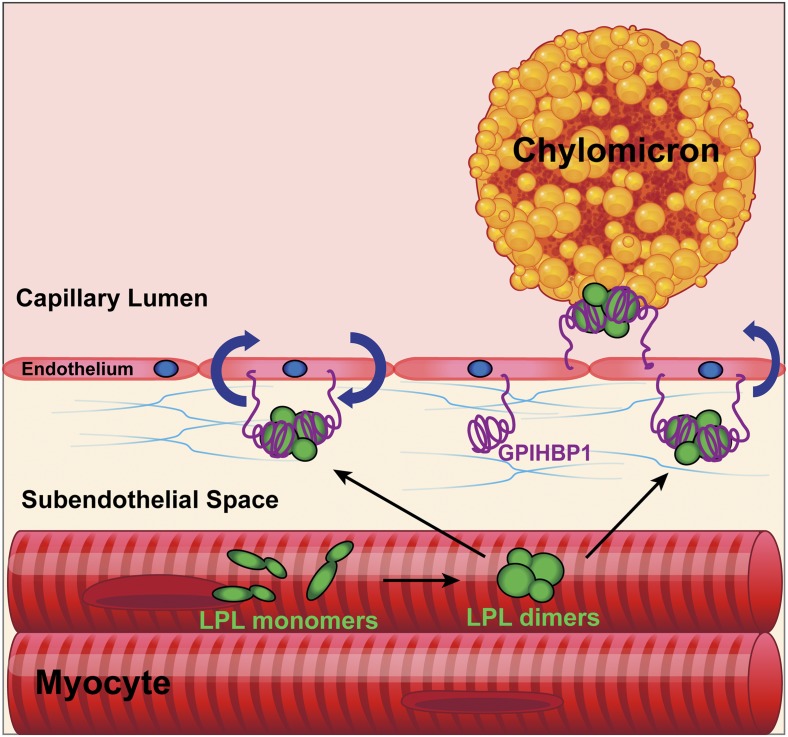
GPIHBP1 was initially cloned in 2003 and belongs to the lymphocyte antigen 6/urokinase-type plasminogen activator receptor (Ly6/uPAR; LU) superfamily, of which there are 35 members in humans (6, 10, 11). GPIHBP1’s function in triglyceride metabolism was first noted by Beigneux et al. (6) in 2007 while studying GPIHBP1-knockout mice. The knockout mice exhibited plasma triglyceride levels >5,000 mg/dL while consuming a chow diet (a dramatic phenotype that could be explained only by defective LPL function). A series of studies conducted by Young, Beigneux, Fong, Ploug, and colleagues (6, 12–14) clarified the mechanism by which GPIHBP1 affects LPL activity. These researchers showed that GPIHBP1 is expressed exclusively in endothelial cells and that it binds LPL with high affinity and then transports LPL to the capillary lumen (Fig. 1) (6, 12). They also showed that the LPL–GPIHBP1 complex on endothelial cells is essential for the docking of lipoproteins, allowing LPL-mediated triglyceride hydrolysis to proceed (13). Finally, and most importantly for the realization of the current report (7), they established that GPIHBP1 stabilizes LPL’s structure and preserves its catalytic activity (14).
Fig. 1.
LPL and GPIHBP1 itinerary and function. LPL is synthesized by parenchymal cells as dimers that are secreted into the subendothelial space where one GPIHBP1 molecule on the surface of the endothelial cell binds one molecule of LPL. The LPL–GPIHBP1 complex is then moved across the cell in vesicles to the capillary lumen. GPIHBP1 anchors LPL to the endothelial cell and is required for margination of chylomicrons and VLDL particles, which permits the hydrolysis of triglycerides by LPL.
GPIHBP1 has three important domains. The first is a disordered acidic domain in which 21 of 26 amino acids are aspartates or glutamates. The second is a three-fingered LU domain containing 10 cysteines, all disulfide bonded. The third is a carboxyl-terminal signal sequence that triggers the addition of a GPI anchor (1). Early studies revealed that GPIHBP1’s LU domain is critical for LPL binding (15), with the acidic domain playing only an accessory role in LPL binding (14). Interestingly, however, GPIHBP1’s acidic domain plays a crucial role in stabilizing LPL structure (14). Purified preparations of LPL lose catalytic activity within minutes, a consequence of spontaneous unfolding of LPL’s hydrolase domain. This instability of LPL has foiled previous efforts to obtain a crystal structure. The recognition that GPIHBP1, and in particular GPIHBP1’s acidic domain, prevents LPL unfolding raised the possibility that recombinant GPIHBP1 might be the key to successful X-ray crystallography studies. As shown by the studies of Birrane et al. (7), this proved to be the case!
The structure of the LPL–GPIHBP1 complex by Birrane et al. (7) reveals that one GPIHBP1 molecule binds to each LPL molecule. One entire side of GPIHBP1’s LU domain binds to LPL, with binding mediated almost exclusively by hydrophobic contacts. An improved understanding of the LPL–GPIHBP1 binding interface represents an important step forward inasmuch as site-directed mutagenesis studies of LPL–GPIHBP1 interactions were complicated by the complex effects they had on protein conformation and protein–protein interactions.
Consistent with prevailing dogma holding that LPL is a homodimer, the LPL–GPIHBP1 crystal (7) revealed two LPL molecules oriented in a head-to-tail orientation. While the structure would seemingly solidify the idea that LPL is a homodimer, caution is warranted. The crystal structure showed that the two LPL monomers interact, in a reciprocal fashion, between a hydrophobic lipoprotein-binding motif in the carboxyl-terminal domain of one LPL monomer and the catalytic pocket in the amino-terminal domain of the partner monomer. It seems somewhat unlikely that the LPL–LPL interactions observed in the crystal structure would be compatible with either lipoprotein binding or triglyceride hydrolysis, but it is still quite possible that those interactions are physiologically relevant when triglyceride-rich lipoproteins are absent, such as in the subendothelial spaces (7). Another member of the lipase family, pancreatic lipase, was previously crystallized and has many structural similarities to LPL, but pancreatic lipase functions as a monomer (16). Additional studies will be needed to determine whether catalytically active LPL is truly in the form of homodimers and, if so, whether the conformation of active homodimers resembles those in the present crystal structure.
In PNAS, Birrane et al. report the crystal structure of the LPL–GPIHBP1 complex. This accomplishment culminates a decade of collaborative studies by Stephen G. Young and collaborators that have focused on defining GPIHBP1 function in triglyceride metabolism.
As noted earlier, intravascular triglyceride hydrolysis is important for the delivery of lipids to tissues, and defects in triglyceride hydrolysis cause chylomicronemia. The LPL–GPIHBP1 crystal structure (7) provided the molecular mechanisms by which LPL missense mutations cause chylomicronemia: One mutation introduced a polar residue into the hydrophobic LPL–GPIHBP1 binding interface, abolishing LPL’s ability to bind GPIHBP1; the second eliminated a residue involved in coordinating LPL’s calcium ion. Birrane et al. (7) show that mutations in calcium-coordinating residues abolish LPL secretion from cells. These studies define the mechanisms for two mutations, but they only scratch the surface in understanding the dozens of other LPL and GPIHBP1 mutations associated with chylomicronemia. It seems highly likely, however, that the LPL–GPIHBP1 structure, combined with simple laboratory studies, will make it possible to define mechanisms for the vast majority of clinically important LPL and GPIHBP1 mutations.
Seldom can a structure answer all the issues regarding a protein’s function, and this is also the case for the LPL–GPIHBP1 complex. First, GPIHBP1’s highly disordered acidic domain was not visualized in the crystal structure but appeared to be positioned to project across, and interact with, a large basic patch in LPL. It would be desirable to better define the interactions of the acidic domain with LPL and elucidate how it protects LPL from unfolding. Second, apo-CII and apo-AV, small apolipoproteins carried by triglyceride-rich lipoproteins, activate LPL-mediated triglyceride hydrolysis, but how they influence LPL activity remains largely unknown—even with the LPL crystal structure in hand. Third, the crystal structure does not provide immediate insight into how ANGPTL3, ANGPTL4, and ANGPTL8 inhibit LPL activity (9, 17). The ANGPTLs inhibit LPL activity, and deficiencies in these proteins cause abnormally low plasma lipid levels (18). Mysling et al. (19) demonstrated that ANGPTL4 catalyzes the unfolding of LPL’s hydrolase domain, but how ANGPTLs interact with LPL to catalyze LPL unfolding is unknown. A detailed understanding of how ANGPTLs inactivate LPL is not only an interesting biological question, but is one that could lead to the development of drugs to enhance triglyceride hydrolysis and lower plasma lipid levels.
Despite the remaining questions detailed above, solving the crystallographic structure for two critical proteins in plasma triglyceride metabolism represents a huge step forward. There is no doubt that this structure will now serve as a roadmap to further clarify how amino acid changes in LPL and/or GPIHBP1 influence intravascular triglyceride hydrolysis, and it will also likely serve as a nidus to ultimately define the mechanisms by which apolipoproteins and ANGPTLs regulate LPL activity.
Footnotes
The author declares no conflict of interest.
See companion article on page 1723.
References
- 1.Fong LG, et al. GPIHBP1 and plasma triglyceride metabolism. Trends Endocrinol Metab. 2016;27:455–469. doi: 10.1016/j.tem.2016.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gehrisch S. Common mutations of the lipoprotein lipase gene and their clinical significance. Curr Atheroscler Rep. 1999;1:70–78. doi: 10.1007/s11883-999-0052-4. [DOI] [PubMed] [Google Scholar]
- 3.Dallinga-Thie GM, Kroon J, Borén J, Chapman MJ. Triglyceride-rich lipoproteins and remnants: Targets for therapy? Curr Cardiol Rep. 2016;18:67. doi: 10.1007/s11886-016-0745-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khera AV, et al. Myocardial Infarction Genetics Consortium, DiscovEHR Study Group, CARDIoGRAM Exome Consortium, and Global Lipids Genetics Consortium Association of rare and common variation in the lipoprotein lipase gene with coronary artery disease. JAMA. 2017;317:937–946. doi: 10.1001/jama.2017.0972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beigneux AP, et al. Chylomicronemia with a mutant GPIHBP1 (Q115P) that cannot bind lipoprotein lipase. Arterioscler Thromb Vasc Biol. 2009;29:956–962. doi: 10.1161/ATVBAHA.109.186577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beigneux AP, et al. Glycosylphosphatidylinositol-anchored high-density lipoprotein-binding protein 1 plays a critical role in the lipolytic processing of chylomicrons. Cell Metab. 2007;5:279–291. doi: 10.1016/j.cmet.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Birrane G, et al. Structure of the lipoprotein lipase–GPIHBP1 complex that mediates plasma triglyceride hydrolysis. Proc Natl Acad Sci USA. 2019;116:1723–1732. doi: 10.1073/pnas.1817984116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hahn PF. Abolishment of alimentary lipemia following injection of heparin. Science. 1943;98:19–20. doi: 10.1126/science.98.2531.19. [DOI] [PubMed] [Google Scholar]
- 9.Kersten S. Physiological regulation of lipoprotein lipase. Biochim Biophys Acta. 2014;1841:919–933. doi: 10.1016/j.bbalip.2014.03.013. [DOI] [PubMed] [Google Scholar]
- 10.Ioka RX, et al. Expression cloning and characterization of a novel glycosylphosphatidylinositol-anchored high density lipoprotein-binding protein, GPI-HBP1. J Biol Chem. 2003;278:7344–7349. doi: 10.1074/jbc.M211932200. [DOI] [PubMed] [Google Scholar]
- 11.Loughner CL, et al. Organization, evolution and functions of the human and mouse Ly6/uPAR family genes. Hum Genomics. 2016;10:10. doi: 10.1186/s40246-016-0074-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davies BS, et al. GPIHBP1 is responsible for the entry of lipoprotein lipase into capillaries. Cell Metab. 2010;12:42–52. doi: 10.1016/j.cmet.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goulbourne CN, et al. The GPIHBP1-LPL complex is responsible for the margination of triglyceride-rich lipoproteins in capillaries. Cell Metab. 2014;19:849–860. doi: 10.1016/j.cmet.2014.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mysling S, et al. The acidic domain of the endothelial membrane protein GPIHBP1 stabilizes lipoprotein lipase activity by preventing unfolding of its catalytic domain. eLife. 2016;5:e12095. doi: 10.7554/eLife.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beigneux AP, et al. Highly conserved cysteines within the Ly6 domain of GPIHBP1 are crucial for the binding of lipoprotein lipase. J Biol Chem. 2009;284:30240–30247. doi: 10.1074/jbc.M109.046391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Tilbeurgh H, Sarda L, Verger R, Cambillau C. Structure of the pancreatic lipase-procolipase complex. Nature. 1992;359:159–162. doi: 10.1038/359159a0. [DOI] [PubMed] [Google Scholar]
- 17.Kersten S. Angiopoietin-like 3 in lipoprotein metabolism. Nat Rev Endocrinol. 2017;13:731–739. doi: 10.1038/nrendo.2017.119. [DOI] [PubMed] [Google Scholar]
- 18.Romeo S, et al. Rare loss-of-function mutations in ANGPTL family members contribute to plasma triglyceride levels in humans. J Clin Invest. 2009;119:70–79. doi: 10.1172/JCI37118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mysling S, et al. The angiopoietin-like protein ANGPTL4 catalyzes unfolding of the hydrolase domain in lipoprotein lipase and the endothelial membrane protein GPIHBP1 counteracts this unfolding. eLife. 2016;5:e20958. doi: 10.7554/eLife.20958. [DOI] [PMC free article] [PubMed] [Google Scholar]